?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Chocolate beverages made from cocoa powder contain phenolic compounds, especially flavonoids, as antioxidants, but these compounds are easily degraded. Incorporating clove bud extract is recommended to improve the quality of these beverages. However, encapsulation is necessary to protect bioactive compounds and reduce bitterness. This study investigated the total phenolic and flavonoid content, antioxidant activity, and characteristics of chocolate beverages formulated with encapsulated clove bud extract. Six formulations ranging from 0% to 10% encapsulated extract were evaluated. The results show that the incorporation of the encapsulated extract significantly improved several attributes as the percentage of clove extract increased. Phenolic and flavonoid content, antioxidant activity, hygroscopicity, lightness (L*), total color difference (∆E), viscosity, solubility, and soluble solids increased. In contrast, moisture content, red/green (a*) and blue/yellow (b*) values, sedimentation, pH, and dissolution time decreased. Panelists preferred beverages with up to 4% encapsulated clove extract, suggesting the potential for healthier ready-to-drink chocolates.
Introduction
Chocolate beverages are popular due to their unique taste and aroma (Thaichon et al., Citation2018), and they are beneficial for health due to their raw material, cocoa powder, which contains polyphenolic compounds like flavanols, procyanidins, and catechins (Indiarto et al., Citation2019b; Subroto et al., Citation2022). These compounds improve brain (Lamport et al., Citation2020; Santiago-Rodríguez et al., Citation2018) and skin health (Chalyk et al., Citation2018), lower blood pressure (Tanghe et al., Citation2021), reduce the risk of coronary heart disease, stroke and diabetes (Yuan et al., Citation2017), also improve mood (Shin et al., Citation2022), and contribute to antioxidants (Urbańska & Kowalska, Citation2019), helping prevent degenerative diseases caused by free radicals (DiMattia et al., Citation2017). The cocoa powder production process, including fermentation, drying, roasting, and alkalization, can affect the bioactive compound levels (Alasti et al., Citation2019; Indiarto et al., Citation2021; Subroto et al., Citation2023). However, the relatively low proportion used in the formula may also reduce the number of bioactive compounds, resulting in lower functional properties (Indiarto, Rahimah, et al., Citation2022; Indiarto, Reni, et al., Citation2023).
Chocolate beverages are not only enjoyed for their taste but also their potential health benefits. Natural ingredients like spices can be added to enhance the nutritional value and flavor of these beverages. Clove buds (Syzygium aromaticum) are known for their antioxidant properties and unique flavor and aroma (Shahinuzzaman et al., Citation2017). They contain phenolic compounds such as eugenol, eugenol acetate, flavonoids, caffeic acid, and ferulic acid. Eugenol, the main compound in cloves, offers various health benefits, such as reducing the risk of diabetes, relieving stress, and acting as an antimicrobial agent, where the percentage of eugenol in cloves reaches 89% of the whole clove or around 9,381.70–14,650 mg/100 g of cloves (Cortés-Rojas et al., Citation2014; Singletary, Citation2014). These compounds can be applied in food as extracts, making them a popular choice for their taste and health benefits.
However, when clove bud extract is utilized directly in food products, it can alter its physical and chemical properties and impact the taste and aroma due to its strong and bitter flavor. Additionally, the bioactive compounds in cloves are sensitive to factors like temperature, light, and oxygen, which can degrade their quality (Indiarto, Indriana, et al., Citation2022; Majeed et al., Citation2015). Clove bud extract can be encapsulated to address these challenges using maltodextrin and gum arabic coatings. These encapsulants protect the bioactive compounds, improve stability and solubility, and mask unwanted bitter flavors (Akdeniz et al., Citation2017; Indiarto, Rahimah, et al., Citation2022; Indiarto, Reni, et al., Citation2023). The goal is to add encapsulated clove bud extract to chocolate beverages without changing their physical and chemical characteristics.
Previous studies have investigated the incorporation of clove into chocolate products. In the Praseptiangga et al. (Citation2021) study, chocolate bars with 0.1‒0.3% clove extract showed good overall acceptability with a score of 3.87–4.43, but low antioxidant activity with an IC₅₀ of 1,029.73–1,422.04 ppm. In another study, Aroyeun et al. (Citation2020) found that adding 8% clove powder to chocolate bars had a low overall organoleptic acceptability score of 4.4 out of 9.0, and its antioxidant activity was not investigated. In addition, Nurhafsah et al. (Citation2022) found that higher levels of turmeric powder increased phenolic content in chocolate beverages but decreased organoleptic taste preference.
Research on the encapsulation of clove bud extract in chocolate beverages is limited, and studies of the various levels of encapsulated clove bud extract are needed to understand its effects on physicochemical and organoleptic properties. Therefore, in this study, different levels of encapsulated clove bud extract were incorporated into the chocolate beverage formulation to investigate its effect on the physicochemical and organoleptic properties to obtain the best and panelist-preferred formulation. The research will contribute to developing chocolate beverage products that offer a convenient, delicious, and antioxidant-rich alternative. Furthermore, using a simple and effective encapsulation method allows it to be applied by small-scale producers to produce antioxidant-rich products.
Materials and methods
Materials
Alkalized cocoa powder (pH 7.12) was obtained from CV. Timurasa, Indonesia, with the variety of Forastero from Lampung, Indonesia. Clove buds were obtained from PT Sari Bumbu, Indonesia, with the variety of Zanzibar from Sulawesi, Indonesia. Sugar was obtained from PT Sugar Group Companies, Indonesia. Xanthan gum was obtained from PT Intan Kimindo Pratama, Indonesia. Maltodextrin was obtained from Qinhuangdao Lihua Starch Co.,Ltd., China. Gum arabic was obtained from Ingredion Sweetener and Starch Co.,Ltd., Thailand. Chemical analysis materials (analytical grade from Sigma Aldrich, Merck) include ethanol, DPPH (2.2diphenyl1picrylhydrazyl), methanol, Folin-Ciocalteu reagent, potassium acetate, sodium carbonate, gallic acid standard, aluminium chloride, and quercetin standard.
Preparation of clove bud powder
Clove bud powder was prepared by powdering dried clove buds using a grinder (FCT-Z300, FOMAC, China). Then, the powder is sieved using a 60-mesh sieve to obtain clove bud powder and stored in a closed container.
Clove bud extraction
The extraction process using the maceration method was carried out according to Fauzya et al. (Citation2019), with slight modifications. Briefly, 100 g of clove bud powder with moisture content (% db) 11.55 (0.35) was macerated in 1,000 mL of 70% ethanol (1:10) (w/v) for 24 h at room temperature in a closed and dark room. The extract was then filtered through Whatman No. 1 filter paper and concentrated using a rotary evaporator (R300, BUCHI, Switzerland) at 45°C. A thick extract (27 mL) was obtained and stored in dark vials at 4°C.
Clove bud extract encapsulation
The encapsulation of clove bud extract was carried out according to Kusmayadi et al. (Citation2019), with slight modifications. Encapsulation was performed by mixing the clove bud extract with the coating materials in a ratio of 4:6 (w/w), resulting in an encapsulation solution with a total solids content of 50 g/200 mL. First, maltodextrin and gum arabic were dissolved in a 50:50 ratio with distilled water using a magnetic stirrer (791, Jiangsu Jinyi Instrument Technology Co., Ltd., China) at 3,000 rpm for 2 minutes. The clove bud extract was then diluted 1:10 with distilled water. The coating materials and diluted clove bud extract were mixed using a homogenizer (GLH-850, Omni, U.S.A.) at 10,000 rpm for 15 minutes. The encapsulation solution was dried in a vacuum oven (VD 23, Binder, Germany) at 60°C for 6 hours. The dried, encapsulated clove bud was ground using a grinder (FCTZ300, FOMAC, China) and stored in a closed container at 4°C.
Chocolate beverages formulation
The formulation of chocolate beverages was carried out based on Muhammad et al. (Citation2021), with some modifications. There are six formulations with different levels of encapsulated clove bud extract. Alkalized cocoa powder, encapsulated clove bud extract, sugar, and xanthan gum were each weighed according to the formulation (), then mixed and stirred until homogeneous to obtain a powdered chocolate beverage. Powdered chocolate beverages are brewed using water at a temperature of 80°C with a ratio of 1:5 between powdered chocolate beverages and water. Then, stirred until the powder dissolved.
Table 1. Formulation of chocolate beverages with different levels of encapsulated clove bud extract.
Total phenolic content determination
Total phenolic content (TPC) was determined according to Indiarto et al. (Citation2019a), with some modifications. Approximately 0.5 mL of the extracted powdered chocolate beverage sample, 0.5 mL of the FolinCiocalteu reagent, and 2.5 mL of Na₂CO₃ were added to the volumetric flask and made up to 25 mL with distilled water. The absorbance was measured with a UV spectrophotometer (VIS-7220N, Beijing Rayleigh Analytical Instrument Co., Ltd., China) at a wavelength of 725 nm after incubation for 40 minutes in a dark room. Total phenolic content was measured using a curve with gallic acid as the standard. It was expressed as mg gallic acid equivalents (GAE)/g extract.
Total flavonoid content determination
Total flavonoid content (TFC) determination was performed according to Faiqoh et al. (Citation2021) with some modifications. Approximately 0.5 mL extracted powdered chocolate beverage was taken and added to 3 mL methanol, 0.2 mL AlCl₃ solution, 0.2 mL CH₃COOK solution, and adjusted with distilled water in a 10 mL volumetric flask. The mixture was homogenized for 1 minute. It was then incubated in a dark room for 30 minutes. The absorbance was measured using a UV spectrophotometer (VIS-7220N, Beijing Rayleigh Analytical Instrument Co., Ltd., China) at a wavelength of 415 nm. The total flavonoid content was measured using the quercetin curve as a standard. Values were expressed as mg quercetin equivalent (QE)/g extract.
Antioxidant activity determination
Determining the antioxidant activity was performed according to Zzaman et al. (Citation2014), with modification. A 0.5 mL of DPPH solution 160 ppm dissolved in methanol was added to 2 mL of the extracted powdered chocolate beverage sample. The tube was homogenized using a vortex mixer. The sample was then incubated in a dark room for 30 minutes. The absorbance was measured using a UV-Vis spectrophotometer (VIS7220N, Beijing Rayleigh Analytical Instrument Co., Ltd., China) at a wavelength of 516 nm. The results were expressed in IC₅₀ value, the sample concentration required to inhibit 50% of the free radicals in DPPH. The absorbance value was used to calculate the inhibition DPPH (%) according to the equation:
Sedimentation
Sedimentation was determined according to Cui et al. (Citation2022), with slight modifications. A sample of 10 mL of chocolate beverage was prepared and placed in a centrifuge tube. The samples were centrifuged (Z306, Hermle, U.S.A.) at 4,000 rpm for 15 minutes. The weight of the sediment was then calculated. The percentage of sediment was expressed as the sedimentation index (SI) (%). The sediment weight was compared to the initial weight of the sample.
Color determination
The color was determined according to Aribah et al. (Citation2020), with a slight modification, using a chromameter (CM5, Konica Minolta, Japan) that was calibrated with a white plate. About 3 g of powdered chocolate beverage sample was placed in the cuvette until it covered the surface, then the color was measured. The CIE Lab model, which includes three color components, L*, a*, and b*, is used to present the measurement obtained. Then, the total color difference value (∆E) is calculated using the equation:
The ΔE values are classified into very different (∆E > 3), different (1.5 <∆E < 3), and slightly different (∆E < 1.5) (Tiwari et al., Citation2008).
pH value determination
The pH analysis was carried out as described by Indiarto, Rahimah, et al. (Citation2022), with slight modifications. A sample of 20 mL chocolate beverage was prepared for analysis. Then, the pH meter (PH-201, Lutron, Taiwan) was calibrated with a buffer solution of pH 4 and pH 7. Then, the tip of the electrode rod was inserted into the sample of chocolate beverage.
Viscosity determination
The viscosity was determined according to Faiqoh et al. (Citation2021), with slight modifications. The chocolate beverage sample is prepared and placed in a 100 mL container. The spindle is then paired and immersed in a container containing a sample. Measurements were made by pressing the “on” button on the viscometer (Brookfield Viscometer LVT, Massachusetts, U.S.A.) using spindle #2 at 60 rpm. After one minute of full rotation, the observations were made by reading the scale indicated on the viscometer.
Solubility and dissolution time determination
The solubility was determined according to Dyaningrum et al. (Citation2019) with slight modifications. Approximately 0.75 g of powdered chocolate beverage sample was weighed and dissolved in 10 mL of distilled water. The solution was then agitated on a hot plate at 330 rpm for 5 minutes at 105°C with a magnetic stirrer (79–1, Jiangsu Jinyi Instrument Technology Co., Ltd., China). The solution was then centrifuged (Z-306, Hermle, U.S.A.) at 5000 rpm for 15 minutes. The supernatant was then separated, placed in a Petri dish, and dried in an oven (Daihan WiseVen, SciLab, Korea) at 105 ± 2°C for 4 hours. The percent solubility was calculated by comparing the supernatant’s weight to the sample’s initial weight.
The powdered chocolate beverage sample weighed 5 g and dissolved in 100 mL of water to determine the dissolution time. A stopwatch measured the time required to dissolve the sample in water completely.
Hygroscopicity determination
The hygroscopicity was determined according to the study of Nurhadi et al. (Citation2022), which was slightly modified. In brief, 0.5 g of powdered chocolate beverage sample was placed in a Petri dish, previously weighed at 25°C, and then placed in a desiccator containing a concentrated NaCl (RH 75 ± 2%). The samples were weighed every 60 minutes for 5 hours. Hygroscopicity is expressed as a percentage of the mass of water absorbed/the initial mass of the sample.
Moisture content determination
The moisture content was determined by thermogravimetric method according to Rushchitc et al. (Citation2022), with slight modifications. The porcelain cup was oven dried (Daihan WiseVen, SciLab, Korea) at 105 ± 2°C for 30 min. The cup was then placed in a desiccator for 30 min and then weighed until a constant mass was reached. Approximately 1 g of powdered chocolate beverage sample was weighed into a constant cup. It was then dried in an oven at 105 ± 2°C for 3 hours to a constant weight. It was then cooled in a desiccator for 15 minutes and then weighed. The sample was weighed periodically until a constant mass was obtained (the difference between each weight was constant or less than 0.005 g). The moisture content was calculated on wet basis (wb) and dry basis (db).
Total soluble solids determination
Total soluble solids were determined according to Mahato et al. (Citation2022), with some modifications. The chocolate beverage sample was dissolved in water. It was poured into the refractometer (PAL-1 Alpha Pocket Refractometer, Atago, Japan) until it covered the surface of the sensor. The total dissolved solids can be displayed and expressed in °Brix.
Sensory evaluation
The sensory evaluation was performed using the hedonic test method based on Indiarto, Reni, et al. (Citation2023), with some modifications. This evaluation involved 33 semitrained panelists, consisting of both men and women in the age range of 20–28 years, with the mean of 21.85 (1.46) years. The evaluation conducted in Sensory Evaluation Laboratory Department of Food Industrial Technology, Universitas Padjadjaran, Indonesia on 30 May 2023. The evaluated parameters consisted of color, aroma, taste, aftertaste, and acceptability measured in a 5-point scale, namely dislike very much (1), dislike (2), neither like nor dislike (3), like (4), and like very much (5).
Statistical analysis
One-way analysis of variance (ANOVA) with SPSS 23.0 software was used for statistical data analysis. Tests were performed at the 95% confidence level (α = 0.05). If the probability of significance p value <.05, there is a significant difference; if the p value >.05, there is no significant difference. If there is a significant difference, further tests are performed using the Duncan Multiple Range Test (DMRT) to determine the true treatment difference. The data were measured in triplicates. The value shown represents the mean ± standard deviation.
Results and discussion
Total phenolic, total flavonoid, and antioxidant activity of chocolate beverage in different formulations
The addition of encapsulated clove bud extract is expected to increase the TPC in chocolate beverages. The results obtained for the TPC of chocolate beverages were 19.27–38.20 mg GAE/g extract, then the TFC of chocolate beverages was 2.76–7.94 mg QE/g extract. This can help to fulfill the recommended daily intake of polyphenols which is 1–2 g/day to help prevent chronic disease (Kapolou et al., Citation2021).
Total phenolic content
Phenolic compounds are a class of bioactive compounds consisting of benzene rings with hydroxyl group substitutions. Phenolic compounds in a material can indicate their potential as an antioxidant (Al Mamari, Citation2022). The TPC of clove bud extract was 506.58 (3.01) mg GAE/g extract (). Cloves are rich in phenolic compounds, mainly derived from eugenol, eugenyl acetate, βcaryophyllene and α-humulene (Haro-González et al., Citation2021). TPC in clove bud extracts can be influenced by the maturity of the cloves as well as extraction conditions such as solvent type and concentration (Ahmed et al., Citation2022). Maturity affects the content of phenolic compounds such as eugenol, with more mature cloves tending to have higher eugenol content. The more mature the clove plant, the more flower buds grow. During the bud growth period, nutrients in the clove plant are gathered and directed to the flower for development. Thus, with the increase of nutrients absorbed in the flower buds, higher levels of bioactive compounds are collected (Alfikri et al., Citation2020).
Table 2. Total phenolic content of chocolate beverages with different formulations.
The type of solvent used in extraction affects the TPC in clove bud extracts. Phenolic compounds in cloves, such as eugenol, are less soluble in water but more soluble in organic solvents such as alcohol (Tursiloadi et al., Citation2015). Solvent concentration also affects the extraction of bioactive compounds in cloves. According to Cortés-Rojas et al. (Citation2015), using ethanol at a concentration of 70% results in a better extraction of bioactive compounds in cloves than at a concentration of 96%. This is because the ethanol concentration of 60–80% is more optimal to penetrate the cell wall in cloves than 96% concentration, which tends to cause protein coagulation in the cell wall, which causes inhibition of solvent penetration into the cell, thus reducing the solubility of bioactive compounds. Then, using a 1:10 ratio of cloves to solvent in the extraction also increases the extracted bioactive compounds due to the increased solvent penetration into the clove tissue (Aziz et al., Citation2023).
The TPC in the encapsulated clove bud extract, which is 65.39 (0.38) mg GAE/g extract (), was lower than the clove bud extracts due to the low content of clove bud extract in the encapsulant, 40%, compared to the 60% of the coating, in which the clove bud extract was dissolved in distilled water in a ratio of 1:10 before mixing with the coating. The coating material also affects TPC retention in the clove bud extract. The use of gum arabic as a coating mixture is effective in maintaining the phenolic compounds. This is due to the nature of gum arabic as an emulsifier that can maintain evaporation and oxidation of volatile bioactive compounds (Amaral et al., Citation2019).
Based on , variations in encapsulated clove bud extract levels significantly affect TPC in chocolate beverages (p < .05), where the TPC of each sample is significantly different. TPC in chocolate beverages ranged from 19.27–38.20 mg GAE/g extract. The higher the addition of encapsulated extract, the higher the TPC in chocolate beverages. The increase in TPC in chocolate beverages is due to the content of phenolic compounds in cloves, as stated by Haro-González et al. (Citation2021). In addition, the cocoa powder itself is rich in phenolic compounds such as catechins, flavonol glycosides, anthocyanins, and procyanidin (Afoakwa et al., Citation2015). However, steps such as roasting and alkalization can decrease phenolic compounds by up to 14% and 64%, respectively (Mazor Jolić et al., Citation2011). When compared, the total phenolic content of chocolate beverages by analysis is higher than theoretical. This may be because polyphenols contain various functional groups, allowing covalent bonding between polyphenols and functional groups on compounds in chocolate beverages such as carbohydrates, proteins, or fats, resulting in phenol conjugates (Siemińska-Kuczer et al., Citation2022). In addition, total phenolics analytically involve all ingredients used in the formulation of chocolate beverages, in contrast to theoretically, which is only influenced by adding encapsulated clove bud extract, thus allowing for differences between analytical and theoretical (Indiarto, Reni, et al., Citation2023).
Total flavonoid content
Flavonoids are part of polyphenols, which are secondary plant metabolites. As part of polyphenols, flavonoids also play a role in health because they can act as antioxidants, anti-inflammatory, neuroprotective, and more (Mutha et al., Citation2021). This study’s TFC of clove bud extract was 27.46 (0.49) mg QE/g extract (), with cloves rich in flavonoid compounds such as kaempferol and quercetin (Cortés-Rojas et al., Citation2014). Solvent type in clove extraction affects TFC. Semipolar solvents can improve the extraction of flavonoid compounds in cloves. This is because mixing polar solutions such as water can increase the breaking of hydrogen bonds in the structure of phenolic compounds, thereby increasing the solubility of bioactive compounds (Mikucka et al., Citation2022).
Table 3. Total flavonoid content of chocolate beverages with different formulations.
The TFC of the encapsulated clove bud extract was 14.54 (0.31) mg QE/g extract (). Coating materials such as maltodextrin and gum arabic can protect the degradation of flavonoid compounds in clove bud extracts by forming a matrix to avoid direct exposure of bioactive compounds to the environment. Gum arabic, as a mixture of maltodextrin in the coating, can provide an emulsion effect that resists the degradation of volatile components (Nurhadi et al., Citation2020).
Based on , the variation of encapsulated clove bud extract levels significantly affects the TFC of chocolate beverages (p < .05), where the TFC of each sample is significantly different. The TFC in chocolate beverages ranged from 2.76 to 7.94 mg QE/g extract. The higher the addition of encapsulated extract, the higher the TFC of chocolate beverages. In addition, cocoa powder is also rich in flavonoid compounds, mainly consisting of flavanols such as epicatechin, catechin, and procyanidin, which have health benefits as neuroprotective and antioxidant (Goya et al., Citation2022). In comparison, the total flavonoid content of chocolate beverages is higher than theoretically expected by analysis. Similar to phenolic compounds, this may be due to interactions between functional groups on flavonoids and compounds in chocolate beverages (Lund, Citation2021).
Antioxidant activity
Antioxidant activity in this study is expressed in IC₅₀ (inhibition concentration, ppm), the concentration required for a material to inhibit 50% of the free radicals in DPPH. The lower the IC₅₀ value, the lower the concentration required, indicating stronger antioxidant activity (Sirait et al., Citation2023). The IC₅₀ value of clove bud extract is 9.31 (0.01) ppm (), which is classified as a very strong antioxidant because it has an IC₅₀ value ≤ 50 ppm (Nurhadi et al., Citation2020). Cloves contain bioactive compounds, mainly eugenol, eugenyl acetate and β-caryophyllene, which act as antioxidants. Eugenol contains hydroxyl groups in its structure that can donate H+ to a free radical (Gengatharan & Rahim, Citation2023). According to Cortés-Rojas et al. (Citation2014), eugenol can reduce two or more free radicals even though it has only one hydrogen in its hydroxyl group. TPC and TFC may also influence the antioxidant activity (Hussain et al., Citation2017). Therefore, extraction conditions such as solvent, time and temperature also affect the antioxidant activity by influencing TPC and TFC (Hala, Citation2011).
Table 4. Antioxidant activity as IC₅₀ (ppm) of chocolate beverages with different formulations.
The IC₅₀ value of encapsulated clove bud extract is 104.14 (0.54) ppm (), classified as a strong antioxidant because it has an IC₅₀ value > 50–200 ppm (Nurhadi et al., Citation2020). Encapsulating materials such as maltodextrin and gum arabic can protect bioactive compounds in cloves from environmental influences to maintain antioxidant abilities (Idowu et al., Citation2021). The drying process may affect the antioxidant activity of the encapsulant. Cloves contain sugar and protein in their compounds (Idowu et al., Citation2021). Therefore, during the drying process in encapsulation, exposure to high temperatures can trigger the Maillard reaction, which produces intermediate compounds that can affect antioxidant activity. The resulting intermediate compounds, such as pyrazinium and pyridinium, are reactive, so the longer the drying time and the higher the temperature used, the more reactive compounds will be produced, reducing the antioxidant activity of the encapsulant (Salim et al., Citation2015).
Based on , the variation of encapsulated clove bud extract levels significantly affects the antioxidant activity of chocolate beverages (p < .05). There is a decreasing trend of the IC₅₀ value with increased encapsulated extract levels, indicating stronger antioxidant activity. In the Duncan Multiple Range Test (DMRT), the IC₅₀ value of sample C was not significantly different from sample D but significantly different from other samples. This indicates no significant difference between 4% and 6% encapsulated clove bud extract in chocolate beverages. This was also found by Faiqoh et al. (Citation2021) that there was no significant difference in antioxidant activity between the addition of 4% and 6% ginger. The antioxidant activity as IC₅₀ value in chocolate beverages ranged from 126.78–167.53 ppm, so overall, chocolate beverages in this study can be classified as strong antioxidants because they have IC₅₀ values > 50–200 ppm (Nurhadi et al., Citation2020).
Adding encapsulated clove bud extract increases the antioxidant activity of chocolate beverages. This is due to the bioactive compounds such as eugenol, βcaryophyllene, αhumulene, eugenyl acetate, eugenitin, hydroxycinnamic acid, gallic acid, ellagic acid, kaempferol, oleanolic acid, stigmasterol, etc., which act as antioxidants (Saeed et al., Citation2021). Bioactive compounds such as phenolics and flavonoids can act as antioxidants. Therefore, the higher the total phenolic and flavonoid content in chocolate beverages, the stronger the antioxidant activity (Hussain et al., Citation2017). In addition, cocoa powder is rich in phenolic compounds (methylxanthines, catechins, and procyanidins), flavonoids (proanthocyanidins, monomeric flavan-3-ols, and anthocyanins), and other compounds such as tocopherols that can inhibit free radicals (Oracz & Żyżelewicz, Citation2020).
Characteristics of powdered chocolate beverages (before brewing)
Color
shows that adding different amounts of encapsulated clove bud extract changes the color parameters (L*, a*, b*) of chocolate beverages in a way that is statistically significant (p < .05). There was an increasing trend in the L* value with the encapsulated extract added. However, it was inversely proportional to the a* and b* values, which decreased. All a* and b* values were positive. It indicates that the color of the chocolate beverage tends to be reddish-yellow.
Table 5. Characteristics of powdered chocolate beverages (before brewing).
The alkalized cocoa powder and the clove extract coating material affected the chocolate beverages’ lightness (L* value). In comparison, the cocoa powder is alkalized and heated to between 60 and 100°C. Polyphenolic compounds like anthocyanins, procyanidins, and catechins oxidize, polymerizing them into darker quinones (Li et al., Citation2014). The Maillard reaction, which does not use enzymes, also creates pyrrole and pyridine compounds, which combine to form brownish melanoidin pigments. Therefore, the higher the pH and alkalization temperature, the darker the color of cocoa powder with lower L* values (Sioriki et al., Citation2021).
When encapsulated extract encapsulant is added to chocolate beverages, the coating material, a mix of maltodextrin and gum arabic, changes color. It makes the chocolate beverages lighter (L* value), darker (a* value), and yellower (b* value). Maltodextrin is white, while gum arabic is pale yellow to white color, so its addition to chocolate beverages can increase the color lightness and impact a* and b* values (Dauqan & Abdullah, Citation2013).
A chocolate beverage without added encapsulated clove bud extract is called sample A. The total color difference (∆E) value compares the color of the treated samples (B, C, D, and E) to sample A, which has not been treated. shows that the amount of encapsulated clove bud extract added significantly affects the ∆E value of chocolate beverages (p < .05). There is an increasing ∆E value trend with the additional encapsulated clove bud extract level. This is due to the significantly different L*, a*, and b* values, which affect the ∆E value. The ∆E values of the treated samples were > 3. It indicates that the ∆E is very different from the untreated samples.
Moisture content
Based on , variations in encapsulated clove bud extract levels significantly affect the moisture content of chocolate beverages before brewing (p < .05), where the moisture content of each sample is significantly different. There is a decreasing moisture content trend as the encapsulated extract level increases. The highest percentage decrease is found in sample F, which is 18.02%. The moisture content in the chocolate beverages ranges from 3.32 to 4.05%. Based on SNI 01–4320–1996 regarding powdered beverages, it is known that the moisture content in powdered beverages ranges from 3–5%. Thus, the moisture content of chocolate beverages in this study is known to be within the SNI standards. The moisture content in powdered beverages is crucial because it affects the product’s resistance to damage, especially that caused by microorganisms (Chang et al., Citation2019).
The decrease in moisture content with the addition of encapsulated extract is influenced by the type of wall material used. In this study, maltodextrin, and gum arabic were used as wall materials. Maltodextrin is hydrophilic, easily binding water by forming hydrogen bonds with water molecules. The maltodextrin molecules attract water to the surface and form a layer around the chocolate beverage particles when maltodextrin is added to a powdered chocolate beverage formula. As a result, adding maltodextrin increases total solids and decreases water availability for evaporation from the chocolate beverage, resulting in a lower water content (Nawi et al., Citation2015). The same occurs in the production of watermelon powder, where using 5% maltodextrin results in a moisture content of 1.62%, which is lower than using 3% maltodextrin, whose water content is 2.78% (Quek et al., Citation2007).
Gum arabic also affects the moisture content of chocolate beverages. Adding gum arabic tends to increase the moisture content of the ingredients. This is because gum arabic has emulsifying properties, so increasing the viscosity of chocolate beverages can interfere with the rate of water diffusion during evaporation in the oven (Premi & Sharma, Citation2017). In other words, gum arabic can retain the water content in the material. This is also following the study of Sukri et al. (Citation2021), where the use of gum arabic at 3% resulted in a higher moisture content of 7.71% in propolis powder than at 1%, which was 5.39%.
Xanthan gum, used as a stabilizer, also affects the moisture content of chocolate beverages. Xanthan gum is a polysaccharide that acts as a hydrocolloid, a polymer containing hydroxyl groups that can bind water, form colloids, and thicken solutions to function as a stabilizer (Herawati, Citation2018; Muhammad et al., Citation2021). Xanthan gum forms hydrogen bonds with water to form a thick colloid that traps water in chocolate beverages. Like gum arabic, the gel texture produced when xanthan gum binds to water causes water retention and makes it difficult to evaporate water. So, the addition of xanthan gum can increase the moisture content. Adding hygroscopic sugar can increase water absorption, thus reducing the moisture content in chocolate beverages (Dyaningrum et al., Citation2019).
The drying method is also a factor that affects the water content of chocolate beverages. The higher the temperature and the longer the drying time, the lower the moisture content of the ingredients. While drying, water is transferred from the inside of the chocolate beverage to the surface, and then the water on the surface will evaporate in the oven (Fardhyanti et al., Citation2022). Encapsulation of clove bud extract using a vacuum oven can affect the moisture content of chocolate beverages. Drying in a vacuum oven tends to produce a higher moisture content than using a spray dryer. This is due to using a lower temperature in a vacuum oven, which is 70°C, compared to a spray dryer that uses a temperature of 170°C, which reduces the evaporation rate (Shuen et al., Citation2021).
Hygroscopicity
shows that varying the addition of encapsulated clove bud extract significantly affects the hygroscopicity of chocolate beverages before brewing (p < .05), where the hygroscopicity of each sample formula is significantly different. An increasing trend in hygroscopicity with the addition of encapsulated clove bud extract is shown in . The hygroscopicity of the chocolate beverages before brewing ranged from 5.38 to 6.80%. According to Vladić et al. (Citation2022), powdered products can be categorized based on the percentage of hygroscopicity, namely non-hygroscopic (<10%), slightly hygroscopic (10–15%), hygroscopic (15–20%), and highly hygroscopic (20–25%). Therefore, based on these categories, the pre-brewed chocolate beverage used in this study was considered non-hygroscopic. The hygroscopicity increased with the addition of the encapsulating agent. This is due to the maltodextrin and gum arabic used as encapsulants, and both are water-absorbent because they have a functional -OH group that binds to water molecules. However, gum arabic is more hygroscopic due to its branched structure, which makes it easier for hydrogen chains to interact with water (Silva et al., Citation2012). Using a maltodextrin and gum arabic mixture, hygroscopicity can be produced with values that are neither too high nor too low. The use of xanthan gum can also influence hygroscopicity. Xanthan gum is hydrophilic. It binds easily to water, increasing hygroscopicity in chocolate beverages (Bhagya Raj & Dash, Citation2022). In addition, the granulated sugar used in this chocolate beverage ingredient contains hydroxyl groups that easily bind to water, contributing to increased hygroscopicity (Ding & Yang, Citation2021).
Characteristics of chocolate beverages (after brewing)
Sedimentation
Based on , variations in the levels of encapsulated clove bud extract significantly affect the sedimentation of chocolate beverages (p < .05), where the sedimentation of each sample is significantly different. The percentage of sedimentation in chocolate beverages is in the range of 7.49–10.65%, where the higher the addition of encapsulated clove bud extract, the lower the sedimentation formed. Cocoa powder is hydrophobic due to its high-fat content of 15–22%, making it difficult to dissolve in water (Veselá et al., Citation2007). The particle size of alkalized cocoa powder is smaller (18.5 µm) than natural cocoa powder (28.5 µm) (Muhammad et al., Citation2019). Smaller particle sizes increase the contact surface between the solvent and the solid, thus accelerating solubility and decreasing sedimentation (Muhammad et al., Citation2019). Cloves are less soluble in water because they contain 15–20% essential oils consisting of eugenol, eugenyl acetate, beta-caryophyllene, and other non-polar compounds (Mittal et al., Citation2014). In chocolate beverages, high sedimentation will reduce quality as it affects appearance and decreases consumer acceptance (Dogan et al., Citation2013). Maltodextrin and gum arabic are water-soluble by forming hydrogen bonds with the encapsulated material, thereby increasing solubility and reducing sediment formation (Todorović et al., Citation2022). Therefore, the higher the levels of encapsulated extract, the lower the sedimentation in the chocolate beverages. Using xanthan gum as a stabilizer can also bind the fat globules in cocoa powder and interact to form hydrogen bonds with the particles contained in the chocolate beverage ingredients, thereby increasing the stability of the chocolate beverage suspension (Holkar et al., Citation2019; Muhammad et al., Citation2021).
Table 6. Characteristics of chocolate beverages (after brewing).
pH value
Based on , variations in the levels of encapsulated clove bud extract significantly affect the pH of the chocolate beverages (p < .05), where the pH of each sample is significantly different. The pH of chocolate beverages ranged from 6.64 to 7.06, with the higher the addition of encapsulated extract, the lower the pH. Cloves contain phenolic compounds, which include acids such as hydroxycinnamic, hydroxybenzoic, chlorogenic, gallic, caffeic, ellagic, ferulic, salicylic, oleanolic, and many others (Saeed et al., Citation2021). These acidic compounds release H+ ions when dissolved in water, forming bonds with water molecules. The more acidic the material, the more H+ ions are released and the lower the pH (Berahun et al., Citation2022). In addition, maltodextrin and gum arabic affect the pH of chocolate beverages. In general, maltodextrin has a pH of 4–6 (Chavan et al., Citation2015). Gum arabic tends to be more acidic with a pH of 4.5–5.5 (Sharma et al., Citation2021) due to a glucuronic acid content of approximately 9.4% (Praseptiangga et al., Citation2016). Therefore, the higher the encapsulated extract, the lower the pH of the chocolate beverages.
Alkalized cocoa powder also affects the pH of chocolate beverages. The cocoa powder used in this study has a pH of 7.12, which, according to Miller et al. (Citation2008) is classified as a light alkalized cocoa powder (pH 6–7.2). As reported by Valverde García et al. (Citation2020), in chocolate beverages, cocoa powders with pH 5–6 (dark natural) and pH 6–7.2 (light) are generally used to obtain a strong chocolate beverage color, to reduce acidity and astringency, and to increase solubility. Alkalization of cocoa powder involves a neutralization reaction between acidic compounds, such as acetic acid and propanoic acid, and the alkali used to raise the pH (Puchol-Miquel et al., Citation2021).
Viscosity
Based on , variations in the levels of encapsulated clove bud extract significantly affect the viscosity of chocolate beverages (p < .05), where the viscosity of each sample is significantly different. The viscosity of the chocolate beverages ranged from 49.34 to 84.84 mPas, with higher levels of encapsulated extract increasing the viscosity. The amount and size of the solids influence the viscosity. The use of alkalized cocoa powder is known to increase viscosity due to the smaller particle size compared to natural cocoa powder. The small particle size increases the interaction between the particles in the solution, thus increasing the viscosity (Muhammad et al., Citation2019). Sugar also affects the viscosity of chocolate beverages. The hydroxyl group in sugar can bind to water molecules, inhibiting water movement in the solution and decreasing free water (Hedayati et al., Citation2016).
The viscosity of chocolate beverages increases with increasing levels of encapsulated extract due to the presence of gum arabic in the coating material. Gum arabic has a hydrophilic portion in the form of carbohydrates and a hydrophobic portion in the form of proteins. In the encapsulant, the hydrophilic portion will bind to the water in the solution, while the hydrophobic portion will bind to the oil in the clove bud extract. Therefore, gum arabic has emulsifying properties in the encapsulated clove bud extract (Dauqan & Abdullah, Citation2013). Gum arabic can bind to water molecules in chocolate beverages, thus reducing the free water and hindering the movement of molecules in chocolate beverages. As a result, the viscosity of chocolate beverages is increased (Indiarto, Rahimah, et al., Citation2022). As a stabilizer, xanthan gum also affects the viscosity of chocolate beverages. Xanthan gum is a hydrocolloid with branched chains in its structure, which allows it to form more hydrogen bonds with water molecules in chocolate beverages, increasing viscosity (Akkarachaneeyakorn & Tinrat, Citation2015).
Solubility
Based on , variations in the levels of encapsulated clove bud extract significantly affect the solubility of chocolate beverages (p < .05), where the solubility of each sample is significantly different. The solubility of the chocolate beverages ranged from 42.06–54.30%, with the solubility increasing with the addition of encapsulated extract. This is due to the use of coatings, which are maltodextrin and gum arabic. Cocoa powder and clove bud extract are less soluble in water due to the fat content (Veselá et al., Citation2007) and oil in clove bud extract (Mittal et al., Citation2014). Maltodextrin is water soluble and has a low viscosity (Burhan et al., Citation2019). Gum arabic has emulsifying properties that help stabilize chocolate beverages’ fat-soluble and water-soluble components (Shittu & Lawal, Citation2007). Therefore, using a blend of maltodextrin and gum arabic can increase the solubility of chocolate beverages.
The use of alkalized cocoa powder instead of natural cocoa powder also improved solubility. Cocoa powder is less soluble in water due to its insoluble cell wall structure. This is influenced by the roasting process in cocoa bean processing, where proteins and polyphenols in cocoa bind to the cell wall, making it difficult to degrade (Holkar et al., Citation2019). Alkalization of cocoa powder with alkaline materials such as NaOH can help hydrolyze the binding complex with the cell wall, thereby increasing the solubility of cocoa powder (Valverde García et al., Citation2020). Therefore, alkalized cocoa powder is used to increase solubility in the production of chocolate beverages, which can improve physical acceptability. If the solubility value is low, it can lead to the formation of layers, flocs, or sediments in chocolate beverages (Valverde García et al., Citation2020).
Adding sugar also increases solubility. Sugar contains hydroxyl groups that can form hydrogen bonds with water molecules, thereby increasing the solubility of chocolate beverages (Leinonen et al., Citation2011). Xanthan gum as a stabilizer can also increase solubility because it can bind water to particles in chocolate beverage ingredients (Akkarachaneeyakorn & Tinrat, Citation2015).
Dissolution time
Based on , variations in the amount of encapsulated clove bud extract significantly affect the dissolution time of chocolate beverages (p < .05), where the dissolution time of each sample is significantly different. The dissolution time of the chocolate beverages ranged from 55.21–110.82 seconds when dissolved in water at 80°C, with the higher the addition of encapsulated extract, the lower the dissolution time. Maltodextrin as a coating reduces the dissolution time of chocolate beverages because it contains hydroxyl groups (–OH), which are polar, so they easily form hydrogen bonds with water molecules, thus increasing solubility (Marta et al., Citation2017). Gum arabic also contains hydrophilic hydroxyl groups to reduce dissolution time (Chew et al., Citation2018). Sugar also affects dissolution time, with high sugar concentrations accelerating solubility. Sugar is hygroscopic, i.e. it binds easily with water (Dyaningrum et al., Citation2019). As a stabilizer, xanthan gum can bind water with chocolate beverage ingredients, increasing solubility and reducing precipitate formation (Indiarto, Rahimah, et al., Citation2022). Using a vacuum oven while drying the encapsulated extract also affects the dissolution time. The use of a vacuum oven produces encapsulants with higher moisture content and lower hygroscopicity than spray drying methods. The lower hygroscopicity makes it difficult for the material to absorb water, thus reducing solubility. Meanwhile, the high moisture content causes clumping of the powder, which reduces the surface area and thus the interaction between water and chocolate beverage powder (Gopinathan et al., Citation2020).
Total soluble solids
Based on , the variation in the encapsulated clove bud extract level significantly affects the total soluble solids in chocolate beverages (p < .05). The total soluble solids content of the chocolate beverages was 7.77–9.48°Brix. The increase in total soluble solids with increasing levels of encapsulated extract was due to the use of maltodextrin and gum arabic as coatings. Maltodextrin and gum arabic are hydrophilic and therefore bind easily with water because they have many free hydroxyl groups, thus increasing the solubility of solid particles in chocolate beverages (Nawi et al., Citation2015). Sugar can also increase total soluble solids because sugar binds free water, thus increasing soluble materials (Likumahua et al., Citation2022). As a hydrocolloid, xanthan gum can increase total soluble solids by binding the – OH group in the sugar in chocolate beverages to water molecules, causing structural changes (Razak et al., Citation2018). Therefore, the higher concentration of xanthan gum will increase the solubility of the solid particles in chocolate beverages. With the increase in total dissolved solids, the solubility of the chocolate beverages will increase, thus reducing sedimentation, as seen in .
Sensory evaluation of chocolate beverages (after brewing)
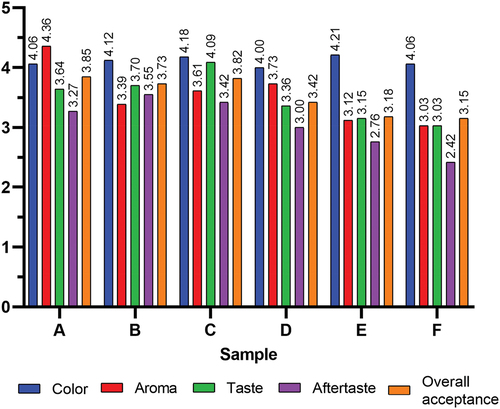
Color
Based on , varying the level of encapsulated clove bud extract does not significantly affect the color of the chocolate beverages (p > .05). This shows no significant difference in the color of each chocolate beverage, with panelists tending to like the color of each sample with a rating range of 4.06–4.21 (like). Color contributes significantly to the product’s visual appeal, which provides a first impression of whether a product will be liked or not (Tarwendah, Citation2017). The color of chocolate beverages is mainly influenced by the alkalized cocoa powder as the main ingredient. The brownish color of cocoa is formed during fermentation, where oxidation of polyphenols to quinine occurs (Peña-Correa et al., Citation2022). A nonenzymatic Maillard browning reaction then occurs during roasting. Further alkalization causes hydrolysis of polyphenols such as anthocyanins, procyanidins, and catechins to quinones, which are brownish (Li et al., Citation2014). Clove buds are initially pale, green, pink, and then red when harvested. The brown color of clove powder is caused by fermentation (Kasai et al., Citation2016). During fermentation, phenolic compounds are degraded to quinones through enzymatic browning reactions by the enzyme polyphenol oxidase (Hasheminejad & Khodaiyan, Citation2020). Cloves tend to be lighter in color when encapsulated with maltodextrin and gum arabic.
Aroma
The aroma of chocolate beverages is derived from volatile compounds that enter the nasal cavity and are then perceived by the olfactory sensory system (Muhammad et al., Citation2022). Based on , varying the encapsulated clove bud extract level significantly affects chocolate beverages’ aroma (p < .05). Overall, panelists liked the chocolate beverages’ aroma, but adding an encapsulated clove too high (>6%) may reduce acceptability due to the clove’s strong and distinctive aroma.
The aroma of cloves is influenced by the presence of volatile components such as eugenol, eugenyl acetate, β-caryophyllene, methyl amyl ketone, kaempferol, vanillin, tannic acid, α-humulene, β-humulene, methyl salicylate, crategolic acid, and benzaldehyde (Mittal et al., Citation2014). These compounds produce sweet, woody, fruity, floral, and spicy aroma sensations in cloves. Eugenol and eugenyl acetate are primarily responsible for sweet, floral, and fruity aromas, vanillin for spicy aromas, and caryophyllene for woody aromas (Karunamay et al., Citation2019; Nowak et al., Citation2012). The volatile components in cloves are in optimal condition when they enter the flowering stage (colored red). This is because, during flowering, the aroma becomes stronger to attract insects for pollination to maintain the plant’s reproductive system (Kasai et al., Citation2016).
The aroma of cocoa powder also affects organoleptic acceptance, where panelists like the aroma of cocoa. The aroma of cocoa powder is influenced by more than 400 aromatic compounds, mainly pyrazines, thiazoles, oxazoles, pyrroles, pyridines, and furans, which cause sweet, chocolate, bitter, fruity, floral and vegetal aromas. These compounds are formed during fermentation, where enzymatic reactions break down sugars into volatile compounds and proteins into amino acids. During roasting, the amino acids and reducing sugars produce aroma compounds in a Maillard reaction (Bonvehí, Citation2005).
Taste and aftertaste
Taste is an impression received by the tongue and transmitted to the nervous system, which is then identified as sweet, salty, sour, bitter, hot, cold, and painful sensations felt by the gustatory sense (Pangestu et al., Citation2016). Meanwhile, aftertaste is the taste that remains in the back of the mouth after tasting and swallowing a food product (Warda et al., Citation2023). Based on , variations in the encapsulated clove bud extract levels significantly affect the taste of chocolate beverages (p < .05). Adding encapsulated clove bud extract at a certain level (4%) can increase the preference for the taste of chocolate beverages. However, the higher the level of encapsulated extract, the lower the preference. As with the taste, the variation in the encapsulated clove bud extract level significantly influenced the aftertaste of chocolate beverages (p < .05).
The taste of chocolate beverages is influenced by alkalized cocoa powder as the main ingredient. Bioactive compounds such as caffeine, theobromine, methylxanthines, diketopiperazines and flavan-3-ols formed during cocoa powder roasting and alkalization give cocoa its bitter taste and aftertaste (Juvinal et al., Citation2023). Alkalization of cocoa powder can reduce astringency due to the breakdown of astringent-producing polyphenols into quinones (Li et al., Citation2014). Adding encapsulated clove bud extract can improve the flavor and aftertaste of chocolate beverages at 2% and 4% additions. Cloves have a sweet, bitter, astringent, and pungent sensation due to the presence of β-caryophyllene and eugenol (Alfikri et al., Citation2020). This sweetness causes the addition of low levels of encapsulated clove bud extract to chocolate beverages to increase preference compared to the control. Cocoa powder has a strong astringent taste and aftertaste caused by theobromine, caffeine, flavan-3-ols, and flavonol glycosides (Stark et al., Citation2006). Encapsulation of clove bud extract can reduce bitter, astringent, and pungent tastes, with the encapsulation wall protecting phenolic compounds such as eugenol (Gottardo et al., Citation2022). This results in a subtle clove flavor in the chocolate beverages. The mouthfeel of the chocolate beverages can then be improved by using xanthan gum as a stabilizer, with increased viscosity and reduced sedimentation.
Overall acceptance
The overall acceptability of chocolate beverages () was determined based on panelists’ liking of the combined sensory attributes of color, aroma, taste, and aftertaste. Based on , variations in encapsulated clove bud extract levels significantly affect the aroma of chocolate beverages (p < .05). Panelists tend to like the cocoa taste and adding up to 4% encapsulated clove bud extract to chocolate beverages does not result in a significant difference in overall acceptability. Each chocolate beverage sample was organoleptically acceptable with an acceptance scale of 3.15–3.85. The high acceptability of sample C was mainly influenced by the taste characteristics, which had the best acceptability among the chocolate beverage samples, with an increase of 12.36% over the control (A) and an increase of 34.98% over the lowest acceptability (F). It was also influenced by the aftertaste, which was not significantly different from the control, and the aroma, which was acceptable (not significantly different from B and D).
Correlation between the variables
This study’s correlation between variables (parameters) was analyzed using Pearson’s correlation. The correlation between parameters is expressed in the correlation coefficient (r) value with a range from − 1 to + 1. If the r value is positive, it can be said that the relationship between parameters is directly proportional, while if the r value is negative, the relationship is inversely proportional (Schober & Schwarte, Citation2018). The correlation coefficient is divided into five categories to express the strength or weakness of the correlation between parameters, namely negligible (very weak) (r = 0.00–0.10), weak (r = 0.11–0.39), moderate (r = 0.40–0.69), strong (r = 0.70 − 0.89), and very strong (r = 0.90–1.00) (De Bruin et al., Citation2021). The results of the correlation coefficient analysis between the parameters in this study are shown in .
Figure 3. Pearson’s correlation coefficient between variables (r). S (sedimentation), pH (power of hydrogen), V (viscosity), SL (solubility), H (hygroscopicity), MC (moisture content), TSS (total soluble solids), DT (dissolution time), TP (total phenolic), TF (total flavonoids), IC₅₀ (inhibitory concentration 50%), L* (lightness), a* (redness), b* (yellowness), and ∆E (total color difference).
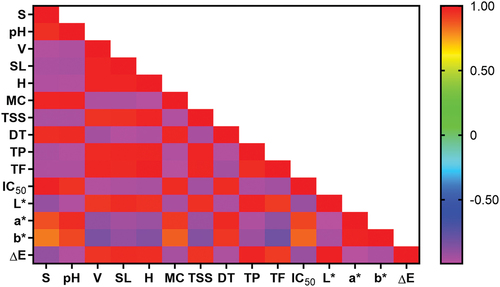
Based on , all parameters in the Pearson correlation analysis are significantly correlated (p < .01), so there is a correlation between each parameter. The correlation between parameters is strong (r = 0.809) to very strong (r = 0.995). In terms of chemical characteristics, the total phenolic content of chocolate beverages was positively correlated with the total flavonoid content (r = 0.952) but negatively correlated with the IC₅₀ value (r = −0.965). Similarly, total flavonoids were negatively correlated with IC₅₀ value (r = −0.943). This is because flavonoids are part of phenolic compounds, so the higher the flavonoid content, the higher the phenolic content. The IC₅₀ value indicates the concentration of the material in inhibiting free radicals. The lower the IC₅₀, the stronger the antioxidant activity of the material. Phenolic compounds, such as phenolic acids and flavonoids, have antioxidant abilities, according to Fitriansyah et al. (Citation2018), where total phenolics and flavonoids were negatively correlated with IC₅₀ in gooseberry extract.
Total phenolic and flavonoid levels correlate positively with solubility (r = 0.970) and viscosity (r = 0.961). This is due to phenolic compounds such as phenolic acids and flavonoids, which are hydrophilic so that they can form hydrogen bonds with water molecules, thereby increasing solubility and viscosity (El Riachy et al., Citation2011).
In physical properties, sedimentation value is positively correlated with pH (r = 0.949), moisture content (r = 0.976), and dissolution time (r = 0.951), but negatively correlated with viscosity (r = −0.983), solubility (r = −0.963), hygroscopicity (r = −0.975) and total soluble solids (r = −0.961). Adding encapsulated clove bud extract to chocolate beverages reduces sedimentation because maltodextrin and gum arabic are water-soluble by forming hydrogen bonds with the encapsulated ingredients. This increases total soluble solids, solubility, and viscosity and decreases sediment formation (Todorović et al., Citation2022). In addition, encapsulated clove bud extract can reduce the pH of chocolate beverages due to the low pH of cloves and the acidic pH of the coating (Sharma et al., Citation2021). Therefore, sedimentation is positively correlated with pH and dissolution time and negatively correlated with viscosity, total soluble solids, and solubility. Sedimentation is positively correlated with moisture content because the higher the moisture content, the more clumping occurs in the powder, resulting in sedimentation (Gopinathan et al., Citation2020). The relationship between moisture content and hygroscopicity is inversely proportional. The higher the hygroscopicity, the easier the material can absorb water from the environment. As a result, water availability for evaporation is reduced, resulting in a lower moisture content (Tonon et al., Citation2008). Therefore, sedimentation is negatively correlated with hygroscopicity.
In the color parameter, L* values were negatively correlated with a* values (r = −0.994) and b* values (r = −0.961) but positively correlated with ∆E (r = 0.995). The alkalized cocoa powder and the encapsulating material in the extract influenced the chocolate beverages’ lightness (L* value). Maltodextrin is white, while gum arabic is pale yellow to white, so when added to chocolate beverages, it can increase the color lightness and affect the a* and b* values (Dauqan & Abdullah, Citation2013). Then, the higher the L* value indicates, the higher the encapsulated clove bud extract adds so that the pH decreases while the total phenolics and flavonoids increase. Therefore, L* is negatively correlated with pH (r = −0.975) but positively correlated with total phenolics (r = 0.983) and total flavonoids (r = 0.924). The positive correlation between L* and ∆E is because the higher the lightness, the higher the difference between the chocolate beverage samples and the control.
Conclusions
Encapsulated clove bud extract levels significantly affected the physicochemical and organoleptic properties of chocolate beverages. Incorporation of clove bud extract in chocolate beverages increased phenolic content, flavonoids, antioxidant activity, hygroscopicity, L* value, ∆E, viscosity, solubility, and total soluble solids. However, chocolate beverage moisture, a* and b* values, sedimentation, pH, and dissolution time decreased. The panelists preferred chocolate beverage formulations with optimal physicochemical and organoleptic properties achieved with a maximum of 4% encapsulated clove bud extract. The panelists were accepting the organoleptic attributes of each of the chocolate beverage samples, thus indicating that a functional antioxidant-rich beverage alternative is available.
Acknowledgments
The authors are grateful to Universitas Padjadjaran and the Directorate General of Higher Education, Research and Technology, Ministry of Education, Culture, Research and Technology of the Republic of Indonesia for all facilities provided.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Afoakwa, E. O., Ofosu-Ansah, E., Budu, A. S., Mensah-Brown, H., & Takrama, J. F. (2015). Roasting effects on phenolic content and free-radical scavenging activities of pulp preconditioned and fermented cocoa (Theobroma cacao) beans. African Journal of Food, Agriculture, Nutrition and Development, 15(1), 9635–15. https://doi.org/10.18697/ajfand.68.13690
- Ahmed, I. A. M., Babiker, E. E., Al-Juhaimi, F. Y., & Bekhit, A. E.-D. A. (2022). Clove polyphenolic compounds improve the microbiological status, lipid stability, and sensory attributes of beef burgers during cold storage. Antioxidants, 11(7), 1354. https://doi.org/10.3390/antiox11071354
- Akdeniz, B., Sumnu, G., & Sahin, S. (2017). The effects of maltodextrin and gum arabic on encapsulation of onion skin phenolic compounds. Chemical Engineering Transactions, 57, 1891–1896. https://doi.org/10.3303/CET1757316
- Akkarachaneeyakorn, S., & Tinrat, S. (2015). Effects of types and amounts of stabilizers on physical and sensory characteristics of cloudy ready-to-drink mulberry fruit juice. Food Science & Nutrition, 3(3), 213–220. https://doi.org/10.1002/fsn3.206
- Al Mamari, H. H. (2022). Phenolic Compounds: Classification, Chemistry, and Updated Techniques of Analysis and Synthesis. In F. A. Badria (Ed.), Phenolic Compounds - Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications (pp. 4). IntechOpen. https://doi.org/10.5772/intechopen.98958
- Alasti, F. M., Asefi, N., Maleki, R., & SeiiedlouHeris, S. S. (2019). Investigating the flavor compounds in the cocoa powder production process. Food Science and Nutrition, 7(12), 3892–3901. https://doi.org/10.1002/fsn3.1244
- Alfikri, F. N., Pujiarti, R., Wibisono, M. G., & Hardiyanto, E. B. (2020). Yield, quality, and antioxidant activity of clove (Syzygium aromaticum L.) Bud Oil at the different phenological stages in young and mature trees. Scientifica, 2020, 1–8. https://doi.org/10.1155/2020/9701701
- Amaral, P. H. R. D., Lopes Andrade, P., & Costa de Conto, L. (2019). Microencapsulation and its uses in food science and technology: A review. Microencapsulation - Processes, Technologies and Industrial Applications, 1–18. https://doi.org/10.5772/intechopen.81997
- Aribah, S. A., Sanjaya, A. P., Muhammad, D. R. A., & Praseptiangga, D. (2020). Sensorial and physical properties of chocolate beverage prepared using low fat cocoa powder. AIP Conference Proceedings, 2219(May). https://doi.org/10.1063/5.0003435
- Aroyeun, S. O., Okunade, A. F., Obatoye, A. O., & Olalekna, M. A. (2020). Nutritional profile and organoleptic qualities of milk chocolate incorporated with different spices. Asian Food Science Journal, 1–8. https://doi.org/10.9734/afsj/2019/v13i430117
- Aziz, A. H. A., Rizkiyah, D. N., Qomariyah, L., Irianto, I., Che Yunus, M. A., & Putra, N. R. (2023). Unlocking the full potential of clove (Syzygium aromaticum) Spice: An overview of extraction techniques, bioactivity, and future opportunities in the food and beverage industry. Processes, 11(8), 2453. https://doi.org/10.3390/pr11082453
- Berahun, M. L., Lindawati, S. A., & Miwada, I. N. S. (2022). Konsentrasi serbuk cengkeh (Syzygium aromaticum) dalam pelumuran daging dan pengaruhnya terhadap karakteristik daging broiler. Majalah Ilmiah Peternakan, 25(1), 6–12. https://doi.org/10.24843/MIP.2022.v25.i01.p02
- Bhagya Raj, G. V. S., & Dash, K. K. (2022). Microencapsulation of betacyanin from dragon fruit peel by complex coacervation: Physicochemical characteristics, thermal stability, and release profile of microcapsules. Food Bioscience, 49(June), 101882. https://doi.org/10.1016/j.fbio.2022.101882
- Bonvehí, J. S. (2005). Investigation of aromatic compounds in roasted cocoa powder. European Food Research and Technology, 221(1–2), 19–29. https://doi.org/10.1007/s00217-005-1147-y
- Burhan, A. M., Abdel-Hamid, S. M., Soliman, M. E., & Sammour, O. A. (2019). Optimisation of the microencapsulation of lavender oil by spray drying. Journal of Microencapsulation, 36(3), 250–266. https://doi.org/10.1080/02652048.2019.1620355
- Chalyk, N., Klochkov, V., Sommereux, L., Bandaletova, T., Kyle, N., & Petyaev, I. (2018). Continuous dark chocolate consumption affects human facial skin surface by stimulating corneocyte desquamation and promoting bacterial colonization. The Journal of Clinical and Aesthetic Dermatology, 11(9), 37–41.
- Chang, L. S., Karim, R., Sabo Mohammed, A., Chai, K. F., & Ghazali, H. M. (2019). Moisture sorption isotherm and shelf-life prediction of anticaking agent incorporated spray-dried soursop (Annona muricata L.) powder. Journal of Food Process Engineering, 42(5), 1–10. https://doi.org/10.1111/jfpe.13134
- Chavan, R. S., Khedkar, C. D., & Bhatt, S. (2015). Fat Replacer. Encyclopedia of Food and Health, 589–595. https://doi.org/10.1016/B978-0-12-384947-2.00271-3
- Chew, S. C., Tan, C. P., & Nyam, K. L. (2018). Microencapsulation of refined kenaf (Hibiscus cannabinus L.) seed oil by spray drying using β-cyclodextrin/gum arabic/sodium caseinate. Journal of Food Engineering, 237, 78–85. https://doi.org/10.1016/j.jfoodeng.2018.05.016
- Cortés-Rojas, D. F., de Souza, C. R. F., & Oliveira, W. P. (2014). Clove (Syzygium aromaticum): A precious spice. Asian Pacific Journal of Tropical Biomedicine, 4(2), 90–96. https://doi.org/10.1016/S2221-1691(14)60215-X
- Cortés-Rojas, D. F., Souza, C. R. F., & Oliveira, W. P. (2015). Surfactant mediated extraction of antioxidants from Syzygium aromaticum. Separation Science and Technology (Philadelphia), 50(2), 207–213. https://doi.org/10.1080/01496395.2014.952305
- Cui, Y., Liu, J., Han, S., Li, P., Luo, D., & Guo, J. (2022). Physical stability of chestnut lily beverages (CLB): Effects of shear homogenization on beverage rheological behavior, particle size, and sensory properties. Foods, 11(20), 3188. https://doi.org/10.3390/foods11203188
- Dauqan, E., & Abdullah, A. (2013). Utilization of gum arabic for industries and human health. American Journal of Applied Sciences, 10(10), 1270–1279. https://doi.org/10.3844/ajassp.2013.1270.1279
- De Bruin, M., Coetzee, D., & Schall, R. (2021). The relationship between core stability and athletic performance in female university athletes. South African Journal of Sports Medicine, 33(1), 1–9. https://doi.org/10.17159/2078-516X/2021/v33i1a10825
- DiMattia, C. D., Sacchetti, G., Mastrocola, D., & Serafini, M. (2017). From cocoa to chocolate: The impact of processing on in vitro antioxidant activity and the effects of chocolate on antioxidant markers in vivo. Frontiers in Immunology, 8(SEP), 1–7. https://doi.org/10.3389/fimmu.2017.01207
- Ding, S., & Yang, J. (2021). The effects of sugar alcohols on rheological properties, functionalities, and texture in baked products – a review. Trends in Food Science and Technology, 111(February), 670–679. https://doi.org/10.1016/j.tifs.2021.03.009
- Dogan, M., Toker, O. S., Aktar, T., & Goksel, M. (2013). Optimization of gum combination in prebiotic instant hot chocolate beverage model system in terms of rheological aspect: Mixture design approach. Food and Bioprocess Technology, 6(3), 783–794. https://doi.org/10.1007/s11947-011-0736-y
- Dyaningrum, E. F., Lutfiyah, R. A., Diasti, D. R., Karyadi, J. N. W., & Saputro, A. D. (2019). Physical characteristics of instanised cocoa drink sweetened with palm sap sugar: A preliminary study. IOP Conference Series: Earth and Environmental Science, 355(1), 012045. https://doi.org/10.1088/1755-1315/355/1/012045
- El Riachy, M., Priego-Capote, F., León, L., Rallo, L., & Luque de Castro, M. D. (2011). Hydrophilic antioxidants of virgin olive oil. Part 1: Hydrophilic phenols: A key factor for virgin olive oil quality. European Journal of Lipid Science and Technology, 113(6), 678–691. https://doi.org/10.1002/ejlt.201000400
- Faiqoh, K. E. N., Muhammad, D. R. A., & Praseptiangga, D. (2021). Ginger-flavoured ready-to-drink cocoa beverage formulated with high and low_fat content powder: Consumer preference, properties and stability. Food Research, 5(2), 7–17. https://doi.org/10.26656/fr.2017.5(S2).004
- Fardhyanti, D. S., Kusumaningrum, M., Jai, J., Andriyani, R., & Rahmahani Putri, M. (2022). Encapsulation of Madeira vine (Anredera cordifolia) leaf oil using maltodextrin and gum Arabic as coating materials. Materials Today: Proceedings, 63, S105–S109. https://doi.org/10.1016/j.matpr.2022.02.046
- Fauzya, A. F., Astuti, R. I., & Mubarik, N. R. (2019). Effect of ethanol-derived clove leaf extract on the oxidative stress response in yeast schizosaccharomyces pombe. International Journal of Microbiology, 2019, 1–7. https://doi.org/10.1155/2019/2145378
- Fitriansyah, S. N., Aulifa, D. L., Febriani, Y., & Sapitri, E. (2018). Correlation of total phenolic, flavonoid and carotenoid content of phyllanthus emblica extract from bandung with DPPH scavenging activities. Pharmacognosy Journal, 10(3), 447–452. https://doi.org/10.5530/pj.2018.3.73
- Gengatharan, A., & Rahim, M. H. A. (2023). The application of clove extracts as a potential functional component in active food packaging materials and model food systems: A mini-review. Applied Food Research, 3(1), 100283. https://doi.org/10.1016/j.afres.2023.100283
- Gopinathan, M., Yusof, Y. A., & Pui, L. P. (2020). Effects of different drying methods on the physicochemical and antioxidant content of “cempedak” (Artocarpus integer L.) powder. Journal of Food Processing and Preservation, 44(12), 0–1. https://doi.org/10.1111/jfpp.14966
- Gottardo, F. M., Biduski, B., dos Santos, L. F., dos Santos, J. S., Rodrigues, L. B., & dos Santos, L. R. (2022). Microencapsulated oregano and cinnamon essential oils as a natural alternative to reduce Listeria monocytogenes in Italian salami. Food Bioscience, 50, 102146. https://doi.org/10.1016/j.fbio.2022.102146
- Goya, L., Kongor, J. E., & de Pascual-Teresa, S. (2022). From cocoa to chocolate: Effect of processing on flavanols and methylxanthines and their mechanisms of action. International Journal of Molecular Sciences, 23(22), 14365. https://doi.org/10.3390/ijms232214365
- Hala, M. A. (2011). Comparative antioxidant activity study of some edible plants used spices in Egypt. Journal of American Science, 7(1), 1118–1122.
- Haro-González, J. N., Castillo-Herrera, G. A., Martínez-Velázquez, M., & Espinosa-Andrews, H. (2021). Clove essential oil (Syzygium aromaticum l. Myrtaceae): Extraction, chemical composition, food applications, and essential bioactivity for human health. Molecules, 26(21), 6387. https://doi.org/10.3390/molecules26216387
- Hasheminejad, N., & Khodaiyan, F. (2020). The effect of clove essential oil loaded chitosan nanoparticles on the shelf life and quality of pomegranate arils. Food Chemistry, 309, 125520. https://doi.org/10.1016/j.foodchem.2019.125520
- Hedayati, S., Shahidi, F., Koocheki, A., Farahnaky, A., & Majzoobi, M. (2016). Comparing the effects of sucrose and glucose on functional properties of pregelatinized maize starch. International Journal of Biological Macromolecules, 88, 499–504. https://doi.org/10.1016/j.ijbiomac.2016.04.026
- Herawati, H. (2018). Potensi hidrokoloid sebagai bahan tambahan pada produk pangan dan nonpangan bermutu. Jurnal Litbang Pertanian, 37(1), 17–25. https://doi.org/10.21082/jp3.v37n1.2018.p17-25
- Holkar, C. R., Jadhav, A. J., & Pinjari, D. V. (2019). A critical review on the possible remediation of sediment in cocoa/coffee flavored milk. Trends in Food Science and Technology, 86(February), 199–208. https://doi.org/10.1016/j.tifs.2019.02.035
- Hussain, S., Rahman, R., Mushtaq, A., & Zerey-Belaskri, A. E. (2017). Clove: A review of a precious species with multiple uses clove: A review of a precious species with multiple uses. International Journal of Chemical and Biochemical Sciences, 11(January), 129–133.
- Idowu, S., Adekoya, A. E., Igiehon, O. O., & Idowu, A. T. (2021). Clove (Syzygium aromaticum) spices: A review on their bioactivities, current use, and potential application in dairy products. Journal of Food Measurement and Characterization, 15(4), 3419–3435. https://doi.org/10.1007/s11694-021-00915-9
- Indiarto, R., Indriana, L. P. A., Andoyo, R., Subroto, E., & Nurhadi, B. (2022). Bottom–up nanoparticle synthesis: A review of techniques, polyphenol-based core materials, and their properties. European Food Research and Technology, 248(1), 1–24. https://doi.org/10.1007/s00217-021-03867-y
- Indiarto, R., Pranoto, Y., Santoso, U., & Supriyanto, S. (2019a). Evaluation of physicochemical properties and antioxidant activity of polyphenol-rich cacao bean extract through water blanching. Pakistan Journal of Nutrition, 18(3), 278–287. https://doi.org/10.3923/pjn.2019.278.287
- Indiarto, R., Pranoto, Y., Santoso, U., & Supriyanto, S. (2019b). In vitro antioxidant activity and profile of polyphenol compounds extracts and their fractions on cacao beans. Pakistan Journal of Biological Sciences, 22(1), 34–44. https://doi.org/10.3923/pjbs.2019.34.44
- Indiarto, R., Rahimah, S., Subroto, E., Putri, N. A. G., & Pangawikan, A. D. (2022). Antioxidant activity and characteristics of a cocoa drink formulated with encapsulated green coffee extract. International Journal of Food Properties, 25(1), 2477–2494. https://doi.org/10.1080/10942912.2022.2144883
- Indiarto, R., Reni, R., Utama, G. L., Subroto, E., Pangawikan, A. D., & Djali, M. (2023). The physicochemical, antioxidant, and sensory properties of chocolate biscuits incorporated with encapsulated mangosteen (Garcinia mangostana L.) peel extract. International Journal of Food Properties, 26(1), 122–138. https://doi.org/10.1080/10942912.2022.2159429
- Indiarto, R., Subroto, E., Sukri, N., & Djali, M. (2021). Cocoa (theobroma cacao L.) beans processing technology: A review of flavonoid changes. Asian Journal of Plant Sciences, 20(4), 684–693. https://doi.org/10.3923/ajps.2021.684.693
- Juvinal, J. G., De Steur, H., Schouteten, J. J., Muhammad, D. R. A., De Leon, A. A., Dewettinck, K., & Gellynck, X. (2023). Physico-chemical property, sensory profile and consumer acceptability of water buffalo (Bubalus bubalis L.) chocolate milk using alkalized and natural cocoa powder. Foods, 12(9), 1797. https://doi.org/10.3390/foods12091797
- Kapolou, A., Karantonis, H. C., Rigopoulos, N., & Koutelidakis, A. E. (2021). Association of mean daily polyphenols intake with Mediterranean diet adherence and anthropometric indices in healthy Greek adults: A retrospective study. Applied Sciences (Switzerland), 11(10), 4664. https://doi.org/10.3390/app11104664
- Karunamay, S., Badhe, S. R., Shukla, V., Singh, N., Lali, K., & Patil, S. (2019). Application of clove essential oil in food industry–A review. Journal of Food Research and Technology, 7(August), 23–25.
- Kasai, H., Shirao, M., & Ikegami-Kawai, M. (2016). Analysis of volatile compounds of clove (Syzygium aromaticum) buds as influenced by growth phase and investigation of antioxidant activity of clove extracts. Flavour and Fragrance Journal, 31(2), 178–184. https://doi.org/10.1002/ffj.3299
- Kusmayadi, A., Adriani, L., Abun, A., Muchtaridi, M., & Tanuwiria, U. H. (2019). The microencapsulation of mangosteen peel extract with maltodextrin from arenga starch: Formulation and characterization. Journal of Applied Pharmaceutical Science, 9(3), 33–40. https://doi.org/10.7324/JAPS.2019.90306
- Lamport, D. J., Christodoulou, E., & Achilleos, C. (2020). Beneficial effects of dark chocolate for episodic memory in healthy young adults: A parallel-groups acute intervention with a white chocolate control. Nutrients, 12(2), 483. https://doi.org/10.3390/nu12020483
- Leinonen, H., Pettersson, M., & Lajunen, M. (2011). Water-soluble carbon nanotubes through sugar azide functionalization. Carbon, 49(4), 1299–1304. https://doi.org/10.1016/j.carbon.2010.11.049
- Likumahua, M. H., Moniharapon, E., & Tuhumury, H. C. D. (2022). Effect of sugar concentration on physical and organoleptic characteristics of lime (Citrus aurantiifolia S.) marmalade. Jurnal Sains Dan Teknologi Pangan, 7(2), 4978–4993. https://doi.org/10.33772/jstp.v7i2.23415
- Li, Y., Zhu, S., Feng, Y., Xu, F., Ma, J., & Zhong, F. (2014). Influence of alkalization treatment on the color quality and the total phenolic and anthocyanin contents in cocoa powder. Food Science and Biotechnology, 23(1), 59–63. https://doi.org/10.1007/s10068-014-0008-5
- Lund, M. N. (2021). Reactions of plant polyphenols in foods: Impact of molecular structure. Trends in Food Science and Technology, 112(February), 241–251. https://doi.org/10.1016/j.tifs.2021.03.056
- Mahato, D. K., Jadhav, S. R., Mukurumbira, A. R., Keast, R., Liem, D. G., Shah, R., & Gamlath, S. (2022). Physicochemical properties and microbial safety of reduced-sugar chocolate-flavored milk. Journal of Food Processing and Preservation, 46(3), 1–12. https://doi.org/10.1111/jfpp.16409
- Majeed, H., Bian, Y.-Y., Ali, B., Jamil, A., Majeed, U., Khan, Q. F., Iqbal, K. J., Shoemaker, C. F., & Fang, Z. (2015). Essential oil encapsulations: Uses, procedures, and trends. RSC Advances, 5(72), 58449–58463. https://doi.org/10.1039/c5ra06556a
- Marta, H., Tensiska, T., & Riyanti, L. (2017). Production and characterisation of maltodextrin from corn starch by enzymatic hydrolysis method. Chimica et Natura Acta, 5(1), 13–20. https://doi.org/10.24198/cna.v5.n1.12816
- Mazor Jolić, S., Radojčic Redovnikovic, I., Marković, K., Ivanec Šipušić, D., & Delonga, K. (2011). Changes of phenolic compounds and antioxidant capacity in cocoa beans processing. International Journal of Food Science and Technology, 46(9), 1793–1800. https://doi.org/10.1111/j.1365-2621.2011.02670.x
- Mikucka, W., Zielinska, M., Bulkowska, K., & Witonska, I. (2022). Recovery of polyphenols from distillery stillage by microwave-assisted, ultrasound-assisted and conventional solid–liquid extraction. Scientific Reports, 12(1), 1–13. https://doi.org/10.1038/s41598-022-07322-0
- Miller, K. B., Hurst, W. J., Payne, M. J., Stuart, D. A., Apgar, J., Sweigart, D. S., & Ou, B. (2008). Impact of alkalization on the antioxidant and flavanol content of commercial cocoa powders. Journal of Agricultural and Food Chemistry, 56(18), 8527–8533. https://doi.org/10.1021/jf801670p
- Mittal, M., Gupta, N., Parashar, P., Mehra, V., & Khatri, M. (2014). Phytochemical evaluation and pharmacological activity of Syzygium aromaticum: A comprehensive review. International Journal of Pharmacy and Pharmaceutical Sciences, 6(8), 67–72.
- Muhammad, D. R. A., Gonzalez, C. G., Sedaghat Doost, A., Van de Walle, D., Van der Meeren, P., & Dewettinck, K. (2019). Improvement of antioxidant activity and physical stability of chocolate beverage using colloidal cinnamon nanoparticles. Food and Bioprocess Technology, 12(6), 976–989. https://doi.org/10.1007/s11947-019-02271-5
- Muhammad, D. R. A., Kongor, J. E., & Dewettinck, K. (2021). Investigating the effect of different types of cocoa powder and stabilizers on suspension stability of cinnamon-cocoa drink. Journal of Food Science and Technology, 58(10), 3933–3941. https://doi.org/10.1007/s13197-020-04855-y
- Muhammad, D. R. A., Marettama, N. M., Fauza, G., & Affandi, D. R. (2022). Can ingredients and information interventions affect the hedonic level and (emo-sensory) perceptions of the milk chocolate and cocoa drink’s consumers? Open Agriculture, 7(1), 847–856. https://doi.org/10.1515/opag-2022-0146
- Mutha, R. E., Tatiya, A. U., & Surana, S. J. (2021). Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future Journal of Pharmaceutical Sciences, 7(1), 1–13. https://doi.org/10.1186/s43094-020-00161-8
- Nawi, N. M., Muhamad, I. I., & Mohd Marsin, A. (2015). The physicochemical properties of microwave-assisted encapsulated anthocyanins from ipomoea batatas as affected by different wall materials. Food Science and Nutrition, 3(2), 91–99. https://doi.org/10.1002/fsn3.132
- Nowak, K., Ogonowski, J., Jaworska, M., & Grzesik, K. (2012). Clove oil - properties and applications. Chemik, 66(2), 149–152.
- Nurhadi, B., Angiputri, F. C., Andoyo, R., Ermawar, R. A., Saputra, R. A., & Hano, C. (2022). Antioxidant stability of moringa leaves extract powders obtained by cocrystallization, vacuum drying, and plating. Journal of Food Quality, 2022, 1–10. https://doi.org/10.1155/2022/3038403
- Nurhadi, B., Suriati, S., Saputra, T., Saputra, R. A., & Sukri, N. (2020). The role of encapsulant materials on the stability of bioactive compounds of red ginger (Zingiber officinale Roscoe. var. Rubrum) extract powder during storage. Food Chemistry, 333, 127490. https://doi.org/10.1016/j.foodchem.2020.127490
- Nurhafsah, N., Laboko, A. I., Rahmi, H., Andriani, I., Fitriawaty, F. W. S., Senge, M., Aziz, A. A., Witono, Y., Sharifuddin, J., Robani, A. B., Krisnamurthi, B., Saiyut, P., Mulyo, J. H., Bin Kamarudin, M. F., & Tjale, M. M. (2022). The effect of adding curcuma powder on the quality of the active components in instant cocoa drinks. E3S Web of Conferences, 361, 361. https://doi.org/10.1051/e3sconf/202236104015
- Oracz, J., & Żyżelewicz, D. (2020). Antioxidants in Cocoa. Antioxidants, 9(12), 1230. https://doi.org/10.3390/antiox9121230
- Pangestu, M. R., Fitri, C. A., & Wajizah, S. (2016). Nilai organoleptik daging ayam broiler dengan penambahan prebiotik immuno forte® pada berbagai level berbeda. Jurnal Ilmiah Mahasiswa Pertanian, 1(1), 731–738. https://doi.org/10.17969/jimfp.v1i1.904
- Peña-Correa, R. F., Ataç Mogol, B., & Fogliano, V. (2022). The impact of roasting on cocoa quality parameters. Critical Reviews in Food Science and Nutrition, 1–14. https://doi.org/10.1080/10408398.2022.2141191
- Praseptiangga, D., Aviany, T. P., & Parnanto, N. H. R. (2016). Pengaruh penambahan gum arab terhadap karakteristik fisikokimia dan sensoris fruit leather nangka (Artocarpus heterophyllus). Jurnal Teknologi Hasil Pertanian, 9(1), 71–83. https://doi.org/10.20961/jthp.v9i2.12858
- Praseptiangga, D., Qomaruzzaman, A. R., & Manuhara, G. J. (2021). The effect of clove leaves essential oil addition on physicochemical and sensory characteristics of milk chocolate bar. International Journal on Advanced Science, Engineering and Information Technology, 11(1), 165–171. https://doi.org/10.18517/ijaseit.11.1.12664
- Premi, M., & Sharma, H. K. (2017). Effect of different combinations of maltodextrin, gum arabic and whey protein concentrate on the encapsulation behavior and oxidative stability of spray dried drumstick (Moringa oleifera) oil. International Journal of Biological Macromolecules, 105, 1232–1240. https://doi.org/10.1016/j.ijbiomac.2017.07.160
- Puchol-Miquel, M., Palomares, C., Fernández-Segovia, I., Barat, J. M., & Perez-Esteve, É. (2021). Effect of the type and degree of alkalization of cocoa powder on the physico-chemical and sensory properties of sponge cakes. LWT, 152(August), 112241. https://doi.org/10.1016/j.lwt.2021.112241
- Quek, S. Y., Chok, N. K., & Swedlund, P. (2007). The physicochemical properties of spray-dried watermelon powders. Chemical Engineering and Processing: Process Intensification, 46(5), 386–392. https://doi.org/10.1016/j.cep.2006.06.020
- Razak, R. A., Karim, R., Sulaiman, R., & Hussain, N. (2018). Effects of different types and concentration of hydrocolloids on mango filling. International Food Research Journal, 25(3), 1109–1119.
- Rushchitc, A. A., Shcherbakova, E. I., & El-Sohaimy, S. A. (2022). Physicochemical and rheological characterization of dumpling’s dough fortified with Jerusalem artichoke (Helianthus tuberosus) powder. Annals of Agricultural Sciences, 67(2), 166–172. https://doi.org/10.1016/j.aoas.2022.11.001
- Saeed, M., Khan, M. S., Alagawany, M., Farag, M. R., Alqaisi, O., Aqib, A. I., Qumar, M., Siddique, F., & Ramadan, M. F. (2021). Clove (Syzygium aromaticum) and its phytochemicals in ruminant feed: An updated review. Rendiconti Lincei, 32(2), 273–285. https://doi.org/10.1007/s12210-021-00985-3
- Salim, A.-B., El-Desouky, T. A., & EL-Sedeek, L. (2015). Clove (Syzygium Aromaticum L.) and their effect on the formation of heterocyclic amines. Journal of Drug Delivery and Therapeutics, 5(5), 33–40. https://doi.org/10.22270/jddt.v5i5.1138
- Santiago-Rodríguez, E., Estrada-Zaldívar, B., & Zaldívar-Uribe, E. (2018). Effects of dark chocolate intake on brain electrical oscillations in healthy people. Foods (Basel, Switzerland), 7(11), 187. https://doi.org/10.3390/foods7110187
- Schober, P., & Schwarte, L. A. (2018). Correlation coefficients: Appropriate use and interpretation. Anesthesia and Analgesia, 126(5), 1763–1768. https://doi.org/10.1213/ANE.0000000000002864
- Shahinuzzaman, M., Rana, S., Yaakob, Z., Azir Uddin, M., Shahinuzzaman, M., & Sohel Rana, M. (2017). Study of chemical composition and medicinal properties of volatile oil from clove buds (Eugenia Caryophyllus). Article in International Journal of Pharmaceutical Sciences and Research, 8(2), 895–899. https://doi.org/10.13040/IJPSR.0975-8232.8(2).895-99
- Sharma, A., Bhushette, P. R., & Annapure, U. S. (2021). Physicochemical and rheological properties of acacia catechu exudate gum. Carbohydrate Polymer Technologies and Applications, 2, 100127. https://doi.org/10.1016/j.carpta.2021.100127
- Shin, J. H., Kim, C. S., Cha, L., Kim, S., Lee, S., Chae, S., Chun, W. Y., & Shin, D. M. (2022). Consumption of 85% cocoa dark chocolate improves mood in association with gut microbial changes in healthy adults: A randomized controlled trial. The Journal of Nutritional Biochemistry, 99, 108854. https://doi.org/10.1016/j.jnutbio.2021.108854
- Shittu, T. A., & Lawal, M. O. (2007). Factors affecting instant properties of powdered cocoa beverages. Food Chemistry, 100(1), 91–98. https://doi.org/10.1016/j.foodchem.2005.09.013
- Shuen, G. W., Yi, L. Y., Ying, T. S., Von Yu, G. C., Binti Yusof, Y. A., & Phing, P. L. (2021). Effects of drying methods on the physicochemical properties and antioxidant capacity of Kuini powder. Brazilian Journal of Food Technology, 24, 1–14. https://doi.org/10.1590/1981-6723.08620
- Siemińska-Kuczer, A., Szymańska-Chargot, M., & Zdunek, A. (2022). Recent advances in interactions between polyphenols and plant cell wall polysaccharides as studied using an adsorption technique. Food Chemistry, 373, 131487. https://doi.org/10.1016/j.foodchem.2021.131487
- Silva, F., Fonseca, C., Alencar, S., Thomazini, M., Balieiro, J., Pittia, P., & Favaro-Trindade, C. (2012). Assessment of production efficiency, physicochemical properties and storage stability of spray-dried propolis, a natural food additive, using gum arabic and OSA starch-based carrier systems. Food and Bioproducts Processing, 91(1), 28–36. https://doi.org/10.1016/j.fbp.2012.08.006
- Singletary, K. (2014). Clove: Overview of potential health benefits. Nutrition Today, 49(4), 207–224. https://doi.org/10.1097/NT.0000000000000036
- Sioriki, E., Lemarcq, V., Alhakim, F., Triharyogi, H., Tuenter, E., Cazin, C. S. J., Nolan, S. P., Pieters, L., Van de Walle, D., & Dewettinck, K. (2021). Impact of alkalization conditions on the phytochemical content of cocoa powder and the aroma of cocoa drinks. LWT, 145, 111181. https://doi.org/10.1016/j.lwt.2021.111181
- Sirait, T. S., Arianto, A., & Dalimunthe, A. (2023). Phytochemical screening of cinnamon bark (Cinnamomum burmanii) (C. Ness & T. Ness) C. Ness ex Blume ethanol extract and antioxidant activity test with DPPH (2,2-diphenyl-1-picrylhydrazyl) method. International Journal of Science Technology & Management, 4(1), 254–259. https://doi.org/10.46729/ijstm.v4i1.739
- Stark, T., Bareuther, S., & Hofmann, T. (2006). Molecular definition of the taste of roasted cocoa nibs (Theobroma cacao) by means of quantitative studies and sensory experiments. Journal of Agricultural and Food Chemistry, 54(15), 5530–5539. https://doi.org/10.1021/jf0608726
- Subroto, E., Andoyo, R., Indiarto, R., Lembong, E., & Rahmani, F. (2022). Physicochemical properties, sensory acceptability, and antioxidant activity of chocolate bar fortified by solid lipid nanoparticles of gallic acid. International Journal of Food Properties, 25(1), 1907–1919. https://doi.org/10.1080/10942912.2022.2115066
- Subroto, E., Djali, M., Indiarto, R., Lembong, E., & Baiti, N. (2023). Microbiological activity affects post-harvest quality of cocoa (Theobroma cacao L.) beans. Horticulturae, 9(7), 805. https://doi.org/10.3390/horticulturae9070805
- Sukri, N., Multisona, R. R., Zaida, Saputra, R. A., Mahani, & Nurhadi, B. (2021). Effect of maltodextrin and arabic gum ratio on physicochemical characteristic of spray dried propolis microcapsules. International Journal of Food Engineering, 17(2), 159–165. https://doi.org/10.1515/ijfe-2019-0050
- Tanghe, A., Heyman, E., Vanden Wyngaert, K., Van Ginckel, A., Celie, B., Rietzschel, E., Calders, P., & Shadid, S. (2021). Evaluation of blood pressure lowering effects of cocoa flavanols in diabetes mellitus: A systematic review and meta-analysis. Journal of Functional Foods, 79, 104399. https://doi.org/10.1016/j.jff.2021.104399
- Tarwendah, I. P. (2017). Jurnal Review: Studi Komparasi Atribut Sensoris Dan Kesadaran Merek Produk Pangan. Jurnal Pangan Dan Agroindustri, 5(2), 66–73.
- Thaichon, P., Jebarajakirthy, C., Tatuu, P., & Gajbhiyeb, R. G. (2018). Are you a chocolate lover? An investigation of the repurchase behavior of chocolate consumers. Journal of Food Products Marketing, 24(2), 163–176. https://doi.org/10.1080/10454446.2017.1266551
- Tiwari, B. K., Muthukumarappan, K., O’Donnell, C. P., & Cullen, P. J. (2008). Effects of sonication on the kinetics of orange juice quality parameters. Journal of Agricultural and Food Chemistry, 56(7), 2423–2428. https://doi.org/10.1021/jf073503y
- Todorović, A., Šturm, L., Salević-Jelić, A., Lević, S., Osojnik Črnivec, I. G., Prislan, I., Skrt, M., Bjeković, A., Poklar Ulrih, N., & Nedović, V. (2022). Encapsulation of bilberry extract with maltodextrin and gum arabic by freeze-drying: Formulation, characterisation, and storage stability. Processes, 10(10), 1991. https://doi.org/10.3390/pr10101991
- Tonon, R. V., Brabet, C., & Hubinger, M. D. (2008). Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae mart.) powder produced by spray drying. Journal of Food Engineering, 88(3), 411–418. https://doi.org/10.1016/j.jfoodeng.2008.02.029
- Tursiloadi, S., Artanti, N., & Sulaswatty, A. (2015). Chemical catalytic and biocatalytic process of clove oil derivatives review. Jurnal Kimia Terapan Indonesia, 17(1), 69–85. https://doi.org/10.14203/jkti.v17i1.24
- Urbańska, B., & Kowalska, J. (2019). Comparison of the total polyphenol content and antioxidant activity of chocolate obtained from roasted and unroasted cocoa beans from different regions of the world. Antioxidants, 8(8), 283. https://doi.org/10.3390/antiox8080283
- Valverde García, D., Pérez Esteve, É., & Barat Baviera, J. M. (2020). Changes in cocoa properties induced by the alkalization process: A review. Comprehensive Reviews in Food Science and Food Safety, 19(4), 2200–2221. https://doi.org/10.1111/1541-4337.12581
- Veselá, A., Barros, A. S., Synytsya, A., Delgadillo, I., Čopíková, J., & Coimbra, M. A. (2007). Infrared spectroscopy and outer product analysis for quantification of fat, nitrogen, and moisture of cocoa powder. Analytica Chimica Acta, 601(1), 77–86. https://doi.org/10.1016/j.aca.2007.08.039
- Vladić, J., Nastić, N., Janković, T., Šavikin, K., Menković, N., Lončarević, I., & Vidović, S. (2022). Microencapsulation of sideritis raeseri Boiss. & Heldr. subsp. raeseri extract using spray drying with maltodextrin and whey protein. Periodica Polytechnica Chemical Engineering, 66(2), 229–238. https://doi.org/10.3311/PPch.19060
- Warda, H., Nawansih, O., Yuliana, N., & Nurdin, S. U. (2023). Customer preferences towards the development of coffee snacks product. Jurnal Agroindustri Berkelanjutan, 2(1), 64–74.
- Yuan, S., Li, X., Jin, Y., & Lu, J. (2017). Chocolate consumption and risk of coronary heart disease, stroke, and diabetes: A meta-analysis of prospective studies. Nutrients, 9(7), 688. https://doi.org/10.3390/nu9070688
- Zzaman, W., Bhat, R., & Yang, T. A. (2014). Effect of superheated steam roasting on the phenolic antioxidant properties of cocoa beans. Journal of Food Processing and Preservation, 38(4), 1932–1938. https://doi.org/10.1111/jfpp.12166