?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Ultrasonic-assisted enzymatic extraction (UAEE) technology was employed to extract the polysaccharides from Dendrobium offcinale Kimura et Migo (D. offcinale). The extraction conditions were optimized by central composite design (CCD) found in the response surface methodology (RSM). The polysaccharide yield (34.05 ± 1.03%) was well matched with the predicted value, as obtained under the optimum parameters: enzyme activity of 2040 U/g, a liquid-solid ratio of 71 mL/g, and a time of 84 min. Compared to other extraction methods, UAEE was able to yield D. offcinale polysaccharide (DOP) highly efficiently in a shorter extraction time period and at a lower extraction temperature. The extracted polysaccharide (DOPUAEE) showcased a better nitrite scavenging ability, a high antioxidant activity, and more potency in inhibiting α-glucosidase, expanding its versatility in food applications.
1. Introduction
Natural polysaccharides are widely present in plants, animals, algae, and microorganisms. Because of their distinctive characteristics of lower toxicity, high pharmacological activity, and fewer adverse reactions, natural polysaccharides are extensively used in pharmaceutical, food, chemical, and other industries (Yu et al., Citation2018). Dendrobium offcinale Kimura et Migo (D. offcinale) belongs to the genus Dendrobium of Orchidaceae and has long been used as herbal medicine and common food in many Oriental countries. Huge market demand contributes greatly to high economic value of D. offcinale. It was an excellent natural polysaccharide resource for the rich contents (25.92–50.89%) presented in the stem (Shan et al., Citation2023). Previous research has confirmed that D. offcinale polysaccharide (DOP) is an important class of characteristic bioactive compounds and acts as an immune enhancer (He et al., Citation2016b; Tao et al., Citation2019), antifatigue (Wei et al., Citation2017), anticarcinogen (Tao et al., Citation2021), antioxidant (Liang et al., Citation2019), and hypoglycemic agent (Kuang et al., Citation2020; Wang et al., Citation2018). It is vital to find D. offcinale polysaccharides with higher efficiency and availability due to their high therapeutic value, great commercial value, and rising demand.
In recent decades, various extraction strategies have been applied to obtain DOP. The conventional method of extracting polysaccharides by using hot water has certain obvious drawbacks, including a low yield, a lengthy extraction period, and a high temperature (Wang et al., Citation2023). To overcome these problems, novel methods, including ultrasonic extraction (Guo et al., Citation2022), microwave extraction (Mirzadeh et al., Citation2020), enzymatic extraction (Pan et al., Citation2015), and freeze-thawing cold-pressing method extraction (He et al., Citation2018) have evolved. Cell walls are broken down during ultrasonic extraction, which also facilitates the decomposition of organic substances within the cells and increases extraction yield, although damage to polysaccharide structure from sustained ultrasound exposure increases (Chen et al., Citation2021). Under the appropriate ultrasonic treatment, enzymatic extraction can significantly shorten the extraction time while maintaining the biological potencies of polysaccharides while gently and effectively destroying cell walls using enzymes. Therefore, ultrasonic-assisted enzymatic extraction (UAEE) has been employed to yield several natural polysaccharides, such as okra polysaccharides (Olawuyi et al., Citation2020), dandelion (Taraxacum officinale) leaf polysaccharides (Wang et al., Citation2019), Corbicula fluminea polysaccharide (Liao et al., Citation2015), and proved to have higher extraction efficiencies. To the best of our knowledge, this extraction method has never been reported in D. offcinale polysaccharide yield.
This study looked into the potential of DOP as a novel functional material for future development as well as a new, effective extraction technique. Effects of five significant variables (enzyme activity, pH value, extraction temperature, extraction time, and liquid–solid ratio) on the process for extracting DOP were investigated by single factor experiments. According to the result, using a response surface methodology (RSM) based on the central composite design (CCD), the ideal UAEE conditions were optimized. In comparison to those obtained by other methods, the bioactivities of polysaccharides derived by UAEE under the ideal extraction conditions were assessed. The findings are expected to provide technical support and a theoretical basis for producing and applying D. offcinale polysaccharide.
2. Materials and methods
2.1. Materials and chemicals
Whole plants of D. offcinale were gathered in Fujian province (24°60′N, 117°96′E), China, and cultivated in the greenhouse of Xiamen Tasman Bioengineering Co. Ltd. (Xiamen, Fujian, China). The plant variety was identified by Professor Chen Hengbin of Xiamen Botanical Garden. The 4-year-old stems were washed, cut into small pieces, deactivated at 105°C for 15 min, and then dried at 45°C for 48 h. The dry film was crushed and filtered through a 40-mesh sieve pending subsequent experimentation. The mean diameter (D50) of the particles was 337.84 μm measured by Malvern Mastersizer 2000.
D-glucose (Glu), ascorbic acid (Vc), acarbose, 2,2’-azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS), α-glucosidase, papain, cellulase were procured from Sigma-Aldrich Chemical Co., Ltd. (St. Louis, MO, U.S.A.). All the chemicals used were of analytical grade.
2.2. Extraction and purification of polysaccharide
2.2.1. Ultrasonic-assisted enzymatic extraction (UAEE)
10.0 g D. offcinale stems pieces were mixed with distilled water in a homogenizer (HGB 550, Waring Commercial, U.S.A.) and homogenized for 2 min. The homogenate was transferred to the ultrasonic extraction device (KQ-400KDE, Kunshan Ultrasound Instrument Co., Ltd., Jiangsu, China) and ultrasound at 40 kHz frequency, 400 W ultrasonic input power for 20 min. The pH value of the homogenate was adjusted followed by the addition of papain and cellulase (1:1, U/U). The mixture was subsequently kept in a water bath for extraction. After boiling for 5 min to inactivate the enzymes, the juice was filtered through 0.45 μm nylon filters under a vacuum. The filtrates were concentrated by a rotary evaporator (N-1100, EYELA, Japan) under vacuum pressure at 60°C and mixed with four times volumes of anhydrous ethanol for precipitation overnight at 4°C, then centrifuged in a refrigerated centrifuge (TDL-8 M, Juliang Co., Ltd., Shanghai, China) at 8000 rpm for 20 min to yield the precipitate. The precipitate was refluxed with ethanol solution until colorless and evaporated dry under vacuum at 40°C. The dry DOP was re-dissolved in water and deproteinized by the Sevage method (Xiong et al., Citation2017), then transferred to ultra centrifugal filter tubes (molecular weight cutoff: 1 kDa, Yuanye Biological Technology Co., Ltd. Shanghai, China) before centrifugation to remove small molecules and freeze-dried to obtain DOP sample (DOPUAEE).
2.2.2. Hot water extraction method (HWE)
10.0 g D. offcinale stem pieces were mixed with distilled water in a homogenizer and homogenized for 2 min. The homogenate was transferred to a reflux device and extracted in a boiling water bath of 95°C to reflux for 3 h. The extract solution was centrifuged, and the residue was extracted again through the same method. The solution extracts were then combined together. The DOP sample (DOPHWE) was collected through precipitation and purification steps similar to that of DOPUAEE.
2.2.3. Ultrasonic-assisted hot water extraction (UAHWE)
The pretreatment process was identical to that of UAEE. The homogenate was then ultrasonically extracted for 1 h at 80°C. The supernatant was centrifuged, filtered, and precipitated as previously described, whereby the residue was extracted. The next step was identical to that of UAEE and the DOP sample (DOPUAHWE) was subsequently obtained.
2.2.4. Ultrasonic-assisted freeze-thaw extraction (UAFTE)
The homogenate was put in a stainless steel bucket and promptly frozen in a liquid nitrogen tank for 2 hours following the pretreatment procedure identical to that of UAEE. The thawing process was conducted in an ultrasonic extraction device at 80°C. The freeze-thawed samples were then centrifuged, and the precipitate was treated through the same method. The following steps were identical to that of UAEE for the DOP sample (DOPUAFTE) obtained.
2.3. Determination of polysaccharide and reducing sugar
The total sugar content was measured by the phenol-sulphuric acid method using D-glucose as a standard (DuBois et al., Citation1956). Briefly, 2 mL DOP solution was mixed with 50 μL 5% phenol. Then, 5 mL concentrated sulfuric acid was added rapidly, directly against the liquid surface. After 10 min, the tubes were shaken and kept for 15 min in a water bath at 25°C. Finally, the mixture was cooled to room temperature, and the absorbance was measured at 490 nm. The blank was subsequently prepared with distilled water.
The 3, 5-dinitrosalicylic acid method was chosen to test the content of reducing sugars (Miller, Citation1959). 2 mL of the DOP solution was pipetted into a colorimetric tube, and 2 mL of the 3, 5-dinitrosalicylic acid solution was added and mixed. The mixture was heated in a boiling water bath for 5 min, cooled and diluted with distilled water to 25 mL in a volumetric flask. Finally, the absorbance was measured at the wavelength of 540 nm.
The polysaccharide content of DOP could be calculated by EquationEquation (1)(1)
(1) .
2.4. Parameter optimization of ultrasonic-assisted enzymatic extraction
Firstly, the effects of five single factors, including enzyme activity (500–3000 U/g), pH (5.0–7.5), extraction temperature (45–80°C), time (20–120 min), and liquid–solid ratio (50–90 mL/g) on the extraction yield of DOP were investigated. Based on the results of single-factor experiments, factor A enzyme activity (1500, 2000 and 2500 U/g), factor B time (60, 80, 100 min), and factor C liquid-solid ratio (70, 80, 90 mL/g) were used for response surface analysis. The Design-Expert program (Version 13.0.1.0) was used to process and analyze the data. For the purpose of statistically optimizing ultrasonic-assisted enzymatic extraction, a 19-run central composite design (CCD) was used ().
Table 1. CCD response surface experimental design scheme and results.
2.5. In vitro bioactivities
2.5.1. Assay of ABTS scavenging activity
The ABTS radical scavenging activities of DOP samples were determined in accordance with the reported method (Erel, Citation2004). An appropriate amount of ABTS was added into 0.5 mmol/L potassium persulfate solution, to yield 0.7 mmol/L ABTS+ solution. The solution was kept in light-deprived conditions for 12 h at room temperature. 2 mL of the sample solution with different DOP concentrations was added into 2 mL ABTS+ solution, the mixture was shaken vigorously, and the absorbance was measured at 734 nm within 6 min. Ascorbic acid (Vc) was used as a positive control set.
2.5.2. Inhibitory activity on α-glucosidase
The inhibitory activity of α-glucosidase was determined using the methods described by Borges et al. (Citation2021) with minor modifications and compared with acarbose as the standard inhibitor. 30 μL DOP solution or acarbose solution and 30 μL 3.0 U/mL α-glucosidase solution were mixed with 110 μL buffer solution (17.003 g sodium acetate tri-hydrate in 250 mL 0.2 mol/L acetic acid until pH 7.0) in a 1.5 mL tube and incubated in a water bath of 37°C for 15 min. The mixture was subsequently mixed with 30 μL 5 mmol/L p-nitrophenyl-α-D-glucopyranoside solution and kept at 37°C. The reaction was terminated by adding 50 μL 2 mmol/L sodium carbonate solution and centrifuging for the supernatant. The absorbance was measured at 405 nm after 5 min.
2.5.3. In vitro nitrite scavenging activity
The method was performed as previously described by Niu et al. (Citation2021) with slight modifications. 1 mL DOP solution was mixed with 1 mL 1 mmol/L sodium nitrite solution, followed by a water bath of 37°C for 1 h for reaction. Then, 1 mL 0.4% sulfanilic acid solution and 2 mL 0.2% N-naphthyl ethylenediamine dihydrochloride were added. The volume of the mixture was subsequently adjusted to 25 mL by deionized water, and the absorbance was recorded at 540 nm. Different nitrite concentrations were used to create a standard curve, and deionized water replaced the sample solution.
2.6. Statistical analysis
All the experiments were performed at least in triplicate. The data were expressed as mean ± standard deviation (S.D.). The comparison between different treatments was performed using a one-way ANOVA using SPSS 23 (SPSS Inc., IBM). A value of p < .05 was considered statistically significant. Excel 2016 (Microsoft, U.S.A.) and Origin 9.1 (Origin Lab, U.S.A.) were used for other image renderings.
3. Results and discussion
3.1. Single-factor experiment analysis
3.1.1. Effect of enzyme activity
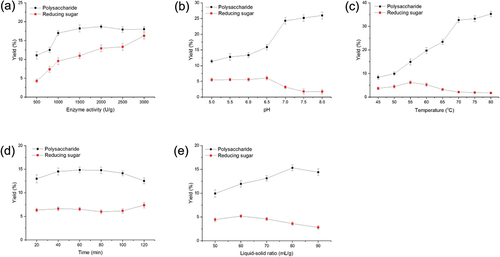
Complex enzymatic hydrolysis can efficiently break down the physiochemical linkages between cellulose, pectin, protein, and other molecules, and facilitate the release of polysaccharides, maximizing the extraction yield (Nadar et al., Citation2018). Compound enzymes (cellulase and papain of 1:1, U/U) were chosen for our study. There was a notable improvement in the extraction yield of reducing sugars with increasing enzyme activity from 500 U/g to 3000 U/g and the polysaccharide yield levels remained at a steady level with a sustained increase in enzymatic activity (). Therefore, 1500 U/g, 2000 U/g and 2500 U/g were chosen for subsequent optimizations.
Figure 1. Effects of different extraction parameters (a) enzyme activity, (b) pH, (c) temperature, (d) time, and (e) liquid-solid radio on extraction yield of polysaccharides and reducing sugars extracted by the method of ultrasonic-assisted enzymatic extraction (n = 3). Each factor was optimized consecutively while keeping rest of the factors constant. The initial constant values of the factors used during the analysis were extraction temperature of 50°C, pH 5.0, time of 2 h, liquid-solid ratio of 50 mL/g, and enzyme activity of 1000 U/g.
3.1.2. Effect of pH value
For the effect of pH values, the extraction yield of polysaccharides increased sharply with an increase in pH value from 5.0 to 7.0. When the pH value exceeded 6.5, the reducing sugar yield decreased. Different enzymes have varying optimal pH values for releasing and hydrolyzing polysaccharides might be because the spatial structure of the enzymes is affected by the change of pH value; thus, the enzyme conformation and enzymatic activity was altered (Pan et al., Citation2015; Zhang et al., Citation2011). Charoensiddhi et al. (Citation2016) reported that pH would influence hydrolysis extents and result in different sugar composition, as well as MW profile of the polysaccharide fractions. The number of hydroxyl group in polysaccharide molecules was associated with the antioxidant activities. According to the yield of reducing sugar, pH 6.5 was optimal in the present experiment ().
3.1.3. Effect of temperature
The effects of extraction temperature on the yield of polysaccharides are illustrated in . When the extraction temperature increased from 50 to 70°C, the yield of DOP increased and reached 33.24 ± 2.09% at 75°C. The high temperature helped the ultrasound to release polysaccharides from cells of raw materials to the solvent, while inactivating enzymes. When the extraction temperature varied from 60 to 80°C, the reducing sugar yield was rapidly decreased, indicating the inactivation of complex enzymes. Considering enzyme activity and polysaccharide yield, the optimum temperature was determined to be 60°C. The data were consistent with the best enzyme-assisted extraction temperature for the Lycium barbarum polysaccharides (Zhang et al., Citation2011).
3.1.4. Effect of extraction time
The required extraction time for the best extraction yield was tested using different extraction times ranging from 20 to 120 min. Early in the extraction process, a large number of polysaccharides were released into the external solution; their concentration peaked at 60 min (). When the extraction time exceeded 60 min, the yield of DOP decreased, while that of reducing sugar increased. DOPs are mainly composed of glucose, mannose, galactose, xylose, arabinose, and rhamnose. The structures have been identified as glucomannan with 1,4-β-D-Manp and 1,4-β-D-Glcp (Chen et al., Citation2021). Long-term extraction resulted in polysaccharide degradation, due to the release of reducing sugar in the enzymatic hydrolysis (Chen et al., Citation2008).
3.1.5. Effect of liquid-solid ratio
The liquid-solid ratio is also an important parameter in the extraction process, which directly affects the dissolution equilibrium of the sample in solution and consequently the extraction yield of the target compound (Shi et al., Citation2022). This study used distilled water as the extraction solvent and different ratios (50, 60, 70, 80, 90 mL/g) were investigated. Because properly increasing the volume of the extraction solvent can enhance the complete dissolution of the target molecule, the polysaccharides acquired a greater extraction yield with the volume of added water rising, as shown in . However, when the ratio was increased to 90 mL/g, a gradual decrease was observed. Therefore, the solid–liquid ratio of 70, 80, and 90 mL/g was further optimized.
3.2. Response surface analysis
To optimize the primary extraction parameters using CCD, 19 sets of experiments were randomly combined. The outcomes are reported in . A mathematical model (EquationEquation (2))(2)
(2) of coded factors predicting the yield of DOP was obtained:
Where Y denotes the yield of DOP, X1 represents the enzyme activity, X2 denotes the time and X3 denotes the liquid-solid ratio. It can be inferred from EquationEquation (2)(2)
(2) that the coefficients of X1, X2, and X3 were all positive, indicating that they played a positive role in the process. Moreover, the absolute values of the coefficients showed X1 > X3 > X2 (p < .05). According to reports, enzyme activity had the biggest impact on enzymatic extraction among these variables (Pan et al., Citation2015).
The results of CCD are shown in . The high value of F (15.92 > 10) and the low p-value (.0002 < .001) indicated that the regression model was highly significant. The “Lack of Fit” (p = .1108) indicated that the model equation was not significant relative to the pure error (Gao et al., Citation2022). The high value of the coefficient of determination (R2 = 0.9409) shows that the model can describe the correlation between the independent variables and the response variables (Guo et al., Citation2022). The R2Adj = 0.8818 also showed the rationality of the model. Moreover, the coefficients of X1, X3, X12, and X32 were significant (p < .05).
Table 2. ANOVA of the experimental results for DOP extraction yield.
A 3D response surface and a 2D contour are created to represent the link between the yields of DOP extraction and the independent factors, as well as the kinds of interactions between the two examined variables (). The interactions between enzyme activity and extraction time as well as between enzyme activity and the liquid-solid ratio were both statistically significant, as indicated in the figure. However, the interaction between time and the liquid-solid ratio was insignificant, as Guo et al. (Citation2022) reported.
Figure 2. Three-dimensional surfaces (A, B and C) and two-dimensional contour plots (a, b and c) showed effects of time, liquid-solid ratio and time on the yield of DOP.
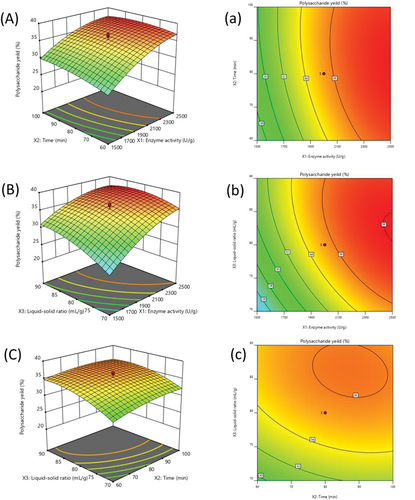
The interactions between the enzyme activity and time on the DOP yield are shown in . The yield of DOP demonstrated a marked increase in response with the increase of enzyme activity at a fixed extraction time. While the yield increased at first and then decreased with the increase of time when enzyme activity was kept at lower level. The elliptical contour plot in indicated significant interaction between enzyme activity and extraction time as well as between enzyme activity and the liquid-solid ratio (Liao et al., Citation2015). When the time was fixed at level 0, enzyme activity and the liquid-solid ratio demonstrated quadratic effects on the extraction yields (). The generation of DOP increased rapidly with the increase of enzyme activity at low liquid-solid ratio. The response surface plot at various times and the liquid-solid ratio was shown in . When the time was varied between 60 and 100 min, the response curves were rather smooth at a greater liquid-solid ratio, indicating a minimal impact on the production yields of DOP. Additionally, even though the shift was negligible, DOP production did gradually rise over time (). A circular contour plot () indicated that the interaction among related variables is insignificant.
3.3. Optimal conditions of DOP extraction and validation of the model
The maximum predicted yield of DOP was determined using a recognized mathematical model. The optimum conditions were as follows: enzyme activity of 2036.640 U/g, a liquid-solid ratio of 71.368 mL/g, and a time of 84.067 min. The estimated value of Y was 33.010% under the aforementioned optimum conditions. The parameters were modified slightly in the verification experiment as follows: enzyme activity of 2040 U/g, the liquid-solid ratio of 71 mL/g, and the time of 84 min. The experimentally obtained value was 34.05 ± 1.03% (n = 3). The experimental value and the projected value did not differ significantly (p > .05), thereby demonstrating that the response surface model was satisfactory and accurate for the expected optimization.
3.4. Effects of different extraction methods on the yield of DOP
Three other extraction methods were performed in our study to compare with UAEE. As shown in , the yield of polysaccharides obtained by different methods listed in the order of UAEE, UHWE, HWE, and UATFTE was 34.05 ± 1.03%, 22.48 ± 1.13%, 11.61 ± 1.80%, 8.45 ± 0.77%, respectively. Under the same liquid-solid ratio, the yield of polysaccharides by UAEE with a shorter extraction time of 84 min and a lower extraction temperature of 60°C was significantly higher than those by UHWE and HWE. UAEE was more efficient compared with UHWE as reported by Wang et al. (Citation2019). Jia et al. (Citation2013) also concluded a similar finding whereby compound enzymes extract possessed higher polysaccharides yield in contrast to that of the hot water extract. The enhancement in extraction efficiency was tentatively attributed to sufficient disintegration of cell wall structure caused by ultrasonication and enzyme hydrolysis (Guo et al., Citation2022). This finding ascertained that UAEE was an effective method for the extraction of polysaccharides from D. officinale. DOPUAFTE yield was the lowest because the dried stem’s cell wall was difficult to break down using this procedure and the solvent could not effectively extract polysaccharides.
Table 3. Analysis of different extraction methods on DOP yield (%).
3.5. In vitro bioactivities of DOPs
3.5.1. ABTS scavenging activity of DOPs
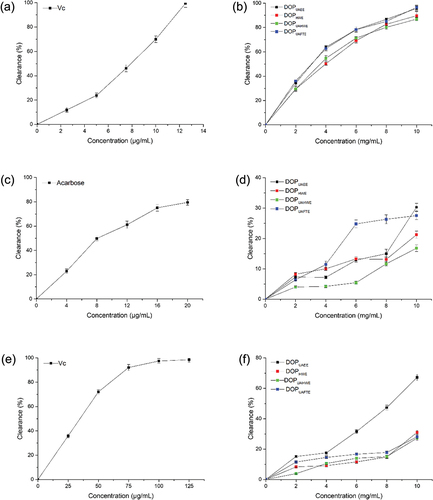
More reactive than DPPH radicals, ABTS+ stable radical cations can swiftly assess the radical-scavenging activity of hydrophilic and lipophilic compounds over a wide pH range in various media (Gao et al., Citation2022). As shown in , all DOP samples exhibited ABTS+ scavenging ability, which could be correlated with DOP concentration. The ABTS+ radical scavenging rate of DOPUAEE gradually increased from 63.85 ± 1.30% to 95.78 ± 1.98% as the concentration increased from 4 to 10 mg/mL. The scavenging rate then remained above 95% when the DOPUAEE dose exceeded 10 mg/mL. The IC50 values of DOPUAEE, DOPHWE, DOPUAHWE, and DOPUAFTE were 3.10 ± 0.04, 3.99 ± 0.66, 3.54 ± 0.33, 2.91 ± 0.31 mg/mL, respectively, indicating their better scavenging ability despite being lower than Ascorbic acid (VC, ). The dose-dependent manner and IC50 values were consistent with the findings of He et al. (Citation2018).
3.5.2. In vitro inhibition of α-glucosidase
α-Glucosidase is a crucial catalytic enzyme in carbohydrate digestion and glucose release. Inhibiting α-glucosidase allows the decreased digestion rate of carbohydrates, thus lowering postprandial blood glucose values (Borges et al., Citation2021). The results of α-glucosidase inhibitory activity () showed that the inhibition rate increased with the increase of DOPs concentration, indicating that the inhibition rate of α-glucosidase depended on the polysaccharide concentration. There was no significant inhibition at the 0–8 mg/mL concentration of DOPUAEE. When the DOPUAEE concentration was 8 mg/mL, the inhibition value of α-glucosidase was 15.00 ± 2.44%, and it rapidly reached 30.27 ± 1.87% at 10 mg/mL. Chu et al. (Citation2019) revealed that crude extracts of the stem of D. officinale had anti-α-glucosidase activity and the inhibitory activity depends on the extract purity. Song et al. (Citation2020) reported that the a-glucosidase inhibition ratio of homogeneous Hypsizygus marmoreus polysaccharide was 1.5 times greater than that of crude polysaccharide at the same concentration. Therefore, the development of DOPUAEE as a α-glucosidase inhibitor warrants further research.
3.5.3. In vitro scavenging capacity against nitrite
In the gastrointestinal environment, NO2− could interact with tertiary or secondary amines and amides leading to the formation of nitrosamine and was reported to be involved in methemoglobinemia syndrome as well as gastric and liver malignancies (Teng et al., Citation2022). As shown in , the nitrite scavenging effects of DOPs were all below 20% at 0–4 mg/mL tested concentrations. Similar results were observed by He et al. (Citation2016a), whereby the nitrite scavenging activities of the four seaweed polysaccharides were weak at 1–4 mg/mL. When the concentration exceeded 4 mg/mL, the scavenging rate of DOPUAEE to nitrite increased gradually and reached 67.19 ± 1.56% at the concentration of 10 mg/mL, which was significantly higher than that of other DOPs (p < .001). The polysaccharide produced by UAEE exhibited a higher NO2− scavenging capacity, which was potentially because DOPUAEE had better solubility in simulated gastric fluid and more exposed functional groups could interact with the nitrite ions. The nitrite scavenging effect of polysaccharides was facilitated by the presence of sulfated groups and hydroxyl groups in their structural makeup (He et al., Citation2016a; Mirzadeh et al., Citation2020).
4. Conclusion
In this study, DOP with a high yield (34.05 ± 1.03%) was developed by the ultrasonic-assisted enzymatic extraction of Dendrobium offcinale Kimura et Migo by RSM for the first time. Compared to the conventional DOP extraction approach, the combined ultrasound-enzymatic treatment produced a greater yield and processed faster. The ABTS scavenging activity, α-glucosidase inhibitory activity, and nitrite scavenging capacity were adopted to evaluate bioactivity. The produced DOPUAEE exhibited good in vitro antioxidant activity and offered effective protection against NO2−, thereby representing a valuable application for further research and development. The extraction method significantly affects the yield, chemical composition, and biological activity of polysaccharides (He et al., Citation2018; Mirzadeh et al., Citation2020). Therefore, it is still necessary to investigate the relationship between extraction parameters, structural characteristics, and product bioactivities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Borges, P. H. O., Pedreiro, S., Baptista, S. J., Geraldes, C., Batista, M. T., Silva, M. M. C., & Figueirinha, A. (2021). Inhibition of alpha-glucosidase by flavonoids of Cymbopogon citratus (DC) Stapf. Journal of Ethnopharmacology, 280, 114470. https://doi.org/10.1016/j.jep.2021.114470
- Charoensiddhi, S., Lorbeer, A. J., Lahnstein, J., Bulone, V., Franco, C. M. M., & Zhang, W. (2016). Enzyme-assisted extraction of carbohydrates from the brown alga ecklonia radiata: Effect of enzyme type, pH and buffer on sugar yield and molecular weight profiles. Process Biochemistry, 51(10), 1503–1510. https://doi.org/10.1016/j.procbio.2016.07.014
- Chen, W. H., Wu, J. J., Li, X. F., Lu, J. M., Wu, W., Sun, Y. Q., Zhu, B., & Qin, L. P. (2021). Isolation, structural properties, bioactivities of polysaccharides from Dendrobium officinale Kimura et. Migo: A review. International Journal of Biological Macromolecules, 184, 1000–1013. https://doi.org/10.1016/j.ijbiomac.2021.06.156
- Chen, M., Zhao, J., & Xia, L. (2008). Enzymatic hydrolysis of maize straw polysaccharides for the production of reducing sugars. Carbohydrate Polymers, 71(3), 411–415. https://doi.org/10.1016/j.carbpol.2007.06.011
- Chu, C., Li, T., Pedersen, H. A., Kongstad, K. T., Yan, J., & Staerk, D. (2019). Antidiabetic constituents of Dendrobium officinale as determined by high-resolution profiling of radical scavenging and α-glucosidase and α-amylase inhibition combined with HPLC-PDA-HRMS-SPE-NMR analysis. Phytochemistry Letters, 31, 47–52. https://doi.org/10.1016/j.phytol.2019.03.002
- DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. T., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356. https://doi.org/10.1021/ac60111a017
- Erel, O. (2004). A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clinical Biochemistry, 37(4), 277–285. https://doi.org/10.1016/j.clinbiochem.2003.11.015
- Gao, J., Hu, D., Shen, Y., Zheng, Y., & Liang, Y. (2022). Optimization of ultrasonic-assisted polysaccharide extraction from Hyperici Perforati Herba using response surface methodology and assessment of its antioxidant activity. International Journal of Biological Macromolecules, 225, 255–265. https://doi.org/10.1016/j.ijbiomac.2022.10.260
- Guo, X., Liu, S., Wang, Z., & Zhang, G. (2022). Ultrasonic-assisted extraction of polysaccharide from Dendrobium officinale: Kinetics, thermodynamics and optimization. Biochemical Engineering Journal, 177, 108227. https://doi.org/10.1016/j.bej.2021.108227
- He, T. B., Huang, Y. P., Yang, L., Liu, T. T., Gong, W. Y., Wang, X. J., Sheng, J., & Hu, J. M. (2016b). Structural characterization and immunomodulating activity of polysaccharide from Dendrobium officinale. International Journal of Biological Macromolecules, 83, 34–41. https://doi.org/10.1016/j.ijbiomac.2015.11.038
- He, J., Xu, Y., Chen, H., & Sun, P. (2016a). Extraction, structural characterization, and potential antioxidant activity of the polysaccharides from four seaweeds. International Journal of Molecular Sciences, 17(12), 1988. https://doi.org/10.3390/ijms17121988
- He, L., Yan, X., Liang, J., Li, S., He, H., Xiong, Q., Lai, X., Hou, S., & Huang, S. (2018). Comparison of different extraction methods for polysaccharides from Dendrobium officinale stem. Carbohydrate Polymers, 198, 101–108. https://doi.org/10.1016/j.carbpol.2018.06.073
- Jia, S., Li, F., Liu, Y., Ren, H., Gong, G., Wang, Y., & Wu, S. (2013). Effects of extraction methods on the antioxidant activities of polysaccharides from Agaricus blazei Murrill. International Journal of Biological Macromolecules, 62, 66–69. https://doi.org/10.1016/j.ijbiomac.2013.08.031
- Kuang, M. T., Li, J. Y., Yang, X. B., Yang, L., Xu, J. Y., Yan, S., Lv, Y. F., Ren, F. C., Hu, J. M., & Zhou, J. (2020). Structural characterization and hypoglycemic effect via stimulating glucagon-like peptide-1 secretion of two polysaccharides from Dendrobium officinale. Carbohydrate Polymers, 241, 116326. https://doi.org/10.1016/j.carbpol.2020.116326
- Liang, J., Zeng, Y., Wang, H., & Lou, W. (2019). Extraction, purification and antioxidant activity of novel polysaccharides from Dendrobium officinale by deep eutectic solvents. Natural Product Research, 33(22), 3248–3253. https://doi.org/10.1080/14786419.2018.1471480
- Liao, N., Zhong, J., Ye, X., Lu, S., Wang, W., Zhang, R., Xu, J., Chen, S., & Liu, D. (2015). Ultrasonic-assisted enzymatic extraction of polysaccharide from Corbicula fluminea: Characterization and antioxidant activity. LWT-Food Science and Technology, 60(2), 1113–1121. https://doi.org/10.1016/j.lwt.2014.10.009
- Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428. https://doi.org/10.1021/ac60147a030
- Mirzadeh, M., Arianejad, M. R., & Khedmat, L. (2020). Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydrate Polymers, 229, 115421. https://doi.org/10.1016/j.carbpol.2019.115421
- Nadar, S. S., Rao, P., & Rathod, V. K. (2018). Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Research International, 108, 309–330. https://doi.org/10.1016/j.foodres.2018.03.006
- Niu, P., Wang, F., Yuan, K., Li, X., Yang, X., & Guo, Y. (2021). Alkaline-extracted thinned young apple polyphenols as an effective scavenger against nitrite in pickles: A comparative study with ethanol-extracted polyphenols. Food Control, 130, 108387. https://doi.org/10.1016/j.foodcont.2021.108387
- Olawuyi, I. F., Kim, S. R., Hahn, D., & Lee, W. Y. (2020). Influences of combined enzyme-ultrasonic extraction on the physicochemical characteristics and properties of okra polysaccharides. Food Hydrocolloids, 100, 105396. https://doi.org/10.1016/j.foodhyd.2019.105396
- Pan, L.-H., Wang, J., Ye, X.-Q., Zha, X.-Q., & Luo, J.-P. (2015). Enzyme-assisted extraction of polysaccharides from Dendrobium chrysotoxum and its functional properties and immunomodulatory activity. LWT-Food Science and Technology, 60(2), 1149–1154. https://doi.org/10.1016/j.lwt.2014.10.004
- Shan, Z., Wang, Y., Jin, Z., Liu, J., Wang, N., Guo, X., Cui, S. W., & Guo, Q. (2023). Insight into the structural and immunomodulatory relationships of polysaccharides from Dendrobium officinale-an in vivo study. Food Hydrocolloids, 139, 108560. https://doi.org/10.1016/j.foodhyd.2023.108560
- Shi, M.-Z., Shi, Y., Jin, H.-F., & Cao, J. (2022). An efficient mixed enzymes-assisted mechanical bio-extraction of polysaccharides from Dendrobium officinale and determination of monosaccharides by HPLC-Q-TOF/MS. International Journal of Biological Macromolecules, 227, 986–1000. https://doi.org/10.1016/j.ijbiomac.2022.11.275
- Song, Q., Teng, A.-G., & Zhu, Z. (2020). Chemical structure and inhibition on α-glucosidase of a novel polysaccharide from Hypsizygus marmoreus. Journal of Molecular Structure, 1211, 128110. https://doi.org/10.1016/j.molstruc.2020.128110
- Tao, S., Huang, C., Tan, Z., Duan, S., Zhang, X., Ren, Z., Zhou, C., Huang, J., Liu, C., & Wei, G. (2021). Effect of the polysaccharides derived from Dendrobium officinale stems on human HT-29 colorectal cancer cells and a zebrafish model. Food Bioscience, 41, 100995. https://doi.org/10.1016/j.fbio.2021.100995
- Tao, S., Lei, Z., Huang, K., Li, Y., Ren, Z., Zhang, X., Wei, G., & Chen, H. (2019). Structural characterization and immunomodulatory activity of two novel polysaccharides derived from the stem of Dendrobium officinale Kimura et Migo. Journal of Functional Foods, 57, 121–134. https://doi.org/10.1016/j.jff.2019.04.013
- Teng, X., Zhang, M., Mujumdar, A. S., & Wang, H. (2022). Inhibition of nitrite in prepared dish of Brassica chinensis L. during storage via non-extractable phenols in hawthorn pomace: A comparison of different extraction methods. Food Chemistry, 393, 133344. https://doi.org/10.1016/j.foodchem.2022.133344
- Wang, C., Li, J., Cao, Y., Huang, J., Lin, H., Zhao, T., Liu, L., Shen, P., McClements, D. L., Chen, J., Liu, C., Liu, J., & Li, Q. (2023). Extraction and characterization of pectic polysaccharides from Choerospondias axillaris peels: Comparison of hot water and ultrasound-assisted extraction methods. Food Chemistry, 401, 134156. https://doi.org/10.1016/j.foodchem.2022.134156
- Wang, L., Li, T., Liu, F., Liu, D., Xu, Y., Yang, Y., Zhao, Y., & Wei, H. (2019). Ultrasonic-assisted enzymatic extraction and characterization of polysaccharides from dandelion (Taraxacum officinale) leaves. International Journal of Biological Macromolecules, 126, 846–856. https://doi.org/10.1016/j.ijbiomac.2018.12.232
- Wang, K., Wang, H., Liu, Y., Shui, W., Wang, J., Cao, P., Wang, H., You, R., & Zhang, Y. (2018). Dendrobium officinale polysaccharide attenuates type 2 diabetes mellitus via the regulation of PI3K/Akt-mediated glycogen synthesis and glucose metabolism. Journal of Functional Foods, 40, 261–271. https://doi.org/10.1016/j.jff.2017.11.004
- Wei, W., Li, Z. P., Zhu, T., Fung, H. Y., Wong, T. L., Wen, X., Ma, D. L., Leung, C. H., & Han, Q. B. (2017). Anti-fatigue effects of the unique polysaccharide marker of Dendrobium officinale on BALB/c mice. Molecules, 22(1), 155. https://doi.org/10.3390/molecules22010155
- Xiong, Q., Huang, S., Chen, J., Wang, B., He, L., Zhang, L., Li, S., Wang, J., Wu, J., Lai, X., & Zhang, D. (2017). A novel green method for deproteinization of polysaccharide from Cipangopaludina chinensis by freeze-thaw treatment. Journal of Cleaner Production, 142, 3409–3418. https://doi.org/10.1016/j.jclepro.2016.10.125
- Yu, Y., Shen, M., Song, Q., & Xie, J. (2018). Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydrate Polymers, 183, 91–101. https://doi.org/10.1016/j.carbpol.2017.12.009
- Zhang, J., Jia, S., Liu, Y., Wu, S., & Ran, J. (2011). Optimization of enzyme-assisted extraction of the Lycium barbarum polysaccharides using response surface methodology. Carbohydrate Polymers, 86(2), 1089–1092. https://doi.org/10.1016/j.carbpol.2011.06.027