ABSTRACT
The transformation of yellow nutsedges into nutritional flour and snack food could be important for expanding consumption and enriching the variety of products. This paper used peeled yellow nutsedges as raw materials to explore the effect of roasting on antioxidant capacity, active ingredients, and aroma. The results showed that the antioxidant capacity, DPPH (2,2-Diphenyl−1-picrylhydrazyl) free radical scavenging rate and ABTS+ (2,2’-azinobis-(3-ethylbenzothiazoline−6-sulfonate)) scavenging rate were significantly improved after baking compared with the control, but the hydroxyl-free radical scavenging rate was not obvious. A total of 6 kinds antimicrobial peptides with high scores were detected, including 2 kinds in the roasted group and four kinds in the control group. ACE (angiotensin converting enzyme) inhibitors, dipeptidyl peptidase IV inhibitors, antimicrobial peptides, antioxidant peptides and blood–brain barrier peptides were changed by roasted treatment. The processing can enhance the kinds of esters from 4 to 8 compared with non-roasted group.
1. Introduction
Yellow nutsedges (Cyperus esculentus), also known as tiger nut, is rich in lipids, starch, dietary fiber, vitamins, minerals in tubers (Zhang et al., Citation2022). In China, the roasted yellow nutsedges tubers are favored by consumers. The protein content of yellow nutsedges is also very high, which can reach 10–15%, and contains 16 kinds of amino acids, including aspartic acid as the main essential amino acid for human body (Sánchez-Zapata et al., Citation2012; Yang et al., Citation2022). In addition, there are a variety of active factors, such as alkaloids, saponins, tannins, and flavonoids (Ekeanyanwu et al., Citation2010). Studies have confirmed that edible yellow nutsedges can help promote blood circulation, which is of great benefit to people with diabetes, cardiovascular diseases and obesity, and can also prevent stroke and respiratory inflammation, reduce the risk of colon cancer and other functions (Ekeanyanwu et al., Citation2010). Because of its rich nutrition, it is very beneficial to human health and is considered to be the most ideal food for children, the elderly and athletes (Defelice, Citation2002; Roselló-Soto et al., Citation2019).
At present, foreign scholars have developed a variety of special foods based on the yellow nutsedges. Badejo et al. (Citation2020) developed a functional drink with antioxidative properties and diabetes prevention based on yellow nutsedges, bitter almonds and bitter melon. With the increasing incidence of inflammatory bowel disease in many countries such as North America and Europe, the demand for gluten-free food is also increasing, and the development and commercial production of high-quality gluten-free food has become a major trend (Demirkesen et al., Citation2011). Ahmed and Hussein (Citation2014) made a gluten-free cookie with excellent appearance, flavor, and taste by mixing corn flour and oil salad flour. Gasparre et al. (Citation2020) found that a crisp and delicious gluten-free snack could be obtained by the mixture of 10% yellow nutsedges flour, rice flour and soluble fiber with a twin-screw extruder. The contents of dietary fiber, fat and minerals of the product were significantly improved after the addition of yellow nutsedges powder, and the pasta after cooking had good color and taste (Albors et al., Citation2016). The roasted tubers of yellow nutsedges have a strong aroma and can be used to make flour or eaten plain in China. However, the nutritional properties of roasted tiger nut such as small-molecule peptide, antioxidant capacity, and esters aroma are unknown. It is necessary to detect nutritional changes of yellow nutsedges after baking, which helps to improve its edible range and enrich the variety of products.
Small molecule active peptide not only has strong bioactive function and diversity, but also has the advantages of abundant source, low cost, high safety and easy to industrialize production. In recent years, some scholars have made a series of achievements in the study of plant active peptides. Angayarkanni and Sridevi (Citation2018) used circular paper chromatography to screen the active peptide in yellow flower, and confirmed that the active peptide could effectively inhibit various gram-negative bacteria and some gram-positive bacteria by disk diffusion method. Liu et al. (Citation2022) discovered a variety of active peptides in walnut by-products by enzymatic hydrolysis, and found that different active peptides showed different structural characteristics and amino acid composition when acting under different conditions. The experiments proved that the active peptides in walnut by-products had anti-hypertension, anti-diabetes, anti-cancer, nerve protection and other effects. Huang et al. (Citation2022) used dimethyl labeling technology to study the active peptide in almond meal protein extract, and revealed that it had a good function of scavenging free radicals in the body, effectively reducing the damage caused by free radical oxidation to the body. Lee et al. (Citation2021) isolated a novel antimicrobial peptide from red pine needles, which can effectively inhibit the activity of food-borne bacteria and can be used as an alternative therapeutic drug in the food industry. Fitzgerald et al. (Citation2012) extracted proteins from large palmitoflagellate algae and enzymatically hydrolyzed them with papain to obtain active peptides, which were identified as renin inhibitory peptides with inhibitory effects on renin enzyme. Based on the above studies, it can be seen that plants are rich in bioactive peptides with diverse functions. However, there are few studies on the active peptide of yellow nutsedges, which is rich in high-quality protein source and has various pharmaceutical and food functions. It has a broad market prospect to develop active peptide with strong function using it as raw material.
Although the proportion of the aroma components is very low, they are the main source of attracting customers. Different processing technology can change the flavor composition and content of food, and then realize the high value of products. Guan et al. (Citation2022) analyzed the effect of baking on the flavor of defatted tiger nut flour by electronic nose and headspace solid-phase microextraction gas chromatography-mass spectrometry, which showed that baking processing can give defatted tiger nut flour special floral and fruity aromas. Ndiaye et al. (Citation2022a) evaluated the effect of roasting on tiger nuts tuber’s physicochemical and biochemical parameters in order to produce high nutritional quality flour. Ndiaye et al. (Citation2022b) optimized temperature and roasting time on Tiger nut’s tubers in order to produce high nutritional tiger nut flour, and found that the optimum roasting process was in a temperature range (130°C−150°C) and in a duration range (20–35 min). However, there are few studies on antioxidant capacity, small peptides species, and volatile aroma during the food processing of yellow nutsedges. The present study is expected to provide a theoretical basis for expanding consumption and enriching the variety of products.
2. Materials and methods
2.1. Material and sampling
No. 4 Yellow nutsedges of China was harvested on 30 September 2021, at the farm of Nongan County, Changchun City, Jilin Province. Yellow nutedges (China No. 4) turned light yellow color and approximately circular shape, which means that it got complete ripeness degree. After harvesting, yellow nutedges were taken to Food Processing Center Laboratory, Jilin University of Agricultural Science and Technology for pretreatment and processing. Yellow nutsedges preserved at room temperature was washed with water to remove the soil, and the fibrous roots were removed. The water content in yellow nutsedges was about 25%. Due to the high fibrosis of yellow nut peels and the poor edibility, therefore, the skin was removed with a satan peeler (6 FT-B7, Heze Engineering, China). Five kilograms of peeled yellow nutsedges were taken and dried in a 40°C oven (DHG−9053A, HengYi, China) for 3 hours. The dried samples were divided into two groups. One group (roasted group, RG) was roasted at 120°C for 80 min in the oven; the other group was kept in a sealed bag at room temperature for natural preservation (non-roasted group, NG). Roasted tubers of yellow nutsedges have a strong aroma and can be used to ground into flour or eaten plain. The flow diagram of yellow nutsedges preparation is shown in . Two groups of samples were used for antioxidant capacity, small-molecule peptide and aroma analysis.
2.2. Analysis of total antioxidant capacity, DPPH scavenging capacity, ABTS+, OH free radical scavenging capacity
The preparation of the analyzed samples in this part was slightly modified by referring to the method which used by Sahreen et al. (Citation2010). The sample was crushed and made into powder. The 1.0 g sample was weighed and placed in a 50 mL triangular flask, 25 mL of 70% ethanol solution was added, shaken well and extracted in an ultrasonic water bath for 30 min, centrifuged at 6500 R for 10 min, and the supernatant was poured into a 50 mL volumetric flask. The remaining residue was added with 25 mL 70% ethanol solution, shaken well and extracted in an ultrasonic water bath for 30 min, then centrifuged at 6500 R for 10 min. The supernatant was merged twice in a 50 mL volumetric flask with 70% ethanol solution. The extract was prepared and used for antioxidant analysis.
2.2.1. Determination of total antioxidant capacity
Total antioxidant capacity was determined by the FRAP (Ferric ion reducing antioxidant power) method which used by Benzie and Strain (Citation1996) with minor modifications. The working solution of FRAP was composed of 300 mmol/L sodium acetate buffer (pH3.6), 10 mmol/L TPTZ solution, and 20 mmol/L FeCl3·6 H2O solution at a volume ratio of 10:1:1. The absorbance value was measured at 593 nm in a 37°C water bath for 30 min. At the same time, water was used as a blank control. According to the above procedure, the standard curve was drawn with the standard solution of FeSO4·7 H2O instead of the sample, and the total antioxidant capacity of the sample was calculated as FeSO4·7 H2O. The test was repeated for 3 times, and the final average was taken in μmol/g.
2.2.2. DPPH clearance rate determination
The test refers to the method which used by Sahreen et al. (Citation2010) with slight modifications. 0.1 mL of the sample solution was tested, and then 2 mL DPPH (0.2 mmol/L) and 3 mL absolute ethanol were added, and then stood still for 30 minutes. The samples were measured at 517 nm. The test was repeated for 3 times, and DPPH scavenging was expressed as a percentage.
2.2.3. ABTS+ for determination of the clearance
The ABTS+ clearance rate was determined according to the method used by Kalt et al. (Citation1999) with slight modifications. 0.1 mL of the solution was tested, 5 mL of ABTS+ working solution was added, and reacted accurately at room temperature for 10 min. The samples absorbance was measured at 734 nm, and its clearance rate was calculated according to the degree of absorbance reduction. Antioxidant standard curves were established with Trolox, and the value of TEAC (Trolox Equivalent Antioxidant Capacity) was calculated. The ABTS scavenging ability of the sample was expressed as milligrams of Trolox equivalent contained in 100 g of sample (mg Trolox/100 g) and the experiment was repeated three times.
2.2.4. Determination of hydroxyl radical scavenging
Hydroxyl radical scavenging was determined according to the method used by H. Li and Wang (Citation2004) with slight modifications. Four milliliters of the assay solution was taken, 1 mL of 6 mmol/L FeSO4 and 1 mL of 8 mmol/L salicylic acid were added, and finally 1 mL of 24 mmol/L H2O2 was added to start the reaction. The reaction was then centrifuged at 4000 R/min for 10 min in a water bath at 37°C. Absorbance was measured at 510 nm. Distilled water instead of extract was used as control. The result of hydroxyl radical scavenging was expressed as a percentage and the test was repeated for 3 times.
2.3. Measurement of acid, peroxide, iodine value, and conjugated diene
The yellow nutsedges was crushed, and powder of 10 g weighed. The powder of yellow nutsedges was extracted with n-hexane at the solid–liquid ratio of 1:7 for 20 min under the condition of ultrasonic frequency of 60 kHz and temperature of 40°C. The extraction was repeated for 2 times and the solvent removed by rotary steaming to obtain the oil to be tested.
2.3.1. Analysis of acid value
Acid value was determined by reference to Chinese Food Safety Standard GB 5009.229–2016. Ten grams of oil, 75 mL of diethyl ether-isopropyl alcohol and 3 drops of phenolphthalein indicator were mixed thoroughly. 0.1 mol/L KOH was used to titrate the solution. When the solution appeared reddish, it indicated that the end point had been determined, and the acid value (mg/g) was calculated as GB 5009.229–2016. The experiment was repeated three times.
2.3.2. Analysis of peroxide value
Chinese Food Safety Standard GB 5009.227–2016 was referred for analyzing the peroxide value. 2.5 g oil sample was taken, put it into 250 mL triangle flask, and then added 30 mL mixture of trichloromethane and glacial acetic acid to make the sample completely dissolved. One-milliliter saturated potassium iodide solution was accurately added into the bottle, shaken gently for 0.5 min, and placed in the dark for 3 min. One hundred-milliliter water was added, immediately titrated the precipitated iodine with 0.01 mol/L sodium thiosulfate standard solution to light yellow, and then added 1 mL starch indicator until the solution blue disappeared as the end point. Finally, the peroxide value (mmol/kg) was calculated as GB 5009.227–2016 and the experiment repeated three times.
2.3.3. Analysis of conjugated diene
Conjugated diene analysis referred to the method used by Seven et al. (Citation2008) with slight modification. The yellow nutsedges were dried and then crushed. The powder 15 g was extracted with n-hexane, solid–liquid ratio was 1:7, and the filtrate was extracted for 15 min at the ultrasonic frequency of 59 kHz and temperature of 40°C. After filtration, the solvent was removed by rotary steaming to obtain yellow nutsedges oil to be tested. The oil sample was dissolved in 10 mL cyclohexane and prepared into a solution with a concentration of 0.03 g/L. The absorbance was measured at 233 nm. The content of conjugated diene in the oil can be calculated as follows:
A233/115·b·c
Where A233 was the absorbance of conjugated diene measured at 233 nm, b was width of absorbency pool 1 cm, c (g/L) was the mass concentration of the sample solution, and 115 was the absorbance coefficient of conjugated diene. The experiment was repeated three times.
2.3.4. Analysis of iodine value
Chinese Food Safety Standard GB 5532–2008 was referred for measuring iodine value. 0.2 g sample and 20 mL cyclohexane acetic acid solution (1:1) were placed into a triangle flask, then 25 mL Wechsler reagent (iodine:chlorine = 1:1) added, and put it in dark place. After dark treatment for 1 hour, 20 mL potassium iodide solution (100 g/L) and 150 mL water were added, then titrated with standard sodium thiosulfate solution (0.1 mol/L) until slightly yellow, adding a few drops of starch indicator solution turned blue, and continued titrating until colorless as the end point. The iodine value (g/100 g) was calculated as GB 5532–2008 and the experiment was repeated three times.
2.4. Analysis of small molecule peptides
2.4.1. Sample pretreatment
The samples were dissolved in 0.1% formic acid and ultrafiltered using a 10 Kd ultrafiltration tube. Subsequently, the sample solution was desalted and lyophilized using a Waters SEP-PAK C18 SPE column. Finally, it was redissolved in 0.1% formic acid water, centrifuged, and the supernatant was taken for detection.
2.4.2. Chromatographic separation
Liquid A was 0.1% formic acid in water, and liquid B was 0.1% formic acid in acetonitrile (80% acetonitrile). The liquid chromatographic column (50 μm × 150 mm, Acclaim PepMapTM RSLC, thermo scientific Technology Inc.) was equilibated with 92% liquid A, and the injection volume of 1 μL was separated by the column. The relevant liquid gradient was set as follows: at 0 min–98 min, the linear gradient of liquid B was from 8% to 28%; 98 min–113 min, the linear gradient of liquid B was from 28% to 37%; 113 min–117 min, the linear gradient of liquid B was from 37% to 100%; 117 min−120 min, B solution was maintained at 100%.
2.4.3. Identification by mass spectrometry
After capillary HPLC separation, Thermo QE HF mass spectrometer (Thermo Fisher) was used for mass spectrometry analysis. The analysis conditions were as follows: analysis time was 120 min and detection method positive ion. The mass charge ratios of peptides and 20 fragment profiles (MS2 Scan) were collected after each full scan. The scan range was 400–1800, the primary index rate was 60,000, the secondary resolution was 15,000, and the collision energy was CE28eV.
2.4.4. Identification of small molecule peptides
The Raw files of mass spectrometry test were retrieved by Proteome Discoverer 2.5, and the identification results of peptides and proteins were obtained.
2.4.5. Function analysis of small peptide
Peptide function analysis was performed based on the previously determined peptide sequences. PeptideRanker was used to predict the functional activity score of peptides, and peptides greater than 0.9 were screened for other functional analysis. ToxinPred and Expasy-compute pI were used to predict the toxicity and isoelectric points of peptides with higher activity values. To predict the activity of small-molecule peptide ACE inhibitor, Dipeptidyl Peptidase IV inhibitor, and antioxidative strategy using BIOPEP-UWM. CAMPR4 (Collection of Anti-Microbial Peptides) and BBPPred (blood–brain barrier peptides) were used to analyze the antimicrobial activity and blood–brain barrier.
2.5. Determination of aroma
About 3 g of samples were weighed and placed in a headspace bottle, sealed, balanced in a water bath at 80°C for 30 min, and then extracted with a solid phase microextraction needle for 30 min. After the extraction, the extraction needle was desorbed at the injection port for 5 min. Chromatographic column: HP−5 MS (30 m × 0.25 mm × 0.25 μm), split ratio: no split, carrier gas flow rate: 1.2 mL/min, inlet temperature: 250°C, scan mode: full scan, ion source temperature: 230°C, four-stage rod temperature: 180°C, heating procedure: initial temperature 50°C, kept for 2 min, then increased to 250°C at the rate of 6°C/min, kept for 5 min. The collected mass spectrograms were retrieved using the NIST (National Institute of Standards and Technology) spectrum library to identify the volatile components in the samples, and the relative contents of each component were analyzed by area normalization method. The experiment was repeated three times.
2.6. Data analysis
Experiments were performed through a completely randomized design. SPSS Software Statics 20.0 was used for analyzing the data. Significant differences of means were analyzed by Duncan’s multiple range tests at the 5% level.
3. Results and discussion
3.1. Effect of baking on the antioxidant activity of yellow nutsedges
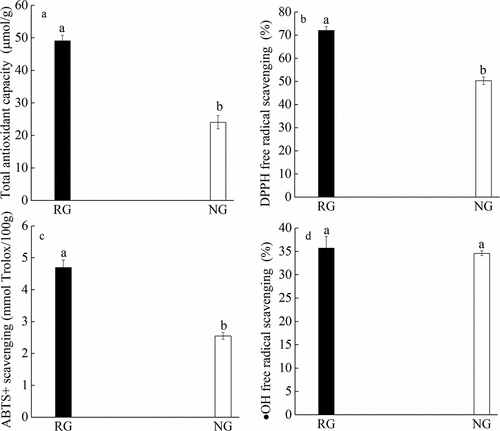
Yellow nutsedges have been applied in the pharmaceutical industry due to its antioxidant and other medicinal properties (Yu et al., Citation2022). Ademosun and Oboh (Citation2015) found that the DPPH and hydroxyl radical scavenging ability of A. chinensis could inhibit the production of MDA and the activities of α-amylase and α-glucosidase. Jing et al. (Citation2013) discovered that the oil of yellow nutsedges has the scavenging capacity of hydroxyl radicals and diphenyl picryl hydrazyl radicals. In this study, it was found that the antioxidant capacity, DPPH-free radical scavenging rate and hydroxyl-free radical scavenging rate were significantly improved after baking compared with those without baking (P < .05), and the ABTS+ scavenging rate was also relatively increased (). This phenomenon may be related to the Maillard reaction that occurs when food is heated, in which antioxidant substances are produced and antioxidant properties are improved. In addition, Djikeng et al. (Citation2022) found that the combination of drying and baking increased the antioxidant activity of yellow nutsedges from 11.00−17.40% to 56.34−96.33%. They also found that with the increase of treatment time, the scavenging rate of DPPH radical and hydroxyl radical would also increase. This is consistent with the research conclusion of this paper.
Figure 2. Compared analysis of antioxidant activity of yellow nutsedges in roasted group (RG) and non-roasted group (NG), total antioxidant capacity (a), DPPH free radical scavenging percentage (b), ·OH free radical scavenging percentage (c) and ABTS+· scavenging (d). The same letter a indicates no significant difference between the two groups. The letters a and b indicate a significant difference between the two groups at 0.05 level.
3.2. Effect of baking on the oxidation and stability degree of yellow nutsedges
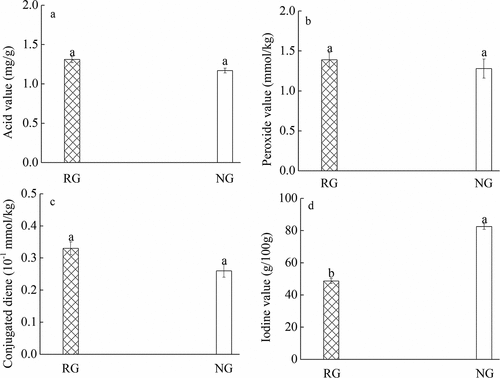
The oxidation degree is closely related to the quality of the product (Cui et al., Citation2022). Plant oils contain a large number of unsaturated fatty acids, which are prone to oxidation and decomposition during storage or processing, leading to rancidity of oils, changing their taste and reducing the quality of products. At the same time, rancidity of oils will produce hydroperoxides, which are easily decomposed into aldehydes and ketones, with potential carcinogenic risks. Therefore, it is important to control the oxidation stability of oils. In the process of forming hydroperoxides, the double bonds of polyunsaturated fatty acids will be rearranged to form conjugated olefin (Yettella et al., Citation2011). Therefore, the change of conjugated diene content in oils can reflect the oxidation degree of polyunsaturated fatty acids in oils. The iodine value reflects the degree of unsaturated fatty acids, and the low iodine value indicates that the baking process changes the characteristics of unsaturated fatty acids and improves the stability of products (An et al., Citation2017). In our study, the oxidation, acid value and conjugated diene of the roasted group did not increase significantly compared with non-roasted group (P ≥ .05), but the iodine value was lower in roasted group than the non-roasted (P < .05), which reveals that the roasted yellow nutsedges has higher food security and stability ().
Figure 3. Compared analysis of oxidation and stability degree of yellow nutsedges in roasted group (RG) and non-roasted group (NG), acid value (a), peroxide value (b), conjugated diene (c) and iodine value (d). The same letter a indicates no significant difference between the two groups. The letters a and b indicate a significant difference between the two groups at 0.05 level.
3.3. Effects of baking on the physicochemical properties of polypeptides from yellow nutsedges
Polypeptide molecules are organic compounds formed by the condensation and linkage of amino acids with antioxidant and antimicrobial activities. Due to their medicinal value, polypeptide molecules have been applied in the treatment of human diseases (Mani et al., Citation2022). Rayaprolu et al. (Citation2017) used peptides obtained from soybean to test the anti-cancer activity of human breast, blood and prostate cancer cells, and the results showed that the inhibition rate of peptides on cancer cells was as high as 50.0% to 68.0%, and soybean peptides could also inhibit gastrointestinal cancer. This effect could potentially make it an alternative food ingredient or nutritional supplement for cancer treatment. L. Feng et al. (Citation2022) isolated crude peptide from Vietnamese camellia, and concluded that active peptide of Vietnamese camellia can protect liver, improve the metabolism of cells damaged by ethanol, and play a certain therapeutic effect on alcohol-induced liver injury. In this study, a total of 19 polypeptide molecules with functional activity score greater than 0.9 were detected, including 17 in the baked group and 8 in the non-baked group. The isoelectric point range of baking group is 5.52–9.63, and the isoelectric point range of non-baking group is 3.80–10.03; In the baking group, 5 kinds of peptides were acidic and 3 kinds were alkaline. In the non-baking group, 13 kinds of peptides were acidic and 4 kinds were alkaline. None of the detected peptide molecules were toxic ().
Table 1. The physical and chemical properties of polypeptides in non-roasted group (NG) and roasted group (RG) with a peptide ranker score greater than 0.9.
3.4. Effect of baking on antimicrobial peptides of yellow nutsedges
Antimicrobial peptides, also known as defense peptides, are generally composed of 10 ~ 50 amino acids and are small molecular peptides that maintain host cells and defend against foreign pathogens. Antimicrobial peptides are different from antibiotics in that they have a wide range of antimicrobial activity, so their main role is to be used as a new class of therapeutic compounds for human use. In addition, it also has anti-inflammatory, healing and immunomodulatory activities (Jaiswal et al., Citation2022). Over the last few decades, antimicrobial peptides have become alternative agents that can meet the need for novel infectious agents and overcome antibiotic resistance. Antimicrobial peptides have potential applications as antimicrobial agents in food, agriculture, environment, animal husbandry and pharmaceutical industries (Erdem & Kesmen, Citation2022). Adelakun et al. (Citation2020) took rats as experimental subjects, and by giving them yellow nutsedges powder every day, it was observed that the mating behavior of rats was greatly improved, which was partly attributed to the increase of serum testosterone level in male rats. Experiments could prove that yellow nutsedges could promote the production of testosterone. It is concluded that the antimicrobial peptides in the soybean powder have anti-inflammatory and anti-apoptotic effects and can effectively prevent testicular dysfunction. Guha and Majumder (Citation2019) identified the active peptides val-pro-tyr and γ-glutamyl isolated from soybean as having anti-inflammatory properties. A total of six antimicrobial peptides with high antimicrobial function score were detected in this study, including two in the baking group and four in the control group. The types of antimicrobial peptides were changed by baking method ().
Table 2. The prediction of Antimicrobial Peptides (AMPs) in non-roasted group (NG) and roasted group (RG).
3.5. Effects of baking on enzyme inhibitors and antioxidant peptides in yellow nutsedges
ACE inhibitors are a class of bioactive substances that can inhibit the activity of ACE. With the continuous development of science and technology, natural and safe food-borne ACE inhibitors are increasingly favored by people and gradually come into people’s view. At present, people have found that legumes have ACE inhibitory activity (S. Li et al., Citation2022). In the study, we discover that the yellow nutsedges have the function of ACE inhibitors, but the baking changes the types of the ACE inhibitors peptides. There are three kinds ACE inhibitors in non-baked group and one kind in baked group (). Dipeptidyl peptidase IV inhibitor is a kind of treatment for type 2 diabetes drugs, which can inhibit glucagon-like peptide 1 (glp−1) promote insulin secretion and glucose dependence polypeptide (GIP) inactivated to improve the level of endogenous glp−1 and GIP islet beta cells release insulin, glucagon secretion and curb islets alpha cells (Kotaro et al., Citation2019). In this way, insulin level can be increased, blood glucose can be lowered, and hypoglycemia and weight gain are not easily caused. In this study, we discover that the yellow nutsedges have the function of DPP-IV, but the baking changes the types of the peptides. Although the Dipeptidyl peptidase IV inhibitor content was reduced in the baked group, it also had this function. Antioxidant peptides with the effect of inhibiting biological macromolecule peroxidation or scavenging free radicals in the body, which can alleviate human peroxidation and neutralize free radicals (Rahimipanah et al., Citation2022). In our study, baking enhances the kinds of antioxidant peptides from 1 to 2, which can effectively explains why the antioxidant activity of the baked group was higher than that of the control group.
Table 3. The prediction of ACE inhibitor, dipeptidyl peptidase IV inhibitor, and antioxidative in non-roasted group (NG) and roasted group (RG).
3.6. Effects of baking on blood–brain barrier peptides in yellow nutsedges
The blood–brain barrier (BBB) is a natural defense mechanism of the brain. It protects the brain through several mechanisms and allows nutrients to enter. It tightly controls the passage of molecules from the blood to the brain. It is an important way to treat brain-related diseases such as central nervous system diseases (Macarena et al., Citation2017). Blood–brain barrier peptides are fully protease resistant peptides that have acquired BBB shuttling capability to transport various cargo to the central nervous system (Prades et al., Citation2015). The incidence of brain diseases has increased significantly over the past few years, and the treatment of brain diseases is highly limited by the presence of the BBB. To overcome the blood–brain barrier impermeability, Cavaco et al. (Citation2021) have generated antibody fragments designed and conjugated to the BBB peptide shuttle that allowed it to penetrate the brain and have demonstrated the potential of Fc-PepH3 as a versatile platform designed to easily accommodate a variety of drugs of therapeutic value to treat different brain diseases. In this study, it was concluded through experiments that the BBB peptide contained in S. sativa had blood–brain barrier peptide-related functions. A total of five blood–brain barrier peptides were detected, among which two peptides were found in the non-baked group, one peptide were found in the baked group and two peptides in both groups (). Therefore, it was concluded that although the type of BBB barrier peptide was changed by baking method, the yellow nutsedges also had BBB function peptide.
Table 4. The prediction of blood–brain barrier peptides (BBPs) in non-roasted group (NG) and roasted group (RG).
3.7. Effect of baking on volatile esters
Aroma is an important factor in determining product quality. Many studies have shown that baking is an effective way to increase the variety of aromas. Ponzoni et al. (Citation2007) used the electronic nose to detect the key aromas peculiar of different stages of the bread baking process, revealing the suitability of the proposed electronic nose to distinguish these volatiles. Yutaka et al. (Citation2016) found that 41 aroma-active compounds were detected in cooked soy miso, and malty, green, roasty and sulfury aromas were identified as the characteristic aromas by the gas chromatography-olfactometry extract dilution assay approach and assessed the contribution to the umami aftertaste of six key compounds with the highest flavour dilution factor. M. M. Feng et al. (Citation2021) found that the contents of volatile flavor compounds were increased in Shanghai smoked fish when white sugar was replaced with glucose, octanol, and 2-pentyl furan for fructose. Guan et al. (Citation2022) analyzed the effect of baking on the flavor of defatted tiger nut flour by electronic nose and headspace solid-phase microextraction gas chromatography-mass spectrometry, which showed that baking processing can give defatted tiger nut flour special floral and fruity aromas. Esters are the second largest group of aromatic substances (Wang et al., Citation2022). Although the content volatile ester is low, but it is one of the important factors of food flavor quality. In this study, it was found that the unbaked yellow nutsedges contained only four kinds of esters, and baking group increased the esters to eight (). Four unique ester aroma substances, ethyl acetate, trichloroacetic acid, undecyl ester, dimethyl phthalate, and heptafluorobutyric acid, pentadecyl ester appeared after baking processing. It is worth noting that the roasting process leads to emergence in the ethyl acetate, which gives the roasted yellow nutsedges special floral and fruity aromas and is consistent with the results of Guan et al. (Citation2022). Therefore, the baking processing changed the types of esters in yellow nutsedges and improved the quality of it. The relative contents of esters in baked yellow nutsedges and the non-baked were 14.17% and 8.67%, respectively, which proved that the baked yellow nutsedges was conducive to the production of volatile aromatic compounds and revealed the reasons for consumers’ preference for baked yellow nutsedges products.
Table 5. The representation of aroma esters in non-roasted group (NG) and roasted group (RG).
4. Conclusions
The antioxidant capacity, DPPH-free radical scavenging rate and ABTS+ scavenging rate in yellow nutsedges were significantly improved by roast. The roasted processing can change the types of polypeptide molecules and potential biological functions. The types of esters are enhanced by roasting treatment. The results of this study will provide new peptide sources and new ideas for the development of leisure baked goods and the preparation of small-molecule peptides from new resources, which will promote the development of the yellow nutsedges industry.
Acknowledgements
Authors are very grateful with Li-ning Kang from Jilin Academy of Agricultural Sciences for his supplying yellow nutsedges.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adelakun, S. A., Akintunde, O. W., & Ogunlade, B. (2020). Fluoride-induced testicular degeneration and sperm quality deteriorations: Salutary role of Cyperus esculentus tubers (tiger nut) extract in animal model. Revista Internacional de Andrología, 19(3), 201–212. https://doi.org/10.1016/j.androl.2020.01.003
- Ademosun, A. O., & Oboh, G. (2015). Inhibition of carbohydrate hydrolyzing enzymes associated with type 2 diabetes and antioxidative properties of some edible seeds in vitro. International Journal of Diabetes in Developing Countries, 35(S3), 516–521. https://doi.org/10.1007/s13410-015-0339-7
- Ahmed, Z. S., & Hussein, A. M. (2014). Exploring the suitability of incorporating tiger nut flour as novel ingredients in gluten free biscuit. Polish Journal of Food and Nutrition Sciences, 64(1), 27–33. https://doi.org/10.2478/v10222-012-0087-z
- Albors, A., Raigon, M., García-Martinez, M., & Martín-Esparza, M. (2016). Assessment of techno-functional and sensory attributes of tiger nut fresh egg tagliatelle. LWT-Food Science and Technology, 74, 183–190. https://doi.org/10.1016/j.lwt.2016.07.047
- Angayarkanni, R., & Sridevi, G. (2018). Partial characterization and antimicrobial property of small peptides isolated from the flowers of millingtonia hortensis. Asian Journal of Pharmaceutical and Clinical Research, 11(12), 354–357. https://doi.org/10.22159/ajpcr.2018.v11i12.28297
- An, Z., Jiang, X., Xiang, G., Fan, L., He, L., & Zhao, W. (2017). A simple and practical method for determining iodine values of oils and fats by the FTIR spectrometer with an infrared quartz cuvette. Analytical Methods, 9(24), 3669–3674. https://doi.org/10.1039/C7AY00727B
- Badejo, A. A., Falarunu, A. J., Duyilemi, T. I., & Fasuhanmi, O. S. (2020). Antioxidative and anti-diabetic potentials of tigernut (Cyperus esculentus) sedge beverages fortified with Vernonia amygdalina and Momordica charantia. Journal of Food Measurement and Characterization, 14(5), 2790–2799. https://doi.org/10.1007/s11694-020-00524-y
- Benzie, I. F. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry, 239(1), 70–76. https://doi.org/10.1006/abio.1996.0292
- Cavaco, M., Frutos, S., Oliete, P., Valle, J., Andreu, D., Castanho, M. A. R. B., VilaPerelló, M., & Neves, V. (2021). Conjugation of a blood brain barrier peptide shuttle to an Fc domain for brain delivery of therapeutic biomolecules. ACS Medicinal Chemistry Letters, 12(11), 1663–1668. https://doi.org/10.1021/acsmedchemlett.1c00225
- Cui, N., Zhao, T., Han, Z., Yang, Z., Wang, G., Ma, Q., & Liang, L. (2022). Characterisation of oil oxidation, fatty acid, carotenoid, squalene and tocopherol components of hazelnut oils obtained from three varieties undergoing oxidation. International Journal of Food Science & Technology, 57(6), 3456–3466. https://doi.org/10.1111/ijfs.15669
- Defelice, M. S. (2002). Yellow nutsedge Cyperus esculentus L.—Snack food of the Gods 1. Weed Technology, 16(4), 901–907.
- Demirkesen, I., Sumnu, G., & Sahin, S. (2011). Quality of gluten-free bread formulations baked in different ovens. Food and Bioprocess Technology, 6(3), 746–753. https://doi.org/10.1007/s11947-011-0712-6
- Djikeng, F. T., Djikeng, C. F. T., Womeni, H. M., Ndefo, D. K. K., Pougoué, A. A. N., Tambo, S. T., & Esatbeyoglu, T. (2022). Effect of different processing methods on the chemical composition, antioxidant activity and lipid quality of tiger nuts (Cyperus esculentus). Applied Food Research, 2(2), 100124. https://doi.org/10.1016/j.afres.2022.100124
- Ekeanyanwu, R. C., Njoku, O., & Ononogbu, I. C. (2010). The phytochemical composition and some biochemical effects of Nigerian tigernut (Cyperus esculentus L .) tuber. Pakistan Journal of Nutrition, 9(7), 709. https://doi.org/10.3923/pjn.2010.709.715
- Erdem, B. M., & Kesmen, Z. (2022). Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. Journal of General and Applied Microbiology, 132(3), 1573–1596. https://doi.org/10.1111/jam.15314
- Feng, L., Chen, J., Yan, W., Ye, Z., Yu, J., Yao, G., Wu, Y., Zhang, J., & Yang, D. (2022). Preparation of active peptides from camellia vietnamensis and their metabolic effects in alcohol-induced liver injury cells. Molecules, 27(6), 1790. https://doi.org/10.3390/molecules27061790
- Feng, M. M., Dai, Z. T., Yin, Z. S., Wang, X. C., Chen, S. S., & Zhang, H. C. (2021). The volatile flavor compounds of Shanghai smoked fish as a special delicacy. Journal of Food Biochemistry, 45(1), e13553. https://doi.org/10.1111/jfbc.13553
- Fitzgerald, C., Mora-Soler, L., Gallagher, E., O’Connor, P., Prieto, J., Soler-Vila, A., & Hayes, M. (2012). Isolation and characterization of bioactive pro-peptides with in vitro renin inhibitory activities from the Macroalga Palmaria palmata. Journal of Agricultural and Food Chemistry, 60(30), 7421–7427. https://doi.org/10.1021/jf301361c
- Gasparre, N., Pan, J., Alves, P. L. D. S., Rosell, C. M., & Berrios, J. D. J. (2020). Tiger nut (Cyperus esculentus) as a functional ingredient in gluten-free extruded snacks. Foods, 9(12), 1770. https://doi.org/10.3390/foods9121770
- Guan, C., Liu, T., Li, Q., Wang, D., & Zhang, Y. (2022). Analyzing the effect of baking on the flavor of defatted tiger nut flour by E-Tongue, E-Nose and HS-SPME-GC-MS. Foods, 11(3), 446. https://doi.org/10.3390/foods11030446
- Guha, S., & Majumder, K. (2019). Structural-features of food-derived bioactive peptides with anti-inflammatory activity: A brief review. Journal of Food Biochemistry, 43(1), e12531. https://doi.org/10.1111/jfbc.12531
- Huang, Y., Dias, F. F. G., Leite, N. M. B. J. M., & Barile, D. (2022). A complete workflow for discovering small bioactive peptides in foods by LC-MS/MS: A case study on almonds. Food Chemistry, 369, 130834. https://doi.org/10.1016/j.foodchem.2021.130834
- Jaiswal, M., Singh, A., & Kumar, S. (2022). PTPAMP: Prediction tool for plant-derived antimicrobial peptides. Amino Acids, 55(1), 1–17. https://doi.org/10.1007/s00726-022-03190-0
- Jing, S., Ouyang, W., & Ren, Z. (2013). The in vitro and in vivo antioxidant properties of Cyperus esculentus oil from Xinjiang, China. Journal of the Science of Food and Agriculture, 93(6), 1505–1509. https://doi.org/10.1002/jsfa.5927
- Kalt, W., Forney, C. F., & Martin, A. (1999). Antioxidant capacity vitamin Cphenolics, and anthocyanins after fresh storage of small fruits. Journal of Agricultural & Food Chemistry, 47(11), 4638–4644. https://doi.org/10.1021/jf990266t
- Kotaro, T., Takao, N., Kimihisa, U., Yuji, N., & Katsuya, H. (2019). Effects of combination treatment with dipeptidyl peptidase IV inhibitor and Sulfonylurea on glucose levels in rats. Journal of Pharmacological Sciences, 95(2), 291–293. https://doi.org/10.1254/jphs.SC0040043
- Lee, J., Kang, H. K., Cheong, H., & Park, Y. (2021). A novel antimicrobial peptides from pine needles of Pinus densiflora Sieb. et Zucc. against foodborne bacteria. Frontiers in Microbiology, 12, 662462. https://doi.org/10.3389/fmicb.2021.662462
- Li, S., Du, G., Shi, J., Zhang, L., Yue, T., & Yuan, Y. (2022). Preparation of antihypertensive peptides from quinoa via fermentation with Lactobacillus paracasei. eFood, 3(3). https://doi.org/10.1002/efd2.20
- Liu, D., Guo, Y., & Ma, H. (2022). Production, bioactivities and bioavailability of bioactive peptides derived from walnut origin by-products: A review. Critical Reviews in Food Science and Nutrition, 11–16. https://doi.org/10.1080/10408398.2022.2054933
- Li, H., & Wang, Q. (2004). Evaluation of free hydroxyl radical scavenging activities of some Chinese herbs by capillary zone electrophoresis with amperometric detection. Analytical & Bioanalytical Chemistry, 378(7), 1801–1805. https://doi.org/10.1007/s00216-004-2509-1
- Macarena, S., Ernest, G., & Meritxell, T. (2017). Blood–brain barrier peptide shuttles. Current Opinion in Chemical Biology, 38, 134–140. https://doi.org/10.1016/j.cbpa.2017.04.019
- Mani, S., Bhatt, S. B., Vasudevan, V., Prabhu, D., Rajamanikandan, S., Velusamy, P., Ramasamy, P., & Raman, P. (2022). The updated review on plant peptides and their applications in human health. International Journal of Peptide Research and Therapeutics, 28(5), 135. https://doi.org/10.1007/s10989-022-10437-7
- National Health Commission of the People’s Republic of China. GB 5009.227-2016.
- National Health Commission of the People’s Republic of China. GB 5009.229-2016.
- National Health Commission of the People’s Republic of China. GB 5532-2008.
- Ndiaye, B., Ndiaye, S., Faye, P. G., Orabueze, I. C., Diouf, A., Cisse, O. I. K., Ndiaye, E. M., Sow, A., Sakho, M., & Asseyou, N. C. M. (2022a). Investigation of physicochemical and biochemical properties of roasted tiger nut (Cyperus esculentus) flour. International Journal of Food Science & Nutrition Engineering, 12(1), 26–35.
- Ndiaye, B., Sow, A., Faye, S., Cissé, O. I. K., Dieye, F., Faye, P. G., Sakho, M., & Ayessou, N. C. (2022b). Optimization of tiger nut (Cyperus esculentus) roasting process using response surface methodology. Food and Nutrition Sciences, 13(10), 811–825. https://doi.org/10.4236/fns.2022.1310058
- Ponzoni, A., Depari, A., Falasconi, M., Comini, E., Flammini, A., Marioli, D., Taroni, A., & Sberveglieri, G. (2007). Bread baking aromas detection by low-cost electronic nose. Sensors & Actuators: B Chemical, 130(1), 100–104. https://doi.org/10.1016/j.snb.2007.07.099
- Prades, R., Oller-Salvia, B., Schwarzmaier, S. M., Selva, J., Moros, M., Balbi, M., Grazú, V., La, F. J. M., Egea, G., Plesnila, N., Teixidó, M., & Giralt, E. (2015). Applying the retro-enantio approach to obtain a peptide capable of overcoming the blood-brain barrier. Angewandte Chemie (International Edition in English), 54(13), 3967–3972. https://doi.org/10.1002/anie.201411408
- Rahimipanah, M., Sadeghi, M. A., Ghorbani, M., Shahiri, T. H., & Nabi, M. M. (2022). Expeller-pressed pomegranate seed (Punica granatum L.) as a protein source for the production of antioxidant peptides. International Journal of Peptide Research and Therapeutics, 28(4). https://doi.org/10.1007/s10989-022-10432-y
- Rayaprolu, S. J., Hettiarachchy, N. S., Horax, R., Phillips, G. K., Mahendran, M., & Chen, P. (2017). Soybean peptide fractions inhibit human blood, breast and prostate cancer cell proliferation. Journal of Food Science and Technology, 54(1), 38–44. https://doi.org/10.1007/s13197-016-2426-2
- Roselló-Soto, E., Garcia, C., Fessard, A., Francisco, J. B., Paulo, E. S. M., Jose, M. L., & Remize, F. (2019). Nutritional and microbiological quality of tiger nut tubers (Cyperus esculentus), derived plant-based and lactic fermented beverages. Fermentation, 5(1), 3. https://doi.org/10.3390/fermentation5010003
- Sahreen, S., Khan, M. R., & Khan, R. A. (2010). Evaluation of antioxidant activities of various solvent extracts of Carissa opaca, fruits. Food Chemistry, 122(4), 1205–1211. https://doi.org/10.1016/j.foodchem.2010.03.120
- Sánchez-Zapata, E., Fernández-López, J., & Pérez-Alvarez, J. A. (2012). Tiger Nut (Cyperus esculentus) commercialization: Health aspects, composition, properties, and food applications. Comprehensive Reviews in Food Science and Food Safety, 11(4), 366–377. https://doi.org/10.1111/j.1541-4337.2012.00190.x
- Seven, A., Guzel, A., Aslan, M., & Hamuryudan, V. (2008). Lipid, protein, DNA oxidation and antioxidant status in rheumatoid arthritis. Clinical Biochemistry, 41(8), 538–543. https://doi.org/10.1016/j.clinbiochem.2008.01.029
- Wang, Z. H., Xiong, A., Yu, Y. G., & Zheng, Q. (2022). Electrochemical esterification in distilled liquor via gold catalysis and its application for enhancing ester aroma of low-alcohol liquor. Current Research in Food Science, 5, 1769–1776. https://doi.org/10.1016/j.crfs.2022.10.003
- Yang, X., Niu, L., Zhang, Y., Ren, W., Yang, C., Yang, J., Xing, G., Zhong, X., Zhang, J., Slaski, J., & Zhang, J. (2022). Morpho-agronomic and biochemical characterization of accessions of tiger nut (Cyperus esculentus) grown in the North Temperate Zone of China. Plants, 11(7), 923. https://doi.org/10.3390/plants11070923
- Yettella, R. R., Henbest, B., & Proctor, A. (2011). Effect of antioxidants on soy oil conjugated linoleic acid production and its oxidative stability. Journal of Agricultural and Food Chemistry, 59(13), 7377–7384. https://doi.org/10.1021/jf2007425
- Yu, Y., Lu, X., Zhang, T., Zhao, C., Guan, S., Pu, Y., & Gao, F. (2022). Tiger nut (Cyperus esculentus L.): Nutrition, processing, function and applications. Foods, 11(4), 601. https://doi.org/10.3390/foods11040601
- Yutaka, I., Sachie, K., Miyu, S., Chihiro, S., Yuriko, O., Yutaro, T., Hirohito, W., & Fumitaka, H. (2016). Analysis of the cooked aroma and odorants that contribute to umami aftertaste of soy miso (Japanese soybean paste). Food Chemistry, 213, 521–528. https://doi.org/10.1016/j.foodchem.2016.06.106
- Zhang, S., Li, P., Wei, Z., Cheng, Y., Liu, J., Yang, Y., Wang, Y., & Mu, Z. (2022). Cyperus (Cyperus esculentus L.): A review of its compositions, medical efficacy, antibacterial activity and allelopathic potentials. Plants, 11(9), 1127. https://doi.org/10.3390/plants11091127