ABSTRACT
Food containing bioactive elements can be exploited to combat cardiovascular diseases like hypertension. This article mainly focuses on the use of dairy peptides and microelements of plants against two major causes of hypertension, e.g. ACE (Angiotensin converting enzyme) inhibition with the help of bioactive peptides and improving endothelial Nitric Oxide (eNO) availability with the help of antioxidants. The main objective of this study is to highlight the bioactive peptides and give a way to produce safe ACE inhibitory food supplements by replacing chemical drugs. The efficiency of milk-based bioactive peptides is discussed in detail from in-vitro to clinical trials, but the data is insufficient to prove the bioactivity of milk protein and peptides to replace drugs. More clinical trials are needed to provide a concrete base for the food and pharmaceutical industry in producing ACE inhibitory peptides. This review will be helpful to produce food-based anti-hypertensive drugs industrially.
RESUMEN
Los alimentos que contienen elementos bioactivos pueden aprovecharse para combatir enfermedades cardiovasculares como la hipertensión. Este artículo se centra, principalmente, en el uso de péptidos lácteos y de microelementos de las plantas para combatir dos de las causas más importantes de hipertensión, esto es, la inhibición de la ECA (enzima convertidora de angiotensina) con la ayuda de péptidos bioactivos y la mejora de la disponibilidad del óxido nítrico endotelial (ONe) con la ayuda de antioxidantes. El objetivo principal de este estudio es poner de relieve los péptidos bioactivos y proporcionar una vía para producir complementos alimenticios inhibidores de la ECA seguros, que sustituyan a los fármacos químicos. Si bien se revisa en detalle la eficacia de los péptidos bioactivos de la leche, desde los ensayos in vitro hasta los clínicos, los datos resultan insuficientes para demostrar la bioactividad de proteínas y péptidos de la leche como sustituta de los fármacos. A fin de proveer una base concreta a las industrias alimentaria y farmacéutica para la producción de péptidos inhibidores de la ECA se necesitan más ensayos clínicos. Esta revisión podrá ser de utilidad para producir industrialmente fármacos antihipertensivos basados en alimentos.
1. Introduction
Hypertension is graded as the most repeatedly cardiovascular risk factor with a frequency of 25–30% across the globe (Kearney et al., Citation2005). Improper management of hypertension claimed the lives of 7 million people with 64 million life-long disabilities worldwide (Perkovic et al., Citation2007). It is defined as a condition in which the systolic blood pressure (SBP), and diastolic blood pressure (DBP) remain ≥140 mmHg, and ≥90 mmHg. Blood pressure is categorized as the speed of blood flow within the walls of blood vessels (Bakris, Citation2015).
Blood pressure keeps changing with the movement and rest; it is low during sleep, increases after waking up, and changes with excitement and anxiety. It also differs with age, race, size, gender, lifestyle, family history of the disease, poorly managed stress, and physical activity. Blood pressure also increases with age because arteries become stiffer and narrower, especially with plaque build-up. Some diseases are also involved in elevating blood pressure, notably diabetes, kidney disease, pheochromocytoma, Cushing syndrome, and congenital adrenal hyperplasia. In comparison, some medications like oral contraceptives, hormone therapy for menopause, and excessive intake of alcohol are a contributor to elevated blood pressure (Bethesda, Citation2015). High blood pressure is considered a basic risk factor for cardiovascular morbidity and mortality. It is associated with coronary artery disease, heart failure, stroke, as well as the progression of chronic kidney disease.
One of the main causative agents of hypertension is ACE; kininase II; EC 3.4.15.1, which is carboxy-dipeptidyl-metallopeptidase and the main linked enzyme of the renin-angiotensin system (RAS). It is involved in the regulation of peripheral blood pressure that mainly catalyzes the production of angiotensin-II from angiotensin-I (a vasoconstrictor) and inactivation of the vasodilator bradykinin (Gobbetti et al., Citation2000; Kim et al., Citation2004).
Another causative agent in growing hypertension is NO (a vasodilator) unavailability to endothelial cells respectively. It is a gaseous and lipophilic signaling molecule that is involved in maintaining blood pressure normal. NO is produced by endothelial NO synthase that is diffused into vascular smooth muscle cells and results in triggering the soluble guanylyl cyclase-cyclic guanosine monophosphate-dependent protein kinase pathway that eventually acts as a vasodilator.
Problems in NO metabolism and production of ACE are the main causative agents of hypertension that need to be addressed with the use of functional foods and bioactive elements.
2. Pathogenesis of hypertension
Arteries carry blood away from the heart to supply it to tissues (with oxygen and nutrients). In the heart, ventricles contract with each heartbeat to push blood to the lungs and through arteries to the whole body. As blood flows through arteries, three main factors affect the pressure on artery walls. The first one is cardiac output as blood pressure goes up as cardiac output increases. The second factor is blood volume, blood pressure goes up as blood volume increases. The third factor is the resistance of anything working against the blood flow through arteries. Resistance of blood flow occurs due to the flexibility of the artery wall (healthy arteries expand with each heartbeat to reduce blood pressure on the wall), the diameter of arteries (the human body is able to increase the diameter of the arteries to decrease the blood pressure or reduce the diameter to increase the blood pressure) and blood viscosity (such as proteins and fats increase blood viscosity). If blood is thicker, blood pressure goes up as the heart works harder to push it through arteries. When the heart beats, the blood pressure on the walls of arteries is called systolic SBP, and when the heart relaxes between beats, pressure on the artery walls is called DBP. Blood pressure may change throughout the day. If SBP frequently stays 140 mmHg or DBP frequently stays above 90, then this condition is considered hypertension. After a certain period, high blood pressure damages the walls of arteries, and as a result, it may become weaker and form an enlargement called an aneurysm, or the walls may burst eventually. This artery damage and increased blood flow lead to conditions such as stroke and heart attack (Oparil et al., Citation2003).
3. Role of RAS in growing hypertension
RAS is an endocrine system that actually regulates blood pressure. Previously, RAS was not considered part of the endocrine system, but with the advancement in molecular biology, it was reported that most of the organs contain those specific genes and mRNAs that are responsible for encoding the polypeptides of the RAS (Miyazaki et al., Citation1984).
The renal artery is a branch of the Aorta and part of RAS that supplies blood from the heart to the kidney. Afferent arteries are also involved to supply blood from the heart to the kidney, as well as to the glomerulus. There are smooth muscle cells in the afferent arteries that sense the reduction in blood pressure and release an enzyme called renin in response [Renin is a soluble protein that was extracted from the kidney of rabbit in the 19th century and its role in regulating arterial blood pressure was observed (Goldblatt et al., Citation1934)]. More renin is released in case of a drop in blood pressure. On the other hand, the liver produces a protein called angiotensinogen (serum α2-globulin in nature) and is released into the circulation (Paul et al., Citation2006; van Haaster et al., Citation2018). When this angiotensinogen combines with renin, it splits into decapeptide, angiotensinogen-1 (Ang-I). When this Ang-I goes into the lungs through blood circulation, it is converted into angiotensinogen-II (Ang-II) by the action of ACE. Ang-II can only be produced if renin and converting enzymes are present in the blood circulation. Ang-I is released only when renin cleaves the LV bond of angiotensinogen while Ang-II is produced as the result of cleaving Phenylalanine and Histidine by ACE (Ferrario, Citation1990). Two G-protein-coupled receptors: AT1R and AT2R are responsible to effectuate the biological character of Ang-II (Henrion et al., Citation2001; Tate, Citation2017; Touyz & Schiffrin, Citation2000; Zhang et al., Citation2017). Ang-II is a vasoconstrictor that constricts peripheral arterial and inactivates a vasodilator called bradykinin. Ang-II also stimulates the secretion of aldosterone from adrenal glands. Being a steroid hormone, it increases the reabsorption of sodium from renal tubules and increases the amount of sodium in the blood (Schütten et al., Citation2017). As sodium is more osmotic, it attracts more water, and that increases intravascular volume. Intravascular volume and venous return are directly proportional to each other. According to the Frank-starling reflex, if venous return increases, the heart pumps more blood. Cardiac output is raised as a result of raised intravascular volume, and ultimately blood pressure increases (Mullens et al., Citation2017).
Some medical developments made it interesting to re-evaluate the role of RAS in growing hypertension. The first development was the preparation of new antihypertensive agents in the form of ACE inhibitors and the second development was gene encoding that was responsible for the production of Ang-II by using genomic tools (Brunner et al., Citation1978; Ertl et al., Citation1983). The preparation of new ACE inhibitors to reduce hypertension is a way to control this cardiovascular disorder. The scientific community is trying to find functional foods with ACE inhibitory microelements, and on the other hand, research is still underway to prepare bioactive elements or peptides from food.
4. Role of functional foods in reducing hypertension
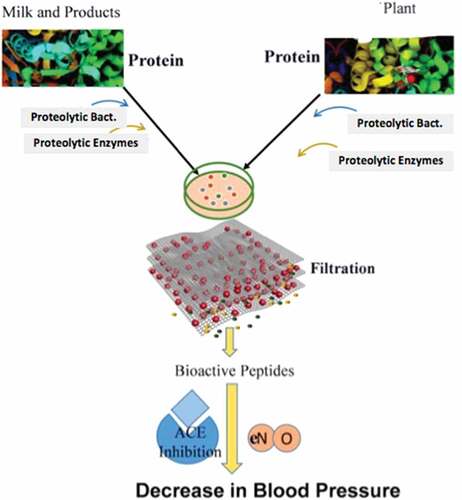
In the case of vascular diseases, the human body responds to certain bioactive micro-elements of plants as well as of animal origin. Among those foods, the role of bioactive molecules in fruits, vegetables, and milk products is promising in reducing hypertension. These bioactive microelements can be derived from food by using different techniques, e.g. hydrolyzing enzymes (pepsin, chymotrypsin, trypsin, alcalase, thermolysin, flavourzyme, and proteinase) and by fermentation (by using microorganisms) as shown in .
Figure 1. Bioactive peptides extracted from milk and plants can be used as anti-hypertensive agents. These functional foods are divided into microelements of plants and bioactive peptides of milk and dairy products.
Figura 1. Los péptidos bioactivos extraídos de la leche y las plantas pueden utilizarse como agentes antihipertensivos. Estos alimentos funcionales se dividen en microelementos de las plantas y péptidos bioactivos de la leche y los productos lácteos.
4.1. Micro-elements of plants
Micro-elements of plants like potassium (K+); sodium (Na+), calcium (Ca+2), vitamins, minerals, co-enzymes, phenolic compounds and antioxidants are most important in maintaining blood pressure normal and are considered as the frontline defender of controlling blood pressure
The quantity of K+, 4700 mg/day with a K+/Na+ ratio of about 4–5 to 1, is recommended as a balanced diet for humans. It was observed that double intake of K+ is associated with a reduction in SBP (4–8 mmHg) and DBP (2.5–4 mmHg) in hypertensive subjects (Whelton & He, Citation1999). Therefore, it can be documented that higher K+ intake is associated with a lower incidence of cardiovascular and cerebrovascular accidents, type 2 diabetes, left ventricular hypertrophy, heart failure, cardiac arrhythmias, and blood pressure reduction (Houston, Citation2011).
Several methods were proposed to control blood pressure by inducing potassium, e.g. excretion of sodium in the urine, regulation of baroreflex sensitivity, reduction in catecholamines, Ang-II sensitivity, the elevation of the sodium-potassium ATPase activity in the vascular smooth muscle cells, amelioration of sympathetic nervous system task, and reduction in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase action. All these methods are involved in reducing hypertension by means of oxidative stress, amelioration of insulin sensitivity, asymmetric dimethylarginine
(ADMA) reduction by falls of intracellular sodium and reduction in the TGF-beta production.
Dietary magnesium plays an important role in controlling hypertension. An antagonistic connection between dietary magnesium intake and blood pressure is well documented in recent studies. A randomized controlled trial indicated that magnesium supplementation is antagonized with the reduction in SBP (3–4 ± 2 mmHg) and DBP (2.5 ± 1 mmHg) (Kass et al., Citation2012). Many techniques have been assumed for magnesium-induced blood pressure reduction, like obstruction of calcium channel activity, enhancement in prostaglandin, and amelioration in NO production (Zhang et al., Citation2016). In the vitamins category, the antagonistic effect of vitamin C is also well documented with respect to blood pressure in humans (Buijsse et al., Citation2015), with less danger in producing cardiovascular diseases (Al-Khudairy et al., Citation2017; Block et al., Citation2008). Low plasma ascorbic level was found in the hypertensive subjects as compared to normal subjects (40 μmol L−1 versus 57 μmol L−1) in a study performed on human subjects (Ness et al., Citation1997). It is recommended to keep plasma ascorbate levels within the range of 100 mmol/L to maintain normal blood pressure in humans (Block et al., Citation2001; Sherman et al., Citation2000). A Meta-analysis was conducted in hypertensive patients, who were administered 500 mg of vitamin C for 56 days and observed reduction in SBP by 4.8 ± 1.2 mmHg (Feringa et al., Citation2011; Juraschek et al., Citation2012). Vitamin C supplementation can increase the effectiveness of antihypertensive drugs such as amlodipine (Mahajan et al., Citation2007). Six hundred milligrams of vitamin C were supplemented in the old patients with refractory hypertension on a daily basis, and a decrease in blood pressure by 20 ± 8/16 ± 5 mmHg was recorded (Sato et al., Citation2006). Many methods like NO and prostaglandin-2 induction, increase in sodium water diuresis, decrease in adrenal steroid preparation, amelioration of sympathovagal balance, superoxide dismutase and cyclic guanosine monophosphate (GMP) activity induction; potassium channels activation, decrease in cytosolic calcium and serum aldehydes, and arterial compliance improvement, have been assumed to increase vitamin C availability and maintain blood pressure normal (Hatzitolios et al., Citation2008; Plantinga et al., Citation2007; Simon, Citation1992). While some recent studies also support the idea of vitamin C supplementation is associated with improvement in blood pressure.
All these microelements play a role in maintaining the blood pressure normal. An increase or decrease in these microelements can lead to condition of hypertension.
4.2. Phenolic compounds and antioxidants
Many dietary flavonoids contain antioxidant and anti-inflammatory properties with the ability to improve NO metabolism and increase arterial stiffness and endothelial function. These flavonoids help in vascular protection and leads to reducing cardiovascular health risks (Habauzit & Morand, Citation2012). Regarding their use, cocoa flavonoids have been extensively used in many clinical trials, and their role in decreasing hypertension is well documented. Dark chocolate flavanols seem to be involved in the NO induction, vascular endothelial protection, cardiovascular disease risk factors reduction, and impairment of endothelial function. Dark chocolate was documented as useful in the elevation of flow-mediated dilation in normotensive and hypertensive patients with and without glucose intolerance (Grassi et al., Citation2012). Randomized clinical trials were performed on 856 healthy individuals. They were administered flavanol-rich cocoa (FRC) products (30–1080 mg of flavanols in 3.6–105 g of cocoa products per day), compared with control in short-term trials of 2–18 weeks. The mean difference in SBP observed in healthy participants was −2.8 mmHg, while the mean difference in DBP was −2.2 mmHg, which indicates the positive effects of FRC in hypertensive patients (Ried et al., Citation2012).
Berried contain lipid phase antioxidant called coenzyme Q10 (Ankola et al., Citation2007) that is involved in lowering blood pressure. It reduces the low-density lipoprotein oxidation and oxidative stress and involves regenerating the other vitamins and antioxidant agents (Langsjoen & Langsjoen, Citation1999). Oral treatment of coenzyme Q10 (100 mg) was administered to participants for three weeks. After treatment, SBP and DBP were measured as >140 mmHg, and >90 mmHg, respectively, and a decrease in blood pressure with mean changes in SBP and DBP (−3.68 and −2.03 mmHg, respectively) was observed.
Phenolic compounds of fruits and vegetables play a useful role in reducing hypertension, e.g. Polyphenol like resveratrol (trans-3, 5,4′- Trihydroxystilbene) is helpful in maintaining blood pressure normal via stimulation of NO induction, and the inhibition of vascular inflammation. It is mainly found in grapes with antioxidant and platelet aggregation prevention properties. The role of resveratrol in reducing hypertension is well documented in some preclinical models (Li et al., Citation2012). After running six randomized clinical trials involving 247 individuals, it was observed that a 150 mg daily dose of resveratrol is helpful in decreasing the SBP up to −11.9 mmHg (Liu et al., Citation2015).
The grape seed extract is also a source of resveratrol and other polyphenols. It is involved in decreasing SBP only (mean difference −1.5 mmHg; 95% CI − 2.8 to −0.2 mmHg; P = 0.02). It was confirmed after running nine double-blind, placebo-controlled randomized clinical trials involving 390 participants (Feringa et al., Citation2011).
Inorganic nitrates in beetroot juice are always used as health-enhancing nutritional supplements. It was found that these nitrates pose favorable effects on cardiovascular health. Inorganic nitrates metabolize into bioactive nitrite after ingestion and finally join the blood circulation, where they serve as nitrogen oxides, including NO (Clements, et al.; V Kapil 2009). Daily utilization (250 ml) of beetroot juice as a source of inorganic nitrates decreases blood pressure via bioconversion of nitrates into NO in normal, pre, and mildly hypertensive subjects (Coles & Clifton, Citation2012; Kapil et al., Citation2015).
Carotenoid lycopene is found abundantly in tomatoes that are part of a western diet. Lycopene is involved in reducing blood pressure. A randomized clinical trial was conducted for 4–12 weeks to evaluate its effect on hypertensive subjects. The observations were recorded with strong SBP-reducing effects. But it is still unclear whether it is more helpful in supplemented form or as a whole fruit (Burton-Freeman & Sesso, Citation2014; Paran et al., Citation2009; Ried and Fakler).
Pycnogenol is a natural ACE inhibitor that can be extracted from Pinus pinaster (French maritime pine). It involves the protection of cell membranes from oxidative stress, induction of NO, reduction in myeloid-peroxidase activity, reduction in urinary albumin excretion, and hsCRP (high-sensitivity C-reactive protein). It improves endothelial function and renal cortical blood flow as well (Maimoona et al., Citation2011). A hundred milligram of pycnogenol for 12 weeks was administered to hypertensive subjects along with many antihypertensive drugs, and its anti-hypertensive effect was observed and reported (Liu et al., Citation2004; Zibadi et al., Citation2008).
Polysulfides like S-allylcysteine are derived from garlic. These are involved in vascular gasotransmitter hydrogen sulfide production and increase the balance of eNO that triggers smooth muscle cell relaxation, vasodilation, and lowering of blood pressure. Several factors influence its effectiveness with which dietary and genetic factors are most dominant. These factors affect the hydrogen sulfide and NO signaling pathways and cause hypertension. Sulfur deficiency may be the causative agent for the development of hypertension and may be reduced by the supplementation of organosulfur compounds in the garlic (Ried & Fakler, Citation2014). Dry garlic performs calcium channel blocking activity, catecholamine sensitivity reduction, the elevation of bradykinin and NO, amelioration of arterial compliance, and ACE inhibitory activity (Butt et al., Citation2009). A randomized clinical trial involving 482 participants was conducted in which participants were administered aged garlic extract for 8 to 26 weeks, and in conclusion, a reduction in SBP (9.1, 95% CI − 12.7 to −5.4 mmHg) and DBP (8, 95% CI − 6.7 to −1.0 mmHg) was observed (Rohner et al., Citation2015). This effect appeared to be the standard antihypertensive therapy (Ried et al., Citation2010, Citation2013; Steiner & Lin, Citation1998).
A study involving 20,343 participants was conducted in which participants were offered polyphenols of extra-virgin olive oil, and concluded its positive effect on the hypertension (Theodora Psaltopoulou 2004). In another trial, 7447 patients with the cardiovascular problem were offered a Mediterranean diet supplemented with extra virgin olive oil (1 liter per week for the participant and their families), and a reduction in DBP was observed in a group supplemented with a Mediterranean diet as compared to the controlled group (−1.5 mmHg; 95% CI − 2.0 to −1.0 mmHg) (Toledo et al., Citation2013). Similarly, monozygotic hypertensive twins were supplemented with olive leaf extract (500–1000 mg per day) for eight weeks, and a reduction in blood pressure was observed at 8th week, which was dose-dependent (low dose groups decreased BP 3/1 mmHg, and the high dose 11/4 mmHg) as compared to the placebo group (Perrinjaquet-Moccetti et al., Citation2008).
Dietary intake of flavonoids appears to decrease the risk of cardiovascular diseases (van Dam et al., Citation2013).
Fruits and vegetables with secret health-promoting compounds are graded beneficial in reducing hypertension, but it is not yet clear in recent studies whether either whole fruit is more active as an antihypertensive agent or in its purified form. Pycnogenol in French Maritime pine and S Allycyteine in garlic are active elements with anti-hypertensive activity. These elements reduce blood pressure by bio-conservation, balance, and induction of endothelial NO, but more clinical trials are needed to support this evidence. Polyphenols and antioxidants in food can have the ability to inhibit ACE and control hypertension to normal blood pressure, as shown in .
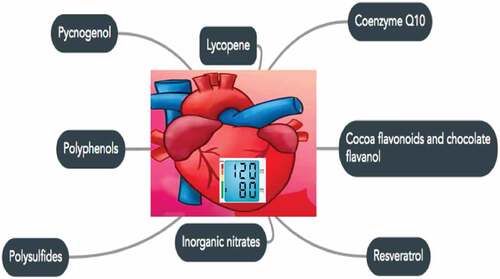
Figure 2. Lycopene, coenzyme, cocoa flavonoids/chocolate flavanols, inorganic nitrates, polysulfides, polyphenols, and pycnogenol are the functional components of food and are helpful in maintaining blood pressure normal.
Figura 2. El licopeno, la coenzima, los flavonoides del cacao/flavanoles del chocolate, los nitratos inorgánicos, los polisulfuros, los polifenoles y el picnogenol son los componentes funcionales de los alimentos y son útiles para mantener la presión arterial en su nivel normal.
4.3. Bioactive peptides of milk protein (Casein and Whey)
Enzymatic digestion of milk proteins and chemical synthesis of peptides are the two most used methods to produce bioactive peptides in the milk protein (Gobbetti et al., Citation2002, Citation2004). Peptides derived from milk protein are either the fragments of casein (casokinins) or whey protein (lactokinins). Many in-vitro and in-vivo studies have confirmed the blood pressure-lowering effect of casein-derived peptides is higher as compared to whey-derived peptides (FitzGerald & Meisel, Citation2000; Meisel & Schlimme, Citation1996). When casein (tryptic digestion) was supplemented to normal and mildly hypertensive subjects, with the dose of 10 g twice a day for four weeks, then a strong anti-hypertensive effect was observed in return (Sekiya et al., Citation1992). Hydrolysis of isoelectric casein with pepsin results in the ACE inhibitory peptides with the sequence of RYLGY, AYFYPEL, and YQKFPQY. These peptides showed strong anti-hypertensive activity when administered to SHR at the dose of 5 mg/kg of body weight. Anti-hypertensive effect of those peptides of casein was almost similar to the tri-peptide (VPP). Hydrolysis of casein with Aspergillus oryzae containing IPP and VPP was tested in a clinical trial. High-normal blood pressure and mild hypertension subjects were involved in the trial. Peptides (IPP and VPP) were supplemented with the daily dose of 1.8 mg and a decrease in SBP was observed after 6 weeks. Eventually, a decrease in SBP was also observed in placebo group subjects who were given 3.6 mg of peptides every day (Mizuno et al., Citation2005).
Casein hydrolysates have also been used to make MPH1 (OrtensVidaTM) and MPH2 as ACE-inhibitor with different bioactive compounds and have been reported to have fewer minerals contrasting ability as compared to other lactotripeptide-based products tested before. MPH1 contained bioactive peptide IPP while MPH2 contained bioactive peptides MAP, IPP, and LPP to inhibit ACE (Allender et al., Citation1996; Elliott et al., Citation2008; Griffith et al., Citation1999; Jee et al., Citation2002; van Mierlo et al., Citation2006; Whelton et al., Citation1997). The safety and tolerability of MPH1 and the usefulness of IPP-rich milk protein hydrolysates in reducing blood pressure were evaluated in a study and observed that MPH1, containing IPP without minerals, is beneficial in stage-1 hypertensive subjects (Boelsma & Kloek, Citation2010). Casein hydrolysate (Casein DP, Kanebo, Ltd., Japan, and C12 peptide, DMV), and whey protein hydrolysate (Biozate, Davisco) are two commercial products of the Netherland and US. Manufacturers claim the anti-hypertensive effect of these products in humans.
Anti-hypertensive peptides from milk and dairy products are not as functional as the drugs, but bioactive peptides are responsible for reducing hypertension to some extent in a safe and natural way. The potential of those peptides depends on the aptitude to reach the target site without degradation. Resistance from the stomach and intestinal enzymes was a prerequisite for anti-hypertensive effects during oral administration (Sharma et al., Citation2011). To highlight the importance of oral administration of bioactive peptides, a study was designed and reported that oral administration of tetra-peptides of β-lactoglobulin in SHR shows significant anti-hypertensive activity (Murakami et al., Citation2004). Furthermore, it was a point of debate between researchers that fermented milk, either in liquid or in tablet form, is more bioavailable to hypertensive patients. To testify this idea, a difference in the mode of action between fermented milk and fermented milk tablets was observed in a trial conducted on human subjects and it was concluded that consumption of fermented milk can decrease SBP (6.7 ± 3.0 mm Hg) and DBP (3.6 ± 1.9 mm Hg) while consumption of fermented milk tablets can decrease only SBP (11.2 ± 3.0 mm Hg) (Aihara et al., Citation2005; Seppo et al., Citation2003).
These data suggest that most of the results are in favor of peptides (derived from food protein) in decreasing blood pressure, while some studies negate this concept, e.g. Lacto-peptides and milk supplemented with whey peptides are considered non-functional in decreasing blood pressure (Lee et al., Citation2007; van der Zander et al., Citation2008). Because those peptides were susceptible to hydrolyze further by the acids or enzymes (pepsin, chymotrypsin, and various peptidases) of the stomach and large intestine. The microbial community of the stomach and large intestine was also involved in the further hydrolysis of peptides, and ultimately those peptides were not bioavailable in the hypertension (FitzGerald & Meisel, Citation2000).
4.3.1. Bioactive peptides of fermented milk
It has been reported extensively that different anti-hypertensive peptides are found in milk and dairy products like yogurt, sour milk, Dahi (a traditional yogurt which is prepared in the Indian continent): kefir (fermented milk drink, prepared from cultured kefir grains), and cheese. All these different forms of milk (Yogurt, sour milk, dahi, and kefir) are considered fermented Milk. When milk is fermented with probiotics (Lb. casei ssp. Rhamnosus strain), it contains many bioactive ACE inhibitory peptides (Rokka et al., Citation1997). Yogurt, made of ovine milk, and kefir made of caprine milk contain anti-hypertensive peptides as well. Bovine fermented milk (Lb. delbrueckii ssp. bulgaricus, Streptococcus thermophilus, and Lc. lactis ssp. lactis biovar diacetylactis) contain a potent ACE inhibitor peptide casokinin SLVTP (Ashar & Chand, Citation2004; Chobert et al., Citation2005). These studies indicate the usefulness of milk and dairy products in reducing blood pressure, and scientists are interested in replacing anti-hypertensive drugs with these functional foods. With this aim, many trials have been conducted.
Milk and fermented dairy products are involved in ACE inhibition and decrease hypertension. National Health and Nutrition Examination Survey (NHANES I) conducted a trial involving 10,000 participants to determine the relationship between milk consumption and blood pressure. They observed that low milk consumption increases the risk of hypertension and high milk consumption decreases blood pressure (McCarron et al., Citation1984). Studies confirmed that individuals with less milk consumption have a greater risk of hypertension. It was reported that men in Puerto Rico with daily consumption of one-liter milk had a 50% lesser chance of developing hypertension as compared to those who drank no milk (Garcia-Palmieri et al., Citation1984).
It is all due to protein content present in milk because milk protein is a bioactive component that is involved in reducing blood pressure by ACE inhibition. Consumption of milk provides functional proteins to the body, and those proteins are directly involved in decreasing hypertension, as stated by the Honolulu Heart Program and the Intersalt studies (Reed et al., Citation1985; Stamler et al., Citation1996). Milk protein is a source of peptides if milk protein is being fermented or hydrolyzed with enzymes or bacteria. It results in the production of smaller peptides like IPP and VPP. These peptides can be detected and purified in fermented and enzymatically treated milk with their pronounced bioactivity in hypertensive subjects (Jauhiainen et al., Citation2005; Sano et al., Citation2005).
It is presumed that small peptides can pass through the digestive enzymes without being decomposed. To evaluate this idea, smaller peptides, especially tri-peptides, were evaluated for their role in reducing hypertension. The hypotensive activity of tri-peptides (IPP and VPP) and fermented milk (Lb. helveticus and Saccharomyces cerevisiae) in spontaneously hypertensive rats were evaluated, and a reduction in SBP was recorded after a single oral dose (5 mL/kg of BW) of fermented milk within 6 to 8 hours. Tri-peptides were also involved in the reduction of SBP after a few hours of ingestion. It was concluded that fermented milk and tri-peptides are only responsive to the state of hypertension (Sharma et al., Citation2011).
With the documentation of smaller peptides and their effectiveness against hypertension, two anti-hypertensive peptides (TP and LVLPVPG) were purified from fermented milk to evaluate their effect on spontaneously hypertensive rats (SHRs). It was observed that those peptides were helpful in decreasing blood pressure in-vivo (Maeno et al., Citation1996; Yamamoto et al., Citation1999). SHRs also respond to the milk peptide α-lactorphin (YGLF) in reducing blood pressure (Nurminen et al., Citation2000).
Foods like sour milk and Swiss cheese contain ACE inhibitory Lacto-tri-peptides like IPP and VPP. Those ACE inhibitory peptides are part of β-casein and κ- casein. Administration of a single dose (5 ml/kg) of those two peptides can decrease the SBP in SHR within 6 to 8 hours (Nakamura et al., Citation1995). Sour milk fermented with the Lb. helveticus and Saccharomyces cerevisiae can be helpful in reducing SBP and DBP in hypertensive patients after supplementation of 8 weeks as well. Sour milk with two tri-peptides is involved in lowering the blood pressure after 4 to 8 weeks with the ACE-inhibitory peptides (1.2 to 1.6 mg/day) (Hata et al., Citation1996). Moreover, Calpis, a Japanese soft drink made of skim milk and fermented by Lb. helveticus and S. cerevisiae, also exhibits anti-hypertensive properties after its regular intake (Nakamura et al., Citation1995).
This idea of bioactive tri-peptides was further evaluated in clinical trials, and similar findings were documented that milk containing the bioactive tri-peptides (VPP and IPP) can reduce the blood pressure in mildly hypertensive patients (Seppo et al., Citation2003; Seppo, Citation2001).
Over 25 human trials were conducted to verify the bioactivity of IPP and VPP to inhibit ACE (Boelsma & Kloek, Citation2008; Xu et al., Citation2008). These studies were performed in different countries and locations in which ten clinical trials were run in Dutch (de Leeuw et al., Citation2009; Engberink et al., Citation2008; Van der Zander et al., Citation2008; van der Zander et al., Citation2008), Finnish (Jauhiainen et al., Citation2005; Seppo et al., Citation2003; Seppo, Citation2001; Tuomilehto et al., Citation2004), Scottish (van Mierlo et al., Citation2008), and American participants; Usefulness of small peptides against hypertension was reported in these studies (Nakamura et al., Citation1995; Neutel et al., Citation2006).
Anti-hypertensive peptides can be prepared with different techniques on a large scale, like ultrasound pretreatment, enzyme treatment, membrane filtration, and drying techniques. But membrane filtration and nano-filtration are considered effective ways to extract bioactive and anti-hypertensive peptides. This fact was established after running experiments on milk protein in which milk protein was hydrolyzed with the help of protease enzymes, and samples were examined after the complete hydrolysis of protein into small peptides. A large number of ACE inhibitory peptides (small peptides with functional amino acid groups) were extracted by using membrane filtration and nano-filtration. On the basis of peptides recovery, it was concluded that membrane filtration and nano-filtration are useful techniques to isolate bioactive peptides (Uluko et al., Citation2014).
While on a small scale, different processes are used to prepare bioactive peptides from the fermented milk. The first process is the exploitation of LABs (lactic acid bacteria) and proteolytic enzymes to hydrolyze protein into peptides and use those peptides to inhibit ACE. The second process is the addition of fermented milk directly with the bioactive peptides produced by enzymatic hydrolysis. The third process is the production of bioactive peptides by the use of microorganisms via recombinant DNA technology. Bioactive peptides and nutraceuticals can be prepared by using any of those processes in sub-industrial level (Hafeez et al., Citation2014). Production and evaluation of these peptides are necessary to point out specific peptides that are beneficial in inhibiting ACE. For this evaluation, in-vitro and in-vivo studies are conducted to report the efficiency of those peptides.
4.3.2. Bioactive peptides of cheese
Various cheese varieties and fermented milk are manufactured with the starter culture in labs as well as on the industrial level. Those fermented dairy products, including cheese, exhibit anti-hypertensive activity in human subjects (Fitzgerald & Murray, Citation2006; Gobbetti et al., Citation2002; Korhonen & Pihlanto, Citation2003, Citation2007). It has been reported earlier about the identification and isolation of bioactive peptides in cheese with ACE inhibitory properties, β- casomorphin, and calcium-binding phosphopeptides properties. Several factors like PH, salt, and type of enzymes control peptides’ stability in the stomach while β- casomorphin can be degraded under normal conditions in Cheddar cheese production (Gobbetti et al., Citation1998; Gómez-Ruiz et al., Citation2004; Jarmołowska et al., Citation1999; Muehlenkamp & Warthesen, Citation1996). Commercially available aged cheese and fresh cheese show mild ACE inhibitory activity, and that activity remained the same or increased after simulated gastrointestinal digestion with pepsin and Corolase PP (from pig pancreas, showing mainly trypsin and chymotrypsin activities). A great variety of bioactive peptides with biological activities is formed during cheese ripening.
Fresh cheese exhibits low ACE inhibitory activity as compared to middle-aged. A study indicated the best ACE inhibitory activity in Gouda cheese aged two years which is a bit opposite from previous reports. Meanwhile, ACE inhibitory peptides like αs1-casein f (1–9) and β-casein f (60–68) were detected in 8-month-old Gouda cheese as well (Saito et al., Citation2000). While after that phenomenon, further studies were conducted and reported that the highest concentration of bioactive peptides can be observed in fifteen days old Manchego cheese and 13-weeks of cheese production that decreases gradually with storage and time (Gómez-Ruiz et al., Citation2002; Ryhänen et al., Citation2001).
Low molecular weight ACE inhibitory peptides were identified in many ripened kinds of cheese, while ACE inhibitory activity elevated during proteolysis development and then decreased after a specific level (Meisel, Citation1998). An In-vitro study described that Emmental cheese contains 28 bioactive peptides with antimicrobial, mineral-carrying, antihypertensive, and immune-stimulatory characteristics (Gagnaire et al., Citation2001). Mature Cheddar cheese does not contain active opioid peptides due to possible degradation during the ripening process (Muehlenkamp & Warthesen, Citation1996). Anyhow, small quantities of β-casomorphin-3 were observed in Edam cheese during the ripening (Sabikhi & Mathur, Citation2001). Forty-four Swiss-origin cheese samples (hard, semi-hard, and soft cheese) were evaluated for the development of IPP and VPP, and observation was varied. Hard and semi-hard cheese varieties (Emmental, Hobelka ̈ se, Gouda, and Gruyere) contained higher amounts (0 to 224 mg/kg for VPP and 0 to 95.4 mg/kg for IPP) of those two peptides as compared to soft cheese samples (Bütikofer et al., Citation2007). Beta casein and αs1—casein of Cheddar cheese (made with starter lactococci) contained many ACE inhibitory peptides. As for as temperature is concerned, 4oC is favorable for the production of ACE inhibitory peptides when probiotic cultures (strains Lb. casei 279 and Lb. casei LAFTI® L26) are added during the cheese ripening (Ong et al., Citation2007). More bioactive peptides are developed in the gastrointestinal tract upon ingestion of cheese (Parrot et al., Citation2003).
Recent studies on goat milk cheese indicate that it is a good source of ACE inhibitory peptides. Small and medium-sized peptides are produced with the addition of probiotic culture, and most of them are part of the αs1-casein, αs2-casein, and β-casein (Atanasova et al., Citation2021; Hernández-Galán et al., Citation2017; Kocak et al., Citation2020).
4.4. Evaluation: ACE inhibitory peptides purified from milk and its products
4.4.1. In-vitro Evaluation
It is a common way to find out ACE inhibitory peptides in-vitro by applying Cushman and Cheung technique. Furthermore, spectrophotometric techniques are used to evaluate those peptides. Scientists have used milk of different origins and co-cultured with probiotic bacteria, yeasts, and proteolytic enzymes with the aim to hydrolyze milk protein. In a study, wild Lactococcus Lactis strains were used for the milk fermentation, and twenty-five peptides were isolated and purified from fermented milk by using Reverse-phase HPLC. Those peptides were tested against ACE in-vitro and reported that small peptides played their role in inhibiting ACE (Rodríguez-Figueroa et al., Citation2012). Leuconostoc lactis has a potential comparable to other lactobacilli in producing potent ACE inhibitory peptides.
More in-vitro trials were reported with higher ACE inhibitory activity, e.g. in a study fermented milk was used to evaluate its ACE inhibitory activity in-vitro. For the fermentation process, Lb. reuteri, Lb. johnsonii and Lb. helveticus were selected to ferment the milk protein. Among all strains, Lb. helveticus exhibited the highest proteolytic activity after 24 hours of incubation and resulted in ACE inhibition (González-Córdova et al., Citation2011). Non-starter lactobacilli, extracted from cheese were used to evaluate ACE-inhibitory activity in combination with bovine milk. Strains were selected on the basis of hydrolytic activity from cheese, and bioactive peptides like VPP and IPP were produced as a result of hydrolysis. L. casei, along with L. rhamnosus showed the highest ACE inhibitory activity in-vitro. Those two strains were reported as potent strains for the production of bioactive peptides that might be used in the industry to produce anti-hypertensive food (Solieri et al., Citation2015). LABs isolated from Greek yogurt and incubated with milk were evaluated for their anti-hypertensive activity. High-performance liquid chromatography was used to check the peptidomic profile hydrolyzed by those strains. Further, the mass spectrometric analysis resulted in the identification of the N- or C-terminal of the isracidin peptide region of aS1-casein that showed a prominent ACE inhibition (Georgalaki et al., Citation2017). The same year, a study was published with the similar aim of producing ACE inhibitory peptides to reduce hypertension. Lb. acidophilus was used to produce fermented milk (Lassi) in that study. Samples were filtered, and reverse phase HPLC was used to purify peptides. Liquid Chromatography-Mass Spectrometry was used to evaluate the peptide sequence of hydrolyzed protein. A total of 14 peptides were extracted from Lassi, and those peptides were the same, which were identified as ACE- inhibitory peptides earlier (Padghan et al., Citation2017).
Recent studies reported that Lb. helveticus and Lb. casei could produce bioactive ACE inhibitory peptides in goat and bovine milk, respectively (Aslam et al., Citation2019). When goat and Cow milk were hydrolyzed with commercial enzymes to evaluate their ACE inhibitory activity, then it was reported that goat milk and cow milk showed the highest ACE inhibitory activity when fermented with Acalase and proteinase K. Efficiency of proteinase k was also reported in whole milk casein of camel with the production of bioactive peptides (Bao et al., Citation2016; Rahimi et al., Citation2016). As viable counts of Lb. plantarum increased, ACE inhibitory activity of fermented goat milk also increased. Fermented samples showed antihypertensive properties in-vitro that were reported in earlier studies. In a way, Lb. plantarum was effective in producing bioactive peptides in goat milk by hydrolyzing protein into peptides and can be used as a functional food (Shu et al., Citation2018). Goat milk fermented with Lb. Plantarum 69 has the ability to inhibit ACE in raw and purified form (Bao et al., Citation2016), as well as fermented with Lb. bulgaricusLB6 is also useful in producing bioactive peptides with antihypertensive activity in-vitro (Shu et al., Citation2017). Goat milk fermented with different lactobacillus strains showed ACE inhibitory activity with varying percentages (Parmar et al., Citation2020). Goat milk Casein hydrolyzed with subtilisin and trypsin has the ability to produce ACE inhibitory activity in-vitro (Espejo-Carpio et al., Citation2014). Casein and Whey protein of goat milk peptide, hydrolyzed with pepsin, can generate more bioactive peptides as compared with un-hydrolyzed protein (Ibrahim et al., Citation2017). Camel milk is mainly consumed in Arab countries and contains an array of bioactive peptides. Fermented camel milk (Lb. acidophilus and Streptococcus thermophilus) is a good source of bioactive peptides with a higher ACE inhibitory activity (Alhaj et al., Citation2018). Fermented Sheep milk (Bacillus sp. 7) has the potential to produce ACE inhibitory peptides as well (Corrêa et al., Citation2011). Yak and donkey’s milk is also tested for their potent role in producing bioactive peptides. Yak milk is, although a minor source of milk but its casein contains ACE inhibitory peptides like LPLPLL, KYIPIQ, and PFPGPIPN (Lin et al., Citation2018). Donkey milk which is considered good for its nutritional profile was used to produce ACE inhibitory peptides. After running chromatographic techniques to purify samples, nano RP-HPLC-MS/MS was used to extract bioactive peptides. In conclusion, two novel bioactive ACE inhibitory peptides (REWFTFLK and MPFLKSPIVPF) were identified. Identification of antihypertensive peptides from donkey milk provided solid ground to use donkey milk as a functional food for the remedy of hypertension (Chiozzi et al., Citation2016). It is observed that lactobacillus like Lb. acidophilus, Lb. helveticus, Lb. casei, Lb. palntarum, and Lb. bulgaricus have the ability to hydrolyze milk protein as compared to the other strains.
Apart from lactobacillus, yeasts were reported to ferment milk for the production of small bioactive peptides. A study was designed in which fermented milk (Kluyveromyces marxianus Z17) was used to extract ACE inhibitory peptides. As a result of gel filtration, two novel peptides (VLSRYP and LRFF) with ACE inhibitory activity were purified and further authenticated with mass spectrometry. Fermentation temperature and PH were reported important during the production of bioactive peptides. The peptide with the best inhibitory activity was identified at PH 6.5 with a 6% inoculum level. Results showed that the production of bioactive ACE inhibitory peptides could be best extracted from the fermented milk by optimizing temperature, PH, and inoculum level (Li et al., Citation2015). An attempt to produce potent ACE inhibitory peptides was made in which the hydrolytic capacity of Aspergillus niger prolyl endoproteinase was evaluated by co-culturing β-casein, purified from milk. By optimizing time, twenty-four hours of incubation was considered best for the hydrolysis of β-casein. Bioactive peptides (IQA and IQP) with anti-hypertensive activity were identified, and that hydrolysate was reported to produce the most potent peptides for the production of functional food (Norris et al., Citation2014) as shown in .
Table 1. Indicates the recent developments in producing ACE inhibitory bioactive peptides.
Tabla 1. Indica los recientes avances en la producción de péptidos bioactivos inhibidores de la ECA.
After analyzing the recently published data, it can be reported that milk protein is the source of bioactive peptides if hydrolyzed with bacterial or commercially available enzymes. Purification and identification of those peptides is an important process, but there is a need to formulate those enzymes into a product that can be used as supplements.
4.4.2. In-vivo Evaluation
The scientific community is conducting in-vivo experiments on SHRs by using milk of different species, co-cultured with proteolytic enzymes.
A trial with the same objective was run in 6-weeks old SHRs to evaluate the effect of fermented milk in decreasing blood pressure. In this trial, three groups were designed. Fermented milk group (fermented milk containing Lb. helveticus LBK16 H enriched with VPP and IPP) Peptide group (VPP and IPP dissolved in water) and control group (pure water). Six-week-old SHRs were supplemented with those experimental groups orally. At the end of the trial, a reduction in SBP was recorded in the fermented milk group and peptide group as compared to the control group. It was concluded on the basis of observations that long-term supplementation of tripeptides (extracted from fermented milk protein) can decrease blood pressure and can be used as a functional food (Sipola et al., Citation2001). A year later, another research work was published with the aim of preparing a fermented milk solution that can reduce hypertension. Four groups were designed according to diet plans in SHRs. Control and blank groups comprised skim milk and water while fermented milk was divided into two groups. The first group contained Lb. helveticus LBK16 H with tripeptides IPP + VPP, and the second group contained Lb. helveticus and Sac. Cerevisiae was evaluated. At the end of the trial, normal blood pressure was recorded in the first and second fermented milk groups as compared to the controlled and blank groups. It was reported that fermented milk could reduce blood pressure, and tri-peptides are the reason to decrease the blood pressure and could be used as a future therapy (Sipola et al., Citation2002).
Functional food with different treatments of experimental foods was prepared and introduced in SHRs to check antihypertensive effects. Four kinds of diet treatments were prepared: (a) tap water, (b) minerals (potassium, calcium, magnesium, and sodium), (c) IPP + VPP and minerals, or (d) milk fermented with Lb. helveticus, IPP + VPP, and minerals. Animals were divided into different groups, and diets were offered accordingly. It was identified that diet (d) was involved in lowering blood pressure as compared to others. In conclusion, milk fermented with Lb. helveticus, IPP + VPP, and minerals could be used as a functional food (Jauhiainen et al., Citation2005). Experiments were conducted on rats to check the ACE inhibitory activity of fermented milk. Different strains (Lb acidophilus, Lb. casei, Lb. helveticus, Lb. jensenii, Lb. reuteri, Lb. rhamnosus, Lactococcus lactis ssp. lactis, Lactococcus. raffinolactis and Leuconostoc mesenteroides ssp. Cremoris) were used. Only seven strains of LABs showed ACE inhibitory activity among tested strains. Change in fermentation conditions or PH had no impact on ACE inhibitory activity while Lb. jensenii was the only strain that showed the best ACE inhibitory activity in-vivo (Pihlanto et al., Citation2010). Two hundred and fifty-nine strains of Lb. helveticus were extracted from Chinese and Mongolian fermented food. Strains were propagated and used to ferment the milk. By optimizing conditions like titratable acidity, free amino nitrogen, and fermentation time, fermented milk was used to evaluate its ACE inhibitory activity. A total of 37 strains were isolated with good ACE inhibitory activity in which IPP and VPP were further evaluated. Finally, after in-vitro identification, three strains were identified with good anti-hypertensive properties. In those three strains, H9 (IMAU60208) was further evaluated in SHRs and showed a promising change in decreasing SBP and DBP (Chen et al., Citation2014).
Whey is a rich source of dairy protein. It was fermented with Lb. casei, Lb. acidophilus, Streptococcus thermophilus, Lb. bulgaricus, and Bifidobacterium. Fermented whey was administered to one group, and skim milk was administered to another group of SHRs. A reduction in SBP was recorded after 4 weeks in the fermented whey group, while a reduction in SBP, as well as DSP, was recorded in the skim milk group. It was concluded that fermented whey could be used as a functional food for hypertensive subjects (Chen et al., Citation2007).
Captopril is a pharmacological drug that is used to reduce hypertension. These studies aimed to see the difference in the mode of action of pharmacological drugs and bioactive peptides against hypertension. ACE inhibitory activity of fermented milk (fermented with LAB isolated from raw bovine milk), Captopril (50 mg/kg of BW; Sigma-Aldrich, St. Louis, MO), water, and skimmed milk was evaluated in SHRs. At the end of the experiments, high blood pressure was observed in the water and skimmed milk group, while low blood pressure in the captopril group and fermented milk group was recorded after 6 and 4 hours, respectively. DBP was the same in Captopril and fermented milk group, while a difference in SBP was observed. Normal rats showed no response to any of the diet groups. It was concluded that fermented milk could reduce blood pressure only in hypertensive subjects (Muguerza et al., Citation2006). At the same time, another trial with a similar objective was conducted. Fermented milk (Lb. helveticus and Saccharomyces cerevisiae), Captopril, and skim milk were evaluated for their effect on maintaining blood pressure normal by ACE inhibition or NO production in SHRs. Blood pressure was recorded normal in the captopril group, while low blood pressure was observed in the fermented milk group as compared to the skim milk group. ACE inhibitory activity was recorded higher in the serum and abdominal aorta tissue, where an extract of the captopril group and fermented milk group was administered. Nitric oxide concentration was recorded higher in the captopril group and fermented milk group as well. It was concluded that fermented milk peptides are anti-hypertensive and can produce vasodilator NO as well (Kim et al., Citation2010). Fermented milk containing Lb. lactis NRRLB -50,571 or NRRLB -50,572 and Captopril were used to evaluate their effect in SHRs. After administering a single dose, blood pressure was recorded four times in a day (2, 4, 6, 24 h). Reduction in SBP and DBP was recorded in Captopril and fermented milk group after 6 hours of administration. Meanwhile, SBP was same after 24 h in Captopril and fermented milk group (Rodríguez-Figueroa et al., Citation2013). It is assumed in the light of previous research that fermented milk containing bioactive peptides has the potential to decrease hypertension but further inline experiments should be designed to verify the comparative effect of tri-peptides and captopril.
4.4.3. Clinical Evaluation
Several clinical trials have been conducted on human subjects with the primary aim to evaluate the efficacy of anti-hypertensive functional foods, but the information related to the use of milk and dairy peptides is limited. Most of the reported research work is related to in-vitro trials, gradually with decreasing trend to in-vivo and finally clinical trials. Some of the recent clinical trials have been discussed, which are helpful to understand the importance of milk-derived bioactive peptides.
A Small trial (17 volunteers) was conducted on hypertensive subjects with the supplementation of fermented milk containing Lb. helveticus LBK16 H with tripeptides (IPP and VPP). This trial was continued for eight weeks while fermented milk was supplemented once a day. A decrease in SBP and DBP was observed in the hypertensive subjects (Seppo, Citation2001).
A randomized, double-blinded, placebo-controlled study was conducted on hypertensive subjects. Participants were allocated into three groups according to the diet plan. They were supplemented with fermented milk containing Lb. helveticus and Saccharomyces Cerevisiae, placed in group-1; fermented with Lb. helveticus CM4 was placed in group-2 and fermented with Lb. bulgaricus and Streptococcus thermophilus were placed in the control group. Reduction in SBP, as well as DBP, was recorded after one week of supplementation in group-1 and two as compared to control. Blood pressure returned to normal after treatment was ended. It was concluded that fermented milk could reduce blood pressure after one week in hypertensive subjects (Kajimoto et al., Citation2002).
The effect of fermented milk (Lb. helveticus, Saccharomyces cerevisiae, and Lb. helveticus CM4) and artificially acidified milk were evaluated in mild or moderate hypertensive subjects for eight weeks. A decrease in SBP was recorded in the fermented and acidified milk group after eight weeks of supplementation. It was concluded that fermented milk could be used as a hypertensive management (Hirata Citation2002).
A study was performed on hypertensive subjects for 21 weeks. Patients were divided into two groups on the basis of diet plans. Group 1 was supplemented with fermented milk (Lb. helveticus LBK16 H; IPP and VPP), and group 2 (control) was supplemented with fermented milk containing Lactococcus spp. Reduction in SBP was observed in group 2 as compared to group 1, while DBP remained the same in both groups (Seppo et al., Citation2003).
A recent study reported the role of milk-based bioactive peptides in reducing the intensity of hypertension in a long-term clinical trial (3.1 year trial including 4378 subjects). It was documented that digestion-resistant smaller peptides can be helpful in reducing hypertension (Barati et al., Citation2021).
Furthermore, it was a point of debate between researchers that fermented milk, either in liquid or in tablet form, is more bioavailable to hypertensive patients. To testify this idea, a difference in the mode of action between fermented milk and fermented milk tablets was observed in a trial conducted on human subjects, and it was concluded that consumption of fermented milk could decrease SBP (6.7 ± 3.0 mm Hg) and DBP (3.6 ± 1.9 mm Hg) while consumption of fermented milk tablets can decrease only SBP (11.2 ± 3.0 mm Hg) (Aihara et al., Citation2005; Seppo et al., Citation2003). Therefore, it can be concluded that fermented milk in liquid form is more useful against hypertension as compared to the tablet form because fermented milk is readily available to the body without further degradation of most of the peptides while in the process of tablet manufacturing and processing, most of the heat-sensitive peptides lose their efficacy. That might be the possible reason for liquid fermented milk’s extra efficiency in reducing hypertension.
Recent data related to the connection of dairy products to hypertension are interesting.
Massimo et al. (Citation2019) documented that the results of supplementing dairy products in minimizing hypertension are inconsistent but a meta-analysis of cohort studies indicates the usefulness of dairy products in lowering SBP and lowering incidents of hypertension. A study involving 715 adults also indicates that daily yogurt consumption can control blood pressure with Lower DBP (Alberto et al., Citation2018).
A cohort study on French women indicates that milk products have no pronounced connection in minimizing hypertension except the processed dairy product “cheese” that is needed to confirm by designing related studies (Villaverde et al., Citation2020). In the same year, a study was conducted on 67,011 university students and it was observed that the consumption of dairy products is inversely proportioned to the incidence of hypertension (Mansouri et al., Citation0000).
The same year, an epidemiological study involving 35–70 years old individuals from 25 countries was conducted with the same objective. The effect of dairy products on the prevalence of metabolic syndrome (hypertension) was evaluated and concluded that a higher intake of dairy products is associated with a lower risk of hypertension (Bhavadharini et al., Citation2020).
If we compare the documented work on SHRs and clinical trials, it is worth mentioning here that clinical data regarding the efficiency of fermented milk products against hypertension are scarce and inconsistent. Most of the studies used fermented milk or processed dairy products with a few studies directly evaluating the effect of purified bioactive peptides. It is suggested to design and run more clinical trials to prove the uselessness of dairy bioactive peptides.
5. General conclusion
Most of the research work related to the use of bioactive peptides in ACE inhibition and controlling hypertension is in-vitro. The promising effect of bioactive elements is documented if we observe the results and observations of in-vitro studies. However, the quality and quantity of documented work is gradually decreasing in-vivo and in clinical trials. In most of the in-vivo and clinical trials, fermented milk was evaluated rather than evaluating the bioactive peptides directly. Bioactive food elements are a better choice for hypertensive subjects than chemical drugs. Among different functional foods, fermented dairy products are gaining popularity due to their ability to produce bioactive peptides to inhibit ACE. Many novel peptides (IPP, VPP) are reported that are helpful in the inhibition of ACE. The role of ACE in maintaining normal blood pressure is prominent and mitigates its deleterious effect. For this purpose, bovine milk was mainly used to ferment with bacterial, commercially available proteolytic enzymes or yeasts. ACE inhibitory activity of reported bioactive peptides or microelements is promising, but there are scarce data related to the use of bioactive microelements in clinical trials. Safety of those bioactive microelements and peptides is necessary before producing it on the industrial level.
Abbreviations
Alanine - A
Arginine - R
Asparagine - N
Aspartic acid - D
Cysteine - C
Glutamine - Q
Glutamic acid - E
Glycine - G
Histidine - H
Isoleucine - I
leucine - L
lysine - K
Methionine - M
Phenylalanine - F
Proline - P
Serine - S
Threonine - T
Tryptophan - W
Tyrosine - Y
Valine - V
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aihara, K., Kajimoto, O., Hirata, H., Takahashi, R., & Nakamura, Y. (2005). Effect of powdered fermented milk with lactobacillus helveticus on subjects with high-normal blood pressure or mild hypertension. Journal of the American College of Nutrition, 24(4), 257–265. https://doi.org/10.1080/07315724.2005.10719473
- Alberto, L., Banegas, J. R., Guallar-Castillón, P., Rodríguez-Artalejo, F., & Lopez-Garcia, E. (2018). Association of dairy consumption and 24-hour blood pressure in older adults with hypertension. The American Journal of Medicine, 131(10), 1238–1249. https://doi.org/10.1016/j.amjmed.2018.04.039
- Alhaj, O. A., Metwalli, A. A., Ismail, E. A., Ali, H. S., Al-Khalifa, A. S., & Kanekanian, A. D. (2018). Angiotensin converting enzyme-inhibitory activity and antimicrobial effect of fermented camel milk (Camelus dromedarius). International Journal of Dairy Technology, 71(1), 27–35. https://doi.org/10.1111/1471-0307.12383
- Al-Khudairy, L., Flowers, N., Wheelhouse, R., Ghannam, O., Hartley, L., Stranges, S., & Rees, K. (2017). Vitamin C supplementation to prevent cardiovascular disease. Cochrane Database of Systemic Reviews, 2017(3). https://doi.org/10.1002/14651858.CD011114.pub2
- Allender, P. S., Cutler, J. A., Follmann, D., Cappuccio, F. P., Pryer, J., & Elliott P. (1996). Dietary calcium and blood pressure: A meta-analysis of randomized clinical trials. Annals of Internal Medicine, 124(9), 825–831. https://doi.org/10.7326/0003-4819-124-9-199605010-00007
- Ankola, D. D., Viswanad, B., Bhardwaj, V., Ramarao, P., & Kumar, M. N. V. R. (2007). Development of potent oral nanoparticulate formulation of coenzyme Q10 for treatment of hypertension: Can the simple nutritional supplements be used as first line therapeutic agents for prophylaxis/therapy? European Journal of Pharmaceutics and Biopharmaceutics, 67(2), 361–369. https://doi.org/10.1016/j.ejpb.2007.03.010
- Ashar, M. N., & Chand, R. (2004). Fermented milk containing ACE-inhibitory peptides reduces blood pressure in middle aged hypertensive subjects. Milchwissenschaft, 59(7–8), 363–366.
- Aslam, M. Z., Aslam, M. S., Firdos, S., Ghous, G., Firdos, G., Hongfei, Z., & Bolin, Z. (2019). Role of bioactive peptides in reducing the severity of hypertension with the inhibition of ACE. International Journal of Peptide Research and Therapeutics, 25(4), 1639–1649. https://doi.org/10.1007/s10989-018-09806-y
- Aslam, M. Z., Shoukat, S., Hongfei, Z., & Bolin, Z. (2019). Peptidomic analysis of ACE inhibitory peptides extracted from fermented goat milk. International Journal of Peptide Research and Therapeutics, 25(4), 1259–1270. https://doi.org/10.1007/s10989-018-9771-0
- Atanasova, J., Dalgalarrondo, M., Iliev, I., Moncheva, P., Todorov, S. D., & Ivanova, I. V. (2021). Formation of free amino acids and bioactive peptides during the ripening of Bulgarian white brined cheeses. Probiotics and Antimicrobial Proteins, 13(1), 261–272. https://doi.org/10.1007/s12602-020-09669-0
- Bakris, G. (2015). Overview of hypertension (G. Bakris and M. Manual, professional edition). Merck & Co.
- Bao, C., Chen, H., Chen, L., Cao, J., & Meng, J. (2016). Comparison of ACE inhibitory activity in skimmed goat and cow milk hydrolyzed by Alcalase, flavourzyme, neutral protease and proteinase K. Acta Universitatis Cibiniensis: Series E: Food Technology, 20(1), 77–84. https://doi.org/10.1515/aucft-2016-0006
- Barati, M., Jabbari, M., Teymoori, F., Farhadnejad, H., Khalili-Moghadam, S., Roshanravan, N., Mosharkesh, E., Kazemian, E., Mirmiran, P., Davoodi, S. H., & Azizi, F. (2021). Dairy-originated digestion-resistant and bioactive peptides increase the risk of hypertension: Tehran lipid and glucose study. Hypertension Research, 44(9), 1194–1204. https://doi.org/10.1038/s41440-021-00692-4
- Bethesda. (2015). What is high blood pressure. In Bethesda, national heart, lung and blood institute, us national institutes of health, information. published online.
- Bhavadharini, B., Dehghan, M., Mente, A., Rangarajan, S., Sheridan, P., Mohan, V., Iqbal, R., Gupta, R., Lear, S., Wentzel-Viljoen, E., Avezum, A., Lopez-Jaramillo, P., Mony, P., Varma, R. P., Kumar, R., Chifamba, J., Alhabib, K. F., Mohammadifard, N., Oguz, A. … Yusuf, S. (2020). Association of dairy consumption with metabolic syndrome, hypertension and diabetes in 147 812 individuals from 21 countries. BMJ Open Diabetes Research and Care, 8(1), e000826. https://doi.org/10.1136/bmjdrc-2019-000826
- Block, G., Jensen, C. D., Norkus, E. P., Hudes, M., & Crawford, P. B. (2008). Vitamin C in plasma is inversely related to blood pressure and change in blood pressure during the previous year in young black and white women. Nutrition Journal, 7(1), 35. https://doi.org/10.1186/1475-2891-7-35
- Block, G., Mangels, A. R., Norkus, E. P., Patterson, B. H., Levander, O. A., & Taylor, P. R. (2001). Ascorbic acid status and subsequent diastolic and systolic blood pressure. Hypertension, 37(2), 261–267.
- Boelsma, E., & Kloek, J. (2008). Lactotripeptides and antihypertensive effects: A critical review. The British Journal of Nutrition, 101(6), 776–786. https://doi.org/10.1017/S0007114508137722
- Boelsma, E., & Kloek, J. (2010). IPP-rich milk protein hydrolysate lowers blood pressure in subjects with stage 1 hypertension, a randomized controlled trial. Nutrition Journal, 9(1), 52. https://doi.org/10.1186/1475-2891-9-52
- Brunner, H.R., Gavras, H., Turini, G. A., Waeber, B., Chappuis, P., & McKinstry, D. N. (1978). Long-term treatment of hypertension in man by an orally active angiotensin-converting enzyme inhibitor. Portland Press Limited.
- Buijsse, B., Jacobs, D. R., Steffen, L. M., Kromhout, D., & Gross, M. D. (2015). Plasma ascorbic acid, a priori diet quality score, and incident hypertension: A prospective cohort study. PLoS One, 10(12), e0144920. https://doi.org/10.1371/journal.pone.0144920
- Burton-Freeman, B. M., & Sesso, H. D. (2014). Whole food versus supplement: Comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Advances in Nutrition: An International Review Journal, 5(5), 457–485. https://doi.org/10.3945/an.114.005231
- Bütikofer, U., Meyer, J., Sieber, R., & Wechsler, D. (2007). Quantification of the angiotensin-converting enzyme-inhibiting tripeptides Val-Pro-Pro and Ile-Pro-Pro in hard, semi-hard and soft cheeses. International Dairy Journal, 17(8), 968–975. https://doi.org/10.1016/j.idairyj.2006.11.003
- Butt, M. S., Sultan, M. T., Butt, M. S., & Iqbal, J. (2009). Garlic: Nature’s protection against physiological threats. Critical Reviews in Food Science and Nutririon, 49(6), 538–551. https://doi.org/10.1080/10408390802145344
- Chen, Y., Liu, W., Xue, J., Yang, J., Chen, X., Shao, Y., Kwok, L.-Y., Bilige, M., Mang, L., & Zhang, H. (2014). Angiotensin-converting enzyme inhibitory activity of lactobacillus helveticus strains from traditional fermented dairy foods and antihypertensive effect of fermented milk of strain H9. Journal of Dairy Science, 97(11), 6680–6692. https://doi.org/10.3168/jds.2014-7962
- Chen, G.-W., Tsai, J.-S., & Sun Pan, B. (2007). Purification of angiotensin I-converting enzyme inhibitory peptides and antihypertensive effect of milk produced by protease-facilitated lactic fermentation. International Dairy Journal, 17(6), 641–647. https://doi.org/10.1016/j.idairyj.2006.07.004
- Chiozzi, R. Z., Capriotti, A. L., Cavaliere, C., La Barbera, G., Piovesana, S., Samperi, R., & Laganà, A. (2016). Purification and identification of endogenous antioxidant and ACE-inhibitory peptides from donkey milk by multidimensional liquid chromatography and nanoHPLC-high resolution mass spectrometry. Analytical and Bioanalytical Chemistry, 408(20), 5657–5666. https://doi.org/10.1007/s00216-016-9672-z
- Chobert, J.-M., El-Zahar, K., Sitohy, M., Dalgalarrondo, M., Métro, F., Choiset, Y., & Haertlé, T. (2005). Angiotensin I-converting-enzyme (ACE)-inhibitory activity of tryptic peptides of ovine β -lactoglobulin and of milk yoghurts obtained by using different starters. Le Lait, 85(3), 141–152. https://doi.org/10.1051/lait:2005005
- Coles, L. T., & Clifton, P. M. (2012). Effect of beetroot juice on lowering blood pressure in free-living, disease-free adults: A randomized, placebo-controlled trial. Nutrition Journal, 11(1), 106. https://doi.org/10.1186/1475-2891-11-106
- Corrêa, A. P. F., Daroit, D. J., Coelho, J., Meira, S. M., Lopes, F. C., Segalin, J., Risso, P. H., & Brandelli, A. (2011). Antioxidant, antihypertensive and antimicrobial properties of ovine milk caseinate hydrolyzed with a microbial protease. Journal of the Science of Food and Agriculture, 91(12), 2247–2254. https://doi.org/10.1002/jsfa.4446
- de Leeuw, P. W., van der Zander, K., Kroon, A. A., Rennenberg, R. M. W., & Koning, M. M. G. (2009). Dose‐dependent lowering of blood pressure by dairy peptides in mildly hypertensive subjects. Blood Pressure, 18(1–2), 44–50. https://doi.org/10.1080/08037050902761209
- Elliott, P., Kesteloot, H., Appel, L. J., Dyer, A. R., Ueshima, H., Chan, Q., Brown, I. J., Zhao, L., & Stamler, J. (2008). Dietary phosphorus and blood pressure: International study of macro-and micro-nutrients and blood pressure. Hypertension, 51(3), 669–675. https://doi.org/10.1161/HYPERTENSIONAHA.107.103747
- Engberink, M. F., Schouten, E. G., Kok, F. J., van Mierlo, L. A. J., Brouwer, I. A., & Geleijnse, J. M. (2008). Lactotripeptides show no effect on human blood pressure: Results from a double-blind randomized controlled trial. Hypertension, 51(2), 399–405. https://doi.org/10.1161/HYPERTENSIONAHA.107.098988
- Ertl, G., Alexander, R.W., & Kloner, R.A. (1983). Interactions between coronary occlusion and the renin-angiotensin system in the dog. Basic Research in Cardiology, 78(5), 518–533. https://doi.org/10.1007/BF01906463
- Espejo-Carpio, F. J., Pérez-Gálvez, R., Almécija, M. D. C., Guadix, A., & Guadix, E. M. (2014). Production of goat milk protein hydrolysate enriched in ACE-inhibitory peptides by ultrafiltration. The Journal of Dairy Research, 81(4), 385–393. https://doi.org/10.1017/S0022029914000284
- Feringa, H. H., Laskey, D. A., Dickson, J. E., & Coleman, C. I. (2011). The effect of grape seed extract on cardiovascular risk markers: A meta-analysis of randomized controlled trials. Journal of the American Dietetic Association, 111(8), 1173–1181. https://doi.org/10.1016/j.jada.2011.05.015
- Ferrario, C. M. (1990). Importance of the renin-angiotensin-aldosterone system (RAS) in the physiology and pathology of hypertension. Springer.
- FitzGerald, R. J., & Meisel, H. (2000). Milk protein-derived peptide inhibitors of angiotensin-I-converting enzyme. The British Journal of Nutrition, 84(S1), 33–37. https://doi.org/10.1017/S0007114500002221
- Fitzgerald, R. J., & Murray, B. A. (2006). Bioactive peptides and lactic fermentations. International Journal of Dairy Technology, 59(2), 118–125. https://doi.org/10.1111/j.1471-0307.2006.00250.x
- Gagnaire, V., Mollé, D., Herrouin, M., & Léonil, J. (2001). Peptides identified during emmental cheese ripening: Origin and proteolytic systems involved. Journal of Agricultural and Food Chemistry, 49(9), 4402–4413. https://doi.org/10.1021/jf000895z
- Garcia-Palmieri, M. R., Costas, R., Cruz-Vidal, M., Sorlie, P. D., Tillotson, J., & Havlik, R. J. (1984). Milk consumption, calcium intake, and decreased hypertension in Puerto rico. Puerto Rico Heart Health Program Study: Hypertension, 6(3), 322–328. https://doi.org/10.1161/01.HYP.6.3.322
- Georgalaki, M., Zoumpopoulou, G., Mavrogonatou, E., Van Driessche, G., Alexandraki, V., Anastasiou, R., Papadelli, M., Kazou, M., Manolopoulou, E., Kletsas, D., Devreese, B., Papadimitriou, K., & Tsakalidou, E. (2017). Evaluation of the antihypertensive angiotensin-converting enzyme inhibitory (ACE-I) activity and other probiotic properties of lactic acid bacteria isolated from traditional Greek dairy products. International Dairy Journal, 75, 10–21. https://doi.org/10.1016/j.idairyj.2017.07.003
- Gobbetti, M., Corsetti, A., Smacchi, E., Zocchetti, A., & De Angelis, M. (1998). Production of Crescenza cheese by incorporation of bifidobacteria. Journal of Dairy Science, 81(1), 37–47. https://doi.org/10.3168/jds.S0022-0302(98)75548-1
- Gobbetti, M., Ferranti, P., Smacchi, E., Goffredi, F., & Addeo, F. (2000). Production of Angiotensin-I-Converting-Enzyme-Inhibitory peptides in fermented milks started by lactobacillus delbrueckii subsp. bulgaricus SS1 and lactococcus lactis subsp. cremoris FT4. Applied and Environmental Microbiology, 66(9), 3898–3904. https://doi.org/10.1128/AEM.66.9.3898-3904.2000
- Gobbetti, M., Minervini, F., & Rizzello, C. G. (2004). Angiotensin i‐converting‐enzyme‐inhibitory and antimicrobial bioactive peptides. International Journal of Dairy Technology, 57(2‐3), 173–188. https://doi.org/10.1111/j.1471-0307.2004.00139.x
- Gobbetti, M., Stepaniak, L., De Angelis, M., Corsetti, A., & DiCagno, R. (2002). Latent bioactive peptides in milk proteins: Proteolytic activation and significance in dairy processing. Critical Reviews in Food Science and Nutrition, 42(3), 223–239. https://doi.org/10.1080/10408690290825538
- Goldblatt, H., Lynch, J., Hanzal, R. F., & Summerville, W. W. (1934). Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. The Journal of Experimental Medicine, 59(3), 347–379. https://doi.org/10.1084/jem.59.3.347
- Gómez-Ruiz, J. Á., Ramos, M., & Recio, I. (2002). Mercedes Ramos, and isidra recio 2002 angiotensin-converting enzyme-inhibitory peptides in manchego cheeses manufactured with different starter cultures. International Dairy Journal, 12(8), 697–706. https://doi.org/10.1016/S0958-6946(02)00059-6
- Gómez-Ruiz, J. A., Recio, I., & Belloque, J. (2004). ACE-Inhibitory activity and structural properties of peptide Asp-Lys-Ile-His-Pro [β-CN f (47− 51)]. Study of the peptide forms synthesized by different methods. Journal of Agricultural and Food Chemistry, 52(20), 6315–6319. https://doi.org/10.1021/jf049532f
- González-Córdova, A.F., Torres-Llanez, M. J., Rodríguez-Figueroa, J. C., Espinosa de Los-Monteros, J. J., Garcia, H. S., & Vallejo-Córdoba, B. (2011). Actividad inhibidora de la enzima convertidora de angiotensina en leches fermentadas con cepas de lactobacillus angiotensin converting enzyme inhibitory activity in milks fermented by lactobacillus strains. CyTa-Journal of Food, 9(2), 146–151. https://doi.org/10.1080/19476337.2010.499568
- Grassi, D., Desideri, G., Necozione, S., Ruggieri, F., Blumberg, J. B., Stornello, M., & Ferri, C. (2012). Protective effects of flavanol-rich dark chocolate on endothelial function and wave reflection during acute hyperglycemia. HYPERTENSIONAHA. 112.193995.
- Griffith, L. E., Guyatt, G. H., Cook, R. J., Bucher, H. C., & Cook, D. J. (1999). The influence of dietary and nondietary calcium supplementation on blood pressure: An updated meta-analysis of randomized controlled trials. American Journal of Hypertension, 12(1), 84–92. https://doi.org/10.1016/S0895-7061(98)00224-6
- Habauzit, V., & Morand, C. (2012). Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Therapeutic Advances in Chronic Disease, 3(2), 87–106. https://doi.org/10.1177/2040622311430006
- Hafeez, Z., Cakir-Kiefer, C., Roux, E., Perrin, C., Miclo, L., & Dary-Mourot, A. (2014). Strategies of producing bioactive peptides from milk proteins to functionalize fermented milk products. Food Research International, 63, 71–80. https://doi.org/10.1016/j.foodres.2014.06.002
- Hata, Y., Yamamoto, M., Ohni, M., Nakajima, K., Nakamura, Y., & Takano, T. (1996). A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. The American Journal of Clinical Nutrition, 64(5), 767–771. https://doi.org/10.1093/ajcn/64.5.767
- Hatzitolios, A., Iliadis, F., Katsiki, N., & Baltatzi, M. (2008). Is the anti-hypertensive effect of dietary supplements via aldehydes reduction evidence based? A systematic review. Clinical and Experimental Hypertension, 30(7), 628–639. https://doi.org/10.1080/10641960802443274
- Henrion, D., Kubis, N., & Lévy, B. I. (2001). Physiological and pathophysiological functions of the AT2 subtype receptor of angiotensin II: From large arteries to the microcirculation. Hypertension, 38(5), 1150–1157. https://doi.org/10.1161/hy1101.096109
- Hernández-Galán, L., Cardador-Martínez, A., López-Del-Castillo, M., Picque, D., Spinnler, H. E., & Martín Del Campo, S. T. (2017). Antioxidant and angiotensin-converting enzyme inhibitory activity in fresh goat cheese prepared without starter culture: A preliminary study. CyTa-Journal of Food, 15(1), 49–57. https://doi.org/10.1080/19476337.2016.1202325
- Hirata, H. (2002). Clinical effects of new sour milk drink in mild or moderate hypertensive subjects. J New Rem Clin, 51, 60–66.
- Houston, M. C. (2011). The importance of potassium in managing hypertension. Current Hypertension Reports, 13(4), 309–317. https://doi.org/10.1007/s11906-011-0197-8
- Ibrahim, H. R., Ahmed, A. S., & Miyata, T. (2017). Novel angiotensin-converting enzyme inhibitory peptides from caseins and whey proteins of goat milk. Journal of Advanced Research, 8(1), 63–71. https://doi.org/10.1016/j.jare.2016.12.002
- Jarmołowska, B., Kostyra, E., Krawczuk, S., & Kostyra, H. (1999). β‐Casomorphin‐7 isolated from Brie cheese. Journal of the Science of Food and Agriculture, 79(13), 1788–1792. https://doi.org/10.1002/(SICI)1097-0010(199910)79:13<1788:AID-JSFA436>3.0.CO;2-T
- Jauhiainen, T., Collin, M., Narva, M., Cheng, Z., Poussa, T., Vapaatalo, H., & Korpela, R. (2005). Effect of long-term intake of milk peptides and minerals on blood pressure and arterial function in spontaneously hypertensive rats. Milchwissenschaft-Milk Science International, 60(4), 358–362.
- Jee, S. H., Miller, E. R., Guallar, E., Singh, V. K., Appel, L. J., & Klag, M. J. (2002). The effect of magnesium supplementation on blood pressure: A meta-analysis of randomized clinical trials. American Journal of Hypertension, 15(8), 691–696. https://doi.org/10.1016/S0895-7061(02)02964-3
- Juraschek, S. P., Guallar, E., Appel, L. J., & Miller, E. R. (2012). Effects of vitamin C supplementation on blood pressure: A meta-analysis of randomized controlled trials. The American Journal of Clinical Nutrition, 95(5), 1079–1088. https://doi.org/10.3945/ajcn.111.027995
- Kajimoto, O., Kurosaki, T., Mizutani, J., Nagisa, I., Kaneko, K., Aihara, K., Yabune, M., Nakamura, Y. (2002). Antihypertensive effects of liquid yogurts containing “lactotripeptides (VPP, IPP)” in mild hypertensive subjects. Journal of Nutrition & Food, 5(3), 55–66.
- Kapil, V., Khambata, R. S., Robertson, A., Caulfield, M. J., & Ahluwalia, A. (2015). Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension, 65(2), 320–327. https://doi.org/10.1161/HYPERTENSIONAHA.114.04675
- Kass, L., Weekes, J., & Carpenter, L. (2012). Effect of magnesium supplementation on blood pressure: A meta-analysis. European Journal of Clinical Nutrition, 66(4), 411–418. https://doi.org/10.1038/ejcn.2012.4
- Kearney, P. M., Whelton, M., Reynolds, K., Muntner, P., Whelton, P. K., & He, J. (2005). Global burden of hypertension: Analysis of worldwide data. The Lancet, 365(9455), 217–223. https://doi.org/10.1016/S0140-6736(05)17741-1
- Kim, J. H., Lee, D. H., Jeong, S. C., Chung, K. S., & Lee, J. S. (2004). Characterization of antihypertensive angiotensin I-converting enzyme inhibitor from saccharomyces cerevisiae. Journal of Microbiology and Biotechnology, 16(6), 1318–1323.
- Kim, S. M., Park, S., & Choue, R. (2010). Effects of fermented milk peptides supplement on blood pressure and vascular function in spontaneously hypertensive rats. Food Science and Biotechnology, 19(5), 1409–1413. https://doi.org/10.1007/s10068-010-0201-0
- Kocak, A., Sanli, T., Anli, E. A., & Hayaloglu, A. A. (2020). Role of using adjunct cultures in release of bioactive peptides in white-brined goat-milk cheese. Lwt, 123, 109127. https://doi.org/10.1016/j.lwt.2020.109127
- Korhonen, H., & Pihlanto, A. (2003). Food-derived bioactive peptides-opportunities for designing future foods. Current Pharmaceutical Design, 9(16), 1297–1308. https://doi.org/10.2174/1381612033454892
- Korhonen, H., & Pihlanto, A. (2007). Technological options for the production of health-promoting proteins and peptides derived from milk and colostrum. Current Pharmaceutical Design, 13(8), 829–843. https://doi.org/10.2174/138161207780363112
- Langsjoen, P. H., & Langsjoen, A. M. (1999). Overview of the use of CoQ10in cardiovascular disease. BioFactors, 9(2–4), 273–284. https://doi.org/10.1002/biof.5520090224
- Lee, Y.-M., Skurk, T., Hennig, M., & Hauner, H. (2007). Effect of a milk drink supplemented with whey peptides on blood pressure in patients with mild hypertension. European Journal of Nutrition, 46(1), 21–27. https://doi.org/10.1007/s00394-006-0625-8
- Leggio, M., Tiberti, C., Armeni, M., Limongelli, G., & Mazza, A. (2019). The effects of dairy consumption on blood pressure and risk of hypertension. The American Journal of Medicine, 132(8), e669. https://doi.org/10.1016/j.amjmed.2019.03.028
- Lin, K., Zhang, L.-W., Han, X., Xin, L., Meng, Z.-X., Gong, P.-M., & Cheng, D.-Y. (2018). Yak milk casein as potential precursor of angiotensin I-converting enzyme inhibitory peptides based on in silico proteolysis. Food Chemistry, 254, 340–347. https://doi.org/10.1016/j.foodchem.2018.02.051
- Li, Y., Sadiq, F. A., Liu, T., Chen, J., & He, G. (2015). Purification and identification of novel peptides with inhibitory effect against angiotensin I-converting enzyme and optimization of process conditions in milk fermented with the yeast kluyveromyces marxianus. Journal of Functional Foods, 16, 278–288. https://doi.org/10.1016/j.jff.2015.04.043
- Liu, Y., Ma, W., Zhang, P., He, S., & Huang, D. (2015). Effect of resveratrol on blood pressure: A meta-analysis of randomized controlled trials. Clinical Nutrition, 34(1), 27–34. https://doi.org/10.1016/j.clnu.2014.03.009
- Liu, X., Wei, J., Tan, F., Zhou, S., Würthwein, G., & Rohdewald, P. (2004). Antidiabetic effect of Pycnogenol® French maritime pine bark extract in patients with diabetes type II. Life Sciences, 75(21), 2505–2513. https://doi.org/10.1016/j.lfs.2003.10.043
- Li, H., Xia, N., & Forstermann, U. (2012). Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide: Biology and Chemistry / Official Journal of the Nitric Oxide Society, 26(2), 102–110. https://doi.org/10.1016/j.niox.2011.12.006
- Maeno, M., Yamamoto, N., & Takano, T. (1996). Naoyuki Yamamoto, and Toshiaki Takano 1996 identification of an antihypertensive peptide from casein hydrolysate produced by a proteinase from lactobacillus helveticus CP790. Journal of Dairy Science, 79(8), 1316–1321. https://doi.org/10.3168/jds.S0022-0302(96)76487-1
- Mahajan, A. S., Babbar, R., Kansal, N., Agarwal, S. K., & Ray, P. C. (2007). Antihypertensive and antioxidant action of amlodipine and vitamin C in patients of essential hypertension. Journal of Clinical Biochemistry and Nutrition, 40(2), 141–147. https://doi.org/10.3164/jcbn.40.141
- Maimoona, A., Naeem, I., Saddiqe, Z., & Jameel, K. (2011). A review on biological, nutraceutical and clinical aspects of French maritime pine bark extract. Journal of Ethnopharmacology, 133(2), 261–277. https://doi.org/10.1016/j.jep.2010.10.041
- Mansouri, M., Pahlavani, N., Sharifi, F., Varmaghani, M., Shokri, A., Yaghubi, H., Asbaghi, O., Keshtkar, A., Tabrizi, Y. M., & Sadeghi, O. Dairy consumption in relation to hypertension among a large population of university students: The MEPHASOUS study. Diabetes Metabolic Syndrome and Obesity Targets and Therapy, 13, 1633–1642. https://doi.org/10.2147/DMSO.S248592
- McCarron, D. A., Morris, C. D., Henry, H. J., & Stanton, J. L. (1984). Blood pressure and nutrient intake in the United States. Science, 224(4656), 1392–1398. https://doi.org/10.1126/science.6729459
- Meisel, H. (1998). Overview on milk protein-derived peptides. International Dairy Journal, 8(5–6), 363–373. https://doi.org/10.1016/S0958-6946(98)00059-4
- Meisel, H., & Schlimme, E. (1996). Bioactive peptides derived from milk proteins: Ingredients for functional foods? Kieler Milchwirtschaftliche Forschungsberichte, 48(4), 343–357.
- Miyazaki, H., Fukamizu, A., Hirose, S., Hayashi, T., Hori, H., Ohkubo, H., Nakanishi, S., & Murakami, K. (1984). Structure of the human renin gene. Proceedings of the National Academy of Sciences, 81(19), 5999–6003. https://doi.org/10.1073/pnas.81.19.5999
- Mizuno, S., Matsuura, K., Gotou, T., Nishimura, S., Kajimoto, O., Yabune, M., Kajimoto, Y., & Yamamoto, N. (2005). Antihypertensive effect of casein hydrolysate in a placebo-controlled study in subjects with high-normal blood pressure and mild hypertension. The British Journal of Nutrition, 94(1), 84–91. https://doi.org/10.1079/BJN20051422
- Muehlenkamp, M.R., & Warthesen, J.J. (1996). β-casomorphins: Analysis in cheese and susceptibility to proteolytic enzymes from lactococcus lactis ssp. cremoris1. Journal of Dairy Science, 79(1), 20–26. https://doi.org/10.3168/jds.S0022-0302(96)76329-4
- Muguerza, B., Ramos, M., Sánchez, E., Manso, M. A., Miguel, M., Aleixandre, A., Delgado, M. A., & Recio, I. (2006). Antihypertensive activity of milk fermented by Enterococcus faecalis strains isolated from raw milk. International Dairy Journal, 16(1), 61–69. https://doi.org/10.1016/j.idairyj.2005.01.001
- Mullens, W., Verbrugge, F. H., Nijst, P., & Tang, W. H. W. (2017). Renal sodium avidity in heart failure: From pathophysiology to treatment strategies. European Heart Journal, 38(24), 1872–1882. https://doi.org/10.1093/eurheartj/ehx035
- Murakami, M., Tonouchi, H., Takahashi, R., Kitazawa, H., Kawai, Y., Negishi, H., & Saito, T. (2004). Structural analysis of a new anti-hypertensive peptide (β-lactosin B) isolated from a commercial whey product. Journal of Dairy Science, 87(7), 1967–1974. https://doi.org/10.3168/jds.S0022-0302(04)70013-2
- Nakamura, Y., Yamamoto, N., Sakai, K., Okubo, A., Yamazaki, S., & Takano, T. (1995). Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk. Journal of Dairy Science, 78(4), 777–783. https://doi.org/10.3168/jds.S0022-0302(95)76689-9
- Nakamura, Y., Yamamoto, N., Sakai, K., & Takano, T. (1995). Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin I-converting enzyme. Journal of Dairy Science, 78(6), 1253–1257. https://doi.org/10.3168/jds.S0022-0302(95)76745-5
- Ness, A. R., Chee, D., & Elliott, P. (1997). Vitamin C and blood pressure–an overview. Journal of Human Hypertension, 11(6), 343–350. https://doi.org/10.1038/sj.jhh.1000423
- Neutel, J.M., et al. (2006). The use of amealpeptide in the treatment of patients with stage I and stage II hypertension-data from the AHEAD study. Journal of Hypertension, 24, S84. LIPPINCOTT WILLIAMS & WILKINS 530 WALNUT ST, PHILADELPHIA, PA 19106-3621 USA .
- Norris, R., Poyarkov, A., O’Keeffe, M. B., & FitzGerald, R. J. (2014). Characterisation of the hydrolytic specificity of aspergillus niger derived prolyl endoproteinase on bovine β-casein and determination of ACE inhibitory activity. Food Chemistry, 156, 29–36. https://doi.org/10.1016/j.foodchem.2014.01.056
- Nurminen, M.-L., Sipola, M., Kaarto, H., Pihlanto-Leppälä, A., Piilola, K., Korpela, R., Tossavainen, O., Korhonen, H., & Vapaatalo, H. (2000). α-lactorphin lowers blood pressure measured by radiotelemetry in normotensive and spontaneously hypertensive rats. Life Sciences, 66(16), 1535–1543. https://doi.org/10.1016/S0024-3205(00)00471-9
- Ong, K. L., Cheung, B. M. Y., Man, Y. B., Lau, C. P., & Lam, K. S. L. (2007). Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension, 49(1), 69–75. https://doi.org/10.1161/01.HYP.0000252676.46043.18
- Oparil, S., Amin Zaman, M., & Calhoun, D. A. (2003). Pathogenesis of hypertension. Annals of Internal Medicine, 139(9), 761–776. https://doi.org/10.7326/0003-4819-139-9-200311040-00011
- Padghan, P.V., Mann, B., Sharma, R., Bajaj, R., & Saini, P. (2017). Production of angiotensin-I-converting-enzyme-inhibitory peptides in fermented milks (Lassi) fermented by lactobacillus acidophillus with consideration of incubation period and simmering treatment. International Journal of Peptide Research and Therapeutics, 23(1), 69–79. https://doi.org/10.1007/s10989-016-9540-x
- Paran, E., Novack, V., Engelhard, Y. N., & Hazan-Halevy, I. (2009). The effects of natural antioxidants from tomato extract in treated but uncontrolled hypertensive patients. Cardiovascular Drugs Therapy, 23(2), 145–151. https://doi.org/10.1007/s10557-008-6155-2
- Parmar, H., Hati, S., Panchal, G., & Sakure, A. A. (2020). Purification and production of novel angiotensin I-converting enzyme (ACE) inhibitory bioactive peptides derived from fermented goat milk. International Journal of Peptide Research and Therapeutics, 26(2), 997–1011. https://doi.org/10.1007/s10989-019-09902-7
- Parrot, S., Degraeve, P., Curia, C., Martial-Gros, A. (2003). In vitro study on digestion of peptides in emmental cheese: Analytical evaluation and influence on angiotensin I converting enzyme inhibitory peptides. Molecular Nutrition & Food Research, 47(2), 87–94.
- Paul, M., Poyan Mehr, A., & Kreutz, R. (2006). Physiology of local renin-angiotensin systems. Physiological Reviews, 86(3), 747–803. https://doi.org/10.1152/physrev.00036.2005
- Perkovic, V., Huxley, R., Wu, Y., Prabhakaran, D., & MacMahon, S. (2007). The burden of blood pressure-related disease: A neglected priority for global health. Hypertension, 50(6), 991–997. https://doi.org/10.1161/HYPERTENSIONAHA.107.095497
- Perrinjaquet-Moccetti, T., Busjahn, A., Schmidlin, C., Schmidt, A., Bradl, B., & Aydogan, C. (2008). Food supplementation with an olive (Olea europaea L.) leaf extract reduces blood pressure in borderline hypertensive monozygotic twins. Phytotherapy Research, 22(9), 1239–1242. https://doi.org/10.1002/ptr.2455
- Pihlanto, A., Virtanen, T., & Korhonen, H. (2010). Angiotensin I converting enzyme (ACE) inhibitory activity and antihypertensive effect of fermented milk. International Dairy Journal, 20(1), 3–10. https://doi.org/10.1016/j.idairyj.2009.07.003
- Plantinga, Y., GHIADONI, L., MAGAGNA, A., GIANNARELLI, C., FRANZONI, F., TADDEI, S., & SALVETTI, A. (2007). Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. American Journal of Hypertension, 20(4), 392–397. https://doi.org/10.1016/j.amjhyper.2006.09.021
- Rahimi, M., Ghaffari, S. M., Salami, M., Mousavy, S. J., Niasari-Naslaji, A., Jahanbani, R., Yousefinejad, S., Khalesi, M., & Moosavi-Movahedi, A. A. (2016). ACE-inhibitory and radical scavenging activities of bioactive peptides obtained from camel milk casein hydrolysis with proteinase K. Dairy Science & Technology, 96(4), 489–499. https://doi.org/10.1007/s13594-016-0283-4
- Reed, D., McGee, D., Yano, K., & Hankin, J. (1985). Diet, blood pressure, and multicollinearity. Hypertension, 7(3 Pt 1), 405–410. https://doi.org/10.1161/01.HYP.7.3.405
- Ried, K., & Fakler, P. (2014). Potential of garlic (Allium sativum) in lowering high blood pressure: Mechanisms of action and clinical relevance. Integrated Blood Pressure Control, 71. https://doi.org/10.2147/IBPC.S51434
- Ried, K., Frank, O. R., & Stocks, N. P. (2010). Aged garlic extract lowers blood pressure in patients with treated but uncontrolled hypertension: A randomised controlled trial. Maturitas, 67(2), 144–150. https://doi.org/10.1016/j.maturitas.2010.06.001
- Ried, K., Sullivan, T. R., Fakler, P., Frank, O. R., Stocks, N. P. (2012). Effect of cocoa on blood pressure. Cochrane Database of Systemic Reviews, 8, CD008893.
- Ried, K., Toben, C., & Fakler, P. (2013). Effect of garlic on serum lipids: An updated meta-analysis. Nutrition Reviews, 71(5), 282–299. https://doi.org/10.1111/nure.12012
- Rodríguez-Figueroa, J.C., González-Córdova, A. F., Astiazaran-García, H., & Vallejo-Cordoba, B. (2013). Hypotensive and heart rate-lowering effects in rats receiving milk fermented by specific lactococcus lactis strains. The British Journal of Nutrition, 109(5), 827–833. https://doi.org/10.1017/S0007114512002115
- Rodríguez-Figueroa, J.C., González-Córdova, A. F., Torres-Llanez, M. J., Garcia, H. S., & Vallejo-Cordoba, B. (2012). Novel angiotensin I-converting enzyme inhibitory peptides produced in fermented milk by specific wild lactococcus lactis strains. Journal of Dairy Science, 95(10), 5536–5543. https://doi.org/10.3168/jds.2011-5186
- Rohner, A., Ried, K., Sobenin, I. A., Bucher, H. C., & Nordmann, A. J. (2015). A systematic review and meta-analysis on the effects of garlic preparations on blood pressure in individuals with hypertension. American Journal of Hypertension, 28(3), 414–423. https://doi.org/10.1093/ajh/hpu165
- Rokka, T., Syväoja, E.-L., Tuominen, J., & Korhonen, H. J. T. (1997). Release of bioactive peptides by enzymatic proteolysis of lactobacillus GG fermented UHT-milk.
- Ryhänen, E.-L., Pihlanto-Leppälä, A., & Pahkala, E. (2001). Anne Pihlanto-Leppälä, and Eero Pahkala 2001 a new type of ripened, low-fat cheese with bioactive properties. International Dairy Journal, 11(4–7), 441–447. https://doi.org/10.1016/S0958-6946(01)00079-6
- Sabikhi, L., & Mathur, B.N. (2001). ORIGINAL PAPERS-qualitative and quantitative analysis of b-casomorphins in edam cheese. Milchwissenschaft, 56(4), 198–200.
- Saito, T., Nakamura, T., Kitazawa, H., Kawai, Y., & Itoh, T. (2000). Isolation and structural analysis of antihypertensive peptides that exist naturally in gouda cheese. Journal of Dairy Science, 83(7), 1434–1440. https://doi.org/10.3168/jds.S0022-0302(00)75013-2
- Sano, J., Ohki, K., Higuchi, T., Aihara, K., Mizuno, S., Kajimoto, O., Nakagawa, S., Kajimoto, Y., & Nakamura, Y. (2005). Effect of casein hydrolysate, prepared with protease derived from Aspergillus oryzae, on subjects with high-normal blood pressure or mild hypertension. Journal of Medicinal Food, 8(4), 423–430. https://doi.org/10.1089/jmf.2005.8.423
- Sato, K., Dohi, Y., Kojima, M., Miyagawa, K., Takase, H., Katada, E., & Suzuki, S. (2006). Effects of ascorbic acid on ambulatory blood pressure in elderly patients with refractory hypertension. Arzneimittelforschung, 56(7), 535–540. https://doi.org/10.1055/s-0031-1296748
- Schütten, M. T. J., Houben, A. J. H. M., de Leeuw, P. W., & Stehouwer, C. D. A. (2017). The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology, 32(3), 197–209. https://doi.org/10.1152/physiol.00037.2016
- Sekiya, S., KOBAYASHI, Y., KITA, E., IMAMURA, Y., & TOYAMA, S. (1992). Antihypertensive effects of tryptic hydrolysate of casein on normotensive and hypertensive volunteers. Journal of Japanese Society of Nutrition and Food Science, 45(6), 513–517. https://doi.org/10.4327/jsnfs.45.513
- Seppo, L. (2001). The effect of a lactobacillus helveticus LBK-16 H fermented milk on hypertension-a pilot study on humans. Milchwissenschaft, 77 (2) , 124–127.
- Seppo, L., Jauhiainen, T., Poussa, T., & Korpela, R. (2003). A fermented milk high in bioactive peptides has a blood pressure–lowering effect in hypertensive subjects. The American Journal of Clinical Nutrition, 77(2), 326–330. https://doi.org/10.1093/ajcn/77.2.326
- Sharma, S., Singh, R., & Rana, S. (2011). Bioactive peptides: A review. International Journal Bioautomation, 15(4), 223–250.
- Sherman, D. L., Keaney, J. F., Biegelsen, E. S., Duffy, S. J., Coffman, J. D., & Vita, J. A. (2000). Pharmacological concentrations of ascorbic acid are required for the beneficial effect on endothelial vasomotor function in hypertension. Hypertension, 35(4), 936–941. https://doi.org/10.1161/01.HYP.35.4.936
- Shu, G., Huang, J., Chen, L., Lei, N., & Chen, H. (2018). Characterization of goat milk hydrolyzed by cell envelope proteinases from lactobacillus plantarum LP69: Proteolytic system optimization, bioactivity, and storage stability evaluation. Molecules, 23(6), 1317. https://doi.org/10.3390/molecules23061317
- Shu, G., Shi, X., Chen, H., Ji, Z., & Meng, J. (2017). Optimization of goat milk with ACE inhibitory peptides fermented by lactobacillus bulgaricus LB6 using response surface methodology. Molecules, 22(11), 2001. https://doi.org/10.3390/molecules22112001
- Simon, J. A. (1992). Vitamin C and cardiovascular disease: A review. Journal of the American College of Nutrition, 11(2), 107–125. https://doi.org/10.1080/07315724.1992.12098232
- Sipola, M., FINCKENBERG, P., KORPELA, R., VAPAATALO, H., & NURMINEN, M.-L. (2002). Effect of long-term intake of milk products on blood pressure in hypertensive rats. The Journal of Dairy Research, 69(1), 103–111. https://doi.org/10.1017/S002202990100526X
- Sipola, M., Finckenberg, P., Santisteban, J., Korpela, R., Vapaatalo, H., & Nurminen, M. L. (2001). Long-term intake of milk peptides attenuates development of hypertension in spontaneously hypertensive rats. Journal of Physiology and Pharmacology : An Official Journal of the Polish Physiological Society, 52(4 Pt 2), 745–754.
- Solieri, L., Rutella, G. S., & Tagliazucchi, D. (2015). Impact of non-starter lactobacilli on release of peptides with angiotensin-converting enzyme inhibitory and antioxidant activities during bovine milk fermentation. Food Microbiology, 51, 108–116. https://doi.org/10.1016/j.fm.2015.05.012
- Stamler, J., Elliott, P., Kesteloot, H., Nichols, R., Claeys, G., Dyer, A. R., & Stamler, R. (1996). Inverse relation of dietary protein markers with blood pressure: Findings for 10 020 men and women in the INTERSALT study. Circulation, 94(7), 1629–1634. https://doi.org/10.1161/01.CIR.94.7.1629
- Steiner, M., & Lin, R. S. (1998). Changes in platelet function and susceptibility of lipoproteins to oxidation associated with administration of aged garlic extract. Journal of Cardiovascular Pharmacology, 31(6), 904–908. https://doi.org/10.1097/00005344-199806000-00014
- Tate, C. G. (2017). Structural biology: A receptor that might block itself. Nature, 544(7650), 307. https://doi.org/10.1038/nature21907
- Toledo, E., Hu, F. B., Estruch, R., Buil-Cosiales, P., Corella, D., Salas-Salvadó, J., Covas, M. I., Arós, F., Gómez-Gracia, E., Fiol, M., Lapetra, J., Serra-Majem, L., Pinto, X., Lamuela-Raventós, R. M., Saez, G., Bulló, M., Ruiz-Gutiérrez, V., Ros, E., Sorli, J. V., & Martinez-Gonzalez, M. A. (2013). Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: Results from a randomized controlled trial. BMC Medicine, 11(1), 207. https://doi.org/10.1186/1741-7015-11-207
- Touyz, R. M., & Schiffrin, E. L. (2000). Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacological Reviews, 52(4), 639–672.
- Tuomilehto, J., Lindström, J., Hyyrynen, J., Korpela, R., Karhunen, M.-L., Mikkola, L., Jauhiainen, T., Seppo, L., & Nissinen, A. (2004). Effect of ingesting sour milk fermented using lactobacillus helveticus bacteria producing tripeptides on blood pressure in subjects with mild hypertension. Journal of Human Hypertension, 18(11), 795. https://doi.org/10.1038/sj.jhh.1001745
- Uluko, H., Zhang, S., Liu, L., Li, H., Cui, W., Xue, H., Zhao, L., Sun, Y., Lu, J., & Lv, J. (2014). Pilot-scale membrane fractionation of ACE inhibitory and antioxidative peptides from ultrasound pretreated milk protein concentrate hydrolysates. Journal of Functional Foods, 7, 350–361. https://doi.org/10.1016/j.jff.2014.01.025
- van Dam, R. M., Naidoo, N., & Landberg, R. (2013). Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: Review of recent findings. Current Opinion in Lipidology, 24(1), 25–33. https://doi.org/10.1097/MOL.0b013e32835bcdff
- van der Zander, K., Bots, M. L., Bak, A. A., Koning, M. M., & de Leeuw, P. W. (2008). Enzymatically hydrolyzed lactotripeptides do not lower blood pressure in mildly hypertensive subjects. The American Journal of Clinical Nutrition, 88(6), 1697–1702. https://doi.org/10.3945/ajcn.2008.26003
- Van der Zander, K., Jäkel, M., Bianco, V., & Koning, M. M. G. (2008). Fermented lactotripeptides-containing milk lowers daytime blood pressure in high normal-to-mild hypertensive subjects. Journal of Human Hypertension, 22(11), 804. https://doi.org/10.1038/jhh.2008.59
- van Haaster, M. C., McDonough, A. A., & Gurley, S. B. (2018). Blood pressure regulation by the angiotensin type 1 receptor in the proximal tubule. Current Opinion in Nephrology and Hypertension, 27(1), 1–7. https://doi.org/10.1097/MNH.0000000000000373
- van Mierlo, L. A. J., Arends, L. R., Streppel, M. T., Zeegers, M. P. A., Kok, F. J., Grobbee, D. E., & Geleijnse, J. M. (2006). Blood pressure response to calcium supplementation: A meta-analysis of randomized controlled trials. Journal of Human Hypertension, 20(8), 571. https://doi.org/10.1038/sj.jhh.1002038
- van Mierlo, L. A. J., Koning, M. M., van der Zander, K., & Draijer, R. (2008). Lactotripeptides do not lower ambulatory blood pressure in untreated whites: Results from 2 controlled multicenter crossover studies–. The American Journal of Clinical Nutrition, 89(2), 617–623. https://doi.org/10.3945/ajcn.2008.26918
- Villaverde, P., Lajous, M., MacDonald, C.-J., Fagherazzi, G., Boutron-Ruault, M.-C., & Bonnet, F. (2020). Dairy product consumption and hypertension risk in a prospective French cohort of women. Nutrition Journal, 19(1), 12. https://doi.org/10.1186/s12937-020-0527-2
- Whelton, P. K., & He, J. (1999). Potassium in preventing and treating high blood pressure. Seminars in Nephrology, 19(5), 494–499.
- Whelton, P. K., He, J., Cutler, J. A., Brancati, F. L., Appel, L. J., Follmann, D., & Klag, M. J. (1997). Effects of oral potassium on blood pressure: Meta-analysis of randomized controlled clinical trials. Jama, 277(20), 1624–1632. https://doi.org/10.1001/jama.1997.03540440058033
- Xu, J.-Y., Qin, L.-Q., Wang, P.-Y., Li, W., & Chang, C. (2008). Effect of milk tripeptides on blood pressure: A meta-analysis of randomized controlled trials. Nutrition, 24(10), 933–940. https://doi.org/10.1016/j.nut.2008.04.004
- Yamamoto, N., Maeno, M., & Takano, T. (1999). Purification and characterization of an antihypertensive peptide from a yogurt-like product fermented by Lactobacillus helveticus CPN4. Journal of Dairy Science, 82(7), 1388–1393. https://doi.org/10.3168/jds.S0022-0302(99)75364-6
- Zhang, H., Han, G. W., Batyuk, A., Ishchenko, A., White, K. L., Patel, N., Sadybekov, A., Zamlynny, B., Rudd, M. T., Hollenstein, K., Tolstikova, A., White, T. A., Hunter, M. S., Weierstall, U., Liu, W., Babaoglu, K., Moore, E. L., Katz, R. D., Shipman, J. M. … Cherezov, V. (2017). Structural basis for selectivity and diversity in angiotensin II receptors. Nature, 544(7650), 327. https://doi.org/10.1038/nature22035
- Zhang, X., Li, Y., Del Gobbo, L. C., Rosanoff, A., Wang, J., Zhang, W., & Song, Y. (2016). Effects of magnesium supplementation on blood pressure: A meta-analysis of randomized double-blind placebo-controlled trials. Hypertension, 68(2), 324–333. https://doi.org/10.1161/HYPERTENSIONAHA.116.07664
- Zibadi, S., Rohdewald, P. J., Park, D., & Watson, R. R. (2008). Reduction of cardiovascular risk factors in subjects with type 2 diabetes by pycnogenol supplementation. Nutrition Research, 28(5), 315–320. https://doi.org/10.1016/j.nutres.2008.03.003
