?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Pectin was extracted from onion peels using microwave and ultrasound-assisted natural deep eutectic solvents (NADES) and compared with conventional acid-extracted pectin. The primary screening trials indicated that the suitable combination of hydrogen bond acceptor and donor was choline chloride and tartaric acid, respectively. The Box-Behnken design was further used to optimize processing conditions like microwave intensity (26.903 W/g), microwave processing duration (2.99 min), and sonication duration (10.18 min) for maximizing the pectin yield from onion peel powder. The moisture, ash, and equivalent weight of extracted pectin were in the range specified by the International Pectin Producers Association. The higher anhydrouronic acid content of the NADES-extracted pectin indicated the higher purity of pectin compared to the conventional acid-extracted pectin. The NADES-based extraction was found to be a promising system for extracting pectin with a higher degree of esterification from onion peels.
1. Introduction
Foods contain various nutrients and bioactive compounds which are required to improve human health and well-being. Consumers prefer products and ingredients derived from natural food products instead of chemically synthesized compounds to attain desired health benefits. During the COVID-19 pandemic, bioactive compounds are focused on enhancing the function of the immune system. The pandemic provided opportunities for the commercialization of functional foods rich in bioactive compounds which can be obtained by valorization of food wastes (Galanakis et al., Citation2021). Several epidemiological studies have shown that consumption of target micro and macronutrients can reduce the risk of diabetes, stroke, heart, and several other diseases (Galanakis, Citation2020a; Galanakis et al., Citation2020). The extraction of bioactive components from agro-industrial wastes and incorporation into functional foods provides additional profit for industries, addresses environmental concerns, and advocates circular economy practices. Pectin is a bioactive polysaccharide that can be used as emulsion stabilizers and fat replacers in food products due to its viscoelastic properties. It can also enhance the shelf life of products and reduce the lipid level in blood.
Pectin is conventionally extracted from agricultural wastes (fruit and vegetable peels) by acidic medium. Acid extraction is generally performed on an industrial scale using strong mineral/organic acid solutions at high temperatures. These processes require longer duration and higher energy which also result in serious environmental problems due to the acidic wastewater production and equipment corrosion. Due to the drawbacks of conventional acid extraction, there is rising interest in the application of alternative innovative approaches for pectin extraction. Various thermal technologies (ohmic and microwave heating) and non-thermal processing methods (ultrasound, high pressure, and pulsed electric field) have recently been used for the extraction of pectin (Galanakis, Citation2020b). Ultrafiltration, nanofiltration, and other membrane-based technologies can be used for further purification of pectin (Galanakis, Citation2015). The combination of thermal and non-thermal processing methods can further improve the pectin yield with the desired level of degree of esterification. In addition to these technologies, alternative green approaches like natural deep eutectic solvents (NADES) can also be used as extraction solvents. The application of ultrasound and microwave-assisted NADES can further improve the extraction efficiency. The industrial viability of the NADES-based extraction process can be analysed using a life cycle assessment that offers a wealth of information about energy efficiency and environmental impacts. The viability of NADES-based processing industries is significantly influenced by NADES recovery. Various methods such as distillation, supercritical fluid extraction, membrane filtration, anti-solvent addition, and recrystallization can be used to recover NADES after extraction. During biofuel production, NADES recovered by membrane filtration and recrystallization resulted in a low net energy ratio and green gas emissions (Song et al., Citation2023).
The pectin content varies considerably in apple pomace (10–15%) and citrus peel (25–35%) as reported by Kumar et al. (Citation2020). Alexander and Sulebele (Citation1973) reported ammonium oxalate-based extraction of pectin from onion peel resulted in a pectin yield of 11–12%. Sub-critical water extraction of pectin from onion peels resulted in a pectin yield of 9% (Benito-Román et al., Citation2022). Hot acid and pulsed ultrasound-assisted extraction of pectin from onion peels resulted in 16.22 and 9.83% of pectin yield, respectively (Şen et al., Citation2023). Silva et al. (Citation2020) reported more than 550,000 tonnes of onion waste are generated annually due to human consumption and food processing industries. The valorization of potential bioresources by biochemical and thermochemical processes can increase the generation of bio-based products (Galanakis, Citation2020a; Galanakis, Citation2022). Onion peels have significant valorization potential as a pectin source which can be used in the preparation of various food products. Hence, in this study, we aim to investigate the sequential extraction of pectin using microwave and ultrasound-assisted NADES and compare it with conventional acid extraction. The effect of the processing conditions on the yield, physicochemical, and techno-functional properties of pectin were also studied.
2. Materials and methods
2.1. Materials
Onion peels (Agrifound Rose variety) were collected from the local market and stored at 4°C until further usage. They were collected from the same source to minimize the variation in the product quality. All the solvents and chemicals used in this study are of analytical grade.
2.2. Methods
2.2.1. Onion peel powder preparation
The hot air oven (Technico Laboratory Products Pvt. Ltd., India) was preheated to maintain a temperature of 50 ± 1°C. The collected onion peels were evenly spread on trays as a single layer and dried to a constant weight for about 6 h. The dried peels were ground into a fine powder of particle size less than 1000 µm by using a blender. The onion peel powder was then transferred into an air-tight container and stored in a refrigerator (4 ± 1°C).
2.2.2. Preparation of NADES
The NADES of various compositions were prepared according to the previously published literature reports (Chen & Lahaye, Citation2021; Elgharbawy et al., Citation2018). Different combinations of the hydrogen bond acceptor and the hydrogen bond donor which constitute aqueous NADES were prepared in the molar ratio of 0.5 M (). The hydrogen bond acceptor and the hydrogen bond donor were mixed at a 1:1 volume ratio and heated at 80°C until a homogeneous and stable mixture without any precipitate was formed ().
Table 1. Pectin yield values obtained using different combinations of hydrogen bond acceptors and hydrogen bond donors.
2.2.3. Screening of potential NADES
In the experimental procedure, initially, 15 ml of each NADES was combined with 1 g of onion peel powder. Subsequently, this mixture was subjected to incubation for 30 min in a water bath maintained at a temperature of 90°C. Following the incubation period, centrifugation was employed for 10 min at a rate of 6000×g, to segregate the supernatant from the mixture. To precipitate the acquired supernatant, 95% ethanol equivalent to twice the volume of the supernatant was added, and the resultant solution was allowed to remain undisturbed overnight at a temperature of 4°C. The mixture was subsequently centrifuged at 6000×g for 15 min, facilitating the separation of the precipitated pectin. The collected pectin was washed twice with 70% (v/v) ethanol, and the pectin was dried in a hot air oven at 70°C until constant weight. The pectin yield was calculated using the below equation.
where Mf is the final dried weight of pectin (g) and M0 is the initial weight of onion peel powder (g).
2.2.4. Optimization of the solid–liquid ratio of potential NADES
To maximize the pectin yield, solid – liquid ratio was optimized by dissolving 1 g of onion peel powder in the different volumes (10–60 ml) of the optimized NADES components ().
Table 2. Effect of solid–liquid ratio on the pectin yield from onion peel powder using choline chloride and tartaric acid.
2.2.5. Sequential extraction process optimization using box Behnken design
The potential NADES at the optimized solid–liquid ratio was combined with onion peel powder followed by sequential exposure to microwave followed by ultrasound to improve the pectin extraction efficiency. The single factorial design was used to select the range of process variables such as microwave intensity, microwave duration, and sonication duration. Microwave and ultrasound-assisted extraction were performed using a domestic microwave oven (MS2043DB, LG Electronics India Private Limited, India) and an ultrasonic bath (USB 3.5 L, PCi Analytics, India), respectively. The 3-level-3-factor Box Behnken design together with the desirability function approach was further utilized to optimize the processing conditions of microwave intensity (X1, 16.15–26.82 W/g), microwave duration (X2, 2–3 min), and sonication duration (X3, 10–15 min), on the extraction yield of pectin (Y1, %) using the software Design-Expert 13 (Stat-Ease Inc., MN, U.S.A.). The complete design is comprised of 17 combinations and was performed in random order (). The obtained data were fitted to a second-order polynomial equation to establish the relationship between the independent variables and the responses. The adequacy of the developed model was validated by predicting the response values and comparing them with the experimental results (Gharibzahedi et al., Citation2019; Jabbar et al., Citation2015)
Table 3. Box-Behnken design with the experimental and predicted values of pectin yield (%) from onion peel powder.
2.2.6. Conventional acid extraction
The onion peel powder was mixed with hydrochloric acid (pH 2.5) in the ratio of 1:25 (w/v) followed by incubation for 60 min at 95°C. The mixture was mixed with twice the volume of ethanol and left overnight. This was centrifuged for 15 min at 6000×g and the obtained precipitate was washed with 95% ethanol followed by hot air drying at 50°C.
2.2.7. Physicochemical characteristics of pectin
2.2.7.1. pH
The pH of the pectin solution (1% w/v in distilled water) was determined according to the protocol reported by Deb Roy et al. (Citation2014) in triplicates by using a pH meter (µ Ph System 361, Systronics India Limited, Chennai).
2.2.7.2. Moisture and ash content
The moisture (130°C for 3 h) and ash (600°C for 4 h) contents were calculated by gravimetric method (Kurita & Yamada, Citation2008).
2.2.7.3. Protein and total carbohydrate content
Protein and total carbohydrate content were determined using the Bradford method (Bradford, Citation1976) and the phenol-sulphuric acid method, respectively (Masuko et al., Citation2005).
2.2.7.4. Galacturonic acid content
The carbazole-sulfuric acid method was used to determine the galacturonic acid content in the extracted pectin samples (Dische & Borenfreund, Citation1951).
2.2.7.5. Total phenolic content
The total phenolic content of the extracted pectin samples was determined by the Folin–Ciocalteu method using gallic acid as standard (Li et al., Citation2014). An equal volume of pectin solution (1 mg/ml) was mixed with 0.5 ml of tenfold diluted Folin-Ciocalteu reagent. After 5 min of incubation, 0.5 mL of a 7% sodium carbonate solution was added and the mixture was kept at 24°C in the dark for 2 h. The absorbance was measured at 725 nm. The results were expressed as mg gallic acid equivalent (GAE)/g of the sample.
2.2.7.6. Equivalent weight, methoxyl, and anhydrouronic acid content
Equivalent weight, methoxyl content, and anhydrouronic acid were determined by using the titration method (Khamsucharit et al., Citation2018). The following equation was used to determine the equivalent weight:
The methoxyl content was calculated using EquationEquation (3)(3)
(3) shown below.
The anhydrouronic acid was determined by using the below EquationEquation (4)(4)
(4) .
where the molecular weight of one unit of anhydrouronic acid is 176 g, z is the amount of sodium hydroxide from equivalent weight calculation, y is the amount of sodium hydroxide in methoxyl content determination, and w is the weight of the sample.
2.2.7.7. Antioxidant activity
The antioxidant activity of pectin (25 and 100 µg/ml) was analyzed using a 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay, and butylated hydroxytoluene (BHT) was used as the positive control in this experiment in the same doses (Li et al., Citation2014).
2.2.7.8. Degree of esterification (DE)
The DE of pectin was calculated according to the formula reported below (Ranganna,Citation2010).
2.2.7.9. Fourier transform infrared (FTIR) spectroscopy
The FTIR spectrometer (IRAffinity-1, Shimadzu, Japan) was used to obtain the FTIR spectra of pectin samples using the potassium bromide (KBr) disc method in the range of 4000–400 cm−1 with a resolution of 4 cm−1 across 30 scans.
2.2.8. Technofunctional properties of pectin
2.2.8.1. Oil holding capacity (OHC)
Pectin was dissolved in palm oil (1:20 w/v) and incubated for 30 min with continuous stirring. The samples were centrifuged (Neya-16 R, Remi, India) for 30 min at 1006×g. The volume of the supernatant was measured after centrifugation. The OHC was expressed as a gram of oil retained per gram of pectin (Chau & Huang, Citation2003).
2.2.8.2. Water retention capacity (WRC)
The WRC was calculated by incubation of pectin in distilled water (1:10 w/v) for 24 h with continuous stirring followed by centrifugation (Neya-16 R, Remi, India) at 1006×g for 30 min. The WRC was expressed as ml of water held by 1 g of pectin (Chau & Huang, Citation2003).
2.2.8.3. Emulsifying properties
The emulsifying properties defined by the emulsifying capacity and the stability of the emulsion were studied using the protocol provided by Bayar et al. (Citation2018). About 10 mL of 1% pectin solution was mixed with 5 ml of palm oil followed by homogenization for 3 min and centrifugation at 948×g for 5 min.
where Vf is the volume of the emulsion and Vi is the total volume of the mixture. The emulsion was incubated for 30 min at 80°C and then centrifuged at 948×g for 5 min to evaluate the emulsion stability (ES) according to the below equation.
where Vi is the total volume of the mixture Vt is the volume of the emulsion after incubation and centrifugation.
2.2.9. Statistical analysis
The data presented in this article are the mean values of triplicate data obtained during the same trial. A one-way analysis of variance using the Tukey multiple comparison method was used to observe significant (p < .05) differences in the tested parameters using Minitab 21.0 software (2021 Minitab Inc.). The results of means ± standard deviations are reported.
3. Result and discussion
3.1. Single-factor experiment of NADES-based extraction
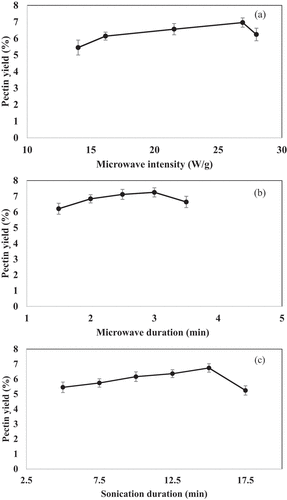
Three different hydrogen bond acceptors (choline chloride, betaine, and proline) and various hydrogen bond donors (tartaric acid, malic acid, malonic acid, fructose, sucrose, and dextrose) in the molar ratio of 0.5 M were used in preliminary trials for the extraction of pectin from onion peel powder. The hydrogen bond donors and hydrogen bond acceptors were mixed in a volume ratio of 1:1. About 1 g of onion peel powder was mixed with 15 ml of NADES and incubated at 90°C for 30 min for the extraction of pectin. NADES directly interacts with the target compounds by hydrogen bonds or by damaging the cell wall leading to their release in the extraction medium. The yield of pectin was higher during the extraction with choline chloride: tartaric acid (). During the extraction process, bonds present between the cell walls of onion peel and pectin are hydrolyzed by NADES which in turn results in the separation of pectin. Soylak and Koksal (Citation2019) also reported that the selection of a type of deep eutectic solvent is critical for the enhanced microextraction of metal ions from oil samples for atomic absorption spectrometric analysis. Following these preliminary trials, the effect of solid–liquid ratios (onion peel powder and choline chloride: tartaric acid) on the extraction of pectin was studied in the range of 1:10 to 1:60 (). The solid–liquid ratio of 1:50 was selected for further optimization studies due to the attained higher yield. Bajkacz and Adamek (Citation2018) also optimized the solid–liquid ratio, composition of NADES, and processing duration for enhancing the extraction of flavonoids from agricultural produce. The volume of deep eutectic solvent played a significant role in the separation and preconcentration of chromotrope 2 R prior to the spectrophotometric analysis (Shah et al., Citation2022). The microwave-assisted extraction using choline chloride and tartaric acid was performed at different microwave intensities in the range of 14–28 W/g for 2 min. The maximum pectin yield was attained at 26.92 W/g, and further optimization studies for microwave processing duration were performed for 1.5–3.5 min. The ultrasound-assisted extraction of pectin was performed for 5–17.5 min. Based on the results obtained from single-factor experiments as shown in , the ranges selected for the response surface methodology are as follows: microwave intensity (16.15–26.92 W/g), microwave duration (2–3 min), and sonication duration (10–15 min).
3.2. Optimisation of NADES-based extraction using Box-Behnken design
The experimental ranges of various factors which exhibited higher responses were utilized in the response surface methodology to obtain the maximum response point. The effect of three different extraction parameters such as microwave intensity (X1), microwave duration (X2), and sonication duration (X3) on pectin yield from onion peel powder was studied using the Box-Behnken design. The experimental design with a total of 17 runs along with the experimental and predicted values are shown in . NADES composed of choline chloride and tartaric acid was sequentially treated with microwave followed by ultrasound. The obtained data show that the experimental and predicted values are in close agreement. Pectin yield varies according to the different extraction parameters and it ranged between 6.13% and 11.11%. The maximum pectin yield was attained at the experimental condition of X1 = 26.92 W/g, X2 = 3 min, and X3 = 12.5 min. To the experimental data of ultrasound and microwave-assisted extraction of pectin from onion peel powder, a second-order polynomial model with significant fit (p < .0001) and non-significant lack-of-fit (p > .05) was effectively constructed (). Multiple regression analysis was used to construct the below quadratic equation for the pectin yield based on the obtained experimental data.
Table 4. ANOVA for the fitted second-order polynomial model of pectin yield (%) from onion peel powder.
The values of model quality indicators for yield including R2 (0.9991), adjusted R2 (0.998), coefficient of variation (0.8112%), PRESS (7.14), and ADP (100.5783) demonstrated that the experimental and predicted data are in good agreement (). The extent of experimental variability is represented by the coefficient of variation. The low coefficient of variation obtained in this model suggested the good precision and high reliability of the experiment. The signal-to-noise ratio is defined as the ADP and a value higher than 4 is appropriate for a good fit of the model (Gharibzahedi et al., Citation2015). The developed quadratic model sufficiently covers the entire experimental range of studies because the predicted values are correlated satisfactorily with the respective observed values. A normal probability plot of the residuals was constructed to evaluate the normality assumption because the adequacy of the developed model can be determined by the residuals from the least square fit as indicated by (Gharibzahedi et al., Citation2015).
Figure 2. (a) Comparison between predicted and actual values of pectin yield obtained by sequential microwave and ultrasound-assisted natural deep eutectic solvents-based extraction (b) normal probability of internally studentized residuals.
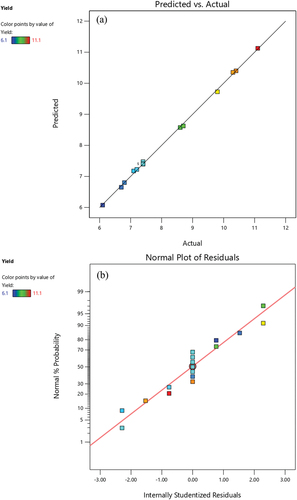
The normality assumption is satisfied because the residual plots in are along a straight line. The internally studentized residuals in display a Gaussian distribution, and the deviations of the values from the diagonal line are small, indicating the high adequacy of the model (Gharibzahedi et al., Citation2015). These results proved that the fitted second-order polynomial model is suitable for studying the maximization of the pectin yield from the onion peel powder by the response surface methodology-Box-Behnken design. The extraction yield of pectin from onion peel powder was affected by the linear effects of MW intensity and MW treatment duration (p < .0001). The quadratic effects of MW intensity (p < .0001), MW treatment duration (p < .0001), and sonication duration (p = .0002) were significant on the extraction yield ().
Figure 3. Three-dimensional response surface plots of pectin yield with three factors (a) microwave intensity vs. microwave duration (b) microwave intensity vs. sonication duration (c) microwave intensity vs. sonication duration.
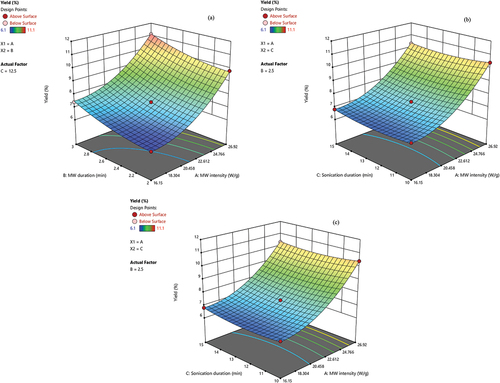
An increase in microwave intensity provides greater exposure to microwave energy that in turn enhances the penetration rate of extraction solvents into the plant matrix. The intense rupture of plant tissue promotes the dissolution of pectin materials into the solution (Rostami & Gharibzahedi, Citation2017). Exposure to ultrasound waves can accelerate the swelling and hydration potential of plant tissues. Cell disruption is accelerated by the collapse of micro-bubbles and micro-jets formation near the plant matrix surface (Gharibzahedi, Citation2017; Moorthy et al., Citation2015). The extraction yield is improved with the increase in sonication duration due to the release of pectin from the solid matrix to the extraction medium (Thirugnanasambandham et al., Citation2015). Lojková et al. (Citation2023) also reported that ultrasound-assisted NADES-based extraction resulted in higher recovery of quercetin from onion peels. The NADES can be effectively used as a recyclable solvent by bubbling carbon dioxide (Wang et al., Citation2024). The optimum parameters to attain the maximum pectin yield from onion peel powder (11.319%) are microwave intensity of 26.903 W/g, microwave duration of 2.99 min, and sonication duration of 10.18 min (Desirability = 1.0). Five additional tests carried out at the predicted optimal conditions resulted in an extraction yield of 11.28 ± 0.08%. This value was found to be very close to the predicted value, which confirms the validity and adequacy of the constructed model.
3.3. Conventional acid extraction
The pectin yield obtained by the conventional hydrochloric acid extraction was lower (3.47 ± 0.26%) than the NADES-assisted sequential extraction, while the value was comparable to the yield (2.59%) obtained from sweet potato peels (Hamidon & Zaidel, Citation2017). Sukor et al. (Citation2021) also reported that deep eutectic solvent-based ultrasound-assisted extraction resulted in a higher yield of tannic acid compared to the conventional method.
3.4. Physicochemical characteristics of pectin
The physicochemical characteristics of pectin obtained using the optimized processing conditions (microwave intensity of 26.903 W/g, microwave duration of 2.99 min, and sonication duration of 10.18 min) and the conventionally extracted pectin were studied in detail.
3.4.1. pH
The pH determines the microbial propagation and plays an important role in determining the suitability of the product for preservation. The pH of the NADES-based extracted pectin from onion peel was 3.5 ± 0.14, which falls in the pH range of 3.2–3.5 as reported by Lochhead (Citation2017). The conventional acid extracted pectin showed a pH of 3.0 ± 0.12 which is slightly higher than the pH (2.81) of Averrhoa bilimbi pectin (Shafie et al., Citation2019). The lower pH of extracted pectin can control the growth of pathogenic microorganisms throughout the storage (Bamba et al., Citation2020).
3.4.2. Moisture and ash content
The NADES-based extracted pectin had a moisture and ash content of 11.5 ± 0.45% and 6.9 ± 0.36%, respectively. The conventional acid-extracted pectin had a moisture content of 9.2 ± 0.12% and ash content of 4.1 ± 0.26%, respectively. These values are in agreement with the previously published moisture content values of pectin from onion peels, i.e. 12.4% (Alexander & Sulebele, Citation1973). The International Pectin Producers Association (IPPA) recommends that the moisture and ash content of pectin should be less than 12% and 10%, respectively. The moisture and ash content of pectin extracted from onion peels by NADES and conventional method are within the permissible limit.
3.4.3. Carbohydrate & protein content determination
The total carbohydrate content of pectin obtained by NADES-based extraction and conventional method was found to be 36 ± 0.85% and 30 ± 0.11%, respectively. The protein content was 0.59 ± 0.06% and 0.32 ± 0.02% for NADES and conventional acid-extracted pectin, respectively. The obtained lower protein content in pectin is advantageous because it further reduces the need for purification methods.
3.4.4. Galacturonic acid content
The extracted pectin using NADES had a galacturonic acid content of 71.28 ± 0.34%, while the conventional acid extracted pectin contained 65.14 ± 0.21%. The capacity of the extraction method to release pectin with higher galacturonic acid from onion peel is demonstrated by the NADES-based extraction of pectin (Garna et al., Citation2004). Santra et al. (Citation2023) also reported that galacturonic acid of pectin extracted from kinnow peel was in the range of 67.56–78.22%.
3.4.5. Total phenolic content
The total phenolic content of the pectin extracted using NADES was 2.42 ± 0.21 mg GAE/g which was similar to the pectin extracted from apple pomace 2.8 mg GAE/g (Konrade et al., Citation2023). Deep eutectic solvent-based extraction also resulted in the improved recovery of phenolic compounds from onion peels (Pal and Jadeja, Citation2019). In contrast, the total phenolic content of the pectin extracted using the conventional method was low (1.17 ± 0.41 mg GAE/g). The higher total phenolic content of NADES-based extracted pectin can further enhance the antioxidant potential of the obtained pectin.
3.4.6. Equivalent weight
The equivalent weight of the NADES (500 ± 1.2 mg) and conventional acid extracted pectin (416.66 ± 0.8 mg) was below the acceptable maximum limit provided by IPPA (<800 mg). The equivalent weight depends on the extraction conditions, number of free acids and source of pectin. Alexander and Sulebele (Citation1973) also reported equivalent weight in the range of 568–758 for white and red onions, respectively. The equivalent weight of pectin from sweet lime peels was found to be in the range of 566.2–795.2 mg (Siddiqui et al., Citation2021).
3.4.7. Methoxyl content
The amount of methylation of the galacturonic acid chain in the pectin molecule is indicated by the methoxyl content. The methoxyl content was found to be 8.06 ± 0.14% and 6.2 ± 0.23% for NADES and conventional acid extracted pectin. These values are comparable to the methoxyl content of pectin from onion, citrus, and mango peels (Abid et al., Citation2009; Alexander & Sulebele, Citation1973; Girma & Teshome Worku, Citation2016).
3.4.8. Anhydrouronic acid
The anhydrouronic acid content of NADES and conventional acid-extracted pectin is 77.44 ± 0.57% and 81.9 ± 0.57%, respectively. The higher anhydrouronic acid content indicates the superior purity of pectin. Alexander and Sulebele (Citation1973) also reported an anhydrouronic acid content of 80.44% for the pectin extracted from onion peels, while Khamsucharit et al. (Citation2018) reported 82.05% for citrus peel pectin. Pectin with anhydrouronic acid content of not less than 65% can be used as a food additive and pharmaceutical ingredient (Food Chemical Codex,Citation1996).
3.4.9. Antioxidant activity
The DPPH assay is a widely used antioxidant assay that is based on the decrease in absorbance of the radical-containing solution (Amorati et al., Citation2013). The DPPH radical scavenging activity of BHT (20 μg/ml), NADES, and conventional acid-extracted pectin are 49.14 ± 0.79, 38.91 ± 0.94, and 31.29 ± 0.11%, respectively. The higher antioxidant activity of pectin obtained by NADES-based extraction can be attributed to the higher phenolic content of the pectin.
3.4.10. Degree of esterification
The degree of esterification of the NADES-based extracted pectin was found to be 56.88 ± 0.96%, while Alexander and Sulebele (Citation1973) also reported a value in the similar range (60.2%) for onion peel pectin. The degree of esterification higher than 50% indicates that the extracted pectin can be classified as high methoxyl pectin. Khamsucharit et al. (Citation2018) reported the degree of esterification of pectin from citrus peel and apple pomace as 62.83% and 58.44%, respectively. In contrast, the conventional acid-extracted pectin has a degree of esterification of 45.46 ± 0.12%, placing it in the low methoxyl pectin category which is comparable to pectin obtained from the lemon peel (Ciriminna et al., Citation2017).
3.4.11. FTIR spectroscopy
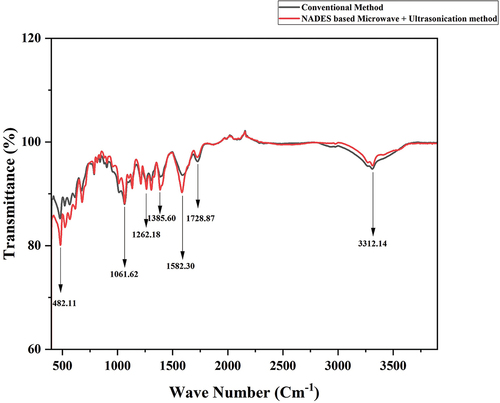
FTIR is a rapid and convenient method for analyzing the functional groups of polysaccharides. The FTIR analysis of NADES and conventional acid-extracted pectin was performed for the identification and understanding of the structural characteristics. The FTIR spectra in the wavelength range from 800 to 1300 cm−1 are known as the “fingerprint” zone for carbohydrates (Kamnev et al., Citation1998). The NADES and conventional acid-extracted pectin have spectra similar to pectin obtained from citrus pomace, and apple pomace in the fingerprint region (Muhammad et al., Citation2014; Oliveira et al., Citation2016). The similarities of the extracted pectin in the fingerprint region prove that the extracted pectin from onion peel powder is effective pectin. The absorption bands at 1730–1760 cm−1 and 1600–1630 cm−1 can be attributed to the stretching vibration of ester carbonyl groups (C=O) and carboxyl groups (COO−), respectively (Kamnev et al., Citation1998). The pectin extracted from onion peel exhibited stronger absorption of ester carbonyl groups with a weaker absorption of the carboxyl stretching band (). Citrus peel and apple pomace pectins also followed a similar trend due to the higher content of anhydrouronic acid indicating that as a high methoxyl pectin (Khamsucharit et al., Citation2018).
3.5. Techno-functional characterisation
3.5.1. Water retention capacity
The amount of bound water per gram of the sample is known as the water retention capacity. The ability of the powder to absorb and retain water is a crucial factor in determining the drying conditions and the choice of appropriate packaging. The water retention capacity of the pectin extracted from onion peel powder using NADES and conventional acid extraction method is 3.2 ± 0.16 and 2.9 ± 0.24 ml of water/g of pectin, respectively. These values are in agreement with the values obtained for pectin extracted from strawberry (2.8 ml/g), red currant (3.4 ml/g), blackberry (3.4 ml/g), and raspberry (3.8 ml/g) as reported by Muñoz-Almagro et al. (Citation2021).
3.5.2. Oil holding capacity
One of the most important characteristics of hydrocolloids is their oil-holding capacity which is the amount of oil that the sample can bind. The oil-holding capacity of pectin extracted from onion peel powder using NADES and HCl was 12 ± 0.86 g of oil/g and 10 ± 0.92 g of oil/g of pectin, respectively. The obtained values are similar to the values of pectin from redcurrant (12.7 g/g) and blackberry (12.2 g/g) as reported by Muñoz-Almagro et al. (Citation2021).
3.5.3. Emulsifying properties
The emulsion capacity was 60 ± 0.94 and 58 ± 0.2% for NADES and conventional acid-extracted pectin. The emulsion stability of NADES and conventional acid-extracted pectin was 46.6 ± 0.87 and 42.2 ± 0.23%, respectively. The values of emulsion capacity (63.7%) and stability (58.3%) obtained for the NADES-based extracted pectin were in agreement with the pectin extracted from pumpkin (Cui & Chang, Citation2014).
4. Conclusion
The pectin extraction from onion peel using choline chloride and tartaric acid resulted in a higher yield compared to other combinations of hydrogen bond donors and acceptors. The optimized extraction condition is processing at a microwave intensity of 26.903 W/g for 2.99 min followed by exposure to sonication for 10.18 min. The developed second-order model for the optimization study using the Box Behnken design predicted yield (%) accurately with adequate determination coefficients. The higher anhydrouronic acid content of the extracted pectin signifies its potential to be used as an alternative food additive source. The yield and other quality parameters of the pectin were enhanced by the NADES-based extraction when compared with the conventional acid-based extraction. The moisture and ash content of NADES-based extracted pectin from onion peels is within the permissible limit provided by IPPA. The lower protein content of pectin indicates the minimal steps required for further purification. Similarities to the fingerprint region for carbohydrates in the FTIR spectrum indicate that the extracted pectin is an effective pectin. The extracted high methoxyl pectin can be used as a gelling agent, thickener, and stabilizer in food products. This study not only contributes to the utilization of onion peels as a potential waste material for pectin extraction but also demonstrates the effectiveness of combining innovative extraction techniques and statistical optimization for enhancing pectin yield and quality. The functional attributes of the extracted pectin underscore its significance as a value-added product derived from agricultural waste and potential applications in various industries, aligning with eco-friendly practices. Further research and applications of the extracted pectin could pave the way for reducing waste and promoting the circular economy.
Acknowledgments
The authors thank the Vellore Institute of Technology, Vellore for providing the ‘VIT SEED Grant – RGEMS Fund (SG20220041)’ for carrying out this research work’.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated during and/or analyzed during the current study are available within this article.
Additional information
Funding
References
- Abid, H., Hussain, A., & Ali, J. (2009). Technique of optimum extraction of pectin from sour orange peels and its chemical evaluation. Journal of the Chemical Society of Pakistan, 31(3), 459–11.
- Alexander, M. M., & Sulebele, G. A. (1973). Pectic substances in onion and garlic skins. Journal of the Science of Food and Agriculture, 24(5), 611–615. https://doi.org/10.1002/jsfa.2740240514
- Amorati, R., Foti, M. C., & Valgimigli, L. (2013). Antioxidant activity of essential oils. Journal of Agricultural and Food Chemistry, 61(46), 10835–10847. https://doi.org/10.1021/jf403496k
- Bajkacz, S., & Adamek, J. (2018). Development of a method based on natural deep eutectic solvents for extraction of flavonoids from food samples. Food Analytical Methods, 11(5), 1330–1344. https://doi.org/10.1007/s12161-017-1118-5
- Bamba, B. S. B., Gouin, J. A., Kouassi, E. K. A., Komenan, A. C. A., Akre, M. S. H., Soro, D., & Soro, Y. R. (2020). Production of pectin as a relevant tool for by-products management resulting from four tropical edible fruits processing: Extraction yield, physicochemical and functional properties of pectin powder. International Journal of Chemical and Process Engineering Research, 7(1), 60–73. https://doi.org/10.18488/journal.65.2020.71.60.73
- Bayar, N., Friji, M., & Kammoun, R. (2018). Optimization of enzymatic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal. Food Chemistry, 241, 127–134. https://doi.org/10.1016/j.foodchem.2017.08.051
- Benito-Román, Ó., Alonso-Riaño, P., Díaz de Cerio, E., Sanz, M., & Beltrán, S. (2022). Semi-continuous hydrolysis of onion skin wastes with subcritical water: Pectin recovery and oligomers identification. Journal of Environmental Chemical Engineering, 10(3), 107439. https://doi.org/10.1016/j.jece.2022.107439
- Bradford, M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254. https://doi.org/10.1016/0003-2697(76)90527-3
- By authority of the U.S. of America legally binding document. (1996). Food chemical codex, academy of sciences ( 4th ed).
- Chau, C. F., & Huang, Y. L. (2003). Comparison of the chemical composition and physicochemical properties of different fibers prepared from the peel of Citrus sinensis L. Cv. Liucheng. Journal of Agricultural and Food Chemistry, 51(9), 2615–2618. https://doi.org/10.1021/jf025919b
- Chen, M., & Lahaye, M. (2021). Natural deep eutectic solvents pretreatment as an aid for pectin extraction from apple pomace. Food Hydrocolloids, 115, 106601. https://doi.org/10.1016/j.foodhyd.2021.106601
- Ciriminna, R., Fidalgo, A., Delisi, R., Tamburino, A., Carnaroglio, D., Cravotto, G., Ilharco, L. M., & Pagliaro, M. (2017). Controlling the degree of esterification of citrus pectin for demanding applications by selection of the source. American Chemical Society Omega, 2(11), 7991–7995. https://doi.org/10.1021/acsomega.7b01109
- Cui, S. W., & Chang, Y. H. (2014). Emulsifying and structural properties of pectin enzymatically extracted from pumpkin. LWT - Food Science and Technology, 58(2), 396–403. https://doi.org/10.1016/j.lwt.2014.04.012
- Deb Roy, S., Biswajit, D., Suvakanta, D., Ramesh Chandra, C., Jashabir, C., & Roy Saumendu, D. (2014). Optimization and characterization of purified polysaccharide from terminalia belarica gum as pharmaceutical excipient. International Journal of Pharmaceutical Research & Allied Sciences, 3(1), 21–29.
- Dische, Z., & Borenfreund, E. (1951). A new spectrophotometric method for the detection and determination of keto sugars and trioses. The Journal of Biological Chemistry, 192(2), 583–587. https://doi.org/10.1016/S0021-9258(19)77782-5
- Elgharbawy, A. A., Hayyan, A., Hayyan, M., Rashid, S. N., Nor, M. R. M., Zulkifli, M. Y., & Mirghani, M. E. S. (2018). Shedding light on lipase stability in natural deep eutectic solvents. Chemical and Biochemical Engineering Quarterly, 32(3), 359–370. https://doi.org/10.15255/CABEQ.2018.1335
- Galanakis, C. M. (2015). Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends in Food Science and Technology, 42(1), 44–63. https://doi.org/10.1016/j.tifs.2014.11.005
- Galanakis, C. M. (2020a). The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods, 9(4), 523. https://doi.org/10.3390/foods9040523
- Galanakis, C. M. (2020b). Functionality of food components and emerging technologies. Foods, 10(1), 128. https://doi.org/10.3390/foods10010128
- Galanakis, C. M. (2022). The “vertigo” of the food sector within the triangle of climate change, the post-pandemic World, and the Russian-Ukrainian war. Foods, 12(4), 721. https://doi.org/10.3390/foods12040721
- Galanakis, C. M., Aldawoud, T. M. S., Rizou, M., Rowan, N. J., & Ibrahim, S. A. (2020). Food ingredients and active compounds against the coronavirus disease (COVID-19) pandemic: A comprehensive review. Foods, 9(1), 1701. MDPI. https://doi.org/10.3390/foods9111701
- Galanakis, C. M., Rizou, M., Aldawoud, T. M. S., Ucak, I., & Rowan, N. J. (2021). Innovations and technology disruptions in the food sector within the COVID-19 pandemic and post-lockdown era. Trends in Food Science & Technology, 110(1), 193–200. https://doi.org/10.1016/j.tifs.2021.02.002
- Garna, H., Mabon, N., Wathelet, B., & Paquot, M. (2004). New method for a two-step hydrolysis and chromatographic analysis of pectin neutral sugar chains. Journal of Agricultural and Food Chemistry, 52(15), 4652–4659. https://doi.org/10.1021/jf049647j
- Gharibzahedi, S. M. T. (2017). Ultrasound-mediated nettle oil nanoemulsions stabilized by purified jujube polysaccharide: Process optimization, microbial evaluation and physicochemical storage stability. Journal of Molecular Liquids, 234(1), 240–248. https://doi.org/10.1016/j.molliq.2017.03.094
- Gharibzahedi, S. M. T., Rostami, H., & Yousefi, S. (2015). Formulation design and physicochemical stability characterization of nanoemulsions of nettle (urtica dioica) essential oil using a model-based methodology. Journal of Food Processing and Preservation, 39(6), 2947–2958. https://doi.org/10.1111/jfpp.12546
- Gharibzahedi, S. M. T., Smith, B., & Guo, Y. (2019). Ultrasound-microwave assisted extraction of pectin from fig (Ficus carica L.) skin: Optimization, characterization and bioactivity. Carbohydrate Polymers, 222, 222. https://doi.org/10.1016/j.carbpol.2019.114992
- Girma, E., & Teshome Worku, M. (2016). Extraction and characterization of Pectin from selected fruit peel waste. International Journal of Scientific and Research Publications, 6(2), 447. www.ijsrp.org
- Hamidon, N. H., & Zaidel, D. N. A. (2017). Effect of extraction conditions on pectin yield extracted from sweet potato peels residues using hydrochloric acid. Chemical Engineering Transactions, 56, 979–984. https://doi.org/10.3303/CET1756164
- Jabbar, S., Abid, M., Wu, T., Hashim, M. M., Saeeduddin, M., Hu, B., Lei, S., & Zeng, X. (2015). Ultrasound-assisted extraction of bioactive compounds and antioxidants from Carrot Pomace: A response surface approach. Journal of Food Processing and Preservation, 39(6), 1878–1888. https://doi.org/10.1111/jfpp.12425
- Kamnev, A. A., Colina, M., Rodriguez, J., Ptitchkina, N. M., & Ignatov, V. V. (1998). Comparative spectroscopic characterization of different pectins and their sources. Food Hydrocolloids, 12(3), 263–271. https://doi.org/10.1016/S0268-005X(98)00014-9
- Khamsucharit, P., Laohaphatanalert, K., Gavinlertvatana, P., Sriroth, K., & Sangseethong, K. (2018). Characterization of pectin extracted from banana peels of different varieties. Food Science and Biotechnology, 27(3), 623–629. https://doi.org/10.1007/s10068-017-0302-0
- Konrade, D., Gaidukovs, S., Vilaplana, F., & Sivan, P. (2023). Pectin from fruit- and berry-juice production by-products: Determination of physicochemical, antioxidant and rheological properties. Foods, 12(8), 1615. https://doi.org/10.3390/foods12081615
- Kumar, S., Ozukum, R., & Mathad, G. M. (2020). Extraction, characterization and utilization of pectin from apple peels. Journal of Pharmacognosy & Phytochemistry, 9(5), 2599–2604. https://doi.org/10.22271/phyto.2020.v9.i5aj.12736
- Kurita, N., & Yamada, H. (2008). The role of local moisture recycling evaluated using stable isotope data from over the middle of the Tibetan Plateau during the monsoon season. Journal of Hydrometeorology, 9(4), 760–775. https://doi.org/10.1175/2007JHM945.1
- Li, J. E., Nie, S. P., Xie, M. Y., & Li, C. (2014). Isolation and partial characterization of a neutral polysaccharide from Mosla chinensis Maxim. cv. Jiangxiangru and its antioxidant and immunomodulatory activities. Journal of Functional Foods, 6, 410–418. https://doi.org/10.1016/j.jff.2013.11.007
- Lochhead, R. Y. (2017). The use of polymers in cosmetic products. In Cosmetic science and technology: Theoretical principles and applications (pp. 171–221). Elsevier Inc. https://doi.org/10.1016/B978-0-12-802005-0.00013-6
- Lojková, L., Pluháčková, H., Benešová, K., Kudláčková, B., & Cerkal, R. (2023). The highest yield, or greener solvents? Latest trends in quercetin extraction methods. TrAc Trends in Analytical Chemistry, 167, 117229. https://doi.org/10.1016/j.trac.2023.117229
- Masuko, T., Minami, A., Iwasaki, N., Majima, T., Nishimura, S. I., & Lee, Y. C. (2005). Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Analytical Biochemistry, 339(1), 69–72. https://doi.org/10.1016/j.ab.2004.12.001
- Moorthy, I. G., Maran, J. P., Surya, S. M. @., Naganyashree, S., & Shivamathi, C. S. (2015). Response surface optimization of ultrasound assisted extraction of pectin from pomegranate peel. International Journal of Biological Macromolecules, 72, 1323–1328. https://doi.org/10.1016/j.ijbiomac.2014.10.037
- Muhammad, K., Nur, N. I., Gannasin, S. P., Adzahan, N. M., & Bakar, J. (2014). High methoxyl pectin from dragon fruit (Hylocereus polyrhizus) peel. Food Hydrocolloids, 42(P2), 289–297. https://doi.org/10.1016/j.foodhyd.2014.03.021
- Muñoz-Almagro, N., Ruiz-Torralba, A., Méndez-Albiñana, P., Guerra-Hernández, E., García-Villanova, B., Moreno, R., Montilla, A., & Montilla, A. (2021). Berry fruits as source of pectin: Conventional and non-conventional extraction techniques. International Journal of Biological Macromolecules, 186, 962–974. https://doi.org/10.1016/j.ijbiomac.2021.07.016
- Oliveira, T. Í. S., Rosa, M. F., Cavalcante, F. L., Pereira, P. H. F., Moates, G. K., Wellner, N., Azeredo, H. M. C., Waldron, K. W., & Azeredo, H. M. C. (2016). Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chemistry, 198, 113–118. https://doi.org/10.1016/j.foodchem.2015.08.080
- Pal, C. B. T., & Jadeja, G. C. (2019). Deep eutectic solvent‐based extraction of polyphenolic antioxidants from onion (<scp>Allium cepa </scp> L.) peel. Journal of the Science of Food and Agriculture, 99(4), 1969–1979. https://doi.org/10.1002/jsfa.9395
- Ranganna, S. (Ed). (2010). Handbook of analysis and quality control for fruit and vegetable products. Tata McGraw Hill education Pvt. Ltd.
- Rostami, H., & Gharibzahedi, S. M. T. (2017). Cellulase-assisted extraction of polysaccharides from malva sylvestris: Process optimization and potential functionalities. International Journal of Biological Macromolecules, 101, 196–206. https://doi.org/10.1016/j.ijbiomac.2017.03.078
- Santra, S., Das, M., Karmakar, S., & Banerjee, R. (2023). NADES assisted integrated biorefinery concept for pectin recovery from kinnow (citrus reticulate) peel and strategic conversion of residual biomass to L(+) lactic acid. International Journal of Biological Macromolecules, 250, 126169. https://doi.org/10.1016/j.ijbiomac.2023.126169
- Şen, E., Göktürk, E., Hajiyev, V., & Uğuzdoğan, E. (2023). Comparisons of pulsed ultrasound-assisted and hot-acid extraction methods for pectin extraction under dual acid mixtures from onion (allium cepa L.) waste. Food Science & Nutrition, 11(11), 7320–7329. https://doi.org/10.1002/fsn3.3657
- Shafie, M. H., Yusof, R., & Gan, C. Y. (2019). Deep eutectic solvents (DES) mediated extraction of pectin from Averrhoa bilimbi: Optimization and characterization studies. Carbohydrate Polymers, 216(1), 303–311. https://doi.org/10.1016/j.carbpol.2019.04.007
- Shah, S. N., Erbas, Z., & Soylak, M. (2022). A novel-easy deep eutectic solvent-based microextraction procedure for the separation, preconcentration and spectrophotometric determination of chromotrope 2R in water, detergent and food samples. International Journal of Environmental Analytical Chemistry, 102(1), 3373–3382. https://doi.org/10.1080/03067319.2020.1768249
- Siddiqui, A., Chand, K., & Shahi, N. C. (2021). Effect of process parameters on extraction of pectin from sweet lime peels. Journal of the Institution of Engineers (India): Series A, 102(2), 469–478. https://doi.org/10.1007/s40030-021-00514-3
- Silva, M. G. R., Skrt, M., Komes, D., Ulrih, N. P., & Pogačnik, L. (2020). Enhanced yield of bioactivities from onion (Allium cepa L.) skin and their antioxidant and anti-α-amylase activities. International Journal of Molecular Sciences, 21(8), 2909. https://doi.org/10.3390/ijms21082909
- Song, W., He, Y., Huang, R., Li, J., Yu, Y., & Xia, P. (2023). Life cycle assessment of deep-eutectic-solvent-assisted hydrothermal disintegration of microalgae for biodiesel and biogas co-production. Applied Energy, 335, 120758. https://doi.org/10.1016/j.apenergy.2023.120758
- Soylak, M., & Koksal, M. (2019). Deep eutectic solvent microextraction of lead(II), cobalt(II), nickel(II) and manganese(II) ions for the separation and preconcentration in some oil samples from Turkey prior to their microsampling flame atomic absorption spectrometric determination. Microchemical Journal, 147, 832–837. https://doi.org/10.1016/j.microc.2019.04.006
- Sukor, N. F., Selvam, V. P., Jusoh, R., Kamarudin, N. S., & Rahim, S. A. (2021). Intensified DES mediated ultrasound extraction of tannic acid from onion peel. Journal of Food Engineering, 296, 110437. https://doi.org/10.1016/j.jfoodeng.2020.110437
- Thirugnanasambandham, K., Sivakumar, V., & Maran, J. P. (2015). Microwave-assisted extraction of polysaccharides from mulberry leaves. International Journal of Biological Macromolecules, 72, 1–5. https://doi.org/10.1016/j.ijbiomac.2014.07.031
- Wang, S., Lei, T., Liu, L., & Tan, Z. (2024). CO2-responsive deep eutectic solvents for the enhanced extraction of hesperidin from fertile orange peel. Food Chemistry, 432, 137255. https://doi.org/10.1016/j.foodchem.2023.137255