?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
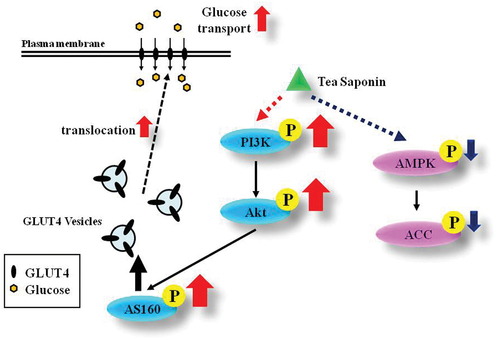
Targeting disorders of glucose metabolism and the occurrence of hyperglycemia provides a potential therapeutic strategy for balancing energy to reduce insulin resistance such as type 2 diabetes. The present study investigated whether theasaponin E1 regulate energy metabolism in skeletal muscle cells. C2C12 mouse myoblasts were differentiated into myotubes . An MTT colorimetric assay verified cell viability. Theasaponin E1 (30, 45 or 60 mg/ ml), or metformin (2.5 mM), insulin (150 nM) for reference were used to treat the C2C12 myotubes. The expressions of energy metabolism were measured by western blotting in C2C12 myotubes. 2-NBDG kit to detect the glucose uptake capacity. Theasaponin E1 Treatment increases glucose uptake,GSK 3 β, as well as increases to the glycogen synthesis through the PI3K/AKT/mTOR signaling pathway, while reducing AMPK and ACC activities, and reducing the adipose synthesis pathway. These results suggest that theasaponin E1 may be increases glucose uptake by improving muscle energy metabolism.
Introduction
Type II diabetes is a chronic disease caused by the body’s own metabolic disorders (Amanat et al., Citation2020), and the incidence of diabetes is increasing significantly worldwide, and the incidence is increasingly tilted towards young people (Chiang et al., Citation2007). Type II diabetes is mainly caused by the body’s intolerance to glucose (Wang et al., Citation2013), resulting sin increased blood sugar, making it difficult to absorb blood sugar (Tulloch & Macintosh, Citation1961), aggravating the metabolic burden on the kidneys (Gu et al., Citation2019), rapidly losing weight (Buriak & Shaban, Citation2007), vision loss and even blindness and a series of complications (Loriz et al., Citation2007). These complications cause great damage to the patient’s mind and body, hence the name of immortal cancer.
Although there have been some reports, diabetes can significantly improve the quality of life of patients with non-pharmacological treatments, such as proper exercise and a moderate diet (Zurlo et al., Citation2019). However, people can’t resist the temptation of food, the patient’s body inertia is not willing to exercise and exercise, It is difficult to improve and cure diseases with the long-term lifestyle. Diabetes has become one of the most threatening metabolic diseases since the 21st century (Borys et al., Citation2018). The study of diabetes in countries around the world has never stopped.
In recent years, treatment options related to Chinese herbal medicine have attracted researchers from all over the world. Food therapy, in particular, has attracted much attention. Plants contain some alkaloids, polyphenols, flavonoids, such as epicatechins (Mi et al., Citation2017), ellagic acid (Liu et al., Citation2021), tea polyphenols (Khan & Mukhtar, Citation2018), capsaicin (Hui et al., Citation2019), etc. can promote the absorption of glucose in the blood and reduce the blood sugar level of the body. This article selects a plant drink that has accompanied China for thousands of years (Wierzejska, Citation2014), namely tea (Snoussi et al., Citation2014), which people have always used to soak in hot water for a period of time and then drink (Chen et al., Citation2018). Studies have found that tea has the effect of reducing fat in mice (Chen et al., Citation2019) and lowering blood sugar (Bulmer et al., Citation2018), but the specific composition is not clear. We chose the tea extract, which is tea saponin (Stavrianidi et al., Citation2018). In contrast, there is little research in the medical direction of cancer and metabolism (Kumar et al., Citation2017), so we chose to explore the theasaponin E1, which has great potential in the treatment of type 2 diabetes (Jia et al., Citation2017).
Therefore, to further evaluate how theasaponin E1 may be beneficial to muscle energy metabolism the present study investigated the regulation of glucose uptake in C2C12 muscle cells via activation of the PI3K/Akt/mTOR signaling pathways theasaponin E1 treatment.
Materials and methods
Chemicals and reagents
theasaponin E1 with >98% purity was purchased from Extrasynthese (Phytopurify, China). C2C12 myoblasts were obtained from Shanghai Institute of Life Sciences, (Shanghai, China). Dimethyl sulfoxide (DMSO), SDS (Solarbio, China), RIPA (Solarbio, China), PMSF (Solarbio, China), Fetal Bovine Serum (BI, U.S.A.), heat-killed horse serum (DES, Invitrogen), BCA/Bradford Protein Assay Kit (Thermo Fisher). SDS-PAGE (Solarbio, China), DMEM (HyClone, 25 mM glucose), 2-NDBG (Thermo Fisher), Anti-AKT (CST), antiphospho-Akt (Ser473) (CST), anti-GLUT4(CST), anti-AMPK(CST), antiphospho-AMPK-T172(CST), anti-mTOR(CST), antiphospho-MTOR(CST), anti-PI3K(CST), antiphospho-PI3K(P85β) (CST), anti-ACC (CST), antiphospho-ACC(S79) (CST), anti-AS160 (CST), antiphospho-AS160(S318) (CST), anti-ACTIN (CST), antimouse and antirabbit IgG antibodies were also from Cell Signaling Technology.
Cell culture
The mouse skeletal muscle C2C12 cells were cultured, and C2C12 cells were seeded at a density of 100 mm plate. They were seeded into 100 mm plate or 6-well plates and treated for subsequent experiments. The proliferation medium DMEM was added to 10% (v/v) FBS, streptomycin (100 U/ml) and penicillin (100 U/ml) under conventional culture conditions: 5% (v/v) CO2 and 37°C incubator. When the cell density increases to 70%~80%, turning to cell differentiation: cell culture medium (DMEM) contains 2% (v/v) DES and same antibiotics, the cell growth reached 80–90%, replacing horse serum medium, Fresh medium was replaced every 2 days. Contractile myotube can be formed 7 days after cell differentiation. The differentiated cells were cultured in serum-free DMEM for 4 h and used for the following experiment.
Cell viability analysis
The cytotoxicity of theasaponin E1 was assessed by MTT. Briefly, undifferentiated C2C12 myotubes were incubated with 100 μL samples in serum-free DMEM at various concentrations (0,8,16,32,64,128,150 and 200 μg/mL). After 24 and 48 hours of incubation, MTT solution (16 μL, 3 mg/mL) was added and incubated for 4 hours at 37°C. Then, the MTT solution was discarded and 160 μL of DMSO was added, and the optical density per well was measured by Multiskan Spectrum (DNM-9602, Perlong Scientific, China) at 492 nm.
Western blot analysis
The cultured cells were in RIPA lysis buffer (50mMTris (pH 7.4), 150 mMNaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) containing a protease inhibitor cocktail (PMSF). Protein concentration was determined using a BCA/Bradford Protein Assay Kit (Thermo Fisher). Protein samples were separated on a 10% SDS-PAGE gel using standard procedures.
Real-time quantitative RT-PCR
RNA isolation, reverse transcription, and quantitative PCR Total RNA from C2C12 myotubes was extracted using TRIzol Reagent (Life Technologies, Waltham, MA, U.S.A.). Isolated RNA was reverse-transcribed into cDNA with a PrimeScript RT Reagent Kit (TaKaRa, Otsu, Shiga, Japan). Target mRNA quantification was measured by real-time PCR using SYBR Premix Ex TaqTM(TaKaRa, Otsu, Shiga, Japan) and a ABI 7900 detection PCR system (Roche, Foster City, CA, U.S.A.). The ACTIN as reference housekeeping gene, Quantification was performed using the 2(−ΔΔCt) method. The primer sequences are shown in .
Table 1. RT-PCR primers used in this study.
Glucose uptake analysis
The cells were inoculated in a sterile 96-well plate, 8 × 103 wells per hole, cultured for 12 hours, washed twice with PBS, and cultured in horse serum medium for 7 days. Differentiated C2C12 cells were starved in serum-free DMEM for 3 hours and washed twice with PBS. Different concentrations of theasaponin E1 (0, 30 μg/L, 45 μg/L, 60 μg/L) and fluorescent incubation glucose were used to simulate the low glucose medium of 2-NBDG. A positive control was performed with insulin (150 nm/L) and 2-NDBG (final concentration 200 μM/L). After treatment, the cells were washed with PBS for 3 times to remove the excess 2-NBDG, and 200 μL DMSO solution was added to each hole. Fluorescence intensity of cells was measured with fluorescence microplate reader at 485 nm excitation wavelength and 538 nm emission wavelength. The control glucose uptake was 100% 2-NBDG uptake in the group without insulin treatment. The uptake ratio of 2-NBDG was calculated. The following equation:
Statistical analysis
Analysis of differences between the two groups using SPSS 17.0(* p < .05, ** p < .01 and *** p < .005). Data are expressed as mean ±SEM. GraphPad Prism 5.0 software is used for all statistical analyses.
Results
Potential of theasaponin E1 to promote proliferation of C2C12 skeletal muscle cells
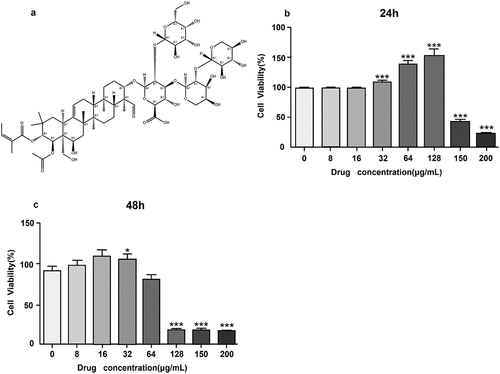
Theasaponin E1 has strong antioxidant capabilities and comprises a range of phenolic hydroxyl groups (). An established and widely used MTT test was utilized to determine the proliferative capacity of theasaponin E1 on the skeletal muscle cells of C2C12 mice. At 24 and 48 hours, theasaponin E1 concentrations were treated (). The findings demonstrated that short-term, low-dose treatment of theasaponin E1 did not appear to have any cytotoxic effects on the skeletal muscle cells of C2C12 mice and may perhaps have encouraged their proliferation.
Figure 1. Effect of theasaponin E1 on cell viability. (a) Molecular structure of theasaponin E1 (b)(c) theasaponin E1 is cytotoxic to C2C12 myotubes. Incubate cells with theasaponin E1 24, 48 hours, specified concentrations, 0, 8 μg/L, 16 μg/L, L, 32 μg/L, 64 μg/L, 128 μg/L, 150 μg/L; 200 μg/L. All data were expressed as mean ± S.D. *P < .05, **P < .01 represented statistical differences between the groups. All measurements are made in triplicate (n = 3).
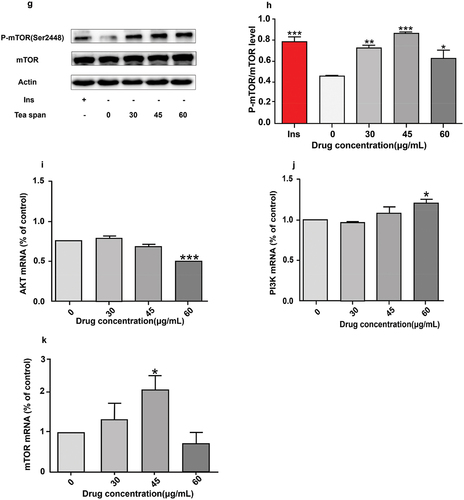
Theasaponin E1 promotes phosphorylation of PI3K/AKT/mTOR in C2C12 myoblasts
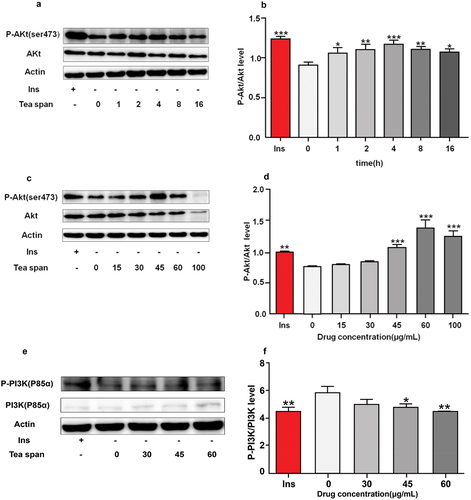
Protein kinase B is Akt, also known as PKB or Rac, which is one of the key proteins in maintaining the physiological activity of cells (Sun et al., Citation2020), It plays an important physiological function in cell proliferation, differentiation, apoptosis etc (Isobe et al., Citation2018). Activation of the PI3K/Akt/mTOR signaling pathway is known to play an important role in many cell survival and growth conditions (Zhong et al., Citation2019). Insulin promotes the uptake of blood glucose by cells through the influence of PI3K/AKT/mTOR signaling pathway, which is also one of the key mechanisms to promote blood glucose absorption (Shahcheraghi et al., Citation2020). We treated C2C12 myoblasts with insulin as positive control at different times () and at different concentrations () to investigate the phosphorylation level of AKT in C2C12 myoblasts after theasaponin E1 treatment and the effect of theasaponin E1 on PI3K/AKT/mTOR signaling pathway. The results showed that theasaponin E1 treated C2C12 myoblasts promoted the phosphorylation of AKT in C2C12 myoblasts, and the phosphorylation of AKT in C2C12 myoblasts was time-dependent. p-PI3K phosphorylation levels were significantly reduced in the drug treatment group (). The phosphorylation level of mTOR was significantly increased in the drug treatment group (). PI3K mRNA, AKtmRNA and mTOR mRNA were detected under the same treatment conditions, and PI3K mRNA and mTOR mRNA were significantly increased (). The results showed that tea saponin E1 could activate PI3K/Akt/mTOR signaling pathway.
Figure 2. Effects of saponin E1 on PI3K/AKT/mTOR signaling pathway. (a) Saposide E1 treated C2C12 cells for 1 h, 2 h, 4 h, 8 h, 16 h. (c) 1 μM/L insulin was used as positive control, and the cells were treated with 0, 15, 30, 45, 60, 100 μg/mL for 4 h, respectively. (b) (d) Quantitative determination of Akt phosphorylation levels in at least three independent experiments. (e). (g) C2C12 cells were treated with theasaponin E1 (30, 45, 60 μg/mL) for 4 H, and western blot clipping was displayed. (f) (h) quantitative western blot analysis was performed. The map also measured total PI3K/mTOR and beta-actin levels. Comparison of PI3K/mTOR mRNA expression levels between (i), (j) (k) and untreated cells All data were expressed as mean ± sd, *P < .05, **P < .01 was statistically significant difference between groups. All measurements are in triplicate (n = 3).
Theasaponin E1 promotes glucose uptake in C2C12 myoblasts
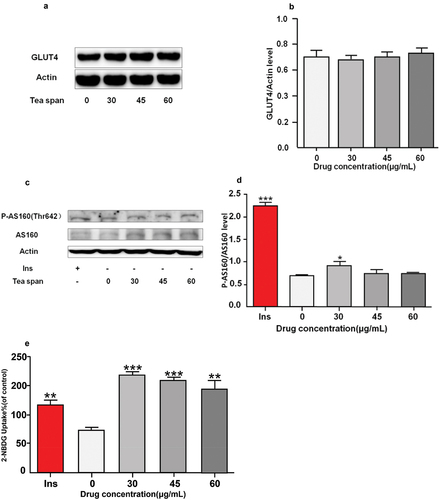
GLUT4 is a major transporter in muscle and directly affects glucose uptake (Tsuji et al., Citation2019). In order to investigate the effect of theasaponin E1 on the expression of GLUT4 protein in C2C12 cells, Western blot was used to detect the expression level of GLUT4. AS160 is an important protein regulating glucose metabolism, which can improve glucose transport efficiency and promote glucose absorption. The glucose uptake level of C2C12 myoblasts was also measured by fluorescence glucose analogue 2-NBDG (Sun et al., Citation2021). The results showed that compared with blank cells, the expression level of GLUT4 protein was not significantly increased (). AS160 (Thr642) has elevated phosphorylation levels (). The highest fluorescence level and glucose uptake capacity were found at 45 μg/mL ().
Figure 3. Effects of saponin E1 on glucose uptake in C2C12 cells. (a) (c) C2C12 cells were treated with (30, 45, 60 μg/mL) theasaponin E1 for 4 h and insulin 1 μM/L as positive control. (b) (d) Quantitative analysis of western blotting. The map also measures total AS160 and beta-actin levels as controls for protein load. (e) Incubate with 2-NBDG or 2-NDBG and different concentrations of saponin E1 for 4 min. All data were expressed as mean ± sd *P < .05, **P < .01 was statistically significant difference between groups. All measurements are in triplicate (n = 3).
Theasaponin E1 inhibits phosphorylation of AMPK/ACC in C2C12 myoblasts
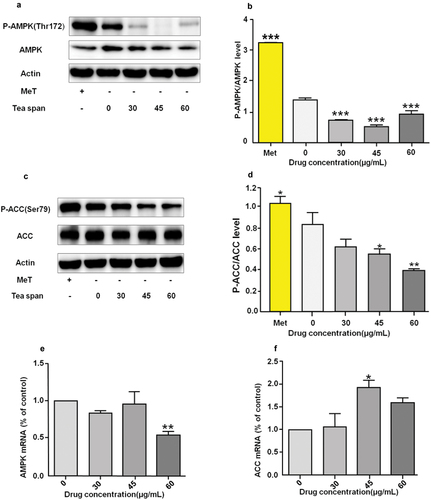
AMPK is an important key enzyme in cell energy metabolism (Trefts & Shaw, Citation2021). We used metformin as a positive control and treated with theasaponin E1 for immunoblotting (). ACC is a key kinase for fatty acid and cholesterol synthesis. We used metformin as a positive control. For different concentrations of theasaponin E1 treatment (), AMPK, ACC expression level mRNA () showed that the AMPK phosphorylation level was significantly reduced in the drug-administered group compared with the blank control group. The phosphorylation level of ACC was significantly decreased, and the expression level of AMPK and ACC was increased.
Figure 4. Effect of theasaponin E1 on AMPK/ACC. (a) and (c) treatment of C2C12 cells with different theasaponin E1 concentrations for a specified period of time, metformin 2.5 mM as a positive control, showing a cropped image of AMPK/ACC Western blot, (b) and (d) P-ACC/ACC immunoblotting quantitative maps were analyzed, while the level of β-Actin was also measured as a control for protein loading. (e) and (f) AMPK, ACC expression levels of mRNA compared to untreated cells, All data were expressed as mean ±S.D. *P < .05, **P < .01 represented statistical differences between the groups. All measurements are made in triplicate (n = 3).
Theasaponin E1 promotes phosphorylation of GSK.P70S6K in C2C12 myoblasts
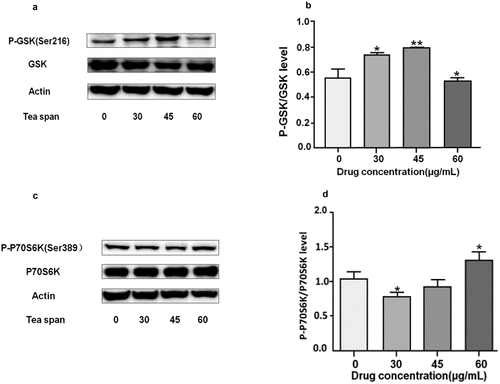
GSK-3β is also known as glycogen synthesis kinase (An et al., Citation2019). We foundP70S6K as a cell growth-related kinase by treatment with theasaponin E1 (Chindemi et al., Citation2019; Gong et al., Citation2019). After treatment with theasaponin E1 (), the results showed that the phosphorylation level of GSK-3β was increased, and there was no significant difference in the phosphorylation level of P70S6K.
Figure 5. Effect of theasaponin E1 on GSK and P-70S6K. (a) and (c) treatment of C2C12 cells with different theasaponin E1 concentrations for a specified time, insulin 1 μM/L as a positive control, showing a cut-off image of GSK, P70S6K Western blot, (b) and (d) GSK, P70S6K immunization Blot analysis quantified the map while also measuring the level of β-Actin as a control for protein loading. All data were expressed as mean ± S.D. *P < .05, **P < .01 represented statistical differences between the groups.All measurements are made in triplicate (n = 3).
Discussion
The discovery of insulin has brought new light to diabetic patients, but insulin receptors tend to reduce insulin sensitivity when stimulated by large amounts of insulin, and increasing insulin dosage will not improve the condition (Yapanis et al., Citation2022), but affects other organs of the body (Crasto et al., Citation2021). It is easy to form organ complications. In order to reduce the symptoms of insulin resistance (Lee et al., Citation2022), scientists have carried out scientific research on metabolic pathways (Freeman & Pennings, Citation2023), insulin structure (Gorai & Vashisth, Citation2022), and successively invented several major compounds such as biguanides (Lee et al., Citation2022), sulfonylureasClass (Zheng et al., Citation2018), thiazolidinediones, α-glycosidase inhibitors (Tong et al., Citation2015), glinides (Taslimi et al., Citation2018). They can be used for different patients with different conditions, and increase the patient’s healing ability, thereby reducing the suffering of the patient.
Research on diabetes in countries around the world has never stopped. The Some researchers tend to rely on the insulin signaling pathway, which affects the downstream phosphatidylinositol kinase PI3K-protein kinase by binding to the insulin receptor IR on the cell surface and the insulin receptor substrate IRS-1 (Li et al., Citation2019). The AKT/mTOR signaling pathway (Liu et al., Citation2018) and ras-mapk (Gao et al., Citation2016) produce physiologically active signaling pathways. The serine/threonine kinase Akt has recently been shown to regulate a variety of cellular functions and play important roles in proliferation (Meng et al., Citation2021), survival (Yu et al., Citation2022), transcription (Thiel et al., Citation2021) and protein synthesis (Wang et al., Citation2018). Murine skeletal muscle C2C12 cells were differentiated into C2C12 myoblasts (Mielcarek et al., Citation2021), and the results showed that 45 μg/mL AKT (Ser473) phosphorylation started to increase significantly. It is well known that activation of the PI3K/Akt/mTOR signaling pathway plays an important role in many cell survival and growth conditions (Li et al., Citation2020). Recent studies have shown that AKT can promote the body’s absorption of glucose through the AKT/mTOR signaling pathway (Hirai et al., Citation2019), reduce blood glucose levels in animals, and alleviate diabetes (Adachi et al., Citation2019). To explore the underlying mechanism of theasaponin E1 metabolism in mouse skeletal muscle C2C12 cells, C2C12 myoblasts were treated with theasaponin E1, and the phosphorylation level of AKT (Ser473) in C2C12 myoblasts was time-dependent and able to Activates the PI3K/AKT/mTOR signaling pathway. GLUT4 is normally located in certain regions of the cytoplasm and appears free in the absence of insulin stimulation. In the activated state of insulin signaling, GLUT4 is regulated and translocated to the cell membrane, where it binds to extracellular glucose and is dragged into the cell membrane (James-Allan et al., Citation2019). This reduces the blood sugar levels of the body while storing and providing energy for the body. AS160 has been shown to activate GLUT4 and translocate GLUT4 to the cell membrane for glucose uptake (Capmany et al., Citation2019; Flores-Opazo et al., Citation2019). This can lower blood sugar levels for the purpose of treating diabetes (Sharma & Dey, Citation2021). When we treated C2C12 myoblasts with theasaponin E1, the expression level of GLUT4 protein was not significantly increased compared with blank cells. AS160 (Thr642) increases phosphorylation levels. The results showed that theasaponin E1 can increase the phosphorylation level of AS160, activate the translocation of GLUT4 to the cell membrane and promote the uptake of extracellular glucose.
As an alkaloid in tea seeds, theasaponin E1 has been reported in previous literature to reduce the weight of mice, but the specific mechanism is not clear. We studied both glucose metabolism and lipid metabolism (Caccese et al., Citation2021; Witt et al., Citation1991). It was found that theasaponin E1 can activate the PI3K/Akt/mTOR signaling pathway to promote AS160 phosphorylation and improve the absorption capacity of cells to extracellular glucose. We found in the energy metabolism pathway that theasaponin E1 can inhibit phosphorylation levels of AMPK (Caccese et al., Citation2021). This finding is likely to be a new AMPK inhibitor. Total ACC levels are also shown to be decreasing, a finding that suggests that theasaponin E1 may be able to promote the breakdown of fatty acids and cholesterol. In turn, it reduces fatty acid synthesis and promotes the decomposition of fatty acids to reduce the fat content in the body. This process is also of research significance. Excessive absorption of glucose reduces The phosphorylation level of in GSK-3β is increased, which reduces the inhibition of glycogen synthesis by GSK-3β (Chauhan et al., Citation2018), It is indicated that theasaponin E1 can promote the synthesis of glycogen and the uptake of sugar into glucose, and store energy for the body.
Conclusion
It is certain that theasaponin E1 can promote glucose uptake by C2C12 cells (). As well as promoting the synthesis of glycogen and fatty acids, it provides a new reference for the treatment of type 2 diabetes.
Author contributions
Ming Zhang, Zhiyun Chen, Di Tian protein assay, qPCR, Western blot and data analysis. ShaningWang, Yujie Huo, Juan Lu helped with protein experiments and some data analysis. Zaiqiao Li, Ling Song helped with qPCR. Xu Ji, Xiao Ma, Mingying Gu and Jun Sheng designed the study. MZ, XJ, XM, wrote this manuscript. All authors read and approved the final manuscript.
Acknowledgments
Thanks to Yunnan Agricultural University, the Key Laboratory of Pu’er Tea Ministry of Education and the Yunnan Plateau Characteristic Agricultural Industry Research Institute for funding and experimental guidance. We express our gratitude to Dr. Xuanjun Wang, Dr. Chongye Fang, and Dr. Yewei Huang for their helpful suggestions.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Data availability statement
Data available on request. The data underlying this article will be shared on reasonable request to the corresponding author.
Additional information
Funding
References
- Adachi, N., Kanazawa, I., Tanaka, K. I., Takeno, A., Notsu, M., Tanaka, S., & Sugimoto, T. (2019). Insulin-like growth factor-I protects against the detrimental effects of advanced glycation end products and high glucose in myoblastic C2C12 cells. Calcified Tissue International, 105(1), 89–96. https://doi.org/10.1007/s00223-019-00537-w
- Amanat, S., Ghahri, S., Dianatinasab, A., Fararouei, M., & Dianatinasab, M. (2020). Exercise and type 2 diabetes. In J. Xiao (Ed.), Advances in experimental medicine and biology (Vol. 1228, pp. 91–105). Springer, Singapore. https://doi.org/10.1007/978-981-15-1792-1_6
- An, Y., Zou, Y., Cao, Y., Yao, M., Ma, N., Wu, Y., Yang, J., Liu, H., & Zhang, B. (2019). The nuclear GSK-3β regulated post-transcriptional processing of mRNA through phosphorylation of SC35. Molecular and Cellular Biochemistry, 451(1–2), 55–67. https://doi.org/10.1007/s11010-018-3393-x
- Borys, S., Hohendorff, J., Koblik, T., Witek, P., Ludwig-Slomczynska, A. H., Frankfurter, C., Kiec-Wilk, B., & Malecki, M. T. (2018). Negative-pressure wound therapy for management of chronic neuropathic noninfected diabetic foot ulcerations – short-term efficacy and long-term outcomes. Endocrine, 62(3), 611–616. https://doi.org/10.1007/s12020-018-1707-0
- Bulmer, J. M., McBain, T. R., & Peart, D. J. (2018). High-intensity interval walking in combination with acute green tea extract supplementation reduces postprandial blood glucose concentrations in physically inactive participants. Nutrition and Health, 24(3), 193–198. https://doi.org/10.1177/0260106018793049
- Buriak, V. N., & Shaban, N. I. (2007). Clinical and pathogenetic mechanisms of the formation of complicated course of I type diabetes mellitus in children. Likars’ka sprava / Ministerstvo okhorony zdorov’ia Ukrainy, 3, 24–29.
- Caccese, J. B., Buckley, T. A., Tierney, R. T., Rose, W. C., Glutting, J. J., & Kaminski, T. W. (2021). Postural control deficits after repetitive soccer heading. Clinical Journal of Sport Medicine: Official Journal of the Canadian Academy of Sport Medicine, 31(3), 266–272. https://doi.org/10.1097/JSM.0000000000000709
- Capmany, A., Gambarte, T. J., Alonso, B. M., & Damiani, M. T. (2019). Akt/AS160 signaling pathway inhibition impairs infection by decreasing Rab14-controlled sphingolipids delivery to chlamydial inclusions. Frontiers in Microbiology, 10, 666. https://doi.org/10.3389/fmicb.2019.00666
- Chauhan, P., Tamrakar, A. K., Mahajan, S., & Prasad, G. (2018). Chitosan encapsulated nanocurcumin induces GLUT-4 translocation and exhibits enhanced anti-hyperglycemic function. Life Sciences, 213, 226–235. https://doi.org/10.1016/j.lfs.2018.10.027
- Chen, Y., Zhou, Y., Zeng, L., Dong, F., Tu, Y., & Yang, Z. (2018). Occurrence of functional molecules in the flowers of tea (Camellia sinensis) plants: Evidence for a second resource. Molecules, 24(1), 23. https://doi.org/10.3390/molecules24010023
- Chen, Q., Zhu, Y., Dai, W., Lv, H., Mu, B., Li, P., Tan, J., Ni, D., & Lin, Z. (2019). Aroma formation and dynamic changes during white tea processing. Food Chemistry, 274, 915–924. https://doi.org/10.1016/j.foodchem.2018.09.072
- Chiang, C., Ho, T., Peng, Y., Hsu, S.-P., Pai, M.-F., Yang, S.-Y., Hung, K.-Y., & Wu, K.-D. (2007). Rosiglitazone in diabetes control in hemodialysis patients with and without viral hepatitis infection. Diabetes Care, 30(1), 3–7. https://doi.org/10.2337/dc06-0956
- Chindemi, C., Cirielli, V., Cima, L., Danzi, O., Raniero, D., Tagliaro, F., Turrina, S., Eccher, A., Ghimenton, C., Bortolotti, F., Brunelli, M., & De Leo, D. (2019). Autophagy pathways in drug abusers after forensic autopsy: LC3B, ph-mTOR and p70S6K analysis. Medicine, Science, and the Law, 59(1), 49–56. https://doi.org/10.1177/0025802419828910
- Crasto, W., Patel, V., Davies, M. J., & Khunti, K. (2021). Prevention of microvascular complications of diabetes. Endocrinology and Metabolism Clinics of North America, 50(3), 431–455. https://doi.org/10.1016/j.ecl.2021.05.005
- Flores-Opazo, M., Raajendiran, A., Watt, M. J., & Hargreaves, M. (2019). Exercise serum increases GLUT4 in human adipocytes. Experimental Physiology, 104(5), 630–634. https://doi.org/10.1113/EP087495
- Freeman, A. M., Acevedo, L. A., & Pennings, N. (2023). Insulin resistance. In StatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK507839/
- Gao, J., Bai, T., Ren, L., Ding, Y., Zhong, X., Wang, H., Guo, Y., Li, J., Liu, Y., & Zhang, Y. (2016). The PLC/PKC/Ras/MEK/Kv channel pathway is involved in uncarboxylated osteocalcin-regulated insulin secretion in rats. Peptides, 86, 72–79. https://doi.org/10.1016/j.peptides.2016.10.004
- Gong, J., Zhou, S., & Yang, S. (2019). Vanillic acid suppresses HIF-1α expression via inhibition of mTor/p70s6k/4E-BP1 and Raf/MEK/ERK pathways in human colon cancer HCT116 cells. International Journal of Molecular Sciences, 20(3), 20. https://doi.org/10.3390/ijms20030465
- Gorai, B., & Vashisth, H. (2022). Progress in simulation studies of insulin structure and function. Frontiers in Endocrinology, 13, 908724. https://doi.org/10.3389/fendo.2022.908724
- Gu, Y., Gong, C. X., & Liang, X. J. (2019). A case of special type diabetes mellitus in infants with acute ischemic stroke. Zhonghua Er Ke Za Zhi, 57(11), 886–888. https://doi.org/10.3760/cma.j.issn.0578-1310.2019.11.015
- Hirai, T., Nomura, K., Ikai, R., Nakashima, K. I., & Inoue, M. (2019). Baicalein stimulates fibroblast growth factor 21 expression by up-regulating retinoic acid receptor-related orphan receptor alpha in C2C12 myotubes. Biomedicine & Pharmacotherapy, 109, 503–510. https://doi.org/10.1016/j.biopha.2018.10.154
- Hui, S., Liu, Y., Chen, M., Wang, X., Lang, H., Zhou, M., Yi, L., & Mi, M. (2019). Capsaicin improves glucose tolerance and insulin sensitivity through modulation of the gut microbiota-bile acid-FXR axis in type 2 diabetic db/db Mice. Molecular Nutrition & Food Research, 63(23), e1900608. https://doi.org/10.1002/mnfr.201900608
- Isobe, M., Lee, S., Waguri, S., & Kametaka, S. (2018). Correction: Clathrin adaptor GGA1 modulates myogenesis of C2C12 myoblasts. PLoS One, 13(12), e207533. https://doi.org/10.1371/journal.pone.0209441
- James-Allan, L. B., Arbet, J., Teal, S. B., Powell, T. L., & Jansson, T. (2019). Insulin stimulates GLUT4 trafficking to the syncytiotrophoblast basal plasma membrane in the human placenta. The Journal of Clinical Endocrinology and Metabolism, 104(9), 4225–4238. https://doi.org/10.1210/jc.2018-02778
- Jia, L. Y., Wu, X. J., Gao, Y., Rankin, G. O., Pigliacampi, A., Bucur, H., Li, B., Tu, Y.-Y., & Chen, Y. C. (2017). Inhibitory effects of total triterpenoid saponins isolated from the seeds of the tea plant (Camellia sinensis) on human ovarian cancer cells. Molecules, 22(10), 22. https://doi.org/10.3390/molecules22101649
- Khan, N., & Mukhtar, H. (2018). Tea polyphenols in promotion of human health. Nutrients, 11(1), 11. https://doi.org/10.3390/nu11010039
- Kumar, M., Kannan, A., Bhar, R., Gulati, A., Gaurav, A., & Sharma, V. K. (2017). Nutrient intake, digestibility and performance of gaddi kids supplemented with tea seed or tea seed saponin extract. Asian - Australasian Journal of Animal Sciences, 30(4), 486–494. https://doi.org/10.5713/ajas.16.0451
- Lee, S. H., Park, S. Y., & Choi, C. S. (2022). Insulin resistance: From mechanisms to therapeutic strategies. Diabetes & Metabolism Journal, 46(1), 15–37. https://doi.org/10.4093/dmj.2021.0280
- Li, Y., Huang, D., Zheng, L., Cao, H., & Fan, Z. (2018). Effect of microRNA-141 on the development of diabetic nephropathy through regulating AKT/AMPK signaling pathway by targeting insulin receptor substrate 2. Journal of Cellular Biochemistry, 120(5), 8008–8015. https://doi.org/10.1002/jcb.28078
- Liu, Y., Li, X., Zhu, Y., Liu, J., & Liu, S. (2021). Subclinical hypothyroidism contributes to poor glycemic control in patients with type 2 diabetes mellitus, and ellagic acid attenuates methimazole-induced abnormal glucose metabolism in mice model. Journal of Food Biochemistry, 45(6), e13753. https://doi.org/10.1111/jfbc.13753
- Liu, L., Wang, H., Cui, J., Zhang, Q., Zhang, W., Xu, W., Lu, H., Liu, S., Shen, S., Fang, F., Li, L., Yang, W., Zhuang, Z., & Li, J. (2018). Inhibition of protein phosphatase 2A sensitizes mucoepidermoid carcinoma to chemotherapy via the PI3K-AKT pathway in response to insulin stimulus. Cellular Physiology & Biochemistry, 50(1), 317–331. https://doi.org/10.1159/000494008
- Li, H., Xie, S., Li, H., Zhang, R., & Zhang, H. (2020). LncRNA MALAT1 mediates proliferation of LPS treated-articular chondrocytes by targeting the miR-146a-PI3K/Akt/mTOR axis. Life Sciences, 254, 116801. https://doi.org/10.1016/j.lfs.2019.116801
- Loriz, P. O., Campos, B. B., Granada, Y. M., Sanmarti, S. A., & Arroyo, B. J. (2007). Detección de diabetes tipo LADA en pacientes diabéticos con exceso ponderal. ¿Es adecuado el tratamiento con metformina? Atención Primaria, 39(3), 133–137. https://doi.org/10.1157/13099560
- Meng, X., Sun, R., Wang, W., Zhang, N., Cao, S., Liu, B., Fang, P., Deng, S., & Yang, S. (2021). ADFP promotes cell proliferation in lung adenocarcinoma via Akt phosphorylation. Journal of Cellular and Molecular Medicine, 25(2), 827–839. https://doi.org/10.1111/jcmm.16136
- Mielcarek, M., Isalan, M., & Tilaoui, M. (2021). Kinetin stimulates differentiation of C2C12 myoblasts. PLoS One, 16(10), e258419. https://doi.org/10.1371/journal.pone.0258419
- Mi, Y., Qi, G., Fan, R., Qiao, Q., Sun, Y., Gao, Y., & Liu, X. (2017). EGCG ameliorates high-fat– and high-fructose-induced cognitive defects by regulating the IRS/AKT and ERK/CREB/BDNF signaling pathways in the CNS. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 31(11), 4998–5011. https://doi.org/10.1096/fj.201700400RR
- Shahcheraghi, S. H., Tchokonte-Nana, V., Lotfi, M., Lotfi, M., Ghorbani, A., & Sadeghnia, H. R. (2020). Wnt/beta-catenin and PI3K/Akt/mTOR Signaling pathways in glioblastoma: Two main targets for drug design: A review. Current Pharmaceutical Design, 26(15), 1729–1741. https://doi.org/10.2174/1381612826666200131100630
- Sharma, M., & Dey, C. S. (2021). AKT ISOFORMS-AS160-GLUT4: The defining axis of insulin resistance. Reviews in Endocrine & Metabolic Disorders, 22(4), 973–986. https://doi.org/10.1007/s11154-021-09652-2
- Snoussi, C., Ducroc, R., Hamdaoui, M. H., Dhaouadi, K., Abaidi, H., Cluzeaud, F., Nazaret, C., Le Gall, M., & Bado, A. (2014). Green tea decoction improves glucose tolerance and reduces weight gain of rats fed normal and high-fat diet. The Journal of Nutritional Biochemistry, 25(5), 557–564. https://doi.org/10.1016/j.jnutbio.2014.01.006
- Stavrianidi, A., Stekolshchikova, E., Rodin, I., Godovikov, I., & Shpigun, O. (2018). Structure elucidation of sweet-tasting cycloartane-type saponins from ginseng oolong tea and Abrus precatorius L. leaves. Natural Product Research, 32(20), 2490–2493. https://doi.org/10.1080/14786419.2017.1416383
- Sun, Y., Hu, M., Wang, F., Tan, H., Hu, J., Wang, X., Wang, B., Hu, J., & Li, Y. (2021). Quantification of 2-NBDG, a probe for glucose uptake, in GLUT1 overexpression in HEK293T cells by LC–MS/MS. Analytical Biochemistry, 631, 114357. https://doi.org/10.1016/j.ab.2021.114357
- Sun, K., Luo, J., Guo, J., Yao, X., Jing, X., & Guo, F. (2020). The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthritis and Cartilage, 28(4), 400–409. https://doi.org/10.1016/j.joca.2020.02.027
- Taslimi, P., Aslan, H. E., Demir, Y., Oztaskin, N., Maraş, A., Gulçin, İ., Beydemir, S., & Goksu, S. (2018). Diarylmethanon, bromophenol and diarylmethane compounds: Discovery of potent aldose reductase, α-amylase and α-glycosidase inhibitors as new therapeutic approach in diabetes and functional hyperglycemia. International Journal of Biological Macromolecules, 119, 857–863. https://doi.org/10.1016/j.ijbiomac.2018.08.004
- Thiel, G., Guethlein, L. A., & Rossler, O. G. (2021). Insulin-responsive transcription factors. Biomolecules, 11(12. https://doi.org/10.3390/biom11121886
- Tong, X., Ma, H., Amadi, S. W., Ma, L., & Wu, G. (2015). Reno-protection of G004, a novel anti-diabetic sulfonylurea in db/db mice. Naunyn-Schmiedeberg’s Archives of Pharmacology, 388(8), 831–841. https://doi.org/10.1007/s00210-015-1112-7
- Trefts, E., & Shaw, R. J. (2021). AMPK: Restoring metabolic homeostasis over space and time. Molecular Cell, 81(18), 3677–3690. https://doi.org/10.1016/j.molcel.2021.08.015
- Tsuji, T., Yamaguchi, M., Kuroyanagi, J., Furuzono, S., Konishi, M., Terayama, K., Tanaka, J., Saito, M., & Kobayashi, Y. (2019). Discovery of novel pyridazine derivatives as glucose transporter type 4 (GLUT4) translocation activators. Bioorganic & Medicinal Chemistry Letters, 29(14), 1785–1790. https://doi.org/10.1016/j.bmcl.2019.05.013
- Tulloch, J. A., & Macintosh, D. (1961). “J”-type diabetes. The Lancet, 278(7194), 119–121. https://doi.org/10.1016/S0140-6736(61)92645-9
- Wang, Z., Wang, J., & Chan, P. (2013). Treating type 2 diabetes mellitus with traditional Chinese and Indian medicinal herbs. Evidence-Based Complementary and Alternative Medicine: eCam, 2013, 343594. https://doi.org/10.1155/2013/343594
- Wang, Y., Wen, L., Zhou, S., Zhang, Y., Wang, X.-H., He, Y.-Y., Davie, A., & Broadbent, S. (2018). Effects of four weeks intermittent hypoxia intervention on glucose homeostasis, insulin sensitivity, GLUT4 translocation, insulin receptor phosphorylation, and Akt activity in skeletal muscle of obese mice with type 2 diabetes. PLoS One, 13(9), e203551. https://doi.org/10.1371/journal.pone.0203551
- Wierzejska, R. (2014). Tea and health--A review of the current state of knowledge. Przeglad epidemiologiczny, 68(3), 501-6, 595–9.
- Witt, D. M., Carter, C. S., & Lnsel, T. R. (1991). Oxytocin receptor binding in female prairie voles: endogenous and exogenous oestradiol stimulation. Journal of Neuroendocrinology, 3(2), 155–161. https://doi.org/10.1111/j.1365-2826.1991.tb00258.x
- Yapanis, M., James, S., Craig, M. E., O’Neal, D., & Ekinci, E. I. (2022). Complications of diabetes and metrics of glycemic management derived from continuous glucose monitoring. The Journal of Clinical Endocrinology and Metabolism, 107(6), e2221–36. https://doi.org/10.1210/clinem/dgac034
- Yu, S., Wang, L., Che, D., Zhang, M., Li, M., Naito, M., Xin, W., & Zhou, L. (2022). Targeting CRABP-II overcomes pancreatic cancer drug resistance by reversing lipid raft cholesterol accumulation and AKT survival signaling. Journal of Experimental & Clinical Cancer Research: CR, 41(1), 88. https://doi.org/10.1186/s13046-022-02261-0
- Zheng, J., Li, H., Zhang, X., Jiang, M., Luo, C., Lu, Z., Xu, Z., & Shi, J. (2018). Prebiotic mannan-oligosaccharides augment the hypoglycemic effects of metformin in correlation with modulating gut microbiota. Journal of Agricultural and Food Chemistry, 66(23), 5821–5831. https://doi.org/10.1021/acs.jafc.8b00829
- Zhong, H., Wu, H., Bai, H., Wang, M., Wen, J., Gong, J., Miao, M., & Yuan, F. (2019). Panax notoginseng saponins promote liver regeneration through activation of the PI3K/AKT/mTOR cell proliferation pathway and upregulation of the AKT/Bad cell survival pathway in mice. BMC Complementary and Alternative Medicine, 19(1), 122. https://doi.org/10.1186/s12906-019-2536-2
- Zurlo, F., Trevisan, C., Vitturi, N., Ravussin, E., Salvò, C., Carraro, S., Siffi, M., Iob, I., Saller, A., Previato, L., Sergi, G., de Kreutzenberg, S., Maran, A., & Avogaro, A. (2019). One-year caloric restriction and 12-week exercise training intervention in obese adults with type 2 diabetes: Emphasis on metabolic control and resting metabolic rate. Journal of Endocrinological Investigation, 42(12), 1497–1507. https://doi.org/10.1007/s40618-019-01090-x