?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
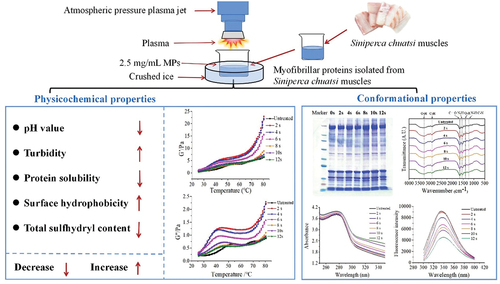
The effects of atmospheric pressure plasma jet (APPJ) treatment on the physicochemical and conformational properties of myofibrillar protein (MP) isolated from mandarin fish. The findings revealed that after exposing MP to APPJ for 0–12 s, there was a significant (P < .05) decline in the solubility and total sulfhydryl concentration. Turbidity and surface hydrophobicity increased significantly, while pH value decreased significantly at first but then stabilized. Gel electrophoresis confirmed that the extracted protein was unaffected. FTIR spectroscopy indicated that the APPJ treatment caused slight changes in secondary structure. However, the UV absorption spectral and intrinsic tryptophan fluorescence demonstrated that the tertiary structure of MP was altered following APPJ treatment. According to the rheological characteristics, APPJ treatment could improve protein cross-linking, raise storage modulus, and promote the development of protein gel network structure. These findings demonstrated that APPJ treatment might improve the functional characteristics of MP isolated from mandarin fish.
Introduction
Mandarin fish (Siniperca chuatsi) is a traditional high-valued fish and widely distributed in China, Korea, Vietnam and other East Asian countries (C. Sun et al., Citation2017). Mandarin fish is highly consumed for its delicate taste and hard texture. Currently, the mandarin fish is a lucrative species of fish that is actively farmed in China, with an annual production of over 370,000 tons in 2022. Therefore, the development of mandarin fish propagation and processing methods is receiving much attention (J. Liu et al., Citation1998; C. F. Sun et al., Citation2015). The main component of mandarin fish protein is myofibrillar protein (MP) which accounts for approximately 55–60% of the total protein (Lin et al., Citation2020). The rheological and heat-induced gelling properties of the meat are affected by the functional characteristics of MP (Niu et al., Citation2018), which are influenced by pH, temperature, ionic strength, and treatment methods such as high pressure (LeBail et al., Citation2002), high-voltage electrostatic field (Jia et al., Citation2018), radio frequency (Bedane et al., Citation2017), ultrasound (Cai et al., Citation2018), vacuum and microwave (Wang et al., Citation2020). Typically, various treatment techniques have the potential to alter the protein’s physicochemical and structural properties, as well as its functional qualities (F. Li et al., Citation2019). Therefore, it is necessary to find an effective processing method that can improve the quality of mandarin fish MP and promote the development of the mandarin fish industry.
Plasma is a unionized gas made up of a variety of reactive components such as electrons and photons (Lu et al., Citation2016), which can disrupt covalent bonds of protein, resulting in structural and physicochemical changes. Cold plasma has been used for sterilization (Kang et al., Citation2022; J. Wang et al., Citation2021), thawing (Liao et al., Citation2020), and food preservation (Chaijan et al., Citation2021) as a novel non-thermal technology. Cold plasma is generated by atmospheric pressure gas discharge, including atmospheric pressure plasma jet (APPJ), dielectric barrier discharge (DBD), and radio frequency discharge (RFD). Several studies have recently been carried out to ascertain the effects of cold plasma on meat components and quality, including modifications in lipids and proteins (Luo et al., Citation2022). Cold plasma was observed to improve the functional features of MP from king prawns (Ekezie et al., Citation2019) through enhanced protein-water and protein-protein interactions. Furthermore, Miao et al. (Citation2020) showed that the gel features of MP in Alaska Pollock may be greatly enhanced by cold plasma (40 kV).
Despite the vast research on microbial decontamination by cold plasma, modification of MP from fish by APPJ has not been reported. In order to explore the interaction of cold plasma on the myofibrillar protein of mandarin fish, the effects of APPJ on the physicochemical and conformational features of MP from mandarin fish was studied. The pH, protein solubility, turbidity, dynamic rheological, primary and secondary structure, surface hydrophobicity, total sulfhydryl content, UV absorption spectra, and fluorescence scan spectra were measured. The findings of this research will aid APPJ in more effectively processing fish products, as well as create the theoretical framework for the development of new products and the optimization of APPJ processing for the fish industry.
Materials and methods
Materials
Fresh mandarin fishes (about 500 g and 12 months old) were acquired from a local supermarket (Hema Fresh supermarket, Hangzhou, China). Upon arrival, the fish muscles were washed with pre-cooled distilled water. Trunk muscles of fish were cut off and divided into small pieces. The muscles were kept in refrigerator (4°C) until protein preparation.
Preparation of MP
MP was isolated from mandarin fish as previously described by K. Li et al. (Citation2020) with minor changes. First, the trunk muscles were homogenized by blending at 10,000 rpm for 60 s with 5 volumes (w/v) of pre-cooled 20 mM Tris-HCl buffer solution (pH 7.0) in a blender (POLYTRON® PT6100D, Kinematica AG, Malters, Switzerland). The supernatant was removed after centrifugation at 4000 × g for 15 min (4°C) using a high-speed refrigerated centrifuge (3K15, Sigma, Osterode am Harz, Germany). The above process was repeated three times. After that, the precipitate was further washed three consecutive times with 5 volumes (w/v) of pre-cooled 0.1 M NaCl solution under the same conditions. The suspension was filtered through 40 mesh strainer to get rid of connective tissue. Afterwards, the filtrate was further centrifuged (4000 × g for 15 min at 4°C), then the pellet was collected and dissolved in pre-cooled 0.6 M NaCl solution. The biuret method was used to calculate the concentration of MP solution in the solution.
APPJ treatment
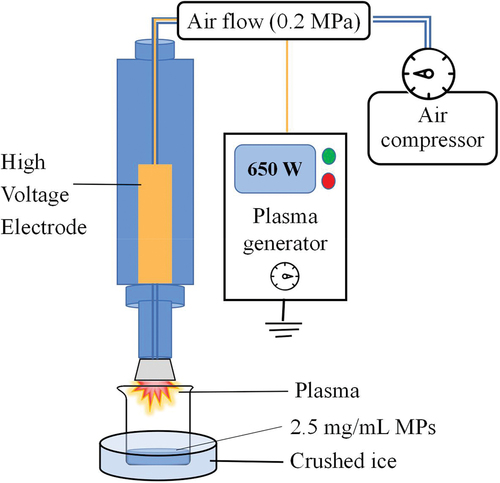
The atmospheric pressure plasma jet (APPJ, Tonson Tech Automation Equipment Co., LtD., Shenzhen, China) was used to treat the MP solution in this experiment. The equipment consisted of a high-pressure generator (power of 650 W), an air compressor, and a rotating plasma discharge nozzle (). For APPJ treatment, 30 mL of MP solution (2.5 mg/mL) isolated from mandarin fish was transferred into a 250 mL glass beaker (6.5 cm × 9.5 cm) with about 1 cm depth, which was placed on crushed ice to keep the sample at a low temperature during processing. The MP solution was discharged by plasma for 0–12 s with 2 s intervals.
Determination of pH value
Samples were equilibrated to room temperature, then a pH meter (PB-10, Beijing Cedis Scientific Instruments Co., Ltd, Beijing, China) was used to measure the pH value of untreated and treated MP solutions and repeated at least three times.
Determination of protein solubility and turbidity
The solubility of MP was determined according to K. Li et al. (Citation2019) with some minor modification. Untreated and treated MP solutions were centrifuged at 5000 × g (4°C, 10 min). Protein solubility was calculated as a ratio of the concentration in the supernatant to the concentration of protein before treatment.
The turbidity of treated and untreated MP solution by APPJ was determined according to F. Li et al. (Citation2019) with some modifications. The absorbance of each sample (2.5 mg/mL) at 660 nm (UV-2600, Shimadzu Co., Kyoto, Japan) was measured a minimum of 3 times used.
Dynamic rheological analysis
A rheometer (Physica MCR 302, Anton Paar, Graz, Austria) with a parallel plate of 25 mm diameter was used to measure the dynamic rheological properties of MP with the temperature increased from 25 to 80°C at a scan rate of 5°C/min, the linear viscoelastic region (LVR) was determined by strain scanning testing, as described previously by K. Li et al. (Citation2019).
SDS-PAGE
The molecular weight of MP following APPJ treatment was determined using gel electrophoresis (SDS-PAGE) in accordance with J. Qian et al. (Citation2021a) with some adjustments. A volume of 20 μL of MP sample was mixed with 5 μL of protein sample loading buffer (5×), then placed in a boiling water bath (100°C) for 3 min. The SDS-PAGE was performed using 4–12% Bis-tris gradient gel (Nanjing ACE Biotechnology CO., Ltd., Nanjing, China), using molecular weights (MWs) of 6.5–240 kDa as the molecular weight standard (Shanghai Beyotime Biotechnology CO., Ltd., Shanghai, China). Protein samples were concentrated for 30 min at 80 V before being switched to 120 V until the bands reached the bottom of the gel. The gel was carefully removed and stained for 4 h using 0.1% coomassie brilliant blue R-250 on a horizontal mixer (Qiwei Instrument Co., Ltd, Hangzhou, China), then unstained for approximately 12 h using deionized water containing 5% ethanol and 7.5% acetic acid on a horizontal shaker.
Fourier transform infrared spectrometer
The MP solution was dried for 24 h in a freeze-dried (SCIENTZ-10N, Ningbo SCIENTZ biotechnology CO. LTD., Ningbo, China). The freeze-dried MP powder was ground in an agate mortar with dried KBr powder before being pressed into a slice. The spectra of sample were recorded from 4000 to 400 cm−1 by an infrared spectrometer (iS50, Thermo Fisher Scientific, Waltham, MA, U.S.A.). Peakfit software (v4.12, SPSS Inc., Chicago,IL, U.S.A.) was used to fit the Gaussian curve of second-derivative peak at 1700–1600 cm−1 (amide Ι) to obtain the information about secondary structure of protein as previously described by K. Li et al. (Citation2020).
Determination of surface hydrophobicity
The surface hydrophobicity of MP was determined according to X. Zhao et al. (Citation2017) with some modifications. An aliquot of 1 mL of MP suspension and 100 μL of bromophenol blue (BPB, 1 mg/mL) were combined. After centrifuging (10000 × g for 5 min), the supernatant was five-fold diluted. The absorbance of the mixture was measured at 595 nm (UV-2600, Shimadzu Co., Kyoto, Japan) and the 0.6 M NaCl buffer without MP was the blank.
Determination of total sulfhydryl content
The total sulfhydryl content (T-SH) was calculated as described by Feng et al. (Citation2017) with slight changes. A volume of 40 μL of Ellman's reagent (DTNB) was added to a mixture of 1 mL MP solution and 4 mL Tris-Glycine buffer (pH 8.0), then incubated for 30 min in the dark. Absorbance was measured at 412 nm with 0.6 M NaCl as the blank. The T-SH was expressed as µmol/g protein.
Fluorescence measurement
The measurement of fluorescence emission spectrum of MP sample was measured by the method of X. Zhao et al. (Citation2018) using a fluorescence spectrophotometer (Infinite E Plex, Tecan Austria GmbH, Grödig, Austria). The fluorescence intensity of the MP sample was determined at an excitation wavelength of 280 nm and an emission wavelength of 300–400 nm, accompanied by a slit width of 2 nm. The emission spectra of all samples was subtracted from that of the buffer solution.
UV absorption spectral analysis
The ultraviolet-visible spectrophotometer (UV-2600, Shimadzu Co., Kyoto, Japan) was used to scan the UV spectrum of untreated and treated MP solution. The spectrum was scanned at medium speed and ranged from 250 to 350 nm (Ekezie et al., Citation2019).
Statistical analysis
SPSS statistical software (SPSS Statistical 22, International Business Machines Co., Armonk, NY, U.S.A.) was used to analyze significant differences in MP of APPJ treatment based on Duncan’s multiple range test from the single component analysis of variance (ANOVA), with P < .05 considered significant.
Results and discussion
Changes in pH
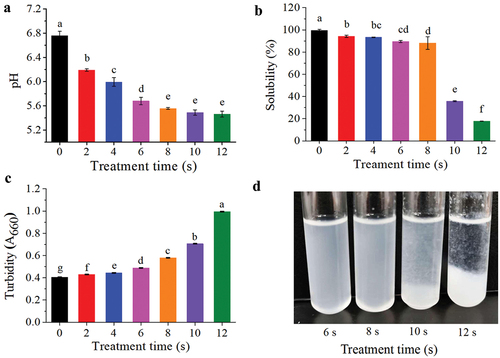
shows the pH values of MP suspensions with different APPJ treatment times. There was a significant decrease (P < .05) from an initial pH of 6.76 ± 0.07 to 5.56 ± 0.015 when the exposure time was increased from 0 to 8 s. After 8 s, the variations in pH value were insignificant (P > .05). The non-covalent bond equilibrium in the polypeptide structure was disturbed when pH value of the solution was close to the isoelectric point (IP) of MP (about pH 5.0–5.2), which caused increased hydrophobic interaction and reduced solubility. These findings corroborated with the trend of MP solubility, turbidity, and surface hydrophobicity. (showed in ). Acidification in the MP solution treated by APPJ could be due to the formation of acidic H3O+ by the reaction of H2O2 with H2O in the air or liquid as well as the generation of HNO2 and HNO3 from NO and NO2 (Traylor et al., Citation2011). Sharifian et al. (Citation2019) found that there was no substantial reduction in pH of MP (solubilized in PBS) before and after treatment by DBD. In the study of Segat et al. (Citation2015), there was a similar tendency, a slight decrease in pH value before 15 min of treatment using PBS as buffer. Other studies reported by Oehmigen et al. (Citation2010) and Y. M. Zhao et al. (Citation2020) found that pH of PBS did not change much with the control while a strong reduction in pH in distilled water or physiological saline was found when using the same plasma treatment conditions. Several factors may contribute to changes in the acidity of plasma treated liquids, including the buffering capacity, the volume treated, sample concentration in solution, as well as the inducer gas employed and type of plasma source.
Figure 2. Changes in the physicochemical parameters of MP from Siniperca chuatsitr untreated and treated with APPJ. (a) pH, (b) Tolubility, (c) Turbidity, (d) MP sample treated by APPJ for 6–12 s. The results are expressed as ,with various letters on the top of the bar chart indicating significant differences (P < .05).
Changes in protein solubility and turbidity
Solubility is an important property of proteins, in which water molecules and protein interact via amino acid side chains (polar, non-polar group and ionization interactions) or peptide bonds (hydrogen bond or dipole-dipole interactions), as it may influence other functional properties (K. Li et al., Citation2019; S. Li et al., Citation2018). shows a significant decrease of protein solubility as exposure time increased (P < .05). The protein solubility decreased to 35.73 ± 0.34% and 17.72 ± 0.07% when the APPJ treatment times were 10 s and 12 s, respectively. When put the treated MP suspension in refrigerator for a period of time, protein aggregation could be clearly seen at the bottom of the tube after 10 s of treatment time (). When the solution was treated by APPJ, some oxidizing active substance (RONS) would be generated in the solution (Hu et al., Citation2021), which may oxidize the active sulfhydryl groups to form disulfide bonds, and cross-linked to form protein polymers. In addition, the pH value of MP solution gradually approached the IP, which also promoted the hydrophobic reaction and precipitation of MP. The secondary and tertiary structures of the protein were extended by changes in the microenvironment and oxidation, exposing substantial sections of the hydrophobic groups that were previously buried in the core of the protein with the folded form. These hydrophobic group interactions led to protein aggregation and precipitation, lowering protein solubility relative to the untreated protein (Bußler et al., Citation2016).
The turbidity was determined through the absorbance value at 660 nm, which can reflect the aggregation state of protein treated by APPJ. The turbidity value of APPJ-treated MP solution was significantly increased (P < .05) from 0.405 ± 0.003 to 0.995 ± 0.003 with the APPJ treatment times increasing from 0 to 12 s (). The turbidity increased due to the unfolding and polymerization of MP molecules, large protein aggregates were produced, which may obstruct light transmission and generate light scattering (D. Jia et al., Citation2015). These results were also consistent with the tendency obtained for the protein solubility treated with APPJ ().
Changes in dynamic rheological property
Rheological property is one of the most significant functional characteristics of protein, mainly affected by molecular weight and structure. The elastic property of protein is expressed as storage modulus (G´), whereas the viscosity property is expressed as the loss modulus (G”) (Zhang et al., Citation2017). So rheological properties are usually used to describe the changes of protein during thermal coagulation. As seen in , untreated MP exhibited a typical rheological graph with transitions between heavy and light meromyosin. There was a small peak, when the temperature increased from 30°C to 54°C. From 54 to 62°C, the G´ and G” decreased slightly and then displayed a steady increase over 62 to 80°C. Chen et al. (Citation2016) also obtained a similar rheological pattern.
Figure 3. Influences of APPJ treatment on (a) Storage modulus (G´) Of mandarin fish MP, (b) Loss modulus (G”) Of mandarin fish MP.
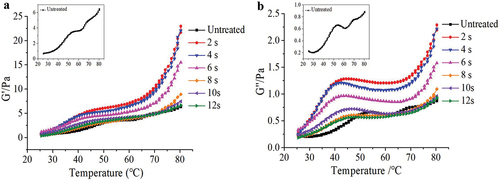
The G´ value changes in all of the samples were similar, the G´ values began to gradually increase from the onset temperature until the temperature reached 80°C after exposure to plasma. The onset temperature of G´ of treated MP was lower than untreated, and the “peak” became inconspicuous. The “peak” G” values of all treated samples were greater than that of untreated sample after exposure to plasma from 2 to 8 s, but lower than that of untreated sample when the MP was exposed to plasma for 10 s and 12 s. It was easy to find that the G´ and G‘ values were maximum at 2 s of treatment time, then decreased when treatment time increased. The G´ and G’ values at 2–6 s were higher than those of untreated MP, demonstrating that mild oxidation was advantageous to promote the generation of gel network structure of MP, and expressed by the storage modulus value which was higher than that of untreated sample (Xiong et al., Citation2010). The increase in gel strength might be due to changes in the cross-linking pattern of MP. Myosin tail disulfide bond crosslinking under oxidized conditions was more stable than myosin head and head crosslinking under non-oxidized conditions, which could promote a more stable gel structure for MP. The G´ and G” values decreased as treatment time increased, due to excessive oxidation of protein, which caused protein denaturation and aggregation, reducing the MP interaction. Furthermore, oxidation mediated by APPJ, particularly when treating for a longer duration, may induce MP cleavage, resulting in the creation of short chain polypeptides that cannot interact efficiently as evidenced, and the gel formation ability was poor. During the gel formation heating process, G” value was lower than G´ value, reflecting that elasticity was more important compared with viscosity during the MP gel formation. The results indicated that APPJ treatment could enhance cross-linking of MP: however, it is necessary to control the treatment time to prevent excessive oxidation of protein.
SDS-PAGE analysis
SDS-PAGE was carried out to measure the effect of APPJ treatment times on the molecular weight of MP in mandarin fish (). The characteristic bands of MP were seen in all samples. Once the MP was treated by different discharge times, there were no disappearance of original protein feature bands and no appearance of new protein feature bands, demonstrating that no remarkable changes were found in the treated MP compared with the control. The comparable expression of MP indicated that there was no change in protein patterns after APPJ treatment or that the influence of APPJ treated on the MP was not visible in the electrophoresis. J. Qian et al. (Citation2021b) also showed that plasma-activated water (PAW) did not change the characteristic bands of MP in chicken breasts. The changes in T-SH (showed in ) may imply the formation of disulphide bonds as a result of SH group recombination or reactive species attachment, however this changes were not visible in the electrophoresis. The FTIR spectrum of MP also depicted that the feature peaks of all proteins treated by different APPJ treatment times were consistent, as seen in .
Figure 4. Electrophoresis pattern of MP extracted from Siniperca chuatsitr after different treatment times by APPJ (0-12s).
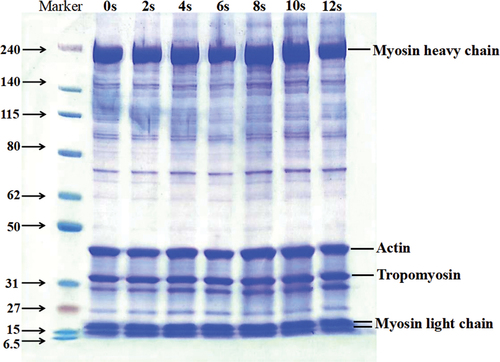
Figure 5. (a) FTIR spectra of MP by different APPJ treatment times; (b) Effects in secondary structure content of MP by different APPJ treatment times.
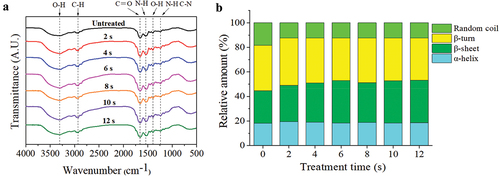
Figure 6. (a) Influences of APPJ treatment on the surface hydrophobicity of mandarin fish MP, (b) Influences of APPJ treatment on the total sulfhydryl content of mandarin fish MP. The findings are expressed as , with various letters on the top of the bars indicating significant differences (P < .05).
FTIR analysis
shows the corresponding FTIR spectra for MP in mandarin fish after various APPJ treatment times compared with the control. The structure remained intact after APPJ treatment, and there was no evidence of new distinctive peaks appearing or the elimination of characteristic peaks.
Among the IR characteristic absorption peaks of protein, Amide Ι band (1600–1700 cm−1) is most frequently used for analyzing the protein secondary structure (Huson et al., Citation2011). As shown in , different plasma treatment times slightly affected the secondary structure of MP in mandarin fish. The percentage of α-helix was increased slightly with the increasing of treatment time. For the 2-s-treated group, the percentage of α-helix reached a high of 19.34%. Dong et al. (Citation2017) observed comparable changes, with the proportion of α-helix of zein in corn increasing after a brief period of atmospheric cold plasma (ACP) treatment. Compared with the untreated group, the proportion of β-sheet was significantly (P < .05) increased, and the proportion of random coils were significantly (P < .05) decreased after treatment. As treatment time increased, there was a slight increase in the concentration of α-helix and a slight decline in the concentration of β-turn. The concentration of β-sheet in MP increased from 26.58 to 34.48%, and the proportion of random coils declined from 18.34 to 12.27%. The hydrogen bonds that are formed in the peptide chain between NH- and C=O were changed by APPJ treatment, inducing slight changes in each component of secondary structure. The disulfide bond was generally generated around the β-turn, there was a similar decreasing trend of sulfhydryl content to the content of β-turn (). In addition, it was known that α-helix was a stretchy and tough component in secondary construction. The enhancement of this section may help MP in mandarin fish having a better gel property. This observation was similar to the rheological properties ().
Changes in total sulfhydryl content and protein surface hydrophobicity
The changes of T-SH can reflect the distribution of hydrophobic residues on the protein surface (J. Jia et al., Citation2017). Protein functionality benefits from an appropriate balance of non-hydrophobic and hydrophobic residue (G. Jia et al., Citation2017). As showed in , the surface hydrophobicity of MP treated with APPJ showed a significant (P < .05) increased from 17.84 ± 1.78 to 62.00 ± 1.68 μg before 6 s of treatment (P < .05) compared to the control, and a minor increase in surface hydrophobicity value after 6 s of treatment time. The plasma treatment improved the surface hydrophobicity of MP in mandarin fish by exposing hydrophobic amino acids such as tryptophan and phenylalanine (Ekezie et al., Citation2019). Plasma discharge treatment can produce a lot of oxidizing reactive species, which may cause the oxidation of protein and improve the surface hydrophobicity. When protein solutions were over-oxidized, the interactions of protein-protein were enhanced which caused the protein denaturation. In addition, the decrease in pH also improved the hydrophobic reaction between proteins and increased the surface hydrophobicity of MP. These results were consistent with the solubility results obtained and the phenomenon of protein stratification as can be seen in the .
The transformation between disulfide linkage and SH groups can be used to determine the change in protein structure (Ekezie et al., Citation2019). The SH group which belong to weak secondary bonds, maintains the tertiary structure of protein. The changing degree of tertiary and quaternary structure can be calculated from the change of SH content (Zhang et al., Citation2017). shows the T-SH of MP in mandarin fish exposed to APPJ at various treatment times. A significant (P < .05) decline in T-SH from 15.03 ± 0.11 to 11.28 ± 0.20 μmol/g after 12 s (P < .05) was observed. This finding was consistent with a previous study of Dong et al. (Citation2017) and Ji et al. (Citation2017), where the T-SH content declined with an increase in exposure time. The T-SH decline may be caused by protein oxidation which was triggered by the attack of oxidizing reactive species (Leygonie et al., Citation2012). Previous research showed that the formation of disulfide bonds caused the reduction of T-SH content in the oxidation system (Chelh et al., Citation2006; Y. Li et al., Citation2013).
Comprehensively, APPJ treatment altered the tertiary and quaternary structure of MP molecules, exposing internal SH groups and hydrophobic groups, and strengthening the generation of disulfide bonds and surface hydrophobicity, which was attributable to protein-protein interactions.
Changes in fluorescence and UV visible spectroscopy
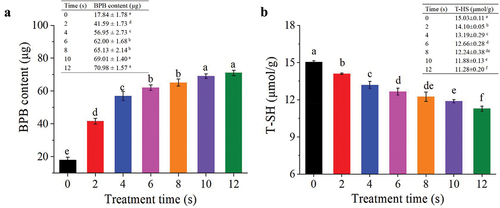
shows the effects of plasma on aromatic amino acid residues in MP from mandarin fish. The UV absorption curve of natural MP showed a main absorption peak inside the ultraviolet region at 276 nm, which was similar with the study reported by Ekezie et al. (Citation2019) for MP in king prawns. It is a typical protein peak (close to 280 nm) including aromatic amino acids, which are most likely linked to the vibration of the tyrosine (Tyr) and tryptophan (Trp) residues, and are the key factors for the UV absorption with peaks near 277 nm and 285 nm, respectively (C. Sun et al., Citation2016). It could be seen that APPJ treatment significantly (P < .05) increased the UV absorbance intensity compared with natural MP, especially during the treatment time of 12 s, which demonstrated the highest absorption value. The increase in absorption intensity revealed a change in MP structure. The changes in fluorescence intensity of MP after exposed to APPJ revealed that a piece of the chromophore rotated to outside, allowing more Tyr and Trp residues to be exposed.
Figure 7. (a) UV absorption spectra of MP from mandarin fish exposed to different APPJ treatment times, (b) Intrinsic emission fluorescence spectra of MP from mandarin fish exposed to different APPJ treatment times.
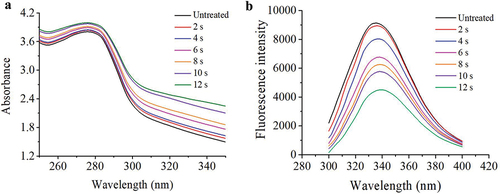
Intrinsic Trp fluorescence properties, which are an important sign of a protein’s tertiary structure, are especially sensitive to the Trp microenvironment (Cao & Xiong, Citation2015). When the Trp residues are buried in the core (a hydrophobic environment) of the protein in its folded state, the intrinsic fluorescence intensity is strong (Cai et al., Citation2018). As shown in , the Trp fluorescence intensity of APPJ-treated MP exhibited a remarkable decline compared with the control, indicating that protein unfolding was attributed to APPJ-induced oxidation of Trp and protein aggregation. Simultaneously, the maximum emission wavelength (λ max) of MP solution increased from 334 to 340 nm, with the increase of treatment time from 0 to 12 s. the slight redshift of λ max demonstrated that the plasma treatment unfolded the tertiary conformation of MP, and promoted the exposure of more aromatic acids to the polar environment. Ekezie et al. (Citation2019) indicated that MP in king prawn was treated by plasma leading to a slight redshift from 333 to 336 nm of λ max, which indicated a certain extent of unfolding of protein. Q. Liu et al. (Citation2015) discovered that with the increased degree of hydroxyl radical-induced oxidation, hydrophobic groups were exposed, which induced the formation of protein aggregation because of hydrophobic interactions. Therefore, APPJ-induced change of tertiary structure in MP extracted from mandarin fish can be understood as follows: APPJ-induced oxidation treatment remarkably increases the range of exposed hydrophobic domains attributed to protein partial unfolding and cross-linking hydrophobic interactions, which leads to MP aggregation.
Conclusion
APPJ treatment decreased pH due to the generation of acidic H3O+ ions, HNO2, and HNO3 in the solution. In addition, plasma treatment could enhance the aggregation of intermolecular proteins, increasing turbidity, precipitating the protein, and decreasing the solubility of protein. The fundamental structure of MP was unaffected, while APPJ treatment changed the secondary structure which increased the ordered structure. As the processing time of plasma increased, the hydrophobicity and maximum UV absorption intensity increased, while the total sulfhydryl content and fluorescence intensity decreased. This is because plasma treatment could unfold MP molecules exposing interior SH groups and hydrophobic groups, increase the generation of disulfide bonds and surface hydrophobicity, and change the tertiary and quaternary structure of MP molecules. In brief, moderate APPJ treatment could accelerate MP oxidation, boost the development of gel network structure of MP, and improve its functional characteristics. Finally, when APPJ technology is used for processing aquatic meat products in the future, the prevention of over-oxidation of MP in fish or other aquatic products should be considered.
Author contributions
Wei Jiang: Conceptualization, Methodology, Software, Data curation, Writing-original draft. Ricardo Antonio Wu: Writing-original draft, Visualization. Xiao Hu: Conceptualization, Methodology. Runrun Zhang: Data curation, Software. Tian Ding: Conceptualization, Supervision, Validation. Jianwei Zhou: Conceptualization, Supervision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bedane, T. F., Chen, L., Marra, F., & Wang, S. (2017). Experimental study of radio frequency (RF) thawing of foods with movement on conveyor belt. Journal of Food Engineering, 201, 17–25. https://doi.org/10.1016/j.jfoodeng.2017.01.010
- Bußler, S., Rumpold, B. A., Fröhling, A., Jander, E., Rawel, H. M., & Schlüter, O. K. (2016). Cold atmospheric pressure plasma processing of insect flour from Tenebrio molitor: Impact on microbial load and quality attributes in comparison to dry heat treatment. Innovative Food Science & Emerging Technologies, 36, 277–286. https://doi.org/10.1016/j.ifset.2016.07.002
- Cai, L., Cao, M., Cao, A., Regenstein, J., Li, J., & Guan, R. (2018). Ultrasound or microwave vacuum thawing of red seabream (Pagrus major) fillets. Ultrasonics Sonochemistry, 47, 122–132. https://doi.org/10.1016/j.ultsonch.2018.05.001
- Cao, Y., & Xiong, Y. L. (2015). Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chemistry, 180, 235–243. https://doi.org/10.1016/j.foodchem.2015.02.036
- Chelh, I., Gatellier, P., & Sante-Lhoutellier, V. (2006). Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Science, 74(4), 681–683. https://doi.org/10.1016/j.meatsci.2006.05.019
- Chen, H., Diao, J., Li, Y., Chen, Q., & Kong, B. (2016). The effectiveness of clove extracts in the inhibition of hydroxyl radical oxidation-induced structural and rheological changes in porcine myofibrillar protein. Meat Science, 111, 60–66. https://doi.org/10.1016/j.meatsci.2015.08.017
- Dong, S., Wang, J. M., Cheng, L. M., Lu, Y. L., Li, S. H., & Chen, Y. (2017). Behavior of zein in aqueous ethanol under atmospheric pressure cold plasma treatment. Journal of Agricultural and Food Chemistry, 65(34), 7352–7360. https://doi.org/10.1021/acs.jafc.7b02205
- Ekezie, F. C., Cheng, J. H., & Sun, D. W. (2019). Effects of atmospheric pressure plasma jet on the conformation and physicochemical properties of myofibrillar proteins from king prawn (Litopenaeus vannamei). Food Chemistry, 276, 147–156. https://doi.org/10.1016/j.foodchem.2018.09.113
- Feng, X., Chen, L., Lei, N., Wang, S., Xu, X., Zhou, G., & Li, Z. (2017). Emulsifying properties of oxidatively stressed myofibrillar protein emulsion gels prepared with (-)-epigallocatechin-3-gallate and NaCl. Journal of Agricultural and Food Chemistry, 65(13), 2816–2826. https://doi.org/10.1021/acs.jafc.6b05517
- Haijan, M., Chaijan, S., Panya, A., Nisoa, M., Cheong, L. Z., & Panpipat, W. (2021). High hydrogen peroxide concentration-low exposure time of plasma-activated water (PAW): A novel approach for shelf-life extension of Asian sea bass (lates calcarifer) steak. Innovative Food Science & Emerging Technologies, 74, 102861. https://doi.org/10.1016/j.ifset.2021.102861
- Hu, X., Zhang, Y., Wu, R. A., Liao, X., Liu, D., Cullen, P. J., Zhou, R.-W., & Ding, T. (2021). Diagnostic analysis of reactive species in plasma-activated water (PAW): Current advances and outlooks. Journal of Physics D: Applied Physics, 55(2), 023002. https://doi.org/10.1088/1361-6463/ac286a
- Huson, M. G., Strounina, E. V., Kealley, C. S., Rout, M. K., Church, J. S., Appelqvist, I. A., Gidley, M. J., & Gilbert, E. P. (2011). Effects of thermal denaturation on the solid-state structure and molecular mobility of glycinin. Biomacromolecules, 12(6), 2092–2102. https://doi.org/10.1021/bm200080h
- Ji, H., Dong, S., Han, F., Li, Y., Chen, G., Li, L., & Chen, Y. (2017). Effects of dielectric barrier discharge (DBD) cold plasma treatment on physicochemical and functional properties of peanut protein. Food and Bioprocess Technology, 11(2), 344–354. https://doi.org/10.1007/s11947-017-2015-z
- Jia, D., You, J., Hu, Y., Liu, R., & Xiong, S. (2015). Effect of CaCl2 on denaturation and aggregation of silver carp myosin during setting. Food Chemistry, 185, 212–218. https://doi.org/10.1016/j.foodchem.2015.03.130
- Jia, G., Liu, H., Nirasawa, S., & Liu, H. (2017). Effects of high-voltage electrostatic field treatment on the thawing rate and post-thawing quality of frozen rabbit meat. Innovative Food Science & Emerging Technologies, 41, 348–356. https://doi.org/10.1016/j.ifset.2017.04.011
- Jia, G., Nirasawa, S., Ji, X., Luo, Y., & Liu, H. (2018). Physicochemical changes in myofibrillar proteins extracted from pork tenderloin thawed by a high-voltage electrostatic field. Food Chemistry, 240, 910–916. https://doi.org/10.1016/j.foodchem.2017.07.138
- Jia, J., Gao, X., Hao, M., & Tang, L. (2017). Comparison of binding interaction between beta-lactoglobulin and three common polyphenols using multi-spectroscopy and modeling methods. Food Chemistry, 228, 143–151. https://doi.org/10.1016/j.foodchem.2017.01.131
- Kang, T., Yim, D., Kim, S. S., Baek, K. H., Kim, H. J., & Jo, C. (2022). Effect of plasma-activated acetic acid on inactivation of Salmonella typhimurium and quality traits on chicken meats. Poultry Science, 101(5), 101793. https://doi.org/10.1016/j.psj.2022.101793
- LeBail, A., Chevalier, D., & Mussa, D. M. (2002). High pressure freezing and thawing of foods a review. International Journal of Refrigeration, 25(5), 504–513. https://doi.org/10.1016/S0140-7007(01)00030-5
- Leygonie, C., Britz, T. J., & Hoffman, L. C. (2012). Impact of freezing and thawing on the quality of meat: Review. Meat Science, 91(2), 93–98. https://doi.org/10.1016/j.meatsci.2012.01.013
- Li, F., Wang, B., Liu, Q., Chen, Q., Zhang, H., Xia, X., & Kong, B. (2019). Changes in myofibrillar protein gel quality of porcine longissimus muscle induced by its structural modification under different thawing methods. Meat Science, 147, 108–115. https://doi.org/10.1016/j.meatsci.2018.09.003
- Li, K., Fu, L., Zhao, Y.-Y., Xue, S.-W., Wang, P., Xu, X.-L., & Bai, Y.-H. (2020). Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocolloids, 98, 105275. https://doi.org/10.1016/j.foodhyd.2019.105275
- Li, K., Liu, J. Y., Fu, L., Zhao, Y. Y., & Bai, Y. H. (2019). Comparative study of thermal gelation properties and molecular forces of actomyosin extracted from normal and pale, soft and exudative-like chicken breast meat. Asian-Australasian Journal of Animal Sciences, 32(5), 721–733. https://doi.org/10.5713/ajas.18.0389
- Li, S., Zheng, Y., Xu, P., Zhu, X., & Zhou, C. (2018). L-Lysine and l-arginine inhibit myosin aggregation and interact with acidic amino acid residues of myosin: The role in increasing myosin solubility. Food Chemistry, 242, 22–28. https://doi.org/10.1016/j.foodchem.2017.09.033
- Li, Y., Kong, B., Xia, X., Liu, Q., & Diao, X. (2013). Structural changes of the myofibrillar proteins in common carp (Cyprinus carpio) muscle exposed to a hydroxyl radical-generating system. Process Biochemistry, 48(5–6), 863–870. https://doi.org/10.1016/j.procbio.2013.03.015
- Liao, X., Xiang, Q., Cullen, P. J., Su, Y., Chen, S., Ye, X., Liu, D., & Ding, T. (2020). Plasma-activated water (PAW) and slightly acidic electrolyzed water (SAEW) as beef thawing media for enhancing microbiological safety. LWT-Food Science and Technology, 17, 108649. https://doi.org/10.1016/j.lwt.2019.108649
- Lin, R., Xiao, X., Yue, Y., Wang, Y., Pan, D., Wang, D., Yang, Q., He, J., & Cao, J. (2020). Myosin affects the structure and volatile flavour compounds binding of G‐actin in grass carp. International Journal of Food Science & Technology, 55(10), 3235–3245. https://doi.org/10.1111/ijfs.14586
- Liu, J., Cui, Y., & Liu, J. (1998). Food consumption and growth of two piscivorous fishes, the mandarin fish and the Chinese snakehead. Journal of Fish Biology, 53(5), 1071–1083. https://doi.org/10.1111/j.1095-8649.1998.tb00464.x
- Liu, Q., Lu, Y., Han, J., Chen, Q., & Kong, B. (2015). Structure-modification by moderate oxidation in hydroxyl radical-generating systems promote the emulsifying properties of soy protein isolate. Food Structure, 6, 21–28. https://doi.org/10.1016/j.foostr.2015.10.001
- Lu, X., Naidis, V. G., Laroussi, M., Reuter, S., & Graves, B. D. (2016). Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Physics Reports, 630, 1–84. https://doi.org/10.1016/j.physrep.2016.03.003
- Luo, J., Xu, W., Liu, Q., Zou, Y., Wang, D., & Zhang, J. (2022). Dielectric barrier discharge cold plasma treatment of pork loin: Effects on muscle physicochemical properties and emulsifying properties of pork myofibrillar protein. LWT-Food Science and Technology, 162, 113484. https://doi.org/10.1016/j.lwt.2022.113484
- Miao, W., Nyaisaba, B. M., Koddy, J. K., Chen, M., Hatab, S., & Deng, S. (2020). Effect of cold atmospheric plasma on the physicochemical and functional properties of myofibrillar protein from Alaska pollock (Theragra chalcogramma). International Journal of Food Science & Technology, 55, 517–525. https://doi.org/10.1111/ijfs.14295
- Niu, H., Xia, X., Wang, C., Kong, B., & Liu, Q. (2018). Thermal stability and gel quality of myofibrillar protein as affected by soy protein isolates subjected to an acidic pH and mild heating. Food Chemistry, 242, 188–195. https://doi.org/10.1016/j.foodchem.2017.09.055
- Oehmigen, K., Hähnel, M., Brandenburg, R., Wilke, C., Weltmann, K. D., & von Woedtke, T. (2010). The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Processes and Polymers, 7(3–4), 250–257. https://doi.org/10.1002/ppap.200900077
- Qian, J., Wang, C., Zhuang, H., Nasiru, M. M., Zhang, J., & Yan, W. (2021a). Evaluation of meat-quality and myofibrillar protein of chicken drumsticks treated with plasma-activated lactic acid as a novel sanitizer. LWT, 138, 110642. https://doi.org/10.1016/j.lwt.2020.110642
- Qian, J., Wang, Y., Zhuang, H., Yan, W., Zhang, J., & Luo, J. (2021b). Plasma activated water-induced formation of compact chicken myofibrillar protein gel structures with intrinsically antibacterial activity. Food Chemistry, 351, 129278. https://doi.org/10.1016/j.foodchem.2021.129278
- Segat, A., Misra, N. N., Cullen, P. J., & Innocente, N. (2015). Atmospheric pressure cold plasma (ACP) treatment of whey protein isolate model solution. Innovative Food Science & Emerging Technologies, 29, 247–254. https://doi.org/10.1016/j.ifset.2015.03.014
- Sharifian, A., Soltanizadeh, N., & Abbaszadeh, R. (2019). Effects of dielectric barrier discharge plasma on the physicochemical and functional properties of myofibrillar proteins. Innovative Food Science & Emerging Technologies, 54, 1–8. https://doi.org/10.1016/j.ifset.2019.03.006
- Sun, C., Dai, L., He, X., Liu, F., Yuan, F., & Gao, Y. (2016). Effect of heat treatment on physical, structural, thermal and morphological characteristics of zein in ethanol-water solution. Food Hydrocolloids, 58, 11–19. https://doi.org/10.1016/j.foodhyd.2016.02.014
- Sun, C., Niu, Y., Ye, X., Dong, J., Hu, W., Zeng, Q., Chen, Z., Tian, Y., Zhang, J., & Lu, M. (2017). Construction of a high-density linkage map and mapping of sex determination and growth-related loci in the mandarin fish (Siniperca chuatsi). BMC Genomics, 18(1), 446. https://doi.org/10.1186/s12864-017-3830-3
- Sun, C. F., Ye, X., Tian, Y. Y., & Dong, J. J. (2015). Simple sequence repeat-based analysis of the genetic diversity and population genetic structure of populations of siniperca chuatsi. Genetics & Molecular Research, 14(3), 9343–9352. https://doi.org/10.4238/2015.August.10.15
- Traylor, M. J., Pavlovich, M. J., Karim, S., Hait, P., Sakiyama, Y., Clark, D. S., & Graves, D. B. (2011). Long-term antibacterial efficacy of air plasma-activated water. Journal of Physics D: Applied Physics, 44(47), 472001. https://doi.org/10.1088/0022-3727/44/47/472001
- Wang, B., Du, X., Kong, B., Liu, Q., Li, F., Pan, N., Xia, X., & Zhang, D. (2020). Effect of ultrasound thawing, vacuum thawing, and microwave thawing on gelling properties of protein from porcine longissimus dorsi. Ultrasonics Sonochemistry, 64, 104860. https://doi.org/10.1016/j.ultsonch.2019.104860
- Wang, J., Han, R., Liao, X., & Ding, T. (2021). Application of plasma-activated water (PAW) for mitigating methicillin-resistant Staphylococcus aureus (MRSA) on cooked chicken surface. LWT-Food Science and Technology, 137, 110465. https://doi.org/10.1016/j.lwt.2020.110465
- Xiong, Y. L., Blanchard, S. P., Ooizumi, T., & Ma, Y. (2010). Hydroxyl radical and ferryl-generating systems promote gel network formation of myofibrillar protein. Journal of Food Science, 75(2), C215–221. https://doi.org/10.1111/j.1750-3841.2009.01511.x
- Zhang, Z., Yang, Y., Zhou, P., Zhang, X., & Wang, J. (2017). Effects of high pressure modification on conformation and gelation properties of myofibrillar protein. Food Chemistry, 217, 678–686. https://doi.org/10.1016/j.foodchem.2016.09.040
- Zhao, X., Bai, Y., Xing, T., Xu, X. L., & Zhou, G. (2018). Use of an isoelectric solubilization/precipitation process to modify the functional properties of PSE (pale, soft, exudative)-like chicken meat protein: A mechanistic approach. Food Chemistry, 248, 201–209. https://doi.org/10.1016/j.foodchem.2017.12.048
- Zhao, X., Xing, T., Chen, X., Han, M., Deng, S., Xu, X., & Zhou, G. (2017). Changes of molecular forces during thermo-gelling of protein isolated from PSE-Like chicken breast by various isoelectric solubilization/precipitation extraction strategies. Food and Bioprocess Technology, 10(7), 1240–1247. https://doi.org/10.1007/s11947-017-1893-4
- Zhao, Y. M., Ojha, S., Burgess, C. M., Sun, D. W., & Tiwari, B. K. (2020). Influence of various fish constituents on inactivation efficacy of plasma‐activated water. International Journal of Food Science & Technology, 55(6), 2630–2641. https://doi.org/10.1111/ijfs.14516