?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Unripe apples contain more functional compounds than ripe apples and thus demonstrate significant potential for use as functional food materials. In this study, the conditions for the extraction of functional compounds from unripe apples were optimized using the response surface methodology. A Box–Behnken design was used to investigate the effects of three independent variables (β-cyclodextrin concentration, extraction time, and extraction temperature) on the total polyphenol content (TPC), chlorogenic acid content (CAC), and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity (RSA). The corresponding optimal extraction conditions were found to be 40 mg/mL, 53.03 min, and 60°C for TPC; 40 mg/mL, 46.67 min, and 60°C for CAC; and 20 mg/mL, 30 min, and 40°C for DPPH RSA. The experimental values obtained under these conditions were consistent with the predicted values, indicating that the model was suitable for optimizing the extraction of functional compounds from unripe apples.
1. Introduction
Apples are the fruits of the apple tree (Malus domestica), a deciduous plant belonging to the Rosaceae family, and are one of the most consumed fruits worldwide (Patocka et al., Citation2020). Apples are rich in vitamins, dietary fibers, and polyphenols and are known to have beneficial effects on human health (Wojdyło et al., Citation2021). In particular, chlorogenic acid, phlorizin, phloretin, catechin, epicatechin, and quercetin, which are the major phenolic compounds in apples, exhibit physiological functions such as antioxidant, antidiabetic, antiobesity, and hepatoprotective activities and alleviate the symptoms of inflammatory bowel disease and cardiovascular disorders (Skinner et al., Citation2018). Unripe apples contain more functional compounds, including polyphenols, than ripe apples and thus demonstrate significant potential for use as functional materials (Zhao et al., Citation2019). Unripe apples are typically generated during the thinning process, which is essential for improving the nutritional content, size, and shape of apples during apple cultivation (Mengyuan et al., Citation2021). Thinned unripe apples generated during this process account for 20–30% of the total apple production annually, and only a fraction of these apples is used as livestock feed or compost, while most of them are discarded (Zheng et al., Citation2012). Therefore, further research is needed to develop strategies for utilizing the unripe apples, which are rich in functional ingredients.
Extraction is required to utilize the functional ingredients in plants, and various techniques have been used for extraction (Gligor et al., Citation2019). Conventional methods, such as solvent extraction, maceration, and Soxhlet extraction, require excessive extraction time, energy, and solvents. Therefore, these extraction methods are uneconomical, and the excessive use of organic solvents causes problems such as environmental pollution. This has resulted in the emergence of economic green extraction techniques such as ultrasound-assisted extraction (UAE), enzyme-assisted extraction, and microwave-assisted extraction using green solvents such as deep eutectic solvents (DESs) and cyclodextrin (CD) (Ameer et al., Citation2017; Xie et al., Citation2019). In particular, UAE is an attractive environmentally friendly method for obtaining bioactive compounds from plants owing to the use of less solvent, low temperature, and reduced extraction time (Maraulo et al., Citation2021). When high-intensity sound waves (ultrasound) are applied to plants, the physical structure of plants is destroyed by cavitation. Cell membrane disruption by cavitation enhances the solubility of the functional compound bound to the cell wall in the solvent, improving the extraction efficiency (Silva et al., Citation2021). Recently, studies on the extracting of functional components from various plants using CD as a solvent to improve the efficiency of UAE have been reported (Alibante et al., Citation2021; Maraulo et al., Citation2020).
CDs are torus-shaped oligosaccharides prepared from starch via a treatment with α-amylase and CD glucosyltransferase. CD is an amphiphilic substance that is hydrophilic on the outside and lipophilic on the inside. Thus, CD can improve the stability and extraction efficiency of the target ingredients by forming inclusion complexes with functional compounds in plants (Matencio et al., Citation2020). In addition, it has advantages such as reduced extraction time and improvement of bioavailability and antioxidant activity of the extract, and it can be used in combination with various extraction methods (Cai et al., Citation2018). β-cyclodextrin (CD) is the most commonly used CD because it is the least expensive (Pinho et al., Citation2014). β-CD has been reported to be useful for extracting polyphenols from biomass, because it interacts with polyphenols by penetrating cell walls and cell membranes to reach target sites (Parmar et al., Citation2015).
Extraction efficiency is affected by several factors such as the solvent type and concentration, extraction time, and temperature employed during the extraction process (Belwal et al., Citation2016). Therefore, it is important to use an appropriate experimental design technique to investigate the interaction between various factors (Hanrahan & Lu, Citation2006; Ćujić et al., Citation2016). Statistical approaches, such as response surface methodology (RSM), can be employed to maximize the production of a specific substance by optimizing operational factors. The Box–Behnken design (BBD) is an appropriate RSM that can be employed to investigate the effects of independent factors and reciprocal actions between the factors on the responses (dependent factors). BBD is considered more efficient and powerful than other designs, such as the three-level full factorial design and central composite design (CCD), despite its poor coverage of the corner in the nonlinear design space (Karmoker et al., Citation2019). In addition, when the number of factors is the same, BBD demonstrates an advantage in that the optimized value can be realized in less experimental runs than those required when using CCD (Yolmeh & Jafari, Citation2017).
Therefore, this study was conducted to obtain a polyphenol-rich extract with high antioxidant activity using β-CD and UAE and to find a use for thinned unripe apples as functional food ingredients. First, a three-factor, three-level BBD was used to select optimal extraction conditions that maximized the total polyphenol content (TPC), chlorogenic acid content (CAC) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity (RSA). Finally, the extraction efficiency of the optimized CD-based UAE was compared with that of hot water extraction, a conventional extraction method.
2. Materials and methods
2.1. Materials
The unripe apples used in this study were of the Miyabi Fuji cultivar type (). They had diameters of 3–4.5 cm and were grown in Cheongsong (Republic of Korea) and were thinned for approximately 65 d after flowering. The unripe apples were washed, cut into quarters using a knife, and freeze-dried. Thereafter, they were ground using a grinder (FM-681C, Hanil, Incheon, Republic of Korea), sieved to a mesh size of 45 mesh, and stored at −30°C. Gallic acid, chlorogenic acid, and DPPH were purchased from Sigma-Aldrich Chemical Co., St. Louis, MO, USA. A Folin–Ciocalteu phenol reagent was purchased from Junsei, Tokyo, Japan, and β-CD was purchased from Daejung, Siheung, Gyeonggi-do, Republic of Korea. All other chemicals used were of analytical grade.
2.2. Experimental design
A three-factor, three-level BBD for RSM was performed to assess the optimal extraction conditions for obtaining the highest recovery of bioactive compounds from unripe apples. The β-CD concentration (X1, 20–40 mg/mL), extraction time (X2, 30–60 min), and extraction temperature (X3, 40–60°C) were selected as independent variables. These three factors and the ranges used were selected based on previous studies (Parmar et al., Citation2015; Rajha et al., Citation2015) and preliminary experiments. The complete BBD design matrix including a total of 15 experiments and experimental results for the TPC, CAC, and DPPH RSA are presented in .
Table 1. Box–Behnken experimental values for the TPC, CAC, and DPPH RSA of unripe apples under different extraction conditions.
2.3. Data analysis
A response surface analysis of the experimental data was performed using the Minitab® 18 software (Minitab Inc., State College, PA, USA). The effect of the interaction between independent variables on the coefficients of the model equation was determined by applying the results of the response variable obtained through the experimental design to the quadratic polynomial model. The model is represented by the following quadratic regression polynomial function (1):
where Y is the response variable, β0 is the constant coefficient, Xi and Xj are independent variables, βi is the linear coefficient, βii is the quadratic coefficient, βij is the interaction coefficient, and k is the number of factors used in the experiment. The regression coefficients of the individual linear, interaction, and quadratic terms were determined using analysis of variance (ANOVA). The fit of the polynomial equation to the responses was determined using the coefficient of determination (R2) of the regression analysis. The influence of the interaction between the independent variables on the response variable was estimated using three-dimensional response surface plots produced using the Design Expert 13 Program (Stat-Ease, Minneapolis, MN, USA).
2.4. Extraction procedure
The extraction of functional ingredients from unripe apples was performed in an ultrasonic bath (24.13 × 13.97 × 10.16 cm, 2210R-DTH, Branson, Danbury, CT, USA) according to the extraction conditions listed in . A powdered sample (5 g) was added to Erlenmeyer flasks and mixed with various concentrations of 50 mL β-CD. UAE was performed after fixing the container to a suitable height using a stand and clamp so that the flask containing the sample did not touch the bottom of the ultrasonic bath during extraction. The intensity of the ultrasonic wave was 90 W, and the frequency was 47 kHz. Extract was centrifuged at 10,000 × g, 20°C for 20 min, and the supernatant was used for the determination of TPC, CAC, and DPPH RSA.
To confirm the efficiency of the optimized β-CD-based UAE approach for the extraction of polyphenol, hot water extraction (HWE), a representative conventional extraction method, was performed for comparative purposes. HWE was performed as follows: a sample (5 g) and 50 mL of distilled water were mixed and extracted using a shaking water bath (BS-11, JeioTech Daejeon, Korea) at 90°C for 1 h.
2.5. Determination of the total polyphenol content
The TPC was determined according to the method described by Folin and Denis (Citation1912), with slight modifications. Briefly, 0.2 mL of Folin–Ciocalteu’s phenol reagent was added to 0.2 mL of the sample and reacted at room temperature for 3 min. Subsequently, 0.4 mL of 10% Na2CO3 and 4 mL of distilled water were added and mixed, the mixture was left at room temperature for 1 h, and the absorbance was measured at 725 nm. The content was calculated from a standard curve obtained using gallic acid, and the results were expressed as mg gallic acid equivalent (GAE)/g dry weight (DW).
2.6. Determination of the chlorogenic acid content
The CAC was measured using high-performance liquid chromatography (HPLC, Waters 2695, Waters Co., Milford, MA, USA) equipped with an ultraviolet detector (UV, Waters 2489, Waters Co., Milford, MA, USA) and an Atlantis® dC18 column (4.6 × 150 mm, 5 μm Waters Co., Milford, MA, USA), according to the method proposed by Lee and Yoon (Citation2021). The samples were filtered through a 0.45 μm syringe filter (SV25P045NL, Hyundai Micro, Seoul, Republic of Korea), injected into the HPLC, and measured at a UV wavelength of 280 nm. The column temperature was maintained at 30°C, and the mobile phase consisted of solvent A (100% acetonitrile) and solvent B (1% phosphoric acid in water, v/v) at a flow rate of 1 mL/min. Chlorogenic acid was identified by comparing its retention time with that of the standard material, and the CAC was calculated from the calibration curve of the standard material.
2.7. Evaluation of antioxidant activity by the 1,1-diphenyl-2-picrylhydrazyl radical scavenging assay
The antioxidant activity of the samples was measured using a method proposed by Kim and Yoon (Citation2020), with slight modifications. The sample (200 μL) and DPPH solution (100 µL, 0.2 mM) were added to a 96-well plate and incubated in a dark room at 37°C for 30 min before the absorbance was measured at 517 nm. The DPPH RSA was calculated using EquationEquation (2)(2)
(2) :
2.8. Statistical analysis for validation
The validation experiments performed to confirm the extraction efficiency of the CD-based UAE method were carried out in triplicate, and the experimental results are expressed as the mean ± standard deviation. The validity of the predicted and experimental values was confirmed using the relative standard deviation (%RSD). Significant differences between the CD-based UAE and HWE extracts at a significance level p < .05 were analyzed by t-test using SPSS (ver. 21, Chicago, IL, USA).
3. Results and discussion
3.1. Fitting of the models
The TPC, CAC, and DPPH RSA values calculated using the three-factor, three-level BBD are presented in . presents the ANOVA results and R2 values for the quadratic polynomial regression of the model. The lack of a fit is an indicator that the null hypothesis is correct; if the p-value is greater than .05, the model is judged to be suitable (Saifullah et al., Citation2020). The lack-of-fit p-values for the TPC, CAC, and DPPH RSA were 0.317, 0.903, and 0.413, respectively. These values are greater than .05, indicating that the model is suitable. The linear effect of the β-CD concentration (X1) was the only parameter that significantly affected all the responses. The linear extraction temperature (X3) had a significant effect on the TPC and DPPH RSA. The quadratic effect of extraction time (X2) and extraction temperature (X3) significantly affected TPC and DPPH RSA, respectively. The interaction effects of the independent variables were significant for some responses. A quadratic polynomial was used to describe the relationship between the independent and response variables. The model polynomial is significant at p < .05, and the R2 values for all equations are 0.9 or higher, indicating that the models can be used to predict the responses.
Table 2. Regression coefficient, R2, and F-test values of the predicted second-order polynomial models for the TPC, CAC, and DPPH RSA of unripe apples.
3.2. Effect of the process variables on the total polyphenol content
The TPC of unripe apple extracts obtained via CD-based UAE through the three-factor, three-level BBD ranges from 3.30–4.66 mg GAE/g DW. The highest TPC (4.66 mg GAE/g DW) was observed in experimental run 15, with a β-CD concentration of 40 mg/mL, extraction time of 45 min, and extraction temperature of 60°C. The lowest TPC (3.30 mg GAE/g DW) was observed in experimental runs 3 and 9 (). The R2 value of the quadratic polynomial equation for the relationship between the TPC and extraction factors is 0.9660, which is considered acceptable (). The results of the multiple regression analysis performed on the experimental TPC data reveal that the linear terms (X1 and X3) and interactive terms (X12 and X13) were significant (p < .05), whereas quadratic (X22) term is not significant (p < .05, ). This is consistent with the results reported by Favre et al. (Citation2020), who reported that when polyphenols were extracted from green peppers using β-CD and ultrasound extraction, the extraction temperature and β-CD concentration exhibited a synergistic effect on the TPC, and the extraction time and extraction temperature exhibited an antagonistic effect on the TPC. The independent variables contributed to the interaction with the TPC, and the quadratic polynomial for the TPC is as follows:
Based on polynomial (3), the influence of the independent variable on the TPC is presented as a three-dimensional response surface plot (). shows the combined effect of the extraction time and β-CD concentration on the TPC. There was a linear increase in the TPC with increasing β-CD concentration and constant extraction time. The effects of the extraction temperature and β-CD concentration on the TPC are shown in . The TPC decreased slowly with increasing extraction temperature at a low β-CD concentration (20 mg/mL), and it increased linearly with increasing extraction temperature at a high β-CD concentration. The effect of the β-CD concentration on the TPC at low temperatures was small, but the TPC increased linearly with increasing β-CD concentration at high temperatures. This result indicates that the β-CD concentration had varying effects on the TPC depending on the temperature. The results reveal that β-CD is effective for extracting phenolic compounds, as the TPC increased with increasing β-CD concentration. This occurred because large quantities of β-CD contained in the extraction solvent lead to the formation of a large quantity of clathrates via the interactions between β-CD and the polyphenols in unripe apples. In addition, polyphenol extraction is accelerated by the transformation and destruction of plant cell walls as the extraction temperature increases (Cai et al., Citation2018; D’Alessandro et al., Citation2012). The combined effect of extraction time and temperature on the TPC of bitter melon extract is illustrated in . The TPC tended to increase as the extraction temperature increased at a constant extraction time. The TPC increased from 30 to 50 min at a constant extraction temperature and decreased thereafter. Extraction at high temperatures destroys the cell wall of plants, increasing the solubility and diffusion of functional components, and reducing the extraction time required to reach the maximum TPC. However, it is important to optimize the extraction time according to the temperature as extending the extraction time at high temperatures may induce decomposition of the bioactive compounds (Aguilera et al., Citation2019).
Figure 2. Response surface plots depicting the effects of the β-cyclodextrin (CD) concentration, extraction time, and extraction temperature on the total polyphenol content (TPC). GAE, gallic acid equivalent; DW, dry weight.
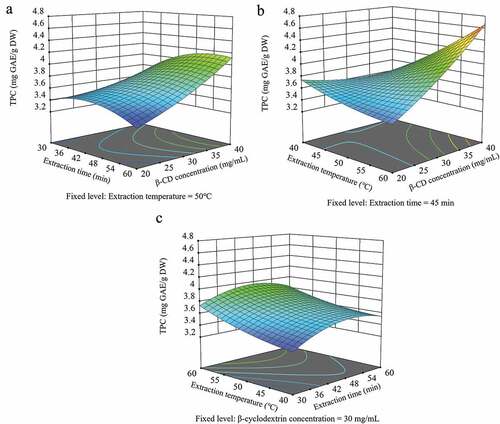
The ANOVA results () reveal that the β-CD concentration and extraction temperature had the most significant influence on the TPC. Liyana-Pathirana and Shahidi (Citation2005) have reported that solvent concentration and extraction temperature play important roles in the extraction of phenolic compounds from wheat, which is consistent with the results of this study.
3.3. Effect of the process variables on the chlorogenic acid content
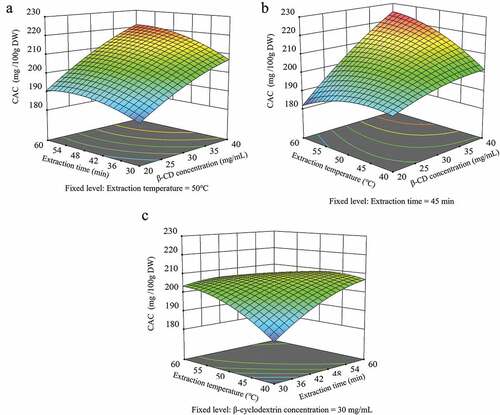
The CAC of unripe apple extracts obtained via the CD-based UAE method designed using a three-factor, three-level BBD ranges from 180.83 to 223.23 mg/100 g DW. The unripe apple extract with the highest CAC was obtained from run 15 (40 mg/mL, 45 min, and 60°C), and the extract with the lowest CAC (180.83 mg/100 g DW) was obtained from run 1 (20 mg/mL, 40 min, and 60°C, ). The ANOVA results indicate that only the linear X1 term had a significant negative effect on the CAC (p < .05). The quadratic terms did not significantly affect the CAC (p > .05), and only the interactive term, X13, had a significant positive effect (p < .05; ). The independent variables contribute to the interaction with the CAC, and the following equation presents the quadratic polynomial for the CAC.
The three-dimensional response surface plot representing the effect of the independent variables on the CAC based on EquationEquation (4)(4)
(4) is illustrated in . The CAC, which was affected by the β-CD concentration, demonstrates a marked increase in CAC with increasing β-CD concentration at a fixed extraction time () and extraction temperature (). In addition, the CAC increased more significantly with increasing extraction time and extraction temperature at high β-CD concentrations than at low β-CD concentrations. The CAC steadily increased as the extraction time increased at low temperatures. However, it decreased after 50 min at high temperatures () because the increased extraction time improved the diffusion of the compound by increasing the contact time between the solvent and solid. However, prolonged extraction at high temperatures destroys polyphenol compounds, which leads to a decrease in the polyphenol content (Ghafoor et al., Citation2009). The CAC is significantly affected by the β-CD concentration () because the higher the β-CD content in the extraction solvent, the more it interacts with the chlorogenic acid in unripe apples to form complexes, which improves the stability (Zhao et al., Citation2010).
3.4. Effect of the process variables on the antioxidant activity
The DPPH RSA of unripe apple extracts obtained via the CD-based UAE through the three-factor, three-level BBD ranges from 75.00 to 87.28%. The unripe apple extract exhibits the highest DPPH RSA (87.28%) in run 12, (40 mg/mL, 60 min, and 50°C) and the lowest activity (75.00%) in run 13 (20 mg/mL, 40 min, and 60°C, ). According to the ANOVA results obtained for the second-order polynomial (), the linear terms, X1 and X3, had significant negative and positive effects on the DPPH RSA, respectively (p < .05). The quadratic X3 term had a significant negative effect (p < .05), and the interactive X12 term had a significant positive effect (p < .05). The independent variables contribute to the interaction with DPPH, and the second-order polynomial equation for the DPPH RSA is described as follows:
depicts a three-dimensional response surface plot to investigate the effect of independent variables on the DPPH RSA according to the polynomial (5). The data shown in reveal that the DPPH RSA decreased with increasing extraction time at a low β-CD concentration (20 mg/mL). In contrast, at high β-CD concentrations, the DPPH RSA increased with increasing extraction time, up to 60 min. , which illustrates the combined effect of the β-CD concentration and extraction temperature, reveals that the DPPH RSA decreased as the extraction temperature increases from 40°C to 60°C, regardless of the β-CD concentration. The maximum DPPH RSA values were obtained at extraction temperatures of 40°C to 50°C and a β-CD concentration of 25 mg/mL. A three-dimensional plot illustrating the effects of extraction time and extraction temperature is shown in . The DPPH RSA increased slightly up to 50°C at a constant extraction time and at temperatures above 50°C, and it decreased rapidly as the temperature increases. The DPPH RSA were the highest at extraction temperatures of 40–45°C and extraction times of 30–35 min.
Figure 4. Response surface plots depicting the effects of β-cyclodextrin (CD) concentration, extraction time, and extraction temperature on the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity (RSA).
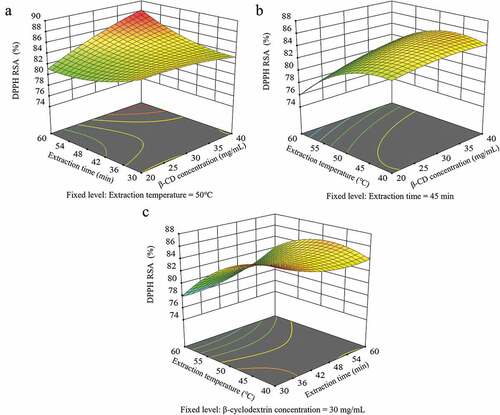
The DPPH RSA was most significantly affected by the β-CD concentration and extraction temperature (). Belwal et al. (Citation2016) demonstrated the manner in which the antioxidant activity of Berberis asiatica could be optimized using CCD, and the optimal conditions were reported to be an extraction time of 30 min and extraction temperature of 80°C. The linear extraction solvent concentration and extraction temperature demonstrated a significant effect on the model, which is consistent with the results of this study. These results indicated that β-CD forms complexes with functional compounds such as polyphenols and promotes the transfer of hydrogen atoms by breaking the hydrogen bonds in the free polyphenol molecule. Therefore, polyphenols are considered to act efficiently as antioxidants (Olga et al., Citation2015). When the extraction temperature increases during sonication, the number of air bubbles inducing cavitation increases, which broadens the surface area. This is effective in promoting the release of physiologically functional compounds from plants (Zhou et al., Citation2013).
3.5. Optimization of the extraction conditions and their validation
The optimal conditions for extracting functional ingredients with high antioxidant activity from unripe apples were determined using the Minitab® 18 software (Minitab Inc.) to maximize all dependent variables. As shown in , the predicted optimal β-CD concentration, extraction time, and extraction temperature for TPC are 40 mg/mL, 53.03 min, and 60°C, respectively, and the predicted TPC under these conditions is 4.72 mg GAE/g DW. Zhang et al. (Citation2018) reported that the TPC of the extract obtained via UAE from apple pomace was 2.11 mg GAE/g, indicating that the CD-based UAE method used in this study is extremely efficient for recovering polyphenols. The optimal conditions for extracting the CAC were a β-CD concentration of 40 mg/mL, an extraction time of 46.67 min, and extraction temperature of 60°C. A yield of 225.18 mg/100 g DW was predicted under these conditions. This value is significantly higher than that reported by Liaudanskas et al. (Citation2015), who extracted 0.76–29.34 mg/g chlorogenic acid from apples of various varieties using UAE with ethanol. The optimal conditions for achieving good DPPH RSA were reported to be a β-CD concentration of 20 mg/mL, extraction time of 30 min, and extraction temperature of 40°C. The predicted DPPH RSA under these conditions was 87.97%. The experimental values obtained under each optimal extraction condition were TPC: 4.70 mg GAE/g DW, CAC: 224.74 mg/100 g DW, and DPPH RSA: 86.49%, which were close to the predicted values. The %RSD, the coefficient of performance, indicating the precision of the experimental value, was 0.65–2.18%. All values were low and within 5%. Therefore, the model designed for extracting functional compounds from unripe apples is suitable, and the optimal conditions were confirmed to have excellent precision and accuracy (Kang & Lee, Citation2013).
Table 3. Validation of predicted and experimental values determined at optimal extraction conditions.
For practical application of the unripe apple extract, the optimal extraction conditions were predicted by superimposing to simultaneously maximize the three dependent variables (Table S1). The predicted optimal conditions were as follows: β-CD concentration, 40 mg/mL; extraction time, 60 min; and extraction temperature, 59.60°C. The experimental values of the extract obtained under the predicted conditions were TPC: 4.71 mg GAE/g DW, CAC: 229.63 mg/100 g DW, and DPPH RSA: 82.85%. The experimental values obtained under the optimal extraction conditions were TPC: 4.71 mg GAE/g DW, CAC: 229.63 mg/100 g DW, and DPPH RSA: 82.85%, which were close to the predicted values. Hernández-Carranza et al. (Citation2016) reported that the TPC of an extract obtained by maceration extraction of apple pomace using distilled water at 51°C for 7.9 h was 389 mg GAE/100 g. The TPC of the extracts obtained by extracting apple by-products using 50% methanol, acetone, and ethanol as solvents were 2.17, 1.00, and 3.31 mg GAE/g, respectively (Rana & Bhushan, Citation2016). From the above results, the TPC of the extract obtained in this study was higher than that reported in previous studies (Hernández-Carranza et al., Citation2016; Rana & Bhushan, Citation2016), confirming that UAE using β-CD was effective for polyphenol extraction.
3.6. Comparison of β-CD-based UAE and HWE
To confirm the extraction efficiency of UAE, the phenolic-rich extract obtained using the optimized β-CD-based UAE was compared with the extract obtained using HWE, which was representative of conventional extractions. The extract obtained by using β-CD-based UAE had significantly higher TPC, CAC, and DPPH RSA values than those obtained using HWE (p < .05; ). For example, the TPC, CAC, and IC50 value for the DPPH RSA of extract obtained by β-CD-based UAE were 17.68 mg GAE/g extract, 5.99 mg/g extract, and 198.07 μg/mL, respectively. TPC, CAC, and IC50 value of DPPH RSA of extract obtained by HWE were 14.19 mg GAE/g extract, 3.39 mg/g extract, and 566.26 μg/mL, respectively. Maraulo et al. (Citation2020) reported that after extracting bioactive compounds from olive pomace using UAE, the extract obtained using β-CD as a solvent had higher TPC and antioxidant activity than the extract obtained using water. Alibante et al. (Citation2021) reported that aqueous β-CD extraction after UAE pretreatment enhanced the TPC and antioxidant activity compared with water extraction, which was similar to our result. These results suggest that ultrasound improves the formation of clathrates of β-CD and polyphenol compounds by releasing bioactive compounds that are tightly bound to plant cell walls. Chlorogenic acid (Shao et al., Citation2014) reported to have improved antioxidant performance when clathrate with CD is compared with the free state. This means that the polyphenol radicals produced via the reaction with other radicals can be more effectively stabilized when encased in CD hydrophobic cavities through resonance by intramolecular hydrogen bonds (Alibante et al., Citation2021). Therefore, it is considered that the CD-based UAE extract has a higher antioxidant activity than the HWE extract owing to its high TPC including chlorogenic acid.
Table 4. Total polyphenol content, chlorogenic acid content, and DPPH radical scavenging activity of polyphenol-rich extract obtained by β-cyclodextrin-based ultrasound-assisted extraction and hot water extraction.
4. Conclusion
In this study, functional compounds were extracted from unripe apples in an environmentally friendly and efficient manner using β-CD and ultrasound. An interaction analysis of the factors affecting the extraction efficiency (β-CD concentration, extraction time, and extraction temperature) was successfully performed using 15 experimental conditions obtained using a three-factor, three-level BBD. The predicted TPC, CAC, and antioxidant activities under each optimal extraction condition were 4.72 mg GAE/g DW, 225.18 mg/100 g DW, and 86.49%, respectively. The experimental values obtained under these conditions were TPC: 4.70 mg GAE/g DW, CAC: 224.74 mg/100 g DW, and DPPH RSA: 86.49%. The predicted superimposed optimal extraction conditions for maximizing all dependent variables were 40 mg/mL, 60 min, and 59.60°C, and the experimental values obtained under these conditions were TPC: 4.65 mg GAE/g DW, CAC: 222.23 mg/100 g DW, and DPPH RSA: 87.97%. There were no significant differences between the predicted and experimental values. The optimized extraction conditions resulted in excellent precision and accuracy, and the model designed for optimization was found to be suitable. The use of the UAE technology with β-CD affected the TPC recovery and led to a high CAC and antioxidant activity. The results of this study demonstrate that extracting by β-CD-based UAE can be applied for the production and retention of bioactive substances.
Supplemental Material
Download MS Word (13.3 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19476337.2022.2156619
Additional information
Funding
References
- Aguilera, Y., Rebollo-Hernanz, M., Cañas, S., Taladrid, D., & Martín-Cabrejas, M. A. (2019). Response surface methodology to optimise the heat-assisted aqueous extraction of phenolic compounds from coffee parchment and their comprehensive analysis. Food & Function, 10(8), 4739–4750. https://doi.org/10.1039/c9fo00544g
- Alibante, A., Lakka, A., Bozinou, E., Chatzilazarou, A., Lalas, S., & Makris, D. P. (2021). Integrated green process for the extraction of red grape pomace antioxidant polyphenols using ultrasound-assisted pretreatment and β-cyclodextrin. Beverages, 7(3), 59. https://doi.org/10.3390/beverages7030059
- Ameer, K., Shahbaz, H. M., & Kwon, J. H. (2017). Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Comprehensive Reviews in Food Science and Food Safety, 16(2), 295–315. https://doi.org/10.1111/1541-4337.12253
- Belwal, T., Dhyani, P., Bhatt, I. D., Rawal, R. S., & Pande, V. (2016). Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chemistry, 207, 115–124. https://doi.org/10.1016/j.foodchem.2016.03.081
- Cai, R., Yuan, Y., Cui, L., Wang, Z., & Yue, T. (2018). Cyclodextrin-assisted extraction of phenolic compounds: Current research and future prospects. Trends in Food Science & Technology, 79, 19–27. https://doi.org/10.1016/j.tifs.2018.06.015
- Ćujić, N., Šavikin, K., Janković, T., Pljevljakušić, D., Zdunić, G., & Ibrić, S. (2016). Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chemistry, 194, 135–142. https://doi.org/10.1016/j.foodchem.2015.08.008
- D’Alessandro, L. G., Kriaa, K., Nikov, I., & Dimitrov, K. (2012). Ultrasound assisted extraction of polyphenols from black chokeberry. Separation and Purification Technology, 93, 42–47. https://doi.org/10.1016/j.seppur.2012.03.024
- Favre, L. C., Rolandelli, G., Mshicileli, N., Vhangani, L. N., Ferreira, C. D. S., Wyk, J. V., & Buera, M. D. P. (2020). Antioxidant and anti-glycation potential of green pepper (Piper nigrum): Optimization of β-cyclodextrin-based extraction by response surface methodology. Food Chemistry, 316, 126280. https://doi.org/10.1016/j.foodchem.2020.126280
- Folin, O., & Denis, W. (1912). On phosphotungstic-phosphomolybdic compounds as color reagents. The Journal of Biological Chemistry, 12(2), 239–243. https://doi.org/10.1016/s0021-9258(18)88697-5
- Ghafoor, K., Choi, Y. H., Jeon, J. Y., & Jo, I. H. (2009). Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. Journal of Agricultural and Food Chemistry, 57(11), 4988–4994. https://doi.org/10.1021/jf9001439
- Gligor, O., Mocan, A., Moldovan, C., Locatelli, M., Crișan, G., & Ferreira, I. C. F. R. (2019). Enzyme-assisted extractions of polyphenols – a comprehensive review. Trends in Food Science & Technology, 88, 302–315. https://doi.org/10.1016/j.tifs.2019.03.029
- Hanrahan, G., & Lu, K. (2006). Application of factorial and response surface methodology in modern experimental design and optimization. Critical Reviews in Analytical Chemistry, 36(3–4), 141–151. https://doi.org/10.1080/10408340600969478
- Hernández-Carranza, P., Ávila-Sosa, R., Guerrero-Beltrán, J. A., Navarro-Cruz, A. R., Corona-Jiménez, E., & Ochoa-Velasco, C. E. (2016). Optimization of antioxidant compounds extraction from fruit by-products: Apple pomace, orange and banana peel. Journal of Food Processing and Preservation, 40(1), 103–115. https://doi.org/10.1111/jfpp.12588
- Kang, K. M., & Lee, S. H. (2013). Effects of extraction methods on the antioxidative activity of Artemisia sp. Journal of the Korean Society of Food Science and Nutrition, 42(8), 1249–1254. https://doi.org/10.3746/jkfn.2013.42.8.1249
- Karmoker, J. R., Hasan, I., Ahmed, N., Saifuddin, M., & Reza, M. S. (2019). Development and optimization of Acyclovir loaded mucoadhesive microspheres by Box–Behnken Design. Dhaka University Journal of Pharmaceutical Sciences, 18(1), 1–12. https://doi.org/10.3329/dujps.v18i1.41421
- Kim, J. M., & Yoon, K. Y. (2020). Determination of protein extraction and trypsin hydrolysis conditions for producing hydrolysates with antioxidant activity from perilla seed meal. Korean Journal of Food Preservation, 27(6), 791–799. https://doi.org/10.11002/kjfp.2020.27.6.791
- Lee, J. J., & Yoon, K. Y. (2021). Optimization of ultrasound-assisted extraction of phenolic compounds from bitter melon (Momordica charantia) using response surface methodology. CyTA – Journal of Food, 19(1), 721–728. https://doi.org/10.1080/19476337.2021.1973110
- Liaudanskas, M., Viškelis, P., Kviklys, D., Raudonis, R., & Janulis, V. (2015). A comparative study of phenolic content in apple fruits. International Journal of Food Properties, 18(5), 945–953. https://doi.org/10.1080/10942912.2014.911311
- Liyana-Pathirana, C., & Shahidi, F. (2005). Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chemistry, 93(1), 47–56. https://doi.org/10.1016/j.foodchem.2004.08.050
- Maraulo, G. E., Ferreira, C. S., & Mazzobre, M. F. (2020). β-cyclodextrin enhanced ultrasound-assisted extraction as a green method to recover olive pomace bioactive compounds. Journal of Food Processing and Preservation, 45(3), e15194. https://doi.org/10.1111/jfpp.15194
- Maraulo, G. E., Ferreira, C. S., & Mazzobre, M. F. (2021). β-cyclodextrin enhanced ultrasound-assisted extraction as a green method to recover olive pomace bioactive compounds. Journal of Food Processing and Preservation, 45(3), e15194. https://doi.org/10.1111/jfpp.15194
- Matencio, A., Navarro-Orcajada, S., García-Carmona, F., & López-Nicolás, J. M. (2020). Applications of cyclodextrins in food science. A review. Trends in Food Science & Technology, 104, 132–143. https://doi.org/10.1016/j.tifs.2020.08.009
- Mengyuan, W., Haoli, W., Tingting, M., Qian, G., Yulin, F., & Xiangyu, S. (2021). Comprehensive utilization of thinned unripe fruits from horticultural crops. Foods, 10(9), 2043. https://doi.org/10.3390/foods10092043
- Olga, G., Styliani, C., & Ioannis, R. G. (2015). Coencapsulation of ferulic and gallic acid in hp-b-cyclodextrin. Food Chemistry, 185, 33–40. https://doi.org/10.1016/j.foodchem.2015.03.058
- Parmar, I., Sharma, S., & Rupasinghe, H. P. V. (2015). Optimization of β-cyclodextrin-based flavonol extraction from apple pomace using response surface methodology. Journal of Food Science and Technology, 52(4), 2202–2210. https://doi.org/10.1007/s13197-014-1282-1
- Patocka, J., Bhardwaj, K., Klimova, B., Nepovimova, E., Wu, Q., Landi, M., Kuca, K., Valis, M., & Wu, W. (2020). Malus domestica: A review on nutritional features, chemical composition, traditional and medicinal value. Plants, 9(11), 1–19. https://doi.org/10.3390/plants9111408
- Pinho, E., Grootveld, M., Soares, G., & Henriques, M. (2014). Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydrate Polymers, 101(1), 121–135. https://doi.org/10.1016/j.carbpol.2013.08.078
- Rajha, H. N., Chacar, S., Afif, C., Vorobiev, E., Louka, N., & Maroun, R. G. (2015). β-Cyclodextrin-assisted extraction of polyphenols from vine shoot cultivars. Journal of Agricultural and Food Chemistry, 63(13), 3387–3393. https://doi.org/10.1021/acs.jafc.5b00672
- Rana, S., & Bhushan, S. (2016). Apple phenolics as nutraceuticals: Assessment, analysis and application. Journal of Food Science and Technology, 53(4), 1727–1738. https://doi.org/10.1007/s13197-015-2093-8
- Saifullah, M., McCullum, R., McCluskey, A., & Vuong, Q. (2020). Comparison of conventional extraction technique with ultrasound assisted extraction on recovery of phenolic compounds from lemon scented tea tree (Leptospermum petersonii) leaves. Heliyon, 6(4), e03666. https://doi.org/10.1016/j.heliyon.2020.e03666
- Shao, P., Zhang, J., Fang, Z., & Sun, P. (2014). Complexing of chlorogenic acid with β-cyclodextrins: Inclusion effects, antioxidative properties and potential application in grape juice. Food Hydrocolloids, 41, 132–139. https://doi.org/10.1016/j.foodhyd.2014.04.003
- Silva, L. C., Viganó, J., de Souza Mesquita, L. M., Dias, A. L. B., de Souza, M. C., Sanches, V. L., Chaves, J. O., Pizani, R. S., Contieri, L. S., & Rostagno, M. A. (2021). Recent advances and trends in extraction techniques to recover polyphenols compounds from apple by-products. Food Chemistry, 12, 100133. https://doi.org/10.1016/j.fochx.2021.100133
- Skinner, R. C., Gigliotti, J. C., Ku, K. M., & Tou, J. C. (2018). A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutrition Reviews, 76(12), 893–909. https://doi.org/10.1093/nutrit/nuy033
- Wojdyło, A., Nowicka, P., Turkiewicz, I. P., Tkacz, K., & Hernandez, F. (2021). Comparison of bioactive compounds and health promoting properties of fruits and leaves of apple, pear and quince. Scientific reports, 11(1), 1–17. https://doi.org/10.1038/s41598-021-99293-x
- Xie, J., Xu, Y., Shishir, M. R. I., Zheng, X., & Chen, W. (2019). Green extraction of mulberry anthocyanin with improved stability using β-cyclodextrin. Journal of the Science of Food and Agriculture, 99(5), 2494–2503. https://doi.org/10.1002/jsfa.9459
- Yolmeh, M., & Jafari, S. M. (2017). Applications of response surface methodology in the food industry processes. Food and Bioprocess Technology, 10(3), 413–433. https://doi.org/10.1007/s11947-016-1855-2
- Zhang, Z., Poojary, M. M., Choudhary, A., Rai, D. K., & Tiwari, B. K. (2018). Comparison of selected clean and green extraction technologies for biomolecules from apple pomace. Electrophoresis, 39(15), 1934–1945. https://doi.org/10.1002/elps.201800041
- Zhao, T., Sun, L., Wang, Z., Nisar, T., Gong, T., Li, D., Niu, P., & Guo, Y. (2019). The antioxidant property and α-amylase inhibition activity of young apple polyphenols are related with apple varieties. LWT, 111, 252–259. https://doi.org/10.1016/j.lwt.2019.05.006
- Zhao, M., Wang, H., Yang, B., & Tao, H. (2010). Identification of cyclodextrin inclusion complex of chlorogenic acid and its antimicrobial activity. Food Chemistry, 120(4), 1138–1142. https://doi.org/10.1016/j.foodchem.2009.11.044
- Zheng, H. Z., Kim, Y. I., & Chung, S. K. (2012). A profile of physicochemical and antioxidant changes during fruit growth for the utilisation of unripe apples. Food Chemistry, 131(1), 106–110. https://doi.org/10.1016/j.foodchem.2011.08.038
- Zhou, J., Zheng, X., Yang, Q., Liang, Z., Li, D., Yang, X., & Xu, J. (2013). Optimization of ultrasonic-assisted extraction and radical-scavenging capacity of phenols and flavonoids from Clerodendrum cyrtophyllum Turcz Leaves. Plos One, 8(7), 1–8. https://doi.org/10.1371/journal.pone.0068392