?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Litopenaeus vannamei easily blackens during cold storage. To inhibit blackening and improve the storage quality of prawns, in this study, konjac glucomman (KGM)-epigallocatechin gallate (EGCG) topological microgel was formed and used for storage and preservation of prawns to improve their antioxidant properties. The effect of KGM-EGCG topological microgel on inhibiting shrimp blackening was studied by measuring the sensory evaluation score, pH value, TVB-N value, K value, PPO activity, and the effect of changes in the total number of bacteria of KGM-EGCG-treated prawns during storage. SEM and FTIR results showed that during the KGM-EGCG binding process, the number of hydrogen bond connections and the intermolecular force increased. Results also showed that the KGM microgel can effectively embed EGCG and enhance its stability to achieve a better controlled release effect. Compared with the EGCG group and untreated group, KGM-EGCG microgel has better anti-PPO activity and can better maintain the organoleptic quality and inhibit bacterial growth of Litopenaeus vannamei. Therefore, the KGM-EGCG microgel could slow down the increase in pH value, the production of volatile base nitrogen, and the increase in K value, and effectively extend the shelf life of Litopenaeus vannamei from 3 d to 6 d, providing a theoretical basis for the research of KGM functional gel and a reference for the deep processing and value enhancement of green tea and shrimp.
RESUMEN
Litopenaeus vannamei se ennegrece fácilmente durante el almacenamiento en frío. Con el propósito de inhibir el ennegrecimiento, mejorar la calidad de conservación y potenciar las propiedades antioxidantes de los langostinos, en este estudio se utilizó un microgel topológico de konjac glucomanano (KGM) y galato de epigalocatequina (EGCG) para almacenar y conservar los mismos. El efecto del microgel topológico KGM-EGCG en la inhibición del ennegrecimiento de los langostinos se estudió midiendo la puntuación de la evaluación sensorial, el valor del pH, el valor TVB-N, el valor K, la actividad PPO y los cambios en el número total de bacterias de los langostinos tratados con KGM-EGCG durante el almacenamiento. Los resultados de SEM y FTIR dieron cuenta de que durante el proceso de unión de KGM-EGCG aumentaron el número de conexiones de enlace de hidrógeno y la fuerza intermolecular. Asimismo, los resultados permitieron constatar que el microgel KGM puede incrustar eficazmente el EGCG y mejorar su estabilidad para lograr un mejor efecto de liberación controlada. En comparación con el grupo de EGCG y el grupo no tratado, el microgel KGM-EGCG muestra una mejor actividad anti-PPO y puede mantener mejor la calidad organoléptica e inhibir el crecimiento bacteriano del Litopenaeus vannamei. Por lo tanto, el microgel KGM-EGCG podría ralentizar el aumento del valor del pH, la producción de nitrógeno base volátil y el aumento del valor K, extendiendo eficazmente la vida útil de Litopenaeus vannamei de tres a seis días. Ello proporciona una base teórica para la investigación del gel funcional KGM y una referencia para el procesamiento profundo y la mejora del valor del té verde y el camarón.
1. Introduction
Shrimp provides the human body with high-quality, rich protein, calcium, and various extractable compounds and minerals while being low in calories and fat (Fisheries Administration of Ministry of Agriculture and Rural Affairs of People’s Republic of China (FAMA), Citation2018). It is one of the most popular arthropods in the world. However, during the shrimp storage process, the blackening of the shrimp body seriously affects the sensory quality of the shrimp, and the polyphenol oxidase (PPO) in the shrimp is the main factor that causes the blackening of the shrimp (Lv, Zhang, et al., Citation2017). Refrigeration or freezing is the traditional method that is used to control bacterial spoilage which helps delay the blackening of shrimps. However, during the period of refrigeration or freezing, the PPO remains active, and the problem of blackening still exists (D. Xu et al., Citation2018), resulting in a decline in the sensory quality of shrimp and the loss of its consumption value. The use of chemical preservatives has good effects but has potential health risks. Its use in food is strictly restricted by regulatory agencies. Therefore, there is an urgent need to find new and safer alternatives.
Extracting natural antioxidants from fruits, vegetables, and medicinal and edible plants provides a new perspective for food preservation. Epigallocate polyphenol gallate (EGCG) is a plant polyphenol, which is an effective natural antioxidant in green tea and has the strongest free radical scavenging and antioxidant ability among tea polyphenols (Lv, Cai, et al., Citation2017). Studies have found that EGCG has good active effects, including antioxidant activity, neuroprotection and cardiovascular and cerebrovascular system activity (Y. Chen et al., Citation2018; Ellinger et al., Citation2012; Xie et al., Citation2010), promotion of immune endocrine (Menegazzi et al., Citation2020), anti-tumor, and antibacterial and metabolic enzyme intervention activities (Gan et al., Citation2018; Kumazoe et al., Citation2017; Rady et al., Citation2018).
EGCG is widely used in food processing and storage because of its strong antioxidant capacity, and studies showed that high-dose EGCG can help increase the availability of fat-soluble antioxidant vitamin E. Therefore, fish fed with high-dose EGCG have lower levels of liver lipid hydrogen peroxide and better antioxidant properties (Thawonsuwan et al., Citation2010). Clinical health care experimental studies showed that EGCG can significantly enhance the body’s antioxidant capacity, effectively reduce the production of peroxides in the body, and significantly improve enzyme-dependent and non-enzyme-dependent antioxidant functions (Cao, Citation2013; Martínez Leal et al., Citation2018). Studies have shown that treatment with EGCG can slow down the pH value change of grass carp fillet and has the effect of slowing down spoilage (Cao et al., Citation2020). It can also effectively delay the degeneration and degradation of myofibril protein, and slow down the oxidative deterioration of surimi, thus being a good new type of surimi antifreeze (Sae-leaw et al., Citation2017).
Studies also showed that EGCG has an inhibitory effect on shrimp blackening (Y. Q. Xu et al., Citation2019), and as the concentration of EGCG increases, the preservation effect improves. However, previous studies showed that the use of EGCG to inhibit shrimp blackening during cold storage is still relatively rare. Moreover, EGCG is unstable under strong acid, strong base, light, and high heat conditions, and its bioavailability in the body is also low, thereby limiting the application of EGCG (Trott & Olson, Citation2010). The use of microgel as a carrier is an effective way to improve the stability and bioavailability of EGCG (Yang, Liu, et al., Citation2017; Huang et al., Citation2010).
Therefore, by constructing a polymer-functional factor conjugate system with konjac glucomman (KGM) topological microgel as a carrier, this study established a stable and reliable gel system of polysaccharide-active substance conjugate structure, and a functional topological KGM microgel was prepared to verify the regulatory effect of functional topological KGM microgel on prawn blackening. The research results will provide theoretical basis and useful supplements for the research of KGM functional gel and a reference for the deep processing of green tea and prawn and the improvement of their practical value.
2. Materials and methods
Litopenaeus vannamei were purchased from the farmer’s market as freshwater cultured prawn without any antimelanotic treatment; konjac powder (KGM purity 95%) was acquired from Guangxi Duohuan Company; cellulase produced by Wuxi Enzyme Reagent Factory was purchased; EGCG was purchased from Chengdu Biopharmaceutical Co., Ltd.; EGCG reference substance was purchased from Shanghai Yuanye Company; adenosine diphosphate (ADP), adenylate (AMP), inosinic acid (IMP), inosine (HxR), and hypoxanthine (Hx) reference substance were purchased from Sigma Company.
2.1. Preparation of KGM-EGCG microgel
2.1.1. Preparation of low molecular weight KGM
KGM was weighed (3.00 g) and placed in a beaker. Then, 100 mL of distilled water was added to fully swell KGM in water to form a gel. Afterward, 180 U/g cellulase was added to the gel. At pH 6.0 and temperature of 50°C, enzymatic hydrolysis was performed for 30 min. Eighty percent ethanol was used as a precipitant, and the substance was stirred continuously until white flocculent precipitates were gradually produced. Ethanol solution was added continuously until no precipitation occurred. A glass rod was used to carefully transfer the white precipitate to a beaker, which was then washed twice with 95% ethanol. Finally, the product was transferred to a watch glass, and the sample was vacuum freeze-dried.
2.1.2. Preparation of KGM-EGCG gel
A certain amount of EGCG (0.2 g) was weighed and dissolved in 1% low molecular weight KGM solution. After the two were mixed at 50°C, stirring was performed for 60 min at a speed of 600 r/min to fully swell KGM and EGCG. The microgel was obtained, which was freeze-dried using a freeze dryer, and then taken out after 12 h to obtain a gel.
2.1.3. Preparation of EGCG solution
A certain amount of EGCG (0.2 g) was weighed and dissolved in 100 mL of aqueous solution, and then mixed thoroughly at 50°C to obtain an EGCG solution.
2.2. Characterization of KGM-EGCG gel
2.2.1. Scanning electron microscope
The surface morphology of KGM powder and KGM-EGCG microgel was observed under a scanning electron microscope (SEM), and the maximum acceleration voltage was 15 kV.
2.2.2. Infrared spectrum scanning
KGM and KGM-EGCG were fully crushed and compressed with KBr powder. The wave number of infrared spectroscopy was 4000 nm–400 nm, the resolution of the instrument was 0.5 nm, and the number of scans was 32/64.
2.3. Fresh-keeping effect of KGM-EGCG microgel on Litopenaeus vannamei
Live prawns were immersed in ice water (1:2 [w/v]) for 30 min. After washing and draining with distilled water, they were soaked in 4°C distilled water (untreated group), EGCG solution group, and KGM-EGCG microgel aqueous solution, respectively. They were then drained, placed in a sterile bag, and refrigerated at 4°C. Samples consisting of 10 prawns were taken every day to eliminate individual differences.
2.3.1. Sensory analysis
Five people in the laboratory provided sensory scores on the texture overall appearance and odor of prawns according to the 10-point score standard of the sensory evaluation table (Huang et al., Citation2010; ). Fresh odor, good overall appearance, and elastic firm flesh were 10 points. Prawns with strong spoilage odor, blackened body surface, and loose meat received 1 point. If the average score of the three items was less than 5 points, then the sample was no longer suitable for consumption.
Table 1. Criteria for sensory evaluation of Litopenaeus vannamei.
Tabla 1. Criterios de evaluación sensorial de Litopenaeus vannamei.
2.3.2. pH value measurement
A total of 5 g prawn muscle tissue of each group were weighed, ground evenly by mashing them with a mortar, and placed in a beaker. Then, 45 mL distilled water was added, and the mixture was stirred evenly with a magnetic force and stood for 30 min. The pH value of the filtrate was filtered and measured with an acidity meter.
2.3.3. Determination of volatile basic nitrogen
The People’s Republic of China standard GB 5009.228–2016 (NHFPC, Citation2016) was used as reference to determine the value of volatile basic nitrogen (TVB-N) in prawn muscle tissue samples.
2.3.4. K value determination
The People’s Republic of China Fisheries Industry Standard SC/T 3048–2014 (Ministry of Agriculture of the People’s Republic of China (MOA), Citation2014) was used as reference to determine the freshness of prawn. The K value is a freshness determination method that uses the decomposition products of nucleotides as an indicator. It is a ratio obtained by quantifying the ATP degradation products. After the fish dies, the ATP will degrade into ADP, AMP, IMP, HxR, and Hx. Two ATP degradation pathways exist in prawn muscle tissue, one of which is the same as that of fish muscle nucleotides. Therefore, the K value formula can be used to express the freshness change of prawn. The calculation formula for K value is as follows:
2.3.5. Determination of PPO activity
The prawn was washed and then 0.05 mol/L pH6.8 pre-cooled disodium hydrogen phosphate-citric acid buffer solution was added. The ratio of material to liquid is 1:1 (w/v), with an even quality. After centrifugation at 10,000 rpm for 20 min at 4°C, the resulting filtrate was discarded. The filter residue was rinsed with 5 times the volume of deionized water, and then 200 mL Tris-HCl (pH 6.8) buffer was added, sonicated for 10 min, and then allowed to stand for 1 h. The substance was then centrifuged at 10,000 rpm at 4°C for 15 min, placed in the refrigerator to stand for 30 min, then incubated in a 35°C water bath for 15 min. Finally, centrifugation was performed at 25°C for 15 min, and the supernatant obtained was PPO, as determined by colorimetry. First, 4 mL 0.2 mol/L phosphate buffer (pH 6.4) was added to 1 mL (0.1 mol/L) catechol solution, then 1 mL of the above extracted PPO solution was added. The substance was mixed well and immediately measured 420 nm at room temperature. The absorbance at the wavelength was measured every 20 s for a total of 3 min. The reaction solution without PPO extract was taken as the control group (control group: 5 mL buffer and 1 mL catechol solution), and then a graph of the relationship between absorbance A and reaction time was drawn. PPO was calculated based on the slope of the first straight line of the curve to reflect the enzyme activity. The enzyme activity of PPO is defined as follows: Under certain temperature and pH conditions, the amount of enzyme required to cause the absorbance value to change by 0.001 per min is one unit of enzyme activity (Q. S. Chen et al., Citation2008).
2.3.6. Total number of colonies
The People’s Republic of China standard GB 4789.2–2016 method (NHFPC, Citation2016) was used as reference. The plate culture counting method was used to determine the total number of colonies on the prawn muscle tissue samples of the control group and the experimental group.
3. Results
3.1. Study on KGM-EGCG microgel
3.1.1. SEM analysis
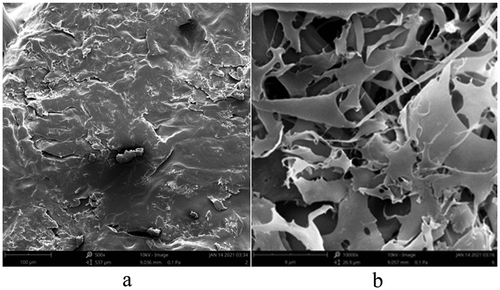
) shows that the KGM microgel had a block-like structure on the outside, which indicated that the molecular weight of KGM was very large, and the molecular chains interacted with each other to form tight molecular chain entanglements. ) shows that KGM-EGCG microgel formed a network structure, indicating that the long KGM molecular chains still existed after degradation. Parts of the long molecular chains were degraded into small molecular chains, which were sterically hindered because of the decrease in molecular weight. This condition is conducive to the formation of non-covalent interactions between KGM-EGCG molecular chains. The hydroxyl groups on the surface of KGM formed a rigid structure with water, forming a hydrophobic cavity or gap inside, and then EGCG was driven into the cavity or gap through hydrophobic interaction, and hydrogen bonds were formed between molecular chains to enhance its binding effect, resulting in an orderly topological entanglement.
3.1.2. FTIR analysis
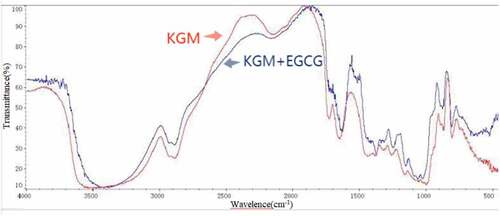
showed that the absorption peak of KGM-EGCG microgel at 3435 cm−1 (ab-sorption peak of -OH) was more intense than that of KGM gel, indicating that more hydrogen bonds were formed in KGM-EGCG microgel. The KGM-EGCG microgel had a more intense absorption peak at 2928 cm−1, which was the absorption peak of -CH in the methyl group. This finding proves that a more orderly formation between the KGM and EGCG molecular chains was found in the KGM-EGCG microgel. The topological entanglement was consistent with the electron microscopy results.
3.2. Preservation effect of KGM-EGCG microgel on Litopenaeus vannamei
3.2.1. Sensory evaluation
Under different pretreatment conditions, Litopenaeus vannamei was stored at a refrigeration temperature of 4°C. Under laboratory conditions, sensorial evaluation experiments were performed under fluorescent lamps to observe and describe the overall appearance, texture, and odor of Litopenaeus vannamei according to the sensory comprehensive score standard. The evaluation results showed that the sensory evaluation items of the untreated group on the fourth day after storage were divided into 4.6 (<5 points) on average, which was not suitable for consumption, and the edible period of the untreated group was 3 days. On the fifth day after storage, the sensory evaluation average score of prawns treated with EGCG was 4.4 (<5 points), so the optimal shelf life of the EGCG-treated group was 4 days. On the 6th day after storage, the average score of sensory evaluation items of prawn treated with KGM-EGCG was 5.11, and on the seventh day after storage, sensory evaluation average score was less than 5. Thus, the consumption period of the KGM-EGCG group was 6 days. Compared with the untreated group, the KGM-EGCG group can effectively extend the storage time of the prawn under refrigeration at 4°C.
The blackspot advancement results of the sensory comprehensive evaluation showed that pretreatment with EGCG solution and KGM-EGCG microgel had a certain inhibitory effect on delaying the blackening of Litopenaeus vannamei under the storage condition of 4°C. The experimental results are shown in . On the third day after storage, large areas of black spots appeared on the body surface of the prawns in the untreated group, and the head–body connection was loose. The prawns pretreated with EGCG showed black spots on the head and tail on the third day after storage, the flesh of the prawn was slightly white, the head and body were tightly connected, the eyeballs were slightly atrophied, and the head and tail had black spots. The prawns pretreated with KGM-EGCG microgel had a stiff texture, and the prawn meat was transparent and shiny. On the fifth day after storage, the prawns pretreated with EGCG had obvious black spots on the head and abdomen, while the KGM-EGCG group showed a few black spots on the head. On the sixth day, the meat quality and odor scores of the KGM-EGCG group were the highest and were statistically significantly higher than those of the control group (P < .05), followed by the EGCG group. On the seventh day after storage, the KGM-EGCG group showed obvious black spots on the prawn head and abdomen. Experiments showed that KGM-EGCG microgel can play a better role in maintaining the overall quality of prawn.
Figure 3. Sensory change of venabian shrimp at 4°C.
Figura 3. Cambio sensorial del camarón “venabian” a 4°C.
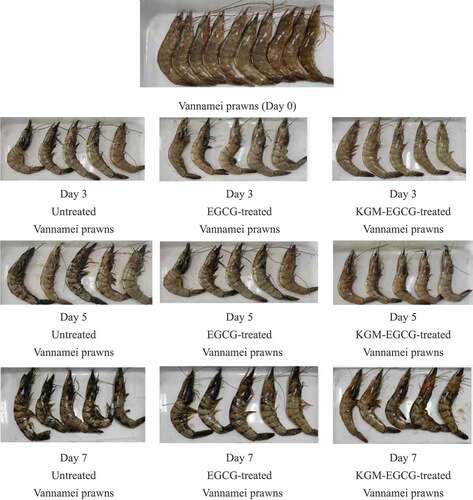
Figure 4. Changes in sensory quality of penaeus vannamei during storage at 4°C: (a) overall appearance; (b) texture; (c) odor.
Figura 4. Cambios en la calidad sensorial de Penaeus vannamei durante el almacenamiento a 4°C: (a) aspecto general; (b) textura; (c) olor.
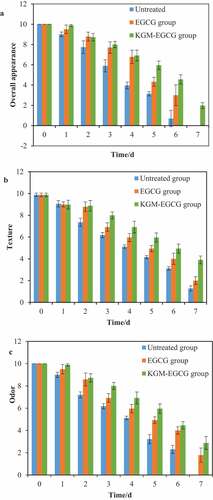
Sensory evaluation was conducted on the texture and odor of the body of Li-topenaeus vannamei. The comparison of sensory scoring results is shown in . Fresh Litopenaeus vannamei had transparent, shiny, and elastic meat; and a hard shell. Touching the prawn body with hands showed that its muscle tissue was firm with no peculiar smell. On the third day after storage, the untreated group of Litopenaeus vannamei had soft meat, a loosely connected shell, and a strong spoilage smell. On the fifth day after storage, in the EGCG group, the meat and prawn shell were significantly softer, and the connection between the meat and the shell was significantly loosened. The sensory evaluation score showed the prawn was not suitable for consumption.
In the KGM-EGCG group, on the third day after storage, the meat of the prawn body was more elastic, and the smell of the prawn was fresh. On the fifth day after storage, the meat became soft, the head and the body remain connected, resulting in a little of peculiar smell, sensory evaluation results showed it was suitable for eating. On the sixth day, the meat quality and smell scores of the KGM-EGCG group were the highest and were significantly higher than those of the control group (P < .05), which was statistically significant, followed by the EGCG group. On the seventh day, the meat was spongy and soft, the meat and shell fell off, and the smell of spoilage was strong. The results showed that KGM-EGCG can effectively maintain the meat quality and sensory quality of prawns.
3.2.2. pH value measurement
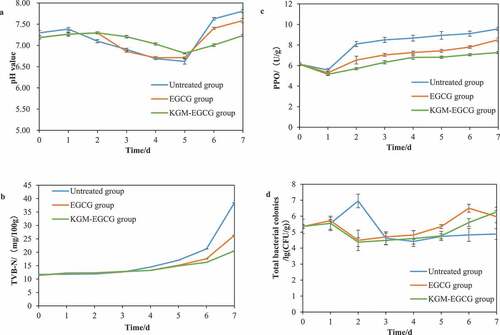
The normal pH value of live prawn meat of Litopenaeus vannamei is between 7.2 and 7.4. After death, the blood circulation and the supply of oxygen in the body stop, glycogen generates pyruvate in the body during the glycolysis process, and the pH value of the body drops after reaching the lowest point. As a result of the rapid growth of microorganisms and the action of its own enzymes, prawn meat degrades and produces alkaline substances such as amines, and the pH value rises accordingly. The higher the pH value at this time, the more spoiled the prawns are (Ezquerra et al., Citation1997). shows that the initial pH values of the experimental group and the untreated group are between 7.2 and 7.4. During the storage period, the pH values of the three groups all decreased first and then continuously increased. At the end of storage, the untreated group reached the highest pH value of 7.93, and the KGM-EGCG group had the lowest pH value of 7.4. The results were consistent with those of the study of Xiong Qing et al. (Citation2014). shows that the pH value of the untreated group changed greatly during storage. The pH value of the storage group decreased significantly on the second day, indicating that the storage and preservation conditions at 4°C have limited inhibition of the growth of prawn microorganisms and the effective preservation period was relatively short. The pH value of the EGCG group was within the normal range for the first two days during storage, indicating that the microbial growth was inhibited under the effect of EGCG, but due to the instability of EGCG, its functional activity decreased on the second day after storage, and the prawn microorganisms began to rapidly destroy the quality of prawn. Compared with the other two groups, the KGM-EGCG-treated group showed that the microgel can effectively control the growth of prawn microorganisms, which can better slow down the increase in the pH value of prawn meat and had a better antibacterial effect.
Figure 5. Factors changed during storage at 4°C: (a) the pH value; (b) the TVB-N content; (c) the PPO content; (d) the total bacterial population of Penaeus.
Figura 5. Factores modificados durante el almacenamiento a 4°C: (a) valor del pH; (b) contenido de TVB-N; (c) contenido de PPO; (d) población bacteriana total de Penaeus.
3.2.3. Determination of TVB-N
In the storage of Litopenaeus vannamei, some Aeromonas, Shewanella, Enterobacter, and Vibrio can decompose the prawn body and produce trimethylamine. At the same time, amino acids are used to produce biogenic amines and other nitrogen-containing volatile substances through decarboxylation (Le et al., Citation2017). Therefore, the content of TVB-N can be used to evaluate the freshness of prawn. shows that at the beginning of storage, the TVB-N of Litopenaeus vannamei was approximately 11 mg/100 g. During the storage process, the TVB-N of the three groups showed an overall upward trend. By the fifth day of storage, three groups did not exceed the national standard GB 2733–2015 for TVB-N (freshwater shrimp ≤20 mg/100 g; The National Health and Family Planning Commission of the People’s Republic of China (NHFPC), Citation2015). On the fifth day after storage, the KGM-EGCG group had the lowest TVB-N content, followed by the EGCG group, and a significant difference was found between the three groups (p < .01). The TVB-N content of the untreated group on the sixth day of storage exceeded the national standard requirement, reaching 20.23 mg/100 g. On the seventh day after storage, the TVB-N content of the KGM-EGCG treatment group was only 20.53 mg/100 g, indicating that the KGM-EGCG group was the most effective in inhibiting the production of TVB-N during storage compared with the other two groups.
3.2.4. Determination of K value
The national standard SC/T 3048–2014 by HPLC (Ministry of Agriculture of the People’s Republic of China (MOA), Citation2014) was used as reference to prepare the test sample and test its K value by HPLC. The sample size was 10 μL, and the sample was injected twice for the determination of ATP, ADP, AMP, HX, HxR, and IMP content. The chromatogram is shown in .
Figure 6. Chromatogram of ATP, ADP, AMP, HX, HxR, IMP reference standard sample, the superimposed chromatogram of reference standard sample and test sample, and the K value: (a) ATP,ADP,AMP,HX,HxR reference standard sample; (b) IMP reference standard sample; (c) Superimposed chromatogram of reference standard sample and test sample; (d) The K value of penaeus vannamei changed during storage at 4°C.
Figura 6. Cromatograma de ATP, ADP, AMP, HX, HxR, muestra estándar de referencia IMP, cromatograma superpuesto de la muestra estándar de referencia y la muestra de prueba, y valor K: (a) Muestra estándar de referencia de ATP, ADP, AMP,HX,HxR; (b) Muestra estándar de referencia de IMP; (c) Cromatograma superpuesto de la muestra estándar de referencia y de la muestra de prueba; (d) El valor K de Penaeus vannamei cambió durante el almacenamiento a 4°C.
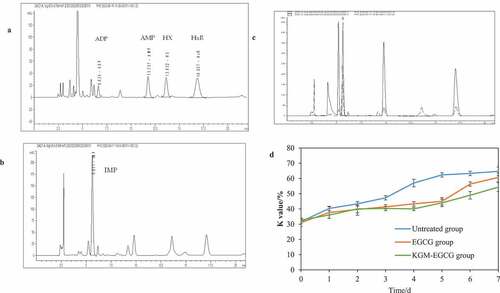
In the early stage of the death of aquatic products, the actions of the prawn’s own enzymes do not stop. At this time, nucleotides are gradually decomposed with the en-ergy substance ATP and ATP. The amount of HxR+Hx is correlated with ATP and the ratio of the total amount is defined as the K value (Saito, Citation1959). The K value can effectively reflect the internal changes of aquatic products and serve as a quality indicator for the freshness of aquatic products. The K value is used as an index to evaluate the freshness of aquatic products. The smaller the value, the better the freshness, and vice versa (Li et al., Citation2017). The K value calculated from the experimental measurement results is shown in , indicating that with the extension of storage time, the K value of Litopenaeus vannamei with different pretreatments showed an upward trend. The untreated group was stored at 4°C, its K value reached 48% on the third day, 56% on the fourth day, and 64% on the seventh day. The growth rate of KGM-EGCG group was slower and remained stable on the second, third, and fourth days, with a K value of 39%, and on the seventh day, the K value reached 53%. On the sixth day after storage, the K value in the KGM-EGCG group was the lowest, and significant differences were found between the three groups (p < .01). This finding indicates that KGM under an ordered network structure had a better embedding effect on EGCG, and the microgel sustained-release system was stable and can have a better sustained-release effect than the EGCG group. Moreover, the KGM-EGCG group had a stronger antibacterial effect, which enhanced the storage and preservation effect of Litopenaeus vannamei.
3.2.5. Determination of PPO activity
The blackening of Litopenaeus vannamei is mainly related to the action of poly-phenol oxidase. Thus, mastering the change of PPO activity during storage is an effective way to study the inhibition of blackening. shows that the PPO activity of the three groups during the storage period showed a change that first decreased and then continuously increased. Experiments showed cold storage had a negligible effect on PPO activity (Bono et al., Citation2010). Under the same conditions, the KGM-EGCG group had a better PPO inhibitory effect than the other two groups. The analysis of the experimental results showed that the PPO activity in the KGM-EGCG group was the lowest. On the fifth day after storage, a statistically significant difference existed between the three groups (p < .01). The KGM-EGCG group encapsulated EGCG well and protected the activity of EGCG because the KGM molecules formed a more stable and orderly topological network structure. Moreover, after KGM embedded EGCG, the hydrogen bond between the two was enhanced, which made the sustained release system more stable and enhanced the sustained release of the microgel.
3.2.6. Determination of total bacterial colonies
After the death of Litopenaeus vannamei, the microorganisms in the prawn body will use the nutrients in the body and multiply, which will accelerate the decline of sensory quality (Aponte et al., Citation2018). shows that the microbial growth rate of the untreated group increased significantly from the second day of storage, and the rapid reproduction reached more than 7 (lg [CFU/g]). As the prawn protein was decomposed completely, the microbial colonies gradually stop to renew and passage, and the total number of colonies decreased, and its refrigerated shelf life was about 3 days. The EGCG and KGM-EGCG groups inhibited the growth of prawn colonies to a certain extent so that the colony growth of Litopenaeus vannamei after the two pretreatments was well controlled, but with the failure of EGCG functional components, the colony activity increased, and the total number of colonies increased slowly. On the sixth day of storage, the total number of colonies in the EGCG group exceeded 6 (lg [CFU/g]), and the colonies multiplied, and then the total number of colonies fell back. The results showed that the KGM-EGCG group had better control of the total number of colonies during storage than the other two groups, followed by the EGCG group, and the untreated group was the worst. The result analysis showed that KGM microgel enhanced the antibacterial ability of EGCG. Through controlled release, EGCG could bind well to the peptidoglycan of the cell wall of Gram-positive bacteria. After the combination, EGCG interfered with the bacterial protein and protein through hydrogen bonding. and peptides gradually dissolved the positive bacteria (Cui et al., Citation2012). On the sixth day, the total number of colonies in the KGM-EGCG group was the lowest, and a statistically significant difference was found among the three groups (p < .01).
4. Discussion
Tea polyphenols are a kind of plant polyphenol, which mainly include EC, EGC, ECG, and EGCG. They are effective natural antioxidants in green tea and can be used as antioxidants and preservatives (Sae-leaw & Benjakul, Citation2019; Shannon et al., Citation2018). EGCG has the strongest ability to inhibit PPO activity, but it is unstable under strong acid, strong alkali, light, and high heat conditions (Maldonado-Valderrama et al., Citation2017; Xiao et al., Citation2018). In the present work, KGM-EGCG, a polymer-functional factor skeleton system, was synthesized with KGM as a carrier, and its effect on blackening control of Litopenaeus prawn was studied to improve the stability of EGCG and increase its bioavailability.
In this study, KGM was degraded by enzymes to reduce the molecular weight, degrading part of the long molecular chains into short chains, reduced the steric hindrance of the network system, and increased the entanglement space within the molecular chains to form an effective topological entanglement so that the ordered entanglement structure was conducive to the preparation of KGM-EGCG microgels and the order of the KGM topology network structure can be regulated. This ordered topological network structure provided a good network framework for EGCG (Wang et al., Citation2020), reduced the friction between KGM and EGCG molecular chains during the EGCG penetration process, and enhanced the intermolecular hydrogen bond force to form a stable network structure to enhance the oxidation inhibition and slow the release of EGCG (Sun et al., Citation2020).
Instrumental analysis proved that the number of methyl groups in the molecular chain of KGM-EGCG microgels, the number of intramolecular and intermolecular hydrogen bonds, and the intermolecular forces all increased, resulting in an ordered network structure (Zhu, Citation2021b). The network structure plays a role in EGCG due to the effect of topological constraints so that the system maintains good stability and controlled release (Wang et al., Citation2020).
The appearance and color of the prawn are of great significance for evaluating the freshness of the prawn and to see whether any bad changes occur. Sensory experiments proved that compared with the EGCG group, KGM-EGCG microgel can better maintain the sensory quality of prawn, effectively delaying the appearance of peculiar smell, softening of the meat, and appearance of black spots. The conformation and aggregation state of the molecular chain will affect the microgel structure (Maldonado-Valderrama et al., Citation2017). Studies have shown that the degradation of the KGM molecular chain contributes to the formation of a regular network, thereby enhancing intermolecular forces, providing a better network support framework for EGCG, and retaining its biological activity, thereby producing a better controlled release effect (Cheng et al., Citation2022).
The stability of EGCG affects its antibacterial ability. Studies showed that KGM-EGCG microgels enhance the stability of EGCG (Cheng et al., Citation2022; Sun et al., Citation2020). Through controlled release, EGCG can bind well to the peptidoglycan of the cell wall of Gram-positive bacteria. After the binding, EGCG interfered with bacterial proteins through hydrogen bonding. With peptides, the positive bacteria were gradually dissolved. During the reaction, EGCG can also destroy some of the polyunsaturated fatty acids on the cell membrane of the Gram-negative bacteria by releasing H2O2, destroy the cell membrane of the bacteria, and achieve the effect of promoting the death of the negative bacteria (Shannon et al., Citation2018). Thus, KGM-EGCG effectively inhibits the growth of bacteria caused by the increase in pH value, the production of nitrogen-containing volatile substances, and the increase in K value.
In this study, no modifiers and catalysts were used to prepare the KGM-EGCG microgels. Thus, no toxic groups were introduced, and no secondary pollution was caused during the preparation. Through reasonable regulation, it produced an orderly topological network structure. The biological activity of KGM was retained, and a better controlled-release effect was produced. Research results showed that KGM microgel can effectively embed EGCG and enhance its stability, achieving a better controlled-release effect while using fewer preservatives. The results of this research provide a theoretical basis and beneficial supplements for the research of KGM functional gel and also provide a reference for the deep processing of green tea and prawn and the improvement of practical value.
Author contributions
Conceptualization, Y.L.W.; methodology, P.L.; software, T.Z.; validation, J.N.W.; formal analysis, T.Z.; investigation, Y.L.W.; resources, J.H. X.; data curation, Y.L.W.; writing—original draft preparation, Y.L.W.; writing—review and editing, J.P.; visualization, T.Z.; supervision, J.P.; project administration, J.P.; funding acquisition, J. H.X. and J.P. All authors have read and agreed to the published version of the manuscript.”
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aponte, M., Anastasio, A., Marrone, R., Mercogliano, R., Peruzy, M. F., & Murru, N. (2018). Impact of gaseous ozone coupled to passive refrigeration system to maximize shelf-life and quality of four different fresh fish products. LWT, 93, 412–419. https://doi.org/10.1016/j.lwt.2018.03.073
- Bono, G., Badalucco, C., Corrao, A., Cusumano, S., Mammina, L., & Palmegiano, G. B. (2010). Effect of temporal variation, gender and size on cuticle polyphenol oxidase activity in deep-water rose shrimp (Parapenaeus longirostris). Food Chemistry, 123(2), 489–493. https://doi.org/10.1016/j.foodchem.2010.04.055
- Cao, H. (2013). Polysaccharides from Chinese tea: Recent advance on bioactivity and function. International Journal of Biological Macromolecules, 62, 76–79. https://doi.org/10.1016/j.ijbiomac.2013.08.033
- Cao, J., Wang, Q., Ma, T., Bao, K., Yu, X., Duan, Z., Shen, X., & Li, C. (2020). Effect of EGCG-gelatin biofilm on the quality and microbial composition of tilapia fillets during chilled storage. Food Chemistry, 305, 125454. https://doi.org/10.1016/j.foodchem.2019.125454
- Chen, Y., Chen, J., Sun, X., Shi, X., Wang, L., Huang, L., & Zhou, W. (2018). Evaluation of the neuroprotective effect of EGCG: A potential mechanism of mitochondrial dysfunction and mitochondrial dynamics after subarachnoid hemorrhage. Food & Function, 9(12), 6349–6359. https://doi.org/10.1039/C8FO01497C
- Chen, Q. S., Yan, Y. L., Yang, X. Q., & Ma, Y. (2008). Effects of different temperatures on polyphenoloxidase activity, microbiological index and freshness of metapenaeus ensis [J]. Food Science, 29, 622–624.
- Cheng, C., Gao, H., McClements, D. J., Zeng, H., Ma, L., Zou, L., Miao, J., Wu, X., Tan, J., Liang, R., & Liu, W. (2022). Impact of polysaccharide mixtures on the formation, stability and EGCG loading of water-in-oil high internal phase emulsions. Food Chemistry, 372, 131225. https://doi.org/10.1016/j.foodchem.2021.131225
- Cui, Y., Oh, Y. J., Lim, J., Youn, M., Lee, I., Pak, H. K., Park, W., Jo, W., & Park, S. (2012). AFM study of the differential inhibitory effects of the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) against Gram-positive and Gram-negative bacteria. Food Microbiology, 29(1), 80–87. https://doi.org/10.1016/j.fm.2011.08.019
- Ellinger, S., Reusch, A., Stehle, P., & Helfrich, H. P. (2012). Epicatechin ingested via cocoa products reduces blood pressure in humans: A nonlinear regression model with a Bayesian approach. The American Journal of Clinical Nutrition, 95(6), 1365–1377. https://doi.org/10.3945/ajcn.111.029330
- Ezquerra, J. M., García-Carreño, F. L., Civera, R., & Haard, N. F. (1997). pH-stat method to predict protein digestibility in white shrimp (Penaeus vannamei). Aquaculture, 157(3–4), 251–262. https://doi.org/10.1016/S0044-8486(97)00058-6
- Fisheries Administration of Ministry of Agriculture and Rural Affairs of People’s Republic of China (FAMA). (2018). China fisheries statistics yearbook. China Agriculture Press.
- Gan, R. Y., Li, H. B., Sui, Z. Q., & Corke, H. (2018). Absorption, metabolism, anti- cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Critical Reviews in Food Science and Nutrition, 58(6), 924–941. https://doi.org/10.1080/10408398.2016.1231168
- Huang, H., Li, L., Yang, X., Hao, S., Shi, H., & Cen, J. (2010). Quality grading factors and evaluation technology of prawn [J]. Journal of Fishery Sciences of China, 17, 1371–1376. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2010&filename=ZSCK201006036&uniplatform=NZKPT&v=zO9S5T6JQUZY6IDWabbXlrcLPEAVo2AaqJvU9DImz_GOrj3oZZvbV5zMrhxtfa3V.
- Kumazoe, M., Yamashita, M., Nakamura, Y., Takamatsu, K., Bae, J., Yamashita, S., Yamada, S., Onda, H., Nojiri, T., Kangawa, K., & Tachibana, H. (2017). Green tea polyphenol EGCG upregulates Tollip expression by suppressing Elf-1 expression. The Journal of Immunology, 199(9), 3261–3269. https://doi.org/10.4049/jimmunol.1601822
- Le, N. T., Doan, N. K., Ba, T. N., & Tran, T. V. T. (2017). Towards improved quality benchmarking and shelf life evaluation of black tiger shrimp (Penaeus monodon). Food Chemistry, 235, 220–226. https://doi.org/10.1016/j.foodchem.2017.05.055
- Li, Y., Yang, Z., & Li, J. (2017). Shelf‐life extension of Pacific white shrimp using algae extracts during refrigerated storage. Journal of the Science of Food and Agriculture, 97(1), 291–298. https://doi.org/10.1002/jsfa.7730
- Lv, Y., Cai, L., Yang, M., Liu, X., Hui, N., & Li, J. (2017). Purification, characterisation, and thermal denaturation of polyphenoloxidase from prawns (Penaeus vannamei). International Journal of Food Properties, 20(sup3), S3345–S3359. https://doi.org/10.1080/10942912.2017.1354019
- Lv, H. P., Zhang, Y., Shi, J., & Lin, Z. (2017). Phytochemical profiles and antioxidant activities of Chinese dark teas obtained by different processing technologies. Food Research International, 100, 486–493. https://doi.org/10.1016/j.foodres.2016.10.024
- Maldonado-Valderrama, J., Del Castillo-santaella, T., Adroher-Benítez, I., Moncho- Jordá, A., & Martín-Molina, A. (2017). Thermoresponsive microgels at the air–water interface: The impact of the swelling state on interfacial conformation. Soft Matter, 13(1), 230–238. https://doi.org/10.1039/C6SM01375A
- Martínez Leal, J., Valenzuela Suárez, L., Jayabalan, R., Huerta Oros, J., & Escalante- Aburto, A. (2018). A review on health benefits of kombucha nutritional compounds and metabolites. CyTA-Journal of Food, 16(1), 390–399. https://doi.org/10.1080/19476337.2017.1410499
- Menegazzi, M., Campagnari, R., Bertoldi, M., Crupi, R., Di Paola, R., & Cuzzocrea, S. (2020). Protective effect of epigallocatechin-3-gallate (EGCG) in diseases with uncontrolled immune activation: Could such a scenario be helpful to counteract COVID-19? International Journal of Molecular Sciences, 21(14), 5171. https://doi.org/10.3390/ijms21145171
- Ministry of Agriculture of the People’s Republic of China (MOA). (2014). Referencing: Determination of K value of fish freshness indexhigh performance liquid chromatography: SC/T 3048-2014. Agriculture Press of China.
- National Health and Family Planning Commission of China (NHFPC), China Food and Drug Administration. (2016). Referencing: national food safety standard food microbiological examination aerobic plate count: GB 4789.2-2016. Standards Press of China.
- The National Health and Family Planning Commission of the People’s Republic of China(NHFPC). (2015). Referencing: National food safety standard: Fresh, frozen aquatic products of animal origin: GB 2733-2015. Standards Press of China.
- National Health and Family Planning Commission of the People’s Republic of China(NHFPC). (2016). Referencing: Determination of volatile base nitrogen in food: GB/5009.228-2016. Standards Press of China.
- Rady, I., Mohamed, H., Rady, M., Siddiqui, I. A., & Mukhtar, H. (2018). Cancer preventive and therapeutic effects of EGCG, the major polyphenol in green tea. Egyptian Journal of Basic and Applied Sciences, 5(1), 1–23. https://doi.org/10.1016/j.ejbas.2017.12.001
- Sae-leaw, T., & Benjakul, S. (2019). Prevention of melanosis in crustaceans by plant polyphenols: A review. Trends in Food Science & Technology, 85, 1–9. https://doi.org/10.1016/j.tifs.2018.12.003
- Sae-leaw, T., Benjakul, S., & Simpson, B. K. (2017). Effect of catechin and its derivatives on inhibition of polyphenoloxidase and melanosis of Pacific white shrimp. Journal of Food Science and Technology, 54(5), 1098–1107. https://doi.org/10.1007/s13197-017-2556-1
- Saito, T. (1959). A new method for estimating the freshness of fish. Nippon Suisan Gakkaishi, 24, 749–750. https://doi.org/10.2331/suisan.24.749
- Shannon, E., Jaiswal, A. K., & Abu-Ghannam, N. (2018). Polyphenolic content and antioxidant capacity of white, green, black, and herbal teas: A kinetic study. Food Research, 2(1), 1–11. https://doi.org/10.26656/fr.2017.2(1).117
- Sun, J., Jiang, H., Li, M., Lu, Y., Du, Y., Tong, C., Pang, J., & Wu, C. (2020). Preparation and characterization of multifunctional konjac glucomannan/carboxymethyl chitosan biocomposite films incorporated with epigallocatechin gallate. Food Hydrocolloids, 105, 105756. https://doi.org/10.1016/j.foodhyd.2020.105756
- Thawonsuwan, J., Kiron, V., Satoh, S., Panigrahi, A., & Verlhac, V. (2010). Epigallocatechin-3-gallate (EGCG) affects the antioxidant and immune defense of the rainbow trout, Oncorhynchus mykiss. Fish Physiology and Biochemistry, 36(3), 687–697. https://doi.org/10.1007/s10695-009-9344-4
- Trott, O., & Olson, A. J. (2010). AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31(2), 455–461. https://doi.org/10.1002/jcc.21334
- Wang, L., Lin, L., Guo, Y., Long, J., Mu, R. J., & Pang, J. (2020). Enhanced functional properties of nanocomposite film incorporated with EGCG-loaded dialdehyde glucomannan/gelatin matrix for food packaging. Food Hydrocolloids, 108, 105863. https://doi.org/10.1016/j.foodhyd.2020.105863
- Xiao, Z., Huang, X., Zang, Z., & Yang, H. (2018). Spatio-temporal variation and the driving forces of tea production in China over the last 30 years. Journal of Geographical Sciences, 28(3), 275–290. https://doi.org/10.1007/s11442-018-1472-2
- Xie, J., Jiang, L., Zhang, T., Jin, Y., Yang, D., & Chen, F. (2010). Neuroprotective effects of Epigallocatechin-3-gallate (EGCG) in optic nerve crush model in rats. Neuroscience Letters, 479(1), 26–30. https://doi.org/10.1016/j.neulet.2010.05.020
- Xiong, Q., Xie, J., Qian, Y. F., & Yang, S. P. (2014). Effect of tea polyphenol complex preservative on the quality of Penaeus vannamei under cold storage. Food Science, 2, 287–291. https://doi:10.7506/spkx1002-6630-201402056
- Xu, D., Sun, L., Li, C., Wang, Y., & Ye, R. (2018). Inhibitory effect of glucose oxidase from Bacillus sp. CAMT22370 on the quality deterioration of Pacific white shrimp during cold storage. LWT, 92, 339–346. https://doi.org/10.1016/j.lwt.2018.02.025
- Xu, Y. Q., Yu, P., & Zhou, W. (2019). Combined effect of pH and temperature on the stability and antioxidant capacity of epigallocatechin gallate (EGCG) in aqueous system. Journal of Food Engineering, 250, 46–54. https://doi.org/10.1016/j.jfoodeng.2019.01.016
- Yang, R., Liu, Y., Gao, Y., Wang, Y., Blanchard, C., & Zhou, Z. (2017). Ferritin glycosylated by chitosan as a novel EGCG nano-carrier: Structure, stability, and absorption analysis. International Journal of Biological Macromolecules, 105, 252–261. https://doi.org/10.1016/j.ijbiomac.2017.07.040
- Zhou, T., Zhu, M., & Liang, Z. (2018b). (-)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson’s disease. Molecular Medicine Reports, 17(4), 4883–4888. https://doi.org/10.3892/mmr.2018.8470
- Zhu, F. (2021b). Polysaccharide based films and coatings for food packaging: Effect of added polyphenols. Food Chemistry, 359, 129871. https://doi.org/10.1016/j.foodchem.2021.129871