ABSTRACT
The aim of this study was to investigate the oxidative status of poultry breast fillets enriched with n-3 from chia seeds, during retail display. Birds were assigned randomly to a control diet (corn-soy), or a corn-soy basal diet with 2.5s%, 5% or 10% chia seed. Lipid oxidation (TBARS), protein oxidation (carbonyls and total sulfhydryls), color, and heme iron content were measured in fresh breasts and after 4 days of display in a refrigerated showcase. Breast fillets from the control group presented higher values of b*, Hue angle, and Chroma than the 10% chia group, and no diet effect was observed on oxidation parameters. An increase in lipid and protein oxidation and in some of the color parameters (b*, Hue angle, and Chroma) were observed after 4 days of display. In conclusion, up to 5% chia seed can be included in the poultry diet without negative effects on meat quality.
Introduction
Several strategies have been tested to improve the nutritional profile of meat to address major public health concerns (Wood et al., Citation2004). A human diet, particularly rich in n-3, has beneficial effects related to obesity, cardiovascular disease, diabetes, and some types of cancer (Pereira da Silva et al., Citation2017). Chicken meat is a source of animal protein worldwide used in the human diet (Mendonça et al., Citation2020) and is highly accepted due to its low energetic content and high level of polyunsaturated fatty acids (PUFA). The level of these unsaturated fatty acids can be increased by incorporating certain ingredients in the diet, like chia seed, which is a promising source of n-3 PUFA, especially α-linolenic acid, which is converted to long-chain ω-3 fatty acids by desaturation and elongation with Δ6 and Δ5 enzymes (Mendonça et al., Citation2020; Woods & Fearon, Citation2009).
Chia seed has been largely used as a food, oil source, and raw material for medicinal compounds (Capitani et al., Citation2013). Its benefits result primarily from the high concentrations of essential fatty acids, dietary fiber, antioxidants, flavonoids, anthocyanins, vitamins, carotenoids, and minerals (Pereira da Silva et al., Citation2017). Protein content in chia seeds (19–23%) is higher than wheat (~14%), corn (~14%), rice (~8.5%), oats (~15.3%), barley (~9.2%), and amaranth (~14.8%) (Mohammed et al., Citation2019). Generally, there are no reports of antinutritive factors in chia seed that could retard its in vitro digestibility such as the presence of protease inhibitors (Ayerza et al., Citation2002).
Previous and recent research showed that the inclusion of chia, as ground whole seed, or seed meal, in the diet of broilers, pigs, rabbits, and lambs was able to increase PUFA concentrations and decrease saturated fatty acid (SFA) content in meat (Ayerza et al., Citation2002; Azcona et al., Citation2008; Da Silva et al., Citation2021; Komprda et al., Citation2013; Peiretti & Meineri, Citation2008; Urrutia et al., Citation2015). However, the increase in PUFA content in meat can enhance its susceptibility to oxidation, because oxidative processes predominantly affect unsaturated fatty acids in lipids, as well as heme groups in pigments, amino acids in proteins, and conjugated double bonds in vitamins (McMillin, Citation1996). Besides, oxidation induces modifications of muscle lipids and proteins and, therefore, affects the organoleptic and nutritional properties of meat and meat products (Insani et al., Citation2008). Lipid oxidation leads to the deterioration of quality and the accumulation of secondary oxidation products, such as aldehydes, alkanes, ketones, and alcohols, which directly affect the flavor, texture, color, and protein stability of meat (Jin et al., Citation2021). Oxidation of meat proteins may reduce sensory quality by decreasing tenderness and juiciness, due to effects on proteolytic enzyme activity, causing changes in flavor and color. Among the oxidative changes in meat proteins, the foremost is the formation of hydroperoxides and carbonyl derivatives, loss of sulfhydryl groups, formation of protein cross-linking occurring thanks to the formation of disulfide bonds (so-called crosslinking), peptide fragmentation, and reduction of protein solubility (Lund et al., Citation2011).
During retail display conditions of meat products, the incidence of light, refrigeration temperature, and the use of aerobic and transparent packages favor the oxidation of pigments (myoglobin) or lipids, which leads to fading or discoloration and to off-odor and off-flavor development (Acton et al., Citation2006). Meat discoloration, due to the oxidation of myoglobin, while in storage or on retail display, leads to significant product discarding (McKenna et al., Citation2005), which carries economic losses. Besides this, color is an important parameter for consumers which has a substantial influence on acceptability and purchasing decisions at retail points (Insani et al., Citation2008).
As research concerning the effect of chia seed inclusion in poultry diet on meat quality characteristics is scarce, the aim of this study was to investigate the incorporation of increasing doses of ground whole chia seeds in a poultry finishing diet, on the oxidative stability of lipids and proteins, color, and heme iron content, in meat under retail display conditions.
Materials and methods
Meat samples, animals and diet treatments
Breast samples (Pectoralis major) were obtained from 64 male Ross 308 broilers which were distributed randomly into four diet treatments (8 cages per treatment with 2 birds in each cage) from day 21 to day 49. The present work was part of an experimental procedure conducted to enrich the poultry meat with DHA and EPA (Da Silva et al., Citation2021). To achieve this, chia seeds were included in the finishing diet at increasing doses, where the control group received a corn-soy basal diet with 0% chia inclusion (n = 16); and the other three groups received a corn-soy basal diet with 2.5% (n = 16), 5% (n = 16) and 10% (n = 16) chia seed inclusion. All diets were formulated with ground corn, soybean meal, meat and bone meal (45% crude protein), high-oleic sunflower oil, salt, and minerals and vitamins premix, resulting in iso-proteic and iso-energetic (). All birds were sacrificed in a commercial slaughterhouse at day 50, following the good animal welfare practices approved by the Honorary Committee for Animal Experimentation (CHEA, protocol N° 702), and after chilling the carcasses at 4°C for 24 hours, they were transported to the laboratory where 16 breasts of each dietary group were separated manually after removing the skin. The longitudinal half of one lateral breast was vacuum packaged (105 µm, Lacor, LR69454, Spain) and frozen at −20°C until determinations in fresh meat (day 0 postmortem samples). The other half was put on food-grade polyfoam trays overwrapped with food-grade oxygen-permeable PVC (polyvinyl chloride) fil͓m (density 1.39 g/cm3; O2 permeability cm3 mm/m2day atm) in a commercial showcase (CE, SS1500 model, 1.25 m height, 90 cm wide and 1.50 m long) with artificial light at 2–8°C, simulating retail display conditions, for 4 days. After this time, breasts were vacuum packaged (105 µm, Lacor, LR69454, Spain) and frozen at −20°C until further analysis (day 4 samples).
Table 1. Ingredients and nutritional composition of the experimental diets.
Color determination
Poultry meat color was determined in breasts from day 0 and day 4 of the display, by the CIELAB method (CIE, Citation2004) using a Minolta CR-10 colorimeter (Konica Minolta, Japan). This method provides three parameters: L* (lightness, 0: black to 100: white component), a* (redness, + red to – green component), and b* (yellowness, + yellow to – blue component). Besides, Hue angle (tone) calculated as tan−1 (b*/a*) and Chroma C* (saturation) calculated as [(a*)2 + (b*)2]1/2 were determined.
Heme iron content determination
Total heme pigments in poultry meat samples from day 0 and day 4 of the display were determined according to Hornsey (Citation1956), and Ramos et al. (Citation2012). Briefly, 2 g meat samples were finely chopped and macerated in 9 ml of acidified acetone (90%) and afterward they were kept for 1 hour in darkness at room temperature. Then, the tubes were stirred in a vortex and filtered with glass filter paper (Whatman GFA). Absorbance was measured at 640 nm (T70 UV/Vis spectrometer, PG Instruments Ltd) in duplicate. Heme iron content was calculated using the factor 0.0882 μg iron/μg hematin and results were expressed as mg/kg meat.
Lipid and protein oxidation determination
Lipid oxidation in breast samples from day 0 and day 4 of the display was determined using the TBARS (thiobarbituric acid reactive species) test described in Terevinto et al. (Citation2019) adapted for poultry meat. First, 10 g of frozen meat was homogenized with 200 ml of an extraction buffer (0.15 M KCl, 0.02 M EDTA, and 0.30 BHT) in a Waring-Blender (Fisher Inc. USA) at 12,000 rpm for 1 min. After centrifugation (Sorvall ST16-R, USA) at 2000 × g for 10 min at 4°C, 1 ml of the supernatant was incubated with 1 ml of 2 TBA-TCA solution (35 mM TBA and 10% TCA in 125 mM HCl) in a boiling water bath for 30 min. Tubes were placed in an ice bath to stop the reaction and then were left at room temperature for 45 min. After adding 3 ml of n-butanol, centrifugation was done for 10 min at 3000 × g and absorbance was measured at 535 nm (T70 UV/Vis spectrometer, PG Instruments Ltd) in duplicate. Results were expressed as mg MDA/kg of meat where MDA concentration was calculated using its molar extinction coefficient (MDA, 156,000 M−1 cm−1).
To assess protein oxidative changes in meat, carbonyl, and sulfhydryl (SH) content were determined in breast samples from day 0 and day 4 of the display. These two methods are the most commonly used in meat. Protein carbonyls were determined following the procedure described in Terevinto et al. (Citation2019) adapted for poultry meat. First, sample homogenates obtained the day before for the TBARS test were thawed and two aliquots of 2 ml were put in two different tubes. One was incubated with 2 ml of 2 M HCl (blank) and the other with 2 ml of 0.02 M dinitrophenylhydrazine (DNPH) in 2 M HCl (sample). Carbonyl groups react with 2,4-dinitrophenylhydrazine (DNPH) to form the corresponding hydrazones. Tubes were left at room temperature and stirred regularly. 2 ml of 20% TCA was added and left 15 min stirring regularly. Then, centrifugation was done for 10 min at 2000 ×g, and pellets were washed with 4 ml ethanol: ethyl acetate (1:1) three times. After each washing procedure, tubes were centrifuged at 2000 × g for 10 min. Pellets were dissolved in 6 ml of 6 M HCl with 0.02 M KH2PO4 (pH 6.5) and stirred regularly for its dissolution. After centrifugation for 10 min at 2400 ×g, the absorbance was measured at 370 nm (T70 UV/Vis spectrometer, PG Instruments Ltd) in duplicate, and results were expressed as nmoles of DNPH/mg of protein. DNPH concentration was calculated using its molar extinction coefficient (DNPH, 22,000 M−1 cm−1). The protein content of each sample was determined at 280 nm using bovine serum albumin (BSA) (Sigma chemicals, USA) as a protein standard, following the Stoscheck (Citation1990) method.
SH content was determined spectrophotometrically following the method described by Jongberg et al. (Citation2013) with modifications (Pirotti, Citation2020) and using Ellman’s reagent 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB) which leads to the formation of a mixed disulfide and release of 5-thio-2-nitrobenzoic acid (Kaczmarek & Muzolf-Panek, Citation2021). First, 1 g of meat sample was homogenized with 20 ml of a solution containing 8 M Urea, 3% SDS, and 0.1 M PBS (pH 7.4) in an Ultra-turrax (IKA T18 basic) at 8000 rpm. Then, tubes were stirred (Vortex-Genie, Model K550-GE, Scientific Industries) and incubated with shaking (Roto-Mix, Thermolyne) at 140 rpm for 1 hour. Samples were filtered (Whatman No. 1) and an aliquot of 40 µL was mixed with 1960 µL Urea-SDS-PBS solution. 600 µL 10 mM DTNB in 0.1 M PBS was added and the tubes were incubated in the dark for 15 min. A blank was done with 2 ml Urea-SDS-PBS solution +600 µL DTNB. Absorbance was measured at 412 nm (T70 UV/Vis spectrometer, PG Instruments Ltd) in duplicate and the molar extinction coefficient of DTNB (13600 M−1cm−1) was used. The corrected absorbance of the sample is given by Abs sample + DTNB - Abs blank. Results were expressed as nmoles SH/mg protein. The protein content of each sample was determined at 280 nm using bovine serum albumin (BSA) (Sigma chemicals, USA) as a protein standard, following the Stoscheck (Citation1990) method.
n-3 fatty acid composition determination
The methodology is detailed in Da Silva (Citation2022). Briefly, intramuscular lipids from breasts of day 0 were extracted following Folch et al. (Citation1957) using chloroform: methanol (2:1). Lipids were submitted previously to a methylation process with methanolic KOH and then injected in a gas chromatograph (Clarus 500, Perkin Elmer Instruments, USA) split/splitless with a fused-silica CPSIL-88 of 100 m capillary column and a FID detector for the determination of fatty acid composition. Fatty acids methylated esters (FAMEs) were determined by comparing the retention time to fatty acids standards (Sigma Corp., USA). From the individual FAME quantified as a percentage of total detected FAMEs reported in Da Silva (Citation2022), individual n-3 fatty acids (C18:3 ALA, C20:3, C20:5 EPA, C 22:5 DPA, and C22:6 DHA) and the sum of PUFA n-3 and total PUFA were expressed as mg of fatty acid per 100 g meat.
Statistical analysis
Results were reported as means ± standard error of the media (SEM) of n = 16. Data of L*, a*, b*, Hue angle, Chroma, heme iron content, TBARS, carbonyls, and sulfhydryls in breast fillets of days 0 and 4 of the display were analyzed by repeated-measures ANOVA and post hoc Tukey-Kramer test. Also, a one way-ANOVA was used to analyze differences between chia doses on day 0 and day 4 of the display. For oxidation data, in addition, a paired Student´s T-test was used to compare means from day 0 and day 4, in each dietary group. The n-3 fatty acid and total PUFA contents at day 0 were analyzed by a one way-ANOVA and post hoc Tukey-Kramer test. The significance level was established at P < .05 and the statistical software used was the NCSS 12.
Results and discussion
When diet effect on poultry meat color was evaluated, no differences were observed in L* (P > .05) parameter (). Lightness is an achromatic contribution to perceived color (Purslow et al., Citation2020) governed by water migration and/or distribution through muscle structure alterations (Kim et al., Citation2017). The L* value has been negatively correlated with the pH 24 h value of the meat with the explanation that a higher pH decline rate may cause a greater denaturation degree of the myoglobin pigments (Suman & Joseph, Citation2013) and greater liquid exudation, promoting greater light dispersion on the surface of the meat (El Rammouz et al., Citation2004). In the study of Mendonça et al. (Citation2020) the experimental diets (soybean oil, chia oil, roasted whole soybean, chia seed) did not influence the L* and Hue parameters in the breast meat of broilers; however, the inclusion of chia seeds in the diet resulted in a higher a* value in the broiler breast, suggesting that part of the chemical compounds present in this feed may have been metabolized and used for the synthesis of myoglobin. Myoglobin is a small globular protein with a central heme iron atom, which maintains oxygen supply in skeletal muscles and other muscle tissue, in mammals (Møller & Skibsted, Citation2006). In our study, no differences were observed between diet treatments in a* value (P > .05) (), which can be associated with no variation in the heme iron content (). Otherwise, the b* parameter (P < .001), Hue angle (P < .01), and Chroma (P < .001) were affected by diet in the present study, where poultry meat from chickens in the control group presented higher values than the 10% chia group (). This is an unfavorable result for the inclusion of 10% chia in the diet of broilers, as the tone and saturation of color were diminished, and this can affect the purchase decision of consumers. The greater b* value in meat from the control group compared with groups with chia may be explained by the high content of carotenoids in corn. Carotenoids consist of a group of more than 500 pigments, and birds use these compounds not only for skin and muscle pigmentation but also to maintain growth and fertility (García et al., Citation2013). Thus, Hue values observed in breast meat were between 70 and 100 degrees, so they can be considered yellow, according to the color scale of the Cie L*a*b* system (Mendonça et al., Citation2020).
Table 2. Effect of chia inclusion in poultry diet on lightness (L*), redness (a*) and yellowness (b*) and calculated Hue (H°) and Chroma (C*), and heme iron content (mg/kg meat) in fresh (day 0) and refrigerated display (day 4 postmortem) breast fillets.
In the present study, poultry breasts were placed in a refrigerated commercial showcase, simulating retail display conditions, for 4 days, because it was previously reported that the shelf life of chicken meat stored aerobically under chilling conditions is only approximately 4 days after slaughtering (Balamatsia et al., Citation2007). This is because chicken meat is highly perishable and contains more pathogenic bacteria than most other types of meat (Azlin-Hasim et al., Citation2018). A longer period would cause meat quality deterioration and consumer rejection. During the retail display time, some significant changes in the color of meat were observed, particularly, an increase in the b* (P < .01) value. This can be related to the increase in lipid oxidation (). The effects of lipid oxidation on the formation of yellow pigments in meat are related to the non-enzymatic browning reactions between lipid oxidation products and the amine in the proteins or in the phospholipid head groups (Xia et al., Citation2009). Amino acid degradations produced by lipid oxidation products are lesser known despite being lipid oxidation a major source of reactive carbonyls in food and result in Strecker aldehydes, α-keto acids, and amines (Hidalgo & Zamora, Citation2016). Therefore, hydroperoxides, the primary products of lipid oxidation, have an alternative way to produce the Strecker degradation of amino acids. This alternative pathway is likely a free radical degradation (Hidalgo & Zamora, Citation2016). In addition, because of oxidative stress, the accumulation of Schiff pigments from lipid oxidation products to protein complexes also contributes to the formation of yellowness (Rodríguez-Carpena et al., Citation2011). In the work of Vorst et al. (Citation2018) in poultry breasts and Wang et al. (Citation2021) in rabbit meat, an increase in b* was also observed with the time of display. In the present study, also an increase in Hue angle (tone) (P < .05) and Chroma (saturation) (P < .01) was found. This may be explained by the increase in b×. Despite this, L* and a* parameters were not affected by the time of display, contrary to what was observed by Vorst et al. (Citation2018) in poultry breasts, where L* diminished and a* increased during 5 days of exposure in retail display conditions, and by Azlin-Hasim et al. (Citation2018) who observed an increase in a* for 4 days in refrigerated conditions. It should be noted that no mention of diet ingredients was done in both investigations. Probably, the non-variation in a* observed in the present study is related to the no variation in heme iron with the time of display, as shown in . In the study of Wang et al. (Citation2021) both L* and a* parameters diminished with the time of display in rabbit meat, which is a redder meat than poultry. It is important to mention that poultry meat from breasts is quite pale because muscle is predominantly formed by white fibers (Ismail & Joo, Citation2017).
Figure 1. Effect of chia inclusion in poultry diet on TBARS (a; mg MDA/kg meat), carbonyls (b; nmoles DNPH/mg protein) and sulfhydryls (c; nmoles SH/mg protein) in fresh (day 0) breast fillets and after 4 days in refrigerated retail display conditions. Results are expressed as means ± SEM (n = 16). Dietary and time effects were analyzed by repeated measures ANOVA and post hoc Tukey-Kramer test (P < .05). * means significant differences between day 0 and day 4 of refrigerated display in each dietary group (P < .05) by a paired Student´s T-test. NS: not significant.
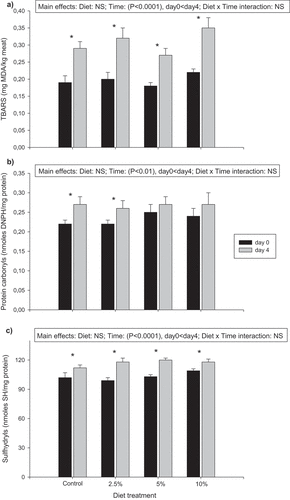
Regarding the oxidation results in poultry breasts (), no diet effect was observed for TBARS, protein carbonyls, and sulfhydryls, which is consistent with the fact that no differences were found between diet treatments in total PUFA content (). Despite this, an increase in total PUFA n-3 can be seen in breast fillets with chia inclusion in the diet, compared with the control group (0% chia). This result is supported by an increase in individual n-3 fatty acid content, like ALA, EPA, DPA, and DHA observed in , and could be useful to reach consumers´ health recommendations, which suggest increasing PUFA n-3 and decreasing PUFA n-6 consumption, to reach an n-6/n-3 ratio of 5/1 (Lunn & Theobald, Citation2006). The strategy of poultry meat n-3 enrichment with chia seed is better than with fish oil (rich in n-3) because in some works (Hugo et al., Citation2009; Saleh et al., Citation2010) an increase in the TBARS values of breast and thigh was observed. According to Marineli et al. (Citation2014), both the chia oil and the seed contain several phenolic compounds with antioxidant activity, especially myricetin, quercetin, kaempferol, chlorogenic acid, and the 3,4-dihydroxy phenyl ethanol-elenolic acid dialdehyde. Therefore, the non-variation in the meat lipid and protein oxidation values can be explained by antioxidant compounds present in the chia seed. Despite this, in another study, but in ground refrigerated rabbit meat (Meineri et al., Citation2010), lower oxidative stability was found when 15% chia seed was included in the diet. These differences in the results found might be explained by the animal specie (rabbit) which has a redder meat (more susceptible to oxidation because of higher content of iron) and that the meat was ground (more susceptible to oxidation because of a greater contact surface with air).
Table 3. Results of n-3 fatty acid and total PUFA content (mg/100 g meat) in fresh breast fillets from birds receiving diets with 0% chia (Control), or 2.5%, 5% and 10% chia.
When evaluating time as a main effect, both lipid and protein oxidation (P < .0001 for TBARS; P < .01 for carbonyls; P < .0001 for sulfhydryls) increased in poultry breasts exposed in a refrigerated showcase. On day 4 of the display, breasts experienced an increase in TBARS () compared to fresh ones (day 0), in all diet treatments, but carbonyl content increased only in control and 2.5% chia groups (). A possible explanation for not observing an increase in protein oxidation expressed as carbonyls in 5% and 10% chia groups at day 4 of display (), is a possible protection against protein oxidative processes, due likely to antioxidants present in whole chia seed. The protective effect of plant extracts rich in phenolics and other phytochemicals is often ambiguous due to the redox activity of such species (Macáková et al., Citation2012). Several studies show both antioxidative and prooxidative effects of plant extract addition to meat or meat products depending on the dose (Estévez & Cava, Citation2006; Utrera et al., Citation2012; Vaithiyanathan et al., Citation2011). For thiols content, breasts from all diet treatment groups showed an increase at day 4 of display in comparison to fresh ones (), as it was observed in TBARS results. This significant difference observed, indicating a protection of residual thiols formed during display because of proteolysis of myofibrillar protein (Baskol et al., Citation2014), is likely due to phytochemicals found in chia. The same thiols intact have, in turn, a protective effect on protein oxidation, since thiols are the major antioxidant coming from protein myofibrils (Hofmann & Hamm, Citation1978) but also it is rapidly oxidized if the prooxidant conditions are present. These results are expected, as lipid and protein oxidation processes are accelerated when meat is exposed to light and refrigerated temperatures. Although an increase in oxidation was observed in poultry meat, the values obtained were far below the maximum limit accepted for lipid oxidation (2 mg MDA/kg meat) (Campo et al., Citation2006), and protein oxidation (3 nmoles DNPH/mg protein) (Estévez, Citation2011). In another study (Azlin-Hasim et al., Citation2018), where chicken breast fillets were wrapped with low-density polyethylene film and refrigerated for 4 days, an increase in TBARS was observed during storage. Concerning rabbit meat where chia seeds were included in the diet, and oxidative stability was evaluated during refrigerated storage, an increase in oxidation was also observed (Meineri et al., Citation2010; Wang et al., Citation2021).
Conclusions
When chia seeds were incorporated into the poultry diet at the finishing phase, an n-3 enrichment was obtained in meat. Despite this, the oxidative stability of meat was not affected, so up to 10% of chia seed can be included in the finishing poultry diet. With this % of chia incorporation, the color might be affected, so a lower % inclusion should be desirable (2.5% or 5%), for greater consumer acceptability. Taking into account that in the 4 days of display some alterations in meat color as a result of oxidation processes occur, this research evidenced that the incorporation of chia in proportions lower than 5% would be ideal, not only for improving the fatty acid profile but for not affecting the characteristic color of poultry meat for consumption.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Acton, J., Kartika, S., Bouster, A., & Dawson, P. (2006, September 10–14). Packaging systems: Effects on poultry product colour and other quality factors [ paper presentation 278]. In 12th European Poultry Conference, Verona, Italy. World’s Poultry Science Association (WPSA).
- Ayerza, R., Coates, W., & Lauria, M. (2002). Chia seed (Salvia hispanica L.) as an ω-3 fatty acid source for broilers: Influence on fatty acid composition, cholesterol and fat content of white and dark meats, growth performance and sensory characteristics. Poultry Science, 81, 826–837. https://doi.org/10.1093/ps/81.6.826
- Azcona, J. O., Schang, M. J., Garcia, P. T., Gallinger, C., Ayerza, R., & Coates, W. (2008). Omega-3 enriched broiler meat: The influence of dietary α-linolenic-ω-3 fatty acid sources on growth, performance and meat fatty acid composition. Canadian Journal of Animal Science, 88(2), 257–269. https://doi.org/10.4141/CJAS07081
- Azlin-Hasim, S., Cruz-Romero, M. C., Morris, M. A., Cummins, E., & Kerry, J. P. (2018). Spray coating application for the development of nanocoated antimicrobial low-density polyethylene films to increase the shelf life of chicken breast fillets. Food Science and Technology International, 24(8), 688–698. https://doi.org/10.1177/1082013218789224
- Balamatsia, C. C., Patsias, A., Kontominas, M. G., & Savvaidis, I. N. (2007). Possible role of volatile amines as quality indicating metabolites in modified atmosphere-packaged chicken fillets: Correlation with microbiological and sensory attributes. Food Chemistry, 104(4), 1622–1628. https://doi.org/10.1016/j.foodchem.2007.03.013
- Baskol, M., Seckin, K. D., & Baskol, G. (2014). Advanced oxidation protein products, total thiol levels and total oxidant/antioxidant status in patients with nash. The Turkish Journal of Gastroenterology, 25(1), 32–37. https://doi.org/10.5152/tjg.2014.4172
- Campo, M., Nute, G., Hughes, S., Enser, M., Wood, J., & Richardson, R. (2006). Flavour perception of oxidation in beef. Meat Science, 72(2), 303–311. https://doi.org/10.1016/j.meatsci.2005.07.015
- Capitani, M. I., Ixtaina, V. Y., Nolasco, S. M., & Tomás, M. C. (2013). Microstructure, chemical composition and mucilage exudation of chia (Salvia hispanica L.) nutlets from Argentina. Argentina Journal of the Science of Food and Agriculture, 93(15), 3856–3862. https://doi.org/10.1002/jsfa.6327
- CIE. (2004). Commisssion Internationale de L`Eleclerige. Technical Report. Third Edition. Colorimetry, 15(3), 82.
- Da Silva, A. (2022). Enriquecimiento de la carne de ave con ácidos grasos poliinsaturados de la serie n-3 utilizando ingeniería nutricional. Tesis de maestría, Universidad de la República (Uruguay), Facultad de Ciencias – PEDECIBA. Repository. https://www.colibri.udelar.edu.uy/jspui/handle/20.500.12008/31611
- Da Silva, A., Cabrera, M. C., & Saadoun, A. (2021). Atherogenic, thrombogenic and hyper/hypo cholesterolemic indices in meat of chickens fed chia (Salvia hispanica) seeds. Archivos Latinoamericanos de Nutrición, 71(1). https://doi.org/10.37527/2021.71.S1
- El Rammouz, R., Babile, R., & Fernandez, X. (2004). Effect of ultimate pH on the physicochemical and biochemical characteristics of turkey breast muscle showing normal rate of postmortem pH fall. Poultry Science, 83(10), 1750–1757. https://doi.org/10.1093/ps/83.10.1750
- Estévez, M. (2011). Protein carbonyls in meat systems: A review. Meat Science, 89(3), 259–279. https://doi.org/10.1016/j.meatsci.2011.04.025
- Estévez, M., & Cava, R. (2006). Effectiveness of rosemary essential oil as an inhibitor of lipid and protein oxidation: Contradictory effects in different types of frankfurters. Meat Science, 72(2), 348–355. https://doi.org/10.1016/j.meatsci.2005.08.005
- Folch, J., Lees, M., & Sloane-Stanley, G. H. (1957). A simple method for the isolation and purification of total lipid from animal tissues. The Journal of Biological Chemistry, 226(1), 497–509. https://pubmed.ncbi.nlm.nih.gov/13428781/
- García, R. G., Mendes, A. A., Almeida Paz, I. C. L., Komiyama, C. M., Caldara, F. R., Nääs, I. A., & Mariano, W. S. (2013). Implications of the use of sorghum in broiler production. Brazilian Journal of Poultry Journal, 15(3), 169–286. https://doi.org/10.1590/S1516-635X2013000300013
- Hidalgo, F. J., & Zamora, R. (2016). Amino acid degradations produced by lipid oxidation products. Critical Reviews in Food Science and Nutrition, 56(8), 1242–1252. https://doi.org/10.1080/10408398.2012.761173
- Hofmann, K., & Hamm, R. (1978). Sulfhydryl and disulfide groups in meats. Advances in Food Research, 24, 1–111. https://doi.org/10.1016/s0065-2628(08)60156-1
- Hornsey, H. C. (1956). The colour of cooked cured pork. I.—Estimation of the nitric oxide-haem pigments. Journal of the Science of Food and Agriculture, 7(8), 534–540. https://doi.org/10.1002/jsfa.2740070804
- Hugo, A., Els, S. P., De Witt, F. H., Van der Merwe, H. J., & Fair, M. D. (2009). Effect of dietary lipid sources on lipid oxidation of broiler meat. South African Journal of Animal Science, 39(1), 149–152. https://doi.org/10.4314/sajas.v39i1.61187
- Insani, E. M., Eyherabide, A., Grigioni, G., Sancho, A. M., Pensel, N. A., & Descalzo, A. M. Oxidative stability and its relationship with natural antioxidants during refrigerated retail display of beef produced in Argentina. (2008). Meat Science, 79(3), 444–452. https://doi.org/10.1016/j.meatsci.2007.10.017
- Ismail, I., & Joo, S. -T. (2017). Poultry meat quality in relation to muscle growth and muscle fiber characteristics. Korean Journal for Food Science of Animal Resources, 37(6), 873–883. https://doi.org/10.5851/kosfa.2017.37.6.87
- Jin, S. -K., Kim, G. -D., & Jeong, J. -Y. (2021). Evaluation of the effect of inhibiting lipid oxidation of natural plant sources in a meat model system. Journal of Food Quality, 2021, 1–8. https://doi.org/10.1155/2021/6636335
- Jongberg, S., Tørngren, M. A., Gunvig, A., Skibsted, L. H., & Lund, M. N. (2013). Effect of green tea or rosemary extract on protein oxidation in Bologna type sausages prepared from oxidatively stressed pork. Meat Science, 93(3), 538–546. https://doi.org/10.1016/j.meatsci.2012.11.005
- Kaczmarek, A., & Muzolf-Panek, M. (2021). Prediction of thiol group changes in minced raw and cooked chicken meat with plant extracts—Kinetic and neural network approaches. Animals, 11(6), 1–15. https://doi.org/10.3390/ani11061647
- Kim, H. -W., Miller, D. K., Yan, F., Wang, W., Cheng, H. -W., & Brad Kim, Y. H. (2017). Probiotic supplementation and fast freezing to improve quality attributes and oxidation stability of frozen chicken breast muscle. LWT – Food Science and Technology, 75, 34–41. https://doi.org/10.1016/j.lwt.2016.08.035
- Komprda, T., Zorníková, G., Rozíková, V., Borkovcová, M., & Przywarová, A. (2013). The effect of dietary Salvia hispánica seed on the content of n-3 long-chain polyunsaturated fatty acids in tissues of selected animal species, including edible insects. Journal of Food Composition and Analysis, 32(1), 36–43. https://doi.org/10.1016/j.jfca.2013.06.010
- Lund, M. N., Heinonen, M., Barón, C. P., & Estévez, M. (2011). Protein oxidation in muscle foods: A review. Molecular Nutrition & Food Research, 55(1), 83–95. https://doi.org/10.1002/mnfr.201000453
- Lunn, J., & Theobald, H. E. (2006). The health effects of dietary unsaturated fatty acids. Nutrition Bulletin, 31(3), 178–224. https://doi.org/10.1111/j.1467-3010.2006.00571.x
- Macáková, K., Mladenka, P., Filipsky, T., Riha, M., Jahodar, L., Trejtnar, F., Bovicelli, P., Silvestri, I. P., Hrdina, R., & Saso, L. (2012). Iron reducing potentials hydroxyl radical formation only in flavonols. Food Chemistry, 135(4), 2584–2592. https://doi.org/10.1016/j.foodchem.2012.06.107
- Marineli, R. S., Moraes, E. A., Lenquiste, A. S., Godoy, A. T., Nogueira, E. M. N., & Marostica, M. R., Jr. (2014). Chemical characterization and antioxidant potential of Chilean chia seeds and oil (Salvia hispánica L.). LWT – Food Science and Technology, 59(2), 1304–1310. https://doi.org/10.1016/j.lwt.2014.04.014
- McMillin, K. (1996, June 9–11). Initiation on oxidative processes in muscle foods. In Proceedings 49th Annual Reciprocal Meat Conference, Kansas City, USA (pp. 53–63).
- McKenna, D. R., Mies, P. D., Baird, B. E., Pfeiffer, K. D., Ellebracht, J. W., & Savell, J. W. (2005). Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Science, 70(4), 665–682. https://doi.org/10.1016/j.meatsci.2005.02.016
- Meineri, G., Cornale, P., Tassone, S., & Peiretti, M. G. (2010). Effects of chia (Salvia hispanica L.) seed supplementation on rabbit meat quality, oxidative stability and sensory traits. Italian Journal of Animal Science, 9(e10), 45–49. https://doi.org/10.4081/ijas.2010.e10
- Mendonça, N. B. D. S. N., Sobrane Filho, S. T., de Oliveira, D. H., Lima, E. M. C., Rosa, P. V. E., Faria, P. B., Naves, L. D. P., & Rodrigues, P. B. (2020). Dietary chia (Salvia hispanica L.) improves the nutritional quality of broiler meat. Asian-Australasian Journal of Animal Science, 33(8), 1310–1322. https://doi.org/10.5713/ajas.19.0608
- Mohammed, O., Bekhet, M., Abd El-Razek, A., & Moharram, Y. (2019). Evaluation of chia (Salvia hispanica L.) seeds meal as a source of bioactive ingredients. Alexandria Science Exchange Journal, 40(January–March), 177–189. https://doi.org/10.21608/ASEJAIQJSAE.2019.29935
- Møller, J. K. S., & Skibsted, L. H. (2006). Myoglobins – the link between discoloration and lipid oxidation in muscle and meat. Química Nova, 29(6), 1678–7064. https://doi.org/10.1590/S0100-40422006000600024
- Peiretti, P. G., & Meineri, G. (2008). Effects on growth performance, carcass characteristics, and the fat and meat fatty acid profile of rabbits fed diets with chia (Salvia hispánica L.) seed supplements. Meat Science, 80(4), 1116–1121. https://doi.org/10.1016/j.meatsci.2008.05.003
- Pereira da Silva, B., Anunciação, P. C., da Silva Matyelka, J. C., Mattos Della Lucia, C., Stampini Duarte Martino, H., & Pinheiro-Sant´ana, H. M. (2017). Chemical composition of Brazilian chia seeds grown in different places. Food Chemistry, 221, 1709–1716. https://doi.org/10.1016/j.foodchem.2016.10.115
- Pirotti, F. (2020). Estatus antioxidante de carne bovina uruguaya: influencia del sistema de producción, tipo de músculo y tiempo de maduración. Tesis de maestría. Universidad de la República (Uruguay), Facultad de Ciencias. Repository. https://www.colibri.udelar.edu.uy/jspui/handle/20.500.12008/27460
- Purslow, P., Warner, R. D., Clarke, F. M., & Hughes, J. M. (2020). Variations in meat colour due to factors other than myoglobin chemistry; a synthesis of recent findings (invited review). Meat Science, 159, 107941. https://doi.org/10.1016/j.meatsci.2019.107941
- Ramos, A., Cabrera, M. C., & Saadoun, A. (2012). Bioaccessibility of Se, Cu, Zn, Mn and Fe, and heme iron content in unaged and aged meat of Hereford and Braford steers fed pasture. Meat Science, 91(2), 116–124. https://doi.org/10.1016/j.meatsci.2012.01.001
- Rodríguez-Carpena, J. G., Morcuende, D. A., & Estévez, M. (2011). Avocado by-products as inhibitors of colour deterioration and lipid and protein oxidation in raw porcine patties subjected to chilled storage. Meat Science, 89(2), 166–173. https://doi.org/10.1016/j.meatsci.2011.04.013
- Saleh, H., Rahimi, S., Torshizi, M. K., & Golian, A. (2010). Effect of dietary fish oil on oxidative stability and lipid composition of broiler chickens breast and thigh meat. Journal of Animal and Veterinary Advances, 9(22), 2877–2882. https://doi.org/10.3923/javaa.2010.2877.2882
- Stoscheck, C. M. (1990). Quantitation of Protein. Methods in Enzymology, 182, 50–68. https://doi.org/10.1016/0076-6879(90)82008-p
- Suman, S. P., & Joseph, P. (2013). Myoglobin chemistry and meat color. Annual Review of Food Science and Technology, 4(1), 79–99. https://doi.org/10.1146/annurev-food-030212-182623
- Terevinto, A., Cabrera, M. C., & Saadoun, A. (2019). Oxidative stability, fatty acid composition and health lipid indices of Longissimus dorsi muscle from Aberdeen Angus steers produced in different feeding systems. Ciência Rural, 49(12), 1–11. https://doi.org/10.1590/0103-8478cr20190537
- Urrutia, O., Soret, B., Insausti, K., Mendizabal, J. A., Purroy, A., & Arana, A. (2015). The effects of linseed or chia seed dietary supplementation on adipose tissue development, fatty acid composition, and lipogenic gene expression in lambs. Small Ruminant Research, 123(2–3), 204–211. https://doi.org/10.1016/j.smallrumres.2014.12.008
- Utrera, M., Rodríguez-Carpena, J. -G., Morcuende, D., & Estévez, M. (2012). Formation of lysine-derived oxidation products and loss of tryptophan during processing of porcine patties with added avocado byproducts. Journal of Agricultural and Food Chemistry, 60(15), 3917–3926. https://doi.org/10.1021/jf3001313
- Vaithiyanathan, S., Naveena, B. M., Muthukumar, M., Girish, P. S., & Kondaiah, N. (2011). Effect of dipping in pomegranate (Punica granatum) fruit juice phenolic solution on the shelf life of chicken meat under refrigerated storage (4°C). Meat Science, 88(3), 409–414. https://doi.org/10.1016/j.meatsci.2011.01.019
- Vorst, K., Shivalingaiah, N., Brenes, A. L. M., Coleman, S., Mendonça, A., Brown, J. W., & Shaw, A. (2018). Effect of display case cooling technologies on shelf-life of beef and chicken. Food Control, 94, 56–64. https://doi.org/10.1016/j.foodcont.2018.06.022
- Wang, Z., Tu, J., Zhou, H., Lu, A., & Xu, B. (2021). A comprehensive insight into the effects of microbial spoilage, myoglobin autoxidation, lipid oxidation, and protein oxidation on the discoloration of rabbit meat during retail display. Meat Science, 172, 108359. https://doi.org/10.1016/j.meatsci.2020.108359
- Wood, J., Richardson, R., Nute, G., Fisher, A., Campo, M., Kasapidou, E., Sheard, P., & Enser, M. (2004). Effects of fatty acids on meat quality: A review. Meat Science, 66(1), 21–32. https://doi.org/10.1016/S0309-1740(03)00022-6
- Woods, V. B., & Fearon, A. M. (2009). Dietary sources of unsaturated fatty acids for animals and their transfer into meat, milk and eggs: A review. Livestock Science, 126(1–3), 1–20. https://doi.org/10.1016/j.livsci.2009.07.002
- Xia, X., Kong, B., Liu, Q., & Liu, J. (2009). Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze–thaw cycles. Meat Science, 83(2), 239–245. https://doi.org/10.1016/j.meatsci.2009.05.003
