?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Nothofagus antarctica (NA) is a native tree of Patagonia. Since ancient times, NA leaves were used in infusions for medical and food purposes, but there are no deep insights on its toxicity. The aim of this work was to assess the safety and antioxidant activity of NA leaves infusion. Mice were used to determine acute and subacute oral toxicity. Total polyphenols, flavonoids and the antioxidant activity of the infusion were assessed, as well as the antioxidant activity in biological samples. Toxicity tests revealed no death or signs of toxicity. No significant differences in biochemical parameters/histological structure were registered. NA infusion exhibited a high content of polyphenols and flavonoids, contributing to its remarkably antioxidant activity. The periodic administration of NA infusion could increase the antioxidant capacity in mice at intestinal level. The results support the safe of consuming NA leaves infusion and suggest their contribution for modulating the intestinal oxidative stress.
1. Introduction
Nothofagus forests are the southernmost broadleaf woodlands of the world. Deciduos Nothofagus antarctica (G.Forst.) Oerst. (NA) covers a wide latitudinal range from 36º30´ to 56º S latitude in mountain regions of Chile and Argentina (Veblen et al., Citation1996). The traditional name for this tree is “ñire”, which means “fox” in Mapuche language (so called because these animals tend to burrow under them) (Sánchez Cabezas, Citation2010). In Patagonia, native ñire forest has been generally used as silvopastoral systems, which support sheep and cattle farming while also yielding various wood products (Peri et al., Citation2016). Ñire forests also provide a variety non-wood products such as fruits, mushrooms and medicinal plants, that could be used in an environmentally and economically sustainable way (Salinas & Uribe, Citation2021). In recent years, governmental and non-governmental organizations in partnership with scientific community, have been advocating for the utilization of N. antarctica as a non-wood forest resource with the goal of making it a key element to alleviate poverty and promote the sustainable management of ecosystems (Rusch et al., Citation2017).
N. antarctica leaves have been used by the indigenous people of Patagonia in folk medicine as a remedy for reducing fever (Barboza et al., Citation2009). Currently, descendants of the native inhabitants continue using leaves, flowers and bark of N. antarctica in infusions and as natural dyes (Salinas & Uribe, Citation2021). Because of its increased promotion, ñire containing preparations have gained significant popularity. Ñire leaves are employed not only for crafting infusions, but have also found applications as an additive in liquor production, as a culinary ingredient, and even in the formulation of cosmetic products.
In spite of its popularity, little is known about the composition, characteristics and effects of ñire infusions. The limited available ethno-medical information suggest that ñire infusions have medicinal properties, especially for its antipyretic effects (Barboza et al., Citation2009). It has been recently reported that ñire extracts contain a high quantity of antioxidant compounds such as flavonoids and polyphenols, as well as essential oils (González et al., Citation2016). Indeed, ñire aqueous extracts exhibited a high antioxidant activity that could be ascribed to the presence of a great diversity of polyphenols and flavonoids (Mattera et al., Citation2022). Therefore, this plant has promising phyto-therapeutic effects, potentially increasing its economic value and further fueling interest in the utilization of ñire forests.
The interest in botanical medicine is on the rise worldwide. Today, consumers are actively seeking natural compounds for treatment or prevention of various diseases. This demand is accompanied by a need of scientific information that can guarantee both their safety and the real bioactive effects of these natural products. However, research on medicinal plants has primarily concentrated in their pharmacological action often neglecting their potential toxic effects. The common misconception that all ‘natural’ products are inherently safe has contributed to a lack of investigation into their potential toxicity (Margină et al., Citation2015). Nowadays, there are increasing proofs indicating that numerous medicinal plants have led to significant toxicity to their consumers, primarily due to the content of pyrrolizidine alkaloids, a diverse group of toxins produced by different plant species (Kharchoufa et al., Citation2020; Knutsen et al., Citation2017; Wojcikowski et al., Citation2004).
Given the therapeutic promise and the growing utilization of N. antarctica (ñire) leaves in various preparations, it becomes crucial to conduct toxicological studies. Therefore, to ensure the safe consumption of N. antarctica (ñire) leaves infusion, this study assessed its potential toxic effects in mice. Additionally, the research aimed to evaluate the antioxidant activity of NA infusion in vitro and in vivo in order to bolster its use as a functional food.
2. Materials and methods
2.1. Chemicals
All chemicals used for assays were of analytical grade. ABTS (2, 2’-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid), chlorogenic acid (1,4,5-trihydroxycyclohexanecarboxylic acid 3-(3,4-dihydroxycinnamate), 3-(3,4-dihydroxycinnamoyl) quinic acid), cathechin,(±cyanidol-3, (2 R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-1(2 H)-benzopyran-3,5,7-triol) and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were purchased from Sigma-Aldrich Chemical, Co (St Louis, MO, U.S.A.). Aluminium chloride (AlCl3), Folin-Ciocalteau reagent and potassium persulfate (K2S2O8) were purchased from Anedra (Buenos Aires, Argentina).
2.2. Plant material
Leaves of N. antarctica were collected in November 2021 after the flowering stage in Tierra del Fuego (Argentina) production forest reserve of Milna river and production forest 110 reserve of Bombilla (−54.362° S, −67.638° W), according to Soler (Citation2013) . The plant material was identified by the forestal engineer S. Farina and the natural resource engineer F. Mattenet. A specimen of NA was deposited at the Herbarium of the Agricultural Experimental Station (Nº 784), INTA, Santa Cruz, Argentina. The scientific plant name was checked and confirmed by the official website Medicinal Plant Name Service (http://mpns.kew.org/). The sample was composed by green leaves and petioles of arboreal (60%) and shrubby (40%) morphotypes of N. antarctica.
2.3. Preparation of the aqueous extract of Nothofagus antarctica
The leaves of N. antarctica (NA) were subjected to air-dried at 22°C for a period of 15 days. Initially, an aqueous extract of NA was prepared by boiling the leaves (5% w/v e.g. 100 g of leaves in 2000 ml of distilled water) for 5 min, followed by allowing the infusion to rest for 20 min to reach a temperature of 45°C. Subsequently, the infusion underwent filtration, freezing at −80°C, and then subjected to a freeze-drying process in a L-A-B4-C equipment (Rificor, Argentina) operating with the condenser set at −45°C and a chamber pressure of 0.04 mbar, for 48 h. The resulting infusion powder was suspended in distilled water to attain the suitable concentration for administration to mice.
2.4. Mice
Male and female Swiss albino mice aged 10–12 weeks and weighing between 25 and 35 g were provided by the Laboratory of Experimental Animals (LAE) at the Faculty of Veterinary Sciences of the National University of La Plata (UNLP). Mice were kept under standard conditions with a temperature of 23°C ± 2°C and a 12 h light-dark cycle. They had unrestricted access to water and food throughout the study. The experimental protocol received the approval from the Institutional Committee for the Care and Use of Laboratory Animals (CICUAL) within the Faculty of Exact Science at UNLP (protocol number: 009-00-21; 11/28/2022).
2.5. Toxicity studies in mice
The acute and subacute oral toxicity studies were performed following the recommendations of the guidelines of the Organization for Economic Cooperation and Development (OECD) (OECD, Citation2002). In the acute toxicity study a group of 6 mice (comprising 3 males and 3 females) received orally a single limit dose of 2000 mg/kg body weight by gavage. Concurrently, a control group consisting of 3 males and 3 females received distilled water. Observations of general behavior and signs of toxicity were conducted continuously during the first hour following the oral treatment. Subsequently, the observations were made every 4 h throughout a 24 h-period. After this initial period, the mice were then observed once daily for up to 14 days with the focus on monitoring behavioral changes and signs of toxicity and any potential occurrences of mortality.
In the subacute toxicity study, two experimental groups were set up each comprising 6 animals (3 males and 3 females). These groups were as follows: control group (receiving distilled water) and test group (receiving a 800 mg/kg body weight dose). Both groups were administrated their respective treatment by gavage every 2 days for a total treatment duration of 14 days. Throughout this 14-day period, the animals were observed daily basis to monitor their health and to detect any clinical signs of toxicity.
In both the acute and subacute studies, the body weight was recorded weekly. Upon the conclusion of both experiments, mice were humanly euthanized by CO2 inhalation. Following euthanasia samples of blood, liver, kidneys, small intestine, and feces were prompted collected for further analysis.
2.6. Histopathological examination
After euthanasia a macroscopic examination of vital organs was conducted. Fragments of the livers, kidneys and small intestine were preserved in a 4% (v/v) buffered formalin solution. Subsequently, they were embedded in paraffin wax, sliced into sections of 3–4 μm in thickness, and stained with hematoxylin and eosin. These histological sections were then observed using an optical microscopy (Leica DM500, Wetzlar, Germany) equipped with a digital camera.
2.7. Plasma biochemistry
The biochemical analysis of plasma samples was carried out using an automatic chemistry analyzer (Metrolab CM 250, Buenos Aires, Argentina). This analysis involved the measurement of various parameters including: alanine aminotransferase activity (ALT), aspartate transaminase activity (AST), total bilirubin levels, cholesterol levels, creatinine levels and urea levels were determined in all the collected plasma samples.
2.8. Total polyphenol content
Content of total polyphenols in NA infusion were determined according to the method described by Horwitz (Horwitz & Latimer, Citation2010). This method relies on the oxidation of the hydroxyl groups present in phenols within a basic solution by the Folin – Ciocalteu reagent. The results were expressed as chlorogenic acid equivalents in mg/g of dry leaves. Calibration curve was prepared using concentrations of chlorogenic acid ranging between 0–30 μg/mL The absorbance at 760 nm was registered in a microplate reader (Synergy HT, Bio-Tek Instruments, Winoski, U.S.A.).
2.9. Total flavonoid content
The total flavonoid content of NA infusion was measured by a colorimetric assay (Zhishen et al., Citation1999). This assayed is based on the generation of a red compound resulting from the interaction of flavonoids with aluminum chloride in the presence of sodium nitrite under alkaline conditions. The absorbance of the resulting reaction was measured at 490 nm in a microplate reader (Synergy HT, Bio-Tek Instruments, Winoski, U.S.A.). Catechin was employed as standard for constructing the calibration curve. The total flavonoid content was expressed as mg of catechin equivalents per gram of dry leaves (mg/g).
2.10. Antioxidant capacity
The assessment of the antioxidant capacity of the samples was carried out using the 2,2-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) ABTS radical scavenging assay (Re et al., Citation1999). The ABTS radical cation (ABTS•+) generated by potassium persulfate (K2S2O8) reacts with the antioxidant compounds present in the sample leading to a reduction in its absorbance. The reaction was monitored at 734 nm after incubation for 8 min at 20°C in a microplate reader (Synergy HT, Bio-Tek Instruments, Winoski, Vermont, U.S.A.).
To assess the antioxidant capacity of NA infusion, the aqueous extract 5% (w/v) was tested with the ABTS•+ reagent. A standard of Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) solution was used for the calibration curve. The results were expressed as mg of Trolox equivalent antioxidant capacity per g of dry leaves.
For the measurement of the antioxidant capacity in biological samples a three-times dilution of plasma or a 10-fold dilution of stool in PBS were mixed with the ABTS•+ reagent. Results were adjusted per mg of fecal content. The ABTS-free radical scavenging activity in plasma and feces was calculated according to EquationEquation 1(1)
(1) :
where Ai is the absorbance of samples mixed with the ABTS reagent and A0, the absorbance of ABTS control.
2.11. Statistical analysis
Data were expressed as mean ± standard deviation. Comparisons between treated and control groups were performed by a two-tailed Student’s t-test. Differences were considered significant when P < .05. The statistical analyses were carried out using the program STATISTIX 8 Software (Analytical Software, Tallahassee, U.S.A.).
3. Results and discussion
3.1. Acute and subacute toxicity
The acute toxicity studies are utilized to investigate the harmful effects of a given agent to the organism when administered as a single or short-term exposure. Following the OECD recommendations for compounds having an empiric evidence of being non-toxic, a unique limit dose of 2000 mg/kg of body weight was chosen for testing acute toxicity of NA infusions (OECD, Citation2002). No signs of toxicity or mortality were observed in treated animals during the experimental period. In addition, no changes in behavior were registered in mice during the toxicity assays. Hence, it can be inferred that, the LD50 of extract may be considered to be higher than 2000 mg/kg. According to the Globally Harmonized System of Classification and Labelling of Chemicals (GHS), the substances having a LD50 value greater than 2000 mg/kg are considered as relatively safe (Nations, Citation2015).
For the subacute test a scheme of repeated doses had been employed to evaluate toxicity. A relative high dose of 800 mg/kg body weight was chosen in this work and no deaths and no clinical signs of local or systemic toxic effects were registered.
It is important to point out, that animals received doses 16-fold (subacute) or 20-fold (acute) higher than the normal NA infusion. The aqueous extraction of NA leaves (5% w/v) exhibited a concentration of solids of 10 mg/mL (w/v) whereas the solutions administrated to the treated animals were 160 mg/mL (w/v) for subacute assay and 200 mg/mL (w/v) for de acute assay. Even at very high doses NA infusion is a safe product. Indeed, in silico studies by ADME/T analysis predicts that the main compounds present in NA infusions such us flavonoids compounds (quercetin and myricetin oligosacacharides derivatives) and chlorogenic acid derivatives are low-toxic, non-mutagenic and non-carcinogenic (Guo & Feng, Citation2017; Harishkumar et al., Citation2019; Hasan et al., Citation2022)
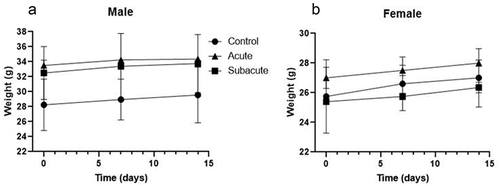
In general, changes in the body weight have been used as indicators of an adverse effect of drugs and chemicals in experimental animals. In the present study, the body weight of acute or subacute treated mice did not differ significantly (P > .05) from controls (). Moreover, the average body weight gain was 515 mg ± 137 mg per week in all groups, in concordance with a normal increase of body mass in Swiss albino mice of that age when fed standard chow ad libitum (Assunção Ferreira et al., Citation2022; Silva-Santana et al., Citation2020). These results indicate that the extract did not affect the appetite or have adverse effects on the animal’s growth.
3.2. Biochemical parameters and histological analysis
The functionality of liver and kidneys is crucial for the metabolism of ingested substances and for excretion of the waste products, respectively. Regarding this, plasma levels of transaminases, cholesterol and total bilirubin were assessed for the evaluation of hepatocellular injury, whereas the renal function was evaluated by the determination of urea and creatinine levels (Abboud & Kaplowitz, Citation2007) ().
Table 1. Biochemical parameters of mice after subacute and acute oral administration of NA infusion. Values are presented as means ± standard deviation.
The biochemical parameters of both acutely and subacutely treated mice showed minor changes (statistically not significant) when compared with controls. Besides, all the values registered entered into the normal range for the Swiss albino mice, indicating that administration of NA infusion did not alter liver or kidney function (Silva-Santana et al., Citation2020) ().
According to the biochemical results, it is reasonable to speculate that no histopathological changes will be observed in kidney and liver. Indeed, histology of the liver sections of both control and treated mice showed normal hepatocellular architecture along with well-preserved hepatocytes, organized hepatic cords, no dilated sinusoids, and central veins with a normal lumen. There was no evidence of lymphoid infiltration or inflammation (). Regarding kidneys, normal histology was found in control and treated mice. Well-organized glomeruli and tubules were observed, with no enlarged interstitial spaces. Blood vessels showed preserved endothelium and no inflammatory infiltrates ().
Figure 2. Histological sections of hematoxylin and eosin staining, as observed by optical microscopy. Liver sections showed well preserved hepatic cords and central vein (*). Intestine sections showed the mucosa with normal length villi. Kidney sections showed conserved glomeruli (circles) and tubules. Magnification 200 X.
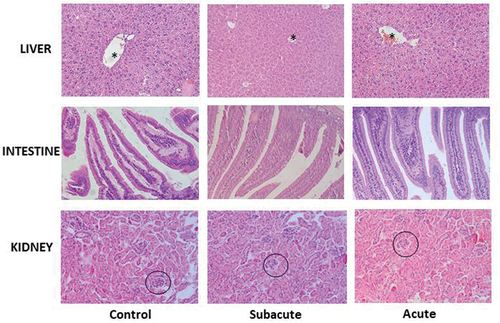
The absence of clinical signs of intestinal disorders (such as diarrhea and weight loss) during the experiment correlated with the lack of histopathological lesions in the intestinal mucosa. Crypts and villi of control and treated animals had a similar length. The intestinal epithelium showed no alterations with regular quantities of calceiform cells ().
3.3. Analysis of composition and antioxidant capacity of NA infusion
The total content of polyphenols and flavonoids, which represent the main compounds responsible for the antioxidant activity in tea, was determined in NA infusion ().
Table 2. Polyphenol, flavonoid content and antioxidant capacity of NA aqueous infusion. The infusion was obtained by boiling 5 g of leaves in 100 mL of water (5% w/v) and had a solid content of 10 mg/L.
NA infusions had a polyphenol content of 48 mg/g and a flavonoid content of 29.54 mg/g. Considering that food incorporation of polyphenols in the world is 900 mg/day, a cup of NA infusion (100 mL) provides 240 mg of polyphenols being the 26, 67% of the polyphenol daily intake (Del Bo’ et al., Citation2019). The levels of polyphenols and flavonoids found in this work are higher than previously reported values. These findings could be related to the latitude of origin of ñire leaves since the specimens used in this study are from Tierra del Fuego and exhibit higher antioxidant activity than northern specimens (de Armas-Ricard et al., Citation2021; Mattera et al., Citation2022). A detailed study of the composition of NA infusions performed by Mattera and colleagues revealed a great diversity of phenolic compounds including seven derivatives of phenolic acids and eight flavonoid derivatives. The main phenolic acid constituents were gallic, ellagic, quinic, caffeic, and coumaric acids whereas in the case of flavonoids, the most important were myricetin and quercetin compounds (Mattera et al., Citation2022). Comparing NA infusions with traditional green and black tea they contain lower levels of polyphenol and similar levels of flavonoids (Rahman et al., Citation2021; Zhao et al., Citation2019). It is interesting to point out that, NA infusions exhibit a polyphenol profile with a great complementarity with the compounds present in green and black tea (Mattera et al., Citation2022).
In this work, the antioxidant capacity of NA infusion was also determined (). The scavenging activity of NA infusion on ABTS•+ radical showed a value of 109.20 mg/g of Trolox equivalents. This value falls between those reported for black tea’s aqueous extracts (314 mg/g) and green tea’s aqueous extract (68.25 mg/g) (Gramza et al., Citation2005). In summary, NA infusion exhibited a high antioxidant activity comparable of that was described by traditional tea infusions, mainly due to the presence of polyphenols and flavonoid compounds.
Considering the results obtained in vitro, in vivo experiments were further performed to assess antioxidant capacity. For this purpose, plasma and feces from animals under study were analyzed. (), showed an increasing trend in the antioxidant activity in feces from acute treated mice. On the other hand, samples from subacute treated animals exhibited a significant difference compared with control (), which could be explained considering the repetitive doses of NA infusion administered during 14 days to mice belonging to this last experimental group. In plasma, treated animals also showed an increasing trend of the antioxidant activity but it did not reach significant level (). It is expected that, changes in the antioxidant activity of the gut are reflected first in the feces than in plasma, since they have a direct correlation with food intake (Garsetti et al., Citation2000).
Figure 3. Antioxidant activity of feces (A) and plasma (B) of mice treated with NA infusion. Asterisks indicate significant differences regarding the control.
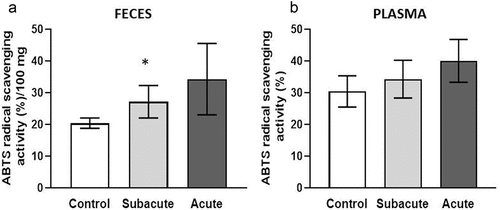
The antioxidant activity in feces reflects the input of different endogenous substances, such as sulfated glycoproteins, uric acid, coproporphyrins and bile pigments. In addition, feces have an important contribution of antioxidant compounds from gut microbiota and from the unabsorbed dietary antioxidants, such as polyphenols (Garsetti et al., Citation2000; Rajoka et al., Citation2021). Absorption of polyphenols in the small intestine is very poor (5–10%), thus, the remaining amounts are metabolized by colonic bacteria or contribute to the overall antioxidant activity of the gut (Sorrenti et al., Citation2020). It is important to note that microbial enzymes also play a significant role in the absorption of polyphenols enhancing their bioavailability (Rajoka et al., Citation2021).
It is known that polyphenols exert a direct antimicrobial activity against some harmful bacteria and also exhibit prebiotic activity for Bifidobacterium and Lactobacillus species (Sorrenti et al., Citation2020). These health-promoting bacteria produce numerous compounds (glutathione, butyrate, folate) with antioxidant properties as well as the capacity to induce expression of the host antioxidant enzymes, namely superoxide dismutase and catalase (Ma et al., Citation2018; Rajoka et al., Citation2021).
The increased level of antioxidant activity observed in feces of subacute treated mice could be ascribed to the direct influence of the polyphenols and flavonoids present in NA infusion or to their indirect impact on gut microbiota or on the modulation of the host antioxidant systems. Targeted studies on microbiota composition and internal antioxidant systems would clarify the contribution of NA infusion to reduce the oxidative stress in the gut.
4. Conclusions
Even though many native plants have a traditional use, it is relevant to study their potential toxicity to guarantee their safe consumption. In this study, the acute and subacute toxicity of an aqueous infusion of NA leaves were assessed using a mouse model. In addition, the antioxidant capacity of NA infusion was evaluated both in vivo and in vitro. Results demonstrated that NA infusion has no toxic effects in mice even at very high doses. Moreover, NA infusion exhibited a high radical scavenging activity comparable with traditional teas and was able to increase the antioxidant activity at intestinal level when periodically administered. The results presented in this work support the use of ñire infusions in different preparations, also contributing for the sustainable management of N. antarctica forests in southern Patagonia.
5. Abbreviations
ABTS: 2,2-azinobis (3-ethylbenzothiazoline-6-sulphonic acid)
GHS: Globally harmonized classification system
LD50: Lethal dose 50%
NA: Nothofagus antarctica
OECD: Organization for Economic Cooperation and Development
Trolox: (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid).
Author contributions
Ayelén A. Hugo: Conceptualization, methodology, investigation, formal analysis, original draft preparation. María de los Ángeles Serradell: Investigation, methodology (animal experiment). Pablo L. Peri and Sebastián Farina: Resources (collection and primary processing of Ñire leaves), funding acquisition. Andrea Gomez-Zavaglia: Investigation, supervision, funding acquisition. All authors contributed to the writing and review process of the manuscript.
Availability of data and materials
Raw datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Laboratory animal protocol
All procedures were approved by the Laboratory Animal Ethic Committee (CICUAL) from the Faculty of Exact Sciences (National University of La Plata).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abboud, G., & Kaplowitz, N. (2007). Drug-induced liver injury. Drug Safety, 30(4), 277–294. https://doi.org/10.2165/00002018-200730040-00001
- Assunção Ferreira, M. R., Daniele-Silva, A., de Almeida, L. F., Felipe dos Santos, E. C., Barbosa Machado, J. C., Macário de Oliveira, A., de Freitas Fernandes Pedrosa, M., Guedes Paiva, P. M., Napoleão, T. H., & Lira Soares, L. A. (2022). Safety evaluation of aqueous extract from Eugenia uniflora leaves: Acute and subacute toxicity and genotoxicity in vivo assays. Journal of Ethnopharmacology, 298(July). https://doi.org/10.1016/j.jep.2022.115668
- Barboza, G., Cantero, J., Núñez, C., Pacciaroni, A., & Ariza Espinar, L. (2009). Medicinal plants: A general review and a phytochemical and ethnopharmacological screening of the native Argentine flora. Kurtziana, 34(1–2), 7–365. http://hdl.handle.net/11336/24328
- de Armas-Ricard, M., Quinán-Cárdenas, F., Sanhueza, H., Pérez-Vidal, R., Mayorga-Lobos, C., & Ramírez-Rodríguez, O. (2021). Phytochemical Screening and antioxidant activity of seven native species growing in the forests of southern Chilean Patagonia. Molecules, 26(21), 6722. https://doi.org/10.3390/molecules26216722
- Del Bo’, C., Bernardi, S., Marino, M., Porrini, M., Tucci, M., Guglielmetti, S., Cherubini, A., Carrieri, B., Kirkup, B., Kroon, P., Zamora-Ros, R., Liberona, N. H., Andres-Lacueva, C., & Riso, P. (2019). Systematic review on Polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients, 11(6), 1355. https://doi.org/10.3390/nu11061355
- Garsetti, M., Pellegrini, N., Baggio, C., & Brighenti, F. (2000). Antioxidant activity in human faeces. British Journal of Nutrition, 84(5), 705–710. https://doi.org/10.1017/s0007114500002051
- González, S. B., Gastaldi, B., Mattenet, F., Peri, P., & van Baren, C. D. L. L. P., & Retta, D. B. A. (2016). Aceites esenciales en partes aéreas de Nothofagus antartica (G.Forst) Oerst de diferentes sitios de la Patagonia. Dominguezia, 32(2), 180.
- Gramza, A., Pawlak-Lemańska, K., Korczak, J., Wasowicz, E., & Rudzinska, M. (2005). Tea extracts as free radical scavengers. Polish Journal of Environmental Studies, 14(6), 861–867.
- Guo, P., & Feng, Y. Y. (2017). Anti-inflammatory effects of kaempferol, myricetin, fisetin and ibuprofen in neonatal rats. Tropical Journal of Pharmaceutical Research, 16(8), 1819–1826. https://doi.org/10.4314/tjpr.v16i8.10
- Harishkumar, R., Reddy, L. P. K., Karadkar, S. H., Murad, M. A., Karthik, S. S., Manigandan, S., Selvaraj, C. I., & Christopher, J. G. (2019). Toxicity and selective biochemical assessment of quercetin, gallic acid, and curcumin in zebrafish. Biological & Pharmaceutical Bulletin, 42(12), 1969–1976. https://doi.org/10.1248/bpb.b19-00296
- Hasan, M. M., Khan, Z., Chowdhury, M. S., Khan, M. A., Moni, M. A., & Rahman, M. H. (2022). In silico molecular docking and ADME/T analysis of quercetin compound with its evaluation of broad-spectrum therapeutic potential against particular diseases. Informatics in Medicine Unlocked, 29, 100894. https://doi.org/10.1016/j.imu.2022.100894
- Horwitz, W., & Latimer, G. W. (2010). Official methods of analysis of AOAC international (18thEd.). AOAC International.
- Kharchoufa, L., Bouhrim, M., Bencheikh, N., El Assri, S., Amirou, A., Yamani, A., Choukri, M., Mekhfi, H., Elachouri, M., & Oliveira, M. S. (2020). Acute and subacute toxicity studies of the aqueous extract from Haloxylon scoparium pomel (Hammada scoparia (Pomel)) by oral administration in rodents. BioMed Research International, 2020, 1–11. https://doi.org/10.1155/2020/4020647
- Knutsen, H. K., Alexander, J., Barregård, L., Bignami, M., Brüschweiler, B., Ceccatelli, S., Cottrill, B., Dinovi, M., Edler, L., Grasl-Kraupp, B., Hogstrand, C., Hoogenboom, L., Nebbia, C. S., Oswald, I. P., Petersen, A., Rose, M., Roudot, A. C., Schwerdtle, T., Vleminckx, C. … Binaglia, M. (2017). Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. The EFSA Journal, 15(7), https://doi.org/10.2903/j.efsa.2017.4908
- Ma, N., Abaker, J. A., Bilal, M. S., Dai, H., & Shen, X. (2018). Sodium butyrate improves antioxidant stability in sub-acute ruminal acidosis in dairy goats. BMC Veterinary Research, 14(1), 1–13. https://doi.org/10.1186/s12917-018-1591-0
- Margină, D., Ilie, M., Grădinaru, D., Androutsopoulos, V. P., Kouretas, D., & Tsatsakis, A. M. (2015). Natural products-friends or foes? Toxicology Letters, 236(3), 154–167. https://doi.org/10.1016/j.toxlet.2015.05.009
- Mattera, M. G., Langenheim, M. E., Reiner, G., Peri, P. L., & Moreno, D. A. (2022). Patagonian ñire (Nothofagus antarctica) combined with green tea - novel beverage enriched in bioactive phytochemicals as health promoters. JSFA Reports, 2(May), 442–451. https://doi.org/10.1002/jsf2.78
- Nations, U. (2015). Globally harmonized system of classification and labelling of chemicals (GHS) - (sixth revised edition ed.). UN. https://doi.org/10.18356/591dabf9-en
- OECD. (2002). Test No. 423: Acute oral toxicity - Acute toxic class method. Oecd Guideline for Testing of Chemicals, December, 1–14. https://doi.org/10.1787/9789264071001-en
- Peri, P. L., Bahamonde, H. A., Lencinas, M. V., Gargaglione, V., Soler, R., Ormaechea, S., & Pastur, G. M. (2016). A review of silvopastoral systems in native forests of nothofagus antarctica in southern Patagonia, Argentina. Agroforestry Systems, 90(6), 933–960. https://doi.org/10.1007/s10457-016-9890-6
- Rahman, M., Jahan, I. A., Ahmed, S., Ahmed, K. S., Roy, M., Zzaman, W., & Ahmad, I. (2021). Bioactive compounds and antioxidant activity of black and green tea available in Bangladesh. Food Research, 5(3), 107–111. https://doi.org/10.26656/fr.2017.5(3).491
- Rajoka, M. S. R., Thirumdas, R., Mehwish, H. M., Umair, M., Khurshid, M., Hayat, H. F., Phimolsiripol, Y., Pallarés, N., Martí-Quijal, F. J., & Barba, F. J. (2021). Role of food antioxidants in modulating gut microbial communities: Novel understandings in intestinal oxidative stress damage and their impact on host health. Antioxidants, 10(10), 1–24. https://doi.org/10.3390/antiox10101563
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9), 1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
- Rusch, V., López, D. R., Cavallero, L., Rusch, G. M., Garibaldi, L. A., Grosfeld, J., & Peri, P. (2017). State-and-transition model of ñire forest in NW Patagonia as a tool for sustainable silvopastoral management. Ecologia Austral, 27(2), 266–278. https://doi.org/10.25260/ea.17.27.2.0.240
- Salinas, J., & Uribe, A. (2021). Productos forestales no madereros presentes en los bosques de ñire (Nothofagus antarctica) de la zona sur austral de Chile. Ciencia & Investigación Forestal, 27(1), 87–100. https://doi.org/10.52904/0718-4646.2021.474
- Sánchez Cabezas, G. (2010). Los mapuchismos en el DRAE. Boletín de Filología, 45(2), 149–256. http://dx.doi.org/10.4067/S0718-93032010000200008
- Silva-Santana, G., Bax, J. C., Fernandes, D. C. S., Bacellar, D. T. L., Hooper, C., Dias, A. A. S. O., Silva, C. B., de Souza, A. M., Ramos, S., Santos, R. A., Pinto, T. R., Ramão, M. A., & Mattos-Guaraldi, A. L. (2020). Clinical hematological and biochemical parameters in Swiss, BALB/c, C57BL/6 and B6D2F1 Mus musculus. Animal Models and Experimental Medicine, 3(4), 304–315. https://doi.org/10.1002/ame2.12139
- Soler, R., Martinez Pastur, G., Peri, P., Lencinas, M. V., & Pulido, F. (2013). Are silvopastoral systems compatible with forest regeneration? An integrative approach in southern Patagonia. Agroforest Systems, 87, 1213–1227. https://doi.org/10.1007/s10457-013-9631-z
- Sorrenti, V., Ali, S., Mancin, L., Davinelli, S., Paoli, A., & Scapagnini, G. (2020). Cocoa polyphenols and gut microbiota interplay: Bioavailability, prebiotic effect, and impact on human health. Nutrients, 12(Issue 7), https://doi.org/10.3390/nu12071908
- Veblen, T. T., Donoso, C., Kitzberger, T., & Rebertus, A. J. (1996). Ecology of southern Chilean and Argentinean Nothofagus forests. In J. Veblen, T.T. Hill, & R.S. Read (Eds.), Ecology and Biogeography of Nothofagus forests. (Issue January, pp. 293–353). Yale University Press.
- Wojcikowski, K., Johnson, D. W., & Gobé, G. (2004). Medicinal herbal extracts - Renal friend or foe? Part one: The toxicities of medicinal herbs. Nephrology, 9(5), 313–318. https://doi.org/10.1111/j.1440-1797.2004.00310.x
- Zhao, C. N., Tang, G. Y., Cao, S. Y., Xu, X. Y., Gan, R. Y., Liu, Q., Mao, Q. Q., Shang, A., & Li, H. B. (2019). Phenolic profiles and antioxidant activities of 30 tea infusions from green, black, oolong, white, yellow and dark teas. Antioxidants, 8(7), 9–13. https://doi.org/10.3390/antiox8070215
- Zhishen, J., Mengcheng, T., & Jianming, W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry, 64(4), 555–559. https://doi.org/10.1016/S0308-8146(98)00102-2