 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study investigated the effects of Aloe vera gel (AVG) based on edible coating solutions on tomatoes to maintain the postharvest quality during storage at 10°C for 30 days. The AVG coating solutions were prepared with different percentages of extracted gel ranging from 0 to 80% with addition to calcium chloride (2%), ascorbic acid (4%), carboxymethyl cellulose (3%), glycerol (2%), and oleic acid (3 mL). Results showed that coating solutions containing 60 to 80% AVG had better results than coating solutions containing 0 to 40% gel. Contents of ascorbic acid, sugar, flavonoids, carotenoids, lycopene, and pectin remained higher, and total microbial count remained lesser in fruits treated with a higher concentration of AVG over the storage time. Tomatoes coated with 60% and 80% Aloe vera gel also showed maximum antioxidant efficiency, absence of E. coli, and no signs of fungal (Botrytis cinerea) growth on tomatoes. In conclusion, applying 60–80% AVG edible coatings might be suggested to maintain the postharvest quality of tomato fruit.
RESUMEN
A fin de mantener la calidad de los tomates durante su almacenamiento poscosecha a 10°C por un periodo de 30 días, el presente estudio investigó los efectos del gel de Aloe Vera (AVG), preparado en soluciones de recubrimiento comestibles que contenían diversas concentraciones del mismo, sobre los tomates. Las soluciones de recubrimiento de AVG se elaboraron con diferentes porcentajes de gel extraído que iban del 0 al 80% a las que se adicionaron cloruro de calcio (2%), ácido ascórbico (4%), carboximetilcelulosa (3%), glicerol (2%) y ácido oleico (3 mL). Se constató que las soluciones de recubrimiento que contienen entre 60 y 80% de AVG proporcionaron mejores resultados que las que contienen entre 0 y 40% de gel. Los contenidos de ácido ascórbico, azúcar, flavonoides, carotenoides, licopeno y pectina se mantuvieron más altos, mientras que el recuento microbiano total siguió siendo menor en las frutas tratadas con soluciones de mayor concentración de AVG durante el tiempo de almacenamiento. Asimismo, los tomates recubiertos con 60% y 80% de gel de Aloe vera mostraron la máxima eficacia antioxidante, ausencia de E.coli y ningún signo de crecimiento de hongos (Botrytis cinerea) en ellos. En conclusión, para mantener la calidad poscosecha de los frutos de tomate, podrían aplicarse recubrimientos comestibles de 60–80% de AVG.
1. Introduction
Climacteric fruits such as tomatoes are highly perishable, have a limited shelf life (7 to 10 days), and are prone to early quality deterioration under ambient conditions. Tomatoes are susceptible to ethylene and tend to ripen sharply, particularly after harvest. That’s why; growers need to slow down the ripening process of tomatoes after harvest to make them available to the commercial markets as wholesome fruit. Tomatoes are a vital source of nutritional and therapeutic compounds, including ascorbic acid, sugars, total phenols, flavonoids, carotenoids, and lycopene (Chrysargyris et al., Citation2016). For maintaining the fruit texture, cell wall compounds such as pectin play essential roles in tomato fruit softening and texture integrity (Wang et al., Citation2018). Meanwhile, undesired storage environments and microbial/fungal attacks may primarily affect such compounds, thus leading to postharvest quality losses of tomatoes.
The edible coating is a robust approach to enhancing the shelf life of the produce by preventing anaerobiosis in perishable fruit like tomatoes (Hasan et al., Citation2021; Iftikhar et al., Citation2022; Wahab et al., Citation2021). Various kinds of biodegradable, edible coatings (i.e. seed mucilage, microbial gums, pectin polysaccharides, corn starch, gum arabic, polyalcohols, etc.) are in practice to overcome postharvest losses in horticultural products (Alizadeh-Sani et al., Citation2019; Anjum et al., Citation2020; Beikzadeh et al., Citation2020; Dhall, Citation2013; Panahirad et al., Citation2021; Raghav et al., Citation2012; Rehman et al., Citation2021; Villafañe, Citation2017). Edible coatings create a modified atmosphere by generating a semi-permeable barrier against O2, CO2, solute, and moisture exchange. Subsequently, oxidation rate, respiration rate, ethylene production, textural strength, flavour quality, and water loss remained controlled, maintaining the fruit quality for a longer time (Ali et al., Citation2010).
Hydro colloidal Aloe vera gel (AVG) can potentially extend the shelf life and maintain the postharvest quality of various perishable products (Chrysargyris et al., Citation2016). The AVG contains 99% gel component along with numerous essential substances such as polysaccharides, amino acids, organic acids, minerals (zinc, calcium, copper, magnesium, manganese, and phosphorus), essential enzymes, sterols, gibberellins, and water- and fat-soluble vitamins and phenolic compounds, many of which have antimicrobial and anti-mutagenic properties (Anjum et al., Citation2020; Ramachandra & Rao, Citation2008). The AVG is generally considered a safe (GRAS) coating material due to its accessible biochemical properties, biodegradability, antimicrobial action, non-toxicity, film-forming properties, and eco-friendly nature. It is bio-preservative, affordable, technologically viable, and easily applicable. The AVG contains various components exhibiting antimicrobial activities, such as anthraquinones that could show the inhibitory potential against Staphylococcus aureus and E. coli. The mechanism behind its antimicrobial action is the inhibition of solute transport through membranes. A previous study reported its effectiveness against food-borne pathogens such as Salmonella typhimurium, Klebsialla pneumonia, Bacillus cereus, and E. coli (Alemdar & Agaoglu, Citation2009).
In contrast, adverse effects of various agrochemicals and synthetic antimicrobial components applied to fruits and vegetables have been reported to affect human health and the environment as well as natural edible coatings (Garcia & Davidov‐pardo, Citation2021; Kahramanoğlu et al., Citation2020; Palou et al., Citation2015; Rehman et al., Citation2022). In this scenario, AVG-based edible coatings seem like a green alternative attributed with cost-effective and readily available than already existing synthetic materials (Chrysargyris et al., Citation2016). Meanwhile, an optimized formulation of the AVG as edible coatings material for tomatoes still needs to be investigated. Thus, the present research was designed to develop and optimize the AVG-based edible coatings with different AVG concentrations as green alternative coating material than synthetic agents and its impact on the quality attributes and microbiological safety of tomatoes during postharvest storage.
2. Material and methods
2.1. Material and preparation of coating solutions
Freshly harvested “Aquila” tomatoes (Solanum lycopersicum L.) were purchased based on regularity in size, shape, color and free of physical damage or absence of fungal attack at the pink stage from King Produce Market (4000 K Airline Dr, Houston, T× 77022, USA) and transported to a laboratory in Department of Horticultural Science, University of Minnesota, USA. All fruit was washed to remove dirt and clean it. Five batches with 26 tomatoes each were formed, and the fruit was stored at 10°C for 30 d to avoid undesirable biochemical changes. Fresh healthy Aloe vera (Aloe vera (L.) Burm.f.) leaves, 76 to 91 cm long, were purchased from San Rey Produce. Inc. (10101 S Keystone Dr, Pharr, T× 78577, USA). All chemicals used in this study were procured from the Alfa Aesar and Sigma Aldrich Company.
Aloe (hydroparenchyma) was separated from the leaves after removing the outer tissue layers. The hydroparenchyma gel matrix was mixed in an electronic blender (Vitamix E310 Explorian Blender) for 15 min at 25°C to get a homogeneous mixture, and the homogeneous mixture was filtered through a filter cloth of normal mesh size (1–2 mm) to get a gel-free for fibers and coarse particles. The gel was heated for pasteurization at 72°C for 45 min in a stainless-steel jar using a heating plate and then stabilized by cooling at room temperature (25°C). All coating solutions of AVG (0%, 20%, 40%, 60%, and 80%) were prepared contained with CaCl2 (2%) as a crosslinking agent, ascorbic acid (4%) as an antioxidant, CMC (3%) as a gelling agent, glycerol (2%), and 3 mL oleic acid to avoid precipitation. Distilled water was added to make the rest of the solution volume. Almost 4 litres of coating solution were prepared separately for 26 tomatoes. The detailed treatment plan is shown in .
Table 1. Aloe vera gel (AVG) based edible coatings formulation: (total volume = 1000 mL).
Tabla 1. Formulación de recubrimientos comestibles a base de gel de Aloe vera (AVG): (volumen total = 1000 mL).
2.2. Homogeneity of coating solutions
Homogeneity was determined to ensure solution stability against gravity assays using a spectrophotometric method described by Mehyar et al. (Citation2012). An aliquot of 10 mL coating solution was added to a plastic test tube, and then test tubes were incubated at 25°C. After 48 h, the samples from the upper and bottom layers of solution were taken to measure optical density (OD) using a spectrophotometer (DU-8200) at the wavelength of 620 nm.
2.3. Application of coating solutions on tomatoes
The AVG coating solutions were applied on tomato surfaces by immersion/dipping for 5 min and then dried at room temperature for 2 to 4 h in the open air.
2.4. Chemical analysis of tomatoes
Ascorbic acid was determined by visual titration of the 2, 6-dichlorophenol indophenol dye method as described by Athmaselvi et al. (Citation2013). An aliquot of 10 mL tomato juice was mixed with 0.4% oxalic acid solution to make the final volume of 100 mL. After that mixture was filtered through a cotton cloth to remove the fibrous material. Finally, 10 mL of filtrate was mixed with 15 mL of 0.4% oxalic acid and titrated against 2, 6-dichlorophenol indophenol dye until light pink color appeared. Contents of AsA were calculated by comparing the known concentrations of standard solution using the following formula:
Where R is the volume of dye used against sample extract titration, V is the volume of sample by adding 0.4% oxalic acid, R1 is the volume of dye used against standard titration, W is the weight of the sample, and V1 is the volume of filtrate used.
Reducing sugars were measured spectrophotometrically by 3,5- DNSA assay as described by Pila and Gol (Citation2010). Two grams of frozen sample in powder form were homogenized with 5 mL of deionized water and then centrifuged at 10,000 × g for 10 min at 4°C. Then, the filtrate was collected for analysis of reducing sugars. The DNSA solution was prepared by mixing 10 g DNSA in 200 mL of 2 mol/L NaOH solution. An aliquot of 2 mL of DNSA reagent was homogenized with 4 mL of sample filtrate in plastic test tubes. The blank mixture was prepared by homogenizing 4 mL of distilled water to 2 mL of DNSA solution. Both blank and sample mixtures were placed in a boiling water bath for 5 min and then cooled at ambient temperature until they appeared red-brown. The colored solution was then mixed with 1 mL 0.5% w/v sodium sulfate to absorb the dissolved oxygen and obtain a stable color solution. Finally, the mixture was run against blank at 540 nm wavelength using a spectrophotometer (UV-1800, Shimadzu, Ltd., Japan). Final results were estimated by comparing the known concentration of reducing sugars in standard solution and expressed in mg/g FW.
Total sugars were estimated by the anthrone method using a spectrophotometer as determined by Harsh et al. (Citation2016). Briefly, 2.0 g of tomato sample was ground in a mortar and pestle using hot aqueous ethanol (v/v 80%). Afterwards, the mixture was centrifuged at 8,000 × g for 12 min at room temperature. An aliquot of 0.5 mL of supernatant was collected in a plastic test tube, and 0.5 mL of deionized water was added to make the final volume up to 1 mL followed by the addition of 0.2% anthrone reagent. After that, the mixture was heated in a boiling water bath for 10 min and then placed at room temperature. The final solution was run at 620 nm wavelength to measure the green to dark green color intensity on a spectrophotometer (UV-1800, Shimadzu, Ltd., Japan). Final results were expressed in mg/g FW.
2.5. Total antioxidant efficiency, total phenols, flavonoids, carotenoids, and lycopene contents
Total antioxidants efficiency or FRAP (Ferric reducing antioxidant power) assays were determined spectrophotometrically as described by Guo et al. (Citation2003). FRAP assay method is based on reducing a ferric-tripyridyltriazine to ferrous colored compound in the presence of antioxidants. Briefly, 2.0 g of tomato sample was ground using a pestle and mortar and added deionized water with a ratio of 1:9 w/v. Afterwards, the mixture was centrifuged at 8,000 × g for 12 min, and the supernatant was collected for FRAP assay. The FRAP mixture was prepared by mixing the 3.0 mL of 10 mmol/L 2,4,6- tripyridy-s-triazine (TPTZ) solution, 20 mL of 0.4 mol/L acetate buffer, 3.0 mL of 20 mmol/L ferric chloride, and 40 mmol/L HCL (pH 3.5). FRAP mixture was prepared freshly and subjected to heat at 37°C in a hot water bath before use. Finally, 50 µL of sample supernatant was mixed with 2 mL FRAP mixture and 0.4 mL distilled water, then subjected to incubation at 37°C for 8 min before running the solution at 593 nm wavelength using a spectrophotometer (UV-1800, Shimadzu, Ltd., Japan). The 1.0 mmol/L FeSO4 was used as the standard solution. The final results were estimated by comparing the known concentration of FeSO3 standard solution (1.0 mmol/L) and expressed in mM FRAP/g FW.
Pectin was isolated and extracted by ultrasound-assisted extraction (UAE) method, as described by Grassino et al. (Citation2016). The UAE process was conducted at 60 and 80°C with a frequency of 37 kHz using an ultrasonic bath (Elmasconic, Germany). The time slots for sonication were 15, 45, and 90 min. Finally, the mixture was filtered through Whatman no.3 under vacuum conditions. The 10 mL of ethanol (96%) precipitated the mixture residues. After washing the residues with ethanol and acetone, pectin contents were dried at 40°C using a vacuum oven. Final results were calculated and expressed in mg/g FW.
Lycopene in tomato juice was determined quantitatively using a slightly modified spectrophotometrical method as described by Choudhari and Ananthanarayan (Citation2007). A 100 µL of homogenized tomato fruit juice was mixed with 8.0 mL of acetone:methanol: hexane (1:1:2) solution in a screw cap tube. Afterwards, 1.0 mL of water was added and vortexed. The mixture was placed at room temperature for at least 10 min to allow separation of mixture phases and the disappearance of air bubbles. Finally, an upper solution layer was run at 503 nm wavelength using a spectrophotometer (UV-1800, Shimadzu, Ltd., Japan). Final results were calculated and expressed in ppm.
Total phenolic contents (TPC) and total flavonoid contents (TFC) were estimated as described by (Manzoor et al., Citation2021). The tomato juice extract was used to determine TPC and TFC. For TPC, 1 ml of sample extract was mixed with 10 mL of deionized water and 1 mL of Folin-Ciocalteu phenol reagent. Afterwards, 10 mL of sodium carbonate (7%) was mixed and raised. The final volume of the mixture was up to 25 mL by adding deionized water. Finally, the mixture was run at 750 nm wavelength using a spectrophotometer (UV-1800, Shimadzu, Ltd., Japan) after incubation for 90 min at room temperature. The TPC was expressed in mg GAE/g FW.
For TFC, 1 ml of sample extract was homogenized with 0.3 mL aluminum chloride (10%), 0.3 mL sodium nitrite (3%), and 4 mL deionized water. After it, the mixed solution was thoroughly agitated with 2 mL of 1 M NaOH for 5 min, and then the final volume was raised to 10 mL by adding deionized water. Finally, the mixture was run at 510 nm on a spectrophotometer (UV-1800, Shimadzu, Ltd., Japan). Final results were expressed in mg QE/g FW.
Total carotenoid contents were determined spectrophotometrically by Sahabi et al. (Citation2012). Briefly, 15 g of tomato flesh was mixed with 3 g of celite 454 using a mortar and pestle. To extract carotenoids, 25 mL of acetone was added to the sample mixture to make the paste and then subjected to a filtration process under a vacuum to obtain the colorless extract. Afterward, the extract was mixed with 40 mL of petroleum ether in a 500 mL separatory funnel. The aqueous phase of the sample mixture was removed, followed by acetone removal by adding ultrapure water to prevent emulsion development. This process was repeated 3 to 4 times until evaporation of the complete solvent was assured. Afterwards, the extract was mixed with 15 g of anhydrous Na2SO4, and the total volume was raised to 50 mL by adding petroleum ether. Finally, the sample mixture was run at 450 nm wavelength using a spectrophotometer (UV-1800, Shimadzu, Ltd., Japan). Final results were calculated and expressed in mg/100 g FW.
2.6. Microbiological analysis
The total microbial count (TMC) of all samples was determined according to the method described by Ribeiro et al. (Citation2007). Tomato samples diluted with a standard saline solution were spread on nutrient agar plates that were incubated for 48 h at 37 ̊C. The average numbers of colonies were counted with the help of the colony counter. The formula used was:
The E-coli was tested by the method described by Cappuccino and Sherman (Citation2005). The samples were diluted using saline solution and then applied on a MacConkey agar plate. Incubation was carried out at 37°C for 24 h. The appearance of red-colored colonies on the MacConkey plate showed the presence of coliforms. Detection of Botrytis cinerea was conducted according to a method described by Cristescu et al. (Citation2002). The infected fungal tissues were cut down with flame sterilized forceps into 2 to 5 mm pieces. Infected tissues were transferred to sterile Petri dishes containing mercuric chloride solution (0.1%) used for surface sterilization of plant tissues for 30 to 60 s. The sterilized pieces (3 to 5) were aseptically transferred to petri dishes containing potato dextrose agar (PDA) supplemented and incubated at ambient temperatures (25–27°C) for pathogen development. The mycelium growth in the medium was marked by a glass marking pencil and observed visually under the microscope (Bio-Rad MRC 1024 confocal laser microscope).
2.7. Statistical analysis
Statistical analysis was conducted by analysis of variance (ANOVA) to denote the significant difference, where HSD Tukey’s test was applied to show the pair-wise comparison among the treatments at each storage time or within treatment with correspondence of storage duration using SPSS 23.0 software (IBM SPSS, Inc., Chicago, IL, USA). Differences at p ≤ 0.05 were considered significant. All data were presented as means ± SD. Figures were generated using Microsoft Excel 2010.
3. Results
3.1. Coating solution analysis
3.1.1. Homogeneity
Data showed the homogeneity of coatings solutions containing 60% and 80% AVG in a stable range over tomato surfaces than that of formulations containing 0%, 20%, and 40% AVG, as shown in .
Table 2. Homogeneity values for edible coating of various AVG concentrations stored at 10°C for up to 30 days.
Tabla 2. Valores de homogeneidad para el recubrimiento comestible de varias concentraciones de AVG almacenado a 10°C durante un máximo de 30 días.
3.2. Chemical analysis of tomatoes
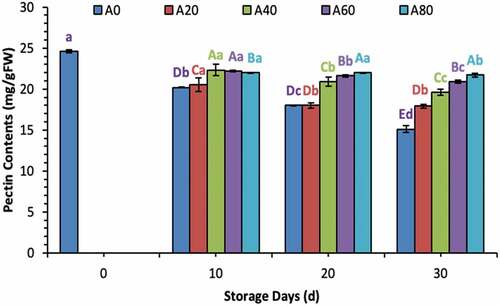
Ascorbic acid contents increased during the first 20 d in all treated tomatoes and then decreased eventually in 20% and 40% AVG-coated tomatoes. Meanwhile, ascorbic acid contents remained stable in tomatoes treated with 60% and 80% AVG coatings at 30 d of storage as well, as shown in . Total sugar and reducing sugar contents increased significantly in tomatoes treated with a coating solution containing lower concentrations (0%, 20%, and 60% in total sugars; and 0% and 20% in reducing sugars) of AVG, as shown in . Pectin contents decreased gradually from 0 d to 30 d in all treated tomatoes. Meanwhile, the declining trend remained lower in all tomatoes treated with higher concentrated AVG coatings. In contrast, tomatoes treated with 0% AVG coating showed a drastic decrease in pectin throughout the storage time, as shown in .
Figure 1. Ascorbic acid contents (mg/g FW) of tomatoes treated with an edible coating of various AVG concentrations stored at 10°C for up to 30 days. Vertical bars represented the standard deviation (SD). Different capital letters (A, B, C etc) indicate significant differences on the same day among the treatments at p ≤ 0.05. Different lower-case letters (a, b, c etc) indicate a significant difference at p ≤ 0.05 within the treatment concerning the storage days (d).
Figura 1. Contenido de ácido ascórbico (mg/g FW) de tomates tratados con un recubrimiento comestible de varias concentraciones de AVG almacenados a 10°C durante un máximo de 30 días. Las barras verticales representan la desviación estándar (SD). Las letras mayúsculas distintas (A, B, C, etc.) indican diferencias significativas (p ≤ 0.05) entre los tratamientos realizados el mismo día. Las letras minúsculas distintas (a, b, c, etc.) indican una diferencia significativa (p ≤ 0.05) en el tratamiento en lo que respecta a los días de almacenamiento (d).
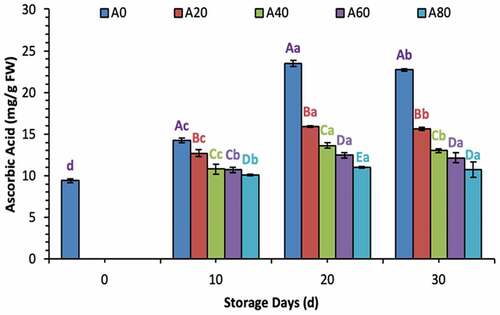
Figure 2. Total sugar contents (mg/g FW) of tomatoes treated with an edible coating of various AVG concentrations stored at 10°C for up to 30 days. Vertical bars represented the standard deviation (SD). Different capital letters (A, B, C etc) indicate significant differences on the same day among the treatments at p ≤ 0.05. Different lower-case letters (a, b, c etc) indicate a significant difference at p ≤ 0.05 within the treatment concerning the storage days (d).
Figura 2. Contenido total de azúcares (mg/g FW) de tomates tratados con un recubrimiento comestible de diversas concentraciones de AVG almacenados a 10°C durante un máximo de 30 días. Las barras verticales representan la desviación estándar (SD). Las letras mayúsculas distintas (A, B, C, etc.) indican diferencias significativas (p ≤ 0.05) entre los tratamientos realizados el mismo día. Las letras minúsculas distintas (a, b, c, etc) indican una diferencia significativa (p ≤ 0.05) en el tratamiento en lo que respecta a los días de almacenamiento (d).
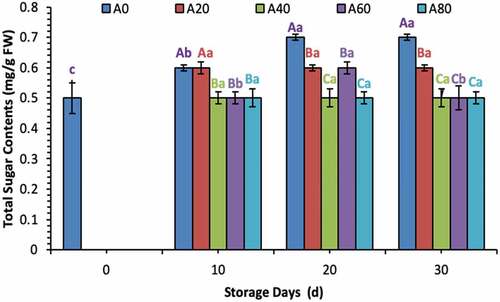
Figure 3. Reducing sugar contents (mg/g FW) of tomatoes treated with an edible coating of various AVG concentrations stored at 10°C for up to 30 days. Vertical bars represented the standard deviation (SD). Different capital letters (A, B, C etc) indicate significant differences on the same day among the treatments at p ≤ 0.05. Different lower-case letters (a, b, c etc) indicate a significant difference at p ≤ 0.05 within the treatment concerning the storage days (d).
Figura 3. Contenido de azúcares reductores (mg/g FW) en tomates tratados con un recubrimiento comestible de diversas concentraciones de AVG almacenados a 10°C durante un máximo de 30 días. Las barras verticales representan la desviación estándar (SD). Las letras mayúsculas distintas (A, B, C, etc.) indican diferencias significativas (p ≤ 0.05) entre los tratamientos realizados el mismo día. Las letras minúsculas distintas (a, b, c, etc) indican una diferencia significativa (p ≤ 0.05) en el tratamiento en lo que respecta a los días de almacenamiento (d).
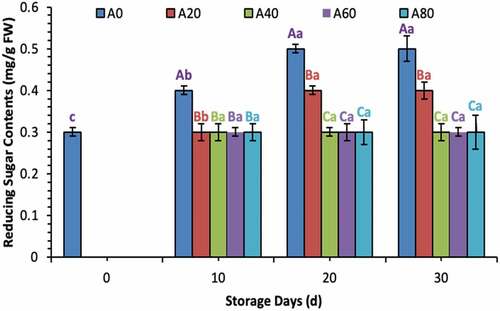
Figure 4. Pectin contents (mg/g FW) of tomatoes treated with an edible coating of various AVG concentrations stored at 10°C for up to 30 days. Vertical bars represented the standard deviation (SD). Different capital letters (A, B, C etc) indicate significant differences on the same day among the treatments at p ≤ 0.05. Different lower-case letters (a, b, c etc) indicate a significant difference at p ≤ 0.05 within the treatment concerning the storage days (d).
Figura 4. Contenido de pectina (mg/g FW) de tomates tratados con un recubrimiento comestible de diversas concentraciones de AVG almacenados a 10°C durante un máximo de 30 días. Las barras verticales representan la desviación estándar (SD). Las letras mayúsculas distintas (A, B, C, etc.) indican diferencias significativas (p ≤ 0.05) entre los tratamientos realizados el mismo día. Las letras minúsculas distintas (a, b, c, etc) indican una diferencia significativa (p ≤ 0.05) en el tratamiento en lo que respecta a los días de almacenamiento (d).
3.3. Total antioxidant efficiency, total phenols, flavonoids, carotenoids, and lycopene contents
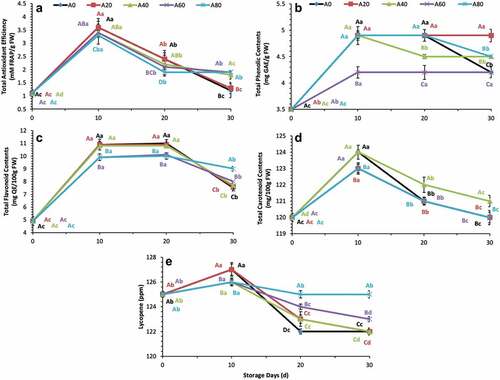
Total antioxidant efficiency increased sharply in all treated tomatoes from 0 d to 10 d, and a drastic decrease was observed. In comparison, maximum total antioxidant efficiency was observed in the tomatoes treated with 0% and 20% AVG coatings during the first 10 d. Meanwhile, higher total antioxidant efficiency was retained in tomatoes treated with 40%, 60% and 80% AVG coatings at 30 d of storage, as shown in . Total phenolic contents increased from 0 d to 10 d in all treated tomatoes and then remained stable until 20 d (in 0% and 80% AVG treatments) and 30 d (in 20% and 60% AVG treatments), followed by a decrease at 20 d in 40% AVG treated tomatoes, and at 30 d in 0% and 80%, AVG treated tomatoes as showed in . Total flavonoid contents increased initially from 0 d to 20 d in all treatments, followed by a decrease at 30 d of storage. Meanwhile, 60% and 80% AVG treatment showed higher total flavonoid contents at 30 d compared with 0%, 20%, and 40% AVG treatments, as shown in . Total carotenoid contents increased sharply from 0 d to 10 d, followed by a continuous decrease until 30 d in all AVG-treated tomatoes, as shown in . Total lycopene contents increased sharply from 0 d to 10 d, followed by a continuous decrease until 30 d in 20%, 40%, and 60% AVG-treated tomatoes. However, 0% and 80% of AVG-treated tomatoes retained the lycopene contents stable more than 20%, 40%, and 60% of AVG-treated tomatoes at 30 d after a drastic decline at 20 d of storage, as shown in .
Figure 5. Total antioxidant efficiency (mM FRAP/g FW), total phenolic contents (mg GAE/g FW), total flavonoid contents (mg QE/100 g FW), total carotenoid contents (mg/100 g FW), and lycopene (ppm) of tomatoes treated with an edible coating of various AVG concentrations stored at 10°C for up to 30 days. Vertical bars represented the standard deviation (SD). Different capital letters (A, B, C etc) indicate significant differences on the same day among the treatments at p ≤ 0.05. Different lower-case letters (a, b, c etc) indicate a significant difference at p ≤ 0.05 within the treatment concerning the storage days (d).
Figura 5. Eficiencia antioxidante total (mM FRAP/g FW), contenido fenólico total (mg GAE/g FW), contenido flavonoide total (mg QE/100 g FW), contenido carotenoide total (mg/100 g FW) y licopeno (ppm) de tomates tratados con un recubrimiento comestible de diversas concentraciones de AVG almacenados a 10°C durante un máximo de 30 días. Las barras verticales representan la desviación estándar (SD). Las letras mayúsculas distintas (A, B, C, etc.) indican diferencias significativas (p ≤ 0.05) entre los tratamientos realizados el mismo día. Las letras minúsculas distintas (a, b, c, etc.) indican una diferencia significativa en el tratamiento (p ≤ 0.05) en lo que respecta a los días de almacenamiento (d).
3.4. Microbiological analysis
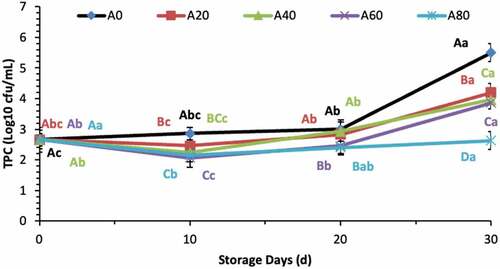
Total viable microbial plate counts increased slowly until 20 d of storage in all treated tomatoes. Afterwards, a sharp increase at 30 d was observed in tomatoes treated with 0%, 20%, 40%, and 60% AVG coating solutions except for 80% AVG-coated tomatoes, as shown in .
Figure 6. Total microbial count (Log10 CFU/mL) of tomatoes treated with an edible coating of various Aloe vera gel contents stored at 10°C for up to 30 days. Vertical bars represented the standard deviation (SD). Different capital letters (A, B, C etc) indicate significant differences on the same day among the treatments at p ≤ 0.05. Different lower-case letters (a, b, c etc) indicate a significant difference at p ≤ 0.05 within the treatment concerning the storage days (d).
Figura 6. Recuento microbiano total (Log10 UFC/mL) de tomates tratados con un recubrimiento comestible de diversos contenidos de gel de Aloe vera almacenados a 10°C durante un máximo de 30 días. Las barras verticales representan la desviación estándar (SD). Las letras mayúsculas distintas (A, B, C, etc.) indican diferencias significativas (p ≤ 0.05) entre los tratamientos realizados el mismo día. Las letras minúsculas distintas (a, b, c etc.) indican una diferencia significativa (p ≤ 0.05) en el tratamiento en lo que respecta a los días de almacenamiento (d).
All the treated tomatoes showed the absence of E. coli throughout the storage time. The colonies of Botrytis cinerea were allowed to grow on Petri plates to measure the radial diameter in all treated tomatoes. No fungal growth was detected until 10 d in tomatoes coated with 0% and 20% AVG solution until 20 d in 40% AVG solution, and until 30 d in 60% and 80% AVG solution, as shown in .
Table 3. Radial diameter of colonies (mm) of Botrytis cinerea in tomatoes treated with edible coating of various Aloe vera gel contents stored at 10°C for up to 30 days.
Tabla 3. Diámetro radial de colonias (mm) de Botrytis cinerea en tomates tratados con recubrimiento comestible de diversos contenidos de gel de Aloe vera almacenados a 10°C durante un máximo de 30 días.
4. Discussion
Stable solutions are characterized by non-significant differences between optical density readings between upper and lower layers of solutions after 48 h of stay time. In this study, coating solutions containing lower AVG showed unstable homogeneity due to the more significant part of added water and less AVG. However, higher concentrations of AVG might be responsible for the uniform distribution of coating solutions over tomato surfaces. In agreement, Mehyar et al. (Citation2012) reported that a higher concentration of coatings material is responsible for an adequate homogeneity of coatings solutions used on walnuts and pine nuts.
The initial increase in ascorbic was presumably due to the ripening process that continues even after harvest (Ali et al., Citation2021). The steady increase of ascorbic acid contents in tomatoes treated with 60% and 80% AVG, e.g. compared to 0–40% AVG coatings, could be due to the uniform distribution of coating solution over fruit surfaces. Moreover, higher concentrated AVG coating solution may result in thick coating layers that could tend to inhibit/reduce respiration rate and biochemical conversions, thus slowing ripening and extending the shelf life of tomatoes. Our findings agree with , who concluded that a higher concentration of AVG resulted in reduced biosynthesis and delayed degradation of ascorbic acid in tomatoes.
The contents of total and reducing sugars are essential parameters for identifying the fruit maturity and ripening stage. The increase in sugar contents in lower AVG concentrated coatings might be associated with increased sugar metabolism-related enzymes such as sucrose synthase/invertase (Khatri et al., Citation2020). In contrast, the non-significant changes in tomatoes treated with higher AVG concentrated coatings sugar contents could be due to reduced respiration rate and biochemical conversions. By our findings, Anjum et al. (Citation2020) also stated that coating treatments may delay the ripening and subsequent senescence along with lower sugar contents due to slowed conversion of starch.
It has been reported that pectin is an essential fruit cell wall component involved in maintaining the fruit texture integrity and strength (Wang et al., Citation2018). However, pectin degradation tends to initiate the fruit softening disorder associated with the activities of pectin degradation-related enzymes such as pectate lyase (PL), pectin methylesterase (PME), and ß-galactosidase (ß-Gal) (Amnuaysin et al., Citation2012). In the present study, coating solutions containing 20%, 40%, 60%, and 80% AVG might inhibit/decrease the PL, PME and ß-Gal enzyme activities in tomatoes, thus resulting in higher pectin contents to avoid the occurrence of fruit softening. In agreement, Benítez et al. (Citation2013) also reported the higher retention of pectin contents under the effect of AVG coating application on fresh-cut kiwifruit.
Higher total antioxidant efficiency is correlated with reduced disease occurrence in various horticultural crops (Khaliq et al., Citation2019; Manzoor et al., Citation2019, Citation2021). Our findings indicate that coating solutions containing a higher concentration of AVG are more effective against disease inhibition/development during the longer storage time for tomatoes. Such findings may contribute to fewer disease attacks and extended shelf life of tomatoes after harvest. A similar finding was reported by Khaliq et al. (Citation2019) concluded that AVG coatings could increase the disease’s resistance by enhancing the antioxidant efficiency in banana fruit. The increase in total antioxidant efficiency in tomatoes is mainly associated with the higher concentration of total phenols, flavonoids, carotenoids, and lycopene contents (Fattore et al., Citation2016; Manzoor et al., Citation2020; Mirdehghan & Valero, Citation2017; Young & Lowe, Citation2018). Presently, findings about higher total antioxidant efficiency in tomatoes are highly correlated with the total flavonoids and lycopene contents (at 30 d in 60% and 80% AVG coatings), as shown in . Meanwhile, total phenols and carotenoid contents do not support this correlation in the current study except at 30 d in 40% AVG coating treatment about carotenoid contents (see ).
The higher concentration of AVG in the coating solution has been shown to reduce the total viable microbial count greatly. Previously, Martínez-Romero et al. (Citation2006) reported AVG extract’s antimicrobial properties against various kinds of yeast, mould, and bacterial growth. The present study also represented that AVG coatings could reduce the total microbial growth, thus improving the tomato’s quality. The microbial inhibition might be associated with the antibiotic effects of functional compounds present in AVG, such as anthraquinones, saponins, and acemannan (Santoso & Rahmat, Citation2012).
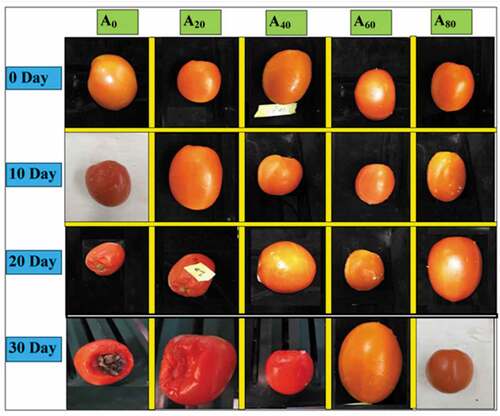
Coating solutions containing a lower concentration of AVG (0%, 20%, and 40%) significantly showed fungal growth during the later storage period in tomatoes. Meanwhile, a complete absence of fungal growth was detected in tomatoes only treated with 60% and 80% AVG solution, thus keeping the fruit safe from decay. The effect of a higher concentration of AVG coatings might be associated with its more prominent antimicrobial and antibiotic action against various plant pathogenic fungi (Chauhan et al., Citation2011; Saks & Barkai-Golan, Citation1995). Overall, a visual representation of all treated tomatoes during 30 d of storage is shown in .
Figure 7. Overall visual representation of tomatoes treated with an edible coating of various AVG concentrations stored at 10°C for up to 30 days.
Figura 7. Representación visual general de tomates tratados con un recubrimiento comestible de varias concentraciones de AVG almacenados a 10°C durante un máximo de 30 días.
5. Conclusion
The AVG-based coatings containing 60–80% gel were observed to be more effective in keeping the postharvest quality of tomatoes. The effectiveness of 60–80% gel coatings might correlate with the higher retention of ascorbic acid, total sugars, pectin, and antioxidant efficiency (associated with total phenols, flavonoids, carotenoids, and lycopene contents) during the ripening of treated tomatoes. Additionally, 60–80% AVG coatings reduced total microbial count, absence of coliforms, and no sign of fungal growth in treated tomatoes, thus decreasing the susceptibility of fruit decay and enhancing the microbial safety over the 30 d of storage period. In short, 60–80% AVG edible coatings might be recommended to maintain the postharvest quality of tomato fruit.
Data availability
The corresponding author’s data supporting this study’s findings are available upon reasonable request.
Supplemental Material
Download MS Word (259.3 KB)Acknowledgement
Higher Education Commission, Pakistan, funded the current work under the International Research Support Initiative Program (IRSIP) slot. The authors acknowledged the guidance and research facilities provided by Prof. Chen, Department of Horticultural Science, College of Food, Agriculture and Natural Resources, University of Minnesota, United States. The authors also want to acknowledge the support of Guangdong Provincial Department of Agriculture and Rural Affairs Agricultural Research and Technology Promotion Demonstration (Project ID:2022KJ144).
Disclosure statement
No potential conflict of interest was reported by the author(s).
supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19476337.2022.2136760
References
- Alemdar, S., & Agaoglu, S. (2009). Investigation of in vitro antimicrobial activity of aloe vera juice. Journal of Animal and Veterinary Advances, 8(1), 99–102.
- Ali, A., Maqbool, M., Ramachandran, S., & Alderson, P. G. (2010). Gum arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest Biology and Technology, 58(1), 42–47. https://doi.org/10.1016/j.postharvbio.2010.05.005
- Ali, M., Raza, M. A., Li, S., Zhou, L., Huan, C., Shuling, S., & Zheng, X. (2021). 1-MCP regulates ethanol fermentation and GABA shunt pathway involved in kiwifruit quality during postharvest storage. Horticultural Plant Journal, 7(1), 23–30. https://doi.org/10.1016/j.hpj.2020.12.006
- Alizadeh-Sani, M., Ehsani, A., Kia, E. M., & Khezerlou, A. (2019). Microbial gums: Introducing a novel functional component of edible coatings and packaging. Applied Microbiology and Biotechnology, 103(17), 6853–6866. https://doi.org/10.1007/s00253-019-09966-x
- Amnuaysin, N., Jones, M. L., & Seraypheap, K. (2012). Changes in activities and gene expression of enzymes associated with cell wall modification in peels of hot water treated bananas. Scientia Horticulturae, 142, 98–104. https://doi.org/10.1016/j.scienta.2012.05.006
- Anjum, M. A., Akram, H., Zaidi, M., & Ali, S. (2020). Effect of gum arabic and Aloe vera gel based edible coatings in combination with plant extracts on postharvest quality and storability of ‘Gola’guava fruits. Scientia Horticulturae, 271, 109506. https://doi.org/10.1016/j.scienta.2020.109506
- Athmaselvi, K., Sumitha, P., & Revathy, B. (2013). Development of Aloe vera based edible coating for tomato. International Agrophysics, 27(4), 369–375. https://doi.org/10.2478/intag-2013-0006
- Beikzadeh, S., Khezerlou, A., Jafari, S. M., Pilevar, Z., & Mortazavian, A. M. (2020). Seed mucilages as the functional ingredients for biodegradable films and edible coatings in the food industry. Advances in Colloid and Interface Science, 280, 102164. https://doi.org/10.1016/j.cis.2020.102164
- Benítez, S., Achaerandio, I., Sepulcre, F., & Pujolà, M. (2013). Aloe vera based edible coatings improve the quality of minimally processed ‘Hayward’kiwifruit. Postharvest Biology and Technology, 81, 29–36. https://doi.org/10.1016/j.postharvbio.2013.02.009
- Cappuccino, J. G., & Sherman, N. (2005). Microbiology: A laboratory manual.
- Chauhan, O., Raju, P., Singh, A., & Bawa, A. (2011). Shellac and aloe-gel-based surface coatings for maintaining keeping quality of apple slices. Food Chemistry, 126(3), 961–966. https://doi.org/10.1016/j.foodchem.2010.11.095
- Choudhari, S. M., & Ananthanarayan, L. (2007). Enzyme aided extraction of lycopene from tomato tissues. Food Chemistry, 102(1), 77–81. https://doi.org/10.1016/j.foodchem.2006.04.031
- Chrysargyris, A., Nikou, A., & Tzortzakis, N. (2016). Effectiveness of Aloe vera gel coating for maintaining tomato fruit quality. New Zealand Journal of Crop and Horticultural Science, 44(3), 203–217. https://doi.org/10.1080/01140671.2016.1181661
- Cristescu, S. M., De Martinis, D., Te Lintel Hekkert, S., Parker, D. H., & Harren, F. J. (2002). Ethylene production by Botrytis cinerea in vitro and in tomatoes. Applied and Environmental Microbiology, 68(11), 5342–5350. https://doi.org/10.1128/AEM.68.11.5342-5350.2002
- Dhall, R. (2013). Advances in edible coatings for fresh fruits and vegetables: A review. Critical Reviews in Food Science and Nutrition, 53(5), 435–450. https://doi.org/10.1080/10408398.2010.541568
- Fattore, M., Montesano, D., Pagano, E., Teta, R., Borrelli, F., Mangoni, A., Seccia, S., & Albrizio, S. (2016). Carotenoid and flavonoid profile and antioxidant activity in “Pomodorino Vesuviano” tomatoes. Journal of Food CompositionAnalysis, 53, 61–68. https://doi.org/10.1016/j.jfca.2016.08.008
- Garcia, F., & Davidov‐pardo, G. (2021). Recent advances in the use of edible coatings for preservation of avocados: A review. Journal of Food Science, 86(1), 6–15. https://doi.org/10.1111/1750-3841.15540
- Grassino, A. N., Brnčić, M., Vikić-Topić, D., Roca, S., Dent, M., & Brnčić, S. R. (2016). Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chemistry, 198, 93–100. https://doi.org/10.1016/j.foodchem.2015.11.095
- Guo, C., Yang, J., Wei, J., Li, Y., Xu, J., & Jiang, Y. (2003). Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutrition Research, 23(12), 1719–1726. https://doi.org/10.1016/j.nutres.2003.08.005
- Harsh, A., Sharma, Y., Joshi, U., Rampuria, S., Singh, G., Kumar, S., & Sharma, R. (2016). Effect of short-term heat stress on total sugars, proline and some antioxidant enzymes in moth bean (Vigna aconitifolia). Annals of Agricultural Sciences, 61(1), 57–64. https://doi.org/10.1016/j.aoas.2016.02.001
- Hasan, M. U., Riaz, R., Malik, A. U., Khan, A. S., Anwar, R., Rehman, R. N. U., & Ali, S. (2021). Potential of Aloe vera gel coating for storage life extension and quality conservation of fruits and vegetables: An overview. Journal of Food Biochemistry, e13640.
- Iftikhar, A., Rehman, A., Usman, M., Ali, A., Ahmad, M. M., Shehzad, Q., Fatim, H., Mehmood, A., Moiz, A., & Shabbir, M. A. (2022). Influence of guar gum and chitosan enriched with lemon peel essential oil coatings on the quality of pears. Food Science Nutrition, 10(7), 2443–2454. https://doi.org/10.1002/fsn3.2851
- Kahramanoğlu, İ., Chen, C., Gan, Z., Chen, J., & Wan, C. (2020). The effects of edible coatings on the postharvest quality of citrus fruits as affected by granulation. Journal of Food Quality, 2020, 1–8. https://doi.org/10.1155/2020/8819233
- Khaliq, G., Abbas, H. T., Ali, I., & Waseem, M. (2019). Aloe vera gel enriched with garlic essential oil effectively controls anthracnose disease and maintains postharvest quality of banana fruit during storage. Horticulture, Environment, and Biotechnology, 60(5), 659–669. https://doi.org/10.1007/s13580-019-00159-z
- Khatri, D., Panigrahi, J., Prajapati, A., & Bariya, H. (2020). Attributes of Aloe vera gel and chitosan treatments on the quality and biochemical traits of postharvest tomatoes. Scientia Horticulturae, 259, 108837. https://doi.org/10.1016/j.scienta.2019.108837
- Manzoor, M. F., Ahmad, N., Ahmed, Z., Siddique, R., Mehmood, A., Usman, M., & Zeng, X. A. (2020). Effect of dielectric barrier discharge plasma, ultra‐sonication, and thermal processing on the rheological and functional properties of sugarcane juice. Journal of Food Science, 85(11), 3823–3832. https://doi.org/10.1111/1750-3841.15498
- Manzoor, M. F., Ahmad, N., Ahmed, Z., Siddique, R., Zeng, X. A., Rahaman, A., Muhammad Aadil, R., & Wahab, A. (2019). Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. Journal of Food Biochemistry, e12974.
- Manzoor, M. F., Hussain, A., Sameen, A., Sahar, A., Khan, S., Siddique, R., Aadil, R. M., & Xu, B. (2021). Novel extraction, rapid assessment and bioavailability improvement of quercetin: A review. Ultrasonics Sonochemistry, 78, 105686. https://doi.org/10.1016/j.ultsonch.2021.105686
- Manzoor, M. F., Hussain, A., Tazeddinova, D., Abylgazinova, A., Xu, B., & Koundal, D. (2022). Assessing the nutritional-value-based therapeutic potentials and non-destructive approaches for mulberry fruit assessment: An overview. Computational Intelligence and Neuroscience, 2022, 1–16. https://doi.org/10.1155/2022/6531483
- Manzoor, M. F., Xu, B., Khan, S., Shukat, R., Ahmad, N., Imran, M., Rehman, A., Karrar, E., Aadil, R. M., & Korma, S. A. (2021). Impact of high-intensity thermosonication treatment on spinach juice: Bioactive compounds, rheological, microbial, and enzymatic activities. Ultrasonics Sonochemistry, 78, 105740. https://doi.org/10.1016/j.ultsonch.2021.105740
- Martínez-Romero, D., Alburquerque, N., Valverde, J., Guillén, F., Castillo, S., Valero, D., & Serrano, M. (2006). Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest Biology and Technology, 39(1), 93–100. https://doi.org/10.1016/j.postharvbio.2005.09.006
- Mehyar, G. F., Al‐ismail, K., Han, J. H., & Chee, G. W. (2012). Characterization of edible coatings consisting of pea starch, whey protein isolate, and carnauba wax and their effects on oil rancidity and sensory properties of walnuts and pine nuts. Journal of Food Science, 77(2), E52–59. https://doi.org/10.1111/j.1750-3841.2011.02559.x
- Mirdehghan, S. H., & Valero, D. (2017). Bioactive compounds in tomato fruit and its antioxidant activity as affected by incorporation of Aloe, eugenol, and thymol in fruit package during storage. International Journal of Food Properties, 20(sup2), 1798–1806.
- Palou, L., Valencia-Chamorro, S. A., & Pérez-Gago, M. B. (2015). Antifungal edible coatings for fresh citrus fruit: A review. Coatings, 5(4), 962–986. https://doi.org/10.3390/coatings5040962
- Panahirad, S., Dadpour, M., Peighambardoust, S. H., Soltanzadeh, M., Gullón, B., Alirezalu, K., & Lorenzo, J. M. (2021). Applications of carboxymethyl cellulose-and pectin-based active edible coatings in preservation of fruits and vegetables: A review. Trends in Food Science & Technology, 110, 663–673. https://doi.org/10.1016/j.tifs.2021.02.025
- Pila, N., & Gol, N. B. (2010). Effect of postharvest treatments on physicochemical characteristics and shelf life of tomato (Lycopersicon esculentum Mill.) fruits during storage.
- Raghav, P. K., Agarwal, N., & Saini, M. (2012). Edible coating of fruits and vegetables: A review. Education, 2014.
- Ramachandra, C., & Rao, P. S. (2008). Processing of Aloe vera leaf gel: A review. American Journal of Agricultural and Biological Sciences, 3(2), 502–510. https://doi.org/10.3844/ajabssp.2008.502.510
- Rehman, A., Qunyi, T., Usman, M., Manzoor, M. F., Zhao, L., Feng, J., & Jafari, S. M. (2022). Nano-enabled agrochemicals for sustainable agriculture. In Nano-enabled agrochemicals in agriculture (pp. 291–306). Elsevier.
- Rehman, A., Tong, Q., Jafari, S. M., Korma, S. A., Khan, I. M., Mohsin, A., Manzoor, M. F., Ashraf, W., Mushtaq, B. S., & Zainab, S. (2021). Spray dried nanoemulsions loaded with curcumin, resveratrol, and borage seed oil: The role of two different modified starches as encapsulating materials. International Journal of Biological Macromolecules, 186, 820–828. https://doi.org/10.1016/j.ijbiomac.2021.07.076
- Ribeiro, C., Vicente, A. A., Teixeira, J. A., & Miranda, C. (2007). Optimization of edible coating composition to retard strawberry fruit senescence. Postharvest Biology and Technology, 44(1), 63–70. https://doi.org/10.1016/j.postharvbio.2006.11.015
- Sahabi, D., Shehu, R., Saidu, Y., & Abdullahi, A. (2012). Screening for total carotenoids and β-carotene in some widely consumed vegetables in Nigeria. Nigerian Journal of Basic and Applied Sciences, 20(3), 225–227.
- Saks, Y., & Barkai-Golan, R. (1995). Aloe vera gel activity against plant pathogenic fungi. Postharvest Biology and Technology, 6(1–2), 159–165. https://doi.org/10.1016/0925-5214(94)00051-S
- Santoso, F., & Rahmat, V. (2012). Safety and quality assurance of tomato using aloe vera edible coating. II Asia Pacific Symposium on Postharvest Research Education and Extension: APS2012 1011.
- Villafañe, F. (2017). Edible coatings for carrots. Food Reviews International, 33(1), 84–103. https://doi.org/10.1080/87559129.2016.1150291
- Wahab, A., Rahim, A. A., Hassan, S., Egbuna, C., Manzoor, M. F., Okere, K. J., & Walag, A. M. P. (2021). Application of nanotechnology in the packaging of edible materials. In Preparation of phytopharmaceuticals for the management of disorders (pp. 215–225).
- Wang, D., Yeats, T. H., Uluisik, S., Rose, J. K., & Seymour, G. B. (2018). Fruit softening: Revisiting the role of pectin. Trends in Plant Science, 23(4), 302–310. https://doi.org/10.1016/j.tplants.2018.01.006
- Young, A. J., & Lowe, G. L. (2018). Carotenoids—antioxidant properties. Antioxidants, 7(2), 28. https://doi.org/10.3390/antiox7020028
