ABSTRACT
In light of the persisting ambiguity surrounding the causality between green tea intake and gastrointestinal health, this study endeavors to elucidate it using mendelian randomization. Leveraging data from the UK Biobank and FinnGen database, instrumental variants were selected from single-nucleotide-polymorphisms associated with green tea intake. The inverse-variance-weighted method served as the primary analytical approach. Rigorous scrutiny of the results encompassed the Egger intercept test, Mendelian Randomization Presso, Cochran Q test, leave-one-out test, and funnel plot. The primary findings underscore a significant association between green tea intake and gastrointestinal diseases (p = 0.001), indicating heightened consumption of green tea could lead to a reduced risk of gastrointestinal diseases (odds ratio = 0.994). Robustness assessments across all measures substantiate the credibility of these outcomes (all p > 0.05). In conclusion, this study supports the assertion that green tea confers beneficial effects on gastrointestinal health.
1. Introduction
Tea is one of the most consumed beverages around the world. It is made from the infusion of the leaves of the Camellia sinensis plant. Tea is first used as beverage in China and expands throughout the rest of the world (Hinojosa et al., Citation2021; Perez et al., Citation2021). According to the processing degree of leaves, teas can be classified into green tea (GT), white tea, yellow tea, dark tea and so on, among which GT is minimalist processed and does not undergo oxidation (Kochman et al., Citation2020). Beyond its delightful flavor, GT is renowned for potential health benefits. It is rich in antioxidants, such as catechins, which are believed to have various health-promoting properties. GT has been associated with benefits like improved heart health, enhanced brain function, weight management, and potential cancer-fighting properties (Kochman et al., Citation2020; Xing et al., Citation2019).
Regarding the impact of GT on gastrointestinal health, various studies presented conflicting outcomes. Some suggested a negative influence on gastrointestinal health, associating GT intake with an elevated risk of certain gastrointestinal diseases (GD). It has been reported that GT intake was associated with chronic atrophic gastritis (Borrelli et al., Citation2004; Kalan et al., Citation2020). Additionally, a previous systematic review has reported that tea, especially hot tea, might be associated with esophageal and gastric cancer (Yi et al., Citation2019). However, other studies argued that GT is a protective factor of some GDs, including chronic atrophic gastritis, ulcerative colitis and kinds of stomach cancer (Almofarreh et al., Citation2022; Borrelli et al., Citation2004; Martimianaki et al., Citation2022; Poorolajal et al., Citation2020). Even for the same disease, conflicting perspectives existed on GT’s effects. Beyond inconsistent results, these observational epidemiological studies were not able to reveal a causal effect of GT on GD, either. The causality between GT and GD will remain unclear until a study dedicated to causation, such as a randomized controlled trial (RCT) or a mendelian randomization (MR) study, is performed on this topic. Therefore, a study in the influence of the intake of GT on GD is needed.
Mendelian randomization is a method widely used in epidemiology to explore potential causal relationships between an exposure and an outcome using genetic variants as instrumental variables (IVs) (Richmond & Smith, Citation2022). MR relies on the principle of genetic IVs, where genetic variants that are associated with the exposure of interest but not directly related to confounding factors are used as proxies to assess causality. This method assumes that these genetic variants influence the exposure but do not directly affect the outcome except through the exposure. Therefore, it can provide evidence for a causal relationship between exposure and outcome. By utilizing large-scale genetic data and appropriate statistical techniques, MR attempts to mimic an RCT by using genetic variants as IVs to estimate the causal effect between the exposure and the outcome (Bowden & Holmes, Citation2019; Davey Smith & Hemani, Citation2014; Emdin et al., Citation2017). Compared with traditional epidemiological research methods, MR has the ability to reveal causal relationships but not only associations.
Since the causality between GT and GD is unclear and MR is efficient in uncovering it, the present study aims to reveal the effect of GT on GD using MR, as well as to give dietary recommendations.
2. Materials and methods
This work was a two sample MR study. It was conducted using summary genome-wide association study (GWAS) data in two open-source databases, UK biobank and FinnGen database. Owing to the use of open data, the study did not require an ethical approval. The report of this study was in line with the reporting of observational studies in epidemiology using mendelian randomization (Skrivankova et al., Citation2021). The checklist was uploaded as supplemental material 1.
The exposure data was sourced from the UK Biobank, while outcome data were obtained from the FinnGen database. The data was available from IEU open GWAS project and the ID of the above GWAS was ukb-b-4078 and finn-b-K11_GIDISEASES, respectively. The former sample had a population of 64,949 and the latter one had 218,792 participants. Both datasets predominantly consisted of European populations, as shown in the information page on the website, thus were ethnically homogeneity. The overlap between the two samples is also negligible, because separate database recruited participants in different countries. Since the two samples are homogeneous and non-overlapping, the conditions for performing two samples MR were satisfied.
The single-nucleotide polymorphisms (SNPs) that were associated with GT intake (p < 5 × 10−8) in the GWAS summary of exposure were extracted. After removal of ones with linkage disequilibrium under a clump distance over 10,000 kb and R2 < 0.001, the information of the remaining ones was also extracted from the GWAS summary of outcome. After orientation adjustment, they were selected as IVs to perform the MR. Furthermore, the F-statistics and variance explained (R2) for each SNP was calculated to assess the instrument variable strength, and ones with F-statistics >10 were considered adequate instruments (Burgess & Thompson, Citation2011).
Inverse variance-weighted (IVW) MR was set as the primary method for this analysis, while MR-Egger and weighted median-based regression were used to evaluate the robustness of the IVW result. To assess potential IV violations, the MR-Egger intercept test, MR presso test and Cochran Q heterogeneity test were also performed (Bowden et al., Citation2017, Citation2019; Verbanck et al., Citation2018). In addition, a leave-one-out test and funnel plot were also employed to ascertain the reliability of the results (Hemani et al., Citation2018).
All statistical analyses were completed in R software, vision 4.1.1, and a “TwoSampleMR” R package was used to assist the analysis. The data extraction and analysis were all through IEU open GWAS website. For all analyses, p < 0.05 was considered significant.
3. Results and discussion
There were 21 SNPs that met the requirements to serve as IVs. The F-statistics for all SNPs were greater than 10, indicating all IVs were able to represent exposure. The IVs used in the analysis were listed in .
Table 1. The instrumental variants used in the mendelian randomization analysis.
The results of IVW analysis, which was preset as the primary results, showed that GT intake was significantly associated with the GD (p = 0.001). Higher GT consumption could lead to lower risk of GD (odds ratio = 0.994). Although analyses using weighted median and MR egger did not reach positive results, both of them indicated higher GT consumption could lead to lower risk of GD as well (odds ratio = 0.996, 0.993 respectively). The results are shown in and visualized in .
Figure 1. Main results of mendelian randomization. The results showed green tea could decrease the risk of the gastrointestinal diseases.
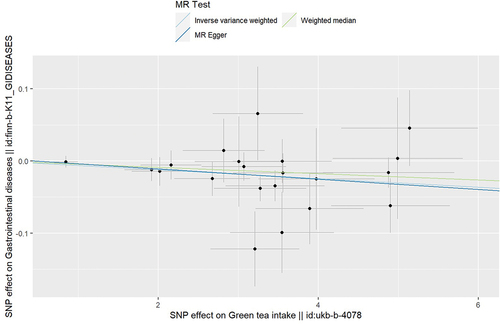
Table 2. The results of mendelian randomization.
The results, passing the heterogeneity test with p-values of 0.777 and 0.730 in the Cochran’s Q test for IVW and MR Egger analyses, indicate homogeneity among different IVs. The results are shown in . The funnel plot confirmed the strength of the IVs as well since they were evenly distributed around the mean (). The MR-Egger intercept test is shown in and did not detect any pleiotropy (p = 0.748). The sensitive analysis showed that removal of any SNPs would not make any difference to the results (). The MR presso test yielded a result of p = 0.809, which suggested no outliers among the selected IVs.
Figure 2. Funnel plot of the included instrumental variants. The instrumental variants evenly distributed around the mean line, indicating no bias of the instrumental variants.
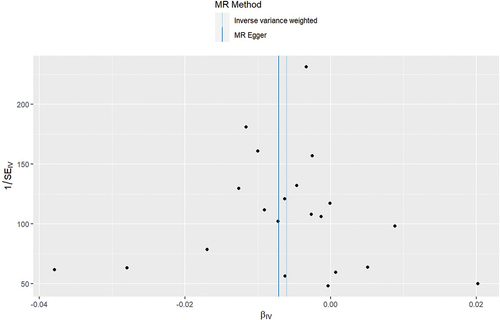
Figure 3. The results of leave one out test. removal of any single-nucleotide polymorphism will not change the initial results.
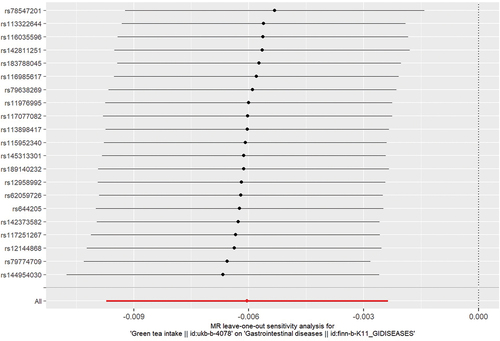
Table 3. The results of Cochran’s Q test.
Table 4. The results of MR-Egger intercept test.
This two sample MR study found that increase in intake of GT would lead to a decrease in the incidence of GD. Since this result passed the heterogeneity test, funnel plot detection, MR-Egger test, sensitive analysis and MR presso test, it should be considered robust and reliable.
Although there were studies that reported hot tea might be a risk factor of some GDs (Kalan et al., Citation2020; Yi et al., Citation2019), recurrent thermal lesion was the major cause of the injury (De Jong et al., Citation1972). There were also studies demonstrating that GT might be a protective factor of some GDs, including chronic atrophic gastritis, ulcerative colitis and kinds of stomach cancer (Almofarreh et al., Citation2022; Borrelli et al., Citation2004; Martimianaki et al., Citation2022; Poorolajal et al., Citation2020). However, these epidemiological studies could only identify that there is association between GT and GD, while the causality between GT and GD is still unclear. Thus, it could not be concluded that whether GT drink should be recommended in order to decrease the risk of GDs based on the existing evidences. Compared to them, the present study revealed the causality between GT and GD by two sample MR, which is a specific method used to reveal the causal correlations between exposure and outcome (Davey Smith & Hemani, Citation2014). MR is based on three principles, the relevance assumption, the independence assumption and the exclusion restriction assumption (Skrivankova et al., Citation2021). In the present study, the IVs extracted from a large genome-wide study were associated with the exposure and they were able to present exposure well. Moreover, the homogeneity and independence of the two samples were ensured since the two samples included the population of the same ethnic but did not overlap each other. In addition, the results passed all following tests detecting the pleiotropy or heterogeneity. Based on these reasons, the results of this study were reliable, and it can be concluded that an increase in GT intake can lead to a decrease in GDs.
The protective influence of GT on GD might come from the gut microbiome. Phenolic compounds constitute most in the dry weight of GT, of which catechins account for the most (Xing et al., Citation2019). Tea catechins can favor the growth of potentially beneficial bacteria and hinder that of some potentially detrimental microbes (Martín & Ramos, Citation2021). Some studies found that in the existence of Bifidobacterium spp., Lactobacillus, and Enterococcus, (−)-epigallocatechin-3-O-gallate (EGCG), gallocatechin gallate and EGCG 3”-methyl could significantly increase the production of short chain fatty acids, the main microbial fermentation product, main energy source for colonocytes, and is beneficial for human health (Rowland et al., Citation2018). Another study also proved this view and pointed out that oral EGCG can ameliorate the colonic inflammation better in the exist of gut microbiota (Wu et al., Citation2021). Obviously, this demonstrated the necessity of gut microbiome in this mechanism. There were also other mechanisms through which GT could do good to gastrointestinal health. In addition to gut microbiome, flavonoids have also been reported to be helpful in the management of inflammatory bowel diseases through its antioxidant, anti-inflammatory, antiviral, anticancer, and neuroprotective effect (Pei et al., Citation2020; Salaritabar et al., Citation2017). This demonstrated that the benefits of GT in gastrointestinal health might come from some independent pathways.
While there were inspiring results from some basic research, they could not provide solid clinical evidence of the benefit of GT. The active ingredients in GT metabolized in vivo may face the problem of low bioavailability, and some ingredients with benefits cannot represent all of GT. The eventual effect of GT on GDs must be verified by studies of a higher level of evidence. Since MR simulates the process of an RCT, it is regarded as slightly inferior to RCT status in evidence-based medicine (Skrivankova et al., Citation2021). Therefore, this study provided more directed evidence of the advantage of GT compared to basic research.
This study is the first to uncover that an increase in GT intake could safeguard against GDs, as indicated by two sample MR. The results stand up well to scrutiny. Moreover, it’s worth noting that this study currently boasts the highest level of evidence available, given the challenges in conducting an RCT on this particular topic.
However, there are some limitations in this study. First, the GDs were not classified, potentially implying that the observed beneficial effects might be attributed to specific types of GDs. Future research should therefore strive to differentiate and identify which specific GDs are mitigated by GT. Second, while the study highlights the positive impact of GT on GD protection, it falls short of unveiling the underlying mechanisms. To delve into the intricacies of these mechanisms, a more pivotal role can be assigned to basic research.
4. Conclusion
In conclusion, GT can be considered a healthy beverage for gastrointestinal health, and the habit of drinking GT is recommended.
Consent to participate
The informed consent has been obtained by UK biobank and the FinnGen project when individual data was collected, and it is unnecessary to inform again for usage of summary data, according to the provisions of the above projects.
Notes on contributor
Design, ZXJ and YL; analysis, ZXJ and RLL; writing, ZXJ and RLL; supervision, YL. All authors have read and agreed to the published version of the manuscript.
Statement of ethics
The data used in this research are publicly available and were obtained with corresponding ethical approval, thereby rendering additional ethical approval unnecessary.
Acknowledgements
The authors would like to thank all the staffs in UK biobank and the FinnGen project. The authors thank Binhong Duan (Huazhong University of Science and Technology Union Shenzhen Hospital) for her contribution in revising the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data is available through corresponding author.
References
- Almofarreh, A., Sheerah, H. A., Arafa, A., Ahamed, S. S., Alzeer, O., Al-Hunaishi, W., Mhimed, M. M., Al-Hazmi, A., & Lim, S. H. (2022). Beverage consumption and ulcerative colitis: A case-control study from Saudi Arabia. International Journal of Environmental Research and Public Health, 19(4), 2287. https://doi.org/10.3390/ijerph19042287
- Borrelli, F., Capasso, R., Russo, A., & Ernst, E. (2004). Systematic review: Green tea and gastrointestinal cancer risk. Alimentary Pharmacology & Therapeutics, 19(5), 497–6. https://doi.org/10.1111/j.1365-2036.2004.01884.x
- Bowden, J., Del Greco, M. F., Minelli, C., Davey Smith, G., Sheehan, N., & Thompson, J. (2017). A framework for the investigation of pleiotropy in two-sample summary data mendelian randomization. Statistics in Medicine, 36(11), 1783–1802. https://doi.org/10.1002/sim.7221
- Bowden, J., Del Greco, M. F., Minelli, C., Zhao, Q., Lawlor, D. A., Sheehan, N. A., Thompson, J., & Davey Smith, G. (2019). Improving the accuracy of two-sample summary-data mendelian randomization: Moving beyond the NOME assumption. International Journal of Epidemiology, 48(3), 728–742. https://doi.org/10.1093/ije/dyy258
- Bowden, J., & Holmes, M. V. (2019). Meta-analysis and mendelian randomization: A review. Research Synthesis Methods, 10(4), 486–496. https://doi.org/10.1002/jrsm.1346
- Burgess, S., & Thompson, S. G. (2011). Avoiding bias from weak instruments in mendelian randomization studies. International Journal of Epidemiology, 40(3), 755–764. https://doi.org/10.1093/ije/dyr036
- Davey Smith, G., & Hemani, G. (2014). Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Human Molecular Genetics, 23(R1), R89–98. https://doi.org/10.1093/hmg/ddu328
- De Jong, U. W., Day, N. E., Mounier-Kuhn, P. L., & Haguenauer, J. P. (1972). The relationship between the ingestion of hot coffee and intraoesophageal temperature. Gut, 13(1), 24–30. https://doi.org/10.1136/gut.13.1.24
- Emdin, C. A., Khera, A. V., & Kathiresan, S. (2017). Mendelian Randomization. JAMA, 318(19), 1925–1926. https://doi.org/10.1001/jama.2017.17219
- Hemani, G., Bowden, J., & Davey Smith, G. (2018). Evaluating the potential role of pleiotropy in mendelian randomization studies. Human Molecular Genetics, 27(R2), R195–r208. https://doi.org/10.1093/hmg/ddy163
- Hinojosa, N. D., Perez, B. S., de la Cueva, S. P., & Rufián-Henares, J. (2021). Green and white teas as health-promoting foods. Food & Function, 12(9), 3799–3819. https://doi.org/10.1039/d1fo00261a
- Kalan, F. K., Mahdavifar, N., Hassanipour, S., & Salehiniya, H. (2020). Epidemiologic study of gastric cancer in Iran: A systematic review. Clinical and Experimental Gastroenterology, 13, 511–542. https://doi.org/10.2147/ceg.S256627
- Kochman, J., Jakubczyk, K., Antoniewicz, J., Mruk, H., & Janda, K. (2020). Health benefits and chemical composition of matcha green tea: A review. Molecules, 26(1). https://doi.org/10.3390/molecules26010085
- Martimianaki, G., Alicandro, G., Pelucchi, C., Bonzi, R., Rota, M., Hu, J., Johnson, K. C., Rabkin, C. S., Liao, L. M., Sinha, R., Zhang, Z. F., Dalmartello, M., Lunet, N., Morais, S., Palli, D., Ferraroni, M., Yu, G. P., Tsugane, S. … La Vecchia, C. (2022). Tea consumption and gastric cancer: A pooled analysis from the stomach cancer pooling (StoP) project consortium. British Journal of Cancer, 127(4), 726–734. https://doi.org/10.1038/s41416-022-01856-w
- Martín, M., & Ramos, S. (2021). Impact of dietary flavanols on microbiota, immunity and inflammation in metabolic diseases. Nutrients, 13(3), 3. https://doi.org/10.3390/nu13030850
- Pei, R., Liu, X., & Bolling, B. (2020). Flavonoids and gut health. Current Opinion in Biotechnology, 61, 153–159. https://doi.org/10.1016/j.copbio.2019.12.018
- Perez, B. S., Navajas-Porras, B., López-Maldonado, A., Hinojosa-Nogueira, D., Pastoriza, S., & Rufián-Henares, J. (2021). Green tea and its relation to human gut microbiome. Molecules, 26(13), 3907. https://doi.org/10.3390/molecules26133907
- Poorolajal, J., Moradi, L., Mohammadi, Y., Cheraghi, Z., & Gohari-Ensaf, F. (2020). Risk factors for stomach cancer: A systematic review and meta-analysis. Epidemiology and Health, 42, e2020004. https://doi.org/10.4178/epih.e2020004
- Richmond, R. C., & Smith, G. D. (2022). Mendelian randomization: Concepts and scope. Cold Spring Harbor Perspectives in Medicine, 12(1), 1. https://doi.org/10.1101/cshperspect.a040501
- Rowland, I., Gibson, G., Heinken, A., Scott, K., Swann, J., Thiele, I., & Tuohy, K. (2018). Gut microbiota functions: Metabolism of nutrients and other food components. European Journal of Nutrition, 57(1), 1–24. https://doi.org/10.1007/s00394-017-1445-8
- Salaritabar, A., Darvishi, B., Hadjiakhoondi, F., Manayi, A., Sureda, A., Nabavi, S. F., Fitzpatrick, L. R., Nabavi, S. M., & Bishayee, A. (2017). Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World Journal of Gastroenterology: WJG, 23(28), 5097–5114. https://doi.org/10.3748/wjg.v23.i28.5097
- Skrivankova, V. W., Richmond, R. C., Woolf, B. A. R., Davies, N. M., Swanson, S. A., VanderWeele, T. J., Timpson, N. J., Higgins, J. P. T., Dimou, N., Langenberg, C., Loder, E. W., Golub, R. M., Egger, M., Davey Smith, G., & Richards, J. B. (2021). Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): Explanation and elaboration. BMJ, 375, n2233. https://doi.org/10.1136/bmj.n2233
- Verbanck, M., Chen, C. Y., Neale, B., & Do, R. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nature Genetics, 50(5), 693–698. https://doi.org/10.1038/s41588-018-0099-7
- Wu, Z., Huang, S., Li, T., Li, N., Han, D., Zhang, B., Xu, Z. Z., Zhang, S., Pang, J., Wang, S., Zhang, G., Zhao, J., & Wang, J. (2021). Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome, 9(1), 184. https://doi.org/10.1186/s40168-021-01115-9
- Xing, L., Zhang, H., Qi, R., Tsao, R., & Mine, Y. (2019). Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. Journal of Agricultural and Food Chemistry, 67(4), 1029–1043. https://doi.org/10.1021/acs.jafc.8b06146
- Yi, M., Wu, X., Zhuang, W., Xia, L., Chen, Y., Zhao, R., Wan, Q., Du, L., & Zhou, Y. (2019). Tea consumption and health outcomes: Umbrella review of meta-analyses of observational studies in humans. Molecular Nutrition & Food Research, 63(16), e1900389. https://doi.org/10.1002/mnfr.201900389
