 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Traditional fermentation using natural microorganisms often results in uncontrolled and inconsistent product quality. Identifying dominant LAB and yeast species through morphological, physiological, and biochemical characterization, this study aimed to optimize the traditional fermentation process of injera dough fermentation. Ersho and dough were prepared in the laboratory, and LAB and yeast were identified and enumerated by smearing on a petri dish at 24-hour intervals from 0 to 96 hours of fermentation. The growth kinetics of both LAB and yeast in fermented teff dough significantly increased (p < 0.05) from 8.91 to 9.97 log CFU/gm and 5.98 to 7.62 log CFU/gm as fermentation time increased. Five dominant LAB species (Lactobacillus fermentum, Lactobacillus Brevis, Lactobacillus Plantarum, Bacillus subtilis, and Enterococcus casseliflavus) and three dominant yeast strains (Saccharomyces cerevisiae, Candida krusei, and Pichia kudriavzevii) were identified. Based on the physicochemical analysis and sensory acceptability result, application of a single starter culture in teff dough fermentation improved fermentation time and acid production.
1. Introduction
Fermentation is one of the most aged traditional and economical techniques of food processing and preserving foods for the improvement of organoleptic properties (Ozturk & Young, Citation2017; Şanlier et al., Citation2019). It is used to produce different flavors of foods, the nutritive value, reducing toxic substances from the products (Ashaolu, Citation2019; Sieuwerts et al., Citation2008). It also helps to break down large organic molecules in to smaller ones through the action of microorganisms for the preservation and transformation of food materials (Nkhata et al., Citation2018; Xiang et al., Citation2019). According to Liu and Pan (Citation2010), fermentation is relatively inexpensive and requires little energy to preserve, extending the shelf life of food products. Fermentation is still part of cultural norms that easily adaptable to local household practices in traditional society (Gawai & Prajapati, Citation2017; Thiele et al., Citation2002). Natural bacteria that have been passed down from generation to generation ferments the majority of native fermented foods (Achi, Citation2005). Before industrialized large-scale manufacturing, the isolation, identification, and starter culture development of a high-efficiency microbial strain for used as inoculum must be approved (Achi, Citation2005). Several microorganisms are expected to play a function in the traditional fermentation (Achi, Citation2005); they used in the preservation and used as a starter culture for the quality and shelf-life improvement of fermented food (Saeed et al., Citation2009). Initially, a wide variety of microorganisms are involved in traditional fermentation those used as a starter culture for the initiation of the first stage of fermentation (Achi, Citation2005; Hotessa et al., Citation2020).
Starter cultures are a microbiological culture which is a preparation to help the starting of the fermentation process in preparation of various fermented foods and beverages which are microorganisms (bacteria, yeasts, and molds or their combinations) that initiate and carry out the desired fermentation essential in manufacturing (Moneim & Sulieman, Citation2018). Starter cultures were first used in the early twentieth century to get better food safety to attain the improvement related to sensorial, nutritional, safety, and technological characteristics that have been widely shown lead to help in terms of final product quality (Baka et al., Citation2011; Bover-Cid et al., Citation2001; Ciuciu Simion et al., Citation2014). These cultures are composed mainly of Lactobacillus spp. and Staphylococcus spp., which have the capacity for improving sensorial and textural characteristics such as color, flavor, taste, hardness, and cohesiveness, among others (Allen Foegeding et al., Citation2003). The most important bacteria in the fermentation of foods are the Lactobacillaceae (Lactobacillus, Pediococcus, Streptococcus, Oenococcus), which can produce lactic acid from carbohydrates; likewise, acetic acid is used to produce Acetobacter mainly from the fermentation of fruit and vegetable and bacillus from the fermentation of legumes species (Joshi & Sharma, Citation2009; Sicard & Legras, Citation2011). Lactic acid bacteria play an essential role in food technology in that they can improve the aroma and texture of food and also inhibit the growth of spoilage bacteria (Zapaśnik et al., Citation2022).
The most common yeast species involved in the preparation of traditional fermented cereal-based foods and beverages are saccharomyces cerevisiae. These play a significant role in providing safety and imparting organoleptic properties to food. The most beneficial yeasts in terms of desirable food fermentations are from the Saccharomyces family, especially S. cerevisiae involved in bread making and alcohol in wine fermentations. Saccharomyces cerevisiae var. ellipsoideus is employed extensively in wine making (Joshi & Sharma, Citation2009) likewise, S. exiguous, Torulopsis holmii, Candida krusei, Pichia norvegensis and Hansenula anomala but S. cerevisiae is frequently present or added in the fermentation of sourdough (Holzapfel, Citation2002). Yeasts carry a significant function in food fermentation through enzyme production that favors desired biochemical reactions, such as production of aroma, alcohol and flavor (Aidoo et al., Citation2006). Most of the favorable yeasts for desirable food fermentation belong to the genera Saccharomyces, especially S. cerevisiae (Sicard & Legras, Citation2011). Food fermentation is dependent on the action of microorganisms or enzymes for the production of pleasant and attractive flavor to the human consumer (Holzapfel, Citation2002).
In traditional fermented foods, microorganisms responsible for the fermentation process are usually the microbial naturally existence on the raw materials (Ashenafi, Citation2006; Hotessa et al., Citation2020; Wedajo Lemi, Citation2020). Microorganisms are naturally ubiquitous and thus observed in raw food materials (Gholami-Shabani et al., Citation2023; Wedajo Lemi, Citation2020) by their metabolic activities, convert these raw materials to the development of characteristic properties such as taste, aroma, visual appearance, texture, shelf life, and safety (Holzapfel, Citation2002; Kimaryo et al., Citation2000). Lactic acid bacteria (LAB) are widely distributed in nature; occur as natural adventitious contaminants in raw milk, yogurt, and sometimes with the coexistence of yeasts, molds, and some other pathogenic microorganisms (Wouters et al., Citation2002). LAB as indigenous flora in raw milk acidifies the milk very slowly due to their low numbers.
In natural fermented beverages and foods, the microorganisms responsible for the fermentation are typically the microorganisms present on the raw substrate. Such microorganisms could be used to develop starter cultures for controlled fermentation of the food products to maintain the quality of the products and used to synthesize certain amino acids and availability of B group vitamins; the result in a reduction in phytate, which increases the amount of soluble iron, zinc, and calcium (Blandino et al., Citation2003; Holzapfel, Citation2002). Several LABs are associated with meat and fish fermentations. L. sakei and L. curvatus have been determined to be superior starter cultures for meat fermentations. Leuconostoc mesenteroides frequently dominate the early stages of most spontaneous fermentations (Wafula et al., Citation2023).
Among the various microbes involved in food fermentation, yeasts play important role in the transformation of the different substrates as yeasts grow and survive under stress conditions. Most of the indigenous or traditional fermented foods are prepared by solid-substrate fermentation in which substrates are allowed to ferment either naturally or by the addition of starter cultures. In food fermentation, yeasts transform the original raw materials physically, nutritionally, and organoleptically into more desirable products. Amongst different yeasts, the genus Saccharomyces has a long history of traditional applications particularly in bread, beer, and wine fermentations (Bhalla & Savitri, Citation2017).
Yeasts and LAB are the most important microorganisms in fermented foods and beverages such as bread, injera, alcoholic beverages, cheeses, and the production of biologically important products like enzymes vitamins, and antibiotics (Ali & Latif, Citation2016; Desiye & Abegaz, Citation2013).
In Ethiopia, most of the fermented food and beverage preparation is predominantly a household phenomenon (Hotessa et al., Citation2020; Tafere, Citation2015). In order to make injera, a starter (Ersho) saved from the previous fermented batter is used to initiate new batches of fermentation (back slopping) and usually involve a spontaneous development of different LAB and yeast (Bultosa et al., Citation2008; Desiye & Abegaz, Citation2013). In Ethiopia, most of the research conducted on the microbial quality, and safety of fermented and non-fermented food products and determination of microorganisms, which are involved in spoilage, impurities and fermenting organism; besides some researchers have done on the importance of nutritional value determination of Ethiopian staple food including injera (Hansen, Citation2002). Moreover, limited studies have showed the presence, isolation, identification and description of different species of LAB and yeast in the fermentation of dough. However, there is no enough investigation on the growth conditions and proportion of the dominant microorganism involved in injera dough fermentation, and also there is a research gap on microbial kinetics growth in ersho which is used as a starter culture for dough fermentation process. Therefore, the aim of this study was to identify dominant species of LAB and yeast for the optimization of traditional fermentation process of injera dough.
Injera is the traditional primary food of the majority of Ethiopians, which is fermented, pancake-like, soft, spongy, sour, circular flatbread), and other food products made from teff (Eragrostis teff), wheat, barley, sorghum, and maize or a combination of some of these cereals (Ashenafi, Citation2008; Bultosa & Taylor, Citation2004). An appropriate amount of flour is mixed with twice its weight of water; this is kneaded thoroughly to produce a thick paste. The gluten-free nature and whole-grain product teff (Eragrostis teff) injera is gaining popularity in developed countries as well. In addition, it has highly nutritious and an important part of Ethiopia’s cultural heritage and national identity (Abiyu et al., Citation2013; Ashenafi, Citation2008; Gizaw, Citation2018; Mulaw & Tesfaye, Citation2017). Fermentation is most of the time initiated spontaneously by the addition of water to teff flour, allowing the naturally existing microorganisms to grow (Baye et al., Citation2013). Microorganisms are naturally ubiquitous and thus observed in raw food materials (Gholami-Shabani et al., Citation2023) by their metabolic activities, convert these raw materials to the development of characteristic properties such as taste, aroma, visual appearance, texture, shelf life, and safety (Holzapfel, Citation2002; Kimaryo et al., Citation2000). The making of teff injera consists of two stages of natural fermentation, which last for about 24 to 72 hours (Ashenafi, Citation2008); likewise, according to Desiye and Abegaz (Citation2013) it is attained after the flour of cereals has been subjected to 24–96 hours of traditional fermentation depending on ambient temperatures.
According to Assefa et al. (Citation2018), the dough was allowed to ferment for 60 hours at room temperature (30 ± 5°C) it is called first fermentation (Tamene, Kariluoto, et al., Citation2019). During the fermentation stages, the main fermenting microorganisms are lactic acid bacteria (Lactobacillus species) (Baye et al., Citation2013) and yeast (Saccharomyces species) (Assefa et al., Citation2018). These microorganisms result in the fall of pH, gas production, and dough rising and are responsible for desired final product flavor and acidity (Assefa et al., Citation2018). The first stage fermentation can also be started by the addition of the starter, ersho, which is a small amount of batter kept from the previous dough (Mengesha et al., Citation2022); then after the liquid present on top of the batter (the supernatant) is discarded and replaced with the same volume of fresh tap water (Tamene, Kariluoto, et al., Citation2019). Inoculation is accomplished by consistently using a partially cleaned fermentation container and by adding some ersho, the clear, yellow liquid that accumulates on the surface of the batter towards the final stage of a previous fermentation (Ashenafi, Citation2008).
After the first stage of fermentation, the portion of equivalent to 10% of the fermented dough is mixed with three parts boiling water, and heated for 15 min with continuous stirring (Assefa et al., Citation2018; Tamene, Kariluoto, et al., Citation2019). This is called “absit”, which is used to ensure that injera will have the proper texture and consistency (Ashenafi, Citation2008). The fermented dough was enhanced and it is mixed back into the fermenting dough, and sufficient potable water was added to make a batter. The batter was left covered for two hours for secondary fermentation. This process is an indication of the second stage of fermentation (Ashenafi, Citation2008; Assefa et al., Citation2018). According to Ashenafi (Citation2008), the dough-rising and gas formation processes are enhanced so they occur in a short time. The maximum dough-rising, which normally takes 30 minutes to 2 hours, signals the termination of fermentation. Besides this, extra water was added to thin and form the right consistency of the batter (Assefa et al., Citation2018). At this stage, half a liter of batter was poured onto the hot clay griddle in a circular form locally known as “mitad” for steam baking into injera. Then, after 2–3 min of cooking, injera was removed and placed in a basket (Ashenafi, Citation2008; Assefa et al., Citation2018).
2. Materials and methods
2.1. Raw material collection and sample preparation
2.1.1. Raw material collection
About 15 kg of white teff grain was obtained from Bahir Dar local market. It was cleaned and ground into flour using attrition mill. The milled flour was sieved and tightly packed in polyethylene bags and stored at a temperature of 4°C until use.
2.1.2. Sample preparation under laboratory
2.1.2.1. Preparation of ersho (starter culture)
Based on the preliminary test and indigenous knowledge, the new starter culture (ersho) was prepared in a lab at different fermentation times (0, 24, 48, 72, and 96 hours). The ersho was prepared by mixing 1 kg of teff flour with 2-L of potable water in a sterilized fermenting plastic jars in 1:2 ratios (m/v) and then equally distributed in five sterilized sample plastic jars and conical flasks left to ferment until to get the desired quality of ersho at room temperature.
The prepared sample was equally distributed into five sterilized sample plastic jars and conical flasks until to get the desired quality of ersho at room temperature (Abiyu et al., Citation2013; Ashenafi, Citation2008; Desiye et al., Citation2017; Mezemir, Citation2015). The physicochemical analysis and microbial kinetics growth of the ersho were studied at different fermentation times (0, 24, 48, 72, and 96 hours).
2.1.2.2. Teff dough preparation
The teff dough was prepared by mixing 2Kg of teff flour with 4-L of portable water, and 10% of the previously prepared starter (ersho) until homogenous dough paste was obtained at different fermentation times (0, 24, 48, 72 and 96); and then the dough paste was equally distributed in five sterilized sample plastic jars allowed to ferment for 3–5 days at room temperature.
2.2. Physicochemical analysis of ersho and dough
2.2.1. Determination of pH of ersho and dough
The pH of ersho and dough was measured according to AOAC method 942.15 (AOAC, Citation2000). About 10-g of ersho and dough samples were homogenized with 100 ml of distilled water and measured the pH value by using scientific electronic bench top pH meter. The pH meter was calibrated before each reading with the standard buffer of pH 4.00 and 7.00.
2.2.2. Determination of titratable acidity of ersho and dough
The total titratable acidity of ersho and dough was determined by using AOAC method 942.15 (AOAC, Citation2000) for both the apparent and produced acidity of the ersho and dough.
A 100 mL of distilled water were added to a sample of 10-g starter (ersho) and dough before being titrated with 0.1 N standardized NaOH. The volume of NaOH used for each titration was recorded and titratable acidity was expressed as % lactic acid (AOAC, Citation1995) using the formula below); where the lactic acid equivalent factor is 0.0908.
2.3. Kinetics growth and enumeration of LAB and yeast in teff ersho and dough
The kinetics growth and enumeration of LAB and yeast in the ersho and dough were enumerated by the standard plate count method (da Silva et al., Citation2013; Roberts & Greenwood, Citation2003).
2.4. Identification of LAB
Morphological, physiological and biochemical characterization were used to identify LAB The bacterial isolates were phenotypically characterized and identified for their presumptive colonies identified and grouped according to the method described by (De Vos et al., Citation2009). The identified organisms were tested for the production of fermented dough that produces unique quality of injera. The pure cultures (LAB) obtained from fermented teff dough was stored at 4°C until use again.
2.4.1. Morphological characterization of LAB
Characterization of the identified LAB isolates were performed based on colony morphology, characteristics of cell type, gram staining, colony characterization, and microscopic observation (De Vos et al., Citation2009). On the MRS agar plate, 15 representative LAB colonies were randomly selected from a total of 256 counted colonies with 107 serial dilutions. The presumptive colonies of LAB were identified based on colony morphology such as color, shape, and size. Each isolate was purified by repeated streak-plating on MRS agar and incubated at 30°C for 24 to 48 hours. The pure cultures obtained from fermented teff dough were stored at 4°C for further characterization.
2.4.1.1. Gram staining and microscopic observation of LAB
The microscopic examination of LAB were performed on gram stained slide preparations of cells by using the method used by Reiner (Citation2010) Each presumptive colony was characterized based on the staining properties.
2.4.1.2. Catalase test for LAB identification
According to the method used by Sultana et al. (Citation2017) and Reiner (Citation2010), a drop of 3% of purified hydrogen peroxide was dropped onto culture on a glass slide. The formation of gas bubbles indicates catalase positive, which shows the presence of catalase enzymes. The colony which does not form bubbles was considered as LAB.
2.4.2. Physiological characterization of LAB species
The physiological test including growth at different pH, temperatures, and salt tolerance were determined using a method described by Tamang et al. (Citation2007). The physiological characterization of lactic acid bacteria LAB isolates were identified according to the method described by De Vos et al. (Citation2009).
2.4.2.1. pH Tolerant LAB species identification
The pH tolerance of LAB was determined according to the method used by Kostinek et al. (Citation2005). The overnight culture of identified LAB was inoculated into MRS broth test tubes at various pH ranges 3, 4.5, 5, 5.5, and 6.2, and incubated at 37°C. The pH of broth medium was adjusted with 1NHCl and 1NNaOH. Uninoculated media was used as the negative control. After 24 and 48 hours of incubation, the turbidity of the culture media was observed as a positive indicator of the acid production (Sultana et al., Citation2017).
2.4.2.2. Testing the growth of identified LAB at different temperature
According to De Vos et al. (Citation2009), the LAB groups grow at the optimum temperature range between 30–40°C. The fresh overnight pure culture of isolated LAB were inoculated into MRS broth and incubated at different temperatures 15, 20, 25, 30, 37, 40, 45°C for 3–5 days. The identified and isolated LAB species growth at the given temperatures were identified by the formation of turbidity on MRS broth.
2.4.2.3. Salt tolerance LAB species identification
The tolerance of LAB in different NaCl concentration was done according to methods used by Mulaw et al. (Citation2019).
The fresh overnight pure culture of the identified and isolated LAB isolates was inoculated into MRS broth with different concentrations (4, 6, 8, and 10%) of NaCl (w/v) and incubated at 37°C for 5 days. The Uninoculated media was used as control. The results were determined by observing the turbidity after 48 and 72-hours.
2.4.2.4. Gas production from glucose for LAB
Carbon dioxide production from glucose was determined by using the method used by Schillinger and Lücke (Citation1987). Fresh overnight culture of LAB isolates was inoculated into 15 ml of MRS broth with inverted Durham tubes and incubated at 30°C and 37°C for 5 days. The gas production during fermentation was indicated by the uplift of the inverted Durham tubes. The accumulation of gas in the Durham’s tube was considered a positive result for gas production.
2.4.3. Biochemical characterization of LAB
Carbohydrate fermentation test was performed according to the method used by Reiner (Citation2012). Sugar (1% w/v) was added into the carbohydrate fermentation broth (18 g/L) with 0.025% Bromocresol purple. Ten ml of the prepared broth was dispensed into the test tubes, and then the inverted Durham tubes were inserted invertible in each of the test tubes and autoclaved at 121°C for 3–5 minutes. Then, the broth was inoculated with LAB isolates and incubated at 37°C for 48 and 72hours. The change of the media from purple to yellow indicated a positive result of fermentation or acidification, whereas absence of color change was considered as negative results. The isolates were tested by using arabinose, galactose, maltose, raffinose, trehalose, sorbitol, xylose, mannose, esculin, fructose, lactose, sucrose, melibiose, ribose and mannitol sugars (De Vos et al., Citation2009).
2.5. Identification of yeast
The identification of yeast isolates was done using the morphological (colony size, color, and shape), physiological and biochemical characterization. Isolates were phenotypically characterized and identified according to the yeast taxonomic study (Cletus Kurtzman & Fell, Citation2011).
2.5.1. Morphological characterization of yeast
Characterization of yeast isolates were performed based on colony morphology, characteristics of cell type, simple gram staining, colony characterization, and microscopic observation.
Out of 115 counted colonies with serial dilution 105, only ten representative colonies of yeast were randomly picked on potato dextrose agar (PDA) plate with countable colonies and subculture on the same media to purify and incubated at 30°C for 24 to 48-hrs. The presumptive colonies of yeast were identified based on their color, shape, and size. The pure isolates were maintained on PDA slants at 4°C until required for further characterization. For this study, only gram-positive, catalase-positive colonies were selected for further characterization.
2.5.1.1. Microscopic observation and catalyst test of identified yeast
The morphological and cultural examination is carried out by using the simple staining method described by Ebabhi et al. (Citation2013); Karki et al. (Citation2017). Thin smears of the identified colonies were prepared on glass slides, and stained with methyl blue for microscopic observation to identify yeast cells morphology. Similarly, the catalyst test was performed using the method described above.
2.5.2. Physiological characterization of yeast
2.5.2.1. pH Tolerant yeast species identification
Fresh culture of the identified yeast isolates was inoculated in malt extract broth in test tubes at various pH of 3, 3.5, 4.5, 5.3, and 6.5 and incubated 30°C for five days. The pH of broth medium was adjusted with 1N HCL and 1NaOH. The Uninoculated media was used as a negative control. Results are obtained by observing turbidity of the media and CO2 gas bubbles after 72- hours (Sultana et al., Citation2017).
2.5.2.2. Testing the growth of yeast at different temperature
The pure culture of isolates were inoculated in malt extract broth incubated at different temperatures 15, 20, 25, 28, 30, 35, 37, and 42°C for 5 days using the modified methods described by Cletus Kurtzman and Fell (Citation2011). Growth at a given temperature was observed by the formation of turbidity and gas in the test tubes.
2.5.2.3. Production of gas from glucose for yeast
The fresh cultures of the identified yeast isolates were transferred into 15–20 ml of glucose-malt extract (2% (w/v) glucose) broth in a test tube with the inverted Durham tubes and incubated at 30°C and 37°C for 7 days. Gas production during fermentation was determined by the uplift of the inverted Durham tubes (Schillinger & Lücke, Citation1987).
2.5.2.4. Ethanol tolerance yeast identification
Ethanol tolerance test of yeast was carried out following the method used by Karki et al. (Citation2017). The isolates were inoculated on glucose malt extract broth at four different concentrations (8%, 10%, 13%, and 15% (v/v)) of ethanol and incubated at 30°C for 72-hours. The result was determined by observing turbidity of the media after 48 and 72-hours of incubation.
2.5.3. Biochemical characterization of yeast
The sugar fermentation test of the isolates was performed using glucose, galactose, maltose, sucrose, trehalose, melibiose, lactose, raffinose, and inulin, starch, and xylose sugars. Carbohydrate utilization test was performed using broth (yeast extract 4.5 g/L, peptone powder 7.5 g/L, 2% (w/v) of sugar; 0.004% Bromocresol green, distilled water 1000 ml). The broth (10 mL) was dispensed into the test tubes and the inverted Durham tubes were inserted in each of the test tubes and autoclaved at 121°C for 3–5 minutes. The fresh culture of isolates were inoculated into the broth test tubes and incubated at 30°C for five to ten days. The change of media from blue green to dark yellow indicates a positive test for fermentation of carbon source. Uninoculated broth was used as a control (Cletus Kurtzman & Fell, Citation2011).
2.6. Experimental design
A controlled experimental design was arranged in completely randomized design (CRD) was used for the experimental design for the microbial analysis of the experiment in triplicate at different fermentation time (0, 24, 48, 72, and 96).
2.7. Data analysis
The analysis of variance (ANOVA) is a statistical decision-making tool used for detecting any differences in average performances of tested parameters. All measurements were analyzed using one-way ANOVA using Minitab 19 statistical software packages (State College, PA). The results were expressed as mean ± standard deviation (SD). Tukey’s multiple comparison tests were used to determine the significance of variation between treatments at a 95% confidence level. The graph plotted techniques were employed by using the Sigma Plot 14.5 software packages.
3. Results and discussions
3.1. Physicochemical analysis of ersho and teff dough prepared under laboratory
3.1.1. The pH and titratable acidity of ersho and fermented dough
The pH and titratable acidity values of collected homemade ersho sample were around 3.65 and 12.05 respectively when it was obtained during the preliminary test. As shown in , the pH of ersho was decreased significantly (P < .05) from 7.20 (0 hrs) to 4.00 (96 hrs) Conversely, the TA of fermented teff ersho was increased (p < .05) from 1.17 to 9.16 at every 24-hrs interval from 0 to 96 hours of fermentation time. The pH of ersho prepared under laboratory condition was high compared with the homemade ersho. However the TA of ersho prepared under laboratory was less compared with the homemade ersho. These might be due to the viability in fermentation time and lack of nutrients in the homemade ersho.
Figure 1. The pH and TA of ersho (a) and pH and TA of fermented teff dough (b) the values expressed were mean ±sd with triplicate experiments and the mean comparison was done by using Tukey’s honestly significant deviation (HSD) test. The mean with different letters in the same line as subscripts are indicated the presence of significant at (p < .05).
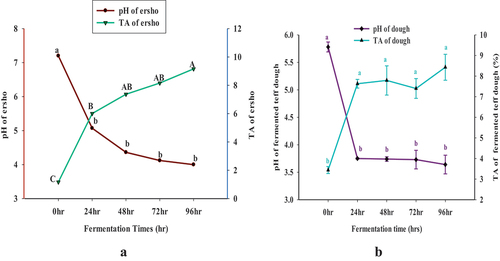
Similarly, the result show in , the pH of dough was decreased significantly (P < .05) from 5.78 to 3.64, whereas the TA of the dough was increased significantly (p < .05) from 3.44 to 8.43 at the every 24hrs intervals from 0 to 96 hours of fermentation time. This was due to the fact that microorganism consumed the nutrients and produced organic acids that were released into the medium. The decrease in pH and an increase in TA of ersho and dough were due to the production of organic acids by LAB and yeasts in fermented ersho and teff dough.
The result of this study was comparable the resulted data that were reported by other studies. According to Urga et al. (Citation2000) reported that the pH of ersho dropped from 3.93 to 3.77 and the titratable acidity of ersho was increased from 1.83% to 2.47% 48 at the 24 hrs intervals from 48 hrs to 96 hrs of fermentation. The other study shown that; the pH of fermented teff was decreased from 6.56 to 3.83 and the titratable acidity of fermented teff dough was increased from 0.26% to 1.31% at the 24 hours intervals from zero to 96 hours of fermentation (Ashenafi, Citation2006; Desiye et al., Citation2017), study showed that the ersho samples were collected from different household the average pH was 3.5 and titratable acidity ranged between 3.1 to 5.7%.
According to Ashenafi (Citation2002), the pH of the fermented teff dough ranged between 6.6 and 3.8 at six hours intervals from 0 to 72 hrs different fermentation time. The study reported by Desiye et al. (Citation2017), at 6 hours interval for 96 hours of fermentation the pH value of fermented teff dough was decreased from pH of 6.66 to 3.44 and the titratable acidity increased from 0.10 to 1.39 during fermentation. The other result reported by Nigatu et al. (Citation2000), that the pH of fermented teff dough was about 4.0 at 72 hour of fermentation.
shows that higher pH and TA values were observed in ersho compared to the fermented teff dough.
This is due to the addition of starter culture (ersho) in the dough during fermentation. In traditional practice a small portion of ersho, which contains complex groups of microorganisms use as a starter to initiate the new fermentation. Hence, the use of ersho as starter culture may accelerate the fermentation process of dough. The other reason is that fermentation temperature is the factor that affects the pH of fermented dough.
In addition, after 24-hour’s fermentation, the pH and TA of teff dough were observed insignificant changes compared to the ersho. This is due to lack of nutrients and they begin to consume the organic acids as nutrients sources.
The values expressed were mean ±sd with triplicate experiments and the mean comparison was done by using Tukey’s honestly significant deviation (HSD) test. The mean with different letters in the same line as subscripts are indicated the presence of significant at (p < .05).
3.2. Kinetics growth of LAB and yeast in ersho and dough
3.2.1. Enumeration of LAB and yeast in ersho and dough
The enumerations of LAB and yeast in homemade ersho were 7.89 and 6.39 log cfu/g, respectively. This preliminary test was performed to determine the concentration of viable LAB and yeast in the teff ersho and decided the starting serial dilution of both LAB and yeast. The growth kinetics of LAB and yeast in ersho at different fermentation times was determined shown in . This study shows that LAB was not detected in the ersho at the initial fermentation time (0 hrs) on the pre-solidified MRS agar. This study shows that LAB was not detected in the ersho at the initial fermentation time (0 hrs) on the pre-solidified MRS agar. This might be due to the physiological adaptation of the cell to the culture conditions. Correspondingly, kinetics growth of yeast in ersho also wasn’t detected at fermentation time from zero to 24 hours on the pre-solidified PDA plates. This might be due to the predominance of LAB, which is naturally present in the fermented dough used to initiate the fermentation.
Figure 2. Kinetics growth of LAB and yeast counts in ersho (a) and fermented teff dough (b) the values expressed were mean ±sd with triplicate experiments and the mean comparison was done by using Tukey’s honestly significant deviation (HSD) test. The mean with different letters in the same line as subscripts are indicated the presence of significant at (p < .05).
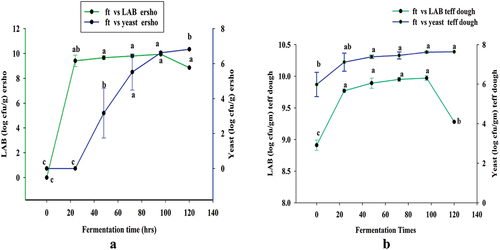
The kinetics growth of LAB in ersho was increase significantly (p < .05) from 9.41 to 9.94 log cfu/g when fermentation time increased from 24 to 96 hours, respectively. Yet, after 120-hrs of fermentation time, the kinetics growth of LAB in ersho was slightly decreased from 9.94 to 8.86 log cfu/g.This might be due to the limitations of nutrients in the medium (Kozlinskis et al., Citation2008). In this study, the kinetics growth of LAB and yeast in collected homemade ersho were slightly less as compared to teff ersho prepared under laboratory. This might be due to the limitation of nutrient and duration of fermentation time.
The kinetics growth of yeast was increased significantly (p < .05) in the ersho from 3.17 to 6.82 log cfu/g at fermentation time from 48 to 120-hours. In the current finding, the yeast in the ersho was start to growth slowly that compared with the growth of LAB in the ersho. This might be due to the domination of LAB in the ersho, and yeast needs acidic condition to grow. However, the beginning of fermentation the acidity of the ersho was near to neutral.
As shown in , the kinetics growth of LAB in fermented teff dough were increase significantly (p < .05) from 8.91 to 9.97 log cfu/g, when fermentation time increased from 0 to 96 hours, respectively.; Yet, the kinetics growth of LAB in fermented teff dough was slightly decreased 9.28 log cfu/g after 120 hours of fermentation time; this might be due to the limitations of nutrients (Kozlinskis et al., Citation2008). This is results in agreement with the result reported by (Ashenafi, Citation1994; Gebru & Sbhatu, Citation2020; Nigatu et al., Citation1997; Tamene, Baye, et al., Citation2019).
This result is in agreement with the result reported by other studies. According to Tamene, Baye, et al. (Citation2019), the report shows that the total count of presumptive LAB ranged from 3.8 × 106 to 1.5 × 108 with an average of 2.5 × 107 colony forming units per gram (cfu/g) of fermented teff dough. In another study that was reported by Desiye et al. (Citation2017), the total counts of LAB in “Kuncho” and “Magna” teff dough samples were ranged from 5.12 to 8.47 log (CFU/gm) and 5.15 to 8.46 in that order (Ashenafi, Citation2002). This study showed that from the total microbial count of teff flour dough, mainly consisting of lactic acid bacteria (103 cfu/g).
Similarly, Dandessa (Citation2019) reported that the total counts of lactic acid bacteria species in the different fermented teff dough samples were ranged between 0.4 and 9.06 × 10−8 cfu/g at the six hours interval of fermentation time from 0 to 72 hrs. According to Nigatu et al. (Citation1997) study that shown the total enumeration of lactic acid bacteria species in fermented teff dough samples were ranged from 104 to 3.3 × 108 CFU/gm at 72 hrs.
In the previous study, Saeed et al. (Citation2009) showed that the lactic acid bacteria load of the laboratory fermented sourdough were ranged between 6.24 × 104 to 6.92 × 107 cfu/g. According to Sevgili et al. (Citation2021) reported the LAB species in the traditional sourdoughs were ranged from 6.58 log (CFU/g to 9.12 log (CFU/g. Resulted data shows that the enumeration of LAB in the ersho was increased with fermentation time increased from 0 to 96 hrs shown in .
Correspondingly, the kinetics growth of yeast in the dough increased significantly (p < .05) from 5.98 to 7.64 from 0 to 96 hours in every 24 hours of fermentation time. These results are in agreed with results of Desiye et al. (Citation2017), Harth et al. (Citation2018), Kozlinskis and Klava (Citation2008), and Moroni et al. (Citation2011). Similarly, Saeed et al. (Citation2009) and Sevgili et al. (Citation2021) reported for the growth of lactic acid bacteria species in the different fermented sourdough at different fermentation time.
In the current study, the enumeration of LAB and yeast in ersho and fermented teff dough were somewhat higher compared to the other study (Koricha et al., Citation2020; Moroni et al., Citation2011). This might be due to the sample preparation and collection conditions, as well as the fermentation time.
3.3. Morphological characterization of LAB
3.3.1. Characteristics of colonial morphology of the identified LAB
In this study, the identified colonies of lactic acid bacteria were observed to have different features of colonial morphology. The representative colonies were showed different cultural characteristics about color (white, light yellow), shape (circular or irregular), and size of colonies arranged small to medium on the MRS agar plate, due to the species of bacteria type (). The result of this study was in line with the results reported by Ewuoso et al. (Citation2020), Saeed et al. (Citation2014), and Tilahun et al. (Citation2018). Similar results were reported by Asnake and Mogessie (Citation2010) the LAB identified from different traditional fermented food.
Table 1. Characteristics of the colonial morphology and catalyst test of LAB.
3.3.2. Catalyst test for the identified LAB
In this study, all the identified LAB species were tested for the catalase reaction. As a result, all the identified LAB isolates were show catalase negative when hydrogen peroxide (H2O2) dropped on the fresh culture of the cell on the microscope glass slide (). The catalase reaction is positive when air bubbles occur, indicating the formation of O2 gas, and negative when air bubbles do not form, indicating the absence of gas bubbles, which is one of the characteristics of lactic acid bacteria (Guesh et al., Citation2019). The result obtained in this study indicated that the identified LAB isolates were unable to produce the catalase enzyme to convert hydrogen peroxide into water and oxygen. The current finding was agreed with the results reported by (Desiye & Abegaz, Citation2013; Guesh et al., Citation2019; Jula, Citation2020; Tafesee et al., Citation2020; Zhao, Citation2011). Hoque et al. (Citation2010) and Mannan et al. (Citation2017), were also report similar report on the LAB species identified from the sample of yogurt and cheese.
3.3.3. Gram staining and microscopic observation of LAB
Gram staining reactions of LAB colonies are shown in . All colony samples tested were gram positive under a compound microscope (100× power magnifications) using oil immersion. The Gram-stained results of the identified LAB species were showed different morphological characteristics, which included color (blue or purple), shape (cocci and rod), and size (short and medium) that belonging to the group of gram-positive bacteria. The shape of all isolated species of LAB were cocci in chain, grouping and single arrangements, and blue/purple color indicates that the isolate species are gram-positive and small and medium in size at zero to 48 hrs of fermentation time. On the other hands, the result showed that the shape, color and size of isolated species were rode, blue-purple, and short and medium at the end of 72 to 96 hours of fermentation time. As shown in , all the identified colonies were Gram-positive under the compound microscope observation. The Gram stained and microscopic observation result of this study was agreed with the same result reported by Asnake and Mogessie (Citation2010), Petkova et al. (Citation2021), Tamene, Baye, et al. (Citation2019), and Tilahun et al. (Citation2018).
Table 2. Gram staining and microscopic observation of identified LAB species.
3.4. Physiological characterization of the identified LAB species
The entire LAB identified from fermented teff dough showed good growth in MRS broth under anaerobic incubation conditions of tightly glass test tubes.
Physiological parameters evaluated included optical growth requirements of pH and Temperature, Salt tolerance at various concentrations and gas production from glucose at 30°C and 37°C. Tests were performed under anaerobic incubation conditions of tightly sealed glass test tubes. Results are shown in Appendix A, .
3.4.1. Identified pH tolerant LAB species
pH tolerance of LAB species was performed using MRS broth which was adjusted with 1HCL and 1NaOH to a range of 3 to 6.2. The results showed that all tested LAB species grew optimally at pH of 5.5 and 6 shown in Appendix A, . This result indicated that the low pH affects the growth of LAB by damaging and loss the viability of the cell. Therefore, these identified and isolated LAB species were able to show signs of very slight turbidity and growth in the low pH MRS broth. The maximum growth of the identified LAB species in both moderate and near to neutral pH of MRS broth medium compared with the low pH of the same medium. The result of the current study was in line the results reported by Hoque et al. (Citation2010), Mannan et al. (Citation2017), and Pundir et al. (Citation2013). On the contrary, the result reported by Desiye and Abegaz (Citation2013) and Guesh et al. (Citation2019), the LAB species identified from fermented teff dough were able to grow at low pH values of MRS broth medium, but unable to grow the alkaline condition, due to the acidophilic properties of the identified species. Similar results reported by Tamang et al. (Citation2007), showed the LAB isolates identified from different cereal product dough’s.
3.4.2. Testing the growth of LAB at different temperature
The temperature is an important factor that affects bacterial growth. As a result shown in Appendix A, , from the total identified LAB isolates, 5(33.3%) and 9(60%) of the identified isolates were able to densely grow and form turbidity at temperatures of 20 and 40°C in that order of inoculation. On the other hand, 7(46.6%), 10 (90.9%), 12 (80%), 11 (73.3%), 11 (73.3%), 3(20%) and 6(40%) isolates of LAB species were able to grow moderately and form more turbidity at 15, 20, 25, 30, 37, 40, and 45°C in order of that inoculation.
Correspondingly, 7(46.6%), 4(26.6%), 1(6.6%), 3(20%), and 6(40%) isolates of LAB species were able to show a sign of turbidity and positively grow at temperatures of 15, 30, 37, 40, and 45°C in order of that inoculation. The remaining of 1(6.6%) and 3(20%) isolates of the LAB species were able to grow weakly at 15 and 37°C in that order of inoculations. On the contrary, 1(6.6%) and 3(20%) isolates of LAB species were unable to grow and form turbidity at 15 and 45°C, respectively; indicating that these were not their favorable temperature to grow. Our finding of optimal temperature range of 20°C to 40°C for growth of LAB species in fermented food corroborates with work done by previous researchers (Ali & Mustafa, Citation2009a; Desiye & Abegaz, Citation2013; Guesh et al., Citation2019; Tamang et al., Citation2007). The other similar result reported by (Abegaz, Citation2007; Akalu et al., Citation2017; Thapa et al., Citation2004) on identified LAB isolates from different fermented foods and sourdough.
3.4.3. Salt tolerance LAB species identification
Results from salt tolerance test showed that all the identified isolates of LAB species were able to grow and form turbidity at different salt concentrations shown in (Appendix A, ), though there were some variations in density of growth and degree of turbidity in broth media. As a result, all the identified isolates of LAB species were able to densely grow and form turbidity in 8% and 10% and were showed positive growth and form slight turbidity in 4% of NaCl salt concentrations in order of that inoculation. Correspondingly, among 15 identified isolates 5(33.33%) of the identified isolates were showed positive growth and tolerated in 6% NaCl salt concentration.; However, 10 (66.67%) of the identified isolates were able to grow moderately (positive) and form more turbidity in 6% NaCl salt concentration inoculation. This suggests that LAB species isolated from fermented teff products were tolerant to range of salt concentration (4% to 10%). This agrees with works done by previous researchers (Desiye & Abegaz, Citation2013; Elyas et al., Citation2015; Guesh et al., Citation2019; Maqsood et al., Citation2013; Wu et al., Citation2014).
3.4.4. Gas production from glucose for LAB
Test for CO2 production from glucose at 30°C and 37°C showed that only 3(20%) of isolates produced CO2 from glucose and thus were hetero-fermentative shown in (Appendix A, ). Such species were considered as Bacillus subtilis. Whereas, 12 (80%) of the isolates didn’t produce CO2, and thus considered as homo-fermentative. These organisms belong to Lactobacillus species. This finding agrees with findings reported by previous researchers (Ali & Mustafa, Citation2009b; Bartkiene et al., Citation2020; Elyas et al., Citation2015; Iruene et al., Citation2021; Wu et al., Citation2014).
3.5. Biochemical characterization of LAB
Results for biochemical tests of LAB species are presented in Appendix B, . All the identified isolates were able to show positive and ferment in glucose monohydrate, D-galactose and D-fructose. In addition, among the 15 isolates 8 (53.33%), 12 (80%), 8 (53.33%), 3(20%), 8 (53.33%), 7 (46.66%), 13 (86.66%), 7 (46.66%), 14 (93.3%), 12 (80%), 6 (40%), 2 (13.3%), and 5 (33.33%) of isolates were able to fermented in L-arabinose, D- (-) arabinose, D-(+)-xylose, D-(-)-ribose, D-mannitol, sorbitol (liquid), aesculin, lactose, D-(+)-maltose monohydrate, sucrose, D (+)-trehalose, D-(+)-melibiose, and D-raffinose in order of that inoculations. Simultaneously, 3 (20%) and 2 (13.3%) of LAB isolates were produced gas in glucose mono hydrate and fructose, and 1 (6.66%) isolates was gas produced in lactose, sucrose, and D-raffinose in order of that inoculation. But, the remaining isolates were unable to fermented and produced gas. This study showed that all the identified LAB isolates were ferment in monosaccharide sugars. On the contrary, most of the identified LAB isolates were not ferment in disaccharides and polysaccharide sugar. This is due to the complexity of the sugar and different identified LAB isolates. The sugar fermentation ability of LAB to disaccharides differ depend on the identified species composition (Gunkova et al., Citation2021; Halász, Citation2009). Hence, the sugar fermentation activity of the identified LAB species slowest in disaccharides and polysaccharide sugar compared to monosaccharide sugars. The result obtained in this study was agreed with the result reported by Akinola and Osundahunsi (Citation2021), Ali and Mustafa (Citation2009a), Desiye and Abegaz (Citation2013), Mannan et al. (Citation2017), Saeed et al. (Citation2009), and Scheirlinck et al. (Citation2007). On contrary, all the identified LAB isolates were unable to ferment on D-(+)-mannose; this might be depend on the incubation time and differ the identified LAB isolates. The time-course of the identified LAB isolate growth was affected by the substrate (sugars) used; in particular the initial rate of growth was mannose alone or mannose plus glucose. The cultures grown on mannose alone from 5 days to 30 days were used to inoculate fresh cultures containing mannose alone (Stewart et al., Citation2000).
3.6. Isolation and identification of LAB
shows species of LAB identified based on all the above tests (gram staining, catalase test, pH, temperature, salt tolerance and gas production from fermentation of various sugars). Based on all the above tests, five dominant LAB species were identified to involve in teff dough fermentation (). These species were percentage occurrence of isolates of LAB species were identified as Lactobacillus fermentum (26.7%), Lactobacillus brevis (20%), Lactobacillus plantarum (13.3%), and Bacillus subtilis (20%), and Enterococcus casseliflavus (20%). The species belonged to three major genera Lactobacillus, bacillus, and enterococcus. Similar results were reported by previous researchers (Asnake & Mogessie, Citation2010; Desiye & Abegaz, Citation2013; Ewuoso et al., Citation2020; Harth et al., Citation2018; Jung et al., Citation2009; Petkova et al., Citation2021; Sevgili et al., Citation2021; Tilahun et al., Citation2018; Zheng et al., Citation2020).
Table 3. Isolated dominant species of LAB involved in teff dough.
3.7. Morphological characterization of yeast
3.7.1. Characteristics of colonial morphology of identified yeast
shows that the results of colony characteristics of identified yeasts. Colonies showed varying morphological characteristics color (creamy and white), shape (circular and irregular) and size of colonies arranged small to medium on the potato dextrose agar (PDA) plate; due to the isolates of different yeast species. The result obtained in this study was in agreed with the results reported by Akinola and Osundahunsi (Citation2021), Beyene et al. (Citation2020), Desiye and Abegaz (Citation2013), Ebabhi et al. (Citation2013), Shona (Citation2020), Sulmiyati et al. (Citation2019), Syal and Vohra (Citation2013), and Tilahun et al. (Citation2019).
Table 4. Characteristics of the colonial morphology of yeast.
3.7.2. Catalyst test for the identified yeast
The results from reaction of yeast colonies to catalase test are shown in . All the tested yeast colonies were catalase positive when hydrogen peroxide (H2O2) dropped on the fresh culture of the cell on the microscope glass slide. The catalase reaction is positive when air bubbles occur, indicating the formation of O2 gas, which indicated the catalyst serves to decompose hydrogen peroxide in to the water and oxygen Fugelsang and Edwards (Citation2007). Hence, this result indicated the identified LAB isolates were able to produce the catalase enzyme to convert hydrogen peroxide into water and oxygen. This result is supported by researchers (Akinola & Osundahunsi, Citation2021; Perricone et al., Citation2014), the dominant yeast species isolated from fermented sourdough. On the contrary, Ewuoso et al. (Citation2020) report showed that all the yeast colonies identified from fermented sorghum sourdough were catalase-negative.
3.7.3. Simple gram staining and microscopic observation of yeast
Cell morphology was done to determine the colored smear culture under a microscope and see how the shape of the cell and the characteristic of the Gram stained yeast. In this study, the compound microscope was used to observe the cell morphology of the identified yeast isolates. The gram stained results of the identified yeast isolate were showed different morphological characteristics, which included colony color (blue), shape (elongated and oval), size (large), and elevation (long to medium) which was observed under a compound microscope (100× power magnifications). All colony samples tested were gram positive (). The shape of all identified yeast isolates were circular in chain, grouping and single arrangements, size (small), and blue color observed under the compound microscope at fermentation time from zero to 48 hours. The blue colors indicate the identified isolates were gram positive; whereas, at the end of fermentation time from 72 to 96 hours the shape (elongated/oval), color (blue), and size (small to medium) were observed.
Table 5. Simple gram staining and microscopic observation of identified LAB species.
As shown in , all the identified isolates were gram-positive under the compound microscope observation. The results obtained in this study were supported by Akinola and Osundahunsi (Citation2021), Beyene et al. (Citation2020), Ewuoso et al. (Citation2020), and Shona (Citation2020).
3.8. Physiological characterization of identified yeast
In , the physiological parameters evaluated included optical growth requirements of pH and temperature, ethanol tolerance at various concentrations and gas production from glucose at 30°C and 37°C.
Table 6. Physiological characterization test of yeast.
3.8.1. pH Tolerant yeast species identification
As shown in the , the pH tolerance of yeast species was performed using malt extract broth which was adjusted with 1HCL and 1NaOH to a range of 3 to 6.5. As a result, the growth of all the identified yeast isolates was varied at different pH values from 3 to 6.5 due to different yeast isolates. Hence, all identified yeast isolates were moderately growth (++) and form more turbidity at pH of 4.5 and 5.5. Likewise, 9(90%) of the identified isolates were moderately (++) grow and form more turbidity, whereas only one identified isolates was show slight turbidity and grow (+) at pH of 6.5 malt extract broth it might be due to the types and characteristics of the identified species.
Simultaneously, all the identified isolates were show slight turbidity and grow (+) at pH of 3 and 3.5. This result show that, all the identified yeast isolates were grow at optimum pH of range from 4.5 to 6.5 malt extract broth. This result indicated that at very low (acidic) and high (alkaline) pH values affect the growth of yeast by causing chemical stress on the cell, damaging and loss the viability of the cell (Yalcin & Ozbas, Citation2008). This finding show that yeast tolerates acidic condition more compared with identified LAB. Consequently, these identified yeast isolates were able to show a signs of very slight turbidity and growth in the low pH of malt extract broth. This study observed the maximum growth of the identified yeast isolates in both moderate and near to neutral pH of malt extracts broth compared to the very low pH of the same medium. Overall, the identified yeast isolate was tolerating the optimum pH of malt extract broth range from 4.5 to 6.5. The result obtained in this study was supported by Brandt et al. (Citation2004), Elsa et al. (Citation2019), Martorana et al. (Citation2018), Ogunsakin et al. (Citation2017), Perricone et al. (Citation2014), Shona (Citation2020), Tadesse et al. (Citation2021), Vrancken et al. (Citation2010), and Yang et al. (Citation2021).
3.8.2. Testing the growth of yeast at different temperature
The reason for choosing this temperature range was to determine the optimum growth temperature whether the isolated yeast species were able to grow within this range of temperature or not.
As shown in , all isolates were able to show positive growth and turbidity at a temperature of 15, 25, 30, 35, and 37°C. Among ten isolates of yeast 4(40%), 2(20%), and 4(40%) isolates of yeast species were able to grow moderately (++) and form more turbidity at 20, 28, and 42°C in order of that inoculation. Similarly, 6(60%), 7(70%), and 8(80%) isolates were able to grow (+) and form slight turbidity at 20, 25, and 28°C in order of that inoculation. On contrary, 6(60%) of the isolated yeast species were able to show weakly growth (+w) and sign of turbidity at 42°C of incubation. Our finding of optimal temperature range of 15–37°C, and survive and form slight turbidity show at 42°C due to the different isolates of the identified yeast species. The result of the current study was corroborate with work done by previous researchers (Akinola & Osundahunsi, Citation2021; Ewuoso et al., Citation2020; Perricone et al., Citation2014; Shona, Citation2020; Simonson et al., Citation2003; Tadesse et al., Citation2021).
3.8.3. Ethanol tolerance yeast species identification
All the identified yeast isolates were tested for their ethanol tolerance and ability to grow to at different ethanol concentration of (8%, 10%, 13%, and 15%). As a result in , all the identified yeast isolates were able to showed good growth at 8% of ethanol concentration, whereas failed to grow at 10%, 13%, and 15% of ethanol concentration, due to the different identified yeast species and their sensitivity to ethanol concentration. This indicated that at high ethanol concentrations reduce cell vitality and it leads to increase cell death at high ethanol concentration. Our finding of optimal ethanol concentration for yeast growth was 8% of ethanol concentration at 30°C incubation. The result obtained in this study was in agreement with the result reported by Desiye and Abegaz (Citation2013), Htet et al. (Citation2018), Kumari et al. (Citation2019), Negera (Citation2017), Tikka et al. (Citation2013), and Tsegaye et al. (Citation2018).
3.8.4. Production of gas from glucose for yeast
As shown in , the entire identified and isolated yeast species varied were produced gas (CO2) from glucose at 30°C and 37°C of incubation.
Test for CO2 production from glucose at 30°C and 37°C showed that only 4(40%) of yeast isolates densely produced CO2 from glucose at both 30°C and 37°C of incubation shown in . Similarly, 3(30%), and 2 (20%) isolates, and 2(20%) and 3(30%) of yeast isolates were moderately and slightly produced CO2 from glucose at 30°C and 37°C in that order of inoculation, whereas only one isolate showed weakly gas produced from glucose at 37°C of incubations. Our finding was in line with the results reported by Beyene et al. (Citation2020), Ogunsakin et al. (Citation2017), Sevgili et al. (Citation2021), and Tilahun et al. (Citation2019). The gas production during fermentation was indicated by the uplift of the inverted Durham tubes.
3.9. Biochemical characterization test for yeast
The biochemical characterization tests of the identified and isolated yeast isolates were performed by using 12 different carbon source sugars.
The results for biochemical tests of yeast species are presented in Appendix A, . As a result, all isolates were densely fermented on galactose, glucose, and fructose, whereas failed to ferment on D-xylose and D-(+)-maltose. Similarly, 2(20%) and 10% of isolates of yeast were fermented on sucrose, and trehalose, lactose, raffinose, inulin, and starch, and 3(30%) isolates and only one isolate was weakly fermented on raffinose and inulin, and starch, whereas 8(80%), 9(90%), and 6(60) isolates of yeast were failed to ferment in sucrose and starch, trehalose and lactose, and raffinose and inulin after 48 hours of incubation at a temperature of 30°C.
The sugar fermentation of the identified yeast isolate was depending on the complexity of the sugar. The simpler chemical structures of monosaccharide have a higher rate of fermentation than a disaccharide due the hydrogen bonds in the sugar, monosaccharides contain fewer bonds than disaccharides, and thus, enzymes in yeast can break monosaccharides down faster, but the more complex structure of disaccharides, they are harder to break down (Burnison et al., Citation2018; Webster et al., Citation2019). The results obtained in this study was supported by Akinola and Osundahunsi (Citation2021), Ebabhi et al. (Citation2013), Martorana et al. (Citation2018), Shona (Citation2020), Valmorri et al. (Citation2010), Vrancken et al. (Citation2010), and Yang et al. (Citation2021).
3.10. Identification of yeast
shows the species of yeast identified based on all the above tests (gram staining, catalyst test, pH, temperature, ethanol tolerance and gas production from different sugars).
Table 7. Isolated dominant yeast species involved in teff dough.
As a result, three dominant yeast species were identified at the end of fermentation time; which was involved in teff dough fermentation. The percentage occurrence of the yeast involved in the fermented teff dough was identified and isolated as Saccharomyces cerevisiae (40%), Candida krusei (40%), and Pichia kudriavzevii (20%). The result obtained in this study was supported by Ali and Mustafa (Citation2009b), Beyene et al. (Citation2020), Desiye and Abegaz (Citation2013), Ewuoso et al. (Citation2020), Meroth et al. (Citation2003), Mugula et al. (Citation2003), Pedersen et al. (Citation2012), Sevgili et al. (Citation2021), Syal and Vohra (Citation2013), and Tadesse et al. (Citation2021).
4. Conclusion
As a conclusion, in this study there were the involvements of different dominant yeast and LAB species in both ersho and dough. In morphological characterization, all the identified representatives’ yeast and LAB colonies were showed diverse colony and cell characteristics regard to color (white, gray pigmentation or blue), shape (circular or irregular), and elevation (flat or raised) on the pre-solidified PDA agar and color (white, light yellow), shape (circular or irregular), and size of colonies arranged small to medium on the pre-solidified PDA and MRS agar plate.
In physiological characterization, the identified LAB able to grow at the optimal growth temperature, pH and salt concentration were ranges from 20 to 40°C; 5.5 to 6.2, and 8 to 10%; likewise the yeast able to grow at optimal temperature 15 to 37°C, pH 4.5 to 6.5 and ethanol concentration at 8%. The entire identified yeast species were produced gas, whereas only 3 of LAB species were produced gas from glucose at 30°C and 37°C.
In biochemical characterization, all identified isolates of LAB and yeast were densely growth and ferment in monosaccharide than disaccharide, and polysaccharide sugar, Finally, five dominant LAB (Lactobacillus fermentum, Lactobacillus brevis, Lactobacillus plantarum, Bacillus subtilis, and Enterococcus casseliflavus) species, and three dominant yeast species included Saccharomyces cerevisiae, Candida krusei, and Pichia kudriavzevii were found to be the most dominant LAB and yeast species and could be identified in fermented teff dough samples from the beginning (24-hrs) to the later (96-hrs) fermentation times of teff dough.
Author contributions
Ms. Zinash Tadesse conducted all the experiments and wrote the manuscript. Dr. Metadel Kassahun support, advice, guidance, assistance and constant encouragement during the research work. Dr. Takele Ayanew assist and guidance during the research works; especially during the laboratory works of the research. Mr. Agimassie Agazie support and guidance, and constant encouragement from the initial to final work of the research. Dr. Mesfin W/Mariam, Mr. Deginet Teferi, Abebaw Teshome, Sadik Jemal and Taddele Andarge coordinated the research work.
Acknowledgments
The authors thanks Bahir Dar Institute of Technology (BIT), Faculty of Chemical and Food engineering; especially for all the laboratory assistances (Food microbiology laboratory, Food chemistry and analytical laboratory, and Food process engineering laboratory) for their cooperation, invaluable and timely help during this thesis works. The author also would like to thanks to Food and Beverage Industry Research and Development Center for funding the project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
References
- Abegaz, K. (2007). Isolation, characterization and identification of lactic acid bacteria involved in traditional fermentation of borde, an Ethiopian cereal beverage. African Journal of Biotechnology. https://doi.org/10.5897/AJB2007.000-2208
- Abiyu, H. T., Woldegiorgis, A. Z., & Haki, G. (2013). Preparation of injera from pre-fermented flour: Nutritional and sensory quality. Hiwot, 3(1), 165–175.
- Achi, O. K. (2005). The potential for upgrading traditional fermented foods through biotechnology. African Journal of Biotechnology. https://doi.org/10.5897/AJB2005.000-3070
- Aidoo, K. E., Rob Nout, M. J., & Sarkar, P. K. (2006). Occurrence and function of yeasts in Asian indigenous fermented foods. FEMS Yeast Research, 6(1), 30–39. https://doi.org/10.1111/j.1567-1364.2005.00015.x
- Akalu, N., Assefa, F., & Dessalegn, A. (2017). In vitro evaluation of lactic acid bacteria isolated from traditional fermented shamita and Kocho for their desirable characteristics as probiotics. African Journal of Biotechnology, 16(12), 594–606. https://doi.org/10.5897/AJB2016.15307
- Akinola, S. A., & Osundahunsi, O. F. (2021). Lactic acid bacteria and yeast diversities in spontaneously fermented millet sourdoughs. Journal of Microbiology, Biotechnology and Food Sciences, 6(4), 1030–1035. https://doi.org/10.15414/jmbfs.2017.6.4.1030-1035
- Ali, M. S., & Latif, Z. (2016). Molecular characterization of yeast strains isolated from different sources by restriction fragment length polymorphism. Pakistan Journal of Botany, 8(1), 363–370.
- Ali, A. A., & Mustafa, M. M. (2009a). Isolation, characterization and identification of lactic acid bacteria from fermented sorghum dough used in Sudanese kisra preparation. Pakistan Journal of Nutrition, 8(11), 1814–1818. https://doi.org/10.3923/pjn.2009.1814.1818
- Ali, A. A., & Mustafa, M. M. (2009b). Use of starter cultures of lactic acid bacteria and yeasts in the preparation of kisra, a Sudanese fermented food. Pakistan Journal of Nutrition, 8(9), 1349–1353. https://doi.org/10.3923/pjn.2009.1349.1353
- Allen Foegeding, E., Brown, J., Drake, M., & Daubert, C. R. (2003). Sensory and mechanical aspects of cheese texture. International Dairy Journal, 13(8), 585–591. https://doi.org/10.1016/S0958-6946(03)00094-3
- AOAC. (1995). Official Methods of Analysis (16th ed.). Association of Official Analytical Chemists.
- AOAC. (2000). Official methods of analysis of AOAC. In International 17th Edition.
- Ashaolu, T. J. (2019). A review on selection of fermentative microorganisms for functional foods and beverages: The production and future perspectives. International Journal of Food Science and Technology, 1–9. https://doi.org/10.1111/ijfs.14181
- Ashenafi, M. (1994). Microbial flora and some chemical properties of ersho, a starter for teff (eragrostis tef) fermentation. World Journal of Microbiology & Biotechnology, 10(1), 69–73.
- Ashenafi, M. (2002). The microbiology of Ethiopian foods and beverages: A review. SINET: Ethiopian Journal of Science, 25(1), 1–44. https://doi.org/10.4314/sinet.v25i1.18076
- Ashenafi, M. (2006). A review on the microbiology of indigenous fermented foods and beverages of Ethiopia. Ethiopian Journal of Biological Sciences, 5(2), 189–245. https://doi.org/10.4314/ejbs.v5i2.39036
- Ashenafi, M. (2008). Review article: A review on the Microbiology of indigenous fermented foods and beverages of Ethiopia. Ethiopian Journal of Biological Sciences, 5(2). https://doi.org/10.4314/ejbs.v5i2.39036
- Asnake, D., & Mogessie, A. (2010). Evaluation of the Probiotic Properties and Antibiotic Resistance of Lactic Acid Bacteria Isolated from Awaze, Qotchqotcha and Tef dough, traditional Ethiopia. Internet Journal of Food Safety, 12, 187–191.
- Assefa, Y., Emire, S., Abebe, W., & Ronda, F. (2018). Effect of mill type and mechanical kneading conditions on fermentation kinetics of tef dough during injera making and phytate to mineral molar ratio of injera. Journal of Food Science and Technology, 7(2), 1–23.
- Baka, A. M., Papavergou, E. J., Pragalaki, T., Bloukas, J. G., & Kotzekidou, P. (2011). Effect of selected autochthonous starter cultures on processing and quality characteristics of Greek fermented sausages. LWT - Food Science and Technology, 44(1), 54–61. https://doi.org/10.1016/j.lwt.2010.05.019
- Bartkiene, E., Lele, V., Ruzauskas, M., Domig, K. J., Starkute, V., Zavistanaviciute, P., Bartkevics, V., Pugajeva, I., Klupsaite, D., Juodeikiene, G., Mickiene, R., & Rocha, J. M. (2020). Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal properties evaluation. Microorganisms [Internet], 8(1), 64. https://doi.org/10.3390/microorganisms8010064
- Baye, K., Mouquet-Rivier, C., Icard-Vernière, C., Rochette, I., & Guyot, J.-P. (2013). Influence of flour blend composition on fermentation kinetics and phytate hydrolysis of sourdough used to make injera. Food Chemistry, 138(1), 430–436. https://doi.org/10.1016/j.foodchem.2012.10.075
- Beyene, E., Tefera, A. T., Muleta, D., Fantahun, S. K., & Wessel, G. M. (2020). Molecular identification and performance evaluation of wild yeasts from different Ethiopian fermented products. Journal of Food Science and Technology, 57(9), 3436–3444. https://doi.org/10.1007/s13197-020-04377-7
- Bhalla, T. C., & Savitri. (2017). Yeasts and traditional fermented foods and beverages. Yeast Diversity in Human Welfare. https://doi.org/10.1007/978-981-10-2621-8_3
- Blandino, A., Al-Aseeri, M. E., Pandiella, S. S., Cantero, D., & Webb, C. (2003). Cereal-based fermented foods and beverages. Food Research International, 36(6), 527–543. https://doi.org/10.1016/S0963-9969(03)00009-7
- Bover-Cid, S., Izquierdo-Pulido, M., & Vidal-Carou, M. C. (2001). Effectiveness of a Lactobacillus sakei starter culture in the reduction of biogenic amine accumulation as a function of the raw material quality. Journal of Food Protection, 64(3), 367–373. https://doi.org/10.4315/0362-028X-64.3.367
- Brandt, M. J., Hammes, W. P., & Gänzle, M. G. (2004). Effects of process parameters on growth and metabolism of Lactobacillus sanfranciscensis and Candida humilis during rye sourdough fermentation. European Food Research and Technology, 218(4), 333–338. https://doi.org/10.1007/s00217-003-0867-0
- Bultosa, G., Hamaker, B. R., & Bemiller, J. N. (2008). An SEC-MALLS study of molecular features of water-soluble amylopectin and amylose of tef [eragrostis tef (zuce.) trotter] starches. Starch/Staerke, 60(1), 8–22. https://doi.org/10.1002/star.200700642
- Bultosa, G., & Taylor, J. R. N. (2004). Teff. Encyclopedia of Grain Science. https://doi.org/10.1016/b0-12-765490-9/00172-5
- Burnison, H., Granato, T., King, M., Peoples, H., & Hallidayschult, T. (2018). The Effect of monosaccharides versus disaccharides on the rate of CO2 production. Journal of Undergraduate Biology Laboratory Investigations, 1(1), 1–4.
- Ciuciu Simion, A. M., Vizireanu, C., Alexe, P., Franco, I., & Carballo, J. (2014). Effect of the use of selected starter cultures on some quality, safety and sensorial properties of dacia sausage, a traditional Romanian dry-sausage variety. Food Control, 35(1), 123–131. https://doi.org/10.1016/j.foodcont.2013.06.047
- Cletus Kurtzman, J. W., & Fell, T. B. (2011). The yeasts: A taxonomic study (Vol. 1). Elsivier.
- Dandessa, C. (2019). Review on Ethiopian traditional fermented foods, its microbial ecology and nutritional value. International Journal of Current Research and Academic Review, 7(5), 13–27.
- da Silva, N., Taniwaki, M. H., Junqueira, V. C. A., Silveira, N., Okazaki, M. M., & Gomes, R. A. R. (2013). Microbiological examination methods of Food and water a laboratory Manual (2nd ed.). CRC Press.
- Desiye, A., & Abegaz, K. (2013). Isolation, characterization and identification of lactic acid bacteria and yeast involved in fermentation of teff (Eragrostis tef) batter. Advanced Research in Biological Sciences, 1(3), 35–44.
- Desiye, A., Abegaz, K., Negera, E., & Gobena, E. (2017). The Microbiology of teff (Eragrostis tef) enjera. The Microbiology of Teff (Eragrostis Tef) Enjera, 2(2), 115–120.
- De Vos, P., Garrity, G. M., Jones, D., Krieg, N. R., Ludwig, W., Rainey, F. A., Schleifer, K.-H., & Whitman, W. B. (2009). Bergey’s manual of systematic bacteriology volume three the firmicutes. In Bergey’s Manual of Systematic Bacteriology (2nd ed.). Springer International Publishing.
- Ebabhi, A. M., Adekunle, A. A., Okunowo, W. O., & Osuntoki, A. A. (2013). Isolation and characterization of yeast strains from local food crops. Journal of Yeast and Fungal Research, 4(4), 38–43.
- Elsa, B. G., Anteneh, T. T., Diriba, M., Solomon, K. F., & Gary, M. W. (2019). Optimization of the cultivation conditions of indigenous wild yeasts and evaluation of their leavening capacity. BioRxiv, 6(10), 1–25.
- Elyas, Y. Y. A., Yousif, N. M. E., & Ahmed, I. A. M. (2015). Screening of lactic acid bacteria from Sudanese fermented foods for bacteriocin production. Journal of Microbiology, Biotechnology and Food Sciences, 4(5), 373–378. https://doi.org/10.15414/jmbfs.2015.4.5.373-378
- Ewuoso, M. O., Animashaun, O. H., & Adejumo, A. A. (2020). Lactic acid bacteria and yeasts in spontaneously fermented sorghum sourdough. American Journal of Microbiological Research, 8(2), 63–72.
- Fugelsang, K. C., & Edwards, C. G. (2007). Wine microbiology: Practical applications and procedures. Springer.
- Gawai, K. M., & Prajapati, J. B. (2017). Safety aspects of fermented and probiotic foods. International Journal of Fermented Foods, 6(1), 45. https://doi.org/10.5958/2321-712x.2017.00005.9
- Gebru, Y. A., & Sbhatu, D. B. (2020). Isolation and characterization of probiotic LAB from kimchi and spontaneously fermented teff (Eragrostis tef (Zucc.) Trotter) batter: Their effects on phenolic content of teff during fermentation. BioMed Research International, 2020, 1–9. https://doi.org/10.1155/2020/4014969
- Gholami-Shabani, M., Shams-Ghahfarokhi, M., & Razzaghi-Abyaneh, M. (2023). Food microbiology: Application of microorganisms in Food Industry. IntechOpen. https://doi.org/10.5772/intechopen.109729
- Gizaw, B. (2018). Traditional knowledge on teff (eragrostistef) farming practice and role of Crop Rotation to enrich plant growth promoting microbes for soil fertility in east Showa: Ethiopia. Agricultural Research & Technology: Open Access Journal, 16(5). https://doi.org/10.19080/artoaj.2018.16.556001
- Guesh, M., Tessema, T. S., & Tesfaye, M. D. A., Tesfaye, A. (2019). In vitro evaluation of probiotic properties of lactic acid bacteria isolated from some traditionally fermented Ethiopian food products. International Journal of Microbiology, 2019, 1–11. https://doi.org/10.1155/2019/7179514
- Gunkova, P. I., Buchilina, A. S., Maksimiuk, N. N., Bazarnova, Y. G., & Girel, K. S. (2021). Carbohydrate fermentation test of lactic acid starter cultures. Iop Conference, 852(1), 1–5. https://doi.org/10.1088/1755-1315/852/1/012035
- Halász, A. (2009). Lactic acid bacteria. Food Quality and Standards, 3(2009), 70–82.
- Hansen, E. B. (2002). Commercial bacterial starter cultures for fermented foods of the future. International Journal of Food Microbiology, 78(1–2), 119–131. https://doi.org/10.1016/S0168-1605(02)00238-6
- Harth, H., Van Kerrebroeck, S., & De Vuyst, L. (2018). Impact of process conditions on the microbial community dynamics and metabolite production kinetics of teff sourdough fermentations under bakery and laboratory conditions. Food Science & Nutrition, 6(6), 1438–1455. https://doi.org/10.1002/fsn3.690
- Holzapfel, W. H. (2002). Appropriate starter culture technologies for small-scale fermentation in developing countries. International Journal of Food Microbiology, 75(2002), 197–212. https://doi.org/10.1016/S0168-1605(01)00707-3
- Hoque, M. Z., Akter, F., Hossain, K. M., Rahman, M. S. M., Billah, M. M., & Islam, K. M. D. (2010). Isolation, identification and analysis of probiotic properties of Lactobacillus spp. From selective regional yoghurts. World Journal of Dairy & Food Sciences, 5(1), 39–46.
- Hotessa, N., Robe, J., & Comi, G. (2020). Ethiopian indigenous traditional fermented beverage: The role of the microorganisms toward nutritional and safety value of fermented beverage. International Journal of Microbiology, 2020, 1–11. https://doi.org/10.1155/2020/8891259
- Htet, N. N. W., Hlaing, T. S., Yu, S. Z., & Yu, S. S. (2018). Isolation and characterization of xylose-utilizing yeasts for ethanol production. Journal of Bacteriology & Mycology: Open Access, 6(2), 109–114. https://doi.org/10.15406/jbmoa.2018.06.00186
- Iruene, I. T., Wafula, E. N., Kuja, J., & Mathara, J. M. (2021). Phenotypic and genotypic characterization of lactic acid bacteria isolated from spontaneously fermented vegetable amaranth. African Journal of Food Science, 15(6), 254–261. https://doi.org/10.5897/AJFS2021.2107
- Joshi, V. K., & Sharma, S. (2009). Cider vinegar: Microbiology, technology and quality. Vinegars of the World, 197–207. https://doi.org/10.1007/978-88-470-0866-3_12
- Jula, M. N. (2020). Quality and microbiological study of Bambara groundnut fortified injera, a fermented flat bread [Doctorial Dissertation]. Durban University of Technology, 1–140.
- Jung, S.-W., Kim, W.-J., Lee, K.-G., Kim, C.-W., & Noh, W.-S. (2009). Isolation and identification of lactic acid bacteria from sourdough with high exopolysaccharide production ability. Food Science and Biotechnology, 18(2), 384–389.
- Karki, T. B., Timilsina, P. M., Yadav, A., Pandey, G. R., Joshi, Y., Bhujel, S., Adhikari, R., & Neupane, K. (2017). Selection and characterization of potential Baker’s yeast from indigenous resources of Nepal. Biotechnology Research International, 2017, 1–10. https://doi.org/10.1155/2017/1925820
- Kimaryo, V. M., Massawe, G. A., Olasupo, N. A., & Holzapfel, W. H. (2000). The use of a starter culture in the fermentation of cassava for the production of “kivunde”, a traditional Tanzanian food product. International Journal of Food Microbiology, 56(2–3), 179–190. https://doi.org/10.1016/S0168-1605(00)00159-8
- Koricha, A. D., Han, D., Bacha, K., & Bai, F. (2020). Diversity and distribution of yeasts in indigenous fermented foods and beverages of Ethiopia. Journal of the Science of Food and Agriculture, 100(9), 3630–3638. https://doi.org/10.1002/jsfa.10391
- Kostinek, M., Specht, I., Edward, V. A., Schillinger, U., Hertel, C., Holzapfel, W. H., & Franz, C. M. A. P. (2005). Diversity and technological properties of predominant lactic acid bacteria from fermented cassava used for the preparation of Gari, a traditional African food. Systematic and Applied Microbiology, 28(6), 527–540. https://doi.org/10.1016/j.syapm.2005.03.001
- Kozlinskis, S., & Klava, K. (2008). Characterization of rye sourdough microflora. FOODBALT-2008(1), 89–92.
- Kozlinskis, E., Skudra, L., Klava, D., & Kunkulberga, D. (2008). Lactic acid bacteria in rye sourdough from crude and peeled rye flour. In Research for Rural Development. International Scientific Conference Proceedings (pp. 308–312). Latvia University of Agriculture.
- Kumari, S., Jha, A. K., & Singh, A. K. (2019). Isolation and characterization of temperature and ethanol tolerant strain of Saccharomyces cerevisiae strains from naturally fermented juices. Biosciences, Biotechnology Research Asia, 16(1), 97–103. https://doi.org/10.13005/bbra/2726
- Liu, C. F., & Pan, T. M. (2010). In vitro effects of lactic acid bacteria on cancer cell viability and antioxidant activity. Journal of Food and Drug Analysis. https://doi.org/10.38212/2224-6614.2287
- Mannan, S. J., Rezwan, R., Rahman, M. S., & Begum, K. (2017). Isolation and biochemical characterization of Lactobacillus species from yogurt and cheese samples in Dhaka metropolitan area. Bangladesh Pharmaceutical Journal, 20(1), 27–33. https://doi.org/10.3329/bpj.v20i1.32090
- Maqsood, S., Hasan, F., & Masud, T. (2013). Characterization of lactic acid bacteria isolated from indigenous dahi samples for potential source of starter culture. African Journal of Biotechnology, 12(33), 5226–5231. https://doi.org/10.5897/AJB09.1172
- Martorana, A., Giuffrè, A. M., Capocasale, M., Zappia, C., & Sidari, R. (2018). Sourdoughs as a source of lactic acid bacteria and yeasts with technological characteristics useful for improved bakery products. European Food Research & Technology, 244(10), 1873–1885. https://doi.org/10.1007/s00217-018-3100-x
- Mengesha, Y., Tebeje, A., Tilahun, B., & Owusu-Kwarteng, J. (2022). A review on factors influencing the fermentation process of teff (Eragrostis teff) and other cereal-based Ethiopian injera. International Journal of Food Science, 2022, 1–10. https://doi.org/10.1155/2022/4419955
- Meroth, C. B., Hammes, W. P., & Hertel, C. (2003). Identification and population dynamics of yeasts in sourdough fermentation processes by PCR-denaturing gradient gel electrophoresis. Applied and Environmental Microbiology, 69(12), 7453–7461. https://doi.org/10.1128/AEM.69.12.7453-7461.2003
- Mezemir, S. (2015). Probiotic potential and nutritional importance of teff (eragrostis tef (zucc) trotter) enjerra-a review. African Journal of Food Agriculture Nutrition & Development, 15(2), 9964–9981. https://doi.org/10.18697/ajfand.69.13905
- Moneim, A., & Sulieman, E. (2018). Microbial starter cultures. May.
- Moroni, A. V., Arendt, E. K., & Dal Bello, F. (2011). Biodiversity of lactic acid bacteria and yeasts in spontaneously-fermented buckwheat and teff sourdoughs. Food Microbiology, 28(3), 497–502. https://doi.org/10.1016/j.fm.2010.10.016
- Mugula, J. K., Nnko, S. A. M., Narvhus, J. A., & Sørhaug, T. (2003). Microbiological and fermentation characteristics of togwa, a Tanzanian fermented food. International Journal of Food Microbiology, 80(3), 187–199. https://doi.org/10.1016/S0168-1605(02)00141-1
- Mulaw, G., Sisay Tessema, T., Muleta, D., & Tesfaye, A. (2019). In vitro evaluation of probiotic properties of lactic acid bacteria isolated from some traditionally fermented Ethiopian food products. International Journal of Microbiology, 1–12.
- Mulaw, G., & Tesfaye, A. (2017). Technology and microbiology of traditionally fermented food and beverage products of Ethiopia: A review. African Journal of Microbiology Research, 11(21), 825–844.
- Negera, T. (2017). Isolation and characterization of ethanol, sugar and thermo tolerant yeast isolates in Ethiopia. International Journal of Research Studies in Biosciences, 5(8), 14–20.
- Nigatu, A., Ahrné, S., & Molin, G. (2000). Temperature-dependent variation in API 50 CH fermentation profiles of lactobacillus species. Current Microbiology, 41(1), 21–26. https://doi.org/10.1007/s002840010085
- Nigatu, A., Gashe, B. A., & Ayele, T. (1997). Bacillus spp from fermented tef dough and kocho: Identity and role in two Ethiopian fermented foods. SINET: Ethiopian Journal of Science, 20(1), 101–114.
- Nkhata, S. G., Ayua, E., Kamau, E. H., & Shingiro, J. (2018). Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Science & Nutrition, 6(8), 2446–2458. https://doi.org/10.1002/fsn3.846
- Ogunsakin, A. O., Vanajakshi, V., Anu-Appaiah, K. A., Vijayendra, S. V. N., Walde, S. G., Banwo, K., Sanni, A. I., & Prabhasankar, P. (2017). Evaluation of functionally important lactic acid bacteria and yeasts from Nigerian sorghum as starter cultures for gluten-free sourdough preparation. LWT-Food Science and Technology, 82, 326–334. https://doi.org/10.1016/j.lwt.2017.04.048
- Ozturk, G., & Young, G. M. (2017). Food evolution: The impact of society and science on the fermentation of cocoa beans. Comprehensive Reviews in Food Science and Food Safety, 16(3), 1–10. https://doi.org/10.1111/1541-4337.12264
- Pedersen, L. L., Owusu-Kwarteng, J., Thorsen, L., & Jespersen, L. (2012). Biodiversity and probiotic potential of yeasts isolated from Fura, a West African spontaneously fermented cereal. International Journal of Food Microbiology, 159(2), 144–151. https://doi.org/10.1016/j.ijfoodmicro.2012.08.016
- Perricone, M., Bevilacqua, A., Corbo, M. R., & Sinigaglia, M. (2014). Technological characterization and probiotic traits of yeasts isolated from Altamura sourdough to select promising microorganisms as functional starter cultures for cereal-based products. Food Microbiology, 38, 26–35. https://doi.org/10.1016/j.fm.2013.08.006
- Petkova, M., Stefanova, P., Gotcheva, V., & Angelov, A. (2021). Isolation and characterization of lactic acid bacteria and yeasts from typical Bulgarian sourdoughs. Microorganisms [Internet], 9(7), 1–18. https://doi.org/10.3390/microorganisms9071346
- Pundir, R. K., Rana, S., Kashyap, N., & Kaur, A. (2013). Probiotic potential of lactic acid bacteria isolated from food samples: an in vitro study. Journal of Applied Pharmaceutical Science, 3(3), 85–93.
- Reiner, K. (2010). Catalase test protocol. American Society for Microbiology, 1(1), 1–9.
- Reiner, K. (2012). Carbohydrate fermentation protocol. American Society of Microbiology, 3(5), 1–12.
- Roberts, D., & Greenwood, M. (2003). Practical Food Microbiology (3rd ed.). Blackwell Publishing Inc.
- Saeed, M., Anjum, F. M., Tahir, Z., & Haq, N. (2009). Isolation and characterization of starter culture from spontaneous fermentation of sourdough. International Journal of Agriculture & Biology, 11(3), 329–332.
- Saeed, R. M., Elyas, Y. Y. A., Yousif, N. M. E., Eltayeb, M. M., & Ahmed, I. A. M. (2014). Incidence of antibiotic resistance of lactic acid bacteria (LAB) isolated from various Sudanese fermented foods. Journal of Food & Nutritional Disorders, 6, 2.
- Şanlier, N., Gökcen, B. B., & Sezgin, A. C. (2019). Health benefits of fermented foods. Critical Reviews in Food Science and Nutrition, 59(3), 506–527. https://doi.org/10.1080/10408398.2017.1383355
- Scheirlinck, I., Van der Meulen, R., Van Schoor, A., Cleenwerck, I., Huys, G., Vandamme, P., & De Vuyst, L. (2007). Lactobacillus namurensis sp. nov., isolated from a traditional Belgian sourdough. International Journal of Systematic and Evolutionary Microbiology, 57(7), 1461–1467. https://doi.org/10.1099/ijs.0.64836-0
- Schillinger, U., & Lücke, F. K. (1987). Identification of lactobacilli from meat and meat products. Food Microbiology, 4(3). https://doi.org/10.1016/0740-0020(87)90002-5
- Sevgili, A., Erkmen, O., & Koçaslan, S. (2021). Identification of lactic acid bacteria and yeasts from traditional sourdoughs and sourdough production by enrichment. Czech Journal of Food Sciences, 39(4), 312–318. https://doi.org/10.17221/56/2021-CJFS
- Shona, Y. (2020). Isolation and characterization of yeast as potential probiotics from fermented cereals dough. Journal of Veterinary Medicine and Research, 7(6), 1–7.
- Sicard, D., & Legras, J. L. (2011). Bread, beer and wine: Yeast domestication in the Saccharomyces sensu stricto complex. Comptes Rendus - Biologies, 334(3), 229–236. https://doi.org/10.1016/j.crvi.2010.12.016
- Sieuwerts, S., de Bok, F. A., Hugenholtz, J., & van Hylckama Vlieg, J. E. (2008). Unraveling microbial interactions in food fermentations: From classical to genomics approaches. Applied & Environmental Microbiology, 74(16), 4997–5007. https://doi.org/10.1128/AEM.00113-08
- Simonson, L., Salovaara, H., & Korhola, M. (2003). Response of wheat sourdough parameters to temperature, NaCl and sucrose variations. Food Microbiology, 20(2), 193–199. https://doi.org/10.1016/S0740-0020(02)00117-X
- Stewart, C. S., Duncan, S. H., Richardson, A. J., Calder, A. G., & Dewey, P. J. S. (2000). The effect of the presence of glucose on the fermentation of mannose by the anaerobic fungus neocallimastix frontalis strain RE1. FEMS Microbiology Letters, 127(1–2), 57–63. https://doi.org/10.1111/j.1574-6968.1995.tb07450.x
- Sulmiyati, S., Said, N. S., Fahrodi, D. U., Malaka, R., & Maruddin, F. (2019). The characteristics yeast isolated from commercial kefir grain, Indonesia. Hasanuddin Journal of Animal Science (HAJAS), 1(1), 26–36. https://doi.org/10.20956/hajas.v1i1.6519
- Sultana, M. J., Refaya, R., Shajidur Md, R., & Begum, K. (2017). Isolation and biochemical characterization of lactobacillus species from yogurt and cheese samples in Dhaka Metropolitan Area. Bangladesh Pharmaceutical Journal, 20(1), 27–33. https://doi.org/10.3329/bpj.v20i1.32090
- Syal, P., & Vohra, A. (2013). Probiotic potential of yeasts isolated from traditional Indian fermented foods. International Journal of Microbiology Research, 5(2), 390–398. https://doi.org/10.9735/0975-5276.5.2.390-398
- Tadesse, T., Yimer, D., Tibebu, T., Workie, M., Kebede, B., Abera, S., Tilahun, A., Alemu, M., Daba, T., Eshetu, A., Alemneh, A., Babiye, B., Dida, G., & Abena, T. (2021). Evaluating the probiotic potential of yeasts isolated from Ethiopian traditionally fermented foods and dairy products. 1(1), 1–10. https://doi.org/10.53430/ijsru.2021.1.1.0011
- Tafere, G. (2015). A review on traditional fermented beverages of Ethiopian. Journal of Natural Sciences Research, 5(15), 94–102.
- Tafesee, T., Beyene, F., & Amenu, D. (2020). Evaluation of Lactic Acid Bacteria Isolated from Ethiopian traditional fermented food (“Borde”) and beverage (“Ititu”) as a Starter Culture for Ayib Production. International Journal of Advanced Research in Biological Sciences, 7(9), 42–52.
- Tamang, J. P., Dewan, S., Tamang, B., Rai, A., Schillinger, U., & Holzapfel, W. H. (2007). Lactic acid bacteria in Hamei and Marcha of North east India. Indian Journal of Microbiology, 47(2), 119–125. https://doi.org/10.1007/s12088-007-0024-8
- Tamene, A., Baye, K., Kariluoto, S., Edelmann, M., Bationo, F., Leconte, N., & Humblot, C. (2019). Lactobacillus plantarum P2R3FA isolated from traditional cereal-based fermented food increase folate status in deficient rats. Nutrients, 11(11), 2819. https://doi.org/10.3390/nu11112819
- Tamene, A., Kariluoto, S., Baye, K., & Humblot, C. (2019). Quantification of folate in the main steps of traditional processing of tef injera, a cereal based fermented staple food. Journal of Cereal Science, 87, 225–230. https://doi.org/10.1016/j.jcs.2019.04.005
- Thapa, N., Pal, J., & Tamang, J. P. (2004). Microbial diversity in ngari, hentak and tungtap, fermented fish products of North-east India. World Journal of Microbiology and Biotechnology, 20(6), 599–607. https://doi.org/10.1023/B:WIBI.0000043171.91027.7e
- Thiele, C., Gänzle, M. G., & Vogel, R. F. (2002). Contribution of sourdough lactobacilli, yeast, and cereal enzymes to the generation of amino acids in dough relevant for bread flavor. Cereal Chemistry, 79(1), 45–51. https://doi.org/10.1094/CCHEM.2002.79.1.45
- Tikka, C., Osuru, H. P., Atluri, N., Raghavulu, P. C. V., Yellapu, N. K., Mannur, I. S., Prasad, U. V., Aluru, S., K, N. V., & Bhaskar, M. (2013). Isolation and characterization of ethanol tolerant yeast strains. Bioinformation, 9(8), 421–425. https://doi.org/10.6026/97320630009421
- Tilahun, B., Bahiru, A., Tesfaye, A., Diriba, M., Zewdu, T., & Wessel, G. (2019). Molecular characterization of fermenting yeast species from fermented teff dough during preparation of injera using ITS DNA sequence. International Journal of Food Science, 201, 1–7. https://doi.org/10.1155/2019/1291863
- Tilahun, B., Tesfaye, A., Muleta, D., Bahiru, A., Terefework, Z., & Wessel, G. (2018). Isolation and Molecular identification of lactic acid bacteria using 16s rRNA genes from fermented teff (Eragrostis tef (Zucc.)) dough. International Journal of Food Science, 2018, 1–7. https://doi.org/10.1155/2018/8510620
- Tsegaye, Z., Tefera, G., Gizaw, B., & Abatenh, E. (2018). Characterization of yeast species isolated from local fruits used for bakery industrial application. Journal of Applied Microbiological Research, 1(1), 21–26.
- Urga, K., Fite, A., & Biratu, E. (2000). Effect of natural fermentation on nutritional and antinutritional factors of tef (Eragrostis tef). Ethiopian Journal of Health Development, 11(1), 1–7.
- Valmorri, S., Tofalo, R., Settanni, L., Corsetti, A., & Suzzi, G. (2010). Yeast microbiota associated with spontaneous sourdough fermentations in the production of traditional wheat sourdough breads of the Abruzzo region (Italy). Antonie Van Leeuwenhoek, 97(2), 119–129. https://doi.org/10.1007/s10482-009-9392-x
- Vrancken, G., De Vuyst, L., Meulen, R., Huys, G., Vandamme, P., & Daniel, H.-M. (2010). Yeast species composition differs between artisan bakery and spontaneous laboratory sourdough. FEMS Yeast Research, 10(4), 471–481. https://doi.org/10.1111/j.1567-1364.2010.00621.x
- Wafula, E. N., Kuja, J. O., Wekesa, T. B., & Wanjala, P. M. (2023). Isolation and identification of autochthonous lactic acid bacteria from commonly consumed African indigenous leafy vegetables in Kenya. Bacteria, 2(1), 1–20. https://doi.org/10.3390/bacteria2010001
- Webster, A., Scherer, S., Fabri, K., Doudican, H., & Felder, M. (2019). Saccharomyces cerevisiae ferments monosaccharides faster than disaccharides. Journal of Undergraduate Biology Laboratory Investigations, 2(1), 1–4.
- Wedajo Lemi, B. (2020). Microbiology of Ethiopian traditionally fermented beverages and condiments. International Journal of Microbiology, 2020, 1–8. https://doi.org/10.1155/2020/1478536
- Wouters, J. T. M., Ayad, E. H. E., Hugenholtz, J., & Smit, G. (2002). Microbes from raw milk for fermented dairy products. International Dairy Journal, 12(2–3), 91–109. https://doi.org/10.1016/S0958-6946(01)00151-0
- Wu, R. N., Wu, Z. X., Zhao, C. Y., Lv, C. M., Wu, J. R., & Meng, X. J. (2014). Identification of lactic acid bacteria in suancai, a traditional Northeastern Chinese fermented food, and salt response of Lactobacillus paracasei LN-1. Annals of Microbiology, 64(3), 1325–1332. https://doi.org/10.1007/s13213-013-0776-9
- Xiang, H., Sun-Waterhouse, D., Waterhouse, G. I. N., Cui, C., & Ruan, Z. (2019). Fermentation-enabled wellness foods: A fresh perspective. Food Science and Human Wellness, 8(3), 203–243. https://doi.org/10.1016/j.fshw.2019.08.003
- Yalcin, S. K., & Ozbas, Z. Y. (2008). Effects of pH and temperature on growth and glycerol production kinetics of two indigenous wine strains of Saccharomyces cerevisiae from Turkey. Brazilian Journal of Microbiology, 39(2), 325–332. https://doi.org/10.1590/S1517-83822008000200024
- Yang, Q., Rutherfurd-Markwick, K., & Mutukumira, A. N. (2021). Identification of dominant lactic acid bacteria and yeast in rice sourdough produced in New Zealand. Current Research in Food Science, 4, 729–736. https://doi.org/10.1016/j.crfs.2021.10.002
- Zapaśnik, A., Sokołowska, B., & Bryła, M. (2022). Role of lactic acid bacteria in food preservation and safety. Foods, 11(9), 1283. https://doi.org/10.3390/foods11091283
- Zhao, D. (2011). Isolation of antifungal lactic acid bacteria from food sources and their use to inhibit mold growth in cheese.
- Zheng, J., Wittouck, S., Salvetti, E., Franz, C. M. A. P., Harris, H. M. B., Mattarelli, P., O’toole, P. W., Pot, B., Vandamme, P., Walter, J., Watanabe, K., Wuyts, S., Felis, G. E., Gänzle, M. G., & Lebeer, S. (2020). A taxonomic note on the genus lactobacillus: Description of 23 novel genera, emended description of the genus lactobacillus beijerinck 1901, and union of lactobacillaceae and leuconostocaceae. International Journal of Systematic and Evolutionary Microbiology, 70(4), 2782–2858. https://doi.org/10.1099/ijsem.0.004107
Appendices
Table A1. Physiological characterization of the identified LAB.
Table A2. The biochemical characterization test result of the identified yeast species.
Appendix B
Table B1. The biochemical characterization test result of LAB.
