ABSTRACT
In this study, mice undergoing either short maternal separation (MS) or long MS during early postnatal life and then experiencing chronic unpredictable mild stress (CUMS) in adulthood were established to explore the critical mechanism of the ginseng total saponins (GTS), extractions of ginseng, on early-life stress (ELS) and subsequent susceptibility to depression-like behaviors in adulthood. The behavioral assessment confirmed that GTS could reduce susceptible effects on mice induced by ELS to develop subsequent adult depression-like behaviors. The epigenetic changes of brain-derived neurotrophic factor (BDNF), its receptor tropomyosin-related kinase B (TrkB), and the downstream target cAMP-response element-binding protein (CREB) in the hippocampal tissue were elevated after GTS treatment. Interestingly, the inhibitor of TrKB, K252a further verified the above findings. All evidence showed that GTS had potential neuroprotective effects due to GTS could restore behavioral abnormalities and alter hippocampal protein levels via the CREB-BDNF-TrkB pathway, thereby ameliorating depression-like behaviors induced by MS.
1. Introduction
Early-life stress (ELS) is characterized by stressful adversities with a series of physical and emotional symptoms after exposure to stressful events directly or indirectly (Du et al., Citation2016). It has prominent effects on neuroplasticity and neurobehavioral development throughout a lifetime (Goodwill et al., Citation2019; Saleh et al., Citation2017). ELS exposure e.g. maternal separation (MS) exerts long-term epigenetic alterations on the brain. Some studies said that MS can heighten stress susceptibility to develop adult depression-like behaviors and even increase the risks of a range of psychopathological disorders in adulthood such as depression (Anda et al., Citation2006; Peña et al., Citation2017). However, others said that MS could result in stress resilience to the following unexpected stress later in life. The results were inconsistent. The underlying molecular and functional mechanisms of ELS leading to the susceptibility to depression or depression-like behavior are associated with epigenetic alterations in brain regions (Russo & Nestler, Citation2013; Torres-Berrío et al., Citation2019). Recent evidence demonstrated that ELS increasing stress vulnerability is possibly associated with brain-derived neurotrophic factor (BDNF) epigenetic changes in the hippocampus (Nishinaka et al., Citation2015; Seo et al., Citation2016). Besides, the receptor, tropomyosin-related kinase B (TrkB), and the downstream target cAMP-response element-binding protein (CREB) of BDNF play important roles in the susceptibility to depression (Advani et al., Citation2009; Hoshaw et al., Citation2005).
It is estimated that 970.1 million people worldwide experience mental disorders according to the Global Burden of Disease 2019 Study (Mental & Collaborators, Citation2022). However, only half of these major depression patients are effective after treatments with antidepressants (Williams et al., Citation2016). And the high recurrence rates, irreversible side effects, and slow onset of action (>14 days) of current antidepressants are still barriers to treating depression (Cipriani et al., Citation2018). Moreover, it brings enormous physiological, psychological, and economic burdens on individuals and families. Herein, there is a pressing need to identify more effective and safer antidepressants with multiple targets.
Mounting evidence indicated that natural products such as ginseng with limited side effects and high safety might have potential anti-depressive effects on depression (Jeong et al., Citation2015; Jin et al., Citation2019; Lee et al., Citation2020; Wang et al., Citation2007). Ginseng, the root of Panax ginseng Meyer, is considered the “king of herbs” in traditional Chinese medicine. It is not only widely used to improve mental states and treat some neurological disorders, i.e. neurasthenia, insomnia, depression, and mental tension in East Asian countries, especially in China (Jin et al., Citation2019), but also served as one of the popular herbal foods for healthcare in daily life (Lu et al., Citation2021). Patients experiencing residual symptoms of major depression given 3 g/day Korean red ginseng showed a significant decrease in depressive symptoms (Jeong et al., Citation2015). It is said that ginseng total saponins (GTS) as the major active components of ginseng has potential anti-depressant or anti-stress effects (Chen et al., Citation2014; Dang et al., Citation2009). In addition, a recent study showed that ginsenosides had neuroprotective effects on nervous system diseases (Lu et al., Citation2022). To underlie the crucial effects of GTS on ELS and subsequent susceptibility to depression-like behaviors in adulthood via the CREB/BDNF/TrkB signaling at molecular levels, the sequential models, that is, MS during early postnatal life and chronic unpredictable mild stress (CUMS) during adulthood were established. As we know, MS is a classic model for studying early separation, while CUMS is often employed to stimulate depressive behavior. This novel model could induce behavioral and neurobiological changes modeling that of depressive patients, and could further help us understand the critical effect of early stress on subsequent depression in adults.
2. Materials and methods
2.1. Chemicals and antibodies
The GTS was provided by Shanghai Yuanye Biotechnology Co., Ltd (China). K252a (inhibitor of TrKB) was provided by Beyotime (China). Primary antibodies, i.e. the anti-CREB antibody (bs0035R, Bioss, China), anti-BDNF antibody (bs4989R, Bioss, China), anti-TrkB antibody (bs0175R, Bioss, China), and second antibodies, i.e. the goat anti-rabbit IgG (A0208, Beyotime, China), anti-rabbit IgG (Cy3, CW0159S, CWBIO, China) were used. β-actin (CW0096M) was brought from Beijing ComWin Biotech Co., Ltd (China). 4’,6-diamidino-2-phenylindole (DAPI, C1005) was provided by Beyotime Biotechnology Co., Ltd (China).
2.2. Experimental animals
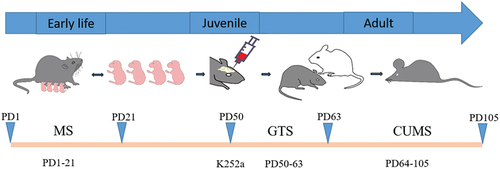
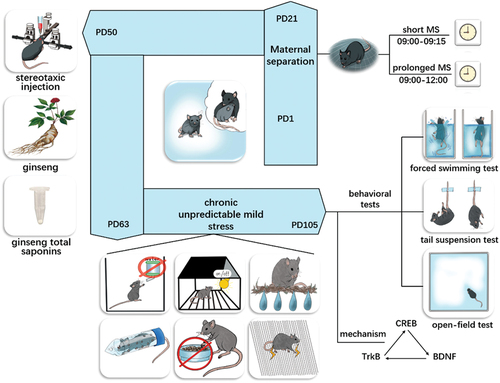
Twelve pregnant C57BL/6N mice were provided by the Experimental Animal Center of Nanjing University of Chinese Medicine. Pregnant mice were bred and maintained with sawdust in the cages individually under a controlled condition (12:12 light/dark cycle, 21–23°C temperature, 50 ± 10% humidity). All mice were fed food and fresh water freely and allowed to habituate to the environment for 7 days. The pregnant mice were randomized to six groups (n = 2 mothers/group), that is, control, CUMS, MS180 (MS for 180 min) + CUMS, MS15 (MS for 15 min) + CUMS, MS180 + GTS + CUMS, MS180 + GTS + K252a + CUMS, respectively. To avoid the influence of sex difference, only male pups were selected at the 21st day postpartum (PD21) for the subsequence analysis in our study. If both two dams in the same group have 6 offspring, only one dam, and its offspring were randomly chosen. If this dam has more than six pups, six of them were randomly chosen for the following study, and the rest pups were euthanized. All manipulations were approved by the Laboratory Animal Management Committee of Nanjing University of Chinese Medicine.
2.3. MS intervention
MS has been widely applied to characterize the neurobehavioural development of early life adverse experiences on altered vulnerability to depression-like behaviors in adulthood (Pryce & Feldon, Citation2003). The MS intervention was described as a previous study reported (Bian et al., Citation2015). Briefly, the mother of the pups was taken out of the cage. To prepare for short and prolonged MS, the experimental pups were housed away from their mother for 180 min (9:00–12:00 a.m.) or 15 min (9:00–9:15 a.m.) daily from the 1st day postpartum (PD1) to PD21 in an incubator (30 ± 2°C). To avoid the possible variations from the tactile stimulation between the littermates (Tractenberg et al., Citation2016), The pups from one dam were separated individually and not allowed to be in contact with their littermates. After the separation, the mother was united with the pups. The control pups were reared with their dam without any interventions under standard conditions.
2.4. CUMS intervention
CUMS is a well-established model of depression and depressive behaviors (Zhu, Shi et al., Citation2014; Zhu, Wang et al., Citation2014). It was described as previously published (Zhao et al., Citation2012). Briefly, experimental mice were singly housed and treated with different mild stressors for 42 consecutive days from the 64th day postpartum (PD64) to the 105th day postpartum (PD105). The stressors for each week were: (1) water deprivation for 24 h, (2) flashing light (stroboscopic light, 60 flashes/min) for 3 h, (3) damp sawdust for 24 h, (4) physical restraint (80 cm3 breathable, transparent and plastic pipe) for 6 h, (5) food deprivation for 24 h, (6) no stressor applied, (7) electric foot-shock (intensity: 1 mA, last; for 4 s with 26 s rest) for 20 min. The above stressors were selected in a random order each day to make sure of the unpredictability of stress. The same stress wasn’t allowed for two consecutive days. The controls were reared without any intervention.
2.5. MS and CUMS sequential model establishment and group treatment
In the CUMS group (n = 6), mice without MS intervention were given saline intragastrically for consecutive 14 days from the 50th day postpartum (PD50) to the 63rd day postpartum (PD63), and given CUMS intervention during PD64-105. In MS180+CUMS group (n = 6), mice were given MS intervention for 180 min daily during PD1-21, saline intragastrically during PD50-63, and CUMS intervention during PD64-PD105. In MS15+CUMS group (n = 6), mice were given MS intervention for 15 min daily during PD1-21, saline intragastrically during PD50-63, and CUMS intervention during PD64-105. Based on the previous study of our team (Chen et al., Citation2017), 50 mg/kg/day GTS (dissolved in distilled water) was administered intragastrically for consecutive 14 days from PD50 to PD63. In MS180 + GTS + CUMS group (n = 6), mice were given MS intervention for 180 min daily during PD1-21, 50 mg/kg/day GTS administered intragastrically during PD50-63, and CUMS intervention during PD64-105. In MS180 + GTS + K252a + CUMS group (n = 6), mice were given MS intervention for 180 min daily during PD1-21, stereotaxic injection of K252a at PD50, 50 mg/kg/day GTS administered intragastrically during PD50-63, and CUMS intervention during PD64-105. Mice in the control group (n = 6) were only given saline intragastrically during PD50-63. All mice were not stressed during handling and experimentation. The protocol was presented in .
2.6. Behavioral assessment
Behavioral tests were carried out after CUMS intervention. Movements and immobility times of behavioral tests were all conducted at PD105 using a smart 3.0 tracking software (Panlab), respectively. The behavioral tests were done as described previously (Bian et al., Citation2019). Each mouse was individually put into a 2000 mL glass container with 20 cm deep cold water (25 ± 1°C) during the test period of 5 min. The forced swimming test (FST) was analyzed by recording the immobility time in the last 4 min by the same trained observer. Immobility was defined as whenever mice stopped swimming, remained floating without struggling, and kept their head out of the water with small limb movements. In terms of the tail suspension test (TST), the tip of the mice’s tail (1 cm) was stuck by tape and the mice were hung upside down 58 cm above the ground. The test lasted for 6 min. The remaining 4 min was measured. Immobility was regarded whenever mice stopped moving their limbs without struggling and maintained a vertical posture. As for the open-field test (OFT), spontaneous activity was recorded. Mice were put separately in the middle of an apparatus (68 × 68 × 50 cm) with 25 small squares. The movement was measured over a 5-min period.
2.7. Stereotaxic injection
The stereotaxic injection was performed as described previously (Lee & Han, Citation2019). In short, mice were anesthetized with 1–1.5% isoflurane and fixed on the stereotaxic frame (RWD Life Science Company, China). Following the cessation of movement, K252a (1.5 µL) was injected into the lateral ventricles at the rate of 0.2 µl per min with a 30-G needle.
2.8. Western blot assays
Hippocampal tissues were collected after mice were sacrificed on the 106th day postpartum (PD106). Protein extraction and qualification were developed as described previously (Bian et al., Citation2015). 10 mg tissue sample with 100 μL RIPA lysis buffer (strong) (Beyotime, China) were homogenized and lysed at 4°C, centrifuged for 30 min, and then quantified. The protein samples with SDS-PAGE (Beyotime, China) at the ratio of 4:1 were protein-denaturated in the incubation at 100°C for 10 min. Each sample was electrophoresed by 12% SDS-PAGE and transferred to a PVDF membrane. Then, the blots were immunoblotted with primary anti-CREB antibody (1:1000), anti-BDNF antibody (1:1000), and anti-TrkB antibody (1:5000) at 4°C overnight. After incubation with the secondary antibody, bands were visualized by a Chemiluminescence Imaging System (Bio-Rad, U.S.A.).
2.9. qRT-PCR assays
Total RNA from brain tissue was prepared using Trizol reagent (Invitrogen, U.S.A.), and RNA concentration was determined using Nanodrop (U.S.A.) following the instructions. Briefly, a 10 mg tissue sample with 200 μL Trizol was homogenized and lysed at 4°C.
After adding 40 μL chloroform and centrifuging for 15 min, the upper solution was transferred to a new EP tube and added with 100 μL isopropyl alcohol. With 75% alcohol washed, RNA was dried and added 40 μL DEPC-treated water (Beyotime, China). Then, RNA was converted to cDNA using the cDNA Synthesis Kit (Yeasen, China). Transcription levels were made with the 2−ΔΔCT value method. GAPDH was employed as the endogenous control. The primer sequences for CREB, BDNF, TrkB, and GAPDH bought from Genscript (Nanjing, China) were listed as follows:
CREB (forward: 5’-TAGTCCCAGCAACCAAGT-3’/reverse: 5’-GGACGCCATAACAACTCCAG-3’),
BDNF (forward: 5’-CGAAGAGCTGCTGGATGAG-3’/reverse: 5’-ATGGGATTACACTTGGTCTCG-3’),
TrkB (forward: 5’-GTGGATTCCGGCTTAAAGTTTGTG-3’/reverse: 5’-AAGTCAAGGTGGCGGAAATG-3’),
GAPDH (forward: 5’-AGGTCGGTGTGAACGGATTTG-3’/reverse: 5’-TGTAGACCATGTAGTTGAGGTCA-3’).
2.10. Immunofluorescence assay
The harvested brain tissues were immersed in 4% paraformaldehyde. After dehydration and embedding, 4 µm thick sections were immersed in 1% bovine serum albumin for 1 h and incubated with primary antibodies: CREB antibody (1:500), BDNF antibody (1:500), and TrkB antibody (1:1000) at 4°C. After washing three times for 5 min, the sections were incubated with Cy3 conjugated goat anti-rabbit IgG (1:2000) at room temperature for 1 h. Then, the nuclei were stained with DAPI at room temperature out of light for another 5 min. Images were viewed under a microscope (Leica DM2500, Germany).
2.11. Statistical analysis
Data were analyzed using SPSS 17.0 statistical software (IBM, U.S.A.) and shown as mean ± standard deviation (SD). Statistical significance was conducted using one-way ANOVA followed by Tukey’s post hoc tests. A P-value less than .05 was used for statistical comparisons.
3. Results
3.1. Prolonged maternal separation induced susceptibility to depression-like behavior in adult mice
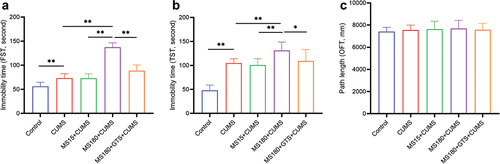
As shown in , the MS180 + CUMS group had more time spent immobile in both FST and TST than the CUMS group (p < .05). However, there were no statistical changes between the MS15+ CUMS group and the CUMS group (p > .05). It indicated that MS for 180 min rather than 15 min in early stressful adversity would be an appropriate time to measure stress-induced alterations later in life. No obvious difference in locomotor activity (the rearing time, central time, and central distance ratio) was found among different groups in OFT (p > .05), as presented in . Our findings indicated that prolonged MS was more susceptible to stress with variable depression-like behaviors in adulthood.
3.2. Prolonged maternal separation altered the hippocampal levels of CREB, BDNF, and TrkB expression
The hippocampal expressions of CREB, BDNF, and TrkB in MS mice that were subjected to unexpected stress later in life were detected by western blot. It indicated that the expressions of CREB, BDNF, and TrkB in the MS180 + CUMS group were markedly down-regulated in comparison with the CUMS group (p < .05, ). The findings of the qRT-PCR analysis and immunofluorescence assay further verified the results above, as presented in . The data highlighted the important role of CREB, BDNF, and TrkB in early stress-induced behavioral alterations and further confirmed the critical role of the hippocampus in the brain region that is susceptible to early life adversity.
Figure 3. The altered hippocampal levels of CREB, BDNF, and TrkB among different groups. (a,b) western blot assay of the altered hippocampal expressions of CREB, BDNF, TrkB. Data were plotted as the mean ± SD. *P-value < .05, **P-value < .01. (c) qRT-PCR analysis of the altered hippocampal mRNA levels of CREB, BDNF, and TrkB. Data were plotted as the mean ± SD. *P-value < .05, **P-value < .01.
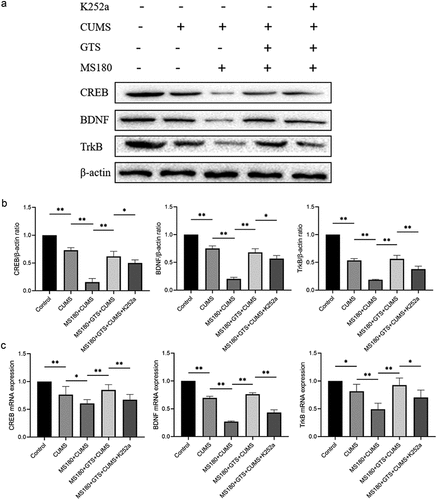
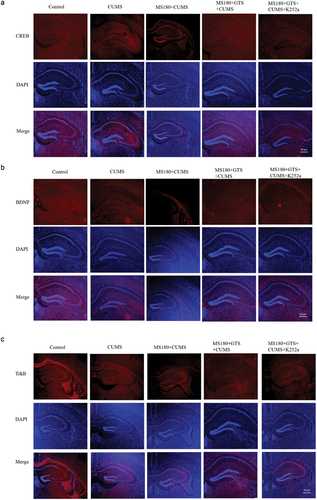
Figure 4. Immunofluorescence assay of the altered hippocampal levels of CREB, BDNF, and TrkB among different groups. All scale bars were × 50 µm. (a) Immunofluorescence assay of the altered hippocampal levels of CREB among different groups. (b) Immunofluorescence assay of the altered hippocampal levels of BDNF among different groups. (c) Immunofluorescence assay of the altered hippocampal levels of TrkB among different groups.
3.3. GTS restored behavioral abnormalities in mice after prolonged maternal separation
To explore the effect of GTS on the vulnerability to adult depression-like behavior in mice with early-life adversity. Mice undergoing maternal separation during early postnatal life were treated with GTS and then subjected to CUMS in adulthood. Afterward, the behavioral tests were measured. The FST and TST results of the MS180 +GTS+ CUMS group indicated that GTS treatment could markedly decrease the immobility time induced by maternal separation in comparison with MS180 + CUMS group, as shown in . But, no obvious changes in spontaneous activity (the rearing time, central time, and central distance ratio) were observed in OFT between the MS180 + CUMS group and the MS180 +GTS+ CUMS group (p > .05), as presented in . All behavioral evidence demonstrated that GTS could reduce susceptible effects on mice induced by early life stress to develop subsequent adult depression-like behaviors.
3.4. GTS rescued the susceptibility to depression-like behavior in mice after prolonged maternal separation through CREB/BDNF/TrkB signaling pathway
Importantly, the relative expressions of CREB, BDNF, and TrkB in the MS180 +GTS+ CUMS group were significantly up-regulated in mice treated with GTS in comparison with MS180+CUMS group (p < .05), as evidenced by the western blot assay (). Similarly, the results of qRT-PCR analysis and immunofluorescence assay further verified the above findings, as presented in . The findings suggested that GTS might activate CREB signaling and play an important role in the susceptible effects on mice induced by maternal separation to develop depression-like behaviors later in life. Then, K252a, an inhibitor of TrKB, was applied in our study to verify our findings. The hippocampal expressions of the above protein were shown in . It indicated that the protein expressions of CREB, BDNF, and TrkB were significantly down-regulated (p < .05) after using the specific tyrosine inhibitor K252a. The results were also in line with those of qRT-PCR analysis and immunofluorescence assay, as presented in . Together, all evidence suggested that GTS could rescue the susceptibility to depression-like behavior in mice after prolonged maternal separation through CREB/BDNF/TrkB signaling pathway.
4. Discussion
Currently, we tried to investigate the underlying effects of GTS on the susceptibility to depression-like behaviors of maternally separated mice that were subsequently subjected to CUMS in adulthood. It is said that MS could evoke stress susceptibility when re-exposure to adult stress (Juruena, Citation2014). However, another study reported that MS could lead to stress resilience when exposed to stress again in adulthood (Macrì et al., Citation2011). The conclusions were contradictory. In this study, we subjected rodents to MS and CUMS stress, which is one of the most reliable models to investigate the impact of early or later life stress on neurobehavioral responses. MS can replicate human ‘reactive’ depression caused by distal stress, while CUMS can model human ‘reactive’ depression caused by proximal stress (Malki et al., Citation2014). The employ of the MS and CUMS sequential models is used to study behavioral and neurobiological changes modeling that of depressive patients. As for the MS paradigm, different MS procedures may have different observations in MS effects. In some studies (Dandekar et al., Citation2022; Jiang et al., Citation2021), the pups were private from maternal care but allowed contact with their littermates. However, in other studies (Bian et al., Citation2015; Vetulani, Citation2013), the pups were housed individually and deprived of their littermates resulting in a complete deprivation of tactile stimuli. It’s said that the last condition may be more stressful because tactile stimulation between mother and infant or between infants is essential to adequate development; its privation could be related to a helplessness condition (Rüedi-Bettschen et al., Citation2004). In our study, we used the last MS paradigm to completely replicate early adversities. The pups were separated from the dam and their littermates so that they could not communicate with, smell, or feel their presence. Our results of behavioral tests (FST and TST) suggested that MS for 180 min daily from PD1 to PD21 could induce depression-like behaviors later in life. However, no obvious difference was observed in mice induced by MS for 15 min daily. The major difference was the duration of MS exposure time. MS lasting for 180 min daily rather than 15 min might be an appropriate time to assess the behavioral outcomes following the second unexpected stress. More time spent immobile in both FST and TST was found in the MS180 + CUMS group than those in the CUMS group. All evidence showed that long-lasting exposure to early life events could trigger susceptibility to depression-like behavioral deficits later in life. The observations were consistent with previous studies (Liu et al., Citation2017, Citation2018).
Growing evidence revealed that the hippocampus is associated with ELS-related psychological disorders (Hanson et al., Citation2015; Wang et al., Citation2018). The hippocampus volumes of depression patients were found smaller than the controls by anatomical MRI assay (Caetano et al., Citation2004). It indicated that the hippocampus might play a pivotal role in stressful susceptibility to depression-like behaviors induced by early life adversities. For this reason, we harvested the hippocampus for further analysis. More importantly, Mi Kyoung Seo et al. (Citation2016) reported that early life stress could increase stress susceptibility by modulating the BDNF gene in the hippocampus. BDNF was found down-regulated in the hippocampus of a suicide patient caused by depression (Karege et al., Citation2005). The knockout experiment demonstrated that BDNF acted with antidepressant-like effects (Advani et al., Citation2009), while peripheral injection of BDNF could evoke these effects in rodent models (Schmidt & Duman, Citation2010). All evidence indicated that BDNF might act as a biomarker in the stress vulnerability induced by ELS. Remarkably, CREB is known to target BDNF (Finkbeiner et al., Citation1997) and TrkB is blind with BDNF (Ye et al., Citation2017), which are critically important to the development of synaptic plasticity in the hippocampus (Henriksson et al., Citation1992). Herein, we hypothesized that BDNF-TrkB-CREB signaling might play pivotal roles in the stress vulnerability induced by early-life events to the following depression-like behaviors. Based on the results of immunofluorescence assay, western blot, and qRT-PCR analysis in our study, we observed the variable changes in CREB, BDNF, and TrkB expression in the hippocampus. As the expressions of the above molecules in the MS180 + CUMS group were obviously lower than those in the CUMS group. These findings further verify our hypothesis about the critical roles of BDNF-TrkB-CREB signaling on the vulnerability to depression-like behavior in mice after prolonged early-life exposure.
A recent study reported that GTS extracted from herbal medicine ginseng exhibited effective anti-depressive effects on mouse chronic unpredictable mild stress models (Zhang et al., Citation2018). However, the critical effects of GTS on the vulnerability induced by early-life events to the following depression-like behaviors are still unclear. To our knowledge, this is the first study exploring GTS on the susceptibility to depression-like behaviors in mice undergoing early life adversity. We found that GTS could significantly decrease the immobility time of FST and TST in prolonged MS mice undergoing CUMS later in life. And GTS could markedly up-regulate the hippocampal levels of CREB, BDNF, and TrkB in prolonged MS mice that were subsequently subjected to CUMS in adulthood. After using the K252a inhibitor, the expressions of the above proteins in the MS180 + GTS + K252a + CUMS group were all reversed. All evidence demonstrated that the application of GTS in the field of stressful susceptibility is effective in prolonged early-life exposure. GTS might have neuroprotective effects because GTS treatment could restore behavioral abnormalities and alter hippocampal protein levels through the CREB-BDNF-TrkB signaling pathway, thereby ameliorating the depression-like behaviors that were induced by maternal separation. Our findings were consistent with the studies, which suggested that GTS had some anti-behavioral abnormalities effects (Dang et al., Citation2009). And the antidepressant-like effects of some metabolites/derivatives such as Rb1 (Wang et al., Citation2019) and Rg1 (Jiang et al., Citation2012) or classic herbal medicine i.e. Danggui Buxue decoction (Wang et al., Citation2021) and Huanglian Jie Du Tang (Ye et al., Citation2017) were due to the activation of the BDNF signaling pathway. Moreover, our findings were consistent with the results of our team, which showed that ginseng had a protective action against hypercortisolism-induced impairment of hippocampal neurons (Wang et al., Citation2011).
In general, the present study demonstrated that GTS plays an important role in rescuing susceptibility to adult depression-like behaviors in mice induced by early-life stress via regulating CREB/BDNF/TrkB signaling (). GTS could serve as a promising neuroprotective target for the treatment of early life stress-associated mental disorders. However, our study comes with several limitations. Firstly, a previous study has shown that early-life maternal separation could induce gender differences (Baugher & Sachs, Citation2022). Sexual alterations associated with pituitary-adrenal activity, vasopressinergic activity, and glucocorticoid receptor expression can influence the outcome of behavioral experiments (Renard et al., Citation2007, Citation2010). However, we only studied the impact of GST on adult stress susceptibility in male mice that experienced early-life maternal separation. Further studies with both male and female mice are needed to obtain more reliable findings. Secondly, we only assessed the hippocampal changes in the CREB/BDNF/TrkB signaling pathway and did not examine the other brain regions, i.e. amygdala, prefrontal cortex, and striatum. Thirdly, there are many ingredients in GTS, such as Ra, Rb, Rc, Rd, Re, Rf, and Rg. We did not investigate which ingredients of GTS act as key roles in the susceptibility of adulthood depression-like behaviors induced by maternal separation. Lastly, we didn’t divide individual litters into half control and half MS, nor did we assign pups to CUMS across litters, leaving maternal care and litter effects as unknown confounding variables. Herein, further studies in both males and females involving more brain regions with fewer confounding variables are required. The exact metabolites/derivatives from GTS on the susceptibility to adult depression-like behaviors in mice undergoing early-life stress need to be fully elucidated in future studies.
Abbreviation
| ELS | = | early-life stress |
| MS | = | maternal separation |
| GTS | = | ginseng total saponins |
| CUMS | = | chronic unpredictable mild stress |
| FST | = | forced swimming test |
| TST | = | tail suspension test |
| OFT | = | open-field test |
| BDNF | = | brain-derived neurotrophic factor |
| TrkB | = | receptor tropomyosin-related kinase B |
| CREB | = | cAMP response element-binding protein |
| PD1 | = | the 1st day postpartum |
| PD21 | = | the 21st day postpartum |
| PD50 | = | the 50th day postpartum |
| PD63 | = | the 63rd day postpartum |
| PD64 | = | the 64th day postpartum |
| PD105 | = | the 105th day postpartum |
| PD106 | = | the 106th day postpartum |
Authors’ contributions
YB and LY conceived and designed this study. YW, PL, WL, and JM helped with the experiment. YG helped with the mechanism figures. BZ analyzed the data. YY revised the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data are available from the corresponding authors on reasonable request.
Additional information
Funding
References
- Advani, T., Koek, W., & Hensler, J. G. (2009). Gender differences in the enhanced vulnerability of BDNF+/− mice to mild stress. The International Journal of Neuropsychopharmacology / Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum (CINP), 12(5), 583–588. https://doi.org/10.1017/S1461145709000248
- Anda, R. F., Felitti, V. J., Bremner, J. D., Walker, J. D., Whitfield, C., Perry, B. D., Dube, S. R., & Giles, W. H. (2006). The enduring effects of abuse and related adverse experiences in childhood: A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience, 256(3), 174–186. https://doi.org/10.1007/s00406-005-0624-4
- Baugher, B. J., & Sachs, B. D. (2022). Early life maternal separation induces sex-specific antidepressant-like responses but has minimal effects on adult stress susceptibility in mice. Frontiers in Behavioral Neuroscience, 16, 1–10. https://doi.org/10.3389/fnbeh.2022.941884
- Bian, Y., Yang, L., Wang, Z., Wang, Q., Zeng, L., & Xu, G. (2015). Repeated three-hour maternal separation induces depression-like behavior and affects the expression of hippocampal plasticity-related proteins in C57BL/6N mice. Neural Plasticity, 2015, 5–11. https://doi.org/10.1155/2015/627837
- Bian, Y., Yang, L., Zhao, M., Li, Z., Xu, Y., Zhou, G., Li, W., & Zeng, L. (2019). Identification of key genes and pathways in post-traumatic stress disorder using microarray analysis. Frontiers in Psychology, 10. https://doi.org/10.3389/fpsyg.2019.00302
- Caetano, S. C., Hatch, J. P., Brambilla, P., Sassi, R. B., Nicoletti, M., Mallinger, A. G., Frank, E., Kupfer, D. J., Keshavan, M. S., & Soares, J. C. (2004). Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Research: Neuroimaging, 132(2), 141–147. https://doi.org/10.1016/j.pscychresns.2004.08.002
- Chen, L., Dai, J., Wang, Z., Zhang, H., Huang, Y., & Zhao, Y. (2014). The antidepressant effects of ginseng total saponins in male C57BL/6N mice by enhancing hippocampal inhibitory phosphorylation of GSK-3β. Phytotherapy Research, 28(7), 1102–1106. https://doi.org/10.1002/ptr.5103
- Chen, L., Wang, X., Lin, Z. X., Dai, J. G., Huang, Y. F., & Zhao, Y. N. (2017). Preventive effects of ginseng total saponins on chronic corticosterone-induced impairment in astrocyte structural plasticity and hippocampal atrophy. Phytotherapy Research, 31(9), 1341–1348. https://doi.org/10.1002/ptr.5859
- Cipriani, A., Furukawa, T. A., Salanti, G., Chaimani, A., Atkinson, L. Z., Ogawa, Y., Leucht, S., Ruhe, H. G., Turner, E. H., Higgins, J. P. T., Egger, M., Takeshima, N., Hayasaka, Y., Imai, H., Shinohara, K., Tajika, A., Ioannidis, J. P. A., & Geddes, J. R. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. The Lancet, 391(10128), 1357–1366. https://doi.org/10.1016/S0140-6736(17)32802-7
- Dandekar, M. P., Palepu, M. S. K., Satti, S., Jaiswal, Y., Singh, A. A., Dash, S. P., Gajula, S. N. R., & Sonti, R. (2022). Multi-strain probiotic formulation reverses maternal separation and chronic unpredictable mild stress-generated anxiety- and depression-like phenotypes by modulating gut microbiome–brain activity in rats. ACS Chemical Neuroscience, 13(13), 1948–1965. https://doi.org/10.1021/acschemneuro.2c00143
- Dang, H., Chen, Y., Liu, X., Wang, Q., Wang, L., Jia, W., & Wang, Y. (2009). Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 33(8), 1417–1424. https://doi.org/10.1016/j.pnpbp.2009.07.020
- Du, L., Wang, J., Meng, B., Yong, N., Yang, X., Huang, Q., Zhang, Y., Yang, L., Qu, Y., Chen, Z., Li, Y., Lv, F., & Hu, H. (2016). Early life stress affects limited regional brain activity in depression. Scientific Reports, 6(1), 6. https://doi.org/10.1038/srep25338
- Finkbeiner, S., Tavazoie, S. F., Maloratsky, A., Jacobs, K. M., Harris, K. M., & Greenberg, M. E. (1997). CREB: A major mediator of neuronal neurotrophin responses. Neuron, 19(5), 1031–1047. https://doi.org/10.1016/S0896-6273(00)80395-5
- Goodwill, H. L., Manzano-Nieves, G., Gallo, M., Lee, H. I., Oyerinde, E., Serre, T., & Bath, K. G. (2019). Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology, 44(4), 711–720. https://doi.org/10.1038/s41386-018-0195-5
- Hanson, J. L., Nacewicz, B. M., Sutterer, M. J., Cayo, A. A., Schaefer, S. M., Rudolph, K. D., Shirtcliff, E. A., Pollak, S. D., & Davidson, R. J. (2015). Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry, 77(4), 314–323. https://doi.org/10.1016/j.biopsych.2014.04.020
- Henriksson, B. G., Söderström, S., Gower, A. J., Ebendal, T., Winblad, B., & Mohammed, A. H. (1992). Hippocampal nerve growth factor levels are related to spatial learning ability in aged rats. Behavioural Brain Research, 48(1), 15–20. https://doi.org/10.1016/S0166-4328(05)80134-2
- Hoshaw, B. A., Malberg, J. E., & Lucki, I. (2005). Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Research, 1037(1–2), 204–208. https://doi.org/10.1016/j.brainres.2005.01.007
- Jeong, H. G., Ko, Y. H., Oh, S. Y., Han, C., Kim, T., & Joe, S. H. (2015). Effect of Korean red ginseng as an adjuvant treatment for women with residual symptoms of major depression. Asia-Pacific Psychiatry, 7(3), 330–336. https://doi.org/10.1111/appy.12169
- Jiang, B., Xiong, Z., Yang, J., Wang, W., Wang, Y., Hu, Z. L., Wang, F., & Chen, J. G. (2012). Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. British Journal of Pharmacology, 166(6), 1872–1887. https://doi.org/10.1111/j.1476-5381.2012.01902.x
- Jiang, Z., Zhu, Z., Zhao, M., Wang, W., Li, H., Liu, D., & Pan, F. (2021). H3K9me2 regulation of BDNF expression in the hippocampus and medial prefrontal cortex is involved in the depressive-like phenotype induced by maternal separation in male rats. Psychopharmacology (Berl), 238(10), 2801–2813. https://doi.org/10.1007/s00213-021-05896-7
- Jin, Y., Cui, R., Zhao, L., Fan, J., & Li, B. (2019). Mechanisms of panax ginseng action as an antidepressant. Cell Proliferation, 52(6). https://doi.org/10.1111/cpr.12696
- Juruena, M. F. (2014). Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy & Behavior: E&B, 38, 148–159. https://doi.org/10.1016/j.yebeh.2013.10.020
- Karege, F., Vaudan, G., Schwald, M., Perroud, N., & La Harpe, R. (2005). Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Molecular Brain Research, 136(1–2), 29–37. https://doi.org/10.1016/j.molbrainres.2004.12.020
- Lee, K. H., Bahk, W. M., Lee, S. J., & Pae, C. U. (2020). Effectiveness and tolerability of Korean red ginseng augmentation in major depressive disorder patients with difficult-to-treat in routine practice. Clinical Psychopharmacology and Neuroscience: The Official Scientific Journal of the Korean College of Neuropsychopharmacology, 18(4), 621–626. https://doi.org/10.9758/CPN.2020.18.4.621
- Lee, Y., & Han, P. L. (2019). Early-life stress in D2 heterozygous mice promotes autistic-like behaviors through the downregulation of the BDNF-TrkB pathway in the dorsal striatum. Experimental Neurobiology, 28(3), 337–351. https://doi.org/10.5607/en.2019.28.3.337
- Liu, H., Atrooz, F., Salvi, A., & Salim, S. (2017). Behavioral and cognitive impact of early life stress: Insights from an animal model. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 78, 88–95. https://doi.org/10.1016/j.pnpbp.2017.05.015
- Liu, H., Patki, G., Salvi, A., Kelly, M., & Salim, S. (2018). Behavioral effects of early life maternal trauma witness in rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 81, 80–87. https://doi.org/10.1016/j.pnpbp.2017.10.013
- Lu, G., Liu, Z., Wang, X., & Wang, C. (2021). Recent advances in panax ginseng C.A. Meyer as a herb for anti-fatigue: An effects and mechanisms review. Foods, 10(5), 1030. https://doi.org/10.3390/foods10051030
- Lu, J., Wang, X., Wu, A., Cao, Y., Dai, X., Liang, Y., & Li, X. (2022). Ginsenosides in central nervous system diseases: Pharmacological actions, mechanisms, and therapeutics. Phytotherapy Research, 36(4), 1523–1544. https://doi.org/10.1002/ptr.7395
- Macrì, S., Zoratto, F., & Laviola, G. (2011). Early-stress regulates resilience, vulnerability and experimental validity in laboratory rodents through mother–offspring hormonal transfer. Neuroscience & Biobehavioral Reviews, 35(7), 1534–1543. https://doi.org/10.1016/j.neubiorev.2010.12.014
- Malki, K., Keers, R., Tosto, M. G., Lourdusamy, A., Carboni, L., Domenici, E., Uher, R., McGuffin, P., & Schalkwyk, L. C. (2014). The endogenous and reactive depression subtypes revisited: Integrative animal and human studies implicate multiple distinct molecular mechanisms underlying major depressive disorder. BMC Medicine, 12(1), 1–14. https://doi.org/10.1186/1741-7015-12-73
- Mental, G. B. D., & Collaborators, D. (2022). Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global burden of disease study 2019. The Lancet Psychiatry, 9, 137–150. https://doi.org/10.1016/S2215-0366(21)00395-3
- Nishinaka, T., Kinoshita, M., Nakamoto, K., & Tokuyama, S. (2015). Sex differences in depression-like behavior after nerve injury are associated with differential changes in brain-derived neurotrophic factor levels in mice subjected to early life stress. Neuroscience Letters, 592, 32–36. https://doi.org/10.1016/j.neulet.2015.02.053
- Peña, C. J., Kronman, H. G., Walker, D. M., Cates, H. M., Bagot, R. C., Purushothaman, I., Issler, O., Eddie Loh, Y. H., Leong, T., Kiraly, D. D., Goodman, E., Neve, R. L., Shen, L., & Nestler, E. J. (2017). Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science, 356(80), 1185–1188. https://doi.org/10.1126/science.aan4491
- Pryce, C. R., & Feldon, J. (2003). Long-term neurobehavioural impact of the postnatal environment in rats: Manipulations, effects and mediating mechanisms. Neuroscience & Biobehavioral Reviews, 27(1–2), 57–71. https://doi.org/10.1016/s0149-7634(03)00009-5
- Renard, G. M., Rivarola, M. A., & Suárez, M. M. (2007). Sexual dimorphism in rats: Effects of early maternal separation and variable chronic stress on pituitary-adrenal axis and behavior. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience, 25(6), 373–379. https://doi.org/10.1016/j.ijdevneu.2007.07.001
- Renard, G. M., Rivarola, M. A., & Suárez, M. M. (2010). Gender-dependent effects of early maternal separation and variable chronic stress on vasopressinergic activity and glucocorticoid receptor expression in adult rats. Developmental Neuroscience, 32(1), 71–80. https://doi.org/10.1159/000280102
- Rüedi-Bettschen, D., Feldon, J., & Pryce, C. R. (2004). Circadian- and temperature-specific effects of early deprivation on rat maternal care and pup development: Short-term markers for long-term effects. Developmental Psychobiology, 45(2), 59–71. https://doi.org/10.1002/dev.20014
- Russo, S. J., & Nestler, E. J. (2013). The brain reward circuitry in mood disorders. Nature Reviews Neuroscience, 14(10), 736–736. https://doi.org/10.1038/nrn3381
- Saleh, A., Potter, G. G., McQuoid, D. R., Boyd, B., Turner, R., MacFall, J. R., & Taylor, W. D. (2017). Effects of early life stress on depression, cognitive performance and brain morphology. Psychological Medicine, 47(1), 171–181. https://doi.org/10.1017/S0033291716002403
- Schmidt, H. D., & Duman, R. S. (2010). Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology, 35(12), 2378–2391. https://doi.org/10.1038/npp.2010.114
- Seo, M. K., Ly, N. N., Lee, C. H., Cho, H. Y., Choi, C. M., Nhu, L. H., Lee, J. G., Lee, B. J., Kim, G. M., Yoon, B. J., Park, S. W., & Kim, Y. H. (2016). Early life stress increases stress vulnerability through BDNF gene epigenetic changes in the rat hippocampus. Neuropharmacology, 105, 388–397. https://doi.org/10.1016/j.neuropharm.2016.02.009
- Torres-Berrío, A., Issler, O., Parise, E. M., & Nestler, E. J. (2019). Unraveling the epigenetic landscape of depression: Focus on early life stress. Dialogues in Clinical Neuroscience, 21(4), 341–357. https://doi.org/10.31887/DCNS.2019.21.4/enestler
- Tractenberg, S. G., Levandowski, M. L., de Azeredo, L. A., Orso, R., Roithmann, L. G., Hoffmann, E. S., Brenhouse, H., & Grassi-Oliveira, R. (2016). An overview of maternal separation effects on behavioural outcomes in mice: Evidence from a four-stage methodological systematic review. Neuroscience & Biobehavioral Reviews, 68, 489–503. https://doi.org/10.1016/j.neubiorev.2016.06.021
- Vetulani, J. (2013). Early maternal separation: A rodent model of depression and a prevailing human condition. Pharmacological Reports, 65(6), 1451–1461. https://doi.org/10.1016/S1734-1140(13)71505-6
- Wang, Z., Dai, J., Chen, L., Huang, Y., & Zhao, Y. (2011). Preventive action of panax ginseng roots in hypercortisolism-induced impairment of hippocampal neurons in male C57BL/6N mice. Phytotherapy Research, 25(8), 1242–1245. https://doi.org/10.1002/ptr.3389
- Wang, G., Lei, C., Tian, Y., Wang, Y., Zhang, L., & Zhang, R. (2019). Rb1, the primary active ingredient in panax ginseng C.A. Meyer, exerts antidepressant-like effects via the BDNF-TrkB-CREB pathway. Frontiers in Pharmacology, 10, 1–12. https://doi.org/10.3389/fphar.2019.01034
- Wang, W., Wang, G. J., Xie, H. T., Sun, J. G., Zhao, S., Jiang, X. L., Li, H., Lv, H., Xu, M. J., & Wang, R. (2007). Determination of ginsenoside Rd in dog plasma by liquid chromatography–mass spectrometry after solid-phase extraction and its application in dog pharmacokinetics studies. Journal of Chromatography B Analytical Technologies in the Biomedical & Life Sciences, 852(1–2), 8–14. https://doi.org/10.1016/j.jchromb.2006.12.046
- Wang, R., Wang, W., Xu, J., Liu, D., Jiang, H., & Pan, F. (2018). Dynamic effects of early adolescent stress on depressive-like behaviors and expression of cytokines and JMJD3 in the prefrontal cortex and hippocampus of rats. Frontiers in Psychiatry / Frontiers Research Foundation, 9. https://doi.org/10.3389/fpsyt.2018.00471
- Wang, W. K., Zhou, Y., Fan, L., Sun, Y., Ge, F., & Xue, M. (2021). The antidepressant-like effects of danggui buxue decoction in GK rats by activating CREB/BDNF/TrkB signaling pathway. Phytomedicine, 89, 153600. https://doi.org/10.1016/j.phymed.2021.153600
- Williams, L. M., Debattista, C., Duchemin, A. M., Schatzberg, A. F., & Nemeroff, C. B. (2016). Childhood trauma predicts antidepressant response in adults with major depression: Data from the randomized international study to predict optimized treatment for depression. Translational Psychiatry, 6(5), e799. https://doi.org/10.1038/tp.2016.61
- Ye, Y. L., Zhong, K., Liu, D. D., Xu, J., Pan, B. B., Li, X., Yu, Y. P., & Zhang, Q. (2017). Huanglian-jie-Du-tang extract ameliorates depression-like behaviors through BDNF-TrkB-CREB pathway in rats with chronic unpredictable stress. Evidence-Based Complementary and Alternative Medicine, 2017, 1–13. https://doi.org/10.1155/2017/7903918
- Zhang, H., Chen, Z., Zhong, Z., Gong, W., & Li, J. (2018). Total saponins from the leaves of panax notoginseng inhibit depression on mouse chronic unpredictable mild stress model by regulating circRNA expression. Brain and Behavior, 8(11), 1–10. https://doi.org/10.1002/brb3.1127
- Zhao, Y., Wang, Z., Dai, J., Chen, L., Huang, Y., & Zhan, Z. (2012). Beneficial effects of benzodiazepine diazepam on chronic stress-induced impairment of hippocampal structural plasticity and depression-like behavior in mice. Behavioural Brain Research, 228(2), 339–350. https://doi.org/10.1016/j.bbr.2011.12.013
- Zhu, S., Shi, R., Wang, J., Wang, J. F., & Li, X. M. (2014). Unpredictable chronic mild stress not chronic restraint stress induces depressive behaviours in mice. Neuroreport, 25(14), 1151–1155. https://doi.org/10.1097/WNR.0000000000000243
- Zhu, S., Wang, J., Zhang, Y., Li, V., Kong, J., He, J., & Li, X. M. (2014). Unpredictable chronic mild stress induces anxiety and depression-like behaviors and inactivates AMP-activated protein kinase in mice. Brain Research, 1576, 81–90. https://doi.org/10.1016/j.brainres.2014.06.002