 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed to investigate the impact of microwave drying (MD), microwave vacuum drying (MVD), freeze drying (FD), vacuum drying (VD), hot air drying (HAD), and sun drying (SD) on the quality attributes of freeze-thaw pretreated beetroots. The quality characteristics including physical properties, bioactive compounds, and antioxidant activities of dried beetroots were evaluated. The results showed that the freeze-thaw pretreated beetroots obtained using FD displayed the lowest shrinkage rate, hardness, and total color difference (ΔE), and the highest rehydration ratio of 6.49. FD, MD, and MVD resulted in porous structures of freeze-thaw pretreated beetroots. The highest betalains, ascorbic acid, total phenolic and total flavonoids contents were found in freeze-thaw pretreated beetroots prepared by MVD. Meanwhile, the largest FRAP value and ABTS free radical scavenging ability of freeze-thaw pretreated beetroots were obtained by MVD. These findings suggested that MVD is a more favorable method for drying freeze-thaw pretreated beetroots.
Introduction
Beetroot is botanically classified as a biennial herb of the Chenopodiaceae family (Varshney & Mishra, Citation2022), which is originated from Eastern and Southern Europe, and Northern Africa (De Oliveira et al., Citation2020). Beetroot is grown in many countries worldwide, and the main producers are France, Germany, the United States, Turkey, Ukraine, Poland, Egypt, the United Kingdom, and China (Chhikara et al., Citation2019). Beetroot contains appreciable amounts of bioactive compounds, including betalains, ascorbic acid, phenolic acids, flavonoids, carotenoids, nitrates and saponins (Lechner & Stoner, Citation2019). In recent years, there has attracted a growing scientific interest in the biological activities of beetroots. It has been reported that bioactive compounds in beetroots exhibit antioxidative, antidiabetic, anticancer, antiviral, anti-inflammation, blood pressure and lipid-lowering, anti-obesity, and immunomodulatory activities (Fu et al., Citation2020; Hadipour et al., Citation2020).
Due to its high moisture content, fresh beetroot deteriorates rapidly, with continuous metabolism and microbial attack, so it cannot be stored for an extended period. Drying is one of the most effective methods of food preservation because the removal of water reduces the volume and weight of the final product, and decreases the availability of water for chemical reactions, enzymatic reactions, and microbial growth, thereby improving the product stability for easy transport and storage (Paula et al., Citation2020). However, the dehydration of thermo-sensitive materials is a highly complex process involving mechanisms of heat and mass transfer, and the applied drying methods and drying parameters often provoke considerable alterations in the physical, organoleptic, biological, and chemical properties of the materials, such as color, texture, aroma, vitamins and phenolic compounds (An et al., Citation2016).
Sun drying (SD) is the most commonly used method worldwide with low cost. However, it is greatly affected by the weather, and the product quality cannot be guaranteed. Hot air drying (HAD) is one of the most widely used drying methods in the food processing industry; however, it has some shortcomings, such as a relatively long drying time, high temperature, degradation of nutritional substances (Marfil et al., Citation2008), color change, and shrinkage (Figiel, Citation2010). As the best dehydration method for heat-sensitive raw materials, freeze drying (FD) produces good-quality food with well-retained flavor and nutritional quality and maintains the material volume with porous structures and good rehydration ability (Janiszewska-Turak et al., Citation2021). It has the disadvantages of high energy consumption, high equipment costs, and long drying time, and is mainly used to produce high-value-added products (Köprüalan et al., Citation2021). Vacuum drying (VD) is an excellent drying technology that offers the advantages of a sealed environment, low oxygen content, and affordability (Dai et al., Citation2021). Several studies have confirmed that VD preserves the nutritional components, physicochemical properties, tissue structure and sensory quality of products well (Liu et al., Citation2021; Mitra et al., Citation2011). Microwave drying (MD), as a single drying method or in combination with other drying methods, is the most rapid and effective processing method used in laboratory and industrial applications for food preservation. MD has several advantages including fast processing (short time), uniform volumetric heating, high efficiency, low processing cost (low energy consumption), and an automatic and automated systems that are compatible with other processing technologies (Dhiman et al., Citation2021). Microwave vacuum drying (MVD) using microwaves under vacuum has the main advantage of preserving bioactive compounds because the desired drying is achieved at a lower temperature. During the MVD process, microwave energy is absorbed by water located in the entire volume of the material being dried (Figiel, Citation2010), and a large vapor pressure is generated in the center of the material, allowing the rapid transfer of moisture to the surrounding vacuum and preventing structural collapse (Lin et al., Citation1998). Different drying methods have significant effects on beetroot quality. Nistor et al. (Citation2017) and Liu et al. (Citation2022) studied the influence of different drying methods on the physicochemical properties and quality attributes of beetroots, respectively.
Freeze-thaw (FT) is a non-thermophysical pretreatment, consisting of two steps: freezing material to its freezing points and thawing the frozen material at higher temperatures (Wu et al., Citation2017). Freeze-thaw pretreatment is an efficient method to improve the drying rate by changing the cell membrane’s permeability and destroying the cell wall’s structure (Ando et al., Citation2016). It has been proven that freeze-thaw pretreatment can significantly improve the performance of different thermal drying processes owing to the rupture of cell membranes and walls, and prevent structural deformation during drying (Feng et al., Citation2020). FT pretreatment of lotus root has been shown to prompt more efficient drying, improve heating uniformity, and display undesirable effects on color, shrinkage, betalains, phenolics and ABTS scavenging activity (Bassey et al., Citation2022). Zhang et al. (Citation2022) reported that freeze-thaw improved drying rate, formed crisp texture of lotus root and saved the total energy consumption. It has been reported that the advantages of freeze-thaw treatment prior to MVD of carrot in terms of increased the drying rate, prevented structural deformation and increased rehydration rate (Ando et al., Citation2019). To date, there have been few thorough studies on the freeze-thaw pre-treatment of beetroots before drying.
To the best of our knowledge, the effects of different drying methods on the quality attributes of freeze-thaw pretreated beetroots have not been studied. This study aimed to evaluate the influence of different drying methods, including microwave drying (MD), microwave vacuum drying (MVD), freeze drying (FD), vacuum drying (VD), hot air drying (HAD), and sun drying (SD), on the physical properties, bioactive compounds, and antioxidant activities of freeze-thaw pretreated beetroots.
Materials and methods
Materials, chemicals, and reagents
Fresh beetroots (Beta vulgaris L. var. conditiva Alef.) were procured from a local market in Xuzhou, China. Fresh beetroots were stored under refrigeration (4°C) until further use. The ascorbic acid assay kit was obtained from the Nanjing Jiancheng Institute of Bioengineering (Nanjing, China). 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS, purity ≥ 98%), 2,4,6-tripyridinyl-1,3,5-triazine (TPTZ, purity ≥ 98.0%), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, purity ≥ 98.0%), gallic acid (purity ≥98%), and rutin (purity ≥98%) were supplied by Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China). All reagents used in this experiment were of analytical grade.
Preparation of freeze-thaw pretreated beetroots
Before experiments, fresh beetroots were washed with tap water to remove surface impurities, peeled and then sliced crosswise into slices with 7.5 cm in diameter and 4 mm in thickness using a stainless steel slicer.
Freeze-thaw pretreatment: Fresh beetroot slices were packed in polyethylene bags (28 × 40 cm, thickness = 0.15 mm). Filled each bag with 1000 g of beetroot slices and sealed them, then frozen them in the refrigerator (−20°C) for 12 h, and finally thawed them in the refrigerator (4°C) for 12 h. The surface moisture of the thawed beetroot slices was absorbed with absorbent paper and dried at different methods.
Drying processing methods
In this study, freeze-thaw pretreated beetroot slices were dried using the following drying methods:
Microwave drying (MD): Freeze-thaw pretreated beetroot slices (300.0 ± 2.0 g) were placed on a circular fiberglass tray (diameter = 30 cm) of microwave drying system (CEM SAM-255, Utah, U.S.A.). MD was performed at 650 W power and 2450 MHz frequency.
Microwave vacuum drying (MVD): Freeze-thaw pretreated beetroot slices (300.0 ± 2.0 g) were placed uniformly on the tray (61 × 43 × 5 cm) of a microwave vacuum dryer (Xinqi WBZ-10, Guiyang, China). MVD was conducted at frequency of 2450 MHz, vacuum degree of 0.09 MPa and cavity dimensions of 50 (diameter) × 85 cm (length). MVD was first performed at microwave power of 1000 W. After drying for 45 min, the microwave power was switched to 500 W to continue drying.
Freeze drying (FD): Freeze-thaw pretreated beetroot slices (300.0 ± 2.0 g) were frozen at −20°C for 12 h and then placed in the cavity (diameter = 20 cm, height = 30 cm) in the freeze dryer (Rikakikai FDU-2110, Tokyo, Japan) at temperature of −80°C and vacuum pressure of 4 Pa.
Vacuum drying (VD): Freeze-thaw pretreated beetroot slices (300.0 ± 2.0 g) were spread evenly on the tray (31 × 29 × 2 cm) of vacuum drying oven (Yiheng BPZ-6033B, Shanghai, China). VD was performed at 60°C and 0.09 MPa.
Hot air drying (HAD): Freeze-thaw pretreated beetroot slices (300.0 ± 2.0 g) were spread evenly on the tray (60 × 50 × 2 cm) of hot air tray dryer (Yiheng DHG-9245A, Shanghai, China). The hot air drying parameters were air speed of 1.0 m/s, relative humidity of 8.0%, and drying temperature of 60°C.
Sun drying (SD): Freeze-thaw pretreated beetroot slices (300.0 ± 2.0 g) were placed on a tray (60 × 50 × 2 cm) and dried in the sun from 8 am to 6 pm. The range of relative humidity was 30% to 70%, and the temperature was in the range of 30 to 35°C.
The drying process was terminated when the final moisture content of beetroot was below 7.00%. All the drying experiments were repeated three times. After drying, the dried samples were cooled to room temperature, put them into packaging bags, and then measured the physical properties of the samples.
Determination of moisture content
The moisture content was determined by a moisture analyzer (Mettler HX204, Zurich, Switzerland) at 105°C. The initial moisture content of fresh beetroots was 90.14% as determined by the moisture analyzer.
Determination of shrinkage rate and rehydration ratio
The shrinkage rate was determined by the solid displacement method using superfine quartz sand. Based on the volume change of the sample, the shrinkage rate was calculated using EquationEquation (1)(1)
(1) (Zielinska et al., Citation2015).
Where SR is shrinkage rate, %, V0 is the volume of fresh beetroots, mL, V1 is the volume of dried beetroots, mL.
The rehydration ratio was determined according to the method given by Bozkir and Ergün (Citation2020). Dried beetroot samples of 2.0 ± 0.1 g were immersed in a beaker with 200 mL of distilled water and the beaker was placed in a water bath at 80°C for 15 min. After rehydration, beetroot samples were removed from liquid, carefully blotted with absorbent papers in order to remove the excess of water and weighed. Rehydration experiments were conducted at least in triplicate for each sample. The rehydration ratio was calculated according to EquationEquation (2)(2)
(2) .
Where RR is rehydration ratio, W1 is the mass of dried beetroot samples, g, W2 is the mass of beetroots after rehydration, g.
Determination of hardness
The textural profile of dried beetroot was analyzed using a texture analyzer (Stable Micro Systems TA.XT PLUS, London, UK) equipped with a cylindrical probe (P/2, 2 mm in diameter). The test parameters were as follows: Test force using puncture mode; pre-speed and test speed of 2 mm/s; post-speed of 10 mm/s and test distance of 10 mm; trigger force of 5.0 g. Ten measurements were performed on each sample and the average was calculated.
Color measurement
In order to ensure the uniformity of color, fresh beetroots were mashed into pulp and then determined, and dried beetroot samples were ground into powder to determine the color. The color parameters were measured using a colorimeter (Konica CR-400, Tokyo, Japan) equipped with D65 illuminant system and 8 mm measuring area in the CIELAB system. Color parameters were expressed as L*, a*, and b*, L* indicates lightness, read from 0 (completely opaque or black) to 100 (completely transparent or white), a positive a* value indicates redness and –a* is greenness, and a positive b* value indicates yellowness (–b* is blueness) on the hue-circle (Pathare et al., Citation2013). Total color change (∆E) represents the magnitude of the color change of the sample after drying. Chroma (C) is the quantitative attribute of colorfulness, which denotes the saturation of the color. Hue angle (H°) indicates the color nuance and defines as follows: 0° (red), 90° (yellow), 180° (green), 270° (blue) (Paciulli et al., Citation2016). The ∆E, C, and H° are calculated according to EquationEquation (3)(3)
(3) , EquationEquation (4)
(4)
(4) , and EquationEquation (5)
(5)
(5) , respectively.
Where L*, a*, and b* are the values of dried samples, L0*, a0*, and b0* are the values of fresh samples.
Microstructure analysis
The micromorphology of dried beetroot was observed using a scanning electron microscope (SEM) (FEI Quanta 450 FEG, Portland, U.S.A.). Dried beetroots were cut into thin slices, fixed on a copper tube with the cross-section upward, and then plated with gold through an ion sputtering apparatus. The scanning was conducted at an accelerating voltage of 20.0 kV and magnification was set at 500 × .
Preparation of beetroot extracts
Dried beetroots obtained from three replicates were mixed and then ground into powder (passed through a 60-mesh sieve) to obtain samples with representative chemical components for each drying method. Two grams of beetroot powder was taken in a 50 mL centrifuge tube and extracted with 50% (v/v) ethanol solution (20 mL) using a vortex mixer (Kylin-Bell VORTEX-5, Jiangsu, China) for 2 min at room temperature. After centrifugation at 5000×g for 10 min using a centrifuge (Xiangyi H1850, Hunan, China), the residue was further extracted twice. The supernatants obtained from the three extractions were combined, and the volume was 100 mL with 50% (v/v) ethanol solution. The extracts were stored in reagent bottles at 4°C for further analysis. All analyses were finished within 7 days after obtaining the extracts.
Determination of betalains and ascorbic acid
Betalains are divided into two main categories: red-violet betacyanins (λ = 538 nm) and yellow-orange betaxanthins (λ = 480 nm). Betalains content was determined using the colorimetric method described by Stintzing et al. (Citation2005) with appropriate modifications. The extract was diluted with 0.05 M phosphate buffer solution (pH 6.5) to obtain absorption values between 0.8 and 1.0 at 538 nm. Then the absorbance of the diluted extract was measured at 480, 538, and 600 nm, respectively.
Ascorbic acid content was determined by colorimetric method using a detection kit (Nanjing Jiancheng Institute of Bioengineering, Nanjing, China). Results were calculated on a dry matter (d.m.) and expressed as milligrams per 100 grams (mg/100 g d.m.).
Measurement of total phenolic content and total flavonoids content
The total phenolic content was determined by the Folin-Ciocalteu method with slight changes (Alvarez-Parrilla et al., Citation2011). Diluted extract (0.5 mL) was mixed with 2.5 mL of 10% (v/v) Folin-Ciocalteu reagent, and then 2 mL of 7.5% (w/v) sodium carbonate was added. The mixture was incubated for 15 min at 50°C and then cooled to room temperature. The absorbance was measured at 760 nm using a visible spectrophotometer (Youke 722N, Shanghai, China) against a blank. A standard curve was obtained using different gallic acid concentrations (0–0.1 mg/mL). The total phenolic content was expressed as milligrams of gallic acid equivalents (GAE) per gram dry matter (mg GAE/g d.m.).
The total flavonoids content was determined using a modified aluminium chloride colorimetric method (Souza et al., Citation2014). The diluted sample extract (0.5 mL) was mixed with 30 µL of 5% (w/v) NaNO2 solution and allowed to stand for 5 min. After that, 30 µL of 10% AlCl3 solution (w/v) was added and mixed for 6 min. Finally, 0.4 mL of 1.0 M NaOH solution and 40 µL of distilled water were added. The mixture was incubated at room temperature for 15 min. The absorbance of the mixture was read at 510 nm. A calibration curve was constructed using different rutin concentrations (0–1.0 mg/mL). Results were expressed as milligrams of rutin equivalents (RE) per gram dry matter (mg RE/g d.m.).
Determination of antioxidant activities
The 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) assay was conducted using the colorimetric method of Re et al. (Citation1999). Equal quantities of 7 mM ABTS and 2.45 mM K2S2O8 solution were mixed to obtain ABTS+· solution. The ABTS+· solution was then incubated for 16 h in the dark at room temperature, and diluted with 80% (v/v) ethanol to obtain an absorbance of 0.70 ± 0.02 at 734 nm before the measurement. Diluted extract (0.4 mL) was reacted with 3.6 mL diluted ABTS+· solution for 6 min at room temperature. The absorbance was recorded at 734 nm. Trolox with 0–0.10 mM concentrations was used as a calibration curve. The results were expressed as milligrams of Trolox equivalents (TE) per gram dry matter (mg TE/g d.m.).
The ferric-reducing antioxidant power (FRAP) assay was performed using a previously described method (Benzie & Strain, Citation1996). First of all, the FRAP reagent was prepared by mixing 0.01 M Tri-2-pyridyl-s-triazine (TPTZ) solution (prepared in 0.04 M HCl), 0.02 M FeCl3 solution and 0.3 M acetate buffer (pH 3.6) at the volumetric ratio of 1:1:10. The diluted extract (0.2 mL) was fully reacted with 6 mL of FRAP reagent. After incubating at 37°C for 10 min, the absorbance at 593 nm was recorded. A calibration curve was obtained using different concentrations (0–0.80 mM) of trolox. All solutions were prepared on the day of use. FRAP values were calculated as milligrams Trolox equivalents (TE) per gram dry matter (mg TE/g d.m.).
Statistical analysis
All experiments were performed in at least triplicate and all data are presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA), one-way multivariate analysis of variance (MANOVA), principal component analysis (PCA), and the Tukey’s multiple range test were computed with the SPSS Statistics Version 20 (IBM Corporation, Chicago, IL, U.S.A.) at a minimum significance level of 95% confidence level (p < .05). All chemical analyses were expressed on a dry weight basis.
Results and discussion
Physical properties of freeze-thaw pretreated beetroots
It is well known that the moisture content is critical for the quality control and stability of dried products. According to the results, the final moisture content of freeze-thaw pretreated beetroots subjected to different drying methods ranged from 5.62% to 6.05%. As shown in , there was no significant difference in the final moisture content of freeze-thaw pretreated beetroots obtained by different drying methods (p > .05), which showed the influence of moisture content on the physicochemical properties of dried beetroots prepared using different drying methods could be neglected.
Figure 1. The drying time and final moisture content of freeze-thaw pretreated beetroots. Means with different letters were significantly different (p < .05).
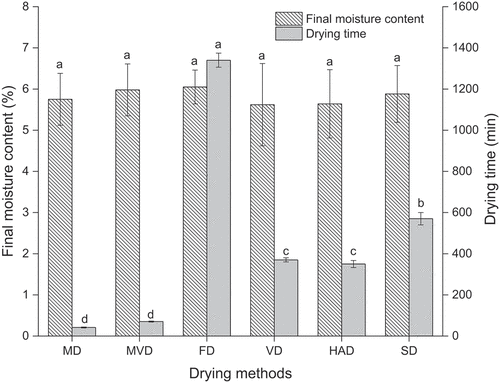
FD required the longest drying time to dry fresh beetroots to the final moisture content (below 7.00%) of 1340.0 min. MD and MVD showed much faster drying rates than FD, SD, HAD, and VD; therefore, shorter drying times were required, but there was no significant difference in drying time between MD and MVD. Meanwhile, the drying times for VD and HAD were also not significantly different (p > .05). The drying time of MD was reduced by 96.90% compared to that of FD, which was only 11.86% for HAD, 11.21% for VD and 7.28% for SD. The drying time required by MVD was approximately 5.25% that of FD and was reduced to 87.67%, 81.0%, and 79.91% compared with SD, VD, and HAD, respectively.
To explain the influence of different drying methods on the quality characteristics of freeze-thaw pretreated beetroots, the microstructures were observed using scanning electron microscopy (SEM), as described above. The microstructures of freeze-thaw pretreated beetroots obtained using different drying methods are depicted in .
Figure 2. SEM micrographs (500 × magnification) of freeze-thaw pretreated beetroots obtained using different drying methods.
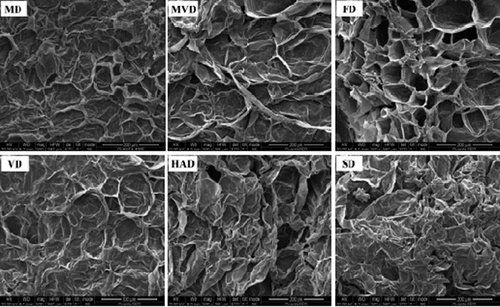
Obvious differences were observed in the microstructures of beetroots pretreated with freeze-thaw using different drying methods. Freeze-thaw pretreated beetroots dried by FD exhibited typical homogenous porous structures, suggesting that FD displayed minimal influence on the cellular structure of beetroot tissue. A previous study confirmed that the porous microstructure of the FD sample was formed by ice sublimation in a vacuum environment without cell shrinkage and external force collapse (Chen et al., Citation2017). During MD and MVD, rapid moisture evaporation caused the microscopic holes, and the freeze-thaw pretreated beetroots prepared by MVD and MD also had porous structures, which was similar to that of FD treatment. Freeze-thaw pretreated beetroots obtained using VD were found to have thin porous walls, invisible cell boundaries, and extensive collapse of cell structures, resulting in negative effects on the texture of the products. Freeze-thaw pretreated beetroots dried by HAD demonstrated tissue shrinkage and collapse of the cell structures, indicating severe damage to the cell structures of beetroots and leading to negative effects on the hardness of the final products. Similar results were reported by Nistor et al. (Citation2017) for convection-free dried beetroots. However, SD led to significant damage to the cell structures of freeze-thaw pretreated beetroots, resulting in complete breakage of the cellular membrane and severe tissue shrinkage and collapse. Heating and moisture loss create pressure in the cellular structures of the material, resulting in microstructural changes and shrinkage. In addition, the microstructures and porosity of the dried products are related to the migration mechanism of moisture and changes in the external pressure (Vadivambal & Jayas, Citation2007).
As presented in , the shrinkage rates of freeze-thaw pretreated beetroots dried by different drying methods ranged from 74.32% to 92.27%. The freeze-thaw pretreated beetroots subjected to FD presented the lowest shrinkage rate, indicating minimal volume change compared to the fresh sample. The highest shrinkage rate of freeze-thaw pretreated beetroots obtained by VD was 92.27%. These results agree with the research of Gong et al. (Citation2022), who reported that shrinkage rate of FD was the lowest, whereas the VD led to the highest shrinkage in garlic slices. However, there were no significant differences in shrinkage rates among the freeze-thaw pretreated beetroots subjected to VD, MD, HAD, and SD (p > .05).
Figure 3. The shrinkage rate and rehydration ratio of freeze-thaw pretreated beetroots. Means with different letters were significantly different (p < .05).
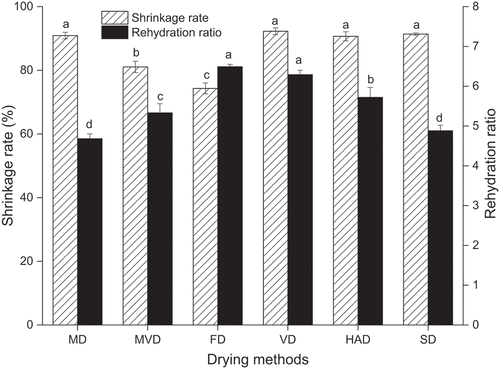
The drying process typically causes irreversible changes in the material’s structure and limits its return to its original shape. The rehydration ratio is a major quality parameter that can indicate the ability of the material to retain its original shape. It can be used to reflect the extent of damage to cellular material during the drying process (Srikanth et al., Citation2020). As shown in , the rehydration ratio of freeze-thaw pretreated beetroots subjected to different drying methods ranged from 4.68 to 6.49. FD led to the highest rehydration ratio of freeze-thaw pretreated beetroots, because FD remained the beetroots structure and large numbers of pores left, and the porous structure contributed to water infiltration during rehydration (Zhang et al., Citation2020). While the difference was not statistically significant (p > .05) in the rehydration ratio of freeze-thaw pretreated beetroots dried using FD and VD. VD resulted in high rehydration ratio of freeze-thaw pretreated beetroots can be explained by the relatively small degree of damage to the cell walls, and the damage to the tissue structure during VD process was confirmed by macroscopic characteristics, including shrinkage or rehydration (Piotrowski et al., Citation2021; Xie et al., Citation2018). The rehydration ratios of freeze-thaw pretreated beetroots obtained from MD and SD were significantly lower than those of other drying methods, indicating that MD and SD caused greater damage to the cell structure of freeze-thaw pretreated beetroots.
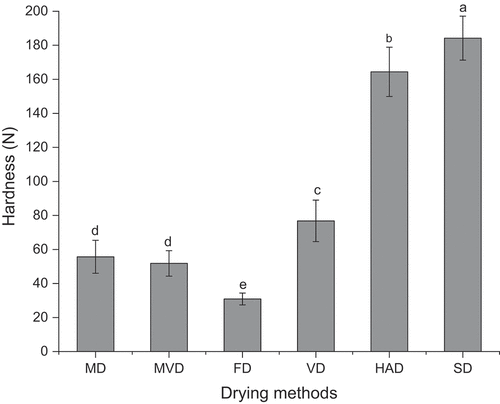
Texture, assessed by hardness, is a critical quality characteristic affecting the acceptability of food. The hardness of the freeze-thaw pretreated beetroots was significantly affected by different drying methods (p < .05). The hardness of freeze-thaw pretreated beetroots subjected to different drying methods ranged from 30.9 to 184.2 N. As observed in , the freeze-thaw pretreated beetroots obtained by SD showed the largest hardness, followed by those of HAD, VD, MD, and MVD. There were many tiny pores in FD freeze-thaw pretreated beetroots (), and the hardness of the freeze-thaw pretreated beetroots obtained by FD was the lowest, indicating that the texture of the freeze-thaw pretreated beetroots prepared by FD was soft. Similar result has been reported that the pores of FD fillets were difficult to recover, so the FD fillets showed the lowest hardness (Zhang et al., Citation2020). There was no significant difference in the hardness of freeze-thaw pretreated beetroots obtained by MD and MVD (p > .05).
Figure 4. Effects of different drying methods on the hardness of freeze-thaw pretreated beetroots. Means with different letters were significantly different (p < .05).
Color is one of the essential visual indicators of dried beetroot quality because beetroot pigments are typically used as natural colorant (Bazaria & Kumar, Citation2018). As shown in , the L* values of freeze-thaw pretreated beetroots dried using different drying methods were observed to be increased compared to that of the fresh sample, indicating that the freeze-thaw pretreated beetroots became brighter after drying than the fresh sample. While, there was no significant difference in L* values of fresh samples and freeze-thaw pretreated beetroots dried using HAD and SD (p > .05). The freeze-thaw pretreated beetroots dried by FD showed the highest L* value of 40.47. The a* values of the freeze-thaw pretreated beetroots after drying were significantly lower than that of the fresh sample (p < .05), indicating that dried beetroots became lighter red color after drying. The possible reason for the lower a* values might be related to the degradation reactions of betacyanins during drying (Prieto-Santiago et al., Citation2020). Compared with other drying methods, a* value of freeze-thaw pretreated beetroots obtained using FD was higher, which was closer to that of fresh sample. Meanwhile, SD led to the lowest a* value of freeze-thaw pretreated beetroots, which was consistent with the high degradation of betacyanins (). The results revealed that freeze-thaw pretreated beetroots after drying presented significantly lower values of b* as compared to that of fresh sample (p < .05). For freeze-thaw pretreated beetroots undergoing MD, FD, and FD, no significant difference was observed in b* values. Compared to other drying methods, SD led to the lowest b* value of freeze-thaw pretreated beetroots.
Figure 5. The betacyanin content and betaxanthin content of freeze-thaw pretreated beetroots. Means with different letters were significantly different (p < .05).
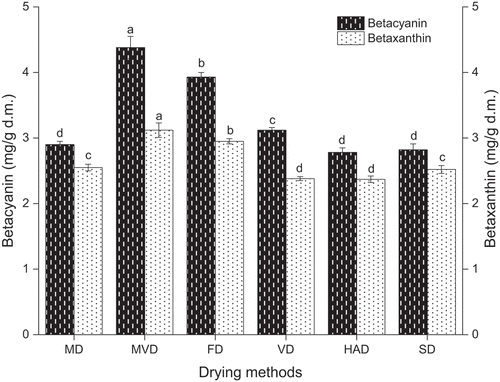
Table 1. Color parameters, principal component scores (F) and ranking of freeze-thaw pretreated beetroots affected by different drying methods.
As shown in , the different drying methods significantly influenced the changes in the C values (p < .05). The C values of all the freeze-thaw pretreated beetroots prepared by different drying methods were lower than that of the fresh sample. The C values of the freeze-thaw pretreated beetroots decreased after drying, resulting in a lower saturation and a duller appearance. Moreover, the freeze-thaw pretreated beetroots prepared by SD displayed the lowest C value of 13.28, indicating the smallest saturation and the dullest appearance. The C value was highest in the freeze-thaw pretreated beetroots dried using FD indicating the most vivid color. It is worth noting that C and a* exhibited similar values. This agrees with several researchers who even considered values of C and a* as indicators describing the thermal degradation of betalains in beetroot products (Mella et al., Citation2022; Prieto-Santiago et al., Citation2020; Seremet et al., Citation2020; Zhang et al., Citation2021). The values of a*, b*, and C in freeze-thaw pretreated beetroots after different drying methods were decreased significantly (p < .05) compared to those of fresh samples. The H° values of freeze-thaw pretreated beetroots obtained by different drying methods were significantly higher than those of fresh samples The H° values of freeze-thaw pretreated beetroots obtained by different drying methods were significantly higher than that of the fresh sample (p < .05). The H°, with values from 7.71 to 31.90, was in the red range to yellow-red for freeze-thaw pretreated beetroots prepared by different drying methods. Freeze-thaw pretreated beetroots obtained by SD displayed the highest H° value, demonstrating that SD caused a great degree of shift to a more yellow-red color. The greatest ΔE (16.34) was observed in the freeze-thaw pretreated beetroots obtained using SD followed by the freeze-thaw pretreated beetroots dried by HAD. It can be observed that FD led to the lowest ΔE (5.61) of freeze-thaw pretreated beetroots followed by MVD. Furthermore, there is no significant difference between the ΔE values of freeze-thaw pretreated beetroots obtained from FD and VD (p > .05).
Principal component analysis (PCA) was based on the color characteristics of freeze-thaw pretreated beetroots, by dimensionality reduction, to construct different color parameters (L*, a*, b*, C, H°, and ΔE) of freeze-thaw pretreated beetroots. The correlation relational model was used to analyze the relationship between color characteristics and color parameters of freeze-thaw pretreated beetroots prepared by different drying methods. Through PCA, six color parameters were constructed into only one principal component (PC1), which explained 82.32% of the variance contribution playing a dominant role in color evaluation. The eigenvalues of the PC1 was 4.939. L*, a*, b*, and C provided positive component matrix, and the values were 0.755, 0.955, 0.939, and 0.958, respectively. While H° and ΔE exhibited negative component matrix, with values of −0.852 and −0.966, respectively. The positive effect was greater than the negative effect, indicating that L*, a*, b*, and C played a decisive role in the PC1 of color characteristics. The principal component score (F) was calculated according to EquationEquation (6)(6)
(6) and results are presented in .
The larger the F value, the better the color of the freeze-thaw pretreated beetroots. FD resulted in the highest F value of 14.31, indicating the comprehensive color characteristics of freeze-thaw pretreated beetroots prepared by FD were the best. The F value of SD was the lowest, so the color characteristics of freeze-thaw pretreated beetroots prepared by SD was the worst. As seen in , the F value of PC1 ranked first and second for FD and MVD.
As a result, freeze-thaw pretreated beetroots dried by FD exhibited the most desirable color among the freeze-thaw pretreated beetroots subjected to six different drying methods, with the lowest ∆E and the highest F, the values of L*, a*, b*, and C closet to those of fresh beetroots. In addition, the worst color of freeze-thaw pretreated beetroots was prepared by SD, indicating that sun drying was extremely unfavorable for the color retention of freeze-thaw pretreated beetroots.
Bioactive compounds of freeze-thaw pretreated beetroots
The betalains and ascorbic acid contents of the freeze-thaw pretreated beetroots prepared by different drying methods are exhibited in . Beetroot is rich in precious betalains, which can be used as a food colorant and additive (Ravichandran et al., Citation2013); but however, betalains are sensitive to exposure to high temperatures and long processing times (Mella et al., Citation2022). Drying changes the content of betalains, and consequently, the color of the products. It can be seen that different drying methods significantly affected the betalains content in the freeze-thaw pretreated beetroots (p < .05). The betacyanin content of freeze-thaw pretreated beetroots obtained using different drying methods ranged from 2.78 to 4.38 mg/g d.m. Meanwhile, the betaxanthin content of frozen-thawed beetroots subjected to different drying methods ranged from 2.37 to 3.12 mg/g d.m. Those values were obviously higher than the range reported by Wruss et al. (Citation2015) for seven beetroots varieties: 2.3 to 3.9 mg/g for betacyanin content, and 1.5 to 2.4 mg/g for betaxanthin content. The results showed that the betacyanin content of freeze-thaw pretreated beetroots dried by MVD was the highest (4.38 mg/g d.m.). In contrast, the lowest betacyanin content of 2.78 mg/g d.m. was observed in freeze-thaw pretreated beetroots dried by HAD. Furthermore, there was no significant difference in betacyanin content between freeze-thaw pretreated beetroots dried by SD and HAD. The betaxanthin content of beetroots obtained by MVD was significantly higher than those of freeze-thaw pretreated beetroots obtained by other drying methods (p < .05). The lowest betaxanthin content was observed in freeze-thaw pretreated beetroots prepared using MVD, and no significant difference was observed in the betaxanthin content between VD and HAD (p > .05).
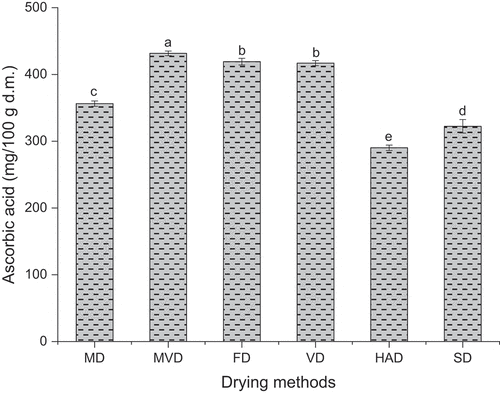
Ascorbic acid is relatively unstable to heat, light, and oxygen and is easily degraded during the drying process. As displayed in , different drying methods had significant effects on the ascorbic acid content of freeze-thaw pretreated beetroots. The highest ascorbic acid content of 431.6 mg/100 g d.m. was observed in freeze-thaw pretreated beetroots obtained by MVD. Moreover, the freeze-thaw pretreated beetroots subjected to HAD showed the lowest ascorbic acid content of 290.2 mg/100 g d.m, indicating that hot air drying resulted in the greatest loss of ascorbic acid in freeze-thaw pretreated beetroots, which can be explained by the fact that the higher the temperature, the longer the drying time, and the more ascorbic acid is degraded in dried fruits and vegetables (Cui et al., Citation2004). In addition, there was no significant difference in the ascorbic acid content of the freeze-thaw pretreated beetroots obtained from FD and VD (p > .05).
Figure 6. The acid content of freeze-thaw pretreated beetroots. Means with different letters were significantly different (p < .05).
The total phenolic content and total flavonoids content of freeze-thaw pretreated beetroots affected by different drying methods are shown in . Different drying methods significantly influenced the total phenolic content of freeze-thaw pretreated beetroots (p < .05). The freeze-thaw pretreated beetroots dried by MVD presented the highest total phenolic content of 8.58 mg GAE/g d.m, followed by the freeze-thaw pretreated beetroots obtained by MD, VD, FD, HAD, and SD, respectively. MVD and MD resulted in higher total phenolic content of freeze-thaw pretreated beetroots, which can be explained by the fact that the microwave irradiation treatments were very short compared to VD, FD, HAD and VD treatments and microwave radiation leads to the release of phenolic compounds from the food matrix (Valadez-Carmona et al., Citation2016). Moreover, the lowest total phenolic content (5.88 mg GAE/g d.m.) was found in freeze-thaw pretreated beetroots prepared by SD. However, the effects of SD and HAD on the total phenolic content of freeze-thaw pretreated beetroots were not significantly different (p > .05). These values were in the range of those proposed by Székely et al. (Citation2019) for dried beetroot species (“Alto F1”, “Cylindra”, “Detroit”) prepared by atmospheric, vacuum and microwave vacuum drying.
Figure 7. The total phenolic content and total flavonoids content of freeze-thaw pretreated beetroots. Means with different letters were significantly different (p < .05).
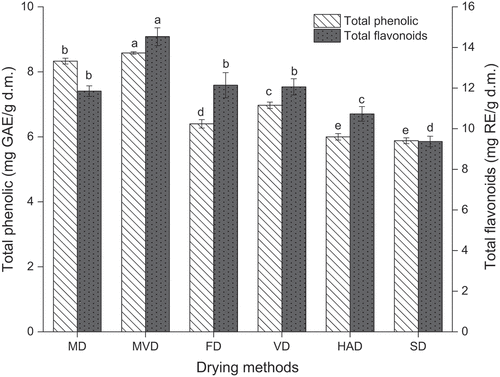
The results showed that the freeze-thaw pretreated beetroots prepared by MVD displayed the highest total flavonoids content of 14.53 mg RE/g d.m, followed by the freeze-thaw pretreated beetroots obtained by FD, VD, MD, HAD, and SD with values of 12.14, 12.06, 11.85, 10.73, and 9.37 mg RE/g d.m, respectively. Those values were significantly lower than the range proposed by Hamid and Nour (Citation2018) of sun-dried, oven-dried, and freeze-dried beetroots with the values of 34.74, 33.28, and 36.11 mg RE/g, respectively. The reason for this difference may be related to the variety and place of production and the pre-treatment of beetroots. Compared to other drying methods, SD resulted in the lowest content of total flavonoids and total phenolic in freeze-thaw pretreated beetroots, indicating that SD was not conducive to the preservation of total flavonoids and total phenolic in beetroots.
Antioxidant activities of freeze-thaw pretreated beetroots
In this study, antioxidant activities of freeze-thaw pretreated beetroots obtained using different drying methods were assessed based on the ABTS radical scavenging ability and FRAP value. The results are presented in .
Figure 8. The ABTS radical scavenging ability and FRAP values of freeze-thaw pretreated beetroots. Means with different letters were significantly different (p < .05).
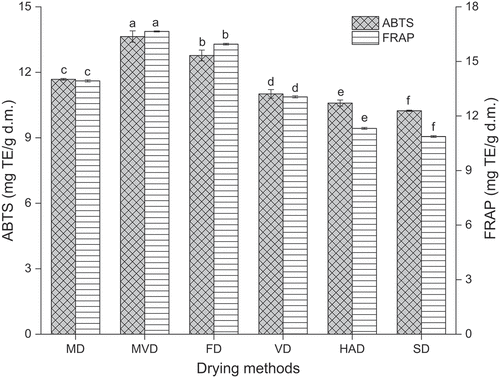
The drying methods significantly affected the ABTS free radical scavenging ability and FRAP values of freeze-thaw pretreated beetroots (p < .05), and the impact trend was consistent. The freeze-thaw pretreated beetroots obtained by MVD showed the highest FRAP value and ABTS radical scavenging ability, which were 16.65 mg TE/g d.m. and 13.64 mg TE/g d.m, respectively. The lowest FRAP value (10.87 mg TE/g d.m.) and ABTS radical scavenging ability (10.24 mg TE/g d.m.) were observed in freeze-thaw pretreated beetroots subjected to SD.
The results revealed that antioxidant activity was related to the content of bioactive compounds in freeze-thaw pretreated beetroots. Changes in antioxidant activity can be attributed to various factors. It has been reported that the degradation caused by enzymes or heating leads to a loss of antioxidant ability. Furthermore, the intermediate degradation products and Maillard reactions during the thermal process can enhance antioxidant activity (Cheigh et al., Citation1995). Moreover, intense or long-term heat treatments may result in the loss of natural antioxidants, most of which are relatively unstable (Lim & Murtijaya, Citation2007). In addition, the enzymatic and non-enzymatic browning reactions during drying may lead to antioxidative characteristics (Samoticha et al., Citation2016).
Conclusions
The results indicated that the drying methods significantly influenced the quality characteristics of freeze-thaw pretreated beetroots. FD led to the longest drying time of 1340.0 min, whereas, MVD and MD required shorter drying times compared to other drying methods. It has been found that the freeze-thaw pretreated beetroots obtained by FD showed the lowest shrinkage rate and hardness, and the highest rehydration ratio of 6.49. The color results demonstrated that the freeze-thaw pretreated beetroots prepared using FD displayed the best color appearance with the lowest ΔE of 5.93 and the highest F value, while SD resulted in the worst color in freeze-thaw pretreated beetroots with the largest ΔE and the lowest F value. According to the microstructural results, the freeze-thaw pretreated beetroots dried by FD, MD, and MVD exhibited porous structures. SD and HAD caused significant damage to the cell structure of freeze-thaw pretreated beetroots, resulting in severe tissue shrinkage and collapse.
For bioactive compounds, freeze-thaw pretreated beetroots obtained using MVD exhibited the highest contents of betacyanin, betaxanthin, ascorbic acid, total phenolic, and total flavonoids, whereas HAD led to the lowest contents of betacyanin, betaxanthin, and ascorbic acid, and SD resulted in the lowest total phenolic and total flavonoids content. It is noted that freeze-thaw pretreated beetroots subjected to MVD presented the highest ABTS radical scavenging ability (13.64 mg TE/g d.m.) and FRAP value (16.65 mg TE/g d.m.).
It can be concluded that MVD is the optimal drying method of freeze-thaw pretreated beetroots with the advantages of high quality properties and low drying time. Further studies should be conducted to investigate how these bioactive compounds affect the antioxidant activity of beetroots, and the application of dried beetroots in the food industry.
Acknowledgements
The authors would like to thank the Guangxi Key Laboratory of Health Care Food Science and Technology and the Institute of Food Science and Engineering Technology of Hezhou University for providing laboratory facilities and technical support during this research work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alvarez-Parrilla, E., de la Rosa, L. A., Amarowicz, R., & Shahidi, F. (2011). Antioxidant activity of fresh and processed Jalapeño and Serrano peppers. Journal of Agricultural and Food Chemistry, 59(1), 163–173. https://doi.org/10.1021/jf103434u
- An, K., Zhao, D., Wang, Z., Wu, J., Xu, Y., & Xiao, G. (2016). Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chemistry, 197, 1292–1300. https://doi.org/10.1016/j.foodchem.2015.11.033
- Ando, Y., Hagiwara, S., Nabetani, H., Sotome, I., Okunishi, T., Okadome, H., Orikasa, T., & Tagawa, A. (2019). Improvements of drying rate and structural quality of microwave-vacuum dried carrot by freeze-thaw pretreatment. LWT-Food Science and Technology, 100, 294–299. https://doi.org/10.1016/j.lwt.2018.10.064
- Ando, Y., Maeda, Y., Mizutani, K., Wakatsuki, N., Hagiwara, S., & Nabetani, H. (2016). Impact of blanching and freeze-thaw pretreatment on drying rate of carrot roots in relation to changes in cell membrane function and cell wall structure. LWT-Food Science and Technology, 71, 40–46. https://doi.org/10.1016/j.lwt.2016.03.019
- Bassey, E. J., Sun, D. W., Esua, O. J., & Cheng, J. H. (2022). Effects of freeze-thaw pretreatments on the drying characteristics, physicochemical and phytochemical composition of red dragon fruit during mid- and near-infrared drying. Drying Technology, 41(4), 561–576. https://doi.org/10.1080/07373937.2022.2109047
- Bazaria, B., & Kumar, P. (2018). Optimization of spray drying parameters for beetroot juice powder using response surface methodology (RSM). Journal of the Saudi Society of Agricultural Sciences, 17(4), 408–415. https://doi.org/10.1016/j.jssas.2016.09.007
- Benzie, I. F. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry, 239(1), 70–76. https://doi.org/10.1006/abio.1996.0292
- Bozkir, H., & Ergün, A. R. (2020). Effect of sonication and osmotic dehydration applications on the hot air drying kinetics and quality of persimmon. LWT-Food Science and Technology, 131(12), 109704. https://doi.org/10.1016/j.lwt.2020.109704
- Cheigh, H. S., Um, S. H., & Lee, C. Y. (1995). Antioxidant characteristics of melanin-related products from enzymatic browning reaction of catechin in a model system. ACS Symposium Series, 600, 200–208. https://doi.org/10.1021/bk-1995-0600.ch016
- Chen, Q., Li, Z., Bi, J., Zhou, L., Yi, J., & Wu, X. (2017). Effect of hybrid drying methods on physicochemical, nutritional and antioxidant properties of dried black mulberry. LWT-Food Science and Technology, 80(3), 178–184. https://doi.org/10.1016/j.lwt.2017.02.017
- Chhikara, N., Kushwaha, K., Sharma, P., Gat, Y., & Panghal, A. (2019). Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chemistry, 272, 192–200. https://doi.org/10.1016/j.foodchem.2018.08.022
- Cui, Z. W., Xu, S. Y., & Sun, D. W. (2004). Effect of microwave-vacuum drying on the carotenoids retention of carrot slices and chlorophyll retention of Chinese chive leaves. Drying Technology, 22(3), 563–575. https://doi.org/10.1081/DRT-120030001
- Dai, Y., Li, M., Li, M., Wang, Z., Lu, J., Zhou, Y., Meng, W., Chen, S., & Li, Q. (2021). Quality study of the preparation of virgin brown sugar powder by vacuum drying. Sugar Technology, 23(4), 1171–1182. https://doi.org/10.1007/s12355-021-00968-6
- De Oliveira, S. P. A., Do Nascimento, H. M. A., Sampaio, K. B., & de Souza, E. L. (2020). A review on bioactive compounds of beet (Beta vulgaris L. subsp. vulgaris) with special emphasis on their beneficial effects on gut microbiota and gastrointestinal health. Critical Reviews in Food Science and Nutrition, 61(12), 2022–2033. https://doi.org/10.1080/10408398.2020.1768510
- Dhiman, A., Suhag, R., Chauhan, D. S., Thakur, D., Chhikara, S., & Prabhakar, P. K. (2021). Status of beetroot processing and processed products: Thermal and emerging technologies intervention. Trends in Food Science and Technology, 114, 443–458. https://doi.org/10.1016/j.tifs.2021.05.042
- Feng, Y., Tan, C. P., Zhou, C., Yagoub, A. E. A., Xu, B., Sun, Y., Ma, H., Xu, X., & Yu, X. (2020). Effect of freeze-thaw cycles pretreatment on the vacuum freeze-drying process and physicochemical properties of the dried garlic slices. Food Chemistry, 324, 126883. https://doi.org/10.1016/j.foodchem.2020.126883
- Figiel, A. (2010). Drying kinetics and quality of beetroots dehydrated by combination of convective and vacuum-microwave methods. Journal of Food Engineering, 98(4), 461–470. https://doi.org/10.1016/j.jfoodeng.2010.01.029
- Fu, Y., Shi, J., Xie, S. Y., Zhang, T. Y., Soladoye, O. P., & Aluko, R. E. (2020). Red beetroot betalains: Perspectives on extraction, processing, and potential health benefits. Journal of Agricultural and Food Chemistry, 68(42), 11595–11611. https://doi.org/10.1021/acs.jafc.0c04241
- Gong, H., Wang, T., Hua, Y., Wang, W. D., Shi, C., Xu, H. X., Li, L. L., Zhang, D. P., Sun, Y. E., & Yu, N. N. (2022). Garlic varieties and drying methods affected the physical properties, bioactive compounds and antioxidant capacity of dried garlic powder. CyTA-Journal of Food, 20(1), 111–119. https://doi.org/10.1080/19476337.2022.2093400
- Hadipour, E., Taleghani, A., Tayarani-Najaran, N., & Tayarani-Najaran, Z. (2020). Biological effects of red beetroot and betalains: A review. Phytotherapy Research, 34(8), 1847–1867. https://doi.org/10.1002/ptr.6653
- Hamid, M. G., & Nour, A. A. A. M. (2018). Effect of different drying methods on quality attributes of beetroot (Beta vulgaris) slices. World Journal of Science, Technology and Sustainable Development, 15(3), 287–298. https://doi.org/10.1108/WJSTSD-11-2017-0043
- Janiszewska-Turak, E., Rybak, K., Grzybowska, E., Konopka, E., & Witrowa-Rajchert, D. (2021). The influence of different pretreatment methods on color and pigment change in beetroot products. Molecules, 26(12), 3683. https://doi.org/10.3390/molecules26123683
- Köprüalan, Ö., Altay, Ö., Bodruk, A., & Kaymak-Ertekin, F. (2021). Effect of hybrid drying method on physical, textural and antioxidant properties of pumpkin chips. Journal of Food Measurement and Characterization, 15(4), 2995–3004. https://doi.org/10.1007/s11694-021-00866-1
- Lechner, J. F., & Stoner, G. D. (2019). Red beetroot and betalains as cancer chemopreventative agents. Molecules, 24(8), 1602–1612. https://doi.org/10.3390/molecules24081602
- Lim, Y. Y., & Murtijaya, J. (2007). Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT-Food Science and Technology, 40(9), 1664–1669. https://doi.org/10.1016/j.lwt.2006.12.013
- Lin, T. M., Durance, T. D., & Scaman, C. H. (1998). Characterization of vacuum microwave, air and freeze dried carrot slices. Food Research International, 31(2), 111–117. https://doi.org/10.1016/S0963-9969(98)00070-2
- Liu, Y., Sabadash, S., Duan, Z., & Deng, C. (2022). The influence of different drying methods on the quality attributes of beetroots. Eastern-European Journal of Enterprise Technologies, 3(11 (117)), 60–68. https://doi.org/10.15587/1729-4061.2022.258049
- Liu, Z. L., Xie, L., Zielinska, M., Pan, Z., Wang, J., Deng, L. Z., Wang, H., & Xiao, H. W. (2021). Pulsed vacuum drying enhances drying of blueberry by altering micro-, ultrastructure and water status and distribution. LWT-Food Science and Technology, 142(8), 111013. https://doi.org/10.1016/j.lwt.2021.111013
- Marfil, P. H. M., Santos, E. M., & Telis, V. R. N. (2008). Ascorbic acid degradation kinetics in tomatoes at different drying conditions. LWT-Food Science and Technology, 41(9), 1642–1647. https://doi.org/10.1016/j.lwt.2007.11.003
- Mella, C., Vega-Gálvez, A., Uribe, E., Pastena, A., Mejias, N., & Quispe-Fuentes, I. (2022). Impact of vacuum drying on drying characteristics and functional properties of beetroot (Beta vulgaris). Applied Food Research, 2(1), 100120. https://doi.org/10.1016/j.afres.2022.100120
- Mitra, J., Shrivastava, S. L., & Rao, P. S. (2011). Process optimisation of vacuum drying of onion slices. Czech Journal of Food Sciences, 29(6), 586–594. https://doi.org/10.17221/162/2010-CJFS
- Nistor, O. V., Seremet, L., Andronoiu, D. G., Rudi, L., & Botez, E. (2017). Influence of different drying methods on the physicochemical properties of red beetroot (Beta vulgaris L. var. Cylindra). Food Chemistry, 236, 59–67. https://doi.org/10.1016/j.foodchem.2017.04.129
- Paciulli, M., Medina-Meza, I. G., Chiavaro, E., & Barbosa-Cánovas, G. V. (2016). Impact of thermal and high pressure processing on quality parameters of beetroot (Beta vulgaris L.). LWT-Food Science and Technology, 68, 98–104. https://doi.org/10.1016/j.lwt.2015.12.029
- Pathare, P. B., Opara, U. L., & Al-Said, F. A. J. (2013). Colour measurement and analysis in fresh and processed foods: A review. Food and Bioprocess Technology, 6(1), 36–60. https://doi.org/10.1007/s11947-012-0867-9
- Paula, R. R., Vimercati, W. C., Araújo, C. D. S., Macedo, L. L., Teixeira, L. J. Q., & Saraiva, S. H. (2020). Drying kinetics and physicochemical properties of whey dried by foam mat drying. Journal of Food Processing and Preservation, 44(10), e14796. https://doi.org/10.1111/jfpp.14796
- Piotrowski, D., Kostyra, E., Grzegory, P., & Janiszewska-Turak, E. (2021). Influence of drying methods on the structure, mechanical and sensory properties of strawberries. European Food Research and Technology, 247(8), 1859–1867. https://doi.org/10.1007/s00217-021-03682-5
- Prieto-Santiago, V., Cavia, M., Alonso-Torre, S., & Carrillo, C. (2020). Relationship between color and betalain content in different thermally treated beetroot products. Journal of Food Science and Technology, 57(9), 3305–3313. https://doi.org/10.1007/s13197-020-04363-z
- Ravichandran, K., Saw, N., Mohdaly, A., Gabr, A., Kastell, A., Riedel, H., Cai, Z., Knorr, D., & Smetanska, I. (2013). Impact of processing of red beet on betalain content and antioxidant activity. Food Research International, 50(2), 670–675. https://doi.org/10.1016/j.foodres.2011.07.002
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9–10), 1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
- Samoticha, J., Wojdyło, A., & Lech, K. (2016). The influence of different the drying methods on chemical composition and antioxidant activity in chokeberries. LWT-Food Science and Technology, 66, 484–489. https://doi.org/10.1016/j.lwt.2015.10.073
- Seremet, L., Nistor, O. V., Andronoiu, D. G., Mocanu, G. D., Barbu, V. V., Maidan, A., Rudi, L., & Botez, E. (2020). Development of several hybrid drying methods used to obtain red beetroot powder. Food Chemistry, 310, 25637. https://doi.org/10.1016/j.foodchem.2019.125637
- Souza, V. R. D., Pereira, P. A. P., Silva, T. L. T. D., Lima, L. C. D. O., Pio, R., & Queiroz, F. (2014). Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chemistry, 156, 362–368. https://doi.org/10.1016/j.foodchem.2014.01.125
- Srikanth, K. S., Sharanagat, V. S., Kumar, Y., Bhadra, R., Singh, L., Nema, P. K., & Kumar, V. (2020). Convective drying and quality attributes of elephant foot yam (Amorphophallus paeoniifolius). LWT-Food Science and Technology, 99, 8–16. https://doi.org/10.1016/j.lwt.2018.09.049
- Stintzing, F. C., Herbac, K. M., Mosshammer, M. R., Carle, R., Yi, W., Sellappan, S., Akoh, C. C., Bunch, R., & Felker, P. (2005). Color, betalain pattern, and antioxidant properties of cactus pear (opuntia spp.) clones. Journal of Agricultural and Food Chemistry, 53(2), 442–451. https://doi.org/10.1021/jf048751y
- Székely, D., Vidák, K., Furulyás, D., Ribárszki, K., & Stéger-Máté, M. (2019). Effect of drying methods on physicochemical parameters of different red beetroots (Beta vulgaris L.) species. Periodica Polytechnica Chemical Engineering, 63(3), 485–490. https://doi.org/10.3311/PPch.13104
- Vadivambal, R., & Jayas, D. S. (2007). Changes in quality of microwave-treated agricultural products—a review. Biosystems Engineering, 98(1), 1–16. https://doi.org/10.1016/j.biosystemseng.2007.06.006
- Valadez-Carmona, L., Cortez-García, R. M., Plazola-Jacinto, C. P., Necoechea-Mondragón, H., & Ortiz-Moreno, A. (2016). Effect of microwave drying and oven drying on the water activity, color, phenolic compounds content and antioxidant activity of coconut husk (cocos nucifera L.). Journal of Food Science and Technology, 53(9), 3495–3501. https://doi.org/10.1007/s13197-016-2324-7
- Varshney, K., & Mishra, K. (2022). An analysis of health benefits of beetroot. International Journal of Innovative Research in Engineering and Management, 9(1), 207–210. https://doi.org/10.55524/ijirem.2022.9.1.39
- Wruss, J., Waldenberger, G., Huemer, S., Uygun, P., Lanzerstorfer, P., Müller, U., Höglinger, O., & Weghuber, J. (2015). Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. Journal of Food Composition and Analysis, 42, 46–55. https://doi.org/10.1016/j.jfca.2015.03.005
- Wu, X. F., Zhang, M., Adhikari, B., & Sun, J. C. (2017). Recent developments in novel freezing and thawing technologies applied to foods. Critical Reviews in Food Science and Nutrition, 57(17), 3620–3631. https://doi.org/10.1080/10408398.2015.1132670
- Xie, L., Zheng, Z. A., Mujumdar, A. S., Fang, X. M., Wang, J., Zhang, Q., Ma, Q., Xiao, H. W., Yan-Hong Liu, Y. H., & Gao, Z. J. (2018). Pulsed vacuum drying (PVD) of wolfberry: Drying kinetics and quality attributes. Drying Technology, 36(12), 1501–1514. https://doi.org/10.1080/07373937.2017.1414055
- Zhang, J., Zhou, D., Zhong, X., Pei, Z., Tian, Y., Xiang, D., Cao, J., Shen, X., & Li, C. (2020). Quality and protein degradation of golden pompano (Trachinotus blochii) fillets during four drying methods. LWT-Food Science and Technology, 130, 109638. https://doi.org/10.1016/j.lwt.2020.109638
- Zhang, L., Yu, X., Arun S, M., & Zhou, C. (2022). Effect of freeze-thaw pretreatment combined with variable temperature on infrared and convection drying of lotus root. LWT-Food Science and Technology, 154, 112804. https://doi.org/10.1016/j.lwt.2021.112804
- Zhang, Y., Sun, B. H., Pei, Y. P., Vidyarthi, S. K., Zhang, W. P., Zhang, W. K., Ju, H. Y., Gao, Z. J., & Xiao, H. W. (2021). Vacuum-steam pulsed blanching (VSPB): An emerging blanching technology for beetroot. LWT-Food Science and Technology, 147(5), 111532. https://doi.org/10.1016/j.lwt.2021.111532
- Zielinska, M., Sadowski, P., & Błaszczak, W. (2015). Freezing/Thawing and microwave-assisted drying of blueberries (Vaccinium corymbosum L.). LWT-Food Science and Technology, 62(1), 555–563. https://doi.org/10.1016/j.lwt.2014.08.002
