 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study was conducted to produce water-soluble polysaccharide extracts (WSP) from perilla seed meal (PSM), a by-product of perilla seed oil extraction, using enzymatic hydrolysis. The aim was to confirm the potential of this method to obtain functional materials from PSM. The cellulose and hemicellulose fractions from PSM were hydrolyzed using Celluclast 1.5 L and Viscozyme L, respectively. The yield of WSP from both fractions increased with an increase in hydrolysis time. Physical properties of WSP, including solubility, oil-holding capacity, and emulsification properties, were increased by enzymatic hydrolysis. WSP produced by hydrolysis had greater antioxidant activity than WSP obtained without hydrolysis; the highest activity was observed after a hydrolysis reaction of 24 h. WSP retarded glucose and bile acid absorption. The data indicate that WSP from PSM have potentially valuable functional and biological activities.
RESUMEN
Este estudio se llevó a cabo para producir, mediante hidrólisis enzimática, extractos de polisacáridos solubles en agua (WSP) a partir de harina de semilla de perilla (PSM), un subproducto de la extracción de aceite de semilla de perilla. El objetivo de este ejercicio era confirmar el potencial de este método para obtener materiales funcionales a partir de la PSM. Las fracciones de celulosa y hemicelulosa de PSM se hidrolizaron utilizando Celluclast 1,5 L y Viscozyme L, respectivamente. El rendimiento de la PSM de ambas fracciones aumentó con el incremento del tiempo de hidrólisis. Las propiedades físicas de la PSM, entre ellas la solubilidad, la capacidad de retención de aceite y las propiedades de emulsificación, también se elevaron con la hidrólisis enzimática. Los WSP producidos por hidrólisis mostraron mayor actividad antioxidante que aquellos obtenidos sin aplicar hidrólisis; la mayor actividad se observó después de una reacción de hidrólisis de 24 horas. Los WSP retardaron la absorción de glucosa y de ácidos biliares. Los datos permiten concluir que los WSP producidos a partir de PSM tienen actividades funcionales y biológicas potencialmente valiosas.
1. Introduction
Plant cell walls are composed of complexes of various polysaccharides such as pectic compounds, hemicellulose, glycoproteins, and cellulose, which is the main constituent. The structure of cellulose consists of long, linear polymers of β-(1-4)-linked glucose residues connected by hydrogen bonds. Hemicellulose is derived from a heterologous group of sugars that includes D-xylose, D-mannose, and D-galactose in the hemicellulose backbone (Misurcova et al., Citation2012). Cellulose and hemicellulose, together with pectin, are considered representative dietary fibers. Polysaccharide-enriched plant cell walls are not digested by humans and are considered a rich source of dietary fiber.
Dietary fiber derived from food is useful in the food industry because of its excellent physiological and physical properties. Dietary fibers are classified into soluble dietary fibers and insoluble dietary fibers according to their physical and chemical characteristics; each type has different physiological effects (Schneeman, Citation1987). Water-soluble dietary fiber is effective in increasing the viscosity of food, improving glucose tolerance, and regulating serum cholesterol. Insoluble fiber has been reported to be effective in increasing fecal volume, facilitating intestinal transit time, and preventing colon cancer (Ebihara & Nakamoto, Citation1998). These functional benefits have led to a continuously increasing demand for food fiber and fiber-containing food products (Chai et al., Citation2003), particularly for water-soluble dietary fiber, which can improve food texture, thickening, emulsification, and stability. Therefore, there is a need to improve the usability of food fiber by converting the insoluble cellulose and hemicellulose dietary fibers, which are enriched in the cell walls of plants, into water-soluble forms.
Perilla (Perilla frutescens var. japonica HARA) is an annual herbaceous plant belonging to the family Labiatae. It is cultivated in Korea, Japan, and China. Perilla seed has a high fat content and a unique aroma; hence, it is used commonly as a seasoning. ω-3 type polyunsaturated fatty acids are the predominant fatty acids found in perilla. The various physiological functions that have been described include anti-inflammatory activity, cognitive improvement, and reduction of the incidence of colon cancer (Kim, Song et al., Citation2015; Park & Yoon, Citation2019). The consumption of perilla oil is gradually increasing in Southeast Asian countries, including Korea.
Perilla seed meal (PSM), which is a by-product of perilla oil extraction, is enriched in protein and carbohydrates and has great potential as an economical functional food material in the food industry (J.M. Kim et al., Citation2019). The purpose of this study was to produce water-soluble polysaccharide extracts (WSP) to improve the availability of PSM as a biofunctional material or functional food ingredient. The cellulose and hemicellulose fractions extracted from PSM were hydrolyzed using enzymes to prepare WSP. In addition, the relationship between the physical properties and physiological activities of WSP, and the hydrolysis time was evaluated.
2. Materials and methods
2.1. Materials
PSM was obtained from Queensbucket Co. (Seoul, Korea), ground, and stored in a model MDF-435 deep freezer (Sanyo, Tokyo, Japan) at – 42°C. The cellulose and hemicellulose fractions were obtained using the method described by Im and Yoon (Citation2015) with slight modifications. Briefly, PSM (37.5 g) was mixed with 500 mL of 1 M NaOH, shaken at 250 rpm for 3 h at 25°C, and centrifuged at 16,270 × g for 20 min. The residue was washed with distilled water (DW) until the pH of washed water became neutral. The residue was then lyophilized and used as the cellulose fraction (CF). The supernatant was neutralized to pH of 7.0 with HCl and used as the hemicellulose fraction (HF).
2.2. Enzymatic hydrolysis
Enzymatic hydrolysis was conducted in 500-mL Erlenmeyer flasks containing the CF or HF to prepare WSP. First, 20 g of the dried CF was blended in 500 mL of 50 mM sodium acetate buffer (pH 5.0). Thirty units of Celluclast 1.5 L (Novozymes A/S, Bagsvaerd, Denmark) was added to the mixture and agitated at 120 rpm in a model BS-11 shaking incubator (JeioTech, Seoul, Korea) for 24, 48, and 72 h at 50°C. For enzymatic hydrolysis of the HF, 30 units of Viscozyme L (Novozymes A/S) was added to 500 mL of the fraction. The flasks were agitated at 150 rpm on a shaking incubator at 50°C and pH 5.0 for 24, 48, and 72 h. The reaction mixture was boiled to inactivate the enzyme activity at 95°C for 3 min, followed by filtering and freeze-drying. The yield of WSP in each fraction is expressed as a percentage of the dry weight of WSP (g) relative to the weight (g) of the fraction before enzymatic hydrolysis.
2.3. Separation of WSP from enzymatic hydrolysate
WSP were separated from the hydrolysate based on their solubilities in alcohol, as described by Oh and Yoon (Citation2018). The lyophilized hydrolysate was mixed with 85% ethanol at 80°C, incubated at 60°C for 40 min, and filtered to remove the low molecular weight saccharides in the hydrolysate. The residue was dissolved in DW and used as WSP for further experiments. WSP obtained from CF and HF were designated as WSP-CF and WSP-HF, respectively.
2.4. Yield and contents of total sugar, uronic acids, and protein
The yields of WSP obtained from PSM were determined based on dry weight. The total sugar content of WSP was determined using the established phenol-sulfuric acid method. Briefly, 0.2 mL of sample, 0.2 mL of 5% phenol solution, and 1 mL of 98% sulfuric acid were added to a test tube and mixed. After 30 min at room temperature, the absorbance was measured at 480 nm using a model U-2900 spectrophotometer (Hitachi, Tokyo, Japan). The total sugar content of CF or HF was calculated from standard curves of glucose and xylose, respectively. The protein content was determined by the Lowry et al. (Citation1951) using bovine serum albumin as the standard. The uronic acid content was measured by the method of Cesaretti et al. (Citation2003) with galacturonic acid as the standard.
2.5. Molecular weight determination
The molecular weight of WSP was determined by gel permeation chromatograph (GPC) system (Breeze System, Waters, Milford, MA, USA), using a Waters Ultrahydrogel 120 column (7.8 mm × 300 mm, 6 μm, Waters, USA). The data were calibrated with PEG standards (peak average molecular weights of 106, 430, 960, 1400, 4290, 6690, 12,600, and 20,600 Da); a refractive index detector (RID) was used. The elution was conducted using 0.2 N NaNO3 at a flow rate of 0.8 mL/min.
2.6. Density
The density of WSP was measured as described by Parrott and Thrall (Citation1978). A defined mass (g) of sample was filled into a graduated cylinder with minimal shaking. The volume was measured to indicate the direct density. For bulk density measurement, a graduated cylinder was filled with a certain amount of sample. Pressure was applied manually until the contents were packed tightly and the volume no longer reduced. The volumetric measurement was then read, and the results are expressed as g/mL.
2.7. Solubility and water swelling capacity (WSC)
Solubility was measured as described by Robertson et al. (Citation2000) with some modifications. Sample (250 mg) and 10 mL of DW were added to a test tube, mixed, and left at room temperature for 30 min. The supernatant obtained by centrifugation (16,000 × g, 15 min) was freeze-dried and weighed. WS was calculated from Equation (1):
Solubility (%) = (dried supernatant weight/dried sample weight) × 100 (1)
WSC was measured as described by Robertson et al. (Citation2000). Each sample (100 mg) was hydrated in a calibrated cylinder containing 0.02% sodium azide in 10 mL of DW. After equilibration at room temperature for 18 h, the increase in volume was measured. WSC was expressed as volume (mL) per gram of sample.
2.8. Water-holding and oil-holding capacities
Water-holding capacity (WHC) was determined as described by Ma and Mu (Citation2016) with slight modifications. Sample (250 mg) was hydrated in 10 mL DW at room temperature for 30 min. After centrifugation at 3,000 × g for 15 min, the residue was weighed and lyophilized. The weight of the lyophilized residue was measured. WHC was calculated using EquationEquation (2)(2)
(2) :
Oil-holding capacity (OHC) was measured as described by Robertson et al. (Citation2000) with some modifications. WSP (0.1 g) was mixed with 100 mL of soybean oil and incubated at room temperature for 1 h. The mixture was centrifuged at 2,000 × g for 20 min, and the centrifuge tube was placed upside-down on a piece of filter paper and left for 1 h to remove grease. The tube was then weighed. OHC is expressed as the amount of oil remaining per gram of sample (g/g).
2.9. Emulsifying properties
Emulsifying capacity (EC) was determined following the method described by Yamauchi et al. (Citation2006) with modifications. Five milliliters of 6% WSP (w/v) was dissolved in DW, and then mixed with 5 mL of corn oil for 60 s to form an emulsion. The emulsion was centrifuged at 1,100 × g for 5 min, and the height of the emulsified layer and the height of the total contents of the tube were determined. EC was calculated from EquationEquation (3)(3)
(3) :
For measurement of emulsion stability (ES), the emulsion was prepared as just described. The emulsion was then heated in a hot water bath at 80°C for 30 min, cooled to 15°C, and centrifuged at 1,100 × g for 5 min. The height of the emulsion layer was measured, and ES was calculated from EquationEquation (4)(4)
(4) :
2.10. Antioxidant activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity was measured by modifying the method described by Jang et al. (Citation2016). Aliquots of sample solution (100 μL/well) were dispensed into a microplate, and 200 μL of 0.2 mM DPPH solution was added to each well. The mixture was reacted at 37°C for 30 min, and the absorbance was measured at 517 nm using an Epoch microplate reader (Biotek Instrument Inc, Winooski, VT, USA).
The ferrous ion (Fe2+) chelating ability of WSP obtained from PSM was measured using the method described by Dinis et al. (Citation1994). One milliliter of sample solution, 25 μL of 2 mM FeCl2 (25 μL), and 25 μL of 5 mM ferrozine [3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4 ‘4ʹ-disulfonic acid] were added. The reaction proceeded at room temperature for 10 min, and the absorbance was measured at 562 nm.
β-Carotene bleaching activity was measured as described by Sila et al. (Citation2014) with some modification. A stock solution of β-carotene/linoleic acid was initially prepared by dissolving 10 mg of β-carotene in 100 mL of chloroform. An aliquot of the β-carotene solution (3 mL) was added to 40 mg of linoleic acid and 400 mg of Tween 40. The chloroform was evaporated using nitrogen gas and 100 mL of DW was added to the mixture. Initial absorbances at 470 nm and 700 nm were immediately recorded. Aliquots of β-carotene/linoleic acid emulsion (2 mL) were mixed with 100 μL of sample solution, and the absorbance of the emulsion was measured at 470 nm. After incubation of the emulsion at 50°C for 2 h, the absorbance measurement was repeated at 470 nm.
2.11. Retarding effect of WSP on glucose and bile acid absorption
The retarding effects of WSP on glucose and bile acid absorption in the gastrointestinal tract was measured by determining the glucose permeation rate (GPR) and bile acid permeation rate (BPR) into the dialysate, as described by Im and Yoon (Citation2015). To measure GPR, sample (0.2 g) and glucose (36 mg) were hydrated with 0.1% sodium azide and added to dialysis bags (D7884: MW cut-off ≤ 1200; Sigma-Aldrich, St. Louis, MO, USA) To measure BPR, 1 L of phosphate buffer (50 mM, pH 7.0) with 0.1% sodium azide and 6 mL of 15 mM taurocholic acid-containing sample (0.2 g) were added to the dialysis bag. Each dialysis bag was immersed in 100 mL of phosphate buffer containing 0.1% sodium azide and dialyzed at 37°C for 12 h. For the measurement of glucose and bile acid, 1 mL of dialysate was taken at regular intervals. The glucose content of the dialysate was analyzed using the 3,5-dinitrosalicylic acid method, and the taurocholic acid content was determined as described by Boyd et al. (Citation1966). The dialysate obtained by the same process but without a sample was used as the control, and the dialysates obtained by adding pectin (Fluka-Biochemika, Buchs, Switzerland) and carboxymethyl cellulose (CMC; Sigma-Aldrich) were used as positive controls. GPR and BPR were calculated from Equationequations (5)(5)
(5) and (Equation6
(6)
(6) ), respectively:
2.12. Statistical analyses
The results are expressed as the mean ± standard deviation of triplicate experiments. Multivariate analysis of variance was conducted using SPSS ver. 21.0 (Chicago, IL, USA). Significant differences between the mean values were identified using Duncan’s multiple range test.
3. Results and discussion
3.1. Yield and chemical composition
The data regarding the yield and total sugar, uronic acids, and protein contents of WSP obtained from PSM for different hydrolysis times are presented in . For a hydrolysis period of 0 h, the yield of WSP-CH was 14.22%. The yield increased as the enzymatic hydrolysis time increased. For example, the yield of WSP-CF obtained after hydrolysis for 48 and 72 h was 18.27 and 18.78%, respectively. The yield of WSP-HF at an enzymatic hydrolysis time of 0 h was 3.38%, and the yield increased significantly as the hydrolysis time increased, with a value of 4.53% after hydrolysis for 72 h (p < .05). This is similar to the prior report that the yield of soluble dietary fiber from Chinese cabbage by-products increased with increasing hydrolysis time (Im & Yoon, Citation2015). The total sugar content of WSP-CF was the lowest, i.e., 38.36% at 0 h, and increased to 69.61% and 69.88% after hydrolysis for 48 and 72 h, respectively. The total sugar content of WSP-HF was the lowest for nonhydrolyzed WSP-HF (24.51%). As the hydrolysis time increased, the total sugar content increased. Park and Yoon (Citation2015) reported that the total sugar content of soluble fiber produced from Chinese cabbage waste was increased by enzymatic hydrolysis, similar to the present results. These results might be due to the increased dissolution of polysaccharides by the conversion of insoluble high molecular weight polysaccharide compounds, which are bound to the cell wall, to low molecular weight polysaccharides (Chae et al., Citation2011). The uronic acid content of WSP was the highest, at 5.67% and 10.30% in CF and HF, respectively, after hydrolysis for 24 h and was not significantly different to WSP produced after hydrolysis for 48 h. It has been reported that uronic acids affect various physiological activities such as antioxidant and anti-whitening (Chen et al., Citation2004) and that WSP obtained after hydrolysis for 24 h may exhibit strong physiological activity. The protein content of WSP tended to increase as the hydrolysis time increased, and after hydrolysis for 72 h, the protein content of WSP-CF and WSP-HF was 8.41 mg/g and 10.87 mg/g, respectively. The protein contained in the polysaccharide fraction interacts with the polysaccharide to show surface activity, thus affecting physical properties such as emulsifying ability (Wang et al., Citation2019).
Table 1. Yield and chemical composition of water-soluble polysaccharides extracts produced from perilla seed meal by enzymatic hydrolysis.
Tabla 1. Rendimiento y composición química de los extractos de polisacáridos solubles en agua producidos por hidrólisis enzimática a partir de harina de semillas de perilla.
3.2. Molecular weight
presents the weight average-molecular weight (Mw), number average-molecular weight (Mn), and polydispersity (Mw/Mn) of WSP. The Mw and Mn of WSP was decreased by enzymatic hydrolysis, and the longer the hydrolysis time, the smaller the molecular weight. The polydispersity of WSP was also decreased with an increase in hydrolysis time, and the polydispersity of WSP-HF was higher than that of WSP-CF. The polydispersity index, an indicator of the degree of homogeneity, is a measurement of the degree of molecular weight distribution in a given polymer. Nonhydrolyzed WSP and WSP-HF exhibited broader molecular weight distribution than hydrolyzed WSP and WSP-CH, respectively.
Table 2. Weight-average (Mw) and number-average (Mn) molecular weights and polydispersity (Mw/Mn) of water-soluble polysaccharides extracts produced from perilla seed meal by enzymatic hydrolysis.
Tabla 2. Pesos moleculares promedio (Mw), promedio numérico (Mn) y polidispersidad (Mw/Mn) de los extractos de polisacáridos solubles en agua producidos por hidrólisis enzimática a partir de harina de semillas de perilla.
3.3. Density
The direct and bulk densities of WSP-CF were increased by enzymatic hydrolysis (). WSP-CF obtained after hydrolysis for 72 h displayed the highest direct density (0.65 g/mL) and bulk density (0.98 g/mL). The direct and bulk densities of WSP-HF were the highest, at 0.89 g/mL and 1.15 g/mL, respectively, after enzymatic hydrolysis for 24 h. Thereafter, there was no significant difference in the values as the reaction time increased. These results agreed with those of Parrott and Thrall (Citation1978), who reported similar trends in the direct and bulk densities of dietary fiber obtained from processed by-products of soybean meal, almond shells, and coconut. Density depends on the particle size and structural characteristics of the dietary fiber, which may be an indicator of the organoleptic properties of the fiber (Hwang et al., Citation1995).
Table 3. Density of water-soluble polysaccharides extracts produced from perilla seed meal by enzymatic hydrolysis.
Tabla 3. Densidad de los extractos de polisacáridos solubles en agua producidos por hidrólisis enzimática a partir de harina de semillas de perilla.
3.4. Hydration properties and oil-holding capacity
depicts the hydration properties of WSP according to enzymatic hydrolysis time of PSM. The solubility of WSP was increased by enzymatic hydrolysis. For example, the solubility of WSP-CF and WSP-HF was 5.28% and 16.97% at 0 h and increased to 26.74 and 26.80% at 72 h, respectively. These values were much higher than the solubility (0.28–4.35%) of dietary fiber obtained from oat, pea, potato, and wheat (Lee et al., Citation2013). This high solubility is considered to be due to degradation of the polymer into low molecular weight polysaccharides by enzymatic hydrolysis. The highest WSC values of WSP-CF and WSP-HF were 7.30 and 11.43 mL/g at 0 h, respectively, and the WSC values decreased with increasing hydrolysis time. Lopez et al. (Citation1996) reported that the WSC of soluble fiber obtained from artichoke was reduced by hydrolysis with cellulase, which was consistent with our results. WHC also decreased from 8.26 mL/g to 1.65 mL/g for WSP-CF and from 20.11 to 7.21 mL/g for WSP-HF with increasing hydrolysis time. The WHC of soluble fiber obtained from artichoke was reduced from 10.97 g/g to 2.83 g/g following enzymatic hydrolysis (Lopez et al., Citation1996). This result might be due to the structural loss of the polymer polysaccharide by the decomposition of the enzyme, thereby reducing the space for water retention (Hassan et al., Citation2011). Although the WHC of WSP was decreased by enzymatic hydrolysis, WSP-HF retained a high WHC, which was much higher than that of dietary fiber obtained from apple (Figuerola et al., Citation2005) and Mangifera pajang Kort. fruit pulp (Al-Sheraji et al., Citation2011). The hydration properties of dietary fibers are influenced by the composition and proportions of the components. In general, soluble dietary fiber has a high hydration capacity and swells to form viscous solutions. Its viscosity impedes the absorption of macronutrients, resulting in increased satiety and decreased energy intake (Al-Sheraji et al., Citation2011).
Table 4. Solubility, water swelling capacity (WSC), water-holding capacity (WHC), and oil-holding capacity (OHC) of water-soluble polysaccharides extracts produced from perilla seed meal by enzymatic hydrolysis.
Tabla 4. Solubilidad, capacidad de hinchamiento de agua (WSC), capacidad de retención de agua (WHC) y capacidad de retención de aceite (OHC) de los extractos de polisacáridos solubles en agua producidos por hidrólisis enzimática a partir de harina de semillas de perilla.
The OHC of WSP increased with an increase in hydrolysis time (). The OHC of WSP obtained by hydrolysis of 72 h was the highest (4.54 and 4.03 g/g for CF and HF, respectively). OHC is related to the nature of the surface and the density or thickness of the particles. Particles with the largest surface area theoretically exhibit great capacity to absorb and bind to components that are oil-based (Lopez et al., Citation1996). The findings indicated the potential value of WSP obtained by enzymatic hydrolysis of PSM in promoting the emulsification of some products.
3.5. Emulsifying properties
The EC of WSP obtained from PSM increased with an increase in hydrolysis time, but there was no significant difference between 48 and 72 h (). The ECs of WSP-CF and WSP-HF obtained after hydrolysis for 72 h were 48.18 and 47.42%, respectively, which was similar to the EC (54.12%) of dietary fiber form Indian jujube (Sangeethapriya & Siddhuraju, Citation2014). In addition, this result was three times higher than the EC (14.43%) of the dietary fiber extracted from rice bran (Abdul-Hmid & Luan, Citation2000). It is considered that the increase in EC induced by hydrolysis is due to the higher protein content compared with the nonhydrolyzed WSP.
Table 5. Emulsifying capacity (EC) and emulsion stability (ES) of water-soluble polysaccharides extracts produced from perilla seed meal by enzymatic hydrolysis.
Tabla 5. Capacidad emulsionante (EC) y estabilidad de la emulsión (ES) de los extractos de polisacáridos solubles en agua producidos por hidrólisis enzimática a partir de harina de semillas de perilla.
or the ES, the WSP-CF was the lowest (40.81%) before hydrolysis and increased significantly, to 43.31–45.17% after hydrolysis. Hydrolysis time did not significantly affect EC. The ES of WSP-HF increased significantly with an increase in hydrolysis time, with the highest value of 45.00% after hydrolysis for 48 h. After hydrolysis for 72 h, ES decreased to 44.14%, but this decrease was not significant. The emulsifying properties of fiber have the advantage of lowering cholesterol in the blood vessels by binding to bile acids and enteric acid to promote excretion (Lopez et al., Citation1996). These findings indicated that WSP from PSM would be expected to reduce blood cholesterol and should be useful in food processing as stable emulsifiers owing to their notable emulsifying capability.
3.6. Antioxidant activity
The DPPH radical scavenging, Fe2+ chelation, and β-carotene bleaching activities of WSP obtained from PSM were measured. The IC50 values are summarized in . The WSP obtained after hydrolysis for 24 to 48 h displayed higher antioxidant activity than nonhydrolyzed WSP. WSP obtained after hydrolysis for 24 h displayed the highest antioxidant activity for both CF and HF. For example, nonhydrolyzed WSP-CF and WSP-HF displayed the lowest DPPH radical scavenging activities with the highest IC50 values of 3.04 mg/mL and 2.39 mg/mL, respectively. The IC50 values of WSP-CF and WSP-HF obtained after hydrolysis for 24 h were significantly lower (1.71 mg/mL and 1.57 mg/mL, respectively) compared with those of WSP obtained after hydrolysis for 48 h and 72 h. In a previous study, the DPPH radical scavenging activities of 2,000 mg/mL WSP prepared from pistachio and almond by-product were 40.08% and 56.78% (Sila et al., Citation2014). The DPPH radical scavenging activity of crude polysaccharides obtained from Hyriopsis cumingii was 21.43% at 2,000 mg/mL (Qiao et al., Citation2009). WSP from PSM displayed markedly higher radical scavenging activity than these prior results. The scavenging ability of polysaccharides may be due to the presence of hydrogen from the specific monosaccharide compositions and their side chain linkages (Kozarski et al., Citation2012). These results imply that WSP from PSM could act as primary antioxidants that can react with radicals by donating hydrogen atoms or electrons to the DPPH radicals to convert them into a stable product.
Table 6. Antioxidant activity of water-soluble polysaccharides extracts produced from perilla seed meal by enzymatic hydrolysis.
Tabla 6. Actividad antioxidante de los extractos de polisacáridos solubles en agua producidos por hidrólisis enzimática a partir de harina de semillas de perilla.
The WSP-CF and WSP-HF obtained after hydrolysis for 24 h also showed the lowest IC50 values of 145.04 μg/mL and 76.32 μg/mL in the Fe2+ chelation assay, respectively. Fe2+ chelation was the highest after 24 h of hydrolysis. Subsequently, the iron chelation ability decreased in the following order, after hydrolysis for 0, 48, and 72 h. The IC50 values of the Fe2+ chelation ability of polysaccharide extracts commonly obtained from mushrooms ranged from 3.58 mg/mL to >20 mg/mL (Kozarski et al., Citation2012). Fan et al. (Citation2014) reported an IC50 value of 1,965 μg/mL for the Fe2+ chelation activity of crude polysaccharides fractionated from the leaves of Ilex latifolia Thunb. The corresponding IC50 values for the chelation of blue, white, and yellow lupin polysaccharides were 3.51, 3.48, and 4.42 mg/mL, respectively (Thambiraj et al., Citation2019). The Fe2+ chelation activity of WSP obtained from PSM was much higher than that reported previously (Fan et al., Citation2014; Kozarski et al., Citation2012). WSP-HF displayed a higher chelating ability than WSP-CH for all hydrolysis times except 72 h. Compounds with structures containing two or more functional groups such as – OH, -COOH, -SH, C = O, and – O- were reported to show metal chelation activity (Yuan et al., Citation2005). Kaplan et al. (Citation1987) reported that the free carboxylic groups contributed by uronic acids and their relatively homogenous distributions in the extracellular polysaccharides play a major role in metal chelation. Lo et al. (Citation2011) found a strong correlation between monosaccharide compositions and chelating ability on Fe2+. The authors showed that the level of rhamnose in the polysaccharide was the dominant component in modulating the chelating response variable. Therefore, it is presumed that the high chelating ability of WSP-HF was due to presence of uronic acid and rhamnose eluted from hemicellulose cell wall components.
After hydrolysis for 24 h, WSP-CF and WSP-HF displayed the highest β-carotene bleaching activity, with IC50 values of 0.85 mg/mL and 0.66 mg/mL, respectively. Subsequently, WSP-CF and WSP-HF activity decreased to 1.72 and 2.15 mg/mL, respectively, at 48 h, and 2.08 and 2.82 mg/mL, respectively, at 72 h, compared with 3.58 and 4.08 mg/mL, respectively, after 0 h of hydrolysis. The β-carotene bleaching activity of WSP obtained from PSM by enzymatic hydrolysis was higher that reported in the study of Sila et al. (Citation2014) – the IC50 values of WSP obtained from pistachio and almond by-products were 4.46 mg/mL and 3.39 mg/mL, respectively, for β-carotene bleaching activity. These findings revealed the potent antioxidant activities of WSP from PSM and indicated their potential value as natural antioxidants.
3.7. Retarding effect of WSP on glucose and bile acid absorption
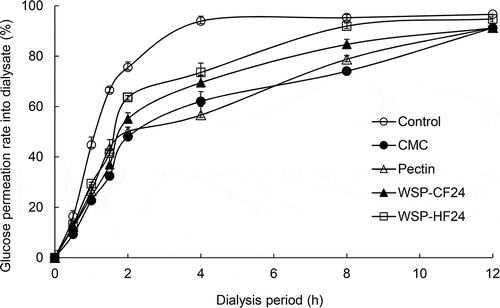
The GPR of WSP obtained from PSM followed the order (lowest to heights) in CMC, WSP-CF24, pectin, WSP-HF24, and control after 1 h of dialysis (). The GPR of WSP-CF24 and WSP-HF24 was 36 and 41% after 1.5 h of dialysis, respectively, which was lower than that of pectin (43.41%). The GPR continued to increase over dialysis time, to 93.30, 73.53, 69.45, 62.04, and 52.32% for the control, WSP-HF24, WSP-CF24, CMC, and pectin, respectively, after 4 h of dialysis. The GPR of WSP obtained from PSM was very low compared with the control, and retarded glucose absorption. Thereafter, the GPR showed a modestly increased rate, in the range of 91.19% to 99.62% after 12 h of dialysis. The retarding effect on absorption of glucose in the human intestine can be determined from the initial dialysis period (Oh & Yoon, Citation2018). WSP obtained from PSM displayed a low GPR until 1.5 h after dialysis. Water-soluble dietary fiber forms a viscous gel, which inhibits glucose uptake by reducing the accessibility of glucose to intestinal epithelial cells (Sangeethapriya & Siddhuraju, Citation2014). Therefore, WSP from PSM are potential hypoglycemic agents owing to their inhibitory effect on absorption through binding to glucose.
Figure 1. Passive transport of glucose in vitro in the presence of water-soluble polysaccharides extracts produced from perilla seed meal by enzymatic hydrolysis. The results are presented as the mean and standard deviation (n = 3). CMC, carboxymethylcellulose; WSP-CF24 and WSP-HF24, water-soluble polysaccharides extracts produced from the cellulose and hemicellulose fractions by enzymatic hydrolysis for 24 h, respectively.
Figura 1. Transporte pasivo de glucosa in vitro en presencia de extractos de polisacáridos hidrosolubles producidos por hidrólisis enzimática a partir de harina de semillas de perilla. Los resultados se presentan como la media y la desviación estándar (n = 3). CMC, carboximetilcelulosa; WSP-CF24 y WSP-HF24, extractos de polisacáridos solubles en agua producidos a partir de fracciones de celulosa y hemicelulosa por hidrólisis enzimática durante 24 horas, respectivamente.
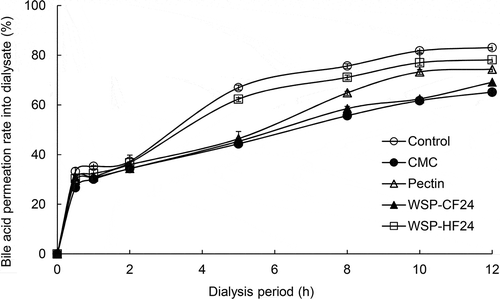
The retardation effect of WSP on bile acid absorption was measured and compared with that of the control. The BPR tended to increase continuously and all samples had a lower BPR compared with the control during the dialysis period (). In particular, the BPR of WSP-CF24 (30.82%) was similar to that of CMC (30.06%) and pectin (30.98%) after dialysis for 1 h, and WSP-CF24 subsequently had a lower BPR compared with pectin after dialysis for 12 h. WSP-CF inhibit bile acid permeation owing to its pronounced affinity with hydrophobic substances due to its high OHC. Plant-derived polysaccharides increase bile acid synthesis (H. Kim et al., Citation2015) and some polysaccharides with cholesterol-lowering effects have also been reported to have bile acid-binding capability. These polysaccharides may form polymers by binding to bile acids and improve the removal of bile acids in the intestine, stimulating the conversion of cholesterol into bile acids in the liver, thereby lowering the total cholesterol level (Wu et al., Citation2019). Therefore, WSP obtained from PSM could be used as effective functional materials to prevent and treat hypercholesterolemia.
Figure 2. Passive transport of bile acid in vitro in the presence of water-soluble polysaccharides extracts produced from perilla seed meal by enzymatic hydrolysis. The results are presented as the mean and standard deviation (n = 3). CMC, carboxymethylcellulose; WSP-CF24 and WSP-HF24, water-soluble polysaccharides extracts produced from the cellulose and hemicellulose fractions of perilla seed meal by enzymatic hydrolysis for 24 h, respectively.
Figura 2. Transporte pasivo de ácido biliar in vitro en presencia de extractos de polisacáridos solubles en agua producidos por hidrólisis enzimática a partir de harina de semillas de perilla. Los resultados se presentan como la media y la desviación estándar (n = 3). CMC, carboximetilcelulosa; WSP-CF24 y WSP-HF24, extractos de polisacáridos solubles en agua producidos por hidrólisis enzimática a partir de las fracciones de celulosa y hemicelulosa de harina de semillas de perilla durante 24 horas, respectivamente.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Abdul-Hmid, A., & Luan, Y. S. (2000). Functional properties of dietary fibre prepared from defatted rice bran. Food Chemistry, 68(1), 15–19. https://doi.org/10.1016/S0308-8146(99)00145-4
- Al-Sheraji, S. H., Ismail, A., Manap, M. Y., Mustafa, S., Yusof, R. M., & Hassan, F. A. (2011). Functional properties and characterization of dietary fiber from Mangifera pajang Kort. fruit pulp. Journal of Agricultural and Food Chemistry, 59(8), 3980–3985. https://doi.org/10.1021/jf103956g
- Boyd, G. S., Eastwood, M. A., & MacLean, N. (1966). Bile acids in the rat: Studies in experimental occlusion of the bile duct. Journal of Lipid Research, 7(1), 83–94. http://www.jlr.org/cgi/content/abstract/7/1/83
- Cesaretti, M., Luppi, E., Maccari, F., & Volpi, N. (2003). A 96-well assay for uronic acid carbazole reaction. Carbohydrate Polymers, 54(1), 59–61. https://doi.org/10.1016/S0144-8617(03)00144-9
- Chae, H. J., Park, D. I., Lee, S. C., Oh, C. H., Oh, N. S., Kim, D. C., Won, S. I., & In, M. J. (2011). Improvement of antioxidative activity by enzyme treatment and lactic acid bacteria cultivation in blank garlic. Journal of the Korean Society of Food Science and Nutrition, 40(1), 660–664. https://doi.org/10.3746/jkfn.2011.40.5.660
- Chai, Y. M., Lim, B. K., Lee, J. Y., Kim, Y. H., & Rhee, S. J. (2003). Preparation of soluble ary fiber from oak wood (Quercus Mongolica) and its physiological function in rat red high cholesterol diets. Korean Journal of Nutrition, 36(1), 9–17. https://www.koreamed.org/article/0124KJN/2003.36.1.9
- Chen, H., Zhang, M., & Xie, B. (2004). Quantification of uronic acids in tea polysaccharide conjugates and their antioxidant properties. Journal of Agricultural and Food Chemistry, 52(11), 3333–3336. https://doi.org/10.1021/jf0349679
- Dinis, T. C. P., Madeira, V. M. C., & Almeida, L. M. (1994). Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Archives of Biochemistry and Biophysics, 315(1), 161–169. https://doi.org/10.1006/abbi.994.1485
- Ebihara, K., & Nakamoto, Y. (1998). Comparative effect of water-soluble and -insoluble dietary fiber on bowel function in rats fed a liquid elemental diet. Nutrition Research, 18(5), 883–891. https://doi.org/10.1016/S0271-5317(98)00073-6
- Fan, J., Wu, Z., Zhao, T., Sun, Y., Ye, H., Xu, R., & Zeng, X. (2014). Characterization, antioxidant and hepatoprotective activities of polysaccharides from Ilex latifolia Thunb. Carbohydrate Polymers, 101, 990–997. https://doi.org/10.1016/j.carbpol.2013.10.037
- Figuerola, F., Hurtado, M. L., Estéve, A. M., Chiffelle, I., & Asenjo, F. (2005). Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chemistry, 91(3), 395–401. https://doi.org/10.1016/j.foodchem.2004.04.036
- Hassan, F. A., Ismail, A., Hamid, A. A., Azlana, A., & Al-sheraji, S. H. (2011). Characterisation of fibre-rich powder and antioxidant capacity of Mangifera pajang K. fruit peels. Food Chemistry, 126(1), 283–288. https://doi.org/10.1016/j.foodchem.2010.11.019
- Hwang, J. K., Kim, C. T., Cho, S. J., & Kim, C. J. (1995). Effects of various thermal treatments on physicochemical properties of wheat bran. Korean Journal of Food Science and Technology, 27(3), 394–403. http://www.koreascience.or.kr/article/JAKO199503042021739.page
- Im, H. J., & Yoon, K. Y. (2015). Production and characterisation of alcohol-Insoluble dietary fibre as a potential source for functional carbohydrates produced by enzymatic depolymerisation of buckwheat hulls. Czech Journal of Food Sciences, 33(5), 449–457. https://doi.org/10.17221/200/2015-CJFS
- Jang, H. L., Liceaga, A. M., & Yoon, K. Y. (2016). Purification, characterisation and stability of an antioxidant peptide derived from sandfish (Arctoscopus japonicus) protein hydrolysates. Journal of Functional Foods, 20, 433–442. https://doi.org/http://dx.doi.10.1016/j.jff.2015.11.020
- Kaplan, D., Christiaen, D., & Arad, S. (1987). Chelating properties of extracellular polysaccharides from Chlorella spp. Applied and Environmental Microbiology, 53(12), 2953–2956. https://doi.org/10.1128/AEM.53.12.2953-2956.1987
- Kim, H., Wang, Q., Shoemaker, C. F., Zhong, F., Bartley, G. E., & Yokoyama, W. H. (2015). Polysaccharide gel coating of the leaves of Brasenia schreberi lowers plasma cholesterol in hamsters. Journal of Traditional and Complementary Medicine, 5(1), 56–61. https://doi.org/10.1016/j.jtcme.2014.10.003
- Kim, J. M., Liceaga, A. M., & Yoon, K. Y. (2019). Purification and identification of an antioxidant peptide from perilla seed (Perilla frutescens) meal protein hydrolysate. Food Science & Nutrition, 7(5), 1645–1655. https://doi.org/10.1002/fsn3.998
- Kim, S. N., Song, B. R., & Ju, J. H. (2015). Antioxidant activities of Perilla frutescens britton seed extract and its inhibitory effects against major characteristics of cancer cells. Journal of the Korean Society of Food Science and Nutrition, 44(2), 208–215. https://doi.org/10.3746/jkfn.2015.44.2.208
- Kozarski, M., Klaus, A., Niksic, M., Vrvic, M. M., Todorovic, N., Jakovljevic, N., & Van Griensven, L. J. L. D. (2012). Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. Journal of Food Composition and Analysis, 26(1–2), 144–153. https://doi.org/10.1016/j.jfca.2012.02.004
- Lee, H. I., Lee, H. G., & Bae, I. Y. (2013). Impact of dietary fibers from various source in wheat flour gel model: Aspect of suitability of processing and in vitro starch digestibility. Food Engineering Progress, 7(4), 297–307. https://doi.org/10.13050/foodengprog.2013.17.4.297
- Lo, -T. C.-T., Chang, C. A., Chiuc, K.-H., Tsayd, P.-K., & Jena, J.-F. (2011). Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydrate Polymers, 86(1), 320–327. https://doi.org/10.1016/j.carbpol.2011.04.056
- Lopez, G., Ros, G., Rincon, F., Periage, M. J., Martinez, M. C., & Ortuno, J. (1996). Relationship between physical and hydration properties of soluble and insoluble fiber of artichoke. Journal of Agricultural and Food Chemistry, 44(9), 2773–2778. https://doi.org/10.1021/jf9507699
- Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193(1), 265–275. https://www.jbc.org/content/193/1/265.full.pdf
- Ma, M., & Mu, T. (2016). Effect of extraction methods and particle size distribution on the structural, physicochemical, and functional properties of dietary fiber from deoiled cumin. Food Chemistry, 194, 237–246. https://doi.org/10.1016/j.foodchem.2015.07.095
- Misurcova, L., Skrovankova, S., Samek, D., Ambrozova, J., & Mach, L. (2012). Health benefits of algal polysaccharides in human nutrition. Advances in Food and Nutrition Research, 66, 75–145. https://doi.org/10.1016/B978-0-12-394597-6.00003-3
- Oh, M. H., & Yoon, K. Y. (2018). Comparison of the biological activity of crude polysaccharide fractions obtained from Cedrela sinensis using different extraction methods. Polish Journal of Food and Nutrition Sciences, 68(4), 327–334. https://doi.org/10.1515/pjfns-2018-0007
- Park, B. Y., & Yoon, K. Y. (2019). Functional properties of enzymatic hydrolysate and peptide fractions from perilla seed meal protein. Polish Journal of Food and Nutrition Sciences, 69(2), 119–127. https://doi.org/10.31883/pjfns-2019-0011
- Park, S. Y., & Yoon, K. Y. (2015). Enzymatic production of a soluble fiber hydrolysate from Chinese cabbage waste and its health-related properties. Journal of Food Biochemistry, 39(3), 274–280. https://doi.org/10.1111/jfbc.12126
- Parrott, M. E., & Thrall, B. E. (1978). Functional properties of various fibers: Physical properties. Journal of Food Science, 43(3), 1365–2621. https://doi.org/10.1111/j.1365-2621.1978.tb02412.x
- Qiao, D., Ke, C., Hu, B., Luo, J., Sun, Y. H., Yan, Y., Zeng, X., & Zeng, X. (2009). Antioxidant activities of polysaccharides from Hyriopsis cumingii. Carbohydrate Polymers, 78(2), 199–204. https://doi.org/10.1016/j.carbpol.2009.03.018
- Robertson, J. A., Monredon, F. D., Dysseler, P., Guillon, F., Amado, R., & Thibault, J. F. (2000). Hydration properties of dietary fiber and resistant starch: A European collaborative study. LWT-Food Science and Technology, 33(2), 72–79. https://doi.org/10.1006/fstl.1999.0595
- Sangeethapriya, M., & Siddhuraju, P. (2014). Health related functional characteristics and antioxidant potential of mucilage (dietary fiber) from Zizyphus mauritiana fruits. Food Science and Human Wellness, 3(2), 79–88. https://doi.org/10.1016/j.fshw.2014.05.003
- Schneeman, B. O. (1987). Soluble vs insoluble fiber: Different physiological responses. Food Technology, 41(2), 81–82.
- Sila, A., Bayar, N., Ghazala, I., Bougatef, A., Ellouz-Ghorbel, R., & Ellouz-Chaabouni, S. (2014). Water-soluble polysaccharides from agro-industrial by-products: Functional and biological properties. International Journal of Biological Macromolecules, 69, 236–243. https://doi.org/10.1016/j.ijbiomac.2014.05.052
- Thambiraj, S. R., Reddy, N., Phillips, M., & Koyyalamudi, S. R. (2019). Biological activities and characterization of polysaccharides from the three Australian Sweet Lupins. International Journal of Food Properties, 22(1), 522–535. https://doi.org/10.1080/10942912.2019.1588298
- Wang, S., Yang, J., Shao, G., Qu, D., Zhao, H., Yang, L., Zhu, L., He, Y., Liu, H., & Zhu, D. (2019). Soy protein isolated-soy hull polysaccharides stabilized O/W emulsion: Effect of polysaccharides concentration on the storage stability and interfacial rheological properties. Food Hydrocolloids, 101, 105490. https://doi.org/10.1016/j.foodhyd.2019.105490
- Wu, Q., Wang, Q., Fu, J., & Ren, R. (2019). Polysaccharides derived from natural sources regulate triglyceride and cholesterol metabolism: A review of the mechanisms. Food & Function, 10(5), 2330–2339. https://doi.org/10.1039/c8fo02375a
- Yamauchi, K., Shimizu, M., & Kamiy, T. (2006). Emulsifying properties of whey protein. Journal of Food Science, 45(2), 1365–2621. https://doi.org/10.1111/j.1365-2621.1980.tb06529.x
- Yuan, Y. Y., Bone, D. E., & Carrington, M. F. (2005). Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chemistry, 91(3), 485–494. https://doi.org/10.1016/j.foodchem.2004.04.039
