ABSTRACT
Kombucha is a fermented tea known for its health-enhancing properties owing to the bioactive compounds generated by acetic acid bacteria (AAB) and lactic acid bacteria (LAB). We compared the distribution of AAB and LAB across nine kombucha products in Singapore (Product A to I) using shotgun metagenomics. High prevalence of Komagataeibacter species including Komagataeibacter saccharivorans (82.93% in B), Komagataeibacter xylinus (93.38% in D), and Komagataeibacter rhaeticus (92.20% and 30.62% in G and I) was detected in AAB-dominant kombucha. LAB-dominant kombucha was represented mainly by Bacillus coagulans (~99% in E and F) and Lactobacillus nagelii (~60% in H). Despite differences in bacterial composition, all kombucha harbour pathways involved in the biosynthesis of short-chain fatty acids (SCFAs), amino acids and vitamin B12. Interestingly, “fatty acid and beta-oxidation II (peroxisome)” and “fatty acid and beta-oxidation I” were only present in LAB-dominant kombucha. Further study is required to elucidate the significance of the discrepancies.
Introduction
Kombucha is a traditionally fermented drink produced from the fermentation of sweetened green, black, or oolong tea by the symbiotic culture of bacteria and yeast (SCOBY) (de Miranda et al., Citation2022; Jayabalan et al., Citation2014). This traditional beverage has been widely consumed worldwide over the last decade due to its purported health benefits. For instance, regular consumption of kombucha is believed to reduce blood pressure, aid in weight loss, improve immunity, facilitate digestion, and prevent the development of metabolic and gastrointestinal diseases as well as cancer (Antolak et al., Citation2021; Jayabalan et al., Citation2014). These positive health effects of kombucha are mainly attributed to the unique microbial consortium and the bioactive compounds these microbes produce during the fermentation process (Villarreal-Soto et al., Citation2018).
The SCOBY in kombucha is primarily made up of yeast, and a large proportion of bacteria called acetic acid bacteria (AAB). AAB are Gram-negative obligate aerobes belonging to the Acetobacteraceae family. They can grow optimally at temperatures of approximately 25°C to 30°C and a pH of 5.0 to 6.5 (Bishop et al., Citation2022; Qiu et al., Citation2021). The most prevalent AAB in kombucha is the species of the genera Komagataeibacter, Gluconobacter, Acetobacter, and Gluconacetobacter (Bishop et al., Citation2022). Common AAB species found in commercial kombucha are Komagataeibacter xylinus, Komagataeibacter saccharivorans, and Gluconobacter oxydans (Antolak et al., Citation2021; Harrison & Curtin, Citation2021; Yang et al., Citation2022). These bacteria produce acetic acid and cellulose through the fermentation process. The metabolic products, in-turn, confer various health-promoting properties such as antimicrobial activities and the prevention of metabolic diseases like diabetes and obesity by decreasing intestinal glucose absorption and converting glucose into cellulose (de Miranda et al., Citation2022; Lavasani et al., Citation2019).
Lactic acid bacteria (LAB) are less frequently detected in kombucha. When present, LAB are usually found in relatively low abundances (Bishop et al., Citation2022; Harrison & Curtin, Citation2021; Yang et al., Citation2022). However, LAB such as Bacillus coagulans and Lactobacillus species are often added to commercial kombucha fermentation to enhance the probiotic potential of the beverage (Nguyen et al., Citation2015; Yang et al., Citation2022). LAB are Gram-positive, facultative anaerobic bacteria belonging to the Firmicutes phylum, which grow optimally at temperatures between 25°C and 40°C and a pH of 4.0 to 6.0. LAB can be categorised into either homofermenters or heterofermenters (Mokoena, Citation2017; Wang et al., Citation2021). Lactobacillus, Lactococcus, and Streptococcus species are among the most common homofermenters utilising glucose to produce lactic acid as the primary end-product of glycolysis. Meanwhile, heterofermenters such as Leuconostoc and Weisella spp. generate lactic acid, carbon dioxide, and ethanol via the pentose phosphate pathway (Bishop et al., Citation2022; Mokoena, Citation2017; Wang et al., Citation2021). Similar to AAB, the metabolites produced by LAB facilitate digestion, prevent infections, and exert immunomodulation properties (Mokoena, Citation2017; Wang et al., Citation2021).
The different preparation and brewing processes have resulted in variable microbial composition across kombucha. In this study, we profiled the microbial composition and functional pathways of nine commercial kombucha products in Singapore using shotgun metagenomics sequencing. We further compared the microbial and functional differences between the AAB- and LAB-dominant.
Methods
Sample procurement and processing
Nine kombucha products were obtained from commercial supermarkets in Singapore. Each product was brewed according to the manufacturer’s instructions. The products were stored at 4°C, with a shelf-life of up to 12 months. Unpasteurised kombucha products were procured and sent to the laboratory for subsequent steps on the same day.
DNA extraction and shotgun metagenomic sequencing
A total of 300–350 mL of each product was centrifuged at 6000 × g. The resulting supernatant was removed, and the pellet was stored at −80°C. The DNA from the frozen pellet was extracted using QIAamp PowerFecal Pro DNA kit according to the manufacturer’s protocol and quantified using Qubit 4 fluorometer. DNA sequencing was performed using Illumina NovaSeq with 2 × 150 bp paired-end configuration and a read depth of 20 million paired-end reads. The raw sequence reads were processed using BioBakery3 workflows (McIver et al., Citation2018). Read-level quality control was conducted using KneadData v0.11.0 with default settings (McIver et al., Citation2018). Taxonomy annotations were performed using MetaPhlan version 3.0, which uses clade-specific markers to identify microbial species and their relative abundances. Meanwhile, functional pathways annotations were conducted using HUMAnN version 3.0 based on the MetaCyc pathway database (McIver et al., Citation2018).
Data analysis
Species and pathway abundances were analysed using phyloseq R package version 1.36.0 (McMurdie & Holmes, Citation2013). Species and functional pathways with <0.1% and <1% relative abundances, respectively, were discarded using genefilter_sample and prune_taxa functions from the phyloseq R package version 1.36.0 (McMurdie & Holmes, Citation2013). The bar plots were generated using ggplot2 R package version 3.3.5, whereas the heatmap was generated using ComplexHeatmap R package version 2.13.1 (Gu et al., Citation2016; Wickham, Citation2022). The products were categorised as AAB- or LAB-dominant if the relative abundances of either AAB or LAB species reached more than 30%. Non-AAB- and LAB-dominant products (relative abundances of AAB, LAB, or other species (<30%)) were categorised as “others”.
Results
All nine products were unpasteurised, and most (n = 5) used either SCOBY or acidifier (n = 2) as the starter culture. Bacillus coagulans which was added in E and F and was detected in our analysis with relative abundances of almost 100% (99.95% and 99.98%, respectively). However, the species of Lactobacillus, Acetobacter, Gluconobacter, and Saccharomyces, which claimed to be added in products D and G by the respective manufacturers were either below the detection limit or present in low abundance (2.06% of Saccharomyces cerevisiae in G) ( and ) .
Figure 1. A. Heatmap of microbial species. The heatmap of 81 microbial species detected across the nine kombucha products after filtering for species with >0.1% relative abundance.
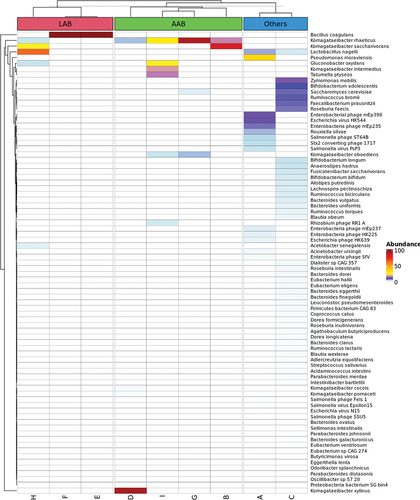
Table 1. Commercial claims and microbiome profiles of the kombucha products (n = 9) included in this study.
Overall, 141 microbial species were detected across all nine products. Eighty-one species remained after filtering for low species abundance (<0.1%) ( and Table S1). Product C had the highest species diversity, followed by product A, and the remaining products contained less than ten species ( and Table S1).
The classification of species from AAB and LAB was based on the definition set by previous literature (Mokoena, Citation2017; Qiu et al., Citation2021). Four of the nine products were predominated by AAB, notably the species from the Komagataeibacter genus (relative abundance >30%). These four products, B, D, G, and I, were predominated by Komagataeibacter saccharivorans (B), Komagataeibacter xylinus (D), and Komagataeibacter rhaeticus (G and I) with relative abundances of 82.93%, 93.38%, 92.20%, and 30.62%, respectively. LAB-dominant products were E, F, and H, with relative abundances of Bacillus coagulans in E and F at 99.95% and 99.98%, respectively, and Lactobacillus nagelli in H at 64.12% ( and Table S1).
After removing functional pathways with <1% relative abundance, 59 out of 433 pathways remained. The top five most abundant functional pathways were the pentose phosphate pathway (non-oxidative branch), aerobic respiration I (cytochrome c), aerobic respiration II (cytochrome c) (yeast), guanosine deoxyribonucleotides de novo biosynthesis II, and adenosine deoxyribonucleotides de novo biosynthesis II ().
Figure 2. (a) Functional pathways. The top 59 functional pathways after removing those with less than 1% relative abundance (the relative abundances were normalised to 100% in this figure) across all the nine kombucha products. (b) Species contribution. The relative abundances of the top 20 functional pathways contributed by three categories of microbes; AAB, LAB and others. Unclassified species contributing to the pathways across the nine products were excluded.
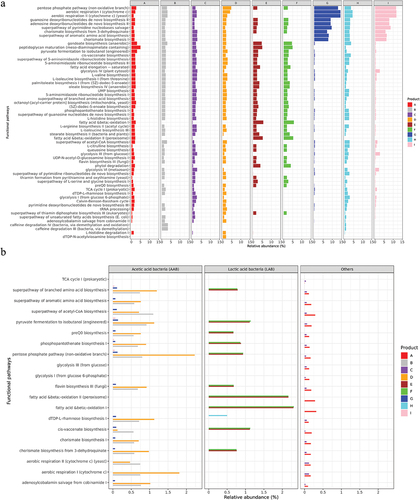
We also mapped 20 health-associated pathways to the microbial species detected from kombucha. AAB harboured 15 out of the 20 selected pathways (75%), while 11 pathways (55%) were detected in LAB. Notably, 2 out of 20 functional pathways (10%) were exclusively contributed by LAB and “others.” These pathways were “fatty acid and beta-oxidation II (peroxisome)” and “fatty acid and beta-oxidation I.” Meanwhile, 6 out of 20 (30%) were exclusively contributed by AAB and “others” ().
Discussion
The growing popularity of kombucha in the last decade has motivated many beverage companies to introduce various new fermentation strategies, starter cultures, and additional flavours, which results in unique microbial and metabolite profiles among different kombucha products. Notably, the bacterial composition in kombucha is comprised mainly of AAB and LAB. These bacteria play an essential role in producing bioactive compounds, thereby contributing to the pro-health effects of kombucha (Antolak et al., Citation2021; Bishop et al., Citation2022). In this study, we analysed nine commercial kombucha products in Singapore and compared the composition of AAB and LAB and their functional potentials. Shotgun metagenomics sequencing revealed that the studied kombucha could be largely classified into three categories, namely the AAB-dominant (B, D, G, and I), LAB-dominant (E, F, and H) and others (A and C).
Consistent with other parallel kombucha studies, Komagateibacter saccharivorans, Komagataeibacter xylinus, and Komagataeibacter rhaeticus are the most abundant and prevalent Komagataeibacter species found in the AAB-dominant products (Arikan et al., Citation2020; Kaashyap et al., Citation2021; Yang et al., Citation2022). Interestingly, the high prevalence of Komagataeibacter spp. was found across geographically distant locations (e.g. Australia, China, Turkey, and U.S.A), suggesting a degree of standardisation in the kombucha preparation (Arikan et al., Citation2020; Harrison & Curtin, Citation2021; Kaashyap et al., Citation2021; Yang et al., Citation2022; Zhao et al., Citation2018). The role of Komagataeibacter xylinus as the main cellulose producer explained its regular presence in kombucha. The species of Komagataeibacter are also reported to exhibit efficient glucose-metabolising activities (e.g. high glucose conversion rate to cellulose), which have a putative role in preventing the onset of metabolic diseases such as type-2 diabetes and obesity (Kaashyap et al., Citation2021; Lavasani et al., Citation2019).
Our study found that the three LAB-dominant products (E, F, and H) were predominated by Bacillus coagulans (~99% relative abundances in E and F) and Lactobacillus nagelii (64.12% relative abundance in H). Our finding is consistent with previous studies conducted in America, which also detected the predominance of these LAB species in their tested kombucha samples (Harrison & Curtin, Citation2021; Yang et al., Citation2022). B. coagulans are often added to food products not only due to their numerous probiotic characteristics but also their high tolerance to extreme environments such as high temperature and acidity. B. coagulans possess a wide range of probiotic attributes, such as promoting intestinal digestion, modulating the immune system, preventing pathogenic bacteria colonisation, and alleviating certain diseases and gastrointestinal tracts (GIT) disorders such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and colorectal cancer (Cao et al., Citation2020; Su & Xu, Citation2014; Yang et al., Citation2022). The predominance of B. coagulans in these products was consistent with the claims made by the product manufacturer. Nonetheless, some probiotic species labelled by the manufacturers were not detectable, likely due to the detection limit of our sequencing approach, or the unfavourable storage conditions. Lactobacillus nagelii, a common Lactobacillus species found in kombucha, predominated product H. The species of Lactobacillus (LAB) are known to enhance the health-promoting properties of kombucha and are widely regarded as probiotics as they can produce short-chain fatty acids (SCFAs), amines, bacteriocins, and vitamins involved in antimicrobial and antioxidant activities as well as gut health maintenance, among others (Nguyen et al., Citation2015; Wang et al., Citation2021).
To evaluate the health benefits of kombucha, we analysed the functional potential of the microbes across the nine products. The top five (out of 59) functional pathways detected were related to sugar metabolism (pentose phosphate pathway (non-oxidative branch), aerobic respiration I (cytochrome c), aerobic respiration II (cytochrome c) (yeast)) and the production of purines (guanosine deoxyribonucleotides de novo biosynthesis II and adenosine deoxyribonucleotides de novo biosynthesis II). Some of the products of these pathways, such as ribose-5-phosphate sugar, nicotinamide adenine dinucleotide phosphate (NADPH), adenosine triphosphate (ATP), and purines, act as essential growth factors and energy sources for the microbes to synthesise bioactive compounds in the kombucha culture (Jayabalan et al., Citation2014).
We further mapped 20 functional pathways that are associated with health-promoting functions, including SCFAs production, biosynthesis of precursors for B vitamins, amino acids, and organic acids (Jayabalan et al., Citation2014; Villarreal-Soto et al., Citation2018; Yang et al., Citation2022) to the microbial species detected in kombucha. We noted that functional pathways such as “fatty acid and beta-oxidation II (peroxisome)” and “fatty acid and beta-oxidation I” were mapped back to LAB but not AAB. These pathways are involved in the anaerobic metabolism of fatty acids to acetyl-CoA. Acetyl-CoA is a precursor for the biosynthesis of acetate, one of the common SCFAs that play an essential role in maintaining gut health, immune homeostasis, and preventing disease development (Tan et al., Citation2014). LAB, which are facultative anaerobes may utilise these pathways to generate SCFAs when the oxygen level is depleted during kombucha fermentation, unlike AAB, which requires oxygen to grow (Bishop et al., Citation2022; PubChem, Citation2022; Schönfeld & Wojtczak, Citation2016; Su & Xu, Citation2014).
In comparison, AAB contributed 20% more pathways involved in sugar metabolism, respiration, biosynthesis of amino acids and B vitamins than LAB. For instance, superpathway of aromatic amino acid biosynthesis, superpathway of acetyl-CoA biosynthesis, chorismate biosynthesis I, aerobic respiration II (cytochrome c) (yeast), aerobic respiration I (cytochrome c), and biosynthesis of adenosylcobalamin (vitamin B12) were among the pathways found in AAB but absent in LAB. Meanwhile, lower abundances of species from the “others” group contributed to the 20 selected pathways as compared to AAB and LAB. This could suggest that AAB and LAB possess a greater capability in producing beneficial metabolites as compared to non-AAB and -LAB species.
Nevertheless, further targeted chemical assays are warranted to profile the metabolite signatures from kombucha. One of the limitations of our study is the small sample size, contributed mainly by the relatively limited number of kombucha brands in Singapore. Future work to assess the stability of the kombucha microbiome over different production batches is warranted to understand the consistency of microbial and chemical compositions in kombucha. Clinical studies, including in vivo tests and bioavailability assessments, are indispensable to providing clinical evidence of kombucha’s biological activities in humans, thereby confirming the positive health implications of this popular beverage.
Conclusion
In this study, we detected a large variety of microbial compositions in commercial kombucha products in Singapore, likely due to the variation in the formula of the started cultures of SCOBY. Nevertheless, the studied kombucha can be largely classified into either AAB- or LAB-dominant categories. Komagataeibacter species, namely Komagateibacter saccharivorans, Komagataeibacter xylinus, and Komagataeibacter rhaeticus are the most prevalent and dominant bacteria present in AAB-dominant products (B, D, G, and I), whereas Bacillus coagulans and Lactobacillus nagelii are the dominant species detected in LAB-dominant products (E, F, and H). Although the variation in functional pathways conferred by AAB and LAB was identified, both categories of kombucha harbour metabolic potential to produce health-promoting chemicals such as organic acids, amino acids, vitamin B compounds, and SCFAs which support the health benefits of kombucha. Further research to characterise the microbial dynamics and the metabolic pathways that affect the acidity, flavour, and aroma of various kombucha products that interest the consumer could be explored more in-depth using both metagenomics and metabolomics approaches.
Author contributions
Conceptualization, GT, TKY, and JL.; Methodology, JD, HSL, CCW, JLWJ; Writing – Original Draft Preparation, JD, HSL.; Writing – Review & Editing, GT, TKY, JL, CCW, JD, HSL. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download MS Excel (13.9 KB)Disclosure statement
SLH, KYT, GT are employees of AMILI Pte. Ltd. JWJL and JL are co-founders of AMILI Pte. Ltd. JD and CWC declare no conflict of interests.
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19476337.2023.2190794.
Additional information
Funding
References
- Antolak, H., Piechota, D., & Kucharska, A. (2021). Kombucha tea—A double power of bioactive compounds from tea and symbiotic culture of bacteria and yeasts (SCOBY). Antioxidants (Basel), 10(10), 1541. https://doi.org/10.3390/antiox10101541
- Arikan, M., Mitchell, A. L., Finn, R. D., & Gurel, F. (2020). Microbial composition of kombucha determined using amplicon sequencing and shotgun metagenomics. Journal of Food Science, 85(2), 455–464. https://doi.org/10.1111/1750-3841.14992
- Bishop, P., Pitts, E. R., Budner, D., & Thompson-Witrick, K. A. (2022). Kombucha: Biochemical and microbiological impacts on the chemical and flavor profile. Food Chemistry Advances, 1, 100025. https://doi.org/10.1016/j.focha.2022.100025
- Cao, J., Yu, Z., Liu, W., Zhao, J., Zhang, H., Zhai, Q., & Chen, W. (2020). Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. Journal of Functional Foods, 64, 103643. https://doi.org/10.1016/j.jff.2019.103643
- de Miranda, J. F., Ruiz, L. F., Silva, C. B., Uekane, T. M., Silva, K. A., Gonzalez, A. G. M., Fernandes, F. F., & Lima, A. R. (2022). Kombucha: A review of substrates, regulations, composition, and biological properties. Journal of Food Science, 87(2), 503–527. https://doi.org/10.1111/1750-3841.16029
- Gu, Z., Eils, R., & Schlesner, M. (2016). Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics (Oxford, England), 32(18), 2847–2849. https://doi.org/10.1093/bioinformatics/btw313
- Harrison, K., & Curtin, C. (2021). Microbial composition of SCOBY starter cultures used by commercial kombucha brewers in North America. Microorganisms, 9(5), 1060. https://doi.org/10.3390/microorganisms9051060
- Jayabalan, R., Malbaša, R. V., Lončar, E. S., Vitas, J. S., & Sathishkumar, M. (2014). A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Comprehensive Reviews in Food Science and Food Safety, 13, 538–550. https://doi.org/10.1111/1541-4337.12073
- Kaashyap, M., Cohen, M., & Mantri, N. (2021). Microbial diversity and characteristics of kombucha as revealed by metagenomic and physicochemical analysis. Nutrients, 13(12), 4446. https://doi.org/10.3390/nu13124446
- Lavasani, P. S., Motevaseli, E., Sanikhani, N. S., & Modarressi, M. H. (2019). Komagataeibacter xylinus as a novel probiotic candidate with high glucose conversion rate properties. Heliyon, 5, e01571. https://doi.org/10.1016/j.heliyon.2019.e01571
- McIver, L. J., Abu-Ali, G., Franzosa, E. A., Schwager, R., Morgan, X. C., Waldron, L., Segata, N., & Huttenhower, C. (2018). BioBakery: A meta’omic analysis environment. Bioinformatics, 34(7), 1235–1237. https://doi.org/10.1093/bioinformatics/btx754
- McMurdie, P. J., & Holmes, S. (2013). Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PloS One, 8(4), e61217. https://doi.org/10.1371/journal.pone.0061217
- Mokoena, M. P. (2017). Lactic acid bacteria and their bacteriocins: Classification, biosynthesis and applications against uropathogens: A mini-review. Molecules, 22(8), 1255. https://doi.org/10.3390/molecules22081255
- Nguyen, N. K., Dong, N. T., Nguyen, H. T., & Le, P. H. (2015). Lactic acid bacteria: Promising supplements for enhancing the biological activities of kombucha. Springerplus, 4, 91. https://doi.org/10.1186/s40064-015-0872-3
- PubChem. Fatty acid oxidation. Retrieved May 25, 2022, from https://pubchem.ncbi.nlm.nih.gov/pathway/PathBank:SMP0000781
- Qiu, X., Zhang, Y., & Hong, H. (2021). Classification of acetic acid bacteria and their acid resistant mechanism. AMB Express, 11(1). https://doi.org/10.1186/s13568-021-01189-6
- Schönfeld, P., & Wojtczak, L. (2016). Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. Journal of Lipid Research, 57(6), 943–954. https://doi.org/10.1194/jlr.R067629
- Su, F., & Xu, P. (2014). Genomic analysis of thermophilic bacillus coagulans strains: Efficient producers for platform bio-chemicals. Scientific Reports, 4(1), 3926. https://doi.org/10.1038/srep03926
- Tan, J., McKenzie, C., Potamitis, M., Thorburn, A. N., Mackay, C. R., & Macia, L. (2014). The role of short-chain fatty acids in health and disease. Advances in Immunology, 91–119. https://doi.org/10.1016/b978-0-12-800100-4.00003-9
- Villarreal-Soto, S. A., Beaufort, S., Bouajila, J., Souchard, J.-P., & Taillandier, P. (2018). Understanding kombucha tea fermentation: A review. Journal of Food Science, 83(3), 580–588. https://doi.org/10.1111/1750-3841.14068
- Wang, Y., Wu, J., Lv, M., Shao, Z., Hungwe, M., Wang, J., Bai, X., Xie, J., Wang, Y., & Geng, W. (2021). Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Frontiers in Bioengineering and Biotechnology, 9. https://doi.org/10.3389/fbioe.2021.612285
- Wickham, H. ggplot2: Elegant graphics for data analysis. Retrieved March 10, 2022, from https://ggplot2.tidyverse.org
- Yang, J., Lagishetty, V., Kurnia, P., Henning, S. M., Ahdoot, A. I., & Jacobs, J. P. (2022). Microbial and chemical profiles of commercial kombucha products. Nutrients, 14, 670. https://doi.org/10.3390/nu14030670
- Zhao, Z., Sui, Y., Wu, H., Zhou, C., Hu, X., & Zhang, J. (2018). Flavour chemical dynamics during fermentation of kombucha tea. Emirates Journal of Food and Agriculture, 30, 732–741. https://doi.org/10.9755/ejfa.2018.v30.i9.1794
