ABSTRACT
Silibinin is a natural flavonoid with anti-diabetic activity. Glucagon-like peptide−1 (GLP−1), an intestinal hormone mainly secreted from L cells, which regulates insulin production and sensitivity, appears to be a potential therapeutic strategy for T2DM. The current study aims to determine the protective effect of silibinin against palmitic acid (PA)-induced damage in GLUTag cells. The results revealed that PA triggered endoplasmic reticulum (ER) stress and apoptosis in GLUTag cells, while silibinin attenuated PA-induced lipotoxicity. Based on the estrogen-like effects of silibinin and the role of estrogen receptors in regulating glycolipid metabolism, the involvement of estrogen receptors in the protective effects of silibinin in GLUTag cells was further investigated. The results showed that estrogen receptor α- and β-specific inhibitors reversed the inhibitory impact of silibinin on ER stress. Our study demonstrated that silibinin protects GLUTag cells from PA-induced injury by decreasing ER stress under the regulation of estrogen receptors α and β.
Introduction
As the most common type of diabetes worldwide, type 2 diabetes mellitus (T2DM) occurs when the body becomes resistant to insulin action or the pancreatic β cells cannot produce sufficient insulin to maintain glucose homeostasis (McAlister et al., Citation2021). Glucagon-like peptide−1 (GLP−1), a peptide hormone produced mainly by intestinal L cells, increases glucose-stimulated insulin secretion, decreases glucagon release and improves insulin resistance by activating the GLP−1 receptor (Bell et al., Citation1983; Meier, Citation2012; Somm et al., Citation2021; E. W. L. Sun et al., Citation2018; Yaribeygi et al., Citation2019). In the early Citation1990s, it was first pointed out that GLP−1 could be a possible target for the therapy of T2DM (Gutniak et al., Citation1992). And the increase in the viability of L cells during the development of T2DM is particularly critical for maintaining endogenous GLP−1 production.
Excessive accumulation of free fatty acids (FFAs) has been demonstrated in several studies to be a major cause of insulin resistance, a phenomenon known as lipotoxicity (Hu et al., Citation2016; Opazo-Rios et al., Citation2020; Ye et al., Citation2019). Palmitic acid (PA, C16:0), one of the most common saturated fatty acids in the diet and serum, has been frequently used to induce the development of lipotoxicity related to T2DM (Barlow et al., Citation2016a; Ly et al., Citation2017; Peng et al., Citation2011; Qureshi et al., Citation2015; Thivolet et al., Citation2017). Endoplasmic reticulum (ER) stress, oxidative stress, mitochondrial damage and inflammation are potential pathways of FFAs-induced β cell death (Lytrivi et al., Citation2020). In a recent investigation, we found that PA treatment caused oxidative stress and apoptosis in the intestinal L cell line GLUTag (J. Wang et al., Citation2020). Thus, we anticipate that PA may generate ER stress in GLUTag cells, which could lead to cell damage.
A buildup of misfolded and unfolded proteins causes ER stress, which triggers the unfolded protein response (UPR), a complicated signaling network (S. J. Wang et al., Citation2020). Upon ER stress, immunoglobulin heavy chain binding protein (BiP) dissociates from protein kinase RNA-like ER kinase (PERK), inositol requiring enzyme−1 (IRE1) and transcription factor 6 (ATF6). Then the sensors activate their respective UPR pathways to relieve ER stress and maintain homeostasis. UPR could shield cells from stress and aid in the restoration of cellular homeostasis. Nonetheless, persistent UPR activation has been linked to a variety of disorders including obesity and T2DM (Kondo, Citation2013; Shah et al., Citation2016; Sozen et al., Citation2015). T2DM is traditionally known as a non-immune disorder, and recent evidence suggests that apoptosis, autophagy and ER stress may be the main factors in the pathogenesis and progression of the chronic metabolic disease (Hoyer-Hansen & Jaattela, Citation2007). Autophagy and apoptosis generally occur together in the same cell, and autophagy occurs first. In some instances, autophagy inhibits apoptosis by degrading misfolded or unfolded proteins, as well as damaged organelles (Maiuri et al., Citation2007; S. L. Song et al., Citation2017).
It has been intensively investigated how estrogen receptors, especially estrogen receptors α and β, affect metabolic pathways in tissues such as the brain, pancreas, and gut (Zhou et al., Citation2018). The function of estrogen receptors α and β in enhancing glucose-stimulated insulin biosynthesis has been reported in several studies, while the functions of estrogen receptors in L cells are poorly understood. Silibinin is a bioactive component of silymarin derived from the seeds of milk thistle (Silibum Marianum). It can activate estrogen receptors to exert a variety of biological activities including hepatoprotective, anti-apoptotic, anti-autophagy, anti-inflammatory and anti-cancer effects (Kumar Singh & Bhushan, Citation2023; Lingling et al., Citation2020; J. Yang et al., Citation2018). Our recent study revealed that the estrogen receptors were involved in the protective effects of silibinin against lipotoxicity in β cells (J. Yang et al., Citation2018). Gut microbiota plays an important role in the pathogenesis and development of various metabolic diseases, such as obesity and diabetes. Recent studies reveal that silibinin can improve liver lipid metabolism and alter gut microbial composition (Sonnenburg & Bäckhed, Citation2016; W.-L. Sun et al., Citation2023). Due to its rapid metabolism and poor solubility, the bioavailability and appropriate therapeutic applications are limited. To accelerate the clinical application of silibinin, the synthesis of nanoformulations and derivatives has been widely studied in recent years. The nanoforms have stronger anti-diabetic and anti-glycating effects due to its better inhibition on α-amylase and α-glucosidase compared to the bulk forms (Khalid & Naseem, Citation2023). In addition, the anti-inflammatory and anti-apoptotic activities of two silibinin derivatives obtained by the Schiff base reaction were significantly improved compared to silibinin (R. Xu et al., Citation2022).
The UPR triggered by ER stress is a defense mechanism in cells, but prolonged ER stress can induce apoptosis and autophagy. In recent years, great progress has been made in the correlation of “endoplasmic reticulum stress-apoptosis-autophagy”, but the specific mechanisms by which ER stress promotes cell survival or apoptosis have not been fully elucidated. In this work, we investigated the involvement of ER stress in PA-induced lipotoxicity and the protective effect of silibinin in GLUTag cells. There are different opinions on whether autophagy plays a protective role in ER-induced apoptosis or further induces apoptosis. Therefore, the role of autophagy in ER stress-induced apoptosis was also investigated. We hypothesized that the effectiveness of silibinin against PA-induced injury is related to the suppression of ER stress through the regulation of estrogen receptors.
Materials and methods
Materials
To generate a 200 mM stock solution of silibinin, 99% pure silibinin (Jurong Best Medicine Material, Jiangsu, China) was dissolved in dimethyl sulfoxide (DMSO). And PA (Sigma-Aldrich, St Louis, MO, U.S.A) was dissolved in 0.1 M sodium hydroxide to generate a 100 mM stock solution. A 40% (w/v) FFA-free bovine serum albumin (BSA) (Sigma-Aldrich, St Louis, MO, U.S.A) solution was made with PBS. The above two solutions were appropriately combined into a 20 mM PA/BSA mixture, and the mixture was conveniently further diluted in low glucose DMEM (Gibco, Grand Island, NY, U.S.A) to obtain the desired final concentrations.
ER-Tracker red and BCA protein assay kits were purchased from Beyotime Institute of Biotechnology (Shanghai, China). Estrogen receptor α-specific antagonist, 1,3-bis(4-hydroxyphenyl)−4-methyl−5-[4-(2-piperidinylethoxy) phenol]−1 H-pyrazole-dihydro-chloride (MPP) and estrogen receptor β full antagonist [2-phenyl−5,7-bis(trifluoromethyl)- pyrazolo[1,5-a] pyrimidin−3-yl] phenol (PHTPP) were obtained from Sigma-Aldrich (St Louis, MO, U.S.A). 4-PBA was purchased from ApexBio (Houston, TX, U.S.A). Hoechst 33,258, acridine orange and 3-methyladenine (3-MA) were obtained from Solarbio (Shanghai, China). Polyclonal rabbit antibodies against BiP and Caspase12 were purchased from Servicebio (Wuhan, China). Monoclonal mouse antibodies against Bax, Bcl2 and phosphorylation of PERK at Thr981 [p-PERK (Thr981)] were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A). Polyclonal rabbit antibodies against CHOP, LC3 and cleaved PARP were obtained from Proteintech Group (Chicago, IL, U.S.A). Polyclonal mouse antibodies against ATF6 and XBP1 were purchased from GeneTex (San Antonio, TX, U.S.A). Polyclonal rabbit antibodies against Beclin1, ATG5, Caspase3 and Caspase9 were obtained from Affinity Biosciences (Cincinnati, OH, U.S.A). 2’,7’-dichlorofluorescein diacetate (DCFH-DA) and Annexin V-FITC/PI were obtained from Meilun Biotech (Dalian, China).
Cell culture and treatment
GLUTag cells were gifted by Prof. Daniel J. Drucker of The University of Toronto, cultured in low glucose DMEM (Gibco, Grand Island, NY, U.S.A) containing 10% heat-inactivated fetal bovine serum (FBS, Clark, Richmond, VA, U.S.A), 100 U/mL penicillin and streptomycin, then incubated at 37°C with a humidified atmosphere containing 5% CO2. The dose and duration of silibinin treatment and PA administration were selected based on our previous study (J. Y. Wang et al., Citation2022).
Hoechst 33258 staining
GLUTag cells were seeded at 5.0 × 104 per well into 24-well cell culture plates and cultured for 24 h. The cells were then washed with PBS three times and stained with Hoechst 33258 (1 μg/mL) at 37°C for 15 min. The apoptotic cells, which showed densely staining nuclei, were observed using a fluorescence microscope (Leica, Wetzlar, Germany).
Acridine orange staining
The treated cells were stained with 1 mg/mL acridine orange (AO) dye solution (Solarbio, Shanghai, China) and incubated at 37°C for 15 minutes. The cells were then washed and acidic vesicular organelles (AVOs) were observed using a confocal microscope (Nikon C2 plus, Tokyo, Japan).
Western blotting analysis
After the treated cells were harvested and lysed with protease inhibitors (Solarbio, Shanghai, China) and RIPA lysis buffer (Solarbio, Shanghai, China) was added, the supernatants were collected by centrifugation at 14,000×g at 4°C for 10 min. Proteins were quantified using a BCA protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China) and electrophoresed on 9%−12% SDS polyacrylamide gels. Subsequently, they were transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, U.S.A). The membranes were blocked with 5% (w/v) milk in tris buffered saline with Tween® 20 (TBST) at room temperature for 2 h and further incubated with the corresponding primary antibody at 4°C overnight. The immunoreactivity was detected using an ECL Kit (Wanleibio, Shenyang, China) after incubation with the corresponding peroxidase-conjugated secondary antibodies at room temperature for 2 h. ImageJ software (Media Cybernetics, Baltimore, MD, U.S.A) was used to assess the protein levels, which were normalized to the relative density of β-actin.
ER-Tracker red staining
The staining assay was carried out according to the manufacturer’s instructions of the ER-Tracker kit (Beyotime Institute of Biotechnology, Shanghai, China) with slight alterations. The cells were rinsed in PBS after staining with ER-Tracker dye solution (1 µM) at 37°C for 15 min, and then fixed in 4% paraformaldehyde at 37°C for 2 minutes and washed with PBS. A confocal microscope (Nikon C2 Plus, Tokyo, Japan) was then used to observe the cells.
Apoptosis detection by flow cytometry
Apoptosis assays were performed as previously described (Tang et al., Citation2022). Briefly, the treated cells were stained with Annexin V-FITC/PI apoptosis kit for 15 min in the dark. Samples were examined using a FACScan flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, U.S.A) and analyzed by FlowJo software (Tomy Digital Biology, Co., Ltd., Japan).
Statistical analysis
Mean ± S.E.M. was used to display results from at least three separate experiments. The one-way ANOVA and Tukey’s post-test were used to assess the statistics by GraphPad Prism 6.0 software (La Jolla, CA, U.S.A). Statistically significant is defined as P < .05.
Results
Silibinin alleviates ER stress and apoptosis of GLUTag cells induced by PA
It is generally believed that the UPR alleviates ER stress. Recent studies have shown that the generation of ER whorls is a new manifestation of ER stress response (F. Xu et al., Citation2021). We used ER-tracker red to observe ER morphology to determine whether PA induced ER stress in GLUTag cells. As shown in , the whorls were observed in the PA-treated group, while they were reduced after silibinin treatment. In addition, there was almost no difference between the cells treated with silibinin alone and the control group.
Figure 1. Silibinin alleviates ER stress and apoptosis of GLUTag cells induced by PA. (a) GLUTag cells were stained with ER-Tracker Red and visualized by confocal microscopy. (b, c) Expression levels of BiP, p-PERK (Thr981), XBP1, ATF6, CHOP and Caspase12 were evaluated by western blotting. (d) Apoptosis was detected by Hoechst 33258 staining. The mean ± S.E.M is shown. *P < .05 versus control group; #P < .05 versus PA group.
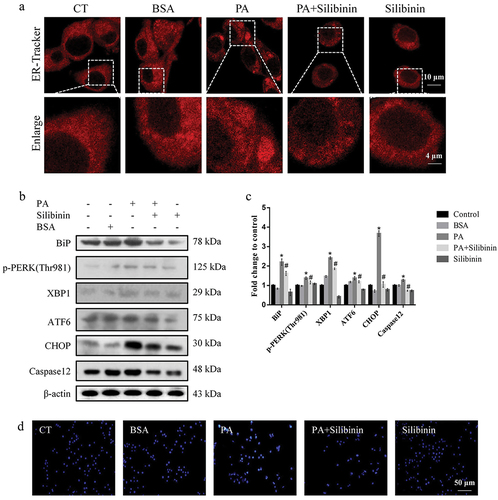
Following confirmation that PA can cause ER stress in GLUTag cells, the activation of the major ER chaperone BiP and three UPR sensor proteins p-PERK (Thr981), ATF6 and XBP1 with indicated treatment was further determined. The results showed that treating cells with PA resulted in a considerable rise in the expression levels of BiP, p-PERK (Thr981), ATF6 and XBP1, while silibinin attenuated the up-regulation of expression of these proteins. In addition, silibinin may block PA-induced ER stress-specific apoptosis by suppressing the expression of downstream factors CHOP and Caspase12 in the ER stress pathway ().
Hoechst 33258 staining was further conducted to confirm whether silibinin could protect GLUTag cells from PA-induced apoptosis. The results showed that PA induced apoptosis compared to the control group, and the apoptosis of silibinin-pretreated cells was lower than that of the PA group. The BSA group showed no obvious changes compared with the control group. In summary, these results demonstrate that silibinin protects GLUTag cells from PA-induced apoptosis via inhibition of ER stress ().
Silibinin reduces PA-induced autophagy in GLUTag cells
Several studies have found a link between ER stress and autophagy in gastrointestinal endocrine cells, with autophagy exhibiting either a protective or cytotoxic function based on gut microenvironments (Larabi et al., Citation2020; Qin et al., Citation2021; Wu et al., Citation2022). As a result, we tried to elucidate if autophagy was involved in PA-induced apoptosis in GLUTag cells when ER stress occurred, as well as determine how silibinin affected autophagy in GLUTag cells. AO is a cell-permeable, green fluorophore that can be protonated and captured in AVOs. The formation of AVOs is a unique process of autophagy (Paglin et al., Citation2001). With AO staining, we investigated the influence of PA on the production of AVOs. As shown in , PA effectively promoted the development of AVOs in GLUTag cells, whereas silibinin markedly attenuated PA-induced AVO formation.
Figure 2. Silibinin reduces PA-induced autophagy in GLUTag cells. (a) the red fluorescence of autophagic morphology stained with AO was visualized by confocal microscopy. (b, c) Expression levels of Beclin1, ATG5 and LC3 were evaluated by western blotting. (d, e) the cells were stained with AV-PI and the ratio (%) of apoptotic cells were analyzed by flow cytometry. (f, g) Expression levels of Bax, Bcl2 and cleaved PARP were evaluated by western blotting. The mean ± S.E.M is shown. *P < .05, **P < .01, ***P < .001 versus BSA group; #P < .05, ##P < .01, ###P < .001, ####P < .0001 versus PA group; &P < .05, &&P < .01, &&&P < .001 versus PA + Silibinin group.
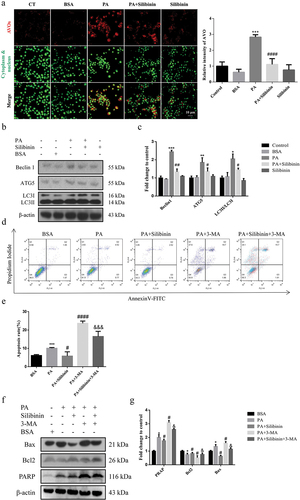
Moreover, we examined the expression levels of autophagy-associated proteins Beclin1, Atg5 and LC3 to explore the role of PA in inducing autophagy. The results showed that PA treatment for 20 h significantly upregulated Beclin1, Atg5 and LC3II expression compared to the control group (). Silibinin effectively reduced PA-upregulated expression of Beclin1, Atg5 and LC3II.
The involvement of autophagy in PA-induced cell damage was further investigated. After inhibiting autophagy in GLUTag cells with the autophagy inhibitor 3-MA, flow cytometry was utilized to evaluate apoptosis. As shown in , with the evidence of Annexin V/PI apoptosis assay, pre-treatment of cells with 3-MA could significantly enhance the apoptotic cell ratio in comparison with cells in the PA group. Furthermore, western blotting analysis confirmed the above-mentioned findings, demonstrating that the combined treatment of PA and 3-MA can further increase the ratio of Bax/Bcl−2 and cleaved PARP expression (). These findings support the hypothesis that autophagy protects GLUTag cells from PA-induced apoptosis.
Silibinin downregulates PA-induced autophagy of GLUTag cells by inhibiting ER stress
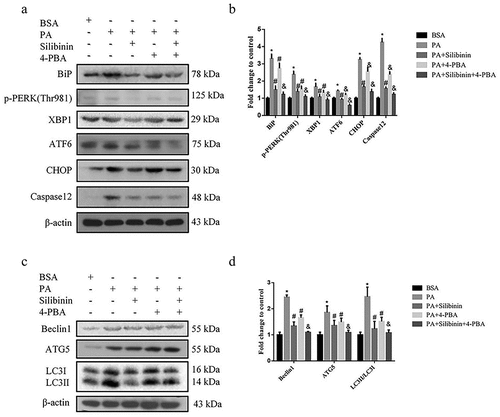
We used the ER stress inhibitor 4-PBA to explore the effect of ER stress on autophagy and further determined whether silibinin protects GLUTag cells by inhibiting ER stress. The results demonstrated that the cells treated with silibinin decreased the expression of ER stress-related proteins and apoptosis-related proteins in GLUTag cells compared with the PA group. Additionally, the combined treatment of silibinin and 4-PBA could further reduce the expression of ER stress-related and apoptosis-related proteins (). Compared with the PA group, Beclin1, ATG5 and LC3II levels were decreased in the silibinin or 4-PBA group (). Additionally, the combined treatment of silibinin and 4-PBA could further reduce the expression of autophagy-related proteins. These results demonstrate that silibinin protects the GLUTag cells from PA-induced injury by inhibiting ER stress, and autophagy also protects the cells in this process. Taken together, silibinin protects GLUTag cells from ER stress and attenuates PA-induced autophagy.
Figure 3. Silibinin downregulates PA-induced autophagy of GLUTag cells by inhibiting ER stress. (a, b) Expression levels of BiP, p-PERK (Thr981), XBP1, ATF6, CHOP and Caspase12 were evaluated by western blotting. (c, d) Expression levels of Beclin1, ATG5 and LC3 were evaluated by western blotting. Data are expressed as mean ± S.E.M. *P < .05 versus BSA group; #P < .05 versus PA group; &P < .05 versus PA + Silibinin group.
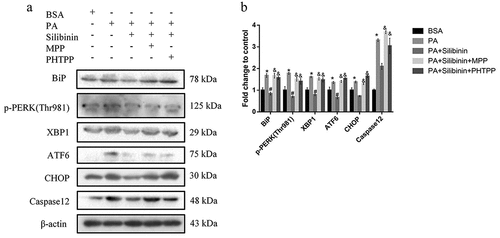
Silibinin ameliorates PA-induced ER stress of GLUTag cells through estrogen receptors α and β
Previously, a study reported that silibinin reduces oxidative stress through estrogen receptors α and β in GLUTag cells (J. Y. Wang et al., Citation2022). To determine whether estrogen receptors mediated the protective effects of silibinin on ER stress, 7.5 μM MPP (an estrogen receptor α antagonist) or 0.9375 μM PHTPP (an estrogen receptor β antagonist) was used with silibinin before cells were treated with PA. The selection of inhibitor dose was based on our previous study (J. Y. Wang et al., Citation2022). Western blotting analysis indicated that the downregulation of p-PERK (Thr981), ATF6, XBP1, CHOP and Caspase12 expression induced by silibinin was markedly reversed by the addition of MPP or PHTPP. These data suggest that silibinin reduces PA-caused GLUTag cell ER stress through the regulation of estrogen receptors α and β ().
Figure 4. Silibinin ameliorates PA-induced ER stress of GLUTag cells through estrogen receptors α and β. Representative western blots (a) and quantitative analysis (b) of BiP, p-PERK (Thr981), XBP1, ATF6, CHOP and Caspase12 in GLUTag cells with indicated treatments. Data are expressed as mean ± S.E.M. *P < .05 versus BSA group; #P < .05 versus PA group; &P < .05 versus PA + Silibinin group.
Discussion
Diabetes has become a major health issue all over the world, and there is an urgent need for the development of novel anti-diabetic medications (Al Lawati, Citation2017). Silibinin, the natural flavonoid widely used clinically for the treatment of acute or chronic liver diseases, has been reported to improve blood glucose homeostasis by improving the activity of pancreatic β cells, increasing insulin sensitivity in liver and muscle cells, and reducing lipid deposition in adipocytes (Chu et al., Citation2018; Enjalbert et al., Citation2002; Mazraesefidi et al., Citation2023; Wadhwa et al., Citation2022; Wah Kheong et al., Citation2017; L. Yang et al., Citation2021). In our previous investigations, we revealed that silibinin protected cells from PA-induced cell damage in both β cells and GLUTag cells (Y. Sun et al., Citation2019). It is well known that excessive accumulation of lipids in β cells can result in irreparable lipotoxicity, which is a primary factor in the development and progression of diabetes (Barlow et al., Citation2016b; Vasu et al., Citation2015; Ye et al., Citation2019). The effects and underlying mechanisms of silibinin on PA-treated GLUTag cells in vitro are investigated in this study. We found that silibinin effectively prevented PA-induced ER stress and downstream apoptosis, possibly through regulating estrogen receptors α and β.
FFAs cause cellular damage by several different pathways, one of which is ER stress, an important cellular stress signal. ER is a multifunctional organelle that is involved in the production and folding of transmembrane proteins. Several cellular stressors and cytotoxic chemicals can cause misfolded and unfolded proteins to accumulate in the ER, triggering the UPR response. BiP is a key ER chaperone that is thought to operate as a primary sensor in the UPR response (Kopp et al., Citation2019). However, in the presence of chronic and severe ER stress, UPR-mediated adaptive responses are insufficient to restore normal cellular function, and apoptosis signaling pathways are then triggered (Nita et al., Citation2017; K. Wang et al., Citation2016). There are two main pathways of ER stress-induced apoptosis, a transcription factor pathway and a caspase-dependent pathway. In the former pathway, IRE1 is thought to exert a pro-apoptotic function by up-regulating the transcription factor CHOP, which may amplify pro-apoptotic signals in turn. This mechanism is achieved by altering the balance between Bcl−2 and Bax. Furthermore, oligomerization of PERK stimulates the activation of transcription factor 4 (ATF4), which then upregulates CHOP, leading to the activation of Caspase12 and the initiation of apoptosis process (Aslani et al., Citation2022; Cui et al., Citation2016; Kamarehei et al., Citation2019). In agreement with previous reports, we found that PA triggered ER stress in GLUTag cells, as evidenced by facilitated production of p-PERK (Thr981), XBP1, ATF6 and BiP. Silibinin significantly suppressed PA-induced expressions of ER stress markers.
The ER accounts for about half of the total eukaryotic cell membrane, and defects in the ER have been shown to be associated with a variety of diseases (Reggiori & Molinari, Citation2022). To study these underlying mechanisms in more detail, ER-specific markers can serve as an important tool. The whorls are formed to resist the expansion of the ER under stress. The ER tracker red results demonstrated that ER whorls were significantly increased after PA treatment, which was reversed by silibinin, indicating that silibinin inhibited PA-induced ER stress. The commonly investigated downstream processes of ER stress are apoptosis and autophagy. In pancreatic β-cells, the regulatory role of ER stress in apoptosis and autophagy has been discovered. Intermittent hypoxia activates the ER-stress-related PERK/eIF2α/ATF4 signaling pathway, which leads to apoptosis and protective autophagy in β cells (S. Song et al., Citation2018).
Pre-treatment with silibinin was also shown to reduce the expression of ER stress-associated pro-apoptotic protein CHOP and Caspase12. And it was shown that silibinin decreased the expression of Beclin1, ATG5 and LC3, and inhibited the formation of AVOs, indicating that silibinin inhibited autophagy in GLUTag cells. PA activates autophagy via the ER-stress-related signaling pathway, as evidenced by the decrease in autophagy in cells co-incubation with the ER stress inhibitor 4-PBA. PA triggered protective autophagy in rat prefrontal cells, according to the study by Xue et al., and apoptosis induced by PA may be boosted by decreasing autophagy (Xue et al., Citation2021). Our study also yielded similar results. Pretreatment with 3-MA further upregulated PA-induced apoptosis, accompanied by increased expression of cleaved PARP and Bax/Bcl2 ratio.
Estrogen receptors are emerging as important molecules for suppressing adipose tissue accumulation and improving insulin secretion and sensitivity (Mauvais-Jarvis, Citation2011). In obese female Wistar rats, activation of estrogen receptors α and β improves systemic insulin sensitivity (Weigt et al., Citation2013). Both GLP−1 and insulin, two key hormones, are necessary for maintaining metabolic homeostasis (Martchenko & Brubaker, Citation2021). Thus, whether estrogen receptors α and β contributed to the effect of silibinin on PA-induced GLUTag cell lipotoxicity was further determined in our study. And the protective effects of silibinin are found to be linked to modulation of estrogen receptors α and β in GLUTag cells. Considering complexity and significance, the particular mechanisms of the activation of estrogen receptors α and β by silibinin remain to be elucidated.
In conclusion, our findings shed light on the relationship between autophagy and apoptosis under PA-induced ER stress conditions. Silibinin protects GLUTag cells from PA-induced ER stress by activating estrogen receptors α and β, and autophagy may protect cells from PA-induced apoptosis.
Author contribution
Conceptualization, X.S., C.C. and F.X.; methodology, X.L., L.Z. and F.X.; data collection, X.S., C.C., X.L. and L.Z.; validation, X.Z. and N.C.; formal analysis, W.L., Z.J. and C.C.; writing-original draft preparation, X.S., C.C. and L.Z.; writing-review and editing, F.X. and T.I.; funding acquisition, F.X.; supervision, F.X. and T.I. All authors have read and agreed to the published version of the manuscript. All data were generated in-house. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Acknowledgements
We thank Dr. Daniel J. Drucker of The University of Toronto for kindly providing GLUTag cell line.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Al Lawati, J. A. (2017). Diabetes mellitus: A local and global public health emergency! Oman Medical Journal, 32(3), 177–179. https://doi.org/10.5001/omj.2017.34
- Aslani, M. R., Amani, M., Masrori, N., Boskabady, M. H., Ebrahimi, H. A., & Chodari, L. (2022). Crocin attenuates inflammation of lung tissue in ovalbumin-sensitized mice by altering the expression of endoplasmic reticulum stress markers. Biofactors, 48(1), 204–215. https://doi.org/10.1002/biof.1809
- Barlow, J., Jensen, V. H., Jastroch, M., & Affourtit, C. (2016a). Palmitate-induced impairment of glucose-stimulated insulin secretion precedes mitochondrial dysfunction in mouse pancreatic islets. Biochemical Journal, 473(4), 487–496. https://doi.org/10.1042/bj20151080
- Barlow, J., Jensen, V. H., Jastroch, M., & Affourtit, C. (2016b). Palmitate-induced impairment of glucose-stimulated insulin secretion precedes mitochondrial dysfunction in mouse pancreatic islets. Biochemical Journal, 473(4), 487–496. https://doi.org/10.1042/BJ20151080
- Bell, G. I., Sanchez-Pescador, R., Laybourn, P. J., & Najarian, R. C. (1983). Exon duplication and divergence in the human preproglucagon gene. Nature, 304(5924), 368–371. https://doi.org/10.1038/304368a0
- Chu, C., Li, D., Zhang, S., Ikejima, T., Jia, Y., Wang, D., & Xu, F. (2018). Role of silibinin in the management of diabetes mellitus and its complications. Archives of Pharmacal Research, 41(8), 785–796. https://doi.org/10.1007/s12272-018-1047-x
- Cui, Y. Y., Zhao, D. M., Sreevatsan, S., Liu, C. F., Yang, W., Song, Z. Q., Yang, L. F., Barrow, P., & Zhou, X. M. (2016). Mycobacterium bovis induces endoplasmic reticulum stress mediated-apoptosis by activating IRF3 in a Murine macrophage cell line. Frontiers in Cellular and Infection Microbiology, 6, Article 182. https://doi.org/10.3389/fcimb.2016.00182
- Enjalbert, F., Rapior, S., Nouguier-Soulé, J., Guillon, S., Amouroux, N., & Cabot, C. (2002). Treatment of amatoxin poisoning: 20-year retrospective analysis. Journal of Toxicology: Clinical Toxicology, 40(6), 715–757. https://doi.org/10.1081/clt-120014646
- Gutniak, M., Orskov, C., Holst, J. J., Ahren, B., & Efendic, S. (1992). Antidiabetogenic effect of glucagon-like peptide-1 (7–36)amide in normal subjects and patients with diabetes mellitus. The New England Journal of Medicine, 326(20), 1316–1322. https://doi.org/10.1056/nejm199205143262003
- Hoyer-Hansen, M., & Jaattela, M. (2007). Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death and Differentiation, 14(9), 1576–1582. https://doi.org/10.1038/sj.cdd.4402200
- Hu, M. L., Yang, S. L., Yang, L., Cheng, Y. Z., Zhang, H., & Pizzo, S. V. (2016). Interleukin-22 alleviated palmitate-induced endoplasmic reticulum stress in INS-1 cells through activation of autophagy. PLoS One, 11(1), e0146818. https://doi.org/10.1371/journal.pone.0146818
- Kamarehei, M., Ardestani, S. K., Firouzi, M., Zahednasab, H., Keyvani, H., & Harirchian, M. H. (2019). Increased expression of endoplasmic reticulum stress-related caspase-12 and CHOP in the hippocampus of EAE mice. Brain Research Bulletin, 147, 174–182. https://doi.org/10.1016/j.brainresbull.2019.01.020
- Khalid, A., & Naseem, I. (2023). Increased therapeutic effect of nanotized silibinin against glycation and diabetes: An in vitro and in silico-based approach. Biochimica et Biophysica Acta (BBA) - General Subjects, 1867(7), 130364. https://doi.org/10.1016/j.bbagen.2023.130364
- Kondo, Y. (2013). A fundamental framework to organize the curriculum of technology education in senior high school. Seminars in Immunopathology, 35(3), 351–373. https://doi.org/10.1007/s00281-013-0370-z
- Kopp, M. C., Larburu, N., Durairaj, V., Adams, C. J., & Ali, M. M. U. (2019). UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor. Nature Structural & Molecular Biology, 26(11), 1053–1062. https://doi.org/10.1038/s41594-019-0324-9
- Kumar Singh, N., & Bhushan, B. (2023). Preclinical evidence-based neuroprotective potential of silibinin. Current Drug Research Reviews, 15. https://doi.org/10.2174/2589977515666230327154800
- Larabi, A., Barnich, N., & Nguyen, H. T. T. (2020). New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy, 16(1), 38–51. https://doi.org/10.1080/15548627.2019.1635384
- Lingling, S., Jianing, F., Liu, W., Toshihiko, H., & Yuheng, N. (2020). Silibinin inhibits migration and invasion of breast cancer MDA-MB-231 cells through induction of mitochondrial fusion. Molecular and Cellular Biochemistry, 463(1), 189–201. https://doi.org/10.1007/s11010-019-03640-6
- Lytrivi, M., Castell, A. L., Poitout, V., & Cnop, M. (2020). Recent insights into mechanisms of beta-cell Lipo- and glucolipotoxicity in type 2 diabetes. Journal of Molecular Biology, 432(5), 1514–1534. https://doi.org/10.1016/j.jmb.2019.09.016
- Ly, L. D., Xu, S. H., Choi, S. K., Ha, C. M., Thoudam, T., Cha, S. K., Wiederkehr, A., Wollheim, C. B., Lee, I. K., & Park, K. S. (2017). Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Experimental and Molecular Medicine, 49(2), e291. https://doi.org/10.1038/emm.2016.157
- Maiuri, M. C., Zalckvar, E., Kimchi, A., & Kroemer, G. (2007). Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nature Reviews Molecular Cell Biology, 8(9), 741–752. https://doi.org/10.1038/nrm2239
- Martchenko, A., & Brubaker, P. L. (2021). Effects of obesogenic feeding and free fatty acids on circadian secretion of metabolic hormones: Implications for the Development of type 2 diabetes. Cells, 10(9), 2297. https://doi.org/10.3390/cells10092297
- Mauvais-Jarvis, F. (2011). Estrogen and androgen receptors: Regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends in Endocrinology and Metabolism, 22(1), 24–33. https://doi.org/10.1016/j.tem.2010.10.002
- Mazraesefidi, M., Mahmoodi, M., & Hajizadeh, M. (2023). Effects of silibinin on apoptosis and insulin secretion in rat RINm5F pancreatic β-cells. Biotechnic & Histochemistry, 98(3), 201–209. https://doi.org/10.1080/10520295.2022.2154840
- McAlister, E., Kirkby, M., Dominguez-Robles, J., Paredes, A. J., Anjani, Q. K., Moffatt, K., Vora, L. K., Hutton, A. R. J., McKenna, P. E., Larraneta, E., & Donnelly, R. F. (2021). The role of microneedle arrays in drug delivery and patient monitoring to prevent diabetes induced fibrosis. Advanced Drug Delivery Reviews, 175, 113825. https://doi.org/10.1016/j.addr.2021.06.002
- Meier, J. J. (2012). GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nature Reviews Endocrinology, 8(12), 728–742. https://doi.org/10.1038/nrendo.2012.140
- Nita, I., Hostettler, K., Tamo, L., Medova, M., Bombaci, G., Zhong, J., Allam, R., Zimmer, Y., Roth, M., Geiser, T., & Gazdhar, A. (2017). Hepatocyte growth factor secreted by bone marrow stem cell reduce ER stress and improves repair in alveolar epithelial II cells. Scientific Reports, 7(1), Article 41901. https://doi.org/10.1038/srep41901
- Opazo-Rios, L., Mas, S., Marin-Royo, G., Mezzano, S., Gomez-Guerrero, C., Moreno, J. A., & Egido, J. (2020). Lipotoxicity and diabetic nephropathy: Novel mechanistic insights and therapeutic opportunities. International Journal of Molecular Sciences, 21(7), 2632. https://doi.org/10.3390/ijms21072632
- Paglin, S., Hollister, T., Delohery, T., Hackett, N., McMahill, M., Sphicas, E., Domingo, D., & Yahalom, J. (2001). A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Research, 61(2), 439–444.
- Peng, G., Li, L. H., Liu, Y. B., Pu, J., Zhang, S. Y., Yu, J. H., Zhao, J. J., & Liu, P. S. (2011). Oleate blocks palmitate-induced abnormal lipid distribution, endoplasmic reticulum expansion and stress, and insulin resistance in skeletal muscle. Endocrinology, 152(6), 2206–2218. https://doi.org/10.1210/en.2010-1369
- Qin, H. R., Zhang, H., Zhang, X. P., Zhang, S. W., Zhu, S. W., & Wang, H. (2021). Resveratrol protects intestinal epithelial cells against radiation-induced damage by promoting autophagy and inhibiting apoptosis through SIRT1 activation. Journal of Radiation Research, 62(4), 574–581. https://doi.org/10.1093/jrr/rrab035
- Qureshi, F. M., Dejene, E. A., Corbin, K. L., & Nunemaker, C. S. (2015). Stress-induced dissociations between intracellular calcium signaling and insulin secretion in pancreatic islets. Cell Calcium, 57(5–6), 366–375. https://doi.org/10.1016/j.ceca.2015.03.002
- Reggiori, F., & Molinari, M. (2022). ER-phagy: Mechanisms, regulation, and diseases connected to the lysosomal clearance of the endoplasmic reticulum. Physiological Reviews, 102(3), 1393–1448. https://doi.org/10.1152/physrev.00038.2021
- Shah, A., Vaidya, N. K., Bhat, H. K., & Kumar, A. (2016). HIV-1 gp120 induces type-1 programmed cell death through ER stress employing IRE1α, JNK and AP-1 pathway. Scientific Reports, 6(1), 18929. https://doi.org/10.1038/srep18929
- Somm, E., Montandon, S. A., Loizides-Mangold, U., Gaia, N., Lazarevic, V., De Vito, C., Perroud, E., Bochaton-Piallat, M. L., Dibner, C., Schrenzel, J., & Jornayvaz, F. R. (2021). The GLP-1R agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Translational Research, 227, 75–88. https://doi.org/10.1016/j.trsl.2020.07.008
- Song, S. L., Tan, J., Miao, Y. Y., Li, M. M., & Zhang, Q. (2017). Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. Journal of Cellular Physiology, 232(11), 2977–2984. https://doi.org/10.1002/jcp.25785
- Song, S., Tan, J., Miao, Y., Sun, Z., & Zhang, Q. (2018). Intermittent-hypoxia-induced autophagy activation through the ER-Stress-related PERK/eIf2α/ATF4 pathway is a protective response to pancreatic β-cell apoptosis. Cellular Physiology & Biochemistry, 51(6), 2955–2971. https://doi.org/10.1159/000496047
- Sonnenburg, J. L., & Bäckhed, F. (2016). Diet–microbiota interactions as moderators of human metabolism. Nature, 535(7610), 56–64. https://doi.org/10.1038/nature18846
- Sozen, E., Karademir, B., & Ozer, N. K. (2015). Basic mechanisms in endoplasmic reticulum stress and relation to cardiovascular diseases. Free Radical Biology and Medicine, 78, 30–41. https://doi.org/10.1016/j.freeradbiomed.2014.09.031
- Sun, W.-L., Hua, S., Li, X.-Y., Shen, L., Wu, H., & Ji, H.-F. (2023). Microbially produced vitamin B12 contributes to the lipid-lowering effect of silymarin. Nature Communications, 14(1). https://doi.org/10.1038/s41467-023-36079-x
- Sun, E. W. L., Martin, A. M., Young, R. L., & Keating, D. J. (2018). The regulation of peripheral metabolism by gut-derived hormones. Frontiers in Endocrinology (Lausanne), 9, 754. https://doi.org/10.3389/fendo.2018.00754
- Sun, Y., Yang, J., Liu, W. W., Yao, G. D., Xu, F. X., Hayashi, T., Onodera, S., & Ikejima, T. (2019). Attenuating effect of silibinin on palmitic acid-induced apoptosis and mitochondrial dysfunction in pancreatic beta-cells is mediated by estrogen receptor alpha. Molecular and Cellular Biochemistry, 460(1–2), 81–92. https://doi.org/10.1007/s11010-019-03572-1
- Tang, X. M., Wang, L. S., Ye, H., Zhao, H. Q., & Zhao, L. S. (2022). Biological matrix-derived carbon quantum dots: Highly selective detection of tetracyclines. Journal of Photochemistry and Photobiology A-Chemistry, 424, Article 113653. https://doi.org/10.1016/j.jphotochem.2021.113653
- Thivolet, C., Vial, G., Cassel, R., Rieusset, J., & Madec, A. M. (2017). Reduction of endoplasmic reticulum mitochondria interactions in beta cells from patients with type 2 diabetes. PLoS One, 12(7), Article e0182027. https://doi.org/10.1371/journal.pone.0182027
- Vasu, S., Moffett, R. C., McClenaghan, N. H., & Flatt, P. R. (2015). Differential molecular and cellular responses of GLP-1 secreting L-cells and pancreatic alpha cells to glucotoxicity and lipotoxicity. Experimental Cell Research, 336(1), 100–108. https://doi.org/10.1016/j.yexcr.2015.05.022
- Wadhwa, K., Pahwa, R., Kumar, M., Kumar, S., Sharma, P. C., Singh, G., Verma, R., Mittal, V., Singh, I., Kaushik, D., & Jeandet, P. (2022). Mechanistic insights into the pharmacological significance of silymarin. Molecules, 27(16), 5327. https://doi.org/10.3390/molecules27165327
- Wah Kheong, C., Nik Mustapha, N. R., & Mahadeva, S. (2017). A randomized trial of silymarin for the treatment of nonalcoholic steatohepatitis. Clinical Gastroenterology and Hepatology, 15(12), 1940–1949.e8. https://doi.org/10.1016/j.cgh.2017.04.016
- Wang, K., Zhang, C., Bao, J. L., Jia, X. J., Liang, Y. E., Wang, X. T., Chen, M. W., Su, H. X., Li, P., Wan, J. B., & He, C. W. (2016). Synergistic chemopreventive effects of curcumin and berberine on human breast cancer cells through induction of apoptosis and autophagic cell death. Scientific Reports, 6(1), Article 26064. https://doi.org/10.1038/srep26064
- Wang, J. Y., Zhang, L. X., Cao, H., Shi, X. Y., Zhang, X. R., Gao, Z. H., Ikeda, K., Yan, T. X., Jia, Y., & Xu, F. X. (2022). Silibinin improves L-cell mass and function through an estrogen receptor-mediated antioxidative mechanism. Phytomedicine, 99, 154022. https://doi.org/10.1016/j.phymed.2022.154022
- Wang, J., Zhang, X., Zhang, L., Yan, T., Wu, B., Xu, F., & Jia, Y. (2020). Silychristin a activates Nrf2-HO-1/SOD2 pathway to reduce apoptosis and improve GLP-1 production through upregulation of estrogen receptor alpha in GLUTag cells. European Journal of Pharmacology, 881, 173236. https://doi.org/10.1016/j.ejphar.2020.173236
- Weigt, C., Hertrampf, T., Kluxen, F. M., Flenker, U., Hulsemann, F., Fritzemeier, K. H., & Diel, P. (2013). Molecular effects of ER alpha- and beta-selective agonists on regulation of energy homeostasis in obese female Wistar rats. Molecular and Cellular Endocrinology, 377(1–2), 147–158. https://doi.org/10.1016/j.mce.2013.07.007
- Wu, H. Y., Gao, Y. A., Li, S. L., Bao, X. Y., Wang, J. Q., & Zheng, N. (2022). Lactoferrin alleviated AFM1-induced apoptosis in intestinal NCM 460 cells through the autophagy pathway. Foods, 11(1), 23. https://doi.org/10.3390/foods11010023
- Xu, F., Du, W. Q., Zou, Q., Wang, Y. T., Zhang, X., Xing, X. D., Li, Y., Zhang, D. C., Wang, H. M., Zhang, W. H., Hu, X. Y., Liu, X., Liu, X. L., Zhang, S. J., Yu, J. Q., Fang, J. H., Li, F. J., Zhou, Y. … Yu, L. (2021). COPII mitigates ER stress by promoting formation of ER whorls. Cell Research, 31(2), 141–156. https://doi.org/10.1038/s41422-020-00416-2
- Xue, X. L., Li, F., Cai, M., Hu, J. Y., Wang, Q., Lou, S. J., & Rastegar, M. (2021). Interactions between endoplasmic reticulum stress and autophagy: Implications for apoptosis and neuroplasticity-related proteins in palmitic acid-treated prefrontal cells. Neural Plasticity, 2021, 1–14. Article 8851327. https://doi.org/10.1155/2021/8851327
- Xu, R., Qiu, S., Zhang, J., Liu, X., Zhang, L., Xing, H., You, M., Wang, M., Lu, Y., Zhang, P., & Zhu, J. (2022). Silibinin Schiff base derivatives counteract CCl4-induced acute liver injury by enhancing anti-inflammatory and antiapoptotic bioactivities. Drug Design, Development and Therapy, 16, 1441–1456. https://doi.org/10.2147/dddt.s356847
- Yang, L., Liu, Q., Zhang, H., Wang, Y., Li, Y., Chen, S., Song, G., & Ren, L. (2021). Silibinin improves nonalcoholic fatty liver by regulating the expression of miR‑122: An in vitro and in vivo study. Molecular Medicine Reports, 23(5). https://doi.org/10.3892/mmr.2021.11974
- Yang, J., Sun, Y., Xu, F., Liu, W., Hayashi, T., Onodera, S., Tashiro, S. I., & Ikejima, T. (2018). Involvement of estrogen receptors in silibinin protection of pancreatic beta-cells from TNFalpha- or IL-1beta-induced cytotoxicity. Biomedicine & Pharmacotherapy, 102, 344–353. https://doi.org/10.1016/j.biopha.2018.01.128
- Yaribeygi, H., Sathyapalan, T., & Sahebkar, A. (2019). Molecular mechanisms by which GLP-1 RA and DPP-4i induce insulin sensitivity. Life Sciences, 234, 116776. https://doi.org/10.1016/j.lfs.2019.116776
- Ye, R., Onodera, T., & Scherer, P. E. (2019). Lipotoxicity and beta cell maintenance in obesity and type 2 diabetes. Journal of the Endocrine Society, 3(3), 617–631. https://doi.org/10.1210/js.2018-00372
- Zhou, Z. Q., Ribas, V., Rajbhandari, P., Drew, B. G., Moore, T. M., Fluitt, A. H., Reddish, B. R., Whitney, K. A., Georgia, S., Vergnes, L., Reue, K., Liesa, M., Shirihai, O., van der Bliek, A. M., Chi, N. W., Mahata, S. K., Tiano, J. P., Hewitt, S. C. … Hevener, A. L. (2018). Estrogen receptor alpha protects pancreatic beta-cells from apoptosis by preserving mitochondrial function and suppressing endoplasmic reticulum stress. Journal of Biological Chemistry, 293(13), 4735–4751. https://doi.org/10.1074/jbc.M117.805069
