ABSTRACT
Immunosenescence is the degeneration of immunological organs and cells due to aging. Some plants and fungi may have anti-aging benefits through regulating immunosenescence. In this study, mice were fed with two dietary supplement products: Zeng Jian Health Tonic (ZJ) contains extracts from Lentinula edodes, Poria cocos, Tremella fuciformis, cassia seed, Flammulina velutipes, Fructus lycii, chrysanthemum, and mulberry. Zhen Yuan Capsule (ZY) contains extracts from Ganoderma lucidum, Semen cuscutae, Poria cocos, Schisandra chinensis, Codonopsis pilosula, and Atractylodes macrocephala. In addition, the immune organ index, immune cell functions, inflammatory cytokines, and antioxidant enzyme activities were evaluated. The combination of ZJ and ZY could restore the function of lymphocytes and NK cells; decrease the level of pro-inflammatory cytokines including IL-1α, IL-1β, and GM-CSF; increase the level of anti-inflammatory cytokine IL-5; and enhance superoxide dismutase and glutathione peroxidase activities. Therefore, combining ZJ with ZY has anti-aging effects on elderly mice.
1. Introduction
The rate of global population aging is increasing as medical technology advances. The population aged 65 and older is predicted to rise from 10% in 2022 to 16% in 2050, roughly equaling the population under the age of 12 (United Nations Department of Economic and Social Affairs, Population Division, Citation2022). Therefore, aging will continue to be an economic and social burden. Throughout the course of aging, the immune system undergoes continual remodeling in response to internal and external stresses. This process is known as immunosenescence. Consequently, it increases the inflammatory response and causes oxidative damage, which promotes chronic diseases associated with aging, such as cancer, diabetes, Alzheimer’s disease, cardiovascular disease, osteoporosis, and infectious and autoimmune diseases (Alexander et al., Citation2018; Bauer & Fuente, Citation2016; Santoro et al., Citation2021). As demonstrated by the COVID-19 pandemic, the vast majority of inpatient deaths caused by viral infection occur among the elderly, particularly those with chronic diseases (Y. Chen et al., Citation2021). Hence, using a food supplement with few side effects to delay immunosenescence may be a readily acceptable and effective way of reducing the social and economic burden of aging.
Immunosenescence is characterized by a dysfunction in the innate and adaptive immune systems. NK cells, as a representative of innate immune cells, play a crucial role in combating cancer, illness, aging, and infection. As more researchers examine the relationship between aging and changes in NK cell function, they discover that NK cell cytotoxicity decreases with age, thereby increasing the risk of viral infection and the accumulation of senescent and cancer cells in older people (Brauning et al., Citation2022). Thymus atrophy is a major contributor to adaptive immunological failure because it reduces naïve T-cell generation, T-cell receptor diversity, T-cell proliferative capability, etc. (Alves & Bueno, Citation2019). A decline in innate or adaptive immunity leads to an increase in the pro-inflammatory cytokine level and a decrease in the anti-inflammatory cytokine level. This disorder is known as “inflammaging” (Franceschi et al., Citation2000). Apart from inflammaging, oxidative damage to proteins, lipids, and carbohydrates during aging is also related to immune system failure (X. Li et al., Citation2020). Therefore, immunosenescence can be delayed by managing the aforementioned essential mechanism, such as preserving immune cell activity, balancing the release of pro-inflammatory and anti-inflammatory cytokines, and reducing oxidative damage.
Although Western medicine with an anti-aging effect has made significant progress, its adverse effects and multidrug resistance remain to be an issue. By contrast, food supplement contains extracts from plants or fungi that have a multi-target mechanism, a low rate of side reactions, and other qualities that could lead to anti-aging advancements (Shen et al., Citation2017). Ingredients from plants or fungi can combat aging via four primary mechanisms (1) The antioxidant effect is accomplished by augmenting the concentration and activity of many antioxidant enzymes. Polysaccharides from Tricholoma lobayense and Astragalus could increase the levels of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) in mice liver to limit oxidative damage to tissues and organs (Ding et al., Citation2016; X. T. Li et al., Citation2012). (2) Reducing pro-inflammatory cytokine levels or increasing anti-inflammatory cytokine levels could improve inflammaging. For example, ginsenoside Rg1 reduced the levels of proinflammatory cytokines, such as IL-6, IL-1β, and tumor necrosis factor (TNF-α), to promote healthy aging (Y. Yang et al., Citation2017). In addition, extracts of Apocynum venetum tea containing multiple compounds, such as neochlorogenic acid, rutin, and isoquercitrin among many others, enhanced the equilibrium between pro- and anti-inflammatory responses in mice (C. Li et al., Citation2019). (3) Immune aging could be regulated by restoring immune cell function. Evidence shows that Korean red ginseng, which includes ingredients such as ginsenosides Rg1, Rb1, and Rg3, and carbohydrate, increases the percentage of regulatory T-cells and cytotoxic natural killer (NK) cells in the spleen of elderly mice (Shin et al., Citation2020). Moreover, Dendrobium polysaccharides promotes lymphocyte proliferation in aged mice (Wang et al., Citation2018). (4) The balance of intestinal flora could be regulated to delay the aging of the immune system by taking particular herbal extracts, such as Paeonia lactiflora petal flavonoid extract (L. Liu et al., Citation2021) and bilberry anthocyanin extract (J. Li et al., Citation2019).
This research aimed to evaluate the anti-aging effect of two food supplement items, Zeng Jian Heath Tonic and Zhen Yuan Capsule, on immunosenescence in elderly mice. Previous research has shown that the ingredients in Zeng Jian Heath Tonic (mushroom polysaccharide (S. Chen et al., Citation2020), Poria cocos (X. Li et al., Citation2019), Tremella fuciformis (D. Yang et al., Citation2019), cassia seed (Feng et al., Citation2016), Flammulina velutipes (Tang et al., Citation2016), Fructus lycii (Neelam et al., Citation2021), chrysanthemum (X. Zhang et al., Citation2019), and mulberry (Chang et al., Citation2021)) and the ingredients in Zhen Yuan Capsule (Ganoderma lucidum (Pan & Lin, Citation2019; Yuan et al., Citation2020), Semen cuscutae (Y. Yang et al., Citation2022), Poria cocos (X. Li et al., Citation2019), Schisandrae chinensis (X. Li et al., Citation2020), Codonopsis pilosula (P. Zhang et al., Citation2017), and Atractylodes macrocephala (X. Li et al., Citation2022)) could regulate immune activity to promote healthy aging. However, the synergistic immunomodulatory effect of these ingredients remains unknown. In this study, mice were treated intragastrically with these compounds to detect immune cell activity, inflammatory cytokines, and antioxidant enzymes and determine their effect on aging regulation. In addition, our research provides references for the development of anti-aging dietary supplements and medications.
2. Materials and methods
2.1. Materials and chemicals
Zeng Jian Health Tonic contains extracts from Lentinula edodes, Poria cocos, Tremella fuciformis, cassia seed, Flammulina velutipes, Fructus lycii, chrysanthemum, and mulberry. Zhen Yuan Capsule contains extracts from Ganoderma lucidum, Semen cuscutae, Poria cocos, Schisandra chinensis, Codonopsis pilosula, and Atractylodes macrocephala. The two dietary supplement products were from Infinitus (China) Company Ltd. RPMI 1640 Medium and Fetal bovine serum (FBS) were purchased from Gibco (U.S.A). Phosphate buffer was purchased from Hyclone (U.S.A). MTT Cell Proliferation and Cytotoxicity Assay Kit (C0009M) was supplied by Beyotime Biotechnology (Shanghai, China). Yac-1 cells were provided by Center for excellence in molecular cell science, CAS (TCM28) (Shanghai, China). LDH Cytotoxicity Assay Kit (C0017) and BCA Protein Assay Kit (P0011) were purchased form Beyotime (Shanghai, China). Superoxide Dismutase (SOD), glutathione peroxidase (GSH-Px) kit were the products of Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Bio-Plex Cytokine Express 10-plex was purchased from BIO-RAD (U.S.A). Concanavalin (ConA) was purchased from Sigma-Aldrich Co. (St. Louis, Mo, U.S.A).
2.2. Animal experiment and sample collection
Male and female C57BL/6JNifdc adult (aged four months; n = 5) and aged mice (aged 24 months; n = 5) were purchased from Zhejiang Vital River Laboratory Animal Technology Co., Ltd. and housed in a specific pathogen-free animal facility on a 12 h light/dark cycle with free access to food and water. As shown in , mice were divided into five groups and fed with ZJ(1055 mg/kg), ZY(369 mg/kg), ZJ(1055 mg/kg)+ZY(369 mg/kg) or phosphate-buffered saline (PBS) intragastrically once per day (200 µL/mouse) for 35 days (). Following a course of intragastric treatment lasting 35 days, the blood from the orbital venous plexus, spleen, thymus, and lung tissue was collected from euthanized mice. Furthermore, the immune organ index, the proliferative capacity of spleen lymphocytes, the cytotoxicity of spleen NK cells, the cytokines secreted in serum, and the SOD and GSH-Px enzyme activities in lung tissues were detected. The study protocol was approved by the Institutional Animal Ethics Committee of Foshan Huamio Biotechnology Company Ltd (Approval number: No.00295716).
Table 1. Animal grouping.
Table 2. Conversion dose in mice based on body surface area (The dose used in mice was calculated by multiplying human dose by 12.3 (Food and Drug Administration, Citation2005)).
2.3. Immune organ index
Mice were weighed before euthanasia. After rinsing with PBS, the spleen and thymus were dried and weighed. Thymus and spleen indices of mice were calculated in accordance with the ratio of thymus weight (milligrams)/body weight (kilograms) and spleen weight (milligrams)/body weight (kilograms), respectively.
2.4. Lymphocyte proliferation assay
The freshly isolated spleen was placed in a Petri dish containing 4 mL of RPMI-1640 and was gently grated with the piston of a syringe. The cell suspension was mixed in the dish and transferred to a centrifuge tube containing 4 mL of Ficoll solution. After centrifugation at 400×g for 35 min at 18°C, the milky white layer cells were aspirated into another centrifuge tube. The cell was washed two times with RPMI-1640, and the supernatant was removed after centrifugation. The proliferative capacity of T-cells was measured by the MTT assay. In brief, the cell concentration was adjusted to 1 × 106 cells/mL. Afterward, 100 µL of cell suspension per well was added into a 96-well plate. Then, 20 µL of ConA with a final concentration of 5 μg/mL was added to each well except for the control well (20 μL of medium was added to the control well), and each well was assayed in duplicate. After incubation at 37°C for 48 h, the absorbance value for each well was measured at 570 nm. The proliferative capacity of splenocytes was expressed by using the stimulus index (SI), which was calculated as follows: SI = Absorbance of sample well/Absorbance of control well.
2.5. NK cell cytotoxicity assay
Target cells (YAC-1) were cultured for 24 h and washed three times with RPMI-1640. Afterward, the cell concentration was adjusted to 1 × 105 cells/mL for target cells and 5 × 106 cells/mL for effector cells (splenic lymphocytes). Then, 100 μL of effector cells and 100 μL of target cells (effector cells:target cells = 50:1) were added into each well of a 96-well plate. Two control wells for the natural release of target cells (100 μL of target cells +100 μL of RPMI-1640) and for maximum release (100 μL of target cells +80 μL of RPMI-1640) were set. The plate was incubated at 37°C for 4 h. One hour before the end of incubation, the plate was removed from the incubator, and 20 μL of LDH release reagent was added. Cytotoxicity was measured and calculated as follows: Cytotoxicity (%) = (Absorbance of sample well − Absorbance of natural release well/(Absorbance of maximum release well − Absorbance of natural release well).
2.6. Determination of SOD and GSH-Px activities in lung tissues
For lung homogenate preparation, mouse lungs were washed with PBS to remove blood and then centrifuged at 3000 rpm for 10 min. The supernatant was used to determine the content of SOD and GSH-Px following the instruction of the SOD and GSH-Px kit from Nanjing Jiancheng Bioengineering Institute.
2.7. Cytokines secreted in serum
Mouse peripheral blood was centrifuged at 3500 rpm for 10 min at 4°C after standing for 1 h at room temperature to obtain serum. Cytokine concentrations (GM-CSF, INF-γ, IL-1α, IL-1β, TNF-α, IL-2, IL-6, IL-4, IL-5, IL-10) in serum were detected using the Bio-Plex Cytokine Express 10-plex detection kit.
2.8. Statistical analysis
All data are presented in mean±SD and were analyzed using Prism 9 (GraphPad Software Inc., San Diego, CA). Data were analyzed by one-way ANOVA or Brown – Forsythe and Welch ANOVA tests, followed by Dunnett’s post-hoc test for comparisons among multiple groups. Differences were considered significant when the value of P < .05.
3. Results
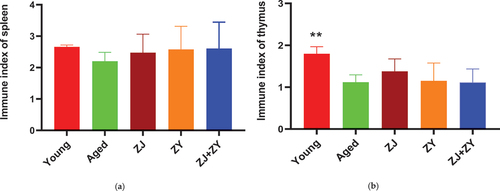
3.1. No major changes in immune organs
The spleen and thymus undergo structural changes as a result of aging, which affect the activity of immune cells and eventually lead to a decline in immunological response and function (Pangrazzi & Weinberger, Citation2020; Sun et al., Citation2018). As shown in , the spleen index of elderly control mice was lower than that of young control mice. The spleen index was higher in the three product-treated groups than in the age-controlled group. However, no statistically significant difference was found. Similarly, the elderly control group had a lower thymus index than the young control group. Compared with the elderly control group, the ZJ group exhibited a slightly greater thymus index, but the difference was not statistically significant (). These results indicated that a single product or a combination of products can mitigate the structural alteration of the spleen associated with aging to a certain extent, but this treatment had almost little effect on the thymus.
Figure 1. Immune organ index. Body weight, spleen weight, and thymus weight of mice were weighed. The spleen index (a) and thymus index (b) were calculated using the following formula: spleen index = spleen weight (milligrams)/body weight (kilograms); thymus index = thymus weight (milligrams)/body weight (kilograms).
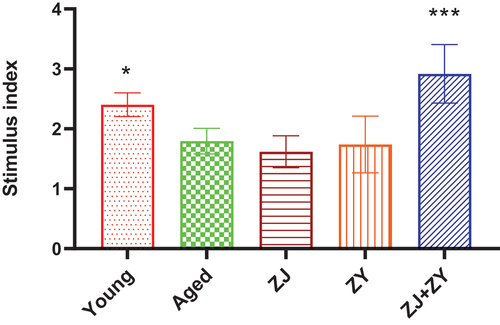
3.2. Combination of ZJ and ZY effectively facilitated T-cell proliferation in aged mice
Aging reduces the proliferative activity of splenic lymphocytes, which reflects the regenerative and rejuvenated function of immune cells (Yousefzadeh et al., Citation2021). As shown in , the ability of aged control mice to proliferate lymphocytes in the spleen was less than that of their younger counterparts. The ZJ+ZY treatment had a substantial influence on the proliferation of splenic lymphocytes, whereas other treatments had no significant effect. Based on these findings, the combination of ZJ and ZY can effectively restore the decrease in lymphocyte activity.
Figure 2. Lymphocyte proliferation in the spleen. Lymphocyte proliferation was detected following the preparation of splenic lymphocytes using the MTT assay. The proliferation ability was expressed by using the stimulus index (Si=absorbance of sample well/Absorbance of control well).
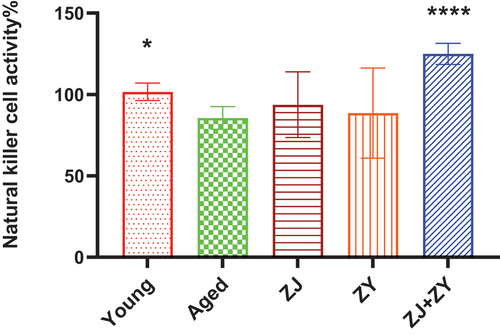
3.3. Combination of ZJ and ZY significantly enhanced the lytic function of NK cells in aged mice
NK cells play a crucial role in the innate immune response as they directly release lysis granzymes or induce apoptosis by expressing Fas ligand (Prager & Watzl, Citation2019; Ritter et al., Citation2022; Terrén et al., Citation2019). Yac-1 cell is a mouse lymphoma cell line that is targeted by NK cells; thus, it is used to investigate the activity of NK cells (Elsner & Dressel, Citation2020). As shown in , elderly animals exhibited impaired NK cell cytotoxicity in the absence of any treatment, which is consistent with previous findings (Chiu et al., Citation2013). The ZJ+ZY treatment considerably increased the activity of NK cells within the spleen compared with the elderly control group. By contrast, a few mice in the two groups treated with a single product displayed NK activation. Based on these results, ZJ+ZY treatment greatly restores the activity of NK cells in aged mice.
Figure 3. Lytic activity of splenic NK cells. NK cells in the spleen (effector cells) were co-cultured with YAC-1 (target cells) for 4 h. Cytotoxicity of NK cells was measured and calculated as follows: Cytotoxicity (%) = (Absorbance of sample well − Absorbance of natural release well/(Absorbance of maximum release well − Absorbance of natural release well). Note: compared with the aged group. *: P < .05, ****: P < .0001.
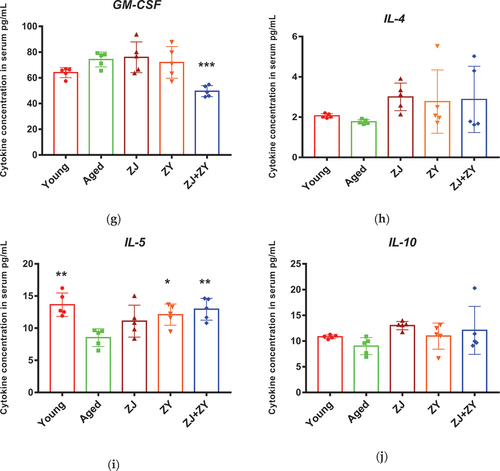
3.4. Combination of ZJ and ZY modulated inflammaging in aged mice
Inflammaging is associated with an increase in the level of pro-inflammatory cytokines and a decrease in the level of anti-inflammatory cytokines during the aging process. With regard to pro-inflammatory cytokines, the ZJ+ZY treatment successfully decreased the levels of IL-1α, IL-1β, and GM-CSF. Meanwhile, ZJ alone suppressed the secretion of IL-1α and IL-1β in the serum of elderly mice. ZY alone showed no effect on other cytokines, except for IL-1α (). With regard to anti-inflammatory cytokines, ZY and ZJ+ZY played a crucial role in boosting IL-5, whereas ZJ alone showed no effect in any cytokine (). The cytokine profiles elicited by single products or product combinations are summarized in . Therefore, ZJ+ZY could observably regulate the equilibrium of inflammatory responses in elderly mice.
Figure 4. Cytokine secretion in serum of mice. Serum was collected following 5-week intragastric administration. Pro-inflammatory cytokines, including IL-1α, IL-1β, IL-2, IL-6, TNF-α, INF-γ, and GM-CSF (a–g), and anti-inflammatory cytokines, including IL-4, IL-5, and IL-10 (h–j), related to aging were detected.
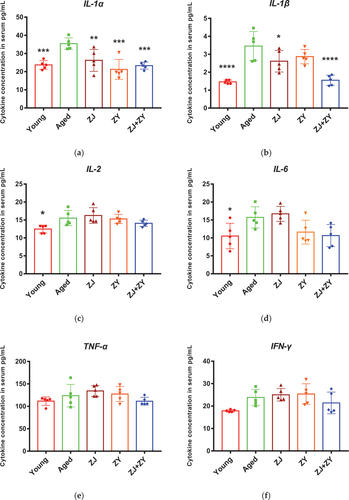
Table 3. Summary of cytokine secretion in food supplement product – treated aged mice.
3.5. Combination products significantly improved the antioxidant enzyme activity in elderly mice
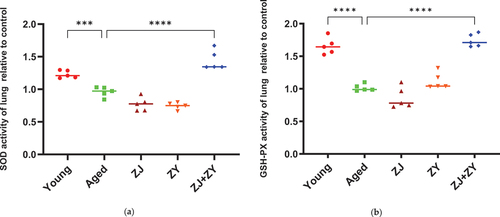
Oxidative damage of proteins, lipids, and carbohydrates, as well as apoptosis caused by high quantities of accumulated oxidative free radicals, are among the causes of immunological failure throughout aging (X. Li et al., Citation2020). SOD is an important antioxidant enzyme that enables the elimination of free radicals during biological oxidation (Sharma et al., Citation2018). GSH-Px is a crucial peroxidase in the body, which can prevent oxidative damage (Mu et al., Citation2021). The results reveal that SOD and GSH-Px enzyme activities of elderly control mice were considerably lower than those of young control mice. In addition, ZJ+ZY treatment remarkably improved the activity of two antioxidant enzymes in elderly mice, whereas the administration of ZJ or ZY alone had little effect on enzyme activity. (). Therefore, a powerful antioxidant effect can be achieved by mixing ZJ with ZY.
Figure 5. Antioxidant enzyme activity in lung tissue of mice. The lungs of mice were collected after 5-week intragastric administration. SOD (a) and GSH-Px (b) enzyme activities were detected using the superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) kit, respectively. Note: compared with the aged group.***: 0.0001 < P < .001, ****: P < .0001.
4. Discussion
The immune system undergoes constant remodeling and adaptation as the body ages. Among the detrimental effects of aging on the immune system are as follows: (1) The gradual atrophy of immunological organs and the rapid degeneration of immune cells. (2)Low-grade, sterile chronic inflammation is generated by the overexpression of pro-inflammatory cytokines and the accumulation of free radicals resulting from the oxidative destruction of proteins, lipids, and carbohydrates (Alexander et al., Citation2018; Santoro et al., Citation2021). Thus, restoring the normal function of immune organs and immune cells, reducing inflammaging, and enhancing the activity of antioxidant enzymes can lessen the harm caused by immunosenescence and promote healthy aging. Based on our study, the combination of ZJ and ZY can repair the function of lymphocytes and NK cells, regulate the balance between pro-inflammatory and anti-inflammatory cytokines, enhance the enzymatic activities of SOD and GSH-Px, and reverse spleen atrophy, thereby promoting healthy aging.
The quality and function of atrophied immunological organs steadily decline with aging. Thus, the immune organ index of the spleen and thymus in mice, which can indicate aging-related changes of immune organs, were measured. It is indicated that the spleen and thymus indices of old mice were lower than those of young mice. Based on previous studies, the spleen and thymus indices can be partially restored by anti-aging Chinese medicine in naturally and chemically induced aged mice models (Dong & Liu, Citation2021; Xi et al., Citation2021; Xiong et al., Citation2011). In this investigation, the spleen index of mice fed with food supplement products or their combination showed only an upward trend probably because the mice used in previous studies were younger than those used in this study, where immunological organ structure is harder to reverse.
In this study, ZJ+ZY had a significantly larger anti-aging effect than either ZJ or ZY alone on all the factors influencing aging. This result may be due to a multi-target mechanism generated by the superposition of herbal and fungal ingredients (Wei et al., Citation2019). Xiangsha Liujunzi could provide patients with functional dyspepsia, a significant symptomatic relief (Lv et al., Citation2017). In addition, the Xiaoyao pill increased the Hamilton Rating Scale score of digestion (Du et al., Citation2014). Therefore, the synergistic effect mechanism must be clarified in the future because of the intricate nature of superposition of herbal and fungal ingredients.
As the subject of research on aging, humans have become complex due to various limiting factors, such as ethical issues, long life spans, and various environmental influences (Mitchell et al., Citation2015). Accordingly, animal models have been established to study aging. Aquatic animals, rodents, and pigs are commonly used to evaluate the effects of herbal medicines. Aquatic animals exhibit the advantages of low breeding cost and high prolificity (Belo et al., Citation2021), and they are suitable for a wide range of studies (Cherr et al., Citation2017; Seto et al., Citation2015; Wang et al., Citation2021; Yu et al., Citation2022). As a representative of aquatic animal models, zebrafish has been used as animal models in the research on aging diseases. However, considering the differences in disease pathogenesis and drug treatment between zebrafish and humans (S. Liu, Citation2022), and the significant environmental effect on their experimental results (Keller & Keller, Citation2018), zebrafish is actually not a good model for human antiaging research. Pigs exhibit the advantage of having certain similarities in anatomy and physiology to humans (Lunney et al., Citation2021; Singh et al., Citation2016). However, given the long breeding cycle and high costs of pigs (Zhu et al., Citation2020), rodents are more commonly used in research on aging diseases than pigs. The genetic proximity of mice to humans, the easy manipulation of the mouse genome, and the availability of many mutants and inbred strains have made mice the most popular mammalian model. In addition, the short life span of mice and the fact that mouse tissues can be analyzed at all stages of aging (Vanhooren & Libert, Citation2013) make mice a potential animal model for studying diseases related to aging.
Institutional review board statement
The study was conducted in accordance with the National Standard of the People’s Republic of China GB/T 35,892–2018 (Laboratory animal-Guideline for ethical review of animal welfare) and approved by the Institutional Animal Ethics Committee of Foshan Huamio Biotechnology Company Ltd. (Approval number: No.00295716).
Acknowledgments
We thank Dong Ding and Zibin Yu for their excellent technical support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data in this study are available on request from the author.
References
- Alexander, J. E., Colyer, A., Haydock, R. M., Hayek, M. G., & Park, J. (2018). Understanding how dogs age: Longitudinal analysis of markers of inflammation, immune function, and oxidative stress. The Journals of Gerontology: Series A, 73(6), 720–728. https://doi.org/10.1093/gerona/glx182
- Alves, A. S., & Bueno, V. (2019). Immunosenescence: Participation of T lymphocytes and myeloid-derived suppressor cells in aging-related immune response changes. Einstein (Sao Paulo), 17(2), eRB4733. https://doi.org/10.31744/einstein_journal/2019RB4733
- Bauer, M. E., & Fuente, M. L. (2016). The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mechanisms of Ageing and Development, 158, 27–37. https://doi.org/10.1016/j.mad.2016.01.001
- Belo, M. A. A., Oliveira, M. F., Oliveira, S. L., Aracati, M. F., Rodrigues, L. F., Costa, C. C., Conde, G., Gomes, J. M. M., Prata, M. N. L., Barra, A., Valverde, T. M., de Melo, D. C., Eto, S. F., Fernandes, D. C., Romero, M. G. M. C., Corrêa Júnior, J. D., Silva, J. O., Barros, A. L. B., Perez, A. C., & Charlie-Silva, I. (2021). Zebrafish as a model to study inflammation: A tool for drug discovery. Biomedicine & Pharmacotherapy, 144, 112310. https://doi.org/10.1016/j.biopha.2021.112310
- Brauning, A., Rae, M., Zhu, G., Fulton, E., Admasu, T. D., Stolzing, A., & Sharma, A. (2022). Aging of the Immune System: Focus on Natural Killer Cells Phenotype and Functions. Cells, 11(6), 1017. https://doi.org/10.3390/cells11061017
- Chang, B. Y., Koo, B. S., & Kim, S. Y. (2021). Pharmacological activities for Morus alba L., focusing on the immunostimulatory property from the fruit aqueous extract. Foods, 10(8), 1966. https://doi.org/10.3390/foods10081966
- Chen, Y., Klein, S. L., Garibaldi, B. T., Li, H., Wu, C., Osevala, N. M., Li, T., Margolick, J. B., Pawelec, G., & Leng, S. X. (2021). Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Research Reviews, 65, 101205. https://doi.org/10.1016/j.arr.2020.101205
- Chen, S., Liu, C., Huang, X., Hu, L., Huang, Y., Chen, H., Fang, Q., Dong, N., Li, M., Tang, W., & Nie, S. (2020). Comparison of immunomodulatory effects of three polysaccharide fractions from Lentinula edodes water extracts. Journal of Functional Foods, 66, 103791. https://doi.org/10.1016/j.jff.2020.103791
- Cherr, G. N., Fairbairn, E., & Whitehead, A. (2017). Impacts of petroleum-derived pollutants on fish development. Annual Review of Animal Biosciences, 5(1), 185–203. https://doi.org/10.1146/annurev-animal-022516-022928
- Chiu, B. C., Martin, B. E., Stolberg, V. R., & Chensue, S. W. (2013). The host environment is responsible for aging-related functional NK cell deficiency. Journal of Immunology, 191(9), 4688–4698. https://doi.org/10.4049/jimmunol.1301625
- Ding, Q., Yang, D., Zhang, W., Lu, Y., Zhang, M., Wang, L., Li, X., Zhou, L., Wu, Q., Pan, W., & Chen, Y. (2016). Antioxidant and anti-aging activities of the polysaccharide TLH-3 from Tricholoma lobayense. International Journal of Biological Macromolecules, 85, 133–140. https://doi.org/10.1016/j.ijbiomac.2015.12.058
- Dong, L., & Liu, C. (2021). Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice. Open Life Sciences, 16(1), 399–407. https://doi.org/10.1515/biol-2021-0046
- Du, H. G., Ming, L., Chen, S. J., & Li, C. D. (2014). Xiaoyao pill for treatment of functional dyspepsia in perimenopausal women with depression. World Journal of Gastroenterology: WJG, 20(44), 16739. https://doi.org/10.3748/wjg.v20.i44.16739
- Elsner, L., & Dressel, R. (2020). 51cr-release to monitor NK cell cytotoxicity. Methods in Enzymology, 631, 497–512. https://doi.org/10.1016/bs.mie.2019.05.037
- Feng, L., Yin, J., Nie, S., Wan, Y., & Xie, M. (2016). Fractionation, physicochemical property and immunological activity of polysaccharides from Cassia obtusifolia. International Journal of Biological Macromolecules, 91, 946–953. https://doi.org/10.1016/j.ijbiomac.2016.05.030
- Food and Drug Administration. (2005). Guidance for industry: Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. https://www.fda.gov/media/72309/download
- Franceschi, C., Bonafè, M., Valensin, S., Olivieri, F., De Luca, M., Ottaviani, E., & De Benedictis, G. (2000). Inflamm‐aging: An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences, 908(1), 244–254. https://doi.org/10.1111/j.1749-6632.2000.tb06651.x
- Keller, J. M., & Keller, E. T. (2018). The use of mature zebrafish (Danio rerio) as a model for human aging and disease. Conn’s Handbook of Models for Human Aging (Second Edition), 351–359. https://doi.org/10.1016/B978-0-12-811353-0.00026-9
- Li, C., Tan, F., Yang, J., Yang, Y., Gou, Y., Li, S., & Zhao, X. (2019). Antioxidant effects of Apocynum venetum tea extracts on d-galactose-induced aging model in mice. Antioxidants, 8(9), 381. https://doi.org/10.3390/antiox8090381
- Li, J., Wu, T., Li, N., Wang, X., Chen, G., & Lyu, X. (2019). Bilberry anthocyanin extract promotes intestinal barrier function and inhibits digestive enzyme activity by regulating the gut microbiota in aging rats. Food & Function, 10(1), 333–343. https://doi.org/10.1039/C8FO01962B
- Li, X., Gao, J., Yu, Z., Jiang, W., Sun, W., Yu, C., Sun, J., Wang, C., Chen, J., Jing, S., & Li, H. (2020). Regulatory effect of anwulignan on the immune function through its antioxidation and anti-apoptosis in D-galactose-induced aging mice. Clinical Interventions in Aging, 15, 97. https://doi.org/10.2147/CIA.S237601
- Li, X., He, Y., Zeng, P., Liu, Y., Zhang, M., Hao, C., Wang, H., Lv, Z., & Zhang, L. (2019). Molecular basis for Poria cocos mushroom polysaccharide used as an antitumor drug in China. Journal of Cellular and Molecular Medicine, 23(1), 4–20. https://doi.org/10.1111/jcmm.13564
- Li, X., Rao, Z., Xie, Z., Qi, H., & Zeng, N. (2022). Isolation, structure and bioactivity of polysaccharides from Atractylodes macrocephala: A review. Journal of Ethnopharmacology, 296, 115506. https://doi.org/10.1016/j.jep.2022.115506
- Li, X. T., Zhang, Y. K., Kuang, H. X., Jin, F.-X., Liu, D.-W., Gao, M.-B., Liu, Z., & Xin, X.-J. (2012). Mitochondrial protection and anti-aging activity of Astragalus polysaccharides and their potential mechanism. International Journal of Molecular Sciences, 13(2), 1747–1761. https://doi.org/10.3390/ijms13021747
- Liu, L., Yuan, Y., & Tao, J. (2021). Flavonoid-rich extract of Paeonia lactiflora petals alleviate d-galactose-induced oxidative stress and restore gut microbiota in ICR mice. Antioxidants, 10(12), 1889. https://doi.org/10.3390/antiox10121889
- Liu, S. (2022). Utilization of zebrafish as a model system in medical research. BIO Integration, 3(4), 188–192. https://doi.org/10.15212/bioi-2022-0019
- Lunney, J. K., Van Goor, A., Walker, K. E., Hailstock, T., Franklin, J., & Dai, C. (2021). Importance of the pig as a human biomedical model. Science Translational Medicine, 13(621). https://doi.org/10.1126/scitranslmed.abd5758
- Lv, L., Wang, F. Y., Ma, X. X., Li, Z.-H., Huang, S.-P., Shi, Z.-H., Ji, H.-J., Bian, L.-Q., Zhang, B.-H., Chen, T., Yin, X.-L., & Tang, X.-D. (2017). Efficacy and safety of Xiangsha Liujunzi granules for functional dyspepsia: A multi-center randomized double-blind placebo-controlled clinical study. World Journal of Gastroenterology, 23(30), 5589. https://doi.org/10.3748/wjg.v23.i30.5589
- Mitchell, S. J., Scheibye-Knudsen, M., Longo, D. L., & de Cabo, R. (2015). Animal models of aging research: Implications for human aging and age-related diseases. Annual Review of Animal Biosciences, 3(1), 283–303. https://doi.org/10.1146/annurev-animal-022114-110829
- Mu, S., Yang, W., & Huang, G. (2021). Antioxidant activities and mechanisms of polysaccharides. Chemical Biology & Drug Design, 97(3), 628–632. https://doi.org/10.1111/cbdd.13798
- Neelam, K., Dey, S., Sim, R., Lee, J., & Au Eong, K.-G. (2021). Fructus lycii: A natural dietary supplement for amelioration of retinal diseases. Nutrients, 13(1), 246. https://doi.org/10.3390/nu13010246
- Pan, Y., & Lin, Z. (2019). Anti-aging Effect of Ganoderma (Lingzhi) with Health and Fitness. Advances in Experimental Medicine and Biology, 1182, 299–309. https://doi.org/10.1007/978-981-32-9421-9_13
- Pangrazzi, L., & Weinberger, B. (2020). T cells, aging and senescence. Experimental Gerontology, 134, 110887. https://doi.org/10.1016/j.exger.2020.110887
- Prager, I., & Watzl, C. (2019). Mechanisms of natural killer cell‐mediated cellular cytotoxicity. Journal of Leukocyte Biology, 105(6), 1319–1329. https://doi.org/10.1002/JLB.MR0718-269R
- Ritter, A. T., Shtengel, G., Xu, C. S., Weigel, A., Hoffman, D. P., Freeman, M., Iyer, N., Alivodej, N., Ackerman, D., Voskoboinik, I., Trapani, J., Hess, H. F., & Mellman, I. (2022). ESCRT-mediated membrane repair protects tumor-derived cells against T cell attack. Science, 376(6591), 377–382. https://doi.org/10.1126/science.abl3855
- Santoro, A., Bientinesi, E., & Monti, D. (2021). Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Research Reviews, 71, 101422. https://doi.org/10.1016/j.arr.2021.101422
- Seto, S. W., Kiat, H., Lee, S. M. Y., Bensoussan, A., Sun, Y.-T., Hoi, M. P. M., & Chang, D. (2015). Zebrafish models of cardiovascular diseases and their applications in herbal medicine research. European Journal of Pharmacology, 768, 77–86. https://doi.org/10.1016/j.ejphar.2015.10.031
- Sharma, G. N., Gupta, G., & Sharma, P. (2018). A comprehensive review of free radicals, antioxidants, and their relationship with human ailments. Critical Reviews™ in Eukaryotic Gene Expression, 28(2), 139–154. https://doi.org/10.1615/CritRevEukaryotGeneExpr.2018022258
- Shen, C. Y., Jiang, J. G., Yang, L., Wang, D.-W., & Zhu, W. (2017). Anti‐ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: Pharmacological mechanisms and implications for drug discovery. British Journal of Pharmacology, 174(11), 1395–1425. https://doi.org/10.1111/bph.13631
- Shin, K. K., Yi, Y. S., Kim, J. K., Kim, H., Hossain, M. A., Kim, J.-H., & Cho, J. Y. (2020). Korean red ginseng plays an anti-aging role by modulating expression of aging-related genes and immune cell subsets. Molecules, 25(7), 1492. https://doi.org/10.3390/molecules25071492
- Singh, V. K., Thrall, K. D., & Hauer-Jensen, M. (2016). Minipigs as models in drug discovery. Expert Opinion on Drug Discovery, 11(12), 1131–1134. https://doi.org/10.1080/17460441.2016.1223039
- Sun, J., Zhang, L., Zhang, J., Ran, R., Shao, Y., Li, J., Jia, D., Zhang, Y., Zhang, M., Wang, L., & Wang, Y. (2018). Protective effects of ginsenoside Rg1 on splenocytes and thymocytes in an aging rat model induced by d-galactose. International Immunopharmacology, 58, 94–102. https://doi.org/10.1016/j.intimp.2018.03.017
- Tang, C., Hoo, P. C. X., Tan, L. T. H., Pusparajah, P., Khan, T. M., Lee, L.-H., Goh, B.-H., & Chan, K.-G. (2016). Golden needle mushroom: A culinary medicine with evidenced-based biological activities and health promoting properties. Frontiers in Pharmacology, 7, 474. https://doi.org/10.3389/fphar.2016.00474
- Terrén, I., Orrantia, A., Vitallé, J., Zenarruzabeitia, O., & Borrego, F. (2019). NK cell metabolism and tumor microenvironment. Frontiers in Immunology, 10, 2278. https://doi.org/10.3389/fimmu.2019.02278
- United Nations Department of Economic and Social Affairs, Population Division. (2022). World population prospects 2022: Summary of results. UN DESA/POP/2022/TR/NO.3.
- Vanhooren, V., & Libert, C. (2013). The mouse as a model organism in aging research: Usefulness, pitfalls and possibilities. Ageing Research Reviews, 12(1), 8–21. https://doi.org/10.1016/j.arr.2012.03.010
- Wang, D., Fan, B., Fang, F., Wang, Y., Xing, C., Zhang, L., & Wang, F. (2018). Advances in researches about immunoregulatory functions of Dendrobium Sw. Medicinal Plant, 9(2), 1–4. https://doi.org/10.19600/j.cnki.issn2152-3924.2018.02.001
- Wang, D., Hu, G., Wang, J., Yan, D., Wang, M., Yang, L., Serikuly, N., Alpyshov, E., Demin, K. A., Galstyan, D. S., Amstislavskaya, T. G., de Abreu, M. S., & Kalueff, A. V. (2021). Studying CNS effects of traditional Chinese medicine using zebrafish models. Journal of Ethnopharmacology, 267, 113383. https://doi.org/10.1016/j.jep.2020.113383
- Wei, J., Man, Q., Guo, F., Xian, M., Wang, T., Tang, C., Zhang, Y., Li, D., Tang, D., Yang, H., & Huang, L. (2019). Precise and systematic survey of the efficacy of multicomponent drugs against functional dyspepsia. Scientific Reports, 9(1), 10713. https://doi.org/10.1038/s41598-019-47300-7
- Xi, W., Song, N., Yan, Q., Liang, H., & Zhang, W. (2021). The analysis of the effects of Liuwei Dihuang decoction on aging-related metabolites and metabolic pathways in naturally aging mice by ultra-performance liquid chromatography quadruple time-of-light mass spectrometry. Physiology and Pharmacology, 72(3), 399–409. https://doi.org/10.26402/jpp.2021.3.09
- Xiong, D., Yu, L. X., Yan, X., Guo, C., & Xiong, Y. (2011). Effects of root and stem extracts of Asparagus cochinchinensis on biochemical indicators related to aging in the brain and liver of mice. The American Journal of Chinese Medicine, 39(4), 719–726. https://doi.org/10.1142/S0192415X11009159
- Yang, D., Liu, Y., & Zhang, L. (2019). Tremella polysaccharide: The molecular mechanisms of its drug action. Progress in Molecular Biology and Translational Science, 163, 383–421. https://doi.org/10.1016/bs.pmbts.2019.03.002
- Yang, Y., Ren, C., Zhang, Y., & Wu, X. (2017). Ginseng: An nonnegligible natural remedy for healthy aging. Aging and Disease, 8(6), 708. https://doi.org/10.14336/AD.2017.0707
- Yang, Y., Wei, Q., An, R., Zhang, H.-M., Shen, J.-Y., Qin, X.-Y., Han, X.-L., Li, J., Li, X.-W., Gao, X.-M., He, J., & Mao, H.-P. (2022). Anti-osteoporosis effect of Semen Cuscutae in ovariectomized mice through inhibition of bone resorption by osteoclasts. Journal of Ethnopharmacology, 285, 114834. https://doi.org/10.1016/j.jep.2021.114834
- Yousefzadeh, M. J., Flores, R. R., Zhu, Y. I., Schmiechen, Z. C., Brooks, R. W., Trussoni, C. E., Cui, Y., Angelini, L., Lee, K.-A., McGowan, S. J., Burrack, A. L., Wang, D., Dong, Q., Lu, A., Sano, T., O’Kelly, R. D., McGuckian, C. A., Kato, J. I., Bank, M. P., … Niedernhofer, L. J. (2021). An aged immune system drives senescence and ageing of solid organs. Nature, 594(7861), 100–105. https://doi.org/10.1038/s41586-021-03547-7
- Yu, Z., Zhao, L., Zhao, J. L., Xu, W., Guo, Z., Zhang, A.-Z., & Li, M.-Y. (2022). Dietary Taraxacum mongolicum polysaccharide ameliorates the growth, immune response, and antioxidant status in association with NF-κB, Nrf2 and TOR in Jian carp (Cyprinus carpio var. Jian). Aquaculture, 547, 737522. https://doi.org/10.1016/j.aquaculture.2021.737522
- Yuan, S., Yang, Y., Li, J., Tan, X., Cao, Y., Li, S., Hong, H.-D., Liu, L., & Zhang, Q. (2020). Ganoderma lucidum Rhodiola compound preparation prevent d-galactose-induced immune impairment and oxidative stress in aging rat model. Scientific Reports, 10(1), 1–11. https://doi.org/10.1038/s41598-020-76249-1
- Zhang, P., Hu, L., Bai, R., Zheng, X., Ma, Y., Gao, X., Sun, B., & Hu, F. (2017). Structural characterization of a pectic polysaccharide from Codonopsis pilosula and its immunomodulatory activities in vivo and in vitro. International Journal of Biological Macromolecules, 104, 1359–1369. https://doi.org/10.1016/j.ijbiomac.2017.06.023
- Zhang, X., Wu, J. Z., Lin, Z. X., Yuan, Q.-J., Li, Y.-C., Liang, J.-L., Zhan, J. Y. X., Xie, Y.-L., Su, Z.-R., & Liu, Y.-H. (2019). Ameliorative effect of supercritical fluid extract of Chrysanthemum indicum Linnén against D-galactose induced brain and liver injury in senescent mice via suppression of oxidative stress, inflammation and apoptosis. Journal of Ethnopharmacology, 234, 44–56. https://doi.org/10.1016/j.jep.2018.12.050
- Zhu, X., Wei, Y., Zhan, Q., Yan, A., Feng, J., Liu, L., & Tang, D. (2020). Crispr/Cas9-mediated Biallelic knockout of IRX3 reduces the production and survival of somatic cell-cloned bama minipigs. Animals (Basel), 10(3), 501. https://doi.org/10.3390/ani10030501