ABSTRACT
The efficacy of concomitant administration of metformin and Cnidoscolus aconitifolius (Chaya) teas in type 2 diabetic rat models was evaluated. Diabetic rats were treated for 28 days with metformin and infusions of green (GTC) and black (BTC) chaya teas. Total phenol and flavonoid contents, as the antioxidant capacities of tea infusions, were assessed. Fasting blood glucose (FBG), lipid profile and markers of hepatic and renal functions were assessed. GTC exhibited the highest phenolic and flavonoid contents, as the best antioxidant activities. Daily co-administration of GTC and BTC with metformin significantly (p < .05) lowered the FBG, triglyceride, total cholesterol, LDL cholesterol, ALT, AST, creatinine and urea, as well as a significant increase in the serum protein. The combination of Chaya tea infusions with metformin treatment performed better than metformin taken alone in regulating fasting blood glucose in diabetic rats, with the best results observed in the group receiving GTC infusion.
1. Introduction
Type 2 diabetes mellitus is the most common form of diabetes mellitus and is largely the result of overweight and sedentary lifestyle (Potier, Citation2014). More than 95% of diabetic patients have type 2 diabetes (World Health Organisation [WHO], Citation2022). The International Diabetes Federation (IDF) estimated its global prevalence at 10.5% in 2021. This prevalence could rise to 11.3% in 2030 (International Diabetes Federation [IDF], Citation2021). Estimated at 24 million in 2021, the number of diabetic patients in sub-Saharan Africa will increase to 55 million in 2045, an increase of 134% (FID, 2021). Many strategies have been put in place to manage this disease, including oral antidiabetic drugs and low-calorie diets. Low-calorie diets are slow to produce desired results, while drugs, in addition to their side effects, are expensive (Kuate et al., Citation2015). Furthermore, epidemiological studies and clinical trials have shown that dietary vegetable consumption is correlated with a reduced risk of developing chronic diseases such as cancer, type 2 diabetes and others (Woumbo et al., Citation2017). In the same line, several studies have been carried out on Cnidoscolus aconitifolius, an Euphorbiaceae used as a food and medicinal plant (Iyke et al., Citation2018). It is rich in nutritional compounds and minerals and contains dietary fiber, vitamins A and C, flavonoids and polyphenols (Perez et al., Citation2019). Roy et al. (Citation2016) also demonstrated its in vitro inhibitory activity on α-glucosidase and α-amylase enzymes and antidiabetic potential in type 2 diabetic Wistar rats induced by streptozotocin. Studies have demonstrated beneficial effects of the C. aconitifolius drinks, including reduction of circulating lipids and increased antioxidant activity (Guevara-Cruz et al., Citation2021). Previous studies have established that drinks made from C. aconitifolius only had moderate activity and could not therefore be taken alone for an effective management of type 2 diabetes mellitus, but no previous work has evaluated the synergistic activity of C. aconitifolius drinks taken in combination with a reference oral antidiabetic drug. The present work was aimed at evaluating the efficacy of the concomitant administration of metformin and Chaya teas in type 2 diabetic rats.
2. Materials and methods
2.1. Plant material
The leaves of C. aconitifolius, were harvested in Dschang (5° 27′ north, 10° 04′ east), West region of Cameroon, identified at the National Herbarium of Yaoundé under the voucher N° 6748/HNC. The leaves were transported to the Laboratory of Biochemistry of Medicinal Plants, Food Sciences and Nutrition, Department of Biochemistry of the University of Dschang where they were sorted, washed and divided into several portions to obtain the different teas (green and black).
2.2. Tea production
For obtaining green tea, fresh leaves were first withered for 18 h. After withering, they were subjected to a high temperature (70°C) for 1 h. Dried leaves were ground using an electric grinder (Gaon, 800 W, 5 cycles of 1 min each at full power), sieved (50 μm mesh sieve) and stored in plastic bags for further use. To obtain black tea, the leaves were withered for 18 h, rolled so as to break the cells they contain and thus facilitate oxidation. They were then allowed to rest for 2 h before they were air-dried using an air oven (70°C for 1 h). The dried leaves were treated as per the same protocol described for green tea production.
Then, 2 g of tea powders (“green” or “black”) was respectively infused (4 min) in 100 mL of boiling water. The green tea yielded a pale yellow to orange brew, while black tea will yielded a darker colored tea ranging from amber to dark brown tones.
2.3. Determination of phenolic composition and antioxidant properties
2.3.1. Total phenol content
The total phenol content (TPC) of green and black tea infusions of C. aconitifolius was determined using the Folin–Ciocalteu method as described by Dohou et al. (Citation2003). Distilled water (1.39 mL) and 0.2 mL of a tenfold diluted Folin–Ciocalteu’s reagent were added to 0.01 mL of tea infusion (2 mg/mL) and left for 3 min, 0.4 mL of 20% Na2CO3 was added and the mixture vortexed and incubated for 20 min at 40°C using a water bath, then the absorbance was read against a blank at 760 nm using a BIOMATE spectrophotometer. Quantification of total phenols was made according to a linear calibration curve (y = 0.044x) produced by a standard (gallic acid) at different concentrations under the same conditions as the sample.
2.3.2. Flavonoid content
The total flavonoid content (TFC) of green and black tea infusions was determined according to the method described by Woumbo et al. (Citation2022). Distilled water (1.4 mL) and 0.03 mL of a 5% sodium nitrite (NaNO2) solution were added to 0.1 mL of tea infusion (2 mg/mL). Then, 0.2 mL of a 10% aluminum trichloride (AlCl3) solution was added. After resting for 5 min, 0.2 mL of a 10% sodium hydroxide solution (NaOH) and 0.24 mL of distilled water were, respectively, added. The mixture was vortexed before the absorbance was read against a blank at 510 nm using a spectrophotometer. Quantification of flavonoids was made according to a linear calibration curve (y = 0.319x) produced by a reference standard (catechin).
2.3.3. Antioxidant activity
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
The protocol established by Mensor et al. (Citation2001) was used to assess the scavenging activity of the teas. Briefly, 100 μl of tea infusion was added to 900 μl of DPPH reagent (0.3 mM DPPH (2,2-Diphenyl-1-picrylhydrazyl) dissolved in methanol). After 30 min of incubation at room temperature, the absorbance was read at 517 nm against a blank; butylated hydroxytoluene (BHT) was used as a standard.
Ferrous ion reducing capacity (FRAP)
The ability of the tea to reduce the ferric iron was assayed according to the protocol established by Oyaizu (Citation1986). Briefly, 100 μL of tea infusion was added to 500 μL of a phosphate buffer solution (0.2 M; pH 6.6), followed by 500 μL of aqueous solution of potassium hexacyanoferrate. After 30 min of incubation at 50°C in a water bath, 500 μL of trichloroacetic acid solution (10% v/v) was added. Absorbance was read at 700 nm against a blank; Vitamin C was used as the standard.
2.4. Induction of obesity and diabetes in experimental animals
Three-week-old Wistar rats were obtained from the animal facility of the department of Biochemistry of the University of Dschang and maintained according to the OECD (Citation2008) guidelines. Obesity was induced using a high-fat high-sucrose diet over a period of 12 weeks. The test groups and the positive control (untreated diabetic rats, T+) received a diet containing corn meal (52.9%), fish meal (20%), beef tallow (17.6%), bones (1%), vitamins (0.5%), salt (1%) and sucrose (7%), while the negative control (healthy rats, T-) received a staple diet consisting of corn flour (77.8%), fish meal (20%), bone (0.1%), palm olein (1%), vitamins (0.1%), salt (1%). Animals fed on hypercaloric diet had free access to a 2% sucrose solution and those with a body mass index (BMI) ≥0.68 g/cm2 were considered obese (Novelli et al., Citation2007). These obese animals received a single intraperitoneal dose of 50 mg/kg of streptozotocin solution (dissolved in citrate buffer; 0.1 M, pH 4.5) with the aim of inducing diabetes. Two days after injection of streptozotocin, animals with a fasting blood glucose level above 2 g/L were considered diabetic (Temiz, Citation2021).
The treatment lasted 28 days, and the animals were grouped according to the treatments received as follows: MET (metformin), MET + GTC (metformin + green tea), MET + BTC (metformin + black tea), T- (healthy control) and T+ (untreated diabetic rats). T- and T+ received water. Each group was made up of six rats, individually housed, which had free access to food and water. The treatments were administered once daily using an esophageal tube. The various “teas” of C. acotinifolius were administered at a dose of 6 mL/kg, while metformin was administered at 250 mg/kg. All experiments were performed in accordance with the regulations of the Ethics and Welfare Committee for Experimental Animals.
2.5. Biological parameters: blood sugar and serum lipids, transaminases, total protein, urea and creatinine
Animal blood glucose concentrations were measured, after overnight fasting (8 h) on 5–10 μL of blood obtained from the tail tip after, using a portable glucometer (Accu-Chek) every week during the treatment according to the standard protocols described by Trinder (Citation1969).
The lipid profile was determined by colorimetric methods using Sigma Diagnostic kits. The triglycerides were determined according to the method of Fossati and Principe (Citation1982). Total cholesterol was determined as per the protocol established by Huang et al. (Citation1997) using SIGMA Diagnostics’ EZ HDLTM cholesterol reagent, while LDL cholesterol was estimated using the formula established by Friedewald et al. (Citation1972).
Colorimetric methods (Randox kits) were also used to determine the serum level of alanine aminotransferase and aspartate aminotransferase. The standard protocol described by Gornall et al. (Citation1949) was used to determine total protein as well as Bartels et al. (Citation1972) for creatinine and Marshall (Citation1913) for urea.
2.6. Statistical analysis
Statistical analysis was performed using XLSTAT 2016 software. Results were expressed as mean ± standard deviation. A one-way analysis of variance (ANOVA) with Tukey’s test was used for the statistical analysis of the mean difference between the groups. The differences were considered significant at p < .05.
3. Results and discussion
3.1. Total phenol and flavonoid contents and antioxidant capacity
3.1.1. Total phenols and flavonoid contents
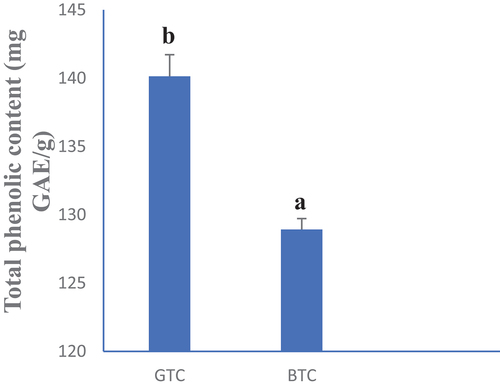
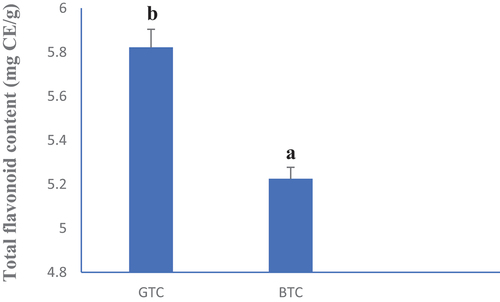
, respectively, show the total phenol and flavonoid contents of GTC and BTC. Green tea (GTC) presented the highest phenol content (140.13 mg EAG/g). The same observation was made with the total flavonoid content where GTC had 5.82 mg CE/g and BTC had 5.22 mg CE/g. Such observations can be explained by the methods of tea production. Hot water used in tea production should have contributed to breaking down the membranes, permitting a better diffusion of bioactive compounds from the biological matrix to the solvent, thus explaining the high content obtained with the different teas in general (Woumbo et al., Citation2021). However, low TPC and TFC from BTC compared to GTC could be due to the implication of these molecules in the enzymatic browning occurring during the fermentation step or to other non-enzymatic processes like the microbial activity (Zhao et al., Citation2019). These results corroborate with those of Veljković et al. (Citation2013) who reported high phenols and flavonoids content in green tea infusions of herbs and fruits consumed in Serbia.
3.1.2. Antioxidant capacity
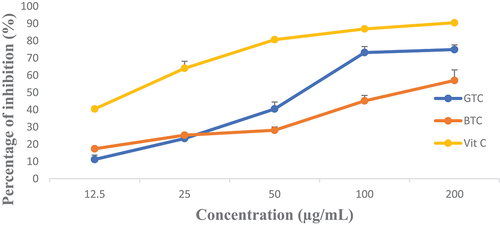
shows the DDPH radical scavenging of the tea infusions at different concentrations compared to vitamin C. GTC exhibited the highest antioxidant activity compared to black tea at concentrations ranging from 50 to 200 µg/mL.
Figure 3. Evolution of the antiradical activity of C. aconitifolius tea infusions at different concentrations compared to vitamin C. GTC: Green tea, BTC: Black tea, Vit C: Vitamin C.
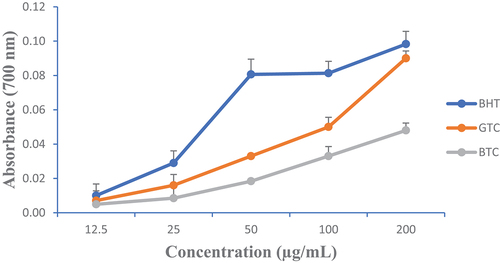
The FRAP assay has been reported to serve as a meaningful indicator of a plant extract antioxidant activity (A. Ojo et al., Citation2019). presents the variations of the iron reducing power of GTC and BTC at different concentrations compared to butylhydroxytoluene (BHT). It also came out that GTC exhibited the best reducing power.
presents the hydroxyl radical inhibitory activities of the different C. aconitifolius tea infusions at different concentrations compared to that of BHT. The highest scavenging ability was observed with GTC at a concentration of 200 µg/mL.
Figure 5. Changes in the hydroxyl radical inhibitory capacity of tea infusion at different concentrations compared to that of BHT.
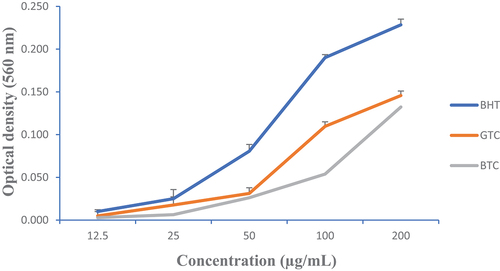
For all the tests used to assess the antioxidant capacity of the teas, GTC exhibited the highest activity. This may be associated with its high content of bioactive compounds such as phenols and flavonoids, which may be able to neutralize the free radical by transferring electrons or hydrogen atoms to DPPH (O. Ojo et al., Citation2014). We also observed the same trends regarding reducing power and phenolic content; this difference could be explained by the presence of phenolic compounds in our different samples as shown by Katalinic et al. (Citation2006).
The ferrous reducing capacity could also be explained by the presence of electron donor compounds which lead to the reduction of Fe3+ to Fe2+. This would be due to their respective contents of total polyphenols and total flavonoids. Indeed, many studies attribute the reducing power to phenolic compounds (Fabri et al., Citation2009; H. Li & Wang, Citation2009). This property may provide protection to affected organs through the ability of the plant extract to scavenge harmful metabolites released during disease states (Ajiboye et al., Citation2016).
3.2. Evolution of the fasting blood glucose
presents the changes in the fasting blood glucose (FBG) concentration in the different groups throughout the treatment period. A significant reduction of the FBG was observed in all groups, except untreated diabetic rats (T+) which experienced a continuous increase in their FBG during the experiment. The concomitant intake of metformin and the different teas of C. aconitifolius significantly decreased the FBG of the diabetic rats than treatment with metformin alone. That reduction can be attributed to the presence of phenolic compounds in our different samples since polyphenols are able to reduce glycaemia by many mechanisms including an increase in insulin sensitivity. R. Li et al. (Citation2006) postulated that green tea catechins could ameliorate insulin resistance by acting as ligands for peroxisome proliferator-activated receptors with a dual alpha/gamma agonist effect. It was also reported that green tea phytochemicals can prevent intestinal glucose absorption by inhibiting the sodium-dependent glucose transporter in rabbit intestinal epithelial cells (Toolsee et al., Citation2013). Combining this effect with the hypoglycaemic activity of metformin explains the better results obtained with the combined treatment over the sole use of metformin.
Table 1. Changes in the FBG (g/L) during the treatment.
3.3. Effect of treatments on the lipid profile
shows the serum levels of triglycerides, total cholesterol, HDL-cholesterol and LDL-cholesterol, respectively, of the animals after 28 days of treatment. The positive control group (untreated diabetics) showed a significant increase in triglyceride, total cholesterol and LDL cholesterol levels compared to the healthy control group. Diabetes mellitus in adults is often associated with hyperlipidemia, either due to inadequate secretion or insulin resistance. It generally results in an increase in the activity of lipases, which is responsible for disorders of lipid metabolism. It is well known that hyperlipidemia is accepted as a risk factor for cardiovascular disorders (Garg & Grundy, Citation1990).
Figure 6. Serum concentration of triglycerides, total cholesterol, HDL cholesterol and LDL cholesterol in animals after 28 days of treatment. T (-): Healthy control; T (+): untreated diabetics; MET: Metformin; GTC: Green tea; BTC: Black tea. a, b, c and d: On the same graph, bands with different letters differ significantly (p < .05).
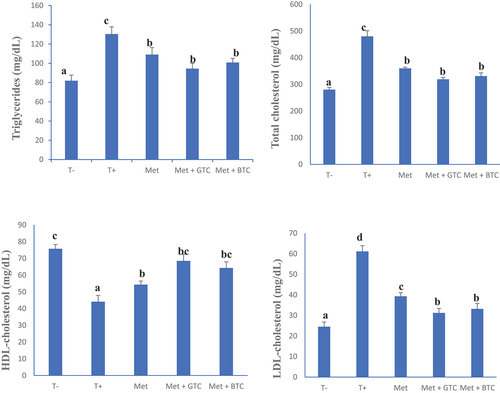
In this study, the treatment of animals by daily administration of different teas (green and black teas) of C. aconitifolius showed a significant decrease in the serum level of triglycerides, total cholesterol and LDL cholesterol, compared to that of the diabetic control group. This decrease could be explained by an inhibition of hepatic synthesis of cholesterol by the phenolic compounds present in the leaves of C. aconitifolius (Oluwatobi et al., Citation2020). In addition, the increase of HDL cholesterol concentrations in animals receiving tea infusions could be related to their polyphenolic content and antioxidant capacity as stated by Asante et al. (Citation2016) who showed that phenolic compounds have the ability to increase serum HDL cholesterol levels. The drop in the LDL level in the animals receiving chaya “teas” could be explained by the presence (in high quantities) of compounds with a strong hypocholesterolemic and hypolipidemic power, in particular phenolic compounds. The drop in LDL levels is in agreement with those of Guevara-Cruz et al. (Citation2021) who showed that chaya leaves lead to a drop in LDL levels in subjects with dyslipidemia.
3.4. Effect of treatments on serum transaminases and total proteins
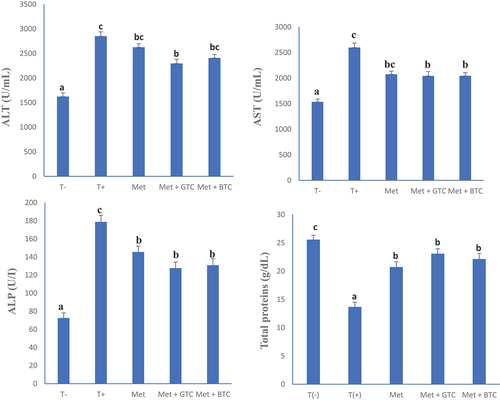
The liver is the central organ involved in metabolic processes in the body and is affected by the complications of diabetes, as increased transaminase activities have been shown to cause liver damage (Hultcrantz et al., Citation1986). shows the serum level of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and total protein of the animals after 28 days of treatment. A significant decrease in the level of serum transaminases and alkaline phosphatase was observed in this work compared to untreated diabetics, which could reflect that the various “teas” of C. aconitifolius would correct the disorders induced by diabetes in rats, this by regulating alterations in liver enzymes and protecting cell membrane permeability or avoiding liver cell rupture (Maria & Maira, Citation2020). These results are in agreement with Chukwu et al. (Citation2020) who reported that the aqueous and ethanolic extracts of the leaves of C. aconitifolius could have dose-dependent hepatoprotective potentials. Untreated animals had significantly elevated serum ALT, AST and alkaline phosphatase levels compared to healthy controls. An increase in the activity of these enzymes reflects active damage and inflammatory hepatocellular disorders (Hultcrantz et al., Citation1986). In addition, an increase in serum protein levels observed in this work would confirm that bioactive compounds present in various “teas” of C. aconitifolius improve diabetes-related disorders. Raza and John (Citation2007) reported that tea catechins can prevent protein degradation by altering subcellular reactive oxygen species production, glutathione metabolism and cytochrome P450 2E1 activity.
Figure 7. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and total protein after 28 days of treatment. T (-): Healthy control; T (+): untreated diabetics; MET: Metformin; GTC: Green tea; BTC: Black tea. a, b and c: On the same graph, bands with different letters differ significantly (p < .05).
3.5. Effect of treatments on serum urea and creatinine
shows serum urea and creatinine concentrations of animals after 28 days of treatment. An increase in the creatinine and urea levels in untreated diabetic animals was observed. This could be linked to renal dysfunction in untreated diabetics, which is due to the nephrotoxicity of streptozotocin.
Figure 8. Serum urea and creatinine concentrations in animals after 28 days of treatment. T (-): Healthy control; T (+): untreated diabetics; MET: Metformin; GTC: Green tea; BTC: Black tea. a and b: On the same graph, bands with different letters differ significantly (p < .05).
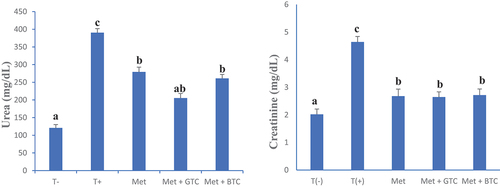
In this work, a significant decrease in the level of urea and serum creatinine was observed in the animals having received the “teas” of C. aconitifolius leaves. This could be justified by the high content of bioactive compounds, in particular phenolic compounds. The present results corroborate those of Oyagbemi & Odetola (Citation2013) who showed that C. aconitifolius has a nephroprotective effect. No significant difference was observed between animals treated with green tea and black tea from C. aconitifolius.
4. Conclusion
Simultaneous intake of green and black teas produced from C. aconitifolius leaves with metformin treatment better improved the health outcomes of type 2 diabetic rats than the treatment with metformin alone. There was a significant decrease in the fasting blood glucose, total and LDL cholesterol and triglyceride levels of diabetic rats after 28 days of treatment compared to animals treated with metformin alone. Green and black tea infusions decreased serum triglycerides, total and LDL cholesterol, ALT, AST, creatinine and urea concentration in the serum of diabetic rats. Furthermore, their consumption also increased the HDL cholesterol and the total protein concentrations. Consumption of black and green teas made from C. aconitifolius leaves potentiates the antidiabetic activity of metformin.
Authors contribution
Woumbo Cerile Ypolyte conceived the work, supervised data analysis and interpretation and read the paper. Mahammad Al-Mansour, Tekou Florian Armel, Kentsop Michel Peguy and Djuine Vanessa collected leaves samples, produced tea infusions, carried out experimentations, analyzed and interpreted data and wrote the paper. Kuate Dieudonné supervised the work and read the paper. All authors have approved the final article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ajiboye, B., Ojo, O., Adeyonu, O., Imiere, O., Olayide, I., Fadaka, A., & Oyinloye, B. (2016). Inhibitory effect on key enzymes relevant to acute type-2 diabetes and antioxidative activity of ethanolic extract of Artocarpus heterophyllus stem bark. Journal of Acute Disease, 5(5), 423–429. https://doi.org/10.1016/j.joad.2016.08.011
- Asante, D. B., Effah-Yeboah, E., Barnes, P., Abban, H. A., Ameyaw, E. O., Boampong, J. N., Ofori, E. G., & Dadzie, J. B. (2016). Antidiabetic effect of young and old ethanolic leaf extracts of Vernonia amygdalina: A comparative study. Journal of Diabetes Research, 2016, 8252741. https://doi.org/10.1155/2016/8252741
- Bartels, H., Böhmer, M., & Heierli, C. (1972). Serum creatinine determination without protein precipitation. Clinica Chimica Acta, 37, 193–197. https://doi.org/10.1016/0009-8981(72)90432-9
- Chukwu, E., Osuocha, K., & Iwueke, A. (2020). Phytochemical profiling, body weight effect and anti-hypercholesterolemia potentials of Cnidoscolus aconitifolius leaf extracts in male albino rat. Journal of Pharmacognosy and Phytotherapy, 12(2), 19–27. https://doi.org/10.5897/JPP2016.0436
- Dohou, N., Yamni, K., Tahrouch, S., Hassani, L. M. I., Badoc, A., & Gmira, N. (2003). Screening of a phytochemical endemic ibero-Moroccan Thymelaea lythroides. Bulletin de la Société de Pharmacie, 142, 61–78.
- Fabri, R., Nogueira, M., Braga, F. G., Coimbra, E. S., & Scio, E. (2009). Mitracarpus frigidus aerial parts exhibited potent antimicrobial, antileishmanial, and antioxidant effects. Bioresource Technology, 100(1), 428–433. https://doi.org/10.1016/j.biortech.2008.05.053
- Fossati, P., & Principe, L. (1982). Serum triacylglycerols determined colorimetrically with an enzyme that produces hydrogen peroxide. Clinical Chemistry, 28(10), 2077–2080. https://doi.org/10.1093/clinchem/28.10.2077
- Friedewald, W., Levy, R., & Frederickson, D. (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry, 18(6), 499–502. https://doi.org/10.1093/clinchem/18.6.499
- Garg, A., & Grundy, S. M. (1990). Management of dyslipidemia in NIDDM. Diabetes Care, 13(2), 153–169. https://doi.org/10.2337/diacare.13.2.153
- Gornall, A., Bardawill, C., & David, M. (1949). Determination of serum proteins by means of the biuret reaction. The Journal of Biological Chemistry, 177(2), 751–766. https://doi.org/10.1016/S0021-9258(18)57021-6
- Guevara-Cruz, M., Medina-Vera, I., Cu-Cañetas, T. E., Cordero-Chan, Y., Torres, N., Tovar, A. R., Márquez-Mota, C., Talamantes-Gómez, J. M., Pérez-Monter, C., Lugo, R., Gutiérrez-Solis, A. L., & Avila-Nava, A. (2021). Chaya leaf decreased triglycerides and improved oxidative stress in subjects with dyslipidemia. Frontiers in Nutrition, 8, 666243. https://doi.org/10.3389/fnut.2021.666243
- Huang, Y. C., Kao, J. T., & Tsai, K. S. (1997). Evaluation of two homogeneous methods for measuring high-density lipoprotein cholesterol. Clinical Chemistry, 43(6 Pt 1), 1048–1055. https://doi.org/10.1093/clinchem/43.6.1048
- Hultcrantz, R., Glaumann, H., Lindberg, G., & Nilsson, L. H. (1986). Liver investigation in 149 asymptomatic patients with moderately elevated activities of serum aminotransferases. Scandinavian Journal of Gastroenterology, 21(1), 109–113. https://doi.org/10.3109/00365528609034632
- International Diabetes Federation. (2021). The IDF diabetes atlas 10th edition 2021. Retrieved February 9, 2022, form www.diabetesatlas.org/fr/.
- Iyke, W., Nwafor, A., Njoku, B., Nwoke, K., & Deebii, N. (2018). Investigation of phytocomponents and hypoglycaemic effect of hydro-methanolic leaf extract of Cnidoscolus aconitifolius (Spinach Tree) in streptozotocin induced-diabetic Wistar rats. Journal of Complementary and Alternative Medical Research, 5(3), 1–9. https://doi.org/10.9734/JOCAMR/2018/26858
- Katalinic, V., Milos, M., & Jukic, M. (2006). Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chemistry, 94(4), 550–557. https://doi.org/10.1016/j.foodchem.2004.12.004
- Kuate, D., Kengne, A., Biapa, C., Azantsa, B., & Muda, W. (2015). Tetrapleura tetraptera spice attenuates high-carbohydrate, high-fat diet-induced obese and type 2 diabetic rats with metabolic syndrome features. Lipids in Health and Diseases, 14(1), 50. https://doi.org/10.1186/s12944-015-0051-0
- Li, R., Douglas, T., Maiyoh, G., Adeli, K., & Theriault, A. (2006). Green tea leaf extract improves lipid and glucose homeostasis in a fructose-fed insulin-resistant hamster model. Journal of Ethnopharmacology, 104(1–2), 24–31. https://doi.org/10.1016/j.jep.2005.08.045
- Li, H., & Wang, X. (2009). Polyphenolic compounds and antioxidant properties of selected China wines. Food Chemistry, 112(2), 454–460. https://doi.org/10.1016/j.foodchem.2008.05.111
- Maria, L., & Maira, R. (2020). Renal and hepatic disease: Cnidoscolus aconitifolius as diet therapy proposal for prevention and treatment. Journal of the American College of Nutrition. https://doi.org/10.1080/07315724.2020.1810171
- Marshall, E. (1913). A new method for the determination of urea in blood. The Journal of Biological Chemistry, 15(3), 487. https://doi.org/10.1016/S0021-9258(18)88512-X
- Mensor, L., Menezez, F., Leitao, G., Reis, A., Dos Santos, T., Coube, C., & Leitao, S. (2001). Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytotherapy Research, 15(2), 127–130. https://doi.org/10.1002/ptr.687
- Novelli, E., Diniz, Y., Galhardi, C., Ebaid, G., Rodrigues, H., Mani, F., Cicogna, A. C., Novelli Filho, J. L. V. B., & Fernandes, A. A. H. (2007). Anthropometrical parameters and markers of obesity in rats. Laboratory Animals, 41(1), 111–119. https://doi.org/10.1258/002367707779399518
- OECD. (2008). Guidelines for chemicals trials: Repeated dose oral toxicity studies for 28 days in rodents. http://www.oecd-ilibrary.org.
- Ojo, A., Ojo, O., Okesola, M., Ajiboye, B., & Oyinloye, B. (2019). Garcinia kola extracts improve biochemical markers associated with erectile function: Possible applications in clinical treatment? Acta Facultatis Naissenis, 36(1), 15–26. https://doi.org/10.2478/afmnai-2019-0002
- Ojo, O., Oloyede, O., Tugbobo, O., Olarewaju, O., & Ojo, A. (2014). Antioxidant and inhibitory effect of scent leaf (Ocimum gratissimum) on Fe2+ and sodium nitroprusside induced lipid peroxidation in rat brain in vitro. Advances in Biological Research, 8(1), 8–17. https://doi.org/10.5829/idosi.abr.2014.8.1.8145
- Oluwatobi, T., Oluseyi, A., Regina, N., & Mopelola, A. (2020). Cnidoscolus aconitifolius leaf extract exhibits comparable ameliorative potentials with ascorbate in dimethylnitrosamine-induced bone marrow clastogenicity and hepatotoxicity. Clinical Nutrition Experimental, 29, 36–48. https://doi.org/10.1016/j.yclnex.2019.11.003
- Oyagbemi, A., & Odetola, A. (2013). Hepatoprotective and nephroprotective effects of Cnidoscolus aconitifolius in protein energy malnutrition induced liver and kidney damage. Pharmacognosy Research, 5(4), 260. https://doi.org/10.4103/0974-8490.118817
- Oyaizu, M. (1986). Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Japanese Journal of Nutrition, 44(6), 307–315. https://doi.org/10.5264/eiyogakuzashi.44.307
- Perez, Z., Macias, M., Hernandez, S., Siordia, A., & Jimenez, M. (2019). Additional compounds and the therapeutic potential of Cnidoscolus chayamansa (McVaugh) against hepatotoxicity induced by antitubercular drugs. Biomedicine and Pharmacotherapy, 117, 109140. https://doi.org/10.1016/j.biopha.2019.109140
- Potier, L. (2014). Role of the kallikrein-kinin system in diabetes and its complications [ Doctoral dissertation, Paris 6].
- Raza, H., & John, A. (2007). In vitro protection of reactive oxygen species-induced degradation of lipids, proteins and 2- deoxyribose by tea catechins. Food and Chemical Toxicology, 45(10), 1814–1820. https://doi.org/10.1016/j.fct.2007.03.017
- Roy, D., Ferdioussi, N., Khatun, T., & Moral, M. (2016). Phytochemical screening, nutritional profile and anti-diabetic effect of ethanolic leaf extract of Cnidoscolus aconitifolius in streptozotocin induce diabetic mice. International Journal of Basic & Clinical Pharmacology, 5(5), 2244–2250. https://doi.org/10.18203/2319-2003.ijbcp20163269
- Temiz, M. (2021). Antioxidant and antihyperglycemic activities of Scorzonera cinerea radical leaves in streptozotocin-induced diabetic rats. Acta Pharmaceutica, 71(4), 603–617. https://doi.org/10.2478/acph-2021-0045
- Toolsee, N. A., Aruoma, O. I., Gunness, T. K., Kowlessur, S., Dambala, V., Murad, F., Googoolye, K., Daus, D., Indelicato, J., Rondeau, P., Bourdon, E., & Bahorun, T. (2013). Effectiveness of green tea in a randomized human cohort: Relevance to diabetes and its complications. BioMed Research International, 2013, 412379. https://doi.org/10.1155/2013/412379
- Trinder, P. (1969). Determination of glucose in blood using glucose oxidase with alternative oxygen acceptor. Annals of Clinical Biochemistry, 6(1), 24–27. https://doi.org/10.1177/000456326900600108
- Veljković, J. N., Pavlović, A., Mitic, S., Tošić, S., Stojanović, G., Kaličanin, B. M., Stanković, D., Stojkovic, M., Mitić, M., & Brcanović, J. M. (2013). Evaluation of individual phenolic compounds and antioxidant properties of black, green, herbal and fruit tea infusions consumed in Serbia: Spectrophotometrical and electrochemical approaches. Journal of Food and Nutrition Research, 52(1), 12–24.
- World Health Organisation. (2022). Diabetes. Retrieved January 14, 2023, from https://www.who.int/news-room/fact-sheets/detail/diabetes.
- Woumbo, C., Kuate, D., Klang, M., & Womeni, H. (2021). Valorization of Glycine max (Soybean) seed waste: Optimization of the microwave-assisted extraction (MAE) and characterization of polyphenols from soybean meal using response surface methodology (RSM). Journal of Chemistry, 2021, 12. https://doi.org/10.1155/2021/4869909
- Woumbo, C. Y., Kuate, D., Metue Tamo, D. G., & Womeni, H. M. (2022). Antioxidant and antidiabetic activities of a polyphenol rich extract obtained from Abelmoschus esculentus (okra) seeds using optimized conditions in microwave-assisted extraction (MAE). Frontiers in Nutrition, 9, 1030385. https://doi.org/10.3389/fnut.2022.1030385
- Woumbo, C., Kuate, D., & Womeni, H. (2017). Cooking methods affect phytochemical composition and anti-obesity potential of soybean (Glycine max) seeds in Wistar rats. Heliyon, 3(8), e00382. https://doi.org/10.1016/j.heliyon.2017.e00382
- Zhao, C. N., Tang, G. Y., Cao, S. Y., Xu, X. Y., Gan, R. Y., Liu, Q., Mao, Q. Q., Shang, A., & Li, H. B. (2019). Phenolic profiles and antioxidant activities of 30 tea infusions from green, black, oolong, white, yellow and dark teas. Antioxidants (Basel, Switzerland), 8(7), 215. https://doi.org/10.3390/antiox8070215