?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study utilized column chromatography and nuclear magnetic resonance (NMR) to isolate and identify 42 compounds in Potentilla fruticosa L. (PL), which compound 10 and 35 were preliminarily identified. Compounds 5–9, 11–13, 16, 17, 20, 23, 24 and 28 (total 14 polyphenols) promoted glucose consumption in IEC cells, exhibiting potential in reducing blood glucose levels. Mechanistic analysis revealed that the 14 polyphenols significantly inhibited α-glucosidase activity, and (expressed as semi-inhibitory concentration values) the capacity ranking is 13 > 9 > 12 > 5 > 16 > 8 > 17 > 7 > 6 > 11 > 28 > 23 > 24 > 20. Molecular docking showed that all 14 polyphenols have an active binding site with α-glucosidase, and the binding energy is all lower than −6.0 kcal·mol−1, thereby inhibiting the entry of p-nitrophenyl-d-glucoside. The results show that PL polyphenols can be used as effective α-glucosidase inhibitors.
1. Introduction
Diabetes is a grave global health affliction, as evidenced by the International Diabetes Federation’s (IDF) recent report indicating that there are 537 million individuals aged between 20 and 79 years old worldwide who suffer from this condition in 2021. The global incidence of diabetes is also surging, with the IDF projecting that the worldwide prevalence of diabetes will reach 578 million by 2030 (Shah et al., Citation2021). In this context, the global health expenditure caused by diabetes is as high as $966 billion every year, which brings a heavy economic burden to all countries (Demir et al., Citation2021). Type 2 diabetes mellitus (T2DM), which accounts for more than 90% of diabetic patients (Meijnikman et al., Citation2017; Saeedi et al., Citation2019), is a chronic metabolic disease that is mainly caused by impaired insulin secretion, increased hepatic glycogen synthesis and impaired glucose utilization in peripheral tissues (Hotamisligil, Citation2006). As a result, T2DM is characterized by hyperglycemia with elevated blood glucose levels, and the management of T2DM patients typically involves glycemic control through reduction of blood glucose levels (Z. Wang et al., Citation2021).
Chemical α-glucosidase inhibitors like acarbose are the major antidiabetic drugs used in the treatment of T2DM. These chemical inhibitors function by impeding the hydrolysis of disaccharide and oligosaccharide substrates into absorbable monosaccharides, thereby retarding intestinal carbohydrate digestion/absorption and ameliorating postprandial hyperglycemia (Herman, Citation2015). However, these chemical inhibitors exhibit several adverse hepatic events and increase hepatic enzyme levels. Therefore, it is imperative to explore alternative potent α-glucosidase inhibitors with reduced adverse effects (D. Li et al., Citation2019; Z. C. Wang et al., Citation2021). Plant polyphenols, primarily sourced from fruits and herbs, encompassing phenolic acids, flavonoids, and tannins, constitute essential constituents of the human diet (Quideau et al., Citation2011). Plant polyphenols exhibit a diverse range of biological functionalities, including antioxidant, antibacterial, antidiarrheal, radioprotective, anticancer, and hypoglycemic effects (L. Li et al., Citation2021). The inhibitory effects of plant polyphenols on digestive enzymes like α-glucosidases have attracted great interests among researchers.
Potentilla fruticosa L. (PL) is a robust deciduous flowering shrub belonging to the Potentilla genus of the Rosaceae family, and it has a wide distribution in western China (Qin et al., Citation2022). The leaves of PL have been widely utilized in Chinese traditional medicine for thousands of years to invigorate the spleen, alleviate heat, regulate menstruation, and as raw materials in food and cosmetic processing. The rhizome and branch of PL also possess potent biological activities, including anti-inflammatory effects as well as glucose- and lipid-lowering effects (Tomczyk & Latté, Citation2009). It is widely acknowledged that the bioactive compounds present in plants contribute significantly to their bioactivity (Qiu et al., Citation2018). However, limited information regarding the chemical composition and α-glucosidase inhibitory activity of polyphenols present in PL is available. Therefore, the objective of this study was to explore the profile of PL polyphenols and their α-glucosidase inhibition activity, which could offer an insight into PL as a therapeutic plant and provide diabetic patients with additional options for plant-derived α-glucosidase inhibitors.
2. Materials and methods
2.1. Plant materials
The fresh leaves of PL were collected from Tibetan Autonomous Prefecture of Haibei (Latitude, 37°8ˊ42“N; Longitude, 101°50ˊ32“E; Altitude, 3006 m), Qinghai Province, China, in 2022. The collected 35 kg of PL leaves were air-dried under shade at room temperature, crushed into powder using a high-speed multi-function grinder (HL-100, Shanghai Senai Machinery Co., Shanghai, China) and sieved through a 60-mesh sieve. The resulting powders were stored at −18°C for further use.
2.2. Chemical reagents
α-Glucosidase (Saccharomyces cerevisiae, EC 3.2.1.20), 4-Nitrophenyl-α-D-glacopyranoside (PNPG), DMSO (Dimethyl sulfoxide) were purchased from Sigma Aldrich (St. Louis, MO, U.S.A.); Acarbose was purchased from Bayer (Leverkusen, Germany); Na2HPO4 was purchased from Shuangshuang Chemical Co., Ltd. (Laiyang, Shandong, China); NaH2PO4 was purchased from Damao Chemical Reagent Manufacturing Co., Ltd. (Tianjin, China).
2.3. Extraction of PL polyphenols
The powders of PL leaves were extracted three times using 95% methanol at a ratio of 1:3 (w:v). The extracts were collected and concentrated using a rotary evaporator (R205, Shanghai Shensheng Technology Co., Shanghai, China) at 50°C to 4.2 kg. The resulting extract was dispersed in water and sequentially extracted with petroleum ether and ethyl acetate for the petroleum ether layer, ethyl acetate layer, and aqueous layer.
2.4. Separation of PL polyphenols
The petroleum ether layer, ethyl acetate layer, and aqueous layer were subjected to deossification and decolorization using Diaion HP20SS methanol/water (1:10) for purification purposes (Samec et al., Citation2016), followed by elution using MCI-gel CPH-20P (MeOH-H2O elution), Rp-18 (MeOH-H2O elution), Toyopearl (MeOH-H2O elution), silica gel (CHCl3-MeOH-H2O elution) on a Sephadex LH-20 column chromatography.
2.5. Identification of PL polyphenols
A Brucker AM-400 NMR instrument equipped with deuterated reagents (CD3OD, DMSO, and CDCl3) was used to identify PL polyphenols in 1D and 2D NMR spectra. The deuterated solvent peak is used as the internal standard, the chemical shift δ is expressed by ppm, and the coupling constant J is expressed by Hz. ESI-MS was determined by liquid phase-ion trap chromatography-mass spectrometry.
2.6. Glucose consumption of small intestinal epithelial cells (IECs)
The IECs were cultured and maintained in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (DMEM-F12) (Life Technologies, Shanghai, China) supplemented with 10% fetal bovine serum (FBS; Gibco, U.S.A) and 0.5% penicillin–streptomycin (Invitrogen). The IECs were enzymatically dissociated and plated onto 96-well culture plates, followed by incubation at 37°C in 5% CO2 until 70% confluence.
After washing the cells twice with the FBS buffer, an aliquot of 200 μL of low-sugar medium (glucose concentration of 1800 mg/L) containing 0.5% penicillin-streptomycin was added. The control group was incubated with dimethyl sulfoxide (DMSO, Hengxing Chemical Preparation Co., Ltd., Tianjin, China) containing 100 μM insulin, and the test sample groups were respectively incubated with the 42 PL polyphenols at a final concentration of 25 μM. There were three replicate holes for each sample. After 24 h of incubation, an aliquot of 10 μL of cell culture medium was drawn to measure the glucose concentration in the culture medium using the glucose oxidase-peroxidase method. At the same time, the remaining cell culture medium was added with 20 μL of MTS, followed by incubation at 37°C for 2 h and recorded at 492 nm to determine whether the compound is toxic to the IECs.
2.7. α-Glucosidase inhibitory activity
The α-glucosidase inhibitory activity of PL polyphenols was measured according to a previous study (Kakarla et al., Citation2016). The assay solution containing 20 μL of PL polyphenols with different concentrations, 100 μL of phosphate buffer (0.05 mol/L, pH 6.8), 20 μL of PNPG (8.92 × 10−3 mol/L), and 20 μL of α-glucosidase (0.05 U/mL) were incubated at 37°C for 20 min. Subsequently, the absorbance of the reaction solution at 400 nm was measured using an automatic enzyme standardizer (MAX190, Molecular Devices, LLC, San Jose, CA, U.S.A.). Distilled water was used to replace the enzyme solution in the blank group. Three parallel tests were performed at the same concentration of each sample, and the average value was obtained. In order to eliminate the influence of sample and substrate PNPG on the measurement results, the background absorption value of the sample and substrate needs to be measured. The samples and substrates were corrected with a phosphate buffer of 0.05 mol/L. The inhibition rate (I, %) of the samples was calculated using the following equation:
where A1 is the original enzyme activity, A2 is the enzyme activity after adding inhibitors, A3 is the background value of PNPG, and A4 is the background value of inhibitors.
The α-glucosidase inhibitory activity of PL polyphenols was expressed as the median effective concentration for inhibitory activity (IC50), which was the concentration required to inhibit 50% of the α-glucosidase activity under the specified assay conditions.
2.8. Inhibitory kinetic analysis
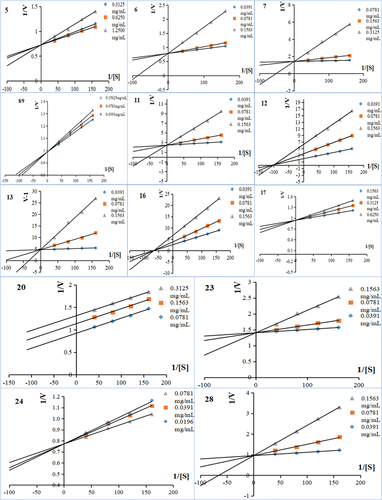
To further understand the inhibitory mechanism of PL polyphenols against α-glucosidase, a kinetic study was carried out according to the previous study (J. Wang et al., Citation2023). The Burke diagram of the PL polyphenols is drawn by the reverse velocity (1/V) and four PNPG concentration (1/[S]), and their Dixon diagram is drawn by the reverse velocity (1/V) of different concentrations of four PNPG. The mixed-type inhibition was evaluated by Lineweaver-Burk plots and described by the following equations (Butterworth, Citation1972; Kakarla et al., Citation2016):
Secondary plots can be constructed as:
and
where Ki and Km represent the inhibition constant and the Michaelis–Menten constant, respectively, m is the enzyme reaction velocity, [I] and [S] denote the concentration of inhibitor and substrate, respectively, and a is the apparent coefficient. The replots of slope and Y-intercept versus [I] were linearly fitted, which suggested that there may be a single inhibition site or a single class of inhibition site (Han et al., Citation2018; Şöhretoğlu et al., Citation2018). The kinetic data were analyzed using a computer program for linear regressions.
2.9. Molecular docking calculation
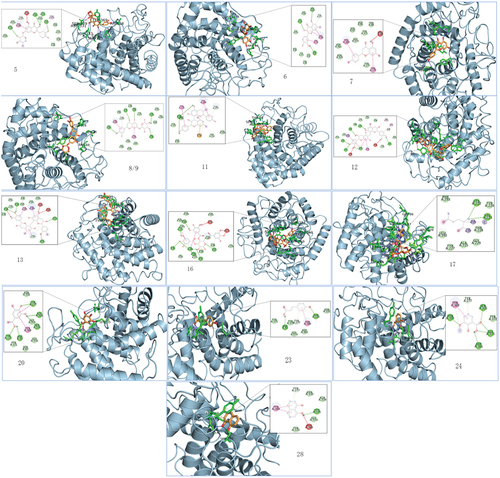
A molecular docking calculation was also performed according to the previous study (J. Wang et al., Citation2023), to investigate the interaction between PL polyphenols and α-glucosidase. The 3D structure of PL polyphenols was constructed by Chem 3D software and saved in mol2 format. The rotatable bond of ligand was determined by Auto Dock Vina 1.1.2 software from the Molecular Graphics Lab. The crystal structure of α-glucosidase enzyme (PDB ID: 1KWFA) was obtained from the Protein Data Bank and further prepared by ionization and minimization. The protein was then dehydrated and ligand removed with PyMOL software. AutoDock software was used for hydrogenation and charge calculation of key targets. AutoDock Vina software was used for molecular docking, and PyMOL software (Schrödinger LLC, New York, NY, U.S.A.) and Discovery Studio 3.5 software (Accelrys Software Inc., San Diego, CA, U.S.A.) were used for visual processing of the results.
2.10. Statistical analysis
All the results are expressed as mean ± standard deviation. A one-way analysis of variance (ANOVA) was performed using SPSS statistics 25.0 (SPSS Inc., Chicago, IL, U.S.A.). Differences were considered significant between the samples at p < .05.
3. Results
3.1. Composition and content of PL polyphenols
According to the mass spectrum and NMR information of the extracts from PL leaves, as shown in Table S1, a total of 42 monomers were isolated and identified by comparing the detailed data with published values (Avelar et al., Citation2014; Boyer & Ducrot, Citation2005; Gai-Mei et al., Citation2010; Ma et al., Citation2016; Olennikov et al., Citation2015; Qiongyu et al., Citation2015; Shakera et al., Citation2015; Yoshiki et al., Citation1990; Zheng, Citation2015). The identification of the PL extracts are presented in , which shows that the PL extracts were composed of 40 known compounds and two new compounds (compounds 10 and 35). Among these compounds, compounds 1–9 belong to flavonoids, compounds 11–17 belong to tannins, compounds 18–34 belong to polyphenols, compounds 37–40 belong to phenylpropanoids and compounds 41–42 belong to triterpenes. In addition, it was observed that compounds 4, 7, 10–12, 14–16, 18, 21–36, 39–42 existed only in a small proportion, while the other compounds, in particularly compound 38, were the dominant polyphenols in PL.
Table 1. Identification and quantification of polyphenols in PL.
3.2. Glucose consumption of IECs
All the isolated 42 polyphenols were subjected to glucose consumption experiment using IECs cells, with the injection insulin and oral acarbose as the positive control drugs. The results showed that 14 polyphenols (compounds 5–9, 11, 13, 16–17, 20, 23–24, and 28) demonstrated significant glucose consumption rates ranging from 28.66% to 24.11%, which are significantly higher than that of the normal group (15.18%) (). The glucose consumption rate of the other compounds is lower than that of the normal group (Table S2), indicating their inability to regulate glucose consumption of IECs. Therefore, the aforementioned 14 polyphenols could be used as promising blood glucose regulators.
Table 2. Effect of PL extraction on glucose consumption of IECs.
3.3. α-Glucosidase inhibition activity
α-Glucosidase is a crucial digestive enzyme located at the brush border of the small intestine. It has the ability to hydrolyze terminal non-reducing (1→4)-linked short chain reducing sugars, such as maltose and oligosaccharides, and releasing a single α-glucose molecule (Z. C. Wang et al., Citation2019). Therefore, to investigate the mechanism that the 14 polyphenols are ability to regulate glucose consumption of IECs, their inhibition activity against α-glucosidase were analyzed. The α-glucosidase inhibitory efficacy of the 14 polyphenols was evaluated by IC50 values. As shown in , their IC50 values were between 0.063 mg/mL and 0.982 mg/mL, with compound 13 exhibiting the lowest IC50 value (0.063 mg/mL). The IC50 values of the 14 polyphenols were all lower than that of the normal group (2.895 mg/mL), indicating their ability to inhibit the activity of α-glucosidase. However, the 14 polyphenols showed weaker inhibitory activities against α-glucosidase as compared to insulin and acarbose as the IC50 values of the 14 polyphenols were higher than that of the insulin and acarbose group (0.02 and 0.047 mg/mL, respectively) (). Considering that insulin and acarbose are relatively expensive and can cause various adverse hepatic events (D. Li et al., Citation2019; Z. C. Wang et al., Citation2021), in recent years, there has been a growing interest in the utilization of dietary polyphenols as effective antidiabetic agents owing to their ability to inhibit digestive enzymes (J. Zhang et al., Citation2019). Therefore, the 14 polyphenols may serve as a promising alternative to synthetic drugs in the prevention and management of T2DM by inhibiting α-glucosidase activity. The potential inhibitory mechanism of 14 polyphenols against α-glucosidase was further investigated.
Table 3. In vitro inhibitory activity of compounds against α-glucosidase, type of inhibition and molecular docking binding energy.
3.4. Inhibition type
The combination of Dixon and Cornish-Bowden plots can be used to analyse the inhibition type of phytochemicals (Dixon & Cornish-Bowden, Citation2010). The Dixon plots of α-glucosidase inhibition by the 14 polyphenols intersected at one point (data not shown), while the Cornish-Bowden plots of α-glucosidase inhibition by compounds intersected at one point but those were found to be parallel (). The results suggested that compounds 5, 6, 11, 12, 17, 20, 23, 24 and 28 were competitive inhibitors, compounds 8, 9, and 13 were mixed inhibitors, and compounds 7 and 16 were non-competitive inhibitors.
3.5. Molecular docking calculation
To provide further evidence of the interactions between α-glucosidase and the14 polyphenols, a molecular docking study was conducted. The findings showed that the polyphenols were docked into the enzymes’ active centres, which are composed of common amino acid residues, through van der Waals forces, hydrogen bonds, and π–π interactions (). These interactions suggest a potential mechanism by which the polyphenols modulate the enzymatic activity of α-glucosidase. Hydrogen bond was the main force that drives the interactions between the polyphenols and α-glucosidase. The number of hydrogen bond of α-glucosidase with the 14 polyphenols (compounds 5, 6, 7, 8, 9, 11, 12, 13, 16, 17, 20, 23, 24 and 28) was 3 (Asn331, Asp365, Ser335, and Ser87), 3 (Asp85, Ser335, and Asn333), 0, 5 (Asn333, Ser87, Arg84, Asp278, and Arg281), 5 (Asn333, Asp365, Ser87, Asp278, Arg281, and Gln95), 2 (Ser88), 5 (Ser335, Asp365, Ser87, Asp278, Asp152, and Tyr277), 3 (Arg84, Gln95, Asn333, and Asn331), 5 (Gln95, Tyr372, Asp365, Ser87, Asp85, and Arg281), 2 (Lys222, Val223), 3 (Tyr277, Ser335, Asp365, Asp85, and Tyr372), 2 (Asp85, Asn333), 3 (Asn333, Ser335, Gly373, and Asp365), 1 (Ser335). As a result, the binding affinities of the 14 polyphenols are −11.9 kcal/mol (compound 13), −11.6 kcal/mol (compound 9), −11.3 kcal/mol (compound 12), −10.9 kcal/mol (compound 5), −10.8 kcal/mol (compound 16), −10.4 kcal/mol (compound 8), −10.3 kcal/mol (compound 17), −10 kcal/mol (compound 7), −9.9 kcal/mol (compound 6), −8.8 kcal/mol (compound 11), −8.5 kcal/mol (compound 28), −7.5 kcal/mol (compound 23), −7.2 kcal/mol (compound 24), −6.9 kcal/mol (compound 20), respectively. A low binding affinity of polyphenols with enzyme represents a stable conformation; therefore, the results suggested that the α-glucosidase inhibition by the 14 polyphenols followed the order of (13 > 9 > 12 > 5 > 16 > 8 > 17 > 7 > 6 > 11 > 28 > 23 > 24 > 20). The findings are consistent with the results of the α-glucosidase inhibition assay.
4. Discussion
α-Glucosidase hydrolyses starch initial hydrolysate maltooligosaccharides to glucose, playing an important role in deciding postprandial blood sugar level (B. Zhang et al., Citation2015). Therefore, inhibiting the enzyme activity by applying/in taking dietary polyphenols has been reported to potentially postpone the increase in blood glucose level after meal (Deng et al., Citation2015; Papoutsis et al., Citation2021). In this study, 42 compounds were isolated from PL by column chromatography and identified compositionally by nuclear magnetic resonance (NMR). This study used column chromatography to isolate 42 compounds from PL and analyzed 7 flavonoids, 20 tannins, 17 polyphenols, 4 phenylpropanoids, 2 triterpenes, and 2 new compounds (10* and 35*) using nuclear magnetic resonance (NMR) for compositional identification. Wang et al. extracted compounds from Perilla frutescens Leaf and found that the ethyl acetate fraction was high in phenols and flavonoids and had good antioxidant capacity and inhibited α-glucosidase better (Z. Wang et al., Citation2021). Among the 42 compounds extracted from PL, 14 compounds showed a good inhibitory effect on α-glucosidase, which are polyphenolic compounds. Therefore, to explore the mechanisms in inhibition of α-glucosidase by the selected polyphenols and how the essential moieties of compounds contributed to the enzyme inhibition, the interactions between 14 compounds and α-glucosidase were further studied through inhibition kinetics, glucose consumption, and molecular docking, and discussed as follows.
The effect of IECs on glucose consumption has been studied (Song et al., Citation2009). After the IECs cells differentiated, the nutrient digestive absorption function of small intestinal epithelial cells was used widely by foreign scholars to repair cells, molecules, and gene mechanisms for small intestinal mucosa repair (Meng et al., Citation2005). By determining the consumption of glucose in the culture medium by determining the consumption of glucose in the culture solution, it is possible to find the function of promoting glucose consumption, which can be used to find functional components for controlling diabetes (Abbasi et al., Citation2016). This study uses the IECs cells to take the glucose model to the 42 compounds separated from PL to blood glucose activity. In 25 μM concentrations for 24 h, the positive control insulin and acarbose showed a significant activity promoting IECs cells to ingest glucose. Compounds 5,6,7,8,9,11,12,13,16,17,20,23,24,28 have significant activity to promote cell-proof glucose, indicating that these compounds may have potential hypoglycemic activity ().
Therefore, the inhibitory activity (characterized as IC50 value) was determined and calculated for the 14 compounds, while the IC50 values of the other compounds were considered relatively high as they were shown with much lower enzyme inhibition effects. The first 10 compounds in the 14 compounds are flavonoids and tannins, and the A ring in the flavonoids of 5–9 is combined with Tyr protein to form a pi–pi Stacked. Other stable keys are pi–donor hydrogen bond, van der Waals, pi–donor hydrogen bond, van der Waals, conventional hydrogen bond, carbon hydrogen bond, and pi–donor hydrogen bond. C5-OH (Ring A) of compound 5 and 7 is formed to form unfavorable donor–donor with ASN333 and Arg84, respectively, which may cause these two compounds’ IC50 values not the highest ().
The tannin compound has a large molecular weight (), with a large bit point in combination with glycosidase, and the various pI bonds formed, and therefore, there is more stable combination with glycosidase. In this study the binding of phenolic compounds and enzymes is the weaker in 14 compounds, which may be because small molecular weight is small, less binding sites, and the aggregation can be relatively weak. Huang et al. investigated the potential α-glucosidase inhibition and hypoglycemic effect of compound 1,2,3,4,6-Penta-O-galloyl-b-D-glucopyranose using techniques such as molecular docking and found multiple hydrogen bonds between phenolic and amino acid residues. Further dynamic simulations showed that 1,2,3,4,6-Penta-O-galloyl-b-D-glucopyranose plays a more critical role with the active site of α-glucosidase (Huang et al., Citation2021).
5. Conclusions
Forty-two compounds were isolated and identified from PL, including two new compounds. Among these 42 compounds, 14 polyphenols have a facilitative effect on glucose consumption in IEC cells. Mechanistic analysis revealed that these 14 polyphenols could inhibit α-glucosidase activity, and molecular docking of 14 polyphenols with α-glucosidase revealed that 14 polyphenols were able to bind to the α-glucosidase active site. This study provides some reference data for the further exploitation of PL in the prevention and treatment of T2DM.
Supplemental Material
Download MS Word (28 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19476337.2023.2245003.
Additional information
Funding
References
- Abbasi, N. N., Purslow, P. P., Tosh, S. M., & Bakovic, M. (2016). Oat β-glucan depresses SGLT1- and GLUT2-mediated glucose transport in intestinal epithelial cells (IEC-6). Nutrition Research, 36(6), 541–552. https://doi.org/10.1016/j.nutres.2016.02.004
- Avelar, L. G., Silva, M. F., Lima, F. W., Takahashi, J. A., Filho, J. D. D. S., & Pimenta, L. P. S. (2014). The first report on flavonoid isolation from Annona crassiflora Mart. Natural Product Research, 28(11), 808–811. https://doi.org/10.1080/14786419.2014.885518
- Boyer, F., & Ducrot, P. (2005). Hydrogenation of substituted aromatic aldehydes: Nucleophilic trapping of the reaction intermediate, application to the efficient synthesis of methylene linked flavanol dimers. Tetrahedron Letters, 46(31), 5177–5180. https://doi.org/10.1016/j.tetlet.2005.05.132
- Butterworth, P. J. (1972). The use of Dixon plots to study enzyme inhibition. Biochimica Et Biophysica Acta (BBA) - Enzymology, 289(2), 251–253. https://doi.org/10.1016/0005-2744(72)90074-5
- Demir, S., Nawroth, P. P., Herzig, S., & Ekim Üstünel, B. (2021). Emerging targets in type 2 diabetes and diabetic complications. Advanced Science, 8(18), e2100275. https://doi.org/10.1002/advs.202100275
- Deng, Y. T., Lin-Shiau, S. Y., Shyur, L. F., & Lin, J.-K. (2015). Pu-erh tea polysaccharides decrease blood sugar by inhibition of α-glucosidase activity in vitro and in mice. Food & Function, 6(5), 1539–1546. https://doi.org/10.1039/C4FO01025F
- Dixon, H. B. F., & Cornish-Bowden, A. (2010). Revision of enzyme nomenclature. Listing enzymes. European Journal of Biochemistry, 133(3), 479–479. https://doi.org/10.1111/j.1432-1033.1983.tb07489.x
- Gai-Mei, S., Jin-Bo, H., Ying-Jun, Z., & Yang, C.-R. (2010). Phenolic constituents from rhopalocnemis phalloides with DPPH radical scavenging activity. Pharmaceutical Biology, 48(1), 116–119. https://doi.org/10.3109/13880200903032757
- Han, Q. T., Ren, Y., Li, G. S., Xiang, K.-L., & Dai, S.-J. (2018). Flavonoid alkaloids from Scutellaria moniliorrhiza with anti-inflammatory activities and inhibitory activities against aldose reductase. Phytochemistry, 152, 91–96. https://doi.org/10.1016/j.phytochem.2018.05.001
- Herman, W. H. (2015). Response to comment on inzucchi et al. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach. Update to a position statement of the American diabetes association and the European Association for the study of diabetes. Diabetes Care, 38(9), 140–149. https://doi.org/10.2337/dc15-1234
- Hotamisligil, G. S. (2006). Inflammation and metabolic disorders. Nature, 444(7121), 860–867. https://doi.org/10.1038/nature05485
- Huang, C. D., Zheng, H. H., Zhang, X. Y., Liu, D.-Z., Gao, J.-M., & Zhang, Q. (2021). Insight into the α-glucosidase-inhibiting mechanism of β-PGG, a commonly occurring polyphenol in diets. Natural Product Research, 36(5), 1–5. https://doi.org/10.1080/14786419.2021.1873983
- Kakarla, L., Katragadda, S. B., Tiwari, A. K., Kotamraju, K., Madhusudana, K., Kumar, D., & Botlagunta, M. (2016). Free radical scavenging, α-glucosidase inhibitory and anti-inflammatory constituents from Indian sedges, Cyperus scariosus R.Br and Cyperus rotundus L. Pharmacognosy Magazine, 12(Suppl 4), S488–S496. https://doi.org/10.4103/0973-1296.191467
- Li, L., Liu, X., Qiu, Z., & Zhao, G. (2021). Microbial synthesis of plant polyphenols. Sheng Wu Gong Cheng Xue Bao = Chinese Journal of Biotechnology, 37(6), 2050–2076. https://doi.org/10.13345/j.cjb.200747
- Li, D., Sun, L., Yang, Y., Wang, Z., Yang, X., Zhao, T., Gong, T., Zou, L., & Guo, Y. (2019). Young apple polyphenols postpone starch digestion in vitro and in vivo. Journal of Functional Foods, 56, 127–135. https://doi.org/10.1016/j.jff.2019.03.009
- Ma, Q. Y., Chen, Y. C., Huang, S. Z., Kong, F.-D., Zhou, L.-M., Dai, H.-F., Hua, Y., & Zhao, Y.-X. (2016). Chemical constituents from the stems of Daphne holosericea (Diels) Hamaya. Chemistry & Biodiversity, 13(11), 1469–1474. https://doi.org/10.1002/cbdv.201600040
- Meijnikman, A. S., Gerdes, V. E., Nieuwdorp, M., & Herrema, H. (2017). Evaluating causality of gut microbiota in obesity and diabetes in humans. Endocrine Reviews, 39(2), 133. https://doi.org/10.1210/er.2017-00192
- Meng, Q., Choudry, H. A., Souba, W. W., Karinch, A., Huang, J., Lin, C., Vary, T., & Pan, M. (2005). Regulation of amino acid arginine transport by lipopolysaccharide and nitric oxide in intestinal epithelial IEC-6 cells. Journal of Gastrointestinal Surgery, 9(9), 1276–1285. https://doi.org/10.1016/j.gassur.2005.08.008
- Olennikov, D. N., Kashchenko, N. I., Schwabl, H., Vennos, C., & Loepfe, C. (2015). New mucic acid gallates from phyllanthus emblica. Chemistry of Natural Compounds, 51(4), 666–670. https://doi.org/10.1007/s10600-015-1380-y
- Papoutsis, K., Zhang, J., Bowyer, M. C., Brunton, N., Gibney, E. R., & Lyng, J. (2021). Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chemistry, 338, 128119. https://doi.org/10.1016/j.foodchem.2020.128119
- Qin, Y., Liu, W., Zhang, X., Adamowski, J. F., & Biswas, A. (2022). Leaf stoichiometry of Potentilla fruticosa across elevations in China’s Qilian mountains. Frontiers in Plant Science, 13, 814059. https://doi.org/10.3389/fpls.2022.814059
- Qiongyu, Z., Yulin, T., Zhao, C., & Wu H. (2015). Studies on the chemical constituents of rhizomes of Smilax bockii Warb. Journal of Organic Chemistry Research, 3(1), 51–54.
- Qiu, B. L., Wang, L. Y., Xia, G. Y., Zhang, J.-F., Wu, Y.-Z., Li, R., Xiao, B.-B., Zhong, W.-C., Lin, P.-C., & Lin, S. (2018). Study on structural conversion of dihydrochelerythrine in different solvents. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China Journal of Chinese Materia Medica, 43(16), 3315–3321. https://doi.org/10.19540/j.cnki.cjcmm.20180511.002
- Quideau, S., Deffieux, D., Douat, C., & Pouységu L. (2011). ChemInform abstract: Plant polyphenols: Chemical properties, biological activities, and synthesis. ChemInform, 42(17), 586–621. https://doi.org/10.1002/chin.201117261
- Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., Colagiuri, S., Guariguata, L., Motala, A. A., Ogurtsova, K., Shaw, J. E., Bright, D., & Williams, R. (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International diabetes federation diabetes Atlas, 9th edition. Diabetes Research & Clinical Practice, 2019(157), 157. https://doi.org/10.1016/j.diabres.2019.107843
- Samec, D., Valek-Zxulj, L., Artinez, S., Grúz, J., Piljac, A., & Piljac-Žegarac, J. (2016). Phenolic acids significantly contribute to antioxidant potency of Gynostemma pentaphyllum aqueous and methanol extracts. Industrial Crops and Products, 84, 104–107. https://doi.org/10.1016/j.indcrop.2016.01.035
- Shah, N., Abdalla, M. A., Deshmukh, H., & Sathyapalan, T. (2021). Therapeutics for type-2 diabetes mellitus: A glance at the recent inclusions and novel agents under development for use in clinical practice. Therapeutic Advances in Endocrinology and Metabolism, 12, 2239–2251. https://doi.org/10.1177/20420188211042145
- Shakera, K. H., Shehri, B. A., Oteef, M. D. Y., & Mahmoud M. F. (2015). Antioxidant compounds from Euphorbia schimperiana scheelein Aseer region, Saudi Arabia. International Journal of Pharmaceutical Sciences Review and Research, 32(1), 117–122.
- Şöhretoğlu, D., Sari, S., Barut, B., & Özel, A. (2018). Discovery of potent α-glucosidase inhibitor flavonols: Insights into mechanism of action through inhibition kinetics and docking simulations. Bioorganic Chemistry, 79, 257–264. https://doi.org/10.1016/j.bioorg.2018.05.010
- Song, X. Z. Y., Yang X. Wang, T., & Liu F. (2009). Effects of four Chinese herb extracts on cell proliferation in IEC-6 cells of rats and their glucose absorption. Chinese Traditional and Herbal Drugs, 40(8), 1263–1266.
- Tomczyk, M., & Latté, P. K. (2009). Potentilla—A review of its phytochemical and pharmacological profile. Journal of Ethnopharmacology, 122(2), 184–204. https://doi.org/10.1016/j.jep.2008.12.022
- Wang, J., Nisar, T., Fang, Z., Huang, G., Yang, R., Wang, F., Zou, L., Wang, Z.-C., & Lin, Y. (2023). Brown sugar polyphenols as natural inhibitors of α-amylase and α-glucosidase enzymes. Food Science & Technology, 58(8), 4366–4375. https://doi.org/10.1111/ijfs.16541
- Wang, Z. C., Sun L., Fang Z., Nisar T., Zou L., Li D., & Guo Y. (2021). Lycium ruthenicum Murray anthocyanins effectively inhibit α-glucosidase activity and alleviate insulin resistance. Food Bioscience, 41, 100949. https://doi.org/10.1016/j.fbio.2021.100949
- Wang, Z. C., Tanzeela N., Sun, L., Fang Z., Yan Y., Li D., Xie H., Wang H., & Guo, Y. (2019). Effect of in vitro gastrointestinal digestion on the composition and bioactivity of anthocyanins in the fruits of cultivated Lycium ruthenicum murray. CyTA - Journal of Food, 17(1), 552–562. https://doi.org/10.1080/19476337.2019.1613449
- Wang, Z., Tu, Z., Xie, X., Cui H., Kong K. W., & Zhang L. 2021. Perilla frutescens leaf extract and fractions: Polyphenol composition, antioxidant, enzymes (α-glucosidase, acetylcholinesterase, and tyrosinase) inhibitory, anticancer, and antidiabetic activities. Foods, 10(2), 315. https://doi.org/10.3390/foods10020315
- Yoshiki, K., Hiroyuki, I., Keiko, Y., Chen R. F., Nonaka G. I., & Nishioka I. (1990). Tannins and related compounds. XCIII. Occurrence of enantiomeric proanthocyanidins in the leguminosae plants, Cassia fistula L. and C. javanica L. Chemical & Pharmaceutical Bulletin, 38(4), 888–893.
- Zhang, B., Dhital, S., & Gidley, M. J. (2015). Densely packed matrices as rate determining features in starch hydrolysis. Trends in Food Science & Technology, 43(1), 18–31. https://doi.org/10.1016/j.tifs.2015.01.004
- Zhang, J., Sun, L., Dong, Y., Fang, Z., Nisar, T., Zhao, T., Wang, Z.-C., & Guo, Y. (2019). Chemical compositions and α-glucosidase inhibitory effects of anthocyanidins from blueberry, blackcurrant and blue honeysuckle fruits. Food Chemistry, 299(NOV.30), 125102. https://doi.org/10.1016/j.foodchem.2019.125102
- Zheng, C. S. (2015). Chemical constituents of antioxidant species in black soybean. Food Ence, 36(6), 155–160.