?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The aim of this study was to investigate acid hydrolysis-bound phenols from Rosa roxburghii Tratt and their biological activities in vitro. The optimal extraction parameters were as follows: HCl concentration of 7 mol/L, extraction time of 10 h and material-liquid ratio of 1:40 g/mL. The highest yield of bound polyphenols was 495.64 ± 11.66 mg GAE/100 g DW. There were 24 phenolic compounds and 14 flavonoids released by acid hydrolysis. The antioxidant study showed that the RRT-BPs had a certain scavenging ability of free radicals in a dose-dependent manner. RRT-BPs showed good inhibitory activity against α-glucosidase and pancreatic lipase, respectively, and RRT-PBs acted as anticompetitive and mixed inhibitors against α-glucosidase and pancreatic lipase, respectively. Our study suggests that acid-hydrolysed bound phenols have good prebiotic properties and can be used as a supplement to existing alkaline-hydrolysed bound phenols to prevent chronic diseases such as diabetes and obesity.
1. Introduction
The most prevalent secondary metabolite in plants, phenols, are widely consumed as part of a plant-based diet and contain a variety of biological properties, including antioxidant, antidiabetic, and hypolipidaemic activity (Zhang et al., Citation2021). Numerous studies have shown that polyphenols are effective in controlling blood glucose and suppressing obesity. Because polyphenols can limit the activity of α-glucosidase, they can lessen the digestion and absorption of glucose in the intestine, hence regulating the postprandial glycaemic response. In addition, polyphenols can inhibit pancreatic lipase activity to reduce fat absorption in the small intestine, thus helping to reduce caloric intake and prevent excessive weight gain. Compared to drugs that delay and/or prevent the absorption of glucose and fat from meals, natural polyphenols have the advantage of low toxicity and fewer side effects. Therefore, polyphenols, as one of the sources of digestive enzyme inhibitors, have great potential for development.
Both the free form (extractable fraction), which is frequently found in the vesicles of plant cells, and the bound form (nonextractable fraction), which is related to other naturally occurring chemical substances through ester, ether, and glycosidic linkages, are found in plants (Wang et al., Citation2020). It was shown that rutin, quercetin and kaempferol-3-O-rutinoside were present in buckwheat in both free and bound forms, while dihydromyricetin was present only in bound form (Zhu et al., Citation2019). The major predominant bound phenolics in pummelo, shamouti and clementine were ferulic acid (Alu’datt et al., Citation2017). Instead of bound phenols, the majority of recent investigations have concentrated on the structure and functional activity of free phenols. Most of the neglected bound phenols in fruits and vegetables are released in the small intestine and colon during digestion (Saura-Calixto, Citation2012), which may lead to an underestimation of the total phenolic content and neglect of the nutritional function of bound polyphenols. Studies have shown that the content of bound polyphenols is much higher than that of free polyphenols in a variety of plant sources and that bound polyphenols exhibit better biological activity than free polyphenols (Sun et al., Citation2002). For example, bound phenolics from grapefruit peels or barley malts were found to have significantly higher radical (DPPH and ABTS) scavenging ability and a higher cellular antioxidant activity (Ademosun et al., Citation2015; Chen et al., Citation2019). Red quinoa-bound phenol has stronger antioxidant activity and hypoglycaemic activity than free phenol (Zhang et al., Citation2022). Therefore, it is important to account for bound phenolics when estimating the bioactive values of foods. This will contribute to the full development and utilization of natural chemical resources.
Rosa roxburghii Tratt (RRT), a member of the Rosaceae family, is widely distributed in southwestern China. RRT fruit has seen an increase in applications recently in the food sector, where it is used to make preserved meals and desserts, as well as in the beverage industry, where it is used to make wine, beer and soft drinks. As a medicinal and edible resource, RRT has recently received increasing attention because it is rich in nutrients and functional components, such as polysaccharides, ascorbic acid, phenols and superoxide dismutase, which have been shown to have antioxidant, antiatherosclerotic, hypoglycaemic, antiaging and antitumour activities (Wang et al., Citation2021a). Recently, Su et al. (Citation2022) elucidated the liberation and phytochemical profile of bound polyphenols present in dietary fibre (RRDF) isolated from Rosa roxburghii fruit pomace during in vitro-simulated digestion and colonic fermentation, and the results showed that the bound polyphenols were released from RRDF at a higher ratio during colonic fermentation than gastrointestinal digestion. It has also been shown that both free and bound polyphenols in RRT extracts can improve the structure of faecal microbial communities and increase the accumulation of short-chain fatty acids (Zhou et al., Citation2023). Huang et al. (Citation2022) found that alkali-hydrolysed RRT-BPs have better scavenging activity in vitro. Xu et al. (Citation2023) used UHPLC-IM-QTOF and UPLC-QQ to compare and analyse the free and alkaline hydrolysis-bound phenolic compounds in the fruits of three chestnut rose varieties from different regions. In summary, there are many functional and active bound phenols in Rosa roxburghii. However, the characterization and prebiotic properties of RRT-BPs by acid hydrolysis are still limited, especially in terms of their ability to lower blood glucose and inhibit obesity.
In this study, the optimal extraction process of acid hydrolysis released bound phenolic in RRT by orthogonal testing and its characterization were determined by LC–MS. The antioxidation and the effects of RRT-BPs on α-glucosidase and lipase activities were also studied in vitro. This research result can be supplemented by existing studies on alkaline hydrolysis of bound phenols, which can aid in our comprehensive comprehension of the features of various methods for obtaining bound phenols from Rosa roxburghii.
2. Materials and methods
2.1. Materials
Folin-Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), α-glucosidase (1KU/13.4 mg) were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China); p-nitrophenyl-β-D-galactopyranoside (p-NPG), acarbose, type II crude porcine pancreas lipase (30000 U/g), and p-nitrophenyl palmitate (p-NPP) were purchased from Yuanye Biological Co., Ltd. (Shanghai, China); orlistat capsules from Youngsure® were purchased in a local drugstore; and other analytical reagents used in this study were purchased from Fuyu Fine Chemical Co., Ltd. (Tianjin China) or Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All other chemicals and solvents used were analytical grade.
Fresh fruits of Rosa roxburghii Tratt were collected from the Longli County Rosa roxburghii Tratt Planting Base (N 106◦57′20′′ and E26◦33′36′′) in Guizhou Province, China. The cultivated variety Guilong No. 5, 100 kilograms of mature fruits of similar size, free from pests and diseases, and with complete fruit types, were stored at −80°C.
2.2. Sample preparation
The RRT fruits were freeze-dried, ground into a fine powder using a grinder, sieved through a 0.25 mm nylon sieve, and stored at −80°C until further study. The extractable phenolic compounds were extracted according to reported methods with slight modifications (Peng et al., Citation2017). Extractable phenolics were ultrasonically extracted from one gram of RRT powder for 30 min (400 W, 30°C) by using 10 mL of ethanol/water (80:20 v/v). Then, the extract was centrifuged at 1500 × g for 10 min, the supernatant was removed, the residue was extracted five times with the same solvent, and the residue was collected, freeze-dried and stored at −20°C for spare use.
2.3. Optimization of extraction conditions of RRT-BPs by acid hydrolysis
2.3.1. Effect of operating parameters on extraction yield
Extraction of bound phenol fractions (RRT-BPs) from RRT was accomplished using the previously reported acid hydrolysis method (Sun et al., Citation2021). Pretreated sample powder (1.0 g) was mixed with HCl (5 mol/L) at a ratio of 1:30 g/mL and placed in a water bath at 45°C for 2 h. Then, the mixture was adjusted to pH 2.0 with NaOH solution (6 mol/L). The mixture was extracted with ethyl acetate six times, and the organic fractions were collected, concentrated and dried to obtain the RRT-BPs. On this basis, we investigated the effects of hydrolysis time (4, 6, 8, 10, and 12 h), HCl concentration (3, 4, 5, 6, and 7 mol/L) and solid–liquid ratio (1:10, 1:20, 1:30, 1:40, and 1:50 g/mL) on the extraction rate of RRT-BPs.
2.3.2. Orthogonal experimental design
On the basis of the results of the single-factor experiment, three-factor and three-level experimental designs were used for the orthogonal experimental design. The factor levels are shown in .
Table 1. Factors and levels of L9(33) orthogonal experiment.
2.3.3. Determination of phenolic content
The analyses of bound phenolic contents from RRT-PBs were performed using Folin-Ciocalteu’s method according to Peng et al. (Citation2017) with some modifications. Briefly, the solution (200 μL) of samples or standards was mixed with 200 μL of Folin-Ciocalteu reagent. Na2CO3 solution (2.0 mL, 7%) was added after incubation for 6 min and kept in the dark at room temperature for 90 min. Gallic acid was used as the standard in the concentration range from 20 to 300 mg/mL (R2 = 0.9978). After measuring the absorbance value of the mixture at 760 nm (Multiskan SkyHigh, Thermo Fisher Scientific, U.S.A.), the results were expressed as mg of gallic acid equivalents (GAE)/g dry weight (DW).
2.4. Identification of phenolic substances in acid hydrolysis extract by LC–MS
The identification of phenolic substances was performed according to the method described by Zelena et al. (Citation2009) and Zhang et al. (Citation2023). The LC analysis was conducted on an ACQUITY UPLC System (Waters, Milford, MA, U.S.A.). Chromatographic analysis was performed with an ACQUITY UPLC HSS T3 (150 × 2.1 mm, 1.8 µm) (Waters, Milford, MA, U.S.A.). The column was maintained at 40°C. The flow rate and injection volume were set at 0.25 mL/min and 2 µL, respectively. For LC-ESI(-)-MS analysis, the mobile phase consisted of (A) acetonitrile and (B) ammonium formate (5 mM). Separation was carried out with the following gradient: 0 ~ 1 min, 2% A; 1 ~ 9 min, 2 ~ 50% A; 9 ~ 12 min, 50 ~ 98% A; 12 ~ 13.5 min, 98% A; 13.5 ~ 14 min, 98 ~ 2% A; and 14 ~ 17 min, 2% A. For LC-ESI(+)-MS analysis, the mobile phases consisted of (C) 0.1% formic acid acetonitrile solution (v/v) and (D) 0.1% formic acid aqueous solution (v/v). Separation was carried out at the following gradient: 0 ~ 1 min, 2% C; 1 ~ 9 min, 2 ~ 50% C; 9 ~ 12 min, 50 ~ 98% C; 12 ~ 13.5 min, 98% C; 13.5 ~ 14 min, 98 ~ 2% C; and 14 ~ 20 min, 2% C (Zelena et al., Citation2009; Zhang et al., Citation2023). Mass spectrometry detection was performed using an ESI ionization source on Q Active (Thermo Fisher Scientific, U.S.A.). Simultaneously, MS1 and MS/MS (Full MS-ddMS2 mode data dependent MS/MS) were used for collection. The parameters were as follows: sheath gas pressure, 30 arb; spray voltage, 3.50 kV and −2.50 kV for ESI(+) and ESI(-), respectively; MS/MS resolving power 17,500 FWHM; auxiliary gas flow rate, 10 arb; capillary temperature, 325°C; MS1 resolving power 70,000 FWHM; MS1 range, m/z 100–1000; number of data-dependent scans per cycle, 10; normalized collision energy, 30 eV; and dynamic exclusion time, automatic.
2.5. Antioxidant activity assays
2.5.1. DPPH radical scavenging activity
The DPPH radical-scavenging assay was performed according to the method described by Wang et al. (Citation2015) with slight modifications. Briefly, 150 μL of 0.1 mM DPPH ethanol solution was mixed with 50 μL of sample solution in a 96-well plate (Asample), and the mixture was reacted in the absence of light for 30 min. The absorbance values were measured at 517 nm, and ascorbic acid was used as a positive control. All measurements were performed in triplicate, and the DPPH radical scavenging rate was measured as follows:
Ab as the absorbance value of the sample solution.
2.5.2. ABTS radical scavenging activity
ABTS was assessed based on a previously reported method (Zhu et al., Citation2020) with slight modifications. The sample solution of each concentration (0.4 mL) was mixed with 2 mL of ABTS working solution and placed in the dark at room temperature for 6 min. The absorbance value of the mixture was measured at 734 nm. Ascorbic acid was used as a positive control. The ABTS radical scavenging activity (%) was calculated by the following formula:
2.5.3. Ferric reducing antioxidant power assay
The reducing power was determined according to a previously published method (Ak & Gülçin, Citation2008). A mixture of 1.0 mL of sample solution (RRT-BPs or ascorbic acid), 2.5 mL of phosphate buffer solution (0.2 mol/L, p H 6.6) and 2.5 mL of 1.0% K3[Fe(CN)6] was prepared and placed in a water bath at 50°C for 20 min. Then, 2.5 mL of 10.0% C2HCl3O2, 2.5 mL of distilled water and 0.5 mL of 0.1% FeCl3 were added, mixed and reacted for 10 min. The absorbance was measured at 700 nm, and a higher absorbance indicated a stronger reducing power.
2.6. Glucosidase inhibition assays
The α-glucosidase inhibitory activity was determined following the method of Chen et al. (Citation2019) with minor modifications. Briefly, 50 μL of α-glucosidase (1 unit mL−1) and 50 μL of sample (RRT-BPs or acarbose) were mixed and incubated for 10 min at 37°C. Then, 50 μL of pNPG (5 mM) was added to initiate the reaction. After another 5 min of incubation at the same temperature, the release of p-nitrophenol from pNPG was measured at 405 nm using an enzyme marker.
Based on Lineweaver–Burk plot analysis and Michaelis–Menten kinetics, the inhibition types of RRT-BPs against α-glucosidase were determined by increasing the concentration of substrate (pNPG 0.5, 1.0, 1.5, 2.0, 2.5 mM).
2.7. Lipase inhibition assays
The pancreatic lipase inhibition rate was determined as described by Liang et al. (Citation2014) with modifications. Briefly, 50 µL of 2 mg/mL pancreatic lipase (dissolved in 0.1 M phosphate buffer solution pH 7.4) was incubated with 100 µL of sample (RRT-BPs or orlistat) at 37°C for 10 min. Then, the reaction was activated by adding 100 µL of 20 mM p-NPP substrate solution, followed by incubation at 37°C for 20 min. Absorbance was detected at 405 nm by a microplate reader.
2.8. Statistical analysis
Data are presented as the means ± standard deviations of triplicate experiments. The response values of the one-way analysis of variance coupled with post hoc least significant difference and Duncan tests were performed using SPSS 25.0 software to compare means among the samples from single-factor experiments. Statistical significance was defined at p less than 0.05.
3. Results and discussion
3.1. Influence of a single factor on RRT-BP yield
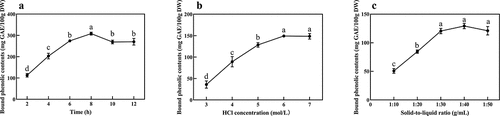
As shown in , the extraction rate of RRT-BPs increased during the hydrolysis treatment, reaching a peak of 298.91 mg GAE/100 g DW at 8 h. Thereafter, the extraction rate showed a decreasing trend (p < .05) as the extraction time continued. This may be because the hydrolysis time is too short to sufficiently break the chemical bonds attached to the bound phenols, while the extraction time is too long, which not only increases the energy loss but also oxidizes and decomposes the bound phenols, resulting in a lower extraction rate (Lu et al., Citation2015). Bound polyphenols cannot be extracted directly by the solvent because they are bound by cell wall substances, so hydrolysis release is a key step in the analysis of bound polyphenols, and acid hydrolysis mainly releases bound polyphenols by breaking glycosidic bonds (Wang et al., Citation2020). As shown in , the extraction rate of RRT-BPs showed a significant increasing trend (p < .05) with increasing HCl concentration, indicating that a high concentration of HCl can promote the breakage of glycosidic bonds and contribute to the release of RRT-BPs. When the concentration of HCl exceeded 6 mol/L, the extraction rate of RRT-BPs did not change significantly (p > .05), indicating that the release of RRT-BPs by HCl had reached saturation at this time. As shown in , the extraction rate reached its maximum as the solid–liquid ratio increased to 1:40, but there was no significant difference compared to 1:30 and 1:50 (p > .05). When the solid–liquid ratio is 1:10 and 1:20, the extraction rate is significantly lower than that at 1:30, 1:40 and 1:50 (p < .05). This may be because the system has high solution viscosity and slow molecular diffusion when the solid–liquid ratio is small (1:10 and 1:20), which makes it difficult to effectively release RRT-BPs from the tissue structure (Wang et al., Citation2021b). As the volume of hydrochloric acid solution increases, the solution can penetrate into the cell to disrupt the tissue structure, thus increasing the extraction rate of RRT-BPs.
Figure 1. Effect of different extraction parameters on bound phenolic contents from Rosa roxburghii Tratt (RRT). (a) Effect of different acidolysis time on bound phenolic contents from RRT. (b) Effect of different concentration of HCl on bound phenolic contents from RRT. (c) Effect of solid-to-liquid ratio on bound phenolic contents from RRT. Different letters (a-d) indicated statistically significant differences (p < .05).
3.2. Orthogonal test
The results of the optimization of the extraction conditions for the orthogonal test of L9 (33) are shown in . According to the R value and deviation squared, the factors affecting the extraction rate of RRT-BPs were ranked as B (HCl concentration) > C (solid–liquid ratio) > A (time). HCl can penetrate and destroy the cell wall so that the polyphenols bound to the matrix can be fully solubilized, so the extraction rate mainly depends on the solubilization ability of the solvent to bound phenols. As shown in , HCl has a significant impact on the extraction effect of bound polyphenols (p < .05). According to extreme difference analysis and variance analysis, the optimal extraction parameters of RRT-BPs were as follows: HCl concentration of 7 mol/L, extraction time of 10 h and solid–liquid ratio of 1:40 g/mL. However, this optimal parameter combination was not included in all tests. To determine the highest yield, a verification test was carried out with three replicate assays under these conditions. The yield of RRT-BPs reached 495.64 ± 11.66 mg GAE/100 g DW, which was higher than that of each treatment in the previous orthogonal experiment, and close to the yield of RRT-bound phenolic fraction by alkaline hydrolysis (532.7 mg GAE/100 g DW) reported by Su et al. (Citation2022). Compared to other substances, the yield of RRT-BPs was higher than that of rice bran-bound phenols (185 to 340 mg GAE/100 g) (Irakli et al., Citation2018), but lower than that of buckwheat-bound phenols (0.63 to 0.96 mg GAE/g DW) (Zhu et al., Citation2019). This indicates that the content of bound phenols may vary depending on different food types.
Table 2. Results and designs of orthogonal test.
Table 3. Analysis of orthogonal test.
3.3. LC–MS analysis of acid-hydrolysed bound phenol composition
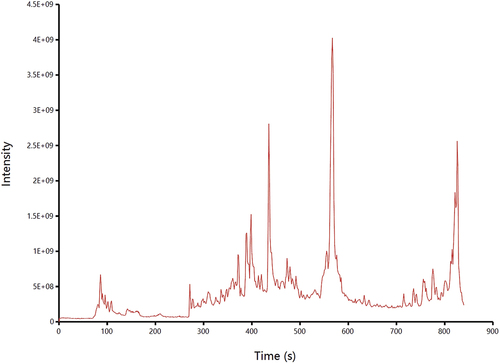
In our study, the acid-hydrolysed bound phenols in RRT were analysed by LC–MS in positive and negative ionization modes. The base peak chromatograms obtained from the RRT extracts are presented in . According to the retention time, precise mass and secondary fragments, a total of 38 polyphenolic compounds were identified. There were 24 phenolic acids and 14 flavonoids released by acid hydrolysis in RRT. The retention times and MS2 fragmentation patterns are shown in . The identified compounds can be divided into two groups, including phenolic acids and their derivatives and flavonoids and their derivatives.
Table 4. The phenolic compositions of the acid hydrolysis bound phenols.
The parent ions of compounds 1 and 15 are located at [M-H]− m/z 163.03, and the characteristic fragment ion is m/z 119, which indicates that these compounds are isomers of coumarinic acid. A comparison of available reports (Sun et al., Citation2020), as well as standard substances, confirms that these three substances are m-coumarinic acid and 2-hydroxycinnamic acid. Compound 2 has a relative molecular mass of 170.0215 and a parent ion of [M-H]− m/z 169.0121 and yields the major fragment ion at m/z 125.0249 ([M-H-CO2]−), which is characteristic of gallic acid. The parent ions of compounds 4 and 21 are located at [M-H]− m/z 167.0321 and 211.0232, respectively, the characteristic ion fragments of compound 4 are m/z 123.04 and m/z 152.01, and the characteristic fragment ions of compound 21 are m/z 123.04 and m/z 167.03. By comparing the database with relevant reports (Dong et al., Citation2021), it was determined that these two compounds were vanillic acid and 5-carboxyvanillic acid. The parent ions [M-H]− of compounds 7 and 10 were m/z 223.0596 and m/z 197.0452, respectively, and their characteristic fragment ions were compared with the reported literature data (Prakash et al., Citation2019; Tang et al., Citation2016) and tentatively determined to be sinapic acid and syringic acid. The parent ions of compounds 8, 11 and 22 were all located at [M-H]− m/z 137.0, and the characteristic fragment ions were all m/z 93.03, which suggested that the compounds were tautomers of hydroxybenzoic acid. The three compounds were identified as 3-hydroxybenzoic acid, 4-hydroxybenzoic acid and salicylic acid by further comparison with the standards. The molecular ion [M-H]− of compound 12 was m/z 193.0514, and the main characteristic ion fragment was m/z 178.03 ([M-H-CH3]−). The substance isoferulic acid was determined by comparison with relevant reports (Zheng et al., Citation2020). Compounds 25–38 belong to the flavonoid group. Based on the parent ion fragments and related characteristic fragments and by comparison with standards, 3,4-dihydroxyhydrocinnamic acid, caffeic acid, ellagic acid, 4-hydroxyphenylpyruvic acid, 3-hydroxyphenylacetic acid, 4-hydroxy-3-methoxymandelic acid, protocatechuic acid in phenolic acids, and arbutin, kaempferide, astragalin, catechin, cyanidin-3-glucoside, catechin, genistein, epicatechin, quercetin in flavonoids are mostly present in the acid hydrolysed bound phenols in RRT. It was shown that the main phenolic acids in RRT-free phenols were hydroxybenzoic acids, including protocatechuic acid (3,4-dihydroxybenzoic acid), gallic acid (3,4-trihydroxybenzoic acid) and ellagic acid (dimeric derivatives of gallic acid), and the main flavonoids were flavonols (poppycin, quercetin and kaempferol) and flavone-3-ols (catechin, epicatechin and its derivatives) (Wang et al., Citation2021b), which were similar to the results identified in this study. In addition, this study also identified components that are different from free phenols, such as perilalin and syringic acid, indicating that the bound phenols released by acid hydrolysis may have different phenolic acid compositions from free phenols and may also lead to different biological activities. Some scholars found approximately seven compounds in the extracts of Kainth-bound phenolics, most of which are flavonoids (Prakash et al., Citation2019). Xu et al. (Citation2023) used UPLC-IM-QTOF and UPLC-QQQ to analyse the free and bound phenolic (alkaline hydrolysis) fractions in three cultivars of chestnut rose fruit from different producing areas and found that catechin, procyanidin B1, gallic acid, ellagic acid and isoquercitrin were the five most abundant phenolics. Nine bound phenolics were also identified from Red Quinoa, of which phenolic acids and flavonoids were the main components (Zhang et al., Citation2022). In addition, alkaline hydrolysis is able to efficiently hydrolyse the ether bond or ester bond between phenolic compounds and food substrates, while acid hydrolysis tends to hydrolyse glycosidic bonds. Moreover, the phenolic extracts after alkaline hydrolysis and acid hydrolysis were almost all individual phenols, with few glycoside phenols, while the soluble phenols had many types of glycoside phenols (Zhu et al., Citation2020), indicating differences in the composition of binding phenols compared to raw materials and extraction methods.
3.4. Antioxidant capacity of RRT-BPs
Excess oxygen free radicals in cells and tissues can lead to oxidative stress, which is implicated in the pathogenesis of several diseases, such as diabetes (Sinan et al., Citation2021). However, to decrease the rate of oxidative damage within our cells, it is suggested that individuals obtain more antioxidants from their diets, and exogenous antioxidants are widely recognized compounds that help reduce or inhibit the production of free radicals, thereby reducing the risk of diseases caused by oxidative stress. Antioxidant activity is based on the ability to electronically neutralize free radicals, and phytochemicals such as polyphenols and polysaccharides have shown such effects (Peng et al., Citation2017). The antioxidant capacity of RRT-BPs was assessed by DPPH and ABTS free radical scavenging activity and total reducing capacity, and the results are shown in .
Figure 3. Antioxidant activities of RRT-BPs in vitro. (a) DPPH radical scavenging activity, (b) ABTS radical scavenging activity, (c) Ferric reducing antioxidant power assay. Different lowercase letters within the same concentration show differences between samples (p < .05). Different uppercase letters within the same sample indicate statistically significant differences (p < .05).
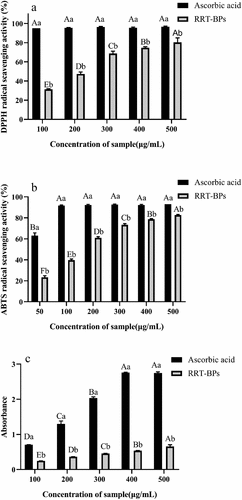
Antioxidants’ ability to deliver hydrogen is frequently assessed based on their DPPH radical scavenging capability. As the concentration of RRT-BPs increased from 100 µg/mL to 500 µg/mL, the scavenging rate increased from 31% to 80%, and the IC50 value was 234.9 µg/mL. When polyphenols scavenge radicals, hydrogen on phenolic hydroxyl is provided to radicals to form more stable phenoxy radicals, which reduces the transmission rate of automatic oxidation chain reactions and thus inhibits further lipid oxidation (Wang et al., Citation2021a). Therefore, the DPPH radical scavenging activity of RRT-BPs was dose dependent. Although the activity of RRT-BPs was lower than that of ascorbic acid, the difference decreased with increasing concentration. The RRT-BPs exhibited higher antioxidant activity than apple-bound phenol DPPH radical scavenging capacity (EC50, 4.55 mg/mL) (Luo et al., Citation2016). He et al. (Citation2016) found that the IC50 values of DPPH radical scavenging capacity of RRT-extractable polyphenols were 0.25–0.49 mg/mL, and the results of the present study were similar to this result, suggesting that acid-hydrolysed bound phenols, as one of the bound phenols in Rosa roxburghii, may contribute to antioxidant effects that cannot be ignored. As shown in , the scavenging of ABTS radicals by RRT-BPs increased with increasing concentration. When the concentration was 500 μg/mL, the scavenging rate of ABTS radicals by RRT-BPs reached 83%. Higher IC50 values (155.3 μg/mL) of the acid-hydrolysed bound phenols were found compared to the IC50 values of apple-bound phenols (0.82 mg/mL) in the ABTS radical scavenging assay.
As shown in , the reducing ability of RRT-BPs was significantly lower than that of ascorbic acid (p < .05), but the reduction ability of RRT-BPs gradually increased with increasing concentration, indicating that RRT-BPs could provide electrons to reduce trivalent ferric ions. Ferric iron ions are associated not only with lipids but also with protein oxidation (de Camargo et al., Citation2017). Hydroxyl radicals are generated by the Fenton reaction (Haber-Weiss cycle) in the presence of hydrogen peroxide and ferrous ions, in addition to the production of ferric ions. In this cycle, a series of dynamic redox reactions continuously take place, in which ferrous ions are oxidized to trivalent iron, while the latter is reduced to gain in the form of ferrous iron. According to relevant reports (Zhang & Tsao, Citation2016), phenolic compounds are able to chelate ferrous ions. Therefore, RRT-BPs can also act as ferrous ion chelators, stopping or delaying the Fenton reaction by reducing iron to ferrous ions and chelating the latter, thus achieving the antioxidant goal.
In all three antioxidant experiments, the acid hydrolysis-bound phenolics demonstrated good antioxidant capacity. This might be due to the higher amount of antioxidant compounds (i.e. phenolics and flavonoids) in the bound form, which probably contributed to the high antioxidant potential. In addition, the concentration and type of phenolic compounds in the sample can affect the level of antioxidant activity. For example, substances such as gallic acid, catechins and epigallocatechin, which contain many hydroxyl groups, can act as good electron donors and have free radical scavenging effects (Jiang et al., Citation2021).
3.5 Inhibition of α-glucosidase by RRT-BPs
α-Glucosidase hydrolyses the glycosidic bonds in oligosaccharides and disaccharides to release D-glucose. Glucosidase inhibitors prevent α-glucosidase from functioning, thereby delaying D-glucose absorption and lowering postprandial blood glucose levels (Kim et al., Citation2010). Thus, lowering blood glucose levels and treating related diseases can be achieved by inhibiting glucosidase activity. In recent years, polyphenols have been reported to have the potential to be alternatives to commercial hypoglycaemic medicines, such as acarbose, due to their inhibitory activity against carbohydrate-hydrolysing enzymes, including α-amylase and α-glucosidase (Zheng et al., Citation2020). As shown in , the inhibition of α-glucosidase by RRT-BPs tended to increase with increasing concentration, but its activity was less than that of acarbose. The IC50 values of RRT-BPs and acarbose were 459.1 μg/mL and 0.018 μg/mL, respectively. Our results showed that the inhibitory effect of RRT-BPs on α-glucosidase was weaker than that of acarbose, but the inhibitory effect of RRT-BPs on α-glucosidase reached more than 80% when the sample concentration reached 0.8 mg/mL, indicating that RRT-BPs have good inhibitory activity against α-glucosidase in a certain concentration range. Quinoa seed-bound phenolic compounds inhibited α-glucosidase with IC50 values ranging from 37.58 to 55.58 mg/mL (Tang et al., Citation2016), indicating that the acid-hydrolysed bound phenols (IC50 459.1 μg/mL) in RRT have markedly higher α-glucosidase inhibition than quinoa seed but lower α-glucosidase inhibition than bound polyphenols (IC50 167.8 μg/mL) extracted from mung bean skin dietary fibre (Zheng et al., Citation2020). According to the study, gallic acid, quercetin, isoquercitrin and catechin inhibited α-glucosidase and significantly reduced the rate of hydrolysis of different starches (Zhou et al., Citation2013). Ellagic acid (180 mg/day for 8 weeks), as a strong α-glucosidase inhibitor (Marella et al., Citation2020), significantly reduced blood glucose levels and insulin resistance in patients with type 2 diabetes (Ghadimi et al., Citation2021). Studies have shown that the structure of phenolic compounds can influence the inhibition of α-glucosidase, quercetin can bind to the active site of α-glucosidase, inhibit its catalytic activity, and exhibit strong α-glucosidase inhibition (Zhu et al., Citation2019). The phenolic acids and flavonoids mentioned above are in agreement with our identification results. Therefore, the α-glucosidase inhibitory activity of RRT-BPs was mainly attributed to the presence of phenolic compounds.
Figure 4. Inhibition of α-glucosidase by RRT-BPs. (a) Inhibitory effect of acarbose on α-glucosidase, (b) Inhibitory effect of RRT-BPs on α-glucosidase, (c) Reversibility of RRT-BPs mediated inhibition, (d) Lineweaver−Burk plots.
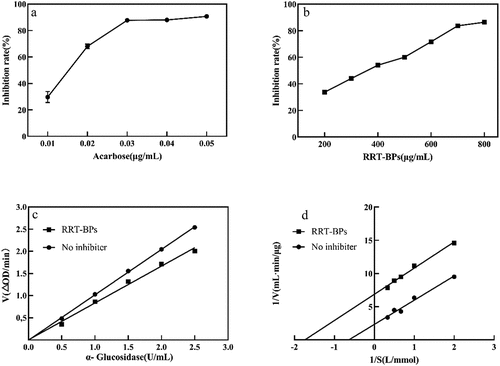
The inhibition kinetic curves of α-glucosidase by RRT-BPs showed that the velocity and enzyme concentration could pass the origin, and the slope of the RRT-BP curve was smaller than that of the control, indicating that RRT-BPs interfered with α-glucosidase by reversible inhibition (). To determine the type of reversible inhibition of α-glucosidase by RRT-BPs, further analysis using the Lineweaver–Burk double inverse curve indicated that the inhibition of α-glucosidase by RRT-BPs was of the anticompetitive inhibition type (). Vmax = 0.43 μg/(mL·min) and Km = 1.567 mmol/L in the no-inhibitor group, while Vmax = 0.146 μg/(mL·min) and Km = 0.575 mmol/L in the RRT-BP group. Both Vmax and Km of RRT-BPs were smaller than those of the no-inhibitor group, which was consistent with the anti-competitive inhibition. This suggests that RRT-BPs cannot bind to the free enzyme but can bind to the complex of enzyme and substrate and prevent product generation, resulting in a reduction in the catalytic activity of the enzyme. Numerous studies have been conducted to report the inhibitory activity of polyphenols on α-glucosidase and their inhibition kinetic patterns. For example, Chen et al. (Citation2019) found that chingiitannin A from the unripe fruit of Rubus Chingii Hu had the highest inhibitory activity against α-glucosidase and had a reversible and noncompetitive inhibition pattern; Kalita et al. (Citation2018) found that potato phenolic acids were mixed inhibitors of α-glucosidase. We found that RRT-BPs are anticompetitive inhibitors of α-glucosidase, and the reason for the different types of inhibition may be that polyphenols of different raw materials have different structures and stabilities. In conclusion, RRT-BPs have promising applications as potential α-glucosidase inhibitors.
3.6. Inhibition of lipase by RRT-BPs
Lipase is secreted in the pancreas as a catalyst to aid in the hydrolysis of triglycerides in the small intestine. It has been estimated that approximately 50–70% of total dietary fats are hydrolysed for absorption by pancreatic lipase (Patil et al., Citation2017; Vijayaraj et al., Citation2019), which emphasizes the importance of this enzyme in calorie release from diets. show that the inhibitory effect of bound phenol extracts on pancreatic lipase increases with increasing mass concentration, and the inhibitory activity of RRT-BPs on lipase was much lower than that of orlistat. Bustanji et al. (Citation2010) isolated rosmarinic acid, gallic acid, caffeic acid, and chlorogenic acid from rosemary and found that they all inhibited the activity of pancreatic lipase. Furthermore, the inhibition kinetic profile of lipase was similar to that of α-glucosidase, suggesting that the mode of lipase inhibition by RRT-BPs is also reversible (). The Lineweaver–Burk double inverse curve of lipase intersected in the second quadrant. In the noninhibited group, Vmax = 0.064 mg/(mL·min) and Km = 0.687 mmol/L, while in the RRT-BP group, Vmax = 0.046 μg/(mL·min) and Km = 0.896 mmol/L. The decrease in Vmax and increase in Km compared to the noninhibitor group suggest that the reversible inhibition of the reversible inhibition of lipase by RRT-BPs can be categorized as mixed inhibition. This indicates that RRT-BPs can not only compete with substrates to bind lipase but also bind to lipase-substrate complexes. It has been shown that phenolic compounds in peppers show mixed inhibitory effects on lipase (Martinez-Gonzalez et al., Citation2017), which is consistent with our findings.
Figure 5. Inhibition of lipase by RRT-BPs. (a) inhibitory effect of orlistat on lipase, (b) inhibitory effect of RRT-BPs on lipase, (c) reversibility of RRT-BPs mediated inhibition, (d) Lineweaver−Burk plots.
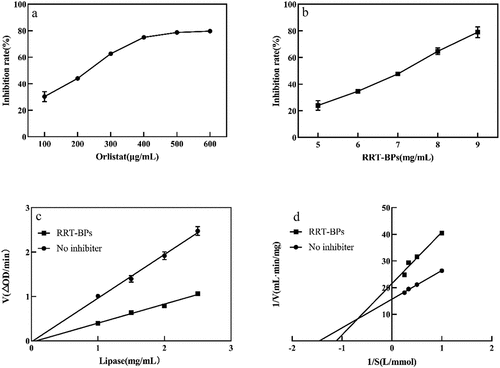
Orlistat is the only FDA-approved drug for inhibiting lipase. According to some reports, orlistat can stop approximately 30% of dietary fat from being absorbed; however, consistent use is linked to some unfavourable side effects, including flatulence, diarrhoea, oily spotting, incontinence, stomach cramping, and faecal urgency (Seyedan et al., Citation2015). As a result, it is critical to search for active lipase inhibitors, particularly natural substances that would be less likely to cause side effects. Although our results suggest that RRT-BPs have lower lipase inhibitory activity than orlistat, it is a side-effect-free natural product that can play an important role in the development of anti-obesity products. Previous studies have shown that flavonoids and tannins have lipase inhibitory activity (Justino et al., Citation2018). These molecules can inactivate lipase by nonspecific binding to the enzyme (Tan et al., Citation2017). In addition, gallic acid has been reported to have high inhibitory activity, and its gallic acyl moiety plays an important role in the inhibition of lipase activity (Rahim et al., Citation2015). In the present study, more species of phenolic acids and flavonoids were identified from RRT-BPs, and the relative content of gallic acid was high. Therefore, RRT-BPs exhibited good lipase inhibitory activity, probably because of their abundant polyphenolic substances. Taken together, these results indicate that RRT-BP exhibited potential prebiotic activity by modulating the antioxidant and against α-glucosidase and lipase, their anti- glucose and anti-lipid activity in vivo and underlying mechanisms will be further investigated in the future work. These findings contribute to acid-hydrolysed bound polyphenols may act as one of the important functional bound phenols contributing to the bioactivity of Rosa roxburghii.
4. Conclusion
In this study, we optimized the process of acid hydrolysis for extracting RRT-BPs as follows: HCl concentration of 7 mol/L, extraction time of 10 h and material-liquid ratio of 1:40 g/mL. The highest yield of bound polyphenols was 495.64 ± 11.66 mg GAE/100 g DW. We identified a total of 38 polyphenols from RT-BPs, and there were 24 phenolic compounds and 14 flavonoids released by acid hydrolysis in RRT-BPs. Moreover, it possessed good antioxidant properties and scavenged free radicals in vitro in a dose-dependent manner, indicating that acid-hydrolysed bound phenols, as a supplement to existing alkaline hydrolyzed bound phenols, have important antioxidant effects and good utilization potential. The inhibition types of RRT-BPs against α-glucosidase and lipase were anticompetitive inhibitors and mixed inhibitors, suggesting that they may have the potential to control postprandial blood glucose levels. The results were helpful for us to better understand acid-hydrolysed bound phenols, as one of the bound phenols in Rosa roxburghii, which may contribute to the improvement of human health and play an important role in the management or prevention of diabetes and obesity.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ademosun, A. O., Oboh, G., Passamonti, S., Tramer, F., Ziberna, L., Boligon, A. A., & Athayde, M. L. (2015). Phenolics from grapefruit peels inhibit HMG-CoA reductase and angiotensin-I converting enzyme and show antioxidative properties in endothelial EA.Hy 926 cells. Food Science and Human Wellness, 4(2), 80–12. https://doi.org/10.1016/j.fshw.2015.05.002
- Ak, T., & Gülçin, İ. (2008). Antioxidant and radical scavenging properties of curcumin. Chemico-Biological Interactions, 174(1), 27–37. https://doi.org/10.1016/j.cbi.2008.05.003
- Alu’datt, M. H., Rababah, T., Alhamad, M. N., Al-Mahasneh, M. A., Ereifej, K., Al-Karaki, G., Al-Duais, M., Andrade, J. E., Tranchant, C. C., Kubow, S., & Ghozlan, K. A. (2017). Profiles of free and bound phenolics extracted from citrus fruits and their roles in biological systems: Content, and antioxidant, anti-diabetic and anti-hypertensive properties. Food & Function, 8(9), 3187–3197. https://doi.org/10.1039/c7fo00212b
- Bustanji, Y., Issa, A., Mohammad, M., Hudaib, M., Tawah, K., Alkhatib, H., Almasri, I., & Al-Khalidi, B. (2010). Inhibition of hormone sensitive lipase and pancreatic lipase by Rosmarinus officinalis extract and selected phenolic constituents. Journal of Medicinal Plants Research, 4(21), 2235–2242. https://doi.org/10.2147/DDDT.S7773
- Chen, Y., Chen, Z., Guo, Q., Gao, X., Ma, Q., Xue, Z., Ferri, N., Zhang, M., & Chen, H. (2019). Identification of ellagitannins in the unripe fruit of Rubus Chingii Hu and evaluation of its potential antidiabetic activity. Journal of Agricultural and Food Chemistry, 67(25), 7025–7039. https://doi.org/10.1021/acs.jafc.9b02293
- Chen, Y., Huang, J., Hu, J., Yan, R., & Ma, X. (2019). Comparative study on the phytochemical profiles and cellular antioxidant activity of phenolics extracted from barley malts processed under different roasting temperatures. Food & Function, 10(4), 2176–2185. https://doi.org/10.1039/c9fo00168a
- de Camargo, A. C., Regitano d’Arce, M. A. B., & Shahidi, F. (2017). Phenolic profile of peanut by-products: Antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Journal of the American Oil Chemists’ Society, 94(7), 959–971. https://doi.org/10.1007/s11746-017-2996-9
- Dong, R., Yu, Q., Liao, W., Liu, S., He, Z., Hu, X., Chen, Y., Xie, J., Nie, S., & Xie, M. (2021). Composition of bound polyphenols from carrot dietary fiber and its in vivo and in vitro antioxidant activity. Food Chemistry, 339, 127879. https://doi.org/10.1016/j.foodchem.2020.127879
- Ghadimi, M., Foroughi, F., Hashemipour, S., Rashidi Nooshabadi, M., Ahmadi, M. H., Ahadi Nezhad, B., & Khadem Haghighian, H. (2021). Randomized double-blind clinical trial examining the ellagic acid effects on glycemic status, insulin resistance, antioxidant, and inflammatory factors in patients with type 2 diabetes. Phytotherapy Research: PTR, 35(2), 1023–1032. https://doi.org/10.1002/ptr.6867
- He, J. Y., Zhang, Y. H., Ma, N., Zhang, X. L., Liu, M. H., & Fu, W. M. (2016). Comparative analysis of multiple ingredients in Rosa roxburghii and R. sterilis fruits and their antioxidant activities. Journal of Functional Foods, 27, 29–41. https://doi.org/10.1016/j.jff.2016.08.058
- Huang, D., Li, C., Chen, Q., Xie, X., Fu, X., Chen, C., Huang, Q., Huang, Z., & Dong, H. (2022). Identification of polyphenols from Rosa roxburghii Tratt pomace and evaluation of in vitro and in vivo antioxidant activity. Food Chemistry, 377, 131922. https://doi.org/10.1016/j.foodchem.2021.131922
- Irakli, M., Kleisiaris, F., Kadoglidou, K., & Katsantonis, D. (2018). Optimizing extraction conditions of free and bound phenolic compounds from rice by-products and their antioxidant effects. Foods, 7(6), 93. https://doi.org/10.3390/foods7060093
- Jiang, S., Deng, N., Zheng, B., Li, T., & Liu, R. H. (2021). Rhodiola extract promotes longevity and stress resistance of Caenorhabditis elegans via DAF-16 and SKN-1. Food & Function, 12(10), 4471–4483. https://doi.org/10.1039/d0fo02974b
- Justino, A. B., Miranda, N. C., Franco, R. R., Martins, M. M., Silva, N. M. D., & Espindola, F. S. (2018). Annona muricata Linn. Leaf as a source of antioxidant compounds with in vitro antidiabetic and inhibitory potential against α-amylase, α-glucosidase, lipase, non-enzymatic glycation and lipid peroxidation. Biomedicine & Pharmacotherapy, 100, 83–92. https://doi.org/10.1016/j.biopha.2018.01.172
- Kalita, D., Holm, D. G., LaBarbera, D. V., Petrash, J. M., Jayanty, S. S., & Li, X.-Q. (2018). Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PLOS ONE, 13(1), e0191025. https://doi.org/10.1371/journal.pone.0191025
- Kim, M. H., Jo, S. H., Jang, H. D., Lee, M. S., & Kwon, Y.-I. (2010). Antioxidant activity and α-glucosidase inhibitory potential of onion (Allium cepa L.) extracts. Food Science and Biotechnology, 19(1), 159–164. https://doi.org/10.1007/s10068-010-0022-1
- Liang, L., Wang, T., Cai, Y., He, W., Sun, P., Li, Y., Huang, Q., Taglialatela-Scafati, O., Wang, H., & Guo, Y. (2014). Brominated polyunsaturated lipids from the Chinese sponge Xestospongia testudinaria as a new class of pancreatic lipase inhibitors. European Journal of Medicinal Chemistry, 79, 290–297. https://doi.org/10.1016/j.ejmech.2014.04.003
- Luo, J., Zhang, P., Li, S., & Shah, N. P. (2016). Antioxidant, antibacterial, and antiproliferative activities of free and bound phenolics from peel and flesh of fuji apple. Journal of Food Science, 81(7), M1735–1742. https://doi.org/10.1111/1750-3841.13353
- Lu, W.-R., Pan, D., Chen, J.-W., Chi, C.-D., & Chen, J.-C. (2015). Study on the ultrasonic-assisted extraction of the bound phenolics from quince peer. The Food Industry, 36(8), 86–90.
- Marella, S., Hema, K., Shameer, S., & Prasad, T. N. V. K. V. (2020). Nano-ellagic acid: Inhibitory actions on aldose reductase and α-glucosidase in secondary complications of diabetes, strengthened by in silico docking studies. Biotechnology, 10(10), 439. https://doi.org/10.1007/s13205-020-02411-1
- Martinez-Gonzalez, A. I., Alvarez-Parrilla, E., Díaz-Sánchez, Á. G., de la Rosa, L. A., Núñez-Gastélum, J. A., Vazquez-Flores, A. A., & Gonzalez-Aguilar, G. A. (2017). In vitro inhibition of pancreatic lipase by polyphenols: a kinetic, fluorescence spectroscopy and molecular docking study. Food Technology and Biotechnology, 55(4), 519–530. https://doi.org/10.17113/ftb.55.04.17.5138
- Patil, M., Patil, R., Bhadane, B., Mohammad, S., & Maheshwari, V. (2017). Pancreatic lipase inhibitory activity of phenolic inhibitor from endophytic diaporthe arengae. Biocatalysis and Agricultural Biotechnology, 10, 234–238. https://doi.org/10.1016/j.bcab.2017.03.013
- Peng, H., Li, W., Li, H., Deng, Z., & Zhang, B. (2017). Extractable and non-extractable bound phenolic compositions and their antioxidant properties in seed coat and cotyledon of black soybean (Glycinemax (L.) merr). Journal of Functional Foods, 32, 296–312. https://doi.org/10.1016/j.jff.2017.03.003
- Prakash, O., Baskaran, R., & Kudachikar, V. B. (2019). Characterization, quantification of free, esterified and bound phenolics in Kainth (Pyrus pashia Buch.-ham. Ex D.Don) fruit pulp by UPLC-ESI-HRMS/MS and evaluation of their antioxidant activity. Food Chemistry, 299, 125114. https://doi.org/10.1016/j.foodchem.2019.125114
- Rahim, A. T. M. A., Takahashi, Y., & Yamaki, K. (2015). Mode of pancreatic lipase inhibition activity in vitro by some flavonoids and non-flavonoid polyphenols. Food Research International, 75, 289–294. https://doi.org/10.1016/j.foodres.2015.05.017
- Saura-Calixto, F. (2012). Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. Journal of Agricultural and Food Chemistry, 60(45), 11195–11200. https://doi.org/10.1021/jf303758j
- Seyedan, A., Alshawsh, M. A., Alshagga, M. A., Koosha, S., & Mohamed, Z. (2015). Medicinal plants and their inhibitory activities against pancreatic lipase: A review. Evidence-Based Complementary and Alternative Medicine, 14, 1–13. https://doi.org/10.1155/2015/973143
- Sinan, K. I., Zengin, G., Fiorentino, A., D’Abrosca, B., Ak, G., Lobine, D., Etienne, O. K., Subratty, A. H., & Mahomoodally, M. F. (2021). Biological insights and NMR metabolic profiling of different extracts of spermacoce verticillata (L.) G. Mey. Chemistry & Biodiversity, 18(10), e2100371. https://doi.org/10.1002/cbdv.202100371
- Su, J., Fu, X., Huang, Q., Liu, G., & Li, C. (2022). Phytochemical profile, bioactivity and prebiotic potential of bound polyphenols released from Rosa roxburghii fruit pomace dietary fiber during in vitro digestion and fermentation. Food & Function, 17(17), 8880–8891. https://doi.org/10.1039/D2FO00823H
- Sun, J., Chu, Y. F., Wu, X., & Liu, R. H. (2002). Antioxidant and antiproliferative activities of common fruits. Journal of Agricultural and Food Chemistry, 50(25), 7449–7454. https://doi.org/10.1021/jf0207530
- Sun, Y., Deng, Z., Liu, R., Zhang, H., Zhu, H., Jiang, L., & Tsao, R. (2020). A comprehensive profiling of free, conjugated and bound phenolics and lipophilic antioxidants in red and green lentil processing by-products. Food Chemistry, 325, 126925. https://doi.org/10.1016/j.foodchem.2020.126925
- Sun, S., Huang, S., Shi, Y., Shao, Y., Qiu, J., Sedjoah, R.-C.-A.-A., Yan, Z., Ding, L., Zou, D., & Xin, Z. (2021). Extraction, isolation, characterization and antimicrobial activities of non-extractable polyphenols from pomegranate peel. Food Chemistry, 351, 129232. https://doi.org/10.1016/j.foodchem.2021.129232
- Tan, Y., Chang, S. K. C., & Zhang, Y. (2017). Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chemistry, 214, 259–268. https://doi.org/10.1016/j.foodchem.2016.06.100
- Tang, Y., Zhang, B., Li, X., Chen, P. X., Zhang, H., Liu, R., & Tsao, R. (2016). Bound phenolics of quinoa seeds released by acid, alkaline, and enzymatic treatments and their antioxidant and α-glucosidase and pancreatic lipase inhibitory effects. Journal of Agricultural and Food Chemistry, 64(8), 1712–1719. https://doi.org/10.1021/acs.jafc.5b05761
- Vijayaraj, P., Nakagawa, H., & Yamaki, K. (2019). Cyanidin and cyanidin-3-glucoside derived from Vigna unguiculata act as noncompetitive inhibitors of pancreatic lipase. Journal of Food Biochemistry, 43(3), e12774. https://doi.org/10.1111/jfbc.12774
- Wang, W., Chen, W., Yang, Y., Liu, T., Yang, H., & Xin, Z. (2015). New phenolic compounds from Coreopsis tinctoria Nutt. And their antioxidant and angiotensin i-converting enzyme inhibitory activities. Journal of Agricultural and Food Chemistry, 63(1), 200–207. https://doi.org/10.1021/jf504289g
- Wang, Z., Li, S., Ge, S., & Lin, S. (2020). Review of distribution, extraction methods, and health benefits of bound phenolics in food plants. Journal of Agricultural and Food Chemistry, 68(11), 3330–3343. https://doi.org/10.1021/acs.jafc.9b06574
- Wang, L., Lv, M., An, J., Fan, X., Dong, M., Zhang, S., Wang, J., Wang, Y., Cai, Z., & Fu, Y. (2021a). Botanical characteristics, phytochemistry and related biological activities of Rosa roxburghii Tratt fruit, and its potential use in functional foods: A review. Food & Function, 12(4), 1432–1451. https://doi.org/10.1039/d0fo02603d
- Wang, Y., Ouyang, F., Teng, C., & Qu, J. (2021b). Optimization for the extraction of polyphenols from Inonotus obliquus and its antioxidation activity. Preparative Biochemistry & Biotechnology, 51(9), 852–859. https://doi.org/10.1080/10826068.2020.1864642
- Xu, L., Yang, H. Z., Li, C. Z., Liu, S. Y., Zhao, H. D., Liao, X. J., & Zhao, L. (2023). Composition analysis of free and bound phenolics in chestnut rose (Rosa roxburghii Tratt.) fruit by UHPLC-IM-QTOF and UPLC-QQQ. LWT, 185, 115125. https://doi.org/10.1016/j.lwt.2023.115125
- Zelena, E., Dunn, W. B., Broadhurst, D., Francis McIntyre, S., Carroll, K. M., Begley, P., O’Hagan, S., Knowles, J. D., Halsall, A., HUSERMET Consortium, Wilson, I. D., & Kell, D. B. (2009). Development of a robust and repeatable UPLC-MS method for the long-term metabolomic study of human serum. Analytical Chemistry, 81(4), 1357–1364.
- Zhang, Y., Bai, B., Yan, Y., Liang, J., & Guan, X. (2022). Bound polyphenols from red quinoa prevailed over free polyphenols in reducing postprandial blood glucoserises by inhibiting α-glucosidase activity and starch digestion. Nutrients, 14(4), 728. https://doi.org/10.3390/nu14040728
- Zhang, L., Han, Z., & Granato, D. (2021). Polyphenols in foods: Classification, methods of identification, and nutritional aspects in human health. Advances in Food and Nutrition Research, 98, 1–33. https://doi.org/10.1016/bs.afnr.2021.02.004
- Zhang, D., Liu, J. M., Ruan, J. G., Jiang, Z. J., Gong, F. Y., Lei, W. J., Wang, X. Y., Zhao, J., Meng, Q. Y., Xu, M., Tang, X., & Li, H. J. (2023). Combination of millet pepper and garlic water extracts improves the antioxidant capability of myofibrillar protein under malondialdehyde-induced oxidative modification. LWT, 174, 114472. https://doi.org/10.1016/j.lwt.2023.114472
- Zhang, H., & Tsao, R. (2016). Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Current Opinion in Food Science, 8, 33–42. https://doi.org/10.1016/j.cofs.2016.02.002
- Zheng, Y., Liu, S., Xie, J., Chen, Y., Dong, R., Zhang, X., Liu, S., Xie, J., Hu, X., & Yu, Q. (2020). Antioxidant, α-amylase and α-glucosidase inhibitory activities of bound polyphenols extracted from mung bean skin dietary fiber. LWT, 132, 109943. https://doi.org/10.1016/j.lwt.2020.109943
- Zhou, B., Wang, F.-F., & Jang, H.-D. (2013). Enhanced antioxidant and antidiabetic activities of barley and wheat after soaking with tea catechin. Food Science and Biotechnology, 22(6), 1753–1761. https://doi.org/10.1007/s10068-013-0277-4
- Zhou, D., Zhong, J., Huang, Y., & Cheng, Y. (2023). Effect of free and bound polyphenols from Rosa roxburghii Tratt distiller’s grains on moderating fecal microbiota. Food Chemistry: X, 19, 100747. https://doi.org/10.1016/j.fochx.2023.100747
- Zhu, L., Li, W., Deng, Z., Li, H., & Zhang, B. (2020). The composition and antioxidant activity of bound phenolics in three legumes, and their metabolism and bioaccessibility of gastrointestinal tract. Foods, 9(12), 1816. https://doi.org/10.3390/foods9121816
- Zhu, H., Liu, S., Yao, L., Wang, L., & Li, C. (2019). Free and bound phenolics of buckwheat varieties: HPLC characterization, antioxidant activity, and inhibitory potency towards α-glucosidase with molecular docking analysis. Antioxidants, 8(12), 606. https://doi.org/10.3390/antiox8120606