ABSTRACT
Low-density lipoprotein receptor (LDLR) and proprotein convertase subtilisin/kexin type 9 (PCSK9) play a pivotal role by regulating plasma low-density lipoprotein cholesterol (LDL-c) levels. Dihydromyricetin (DMY), the most abundant natural flavonoid in rattan tea, has proven anti-atherogenic effects, but the underlying molecular mechanisms remain poorly understood. Therefore, we studied the effects of DMY on LDLR and PCSK9. The results showed DMY promoted LDLR protein and mRNA expression and increased LDL uptake in HepG2 cells. DMY inhibited intracellular PCSK9 protein and mRNA expression. And it also significantly reduced PCSK9 levels in the cell culture medium. Furthermore, DMY inhibited the expression of PCSK9 through the liver nuclear transcription factor 1 A (HNF1A) and increased the protein expression of LDLR. Taken together, our results support the idea that DMY regulates LDL-c metabolism through PCSK9/LDLR signaling. This study reveals the potential mechanism of DMY’s anti-atherogenic effect and provides a theoretical basis for dietary DMY supplementation.
1. Introduction
Atherosclerotic cardiovascular disease (ASCVD) is a complex and chronic progressive disease characterized by lipid deposition on the intima of arteries and the formation of atherosclerotic plaques, leading to lesions in the corresponding organs (Kanter et al., Citation2012). Moreover, cardiovascular disease development and progression are positively associated with the levels of low-density lipoprotein-cholesterol (LDL-c); indeed, LDL-c is a major independent risk factor for cardiovascular diseases (Wu et al., Citation2021). Therefore, in the current clinical practice, treatments focus on controlling blood lipid levels, especially lowering LDL-c levels, which is the most effective way to reduce the occurrence of cardiovascular diseases.
The catabolism of LDL-c in plasma mainly depends on the regulation of the low-density lipoprotein receptor (LDLR). Around 70% of LDL-c is processed by LDLR in the liver, where it is converted into bile acids or directly excluded by bile ducts (Mady & Shaker, Citation2017). Therefore, increasing liver LDLR levels is a convenient way to reduce LDL-c levels. Proprotein convertase subtilisin/kexin type 9 (PCSK9) plays a key role in the regulation of LDLR levels and cholesterol homeostasis, making it a key factor in the regulation of plasma LDL-c levels (Xiao et al., Citation2019; Yang et al., Citation2015). The liver secretes PCSK9 into the blood, and circulating PCSK9 specifically binds to the epidermal growth factor repeat A (EGF-A) domain of LDLR. The formation of this complex induces LDLR degradation through the lysosomes, thereby reducing LDLR levels on the cell surface (Wang et al., Citation2020). Therefore, reducing plasma LDL-c levels by regulating the PCSK9/LDLR pathway is an effective and reliable method to prevent and treat cardiovascular diseases (Song et al., Citation2018).
In liver tissue, the PCSK9 gene transcription level are mainly regulated by hepatocyte nuclear factor 1 A (HNF1A) (Li et al., Citation2009), forkhead box O3 (FoxO3) (Li & Liu, Citation2018) and other transcription factors. In addition, HNF1A is a liver-enriched transcription factor that activates PCSK9 transcription in the liver (Jeong et al., Citation2008). As a forkhead transcription factor, FoxO3 negatively regulates PCSK9 gene expression (Tao et al., Citation2013). FoxO3 interacts with the insulin response element of the PCSK9 promoter and recruits sirtuin-6 to deacetylate histones and reduce the binding capacity of the HNF1A promoter, reducing PCSK9 gene expression in hepatocytes (Chen et al., Citation2016).
Dihydromyricetin (DMY) is a dihydroflavonol flavonoid abundant in Rattan tea, myriceae, and other plants. It is one of the most potent natural antioxidants, with anti-inflammatory, antibacterial, antihypertensive, and antithrombotic effects (Li et al., Citation2017; Zhang et al., Citation2018). It is also the main component of Rattan tea; it enhances cholesterol efflux, and inhibits foam cell formation in atherosclerosis (Zeng et al., Citation2018). Moreover, DMY can affect blood glucose levels, improve lipid metabolism, and reduce liver injury (Chen et al., Citation2015). It also improved serum lipid indices in high-fat diet-fed mice and inhibited aortic plaque formation in LDLR−/− mice (Liu et al., Citation2017). Recent studies have shown that DMY can alleviate cardiovascular disease and improve lipid regulation by enhancing cholesterol reverse transport in high-fat diet-fed Apoe−/− mice (Zhu et al., Citation2018). DMY also has an anti-atherogenic effect, but mechanism of action remains under-documented. In the current study, we explored the role and molecular mechanism of DMY in LDL-c metabolism regulation through LDLR and PCSK9 using HepG2 cells.
2. Materials and methods
2.1. Reagents
We obtained human hepatocellular carcinoma cells HepG2 from the American Type Culture Collection, Manassas, VA, USA. DMY (98% purity) came from the Source Leaf Company (China). We purchased the rabbit anti-human PCSK9, LDLR, HNF1A, FOXO3, SREBP-1c, SREBP-2, and β-actin primary antibodies from Cell Signaling Technology (USA.). The horseradish peroxidase-labeled sheep anti-rabbit secondary antibody came from Shanghai Youweining Biotechnology Co., Ltd. We obtained fetal bovine serum and Dulbecco’s modified Eagle’s medium from Gibco. We bought penystreptomycin, 3-(4, 5-dimethylthiazol-2-YL)-2, 5-diphenyltetrazolium bromide (MTT) and Dil-LDL from Solarbio Science and Tech. Co. Ltd., Beijing, China. We obtained a human PCSK9 detection kit from Sino Biological (Beijing, China). The TRIzol came from TransGen Biotech Co. Ltd. (Beijing, China) and the reverse transcriptase from Takara (Beijing, China).
2.2. MTT assay
We cultured HepG2 cells in Dulbecco’s modified Eagle’s medium (supplemented with 10% fetal bovine serum and 1% penicptomycin) at 37.5°C under a 5% CO2 atmosphere. We seeded HepG2 cells in 96-well culture plates at a density of 2 × 104 cells/well and cultured them overnight at 37°C in a 5% CO2 incubator. Next, we added 5, 10, 20, or 40 μM DMY and incubated the cells for 24 h. Then, we added 20 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (5 mg/mL MTT) solution to each well, covered the culture plate with tin foil to protect it from light, and incubated the cells for 4 h. We then discarded the medium, added 200 µL of dimethyl sulfoxide to each well, and shook the solution on an oscillator for 10 min until the crystal was completely dissolved. Finally, we measured the absorbance at 490 nm on an enzyme-linked immunodetector (Molecular Devices, Sunnyvale, CA, USA).
2.3. PCSK9 ELISA
We treated HepG2 cells with DMY for 24 h, then collected the cell culture medium. We quantified PCSK9 using a human PCSK9 detection kit according to the manufacturer’s instructions. First, wash each well three times with wash buffer. Add 100 μL of each serially diluted protein standard or test sample per well and incubate 2 h at room temperature. After that, wash each well again and add 100 μL of detection antibody in working concentration to each well. Then incubate for 1 h at room temperature. Then, wash wells and add 200 μL of substrate solution. Incubate for 20 min at room temperature in the dark. Finally, the reaction is stopped by the addition of a stop solution and the intensity of the color can be measured at 450 nm.
2.4. Dil-LDL uptake test
We seeded HepG2 cells in 6-well glass climbing plates and incubated them with DMY for 20 h, then added 25 μg/mL Dil-LDL and incubated again at 37°C for 4 h in the dark. Next, we fixed the cells with 4% paraformaldehyde for 20 min at room temperature, then washed them with phosphate-buffered saline (PBS) three times and stained the nuclei with 4’,6-diamidino-2-phenylindole (DAPI). At the end of the reaction, we washed the cells again with PBS three times, added an anti-fluorescence quencher, and let the plates dry completely. Finally, we photographed the cells under a fluorescence microscope to assess LDL uptake (excitation: 514/549 nm, emission: 565 nm). Then Image pro-plus software 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA) was used to quantitatively analyze the fluorescence intensity.
2.5. Quantitative real-time PCR (qRT-PCR)
We treated HepG2 cells with DMY for 24 h, then added 1 mL Trizol, and collected the homogenate by aspiration with a pipette gun. Then, we added 200 μL chloroform and centrifuged at 12,000 × g and 4°C for 10 min. Next, we collected 200 μL of supernatant and added an equal volume of isopropanol before centrifugation at 12,000 ×g and 4°C for 10 min. We then washed the pellet twice with 75% ethanol. We added 100 μL of DEPC (diethyl pyrocarbonate) water and measured RNA purity and concentration using a spectrophotometer (ND2000, Thermo Fisher Scientific). Finally, we reverse-transcribed RNA into cDNA using reverse transcriptase and amplified it with an amplification reagent. We performed the quantitative polymerase chain reaction (qPCR) using the SYBR Green real-time PCR Master Mix in the LightCycler480 II Real-Time PCR System ( 05015243001, Roche Diagnostics. Co. Ltd., Germany. The length of LDLR amplification product was 92 bp. The length of PCSK9 amplified product was 111 bp. The length of HNF1A amplification product was 101 bp. The length of FoxO3 amplified product is 110 bp.
LDLR: F: CTGAAATCGCCGTGTTACTG; R: GCCAATCCCTTGTGACATCT.
PCSK9: F: CCAAGCCTCTTCTTACTTCACC; R: GCATCGTTCTGCCATCACT.
FoxO3: F: ACATGGGCTTGAGTGAGT; R: GCCTGAGAGAGAGTCCGAGA.
HNF1A: F: GTGGCGAAGATGGTCAAGTCC; R: CCCTT GTTGAGGTGTTGGG.
β-actin: F: CCCTGGCACCCAGCAC; R: GCCGATCCACACGGAGTAC.
2.6. Western blot analysis
We seeded HepG2 cells in Petri dishes, incubated them with DMY for 24 h, and collected them. To extract proteins, we lysed the cells with 1 mM PMSF (phenylmethylsulfonyl fluoride, Solarbio Science and Tech. Co. Ltd., Beijing, China) centrifuged them at 15,000 × g for 20 min at 4°C, and removed the supernatant. We then quantified the proteins using a BCA protein assay kit (Thermo scientific, Waltham, MA). Next, we separated a 50 μg total protein sample by 10% SDS-PAGE, transferred the proteins to a membrane, blocked them with 5% skim milk for 2 h, and then incubated them overnight at 4°C with the corresponding primary antibodies (β-actin, LDLR, PCSK9, 1:1000). We then washed off the primary antibodies with 1× TBST (Tris Buffered Saline with Tween® 20) for 15 min, then added the horseradish peroxidase-labeled sheep anti-rabbit secondary antibody (1:5000) and incubated the membranes for 1 h at room temperature. Finally, we revealed the protein bands using an enhanced chemiluminescence system (4A Biotech Co., Ltd). The quantification of detection protein amounts was determined by Image J software (Wang et al., Citation2020). We used the optical density ratio of the target proteins and β-actin as the relative expression level of each target protein.
2.7. Statistical analysis
Data were expressed as the mean ± standard error of the mean (SEM), all experiments were repeated three times, and data were analyzed with GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA). Protein bands were analyzed using Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). Student’s t-test was performed to determine statistically significant differences within groups. Values of p < .05 indicated statistical significance.
3. Results
3.1. DMY promotes LDLR expression in HepG2 cells
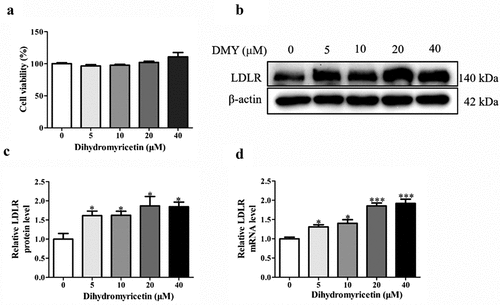
First, we examined the effect of DMY on the viability of HepG2 cells. We treated HepG2 cells with various DMY concentrations (5, 10, 20, 40 μM) for 24 h and measured cell viability using an MTT assay. At the used concentrations, DMY did not significantly affect cell viability (). Thus, we carried out the subsequent experiments at this concentration range. Next, we quantified LDLR protein and mRNA expression in HepG2 cells after incubation with DMY (5, 10, 20, 40 μM) for 24 h. Compared with the control group, 5 μM of DMY treatment significantly increased LDLR protein levels (). In addition, EA upregulated LDLR mRNA levels in a concentration-dependent manner in the range of 5–40 Μm ().
Figure 1. DMY promoted LDLR expression in HepG2 cells. (a) cell viability (MTT assay) of HepG2 cells treated with DMY (5–40 μM) for 24 h. (b, c) Western blot analysis showing LDLR protein levels in HepG2 cells treated with DMY (5–40 µM) for 24 h. (d) LDLR mRNA levels (qRT-PCR) in HepG2 cells treated with DMY (5–40 µM) for 24 h. The values are expressed as means ± SEM (n = 3). *p < .05, **p < .01 and ***p < .001 compared with the control group. DMY, Dihydromyricetin. LDLR, low-density lipoprotein receptor.
3.2. DMY promotes LDL uptake
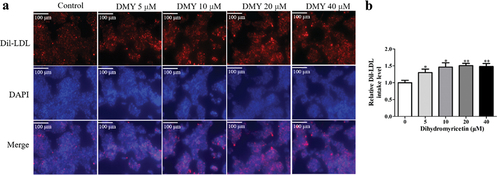
LDLR, on the surface of liver cells, can mediate the degradation of LDL in lysosomes. The above results showed that DMY increased LDLR protein levels in HepG2 cells. Next, we evaluated the effect of DMY on LDL uptake by the cells. We found that DMY significantly increased Dil-LDL uptake in HepG2 cells compared with untreated cells (). Thus, the DMY-induced LDLR protein level increase promotes LDL-c uptake by hepatocytes.
Figure 2. DMY increased LDL uptake in HepG2 cells. (a) Representative fluorescent micrographs show the intake levels of DiI-LDL (red) in HepG2 cells treated with DMY (5–40 µM). (b) Quantitative analysis showing Dil-LDL levels in HepG2 cells treated with DMY (5–40 µM). The values are expressed as means ± SEM (n = 3). *p < .05 and **p < .01 compared with the control group. DMY, Dihydromyricetin. LDL, low-density lipoprotein.
3.3. DMY inhibits PCSK9 expression in HepG2 cells
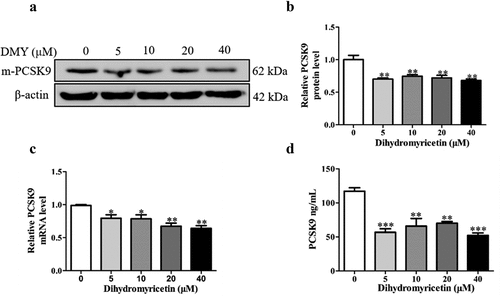
LDLR protein levels can be increased post-transcriptionally (by inhibiting LDLR degradation through PCSK9). Therefore, we next investigated the effect of DMY on PCSK9 expression. We quantified the PCSK9 mRNA and protein levels in HepG2 cells by qRT-PCR and Western blotting. DMY significantly reduced PCSK9 protein and mRNA levels (). Besides, PCSK9 is a secretory protein, so we quantified it in the cell culture medium using ELISA. DMY significantly reduced PCSK9 levels in the cell culture medium (). These results suggest that DMY inhibits PCSK9 expression, promoting LDLR expression at the post-translational level.
Figure 3. DMY inhibited PCSK9 expression in HepG2 cells. (a, b) Western blot analysis showing PCSK9 protein levels in HepG2 cells treated with DMY (5–40 µM) for 24 h. (c) qRT-PCR results showing PCSK9 levels in HepG2 cells treated with DMY (5–40 µM) for 24 h. (d) ELISA results showing PCSK9 content in the cell culture medium with DMY (5–40 µM) for 24 h. The values are expressed as means ± SEM (n = 3). *p < .05 and ***p < .001 compared with the control group. DMY, Dihydromyricetin. PCSK9, proprotein convertase subtilisin/kexin type 9.
3.4. DMY decreases HNF1A expression in HepG2 cells
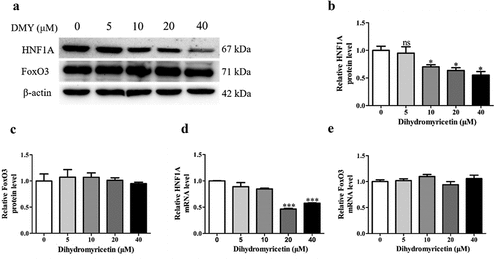
To further investigate the molecular mechanism underlying the DMY-induced decrease in PCSK9 expression, we quantified HNF1A and FoxO3, which are important transcription factors of PCSK9. DMY significantly reduced HNF1A protein levels () but did not affect those of FoxO3 (). DMY also decreased HNF1A mRNA levels (). This suggests that DMY decreases HNF1A protein levels by decreasing its mRNA levels and that DMY induces PCSK9 expression by down-regulating HNF1A.
Figure 4. DMY inhibited the expression of the transcription factors HNF1A in HepG2 cells. (a–c) Western blot analysis showing HNF1A and FoxO3 protein levels in HepG2 cells treated with DMY (5–40 µM) for 24 h. (d, e) qRT-PCR results showing HNF1A and FoxO3 mRNA levels in HepG2 cells treated with DMY (5–40 µM) for 24 h. The values are expressed as means ± SEM (n = 3). *p < .05, **p < .01, and ***p < .001 compared with the control group. DMY, Dihydromyricetin. HNF1A, hepatocyte nuclear factor 1A. FoxO3, forkhead box O3.
4. Discussion
Elevated LDL-c levels are considered an independent risk factor for cardiovascular diseases. Increasing LDLR expression on the hepatocyte surface can reduce LDL-c levels by enhancing LDL uptake and catabolism (Yang et al., Citation2020). PCSK9 is a major regulator of LDLR expression in hepatocytes and can interfere with the recycling of LDLR by binding to it (Huang et al., Citation2022). Studies have shown that PCSK9 is not only associated with autosomal dominant inherited hypercholesterolemia, but also can effectively regulate the level of lipid metabolism in the body and affect the occurrence and development of ASCVD (Seidah et al., Citation2014). PCSK9 regulates lipid metabolism mainly through its specific binding to LDLR on the cell surface to form complexes and transport to lysosomes, leading to accelerated degradation of LDLR and thus increasing plasma LDL-c level. In addition, PCSK9 also regulates LDL-c levels through other mechanisms of action. For example, mutations in the gene PCSK9 have been found to influence LDL-c levels. Gain-of-function mutations in PCSK9 cause AS by reducing LDLR levels, and loss-of-function mutations in PCSK9 are associated with reduced LDL-c levels and risk of ASVCD. PCSK9 has also become a popular target for drug development to lower cholesterol levels. There are currently three PCSK9-targeting drugs on the market, including evolocumab, alirocumab and inclisiran. PCSK9 monoclonal antibodies effectively reduce circulating LDL-c levels with good efficacy and tolerance, confirming PCSK9’s potential as a new therapeutic target for reducing LDL-c levels (Robinson et al., Citation2014). However, considering the high cost of monoclonal antibodies, more effective and convenient small molecule inhibitors have become a research hotspot.
DMY is the most abundant natural flavonoid in rattan tea, but the protective effect against ASCVD is still poorly understood (Sun et al., Citation2022). Liao et al. (Citation2014) have demonstrated that DMY can effectively reduce plasma lipid peroxidation and inhibit the production of malondialdehyde induced by 2,2’-azobis (2-amidinopropane) dihydrochloride (AAPH). Malondialdehyde is a biomarker of oxidative stress and is involved in the formation of AS plaques. Studies have shown that DMY reduces serum total cholesterol (TC), triglycerides (TG), and LDL-c levels, and increases HDL cholesterol (HDL-c) levels in high fat diet (HFD)-fed rats (Le et al., Citation2016). A recent study showed that DMY reduced blood lipid levels in rats fed HFD and prevented plaque formation in the aorta of mice (Williams et al., Citation2015). Together, these studies suggest that DMY ameliorates atherosclerosis and associated metabolic disorders. Liver-expressed LDLR is responsible for the clearance of 70% of LDL-c in the body and plays an essential role in cholesterol metabolism, mediating the absorption and internalization of cholesterol by hepatocytes (Luo et al., Citation2021). Reducing circulating PCSK9 levels may inhibit the PCSK9-mediated degradation of LDLR and thus increase cellular LDLR levels. Therefore, we investigated the effects of DMY on LDLR and PCSK9, and the results showed that DMY increased LDLR expression () and inhibited PCSK9 expression in HepG2 cells (). On the hepatocyte surface, LDLR can bind LDL and mediate LDL degradation by lysosomes. Using Dil-LDL, we assessed the LDL uptake activity of LDLR (Stephan & Yurachek, Citation2013). We found that DMY significantly increased LDL uptake by HepG2 cells (). PCSK9 is mainly synthesized and matured in hepatocytes and secreted by autonomous lysis (Cui et al., Citation2020). PCSK9 secretion is key to its biological activity (Lipari et al., Citation2012). Our results showed that DMY significantly reduced circulating PCSK9 levels (). These results indicate that DMY can promote the liver clearance of plasma LDL-c by inhibiting PCSK9 and thereby increasing LDLR protein levels, which is a potential cholesterol-lowering drug.
Related studies have shown that HNF1A binds to the PCSK9 promoter and promotes PCSK9 expression (Li et al., Citation2020). In contrast, FoxO3 interacts with the PCSK9 promoter and suppresses PCSK9 transcriptional expression (Cui et al., Citation2020). Our data showed that DMY did not regulate FoxO3 expression () but significantly inhibited HNF1A protein expression (). Consistently, DMY also reduced HNF1A mRNA levels (), suggesting that DMY regulates HNF1A expression at the transcriptional level. These results also suggest that DMY inhibits PCSK9 transcription through HNF1A ().
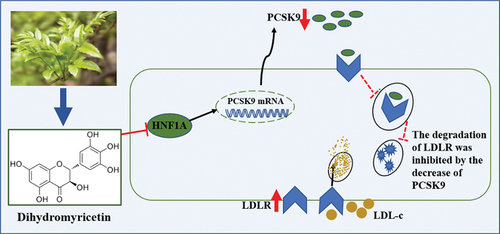
Figure 5. Schematic diagram of the mechanism by which DMY regulates the PCSK9/LDLR pathway. PCSK9, proprotein convertase subtilisin/kexin type 9. LDLR, low-density lipoprotein receptor. HNF1A, hepatocyte nuclear factor 1A. LDL-c, low-density lipoprotein cholesterol.
Vine tea, containing about 60% of DMY, inhibited hepatic lipogenesis by reducing western diet-enhanced the level of SREBP1c and fatty acid synthase (Xie et al., Citation2020). Our results show that DMY promotes LDLR mRNA expression. At the transcriptional level, LDLR is primarily regulated by SREBP-1c and SREBP-2 (Maguire et al., Citation2020). SREBP-2 is mainly responsible for the regulation of LDLR, and SREBP-1c is more closely involved in the regulation of fatty acid synthesis. Further studies determine whether SREBP-2 is involved in DMY’s increase of LDLR mRNA level.
This study systematically investigated how DMY can improve lipid metabolism disorder. We found that DMY regulates LDL-c metabolism by inhibiting PCSK9 expression and promoting LDLR expression. Although our experiments have produced new knowledge, they have some shortcomings. Our study revealed that DMY inhibited LDLR degradation by PCSK9, but determining how DMY affects the interaction between PCSK9 and LDLR needs further study. Studying these questions will help to understand the mechanism by which naturally active compounds improve lipid metabolism.
In conclusion, the present study showed that DMY, a flavonoid compound mainly presented in willow leaf plants, inhibited the expression of PCSK9 protein and the secretion of PCSK9 in culture media via the suppression of HNF1A, thereby increasing LDLR expression and promoting LDL-c uptake. Overall, our study uncovers the mechanisms by which DMY increases LDLR expression and cholesterol uptake and provides a basis for the development of DMY as an anti-atherogenic agent.
Authors’ contributions
Jun Sheng, Ye-wei Huang and Xuan-jun Wang participated in the acquisition of resources and funds. Jun Sheng, Ye-wei Huang and Xuan-jun Wang participated in the conception and review. Li-tian Wang and Dan-dan are responsible for research, formal analysis, visualization, project management and writing of original manuscripts. Huai-liu Yin, Rui Mu, Yu-hang Yang and Run-yu Zhao conducted investigations, data collation and verification. Hua-rong Liu was responsible for the revision of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chen, H.-C., Chen, P.-Y., Wu, M.-J., Tai, M.-H., & Yen, J.-H. (2016). Tanshinone IIA modulates low density lipoprotein uptake via down-regulation of PCSK9 gene expression in HepG2 cells. PloS One, 11(9), e0162414. https://doi.org/10.1371/journal.pone.0162414
- Chen, S., Zhao, X., Wan, J., Ran, L., & Mi, M. (2015). Dihydromyricetin improves glucose and lipid metabolism and exerts anti inflammatory effects in nonalcoholic fatty liver disease: A randomized controlled trial. Pharmacological research: The official journal of the Italian Pharmacological Society, 99, 74–81. https://doi.org/10.1016/j.phrs.2015.05.009
- Yang, H. X., Zhang, M., Long, S. Y., Tuo, Q. H., Tian, Y., Chen, J. X., Zhang, C. P., & Liao, D. F. (2020). Cholesterol in LDL receptor recycling and degradation. Clinica Chimica Acta, 500, 81–86. https://doi.org/10.1016/j.cca.2019.09.022
- Cui, C.-J., Jin, J-L., Guo, L.-N., Sun, J., Wu, N.-Q., Guo, Y.-L., Liu, G., Dong, Q., Li, J.-J. (2020). Beneficial impact of epigallocatechin gallate on LDL-c through PCSK9/LDLR pathway by blocking HNF1α and activating FoxO3a. Journal of Translational Medicine, 18(1), 195. https://doi.org/10.1186/s12967-020-02362-4
- Huang, Y.-W., Zhang, M., Wang, L.-T., Nie, Y., Yang, J.-B., Meng, W.-L., Wang, X.-J., Sheng, J. (2022). 20(S)-protopanaxadiol decreases atherosclerosis in ApoE KO mice by increasing the levels of LDLR and inhibiting its binding with PCSK9. Food & Function, 13(13), 7020–7028. https://doi.org/10.1039/D2FO00392A
- Jeong, H. J., Lee, H.-S., Kim, K.-S., Kim, Y.-K., Yoon, D., & Park, S. W. (2008). Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatory element binding protein-2. Journal of Lipid Research, 49(2), 399–409. https://doi.org/10.1194/jlr.M700443-JLR200
- Kanter, J. E., Kramer, F., Barnhart, S., Averill, M. M., Vivekanandan-Giri, A., Vickery, T., Li, L. O., Becker, L., Yuan, W., Chait, A., Braun, K. R., Potter-Perigo, S., Sanda, S., Wight, T. N., Pennathur, S., Serhan, C. N., Heinecke, J. W., Coleman, R. A., & Bornfeldt, K. E. (2012). Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-coa synthetase 1. Proceedings of the National Academy of Sciences, 109(12), 715–724. https://doi.org/10.1073/pnas.1111600109
- Le, L., Jiang, B., Wan, W., Zhai, W., Xu, L., Hu, K., & Xiao, P. (2016). Metabolomics reveals the protective of Dihydromyricetin on glucose homeostasis by enhancing insulin sensitivity. Scientific Reports, 6(1), 36184. https://doi.org/10.1038/srep36184
- Liao, W., Ning, Z., Ma, L., Yin, X., Wei, Q., Yuan, E., Yang, J., & Ren, J. (2014). Recrystallization of dihydromyricetin from Ampelopsis grossedentata and its anti-oxidant activity evaluation. Rejuvenation Research, 17(5), 422–429. https://doi.org/10.1089/rej.2014.1555
- Li, H., Dong, B., Park, S. W., Lee, H.-S., Chen, W., & Liu, J. (2009). Hepatocyte nuclear factor 1α plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. Journal of Biological Chemistry, 284(42), 28885–28895. https://doi.org/10.1074/jbc.M109.052407
- Li, H., Li, Q., Liu, Z., Chen, K., Chen, Z., Wu, Q., & Wu, L. (2017). The versatile effects of Dihydromyricetin in health. Evidence-Based Complementary and Alternative Medicine, 9, 1–10. https://doi.org/10.1155/2017/1053617
- Li, Z., & Liu, Q. (2018). Hepatitis C virus regulates proprotein convertase subtilisin/kexin type 9 promoter activity. Biochemical & Biophysical Research Communications, 496(4), 1229–1235. https://doi.org/10.1016/j.bbrc.2018.01.176
- Li, H.-H., Li, J., Zhang, X.-J., Li, J.-M., Xi, C., Wang, W.-Q., Lu, Y.-L., & Xuan, L.-J. (2020). 23,24- Dihydrocucurbitacin B promotes lipid clearance by dual transcriptional regulation of LDLR and PCSK9. Acta pharmacologica Sinica, 41(3), 327–335. https://doi.org/10.1038/41401-019-0274-0
- Lipari, M. T., Li, W., Moran, P., Kong-Beltran, M., Sai, T., Lai, J., Lin, S. J., Kolumam, G., Zavala-Solorio, J., Izrael-Tomasevic, A., Arnott, D., Wang, J., Peterson, A. S., & Kirchhofer, D. (2012). Furin-cleaved proprotein convertase subtilisin/kexin type 9 (PCSK9) is active and modulates low density lipoprotein receptor and serum cholesterol levels. Journal of Biological Chemistry, 287(52), 43482–43491. https://doi.org/10.1074/jbc.M112.380618
- Liu, T. T., Zeng, Y., Tang, K., Chen, X., Zhang, W., & Xu, X. L. (2017). Dihydromyricetin ameliorates atherosclerosis in LDL receptor deficient mice. Atherosclerosis, 262, 39–50. https://doi.org/10.1016/j.atherosclerosis.2017.05.003
- Luo, C., Wang, D., Huang, C. W., Song, Y., Ge Change, L., Zhang, X., Yang, L., Lu, J., Tu, X., Chen, C. Q., Yang, J., Xu, C., & Wang, Q. (2021). Feedback regulation of coronary artery disease susceptibility gene ADTRP and LDL receptors LDLR/CD36/LOX-1 in endothelia cell functions involved in atherosclerosis. Biochim Biophys Acta Mol Basis Dis, 1867(7), 166130. https://doi.org/10.1016/j.bbadis.2021.166130
- Mady, F., & Shaker, M. (2017). Enhanced anticancer activity and oral bioavailability of ellagic acid through encapsulation in biodegradable polymeric nanoparticles. International Journal of Offshore and Polar Engineering, 12, 7405–7417. https://doi.org/10.2147/IJN.S147740
- Maguire, M., Larsen, M. C., Vezina, C. M., Quadro, L., Kim, Y.-K., Tanumihardjo, S. A., & Jefcoate, C. R. (2020). Cyp1b1 directs Srebp-mediated cholesterol and retinoid synthesis in perinatal liver; Association with retinoic acid activity during fetal development. PloS One, 15(2), e0228436. https://doi.org/10.1371/journal.pone.0228436
- Sun, Y., Liu, S., Yang, S., Chen, C., Yang, Y., Lin, M., Liu, C., Wang, W., Zhou, X., Ai, Q., Wang, W., & Chen, N. (2022). Mechanism of Dihydromyricetin on inflammatory diseases. Frontiers in Pharmacology, 12, 794563. https://doi.org/10.3389/fphar.2021.794563
- Robinson, J. G., Nedergaard, B. S., Rogers, W. J., Fialkow, J., Neutel, J. M., Ramstad, D., Somaratne, R., Legg, J. C., Nelson, P., Scott, R., Wasserman, S. M., & Weiss, R. (2014). LAPLACE-2 investigators. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-c lowering in patients with hypercholesterolemia: The LAPLACE-2 randomized clinical trial. JAMA, 311(18), 1870–1882. https://doi.org/10.1001/jama.2014.4030
- Seidah, N. G., Awan, Z., Chrétien, M., & Mbikay, M. (2014). Pcsk9: A key modulator of cardiovascular health. Circulation Research, 114(6), 1022–1036. https://doi.org/10.1161/CIRCRESAHA.114.301621
- Song, K. H., Kim, Y. H., Im, A.-R., & Kim, Y. H. (2018). Black raspberry extract enhances LDL uptake in HepG2 cells by suppressing PCSK9 expression to Upregulate LDLR expression. Journal of Medicinal Food, 21(6), 560–567. https://doi.org/10.1089/jmf.2017.4069
- Stephan, Z. F., & Yurachek, E. C. (2013). Rapid fluorometric assay of LDL receptor activity by DiI-labeled LDL. Comparative Study, 34(2), 325–330. https://doi.org/10.1016/S0022-2275(20)40759-X
- Tao, R., Xiong, X., DePinho, R. A., Deng, C.-X., & Dong, X. C. (2013). FoxO3 transcription factor and Sirt6 deacetylase regulate low density lipoprotein (LDL)- cholesterol homeostasis via control of the proprotein convertase subtilisin/kexin type 9 (Pcsk9) gene expression. Journal of Biological Chemistry, 288(41), 29252–29259. https://doi.org/10.1074/jbc.M113.481473
- Wang, X., Chen, X., Zhang, X., Su, C., Yang, M., He, W., Du, Y., Si, S., Wang, L., & Hong, B. (2020). A small-molecule inhibitor of PCSK9 transcription ameliorates atherosclerosis through the modulation of FoxO1/3 and HNF1α. EBioMedicine, 52, 102650. https://doi.org/10.1016/j.ebiom.2020.102650
- Williams, J., Ensor, C., Gardner, S., Smith, R., & Lodder, R. (2015). BSN723T prevents atherosclerosis and weight gain in ApoE knockout mice fed a western diet. Webmedcentral, 6, WMC005034.
- Wu, Y.-R., Li, L., Sun, X.-C., Wang, J., Ma, C.-Y., Zhang, Y., Qu, H.-L., Xu, R.-X., & Li, J.-J. (2021). Diallyl disulfide improves lipid metabolism by inhibiting PCSK9 expression and increasing LDL uptake via PI3K/Akt-SREBP2 pathway in HepG2 cells. Nutrition, Metabolism, and Cardiovascular Diseases, 31(1), 322–332. https://doi.org/10.1016/j.numecd.2020.08.012
- Xiao, J., Bai, X., Liao, L., Zhou, M., Peng, J., Xiang, Q., Ren, Z., Wen, H., Jiang, Z., Tang, Z., Wang, M., & Liu, L. (2019). Hydrogen sulfide inhibits PCSK9 expression through the PI3K/Akt‑SREBP‑2 signaling pathway to influence lipid metabolism in HepG2 cells. International Journal of Molecular Medicine, 43, 2055–2063. https://doi.org/10.3892/ijmm.2019.4118
- Xie, K., He, X., Chen, K., Sakao, K., & Hou, D.-X. (2020). Ameliorative effects and molecular mechanisms of vine tea on western diet-induced NAFLD. Food & Function, 11(7), 5976–5991. https://doi.org/10.1039/D0FO00795A
- Yang, J. H., Bang, M. A., Jang, C. H., Jo, G. H., Jung, S. K., & Ki, S. H. (2015). Alginate oligosaccharide enhances LDL uptake via regulation of LDLR and PCSK9 expression. The Journal of Nutritional Biochemistry, 26(11), 1393–1400. https://doi.org/10.1016/j.jnutbio.2015.07.009
- Zeng, Y., Peng, Y., Tang, K., Wang, Y. Q., Zhao, Z. Y., Wei, X. Y., & Xu, X. L. (2018). Dihydromyricetin ameliorates foam cell formation via LXRα-ABCA1/ABCG1- dependent cholesterol efflux in macrophages. Biomedicine & Pharmacotherapy, 101, 543–552. https://doi.org/10.1016/j.biopha.2018.02.124
- Zhang, J., Chen, Y., Luo, H., Sun, L., Xu, M., Yu, J., Zhou, Q., Meng, G., & Change Yang, S. (2018). Recent update on the pharmacological effects and mechanisms of Dihydromyricetin. Frontiers in Pharmacology, 9, 1204. https://doi.org/10.3389/fphar.2018.01204
- Zhu, J. P., Wei, X. Y., & Xu, X. L. (2018). Effect of dihydromyricetin on reverse cholesterol transport in high-fat feeding ApoE-/- mice. Chinese Pharmacological Bulletin, 34(11), 1610–1616. https://doi.org/10.3969/j.issn.10011978.2018.11.026
