ABSTRACT
The objective of this study was to establish a real-time LAMP assay for authentication of rape (Brassica napus) honey to protect consumers from commercial honey adulteration. The LAMP primers targeting the internal transcribed spacer (ITS) of Brassica napus were designed, and its specificity was tested. The LAMP reaction temperature was also optimized, and the detection limit of the LAMP assay was determined with a serial dilution of genomic DNA from the seeds of Brassica napus. The results showed that the real-time LAMP assay can accurately and specifically detect the rape component in honey, and the detection limit was 10 pg genomic DNA of Brassica napus. Data on monofloral honey samples indicate that the real-time LAMP assay was 100% in concordance with the reported TaqManTM PCR assay. This study provides a promising solution for facilitating the authentication of rape honey in food retail market.
RESUMEN
El presente estudio se propuso establecer un ensayo LAMP en tiempo real destinado a autenticar la miel de colza (Brassica napus) para proteger a los consumidores de la adulteración comercial de la miel. Con esta finalidad se diseñaron cebadores LAMP dirigidos al espaciador transcrito interno (ITS) de Brassica napus y se probó su especificidad. Además, se optimizó la temperatura de reacción del LAMP, determinándose el límite de detección del ensayo LAMP con una dilución en serie de ADN genómico de las semillas de Brassica napus. Los resultados mostraron que dicho ensayo en tiempo real puede detectar con precisión y especificidad el componente de colza en la miel; el límite de detección fue de 10 pg de ADN genómico de Brassica napus. Los datos obtenidos de las muestras de miel monofloral dan cuenta de que el ensayo LAMP en tiempo real concordaba al 100% con el ensayo de PCR TaqManTM notificado. Este estudio ofrece una solución prometedora para facilitar la autenticación de la miel de colza en el mercado minorista de alimentos.
1. Introduction
Honey is a natural sweet substance that is highly welcomed by consumers. The taste, nutritional value and its health benefits make honey a competitive nutritional food on the market (Soares et al., Citation2017); therefore, honey has been a target of food adulteration through mixing with low-priced and low quality honey, as well as sugar syrup (Lara et al., Citation2019). Thus, there has been growing interest in developing methods for the authentication of honey and detection of honey adulterants.
Most of the current studies focus on two aspects: one is the identification of floral honey origin markers, including chiral volatile compounds (e.g., linalool, γ-Nonalactone, farnesol and acetovanillone) (Castro-Vázquez et al., Citation2014), succinate and kynurate (Consonni et al., Citation2019), specific gene fragments (Soares et al., Citation2019), proteins (allergens, venom-like proteins, antibacterial properties, royal jelly proteins, serine proteases, floral nectar chitinase) (Song et al., Citation2019), free amino acids (Azevedo et al., Citation2017), 3-phenyllactic acid, 2′-methoxyacetophenone, 2-methoxybenzoic acid and 4-hydroxyphenyllacetic acid (Burns et al., Citation2018), 5-hydroxymethylfurfural (Turkut et al., Citation2018). The other is the determination of honey adulterants such as starch syrup, inverted syrup, starch or inverted syrup fed to bees, and low-quality honey added to high-priced honey (Naila et al., Citation2018).
Methods used to identify targeted markers of adulteration/authenticity include automatic pulse voltammetric electronic tongue (Sobrino-Gregorio et al., Citation2018), NMR-based metabolomic approach (Consonni et al., Citation2019), rapid evaporative ionization mass spectrometry (H. Wang et al., Citation2019), UPLC-QToF MS (Jandrić et al., Citation2017), ICP-MS/ICP-OES (Enrique et al., Citation2018), SPME-GC-MS (Verzera et al., Citation2014), GC-MS combined with a chemometric approach (Azevedo et al., Citation2017), GC-IMS and FT-MIR (Schwolow et al., Citation2019), LC-MS/MS (Burns et al., Citation2018), FTIR-ATR spectroscopy (Kasprzyk et al., Citation2018), NIR spectroscopy (Guelpa et al., Citation2017), comprehensive proteomic analysis (Erban et al., Citation2019), semiconductor based sequencing (Utzeri et al., Citation2018), DNA-barcoding approach (Soares et al., Citation2018), conventional and real-time PCR (Laube et al., Citation2010; Soares et al., Citation2019), and other advanced molecular, chromatographic, and spectroscopic analytical methods have been established with their own pros and cons (Naila et al., Citation2018). PCR-based methods offer reduced time of analysis and increased level of species discrimination, and hence represent suitable alternatives for authentication of honey and detection of honey adulterants (Soares et al., Citation2017). However, PCR methods are limited by the presence of PCR inhibitors in real biological samples and food samples, and honey is a very complex matrix, mainly composed of different sugars and other substances, such as organic acids, polyphenols, pigments, enzymes, and solid particles such as waxes, which may interfere with PCR reactions (Soares et al., Citation2017). Therefore, it is necessary to develop robust DNA-based methods for botanical honey identification.
Loop-mediated isothermal amplification (LAMP) has advantages over PCR in terms of specificity, sensitivity, rapidity, and cost-effectiveness (Notomi et al., Citation2000). LAMP technology have been widely used to identify meat species (Spielmann et al., Citation2019), to detect foodborne pathogens and viruses (Feng et al., Citation2018; Mei et al., Citation2019), and to verify GMO components (Singh et al., Citation2019). Nevertheless, there is no reported LAMP assay for authentication of floral honey and identification of honey adulterants. The objective of this study was to develop a robust real-time LAMP assay for the authentication of rape honey, to provide a reference for verification of monofloral honey, and to protect consumers from commercial honey adulteration.
2. Materials and methods
2.1. Primer design for LAMP assays
Six LAMP primers targeting the internal transcribed spacer (ITS) of Brassica napus (GenBank accession No. KM892645.1) were designed using PrimerExplorer V5 (http://primerexplorer.jp/e/) and Oligo 7 (Molecular Biology Insights, Inc. Colorado Springs, CO, USA) (D. Wang et al., Citation2019); the TaqMan TM PCR primers for the detection of the Brassica napus lipase gene in honey were the same as those reported by Laube et al. (Citation2010), and all the primer sequences are listed in .
Table 1. The Primer sequences of the established real-time LAMP assay and reported TaqManTM PCR assay.
Tabla 1. Secuencias del cebador del ensayo LAMP en tiempo real y el ensayo PCR TaqManTM reportado
2.2. Genomic DNA extraction
Genomic DNA was extracted from plant seeds and used for temperature optimization and specificity determination. Genomic DNA from seeds of Brassica napus, Ziziphus jujuba, Robinia pseudoacacia, Tilia tuan, Vitex negundo, Zea mays, Oryza sativa, Manihot esculenta, and Beta vulgaris was extracted using the Botanical Genomic DNA Extraction Kit (Tiangen Biotech (Beijing) Co., Ltd, Beijing, China). The rape (Brassica napus) honey, jujube (Ziziphus jujuba) honey, acacia honey (Robinia pseudoacacia), linden honey (Tilia tuan) and chaste honey (Vitex negundo) were the most common monofloral honey products on the retail market, corn (Zea mays) syrup, rice (Oryza sativa) syrup, cassava (Manihot esculenta) syrup and beet (Beta vulgaris) syrup are the most common adulterants frequently added to honey. The seeds were homogenized using a mortar and pestle, and the genomic DNA was extracted according to the manufacturer’s instructions.
Genomic DNA from the honey samples was extracted by the CTAB-based method modified based on previous reports (Kek et al., Citation2018). Briefly, 10 mL of honey sample and 35 mL of deionized water were mixed in a 50-mL tube and incubated at 65°C to reach full dissolution, and then centrifuged at 2656 × g for 30 min. After supernatant removal, the pellets were dissolved in 1 mL of deionized water and transferred to a 2-mL tube, and then centrifuged at 10625 × g for 10 min. The pellets were dissolved in 1 mL of 3% cetyltrimethylammonium bromide (CTAB) buffer containing 0.3% β-mercaptoethanol, incubated at 65°C for 90 min with intermittent shaking, and then centrifuged at 10625 × g for 15 min. The aqueous phase (700 μL) was mixed with an equal volume of phenol: chloroform: isoamylalchohol (25:24:1), and then centrifuged at 10625 × g for 15 min. The aqueous phase (400 μL) was mixed with 2X volume of ethanol (800 μL), precipitated at −20°C for 2 h, and centrifuged at 10625 × g for 15 min. The pellets were washed with 75% ethanol, air-dried, and dissolved in nuclease free water or Tris-EDTA (10:1) buffer until further use.
2.3. Optimization of the real-time LAMP reaction temperature
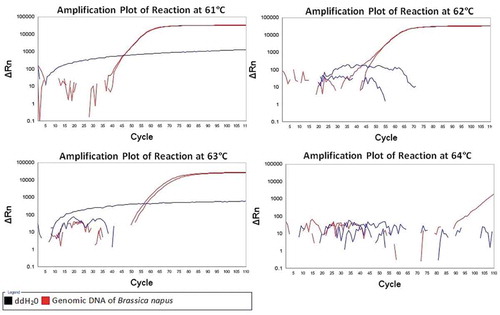
The real-time LAMP assay was performed in a 10-μL reaction mixture containing 0.8 mM each of forward inner primer (FIP) and backward inner primer (BIP), 0.2 mM each of forward outer primer (F3) and backward outer primer (B3), 0.4 mM of forward loop primer (LF) and backward loop primer (LB), 1.0 mM dNTPs, 20 mM Tris-HCl (pH 8.8), 10 mM KCl, 10 mM (NH4)2SO4, 6 mM MgSO4, 0.1% Triton X-100, 7.5% DMSO, 1 × EvaGreen, 1 × Rox, 1 ng DNA template of Brassica napus and 3.2 U Bst 2.0 WarmStart DNA polymerase (New England Biolabs, Beverly, Mass., USA.) (D. Wang et al., Citation2019). The reaction mixture was heated at 61°C, 62°C, 63°C, and 64°C for 60 min (30 s per cycle) using a StepOneTM System (Applied Biosystems, Foster City, CA, USA).
2.4. Specificity determination of the real-time LAMP assay
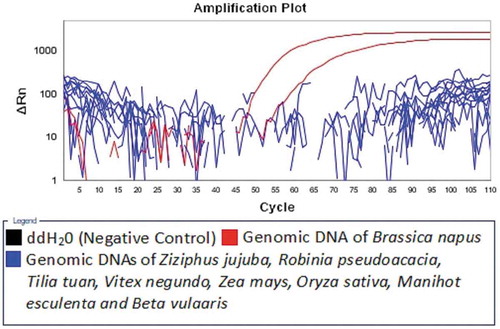
The genomic DNAs from seeds of Brassica napus, Ziziphus jujuba, Robinia pseudoacacia, Tilia tuan, Vitex negundo, Zea mays, Oryza sativa, Manihot esculenta, and Beta vulgaris were used to determine the specificity of the newly established real-time LAMP assay, and the amount of genomic DNA template used was 1 ng per reaction.
2.5. Sensitivity determination of the real-time LAMP assay
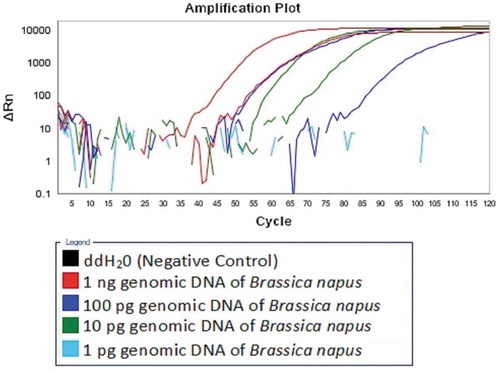
The genomic DNA extracted from the seeds of Brassica napus was tenfold diluted, 1ng/µL, 100 pg/µL, 10 pg/µL and 1 pg/µL, and the detection limit was determined using the above real-time LAMP assay.
2.6. Detection of brassica napus component in monofloral honey samples
A total of 50 monofloral honey samples (10 rape honey samples, 10 jujube honey samples, 10 acacia honey samples, 10 linden honey samples, and 10 chaste honey samples) were analyzed to detect Brassica napus component using our established real-time LAMP assay and compared to the previously reported TaqManTM PCR assay (Laube et al., Citation2010). The 10 µL reaction mixture of TaqManTM PCR contained 1× TaqManTM Buffer A, 1.5 units AmpliTaq Gold® Polymerase, and magnesium chloride buffer, 0.2 mM of each dNTP, forward and reverse primers, dual labelled fluorescent probe, and 2 µL of template DNA solution; and the thermal cycling protocol: 95°C for 10 min followed by 45 cycles of 95°C for 15 s and 60°C for 1 min (Laube et al., Citation2010).
3. Results and analysis
3.1. Optimization of the real-time LAMP reaction temperature
The real-time LAMP reaction with the newly designed primers was carried out at 61°C, 62°C, 63°C, and 64°C for 60 min. As shown in , all positive controls were positive and all the negative controls were negative at 61°C, 62°C and 63°C, while either positive controls or negative controls had no amplification at 64°C. Given the higher the reaction temperature, the less the primer dimers, and the higher specificity of the amplification, the relatively high temperature of 63°C was selected as the optimal temperature.
3.2. Specificity of the real-time LAMP assay
The specificity of the LAMP assay was tested with genomic DNAs extracted from the seeds of Brassica napus, Ziziphus jujuba, Robinia pseudoacacia, Tilia tuan, Vitex negundo, Zea mays, Oryza sativa, Manihot esculenta, and Beta vulgaris, two repeats per test. As shown in , the genomic DNAs extracted from the seeds of Brassica napus were successfully detected, while the genomic DNAs extracted from the seeds of the other species and the negative controls (ddH2O) were negative.
3.3. Sensitivity of the real-time LAMP assay
The sensitivity of the real-time LAMP assay was determined using the tenfold diluted genomic DNA extracted from the seeds of Brassica napus, with the reaction run at 63°C for 60 min. The detection limit was found to be 10 pg genomic DNA of Brassica napus (), and the established real-time LAMP assay was more sensitive than that of the reported TaqManTM PCR assay (Laube et al., Citation2010), 25 pg.
3.4. Detection of the brassica napus target in monofloral honey samples
A total of 50 monofloral honey samples (10 rape honey samples, 10 jujube honey samples, 10 acacia honey samples, 10 linden honey samples, and 10 chaste honey samples) were collected from the retail market of Xuchang City. The test was positive when the threshold of the established real-time LAMP was 1500 or the Ct of the TaqManTM PCR amplification less than 40 (Laube et al., Citation2010), as shown in , all of the 10 rape honey samples tested positive by both the established real-time LAMP assay and the previously reported TaqManTM PCR assay (Laube et al., Citation2010); of the 10 jujube honey samples, 3 samples tested by both methods contained Brassica napus components, and among the 10 acacia honey samples, 3 samples tested positive by our established real-time LAMP assay while 2 samples were positive by the reported TaqManTM PCR assay; of the 10 linden honey samples, 4 samples contained Brassica napus components by both assays, and all of the chaste honey samples tested negative both by the real-time LAMP assay and the reported TaqManTM PCR assay, indicating that mixing of low-priced honey (rape honey) to high-priced honey (jujube honey, acacia honey samples or linden honey) is common in the retail market.
Table 2. Detection of Brassica napus component in monofloral honey samples.
Tabla 2. Detección del componente Brassica napus en muestras de miel monofloral
4. Discussion
Honey has been the target of adulteration for its taste and nutritional value (Lara et al., Citation2019; Naila et al., Citation2018; Sobrino-Gregorio et al., Citation2018), and DNA-based methods are considered as the most reliable and accurate tools for the fraud detection due to the high stability of plant DNA molecules under environmental and production conditions (Soares et al., Citation2017). However, honey is a very complex matrix containing different sugars, organic acids, polyphenols, pigments, enzymes etc., which might interfere with DNA-based analysis (Soares et al., Citation2017), so the DNA extraction is the critical step for reliability and reproducibility. In this study, the CTAB-based method modified upon previous reports has been used to extract genomic DNA from the honey samples as well as the artificially adulterated honey samples (Kek et al., Citation2018). The genomic DNA extracted with the CTAB-based method has favorable performance as the template of real-time LAMP reaction, and the detection limit is 1% rape honey mixed into rice molasses (V/V).
The real-time LAMP assay for authentication of rape honey has been developed in this study, and the number of samples that are positive by the real-time LAMP assay is basically same as that of the reported TaqManTM PCR assay (Laube et al., Citation2010), but the developed real-time LAMP assay has the advantages in sensitivity and rapidity, and this paper has provided the proposal to authenticate other monofloral honeys with real-time LAMP assays.
The nuclear internal transcribed spacer (ITS), a tandem repeat sequence with high copy number in genome, is easily amplified and has high resolution, which has been widely applied in species identification and intraspecific phylogenetic analysis (Downie et al., Citation2000). Therefore, the internal transcribed spacer (ITS) of Brassica napus has been selected as the target sequence to design LAMP primers in the present study, and the LAMP primers can successfully distinguish the genomic DNA of Brassica napus from those of Ziziphus jujuba, Robinia pseudoacacia, Tilia tuan, Vitex negundo, Zea mays, Oryza sativa, Manihot esculenta, and Beta vulgaris.
The detection results of the various monofloral honey samples indicated that honey fraud with the addition of low-price rape honey is very common in the retail market, therefore, for the interest of consumers, there is an urgent need to establish reliable authentication methods for jujube honey, acacia honey, linden honey, chaste honey, and other monofloral honeys with the assay proposed in this paper.
5. Conclusion
A cost-effective, simple, specific, sensitive, real-time LAMP assay for authentication of rape honey was developed in this study, making it suitable for the surveillance of honey fraud in the retail market. However, the numbers of samples used in this study were limited, and thus, confidence in the results of the assay would be increased by verification with a larger set of honey samples.
Acknowledgments
This study was funded by the National Key Research and Development Program of China (2016YFD0500704-4) and Henan Science and Technology Plan Projects (182102110285 and 202102310468).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Azevedo, M. S., Seraglio, S. K. T., Rocha, G., Balderas, C. B., Piovezan, M., Gonzaga, L. V., Falkenberg, D. D. B., Fett, R., Oliveira, M. A. L. D., & Costa, A. C. O. (2017). Free amino acid determination by GC-MS combined with a chemometric approach for geographical classification of bracatinga honeydew honey (mimosa scabrella, bentham). Food Control, 78(8), 383–392. https://doi.org/10.1016/j.foodcont.2017.03.008
- Burns, D. T., Dillon, A., Warren, J., & Walker, M. J. (2018). A critical review of the factors available for the identification and determination of mānuka honey. Food Analytical Methods, 24(4), 1–7. https://doi.org/10.1007/s12161-018-1154-9
- Castro-Vázquez, L., Leon-Ruiz, V., Alañon, M. E., Pérez-Coello, M. S., & González-Porto, A. V. (2014). Floral origin markers for authenticating lavandin honey (Lavandula angustifolia x latifolia). discrimination from lavender honey (lavandula latifolia). Food Control, 37(3), 362–370. https://doi.org/10.1016/j.foodcont.2013.09.003
- Consonni, R., Bernareggi, F., & Cagliani, L. R. (2019). NMR-based metabolomic approach to differentiate organic and conventional Italian honey. Food Control, 98(4), 133–140. https://doi.org/10.1016/j.foodcont.2018.11.007
- Downie, S. R., Katzdownie, D. S., & Spalik, K. (2000). A phylogeny of Apiaceae tribe Scandiceae: Evidence from nuclear ribosomal DNA internal transcribed spacer sequences. American Journal of Botany, 87(1), 76–95. https://doi.org/10.2307/2656687
- Enrique, C. M., Silvia, C., Grazia, F. M., Giustino, M., & Luisa, A. M. (2018). Characterization of Italian multifloral honeys on the basis of their mineral content and some typical quality parameters. Journal of Food Composition and Analysis, 74(12), 102–113. https://doi.org/10.1016/j.jfca.2018.09.002
- Erban, T., Shcherbachenko, E., Talacko, P., & Harant, K. (2019). The unique protein composition of honey revealed by comprehensive proteomic analysis: Allergens, venom-like proteins, antibacterial properties, royal jelly proteins, serine proteases, and their inhibitors. Journal of Natural Products, 82(5), 1217–1226. https://doi.org/10.1021/acs.jnatprod.8b00968
- Feng, J., Dai, Z., Tian, X., & Jiang, X. (2018). Detection of listeria monocytogenes based on combined aptamers magnetic capture and loop-mediated isothermal amplification. Food Control, 85(3), 443–452. https://doi.org/10.1016/j.foodcont.2017.10.027
- Guelpa, A., Marini, F., Du Plessis, A., Slabbert, R., & Manley, M. (2017). Verification of authenticity of South African honey and fraud detection using NIR spectroscopy. Food Control, 73(3), 1388–1396. https://doi.org/10.1016/j.foodcont.2016.11.002
- Jandrić, Z., Frew, R. D., Fernandez-Cedi, L. N., & Cannavan, A. (2017). An investigative study on discrimination of honey of various floral and geographical origins using UPLC-QToF MS and multivariate data analysis. Food Control, 72(2), 189–197. https://doi.org/10.1016/j.foodcont.2015.10.010
- Kasprzyk, I., Depciuch, J., Grabek-Lejko, D., & Parlinska-Wojtan, M. (2018). FTIR-ATR spectroscopy of pollen and honey as a tool for unifloral honey authentication. The case study of rape honey. Food Control, 84(2), 33–40. https://doi.org/10.1016/j.foodcont.2017.07.015
- Kek, S. P., Chin, N. L.,., Tan, S. W., Yusof, Y. A., & Chua, L. S. (2018). Comparison of DNA extraction methods for entomological origin identification of honey using simple additive weighting method. International Journal of Food Science & Technology, 53(1), 2490–2499. https://doi.org/10.1111/ijfs.13840
- Lara, S. G., Santiago, V., Jaime, P., & Isabel, E. (2019). Detection of honey adulteration by conventional and real-time PCR. Food Control, 95(1), 57–62. https://doi.org/10.1016/j.foodcont.2018.07.037
- Laube, I., Hird, H., Brodmann, P., Ullmann, S., Schöne-Michling, M., Chisholm, J., & Broll, H. (2010). Development of primer and probe sets for the detection of plant species in honey. Food Chemistry, 118(4), 979–986. https://doi.org/10.1016/j.foodchem.2008.09.063
- Mei, X., Zhai, X., Lei, C., Ye, X., Kang, Z., Wu, X., Xiang, R., Wang, Y., & Wang, H. (2019). Development and application of a visual loop-mediated isothermal amplification combined with lateral flow dipstick (LAMP-LFD) method for rapid detection of salmonella strains in food samples. Food Control, 104(10), 9–19. https://doi.org/10.1016/j.foodcont.2019.04.014
- Naila, A., Flint, S. H., Sulaiman, A. Z., Ajit, A., & Weeds, Z. (2018). Classical and novel approaches to the analysis of honey and detection of adulterants. Food Control, 90(8), 152–165. https://doi.org/10.1016/j.foodcont.2018.02.027
- Notomi, T., Okayama, H., Masubuchi, H., Yonekawa, T., Watanabe, K., Amino, N., & Hase, T. (2000). Loop-mediated isothermal amplification of DNA. Nucleic Acids Research, 28(12), e63. https://doi.org/10.1093/nar/28.12.e63
- Schwolow, S., Gerhardt, N., Rohn, S., & Weller, P. (2019). Data fusion of GC-IMS data and FT-MIR spectra for the authentication of olive oils and honeys-is it worth to go the extra mile? Analytical and Bioanalytical Chemistry, 411(23), 6005. https://doi.org/10.1007/s00216-019-01978-w
- Singh, M., Pal, D., Sood, P., & Randhawa, G. (2019). Loop-mediated isothermal amplification assays: Rapid and efficient diagnostics for genetically modified crops. Food Control, 106(12), 106759. https://doi.org/10.1016/j.foodcont.2019.106759
- Soares, S., Amaral, J. S., Oliveira, M. B. P. P., & Mafra, I. (2017). A comprehensive review on the main honey authentication issues: Production and origin. Comprehensive Reviews in Food Science and Food Safety, 16(5), 1072–1100. https://doi.org/10.1111/1541-4337.12278
- Soares, S., Grazina, L., Costa, J., Amaral, J. S., Mafra, I., & Mafra, I. (2018). Botanical authentication of lavender (lavandula spp.) honey by a novel DNA-barcoding approach coupled to high resolution melting analysis. Food Control, 86(4), 367–373. https://doi.org/10.1016/j.foodcont.2017.11.046
- Soares, S., Grazina, L., Mafra, I., Costa, J., Pinto, M. A., Beatriz, M., Oliveira, P. P., & Amaral, J. S. (2019). Towards honey authentication: Differentiation of Apis mellifera subspecies in European honeys based on mitochondrial DNA markers. Food Chemistry, 283(6), 294–301. https://doi.org/10.1016/j.foodchem.2018.12.119
- Sobrino-Gregorio, L., Bataller, R., Soto, J., & Escriche, I. (2018). Monitoring honey adulteration with sugar syrups using an automatic pulse voltammetric electronic tongue. Food Control, 91(9), 254–260. https://doi.org/10.1016/j.foodcont.2018.04.003
- Song, Y. Q., Milne, R. I., Zhou, H. X., Ma, X. L., Fang, J. Y., & Zha, H. G. (2019). Floral nectar chitinase is a potential marker for monofloral honey botanical origin authentication: A case study from loquat (Eriobotrya japonica Lindl.). Food Chemistry, 282(6), 76–83. https://doi.org/10.1016/j.foodchem.2018.12.107
- Spielmann, G., Ziegler, S., Haszprunar, G., Busch, U., Huber, I., & Pavlovic, M. (2019). Using loop-mediated isothermal amplification for fast species delimitation in eels (Genus anguilla), with special reference to the European eel (Anguilla anguilla). Food Control, 101(7), 156–162. https://doi.org/10.1016/j.foodcont.2019.02.022
- Turkut, G. M., Degirmenci, A., Yildiz, O., Can, Z., Cavrar, S., Karahalil, F. Y., & Kolayli, S. (2018). Investigating 5-hydroxymethylfurfural formation kinetic and antioxidant activity in heat treated honey from different floral sources. Journal of Food Measurement and Characterization, 12(4), 2358–2365. https://doi.org/10.1007/s11694-018-9852-y
- Utzeri, V. J., Ribani, A., Schiavo, G., Bertolini, F., Bovo, S., & Fontanesi, L. (2018). Application of next generation semiconductor based sequencing to detect the botanical composition of monofloral, polyfloral and honeydew honey. Food Control, 86(4), 342–349. https://doi.org/10.1016/j.foodcont.2017.11.033
- Verzera, A., Tripodi, G., Condurso, C., Dima, G., & Marra, A. (2014). Chiral volatile compounds for the determination of orange honey authenticity. Food Control, 39(3), 237–243. https://doi.org/10.1016/j.foodcont.2013.11.012
- Wang, D., Wang, Y., Zhu, K., Shi, L., Zhang, M., Yu, J., & Liu, Y. (2019). Detection of Cassava Component In Sweet Potato Noodles By Real-Time Loop-Mediated Isothermal Amplification (Real-time LAMP) method. Molecules, 24(11), 2043. https://doi.org/10.3390/molecules24112043
- Wang, H., Cao, X., Han, T., Pei, H., Ren, H., & Stead, S. (2019). A novel methodology for real-time identification of the botanical origins and adulteration of honey by rapid evaporative ionization mass spectrometry. Food Control, 106(12), 106753. https://doi.org/10.1016/j.foodcont.2019.106753