ABSTRACT
A sustainable, low-cost medium based on food waste for cultivating the cyanobacterium Arthrospira platensis (A. platensis) was investigated. Zarrouk medium components, especially phosphate, were substituted with extracts from beetroot peel, brewer’s grains, and walnut press cake. Ion concentrations of magnesium, calcium, iron, potassium, sodium, phos-phate, nitrate, sulfate, and chloride were analyzed to determine the optimal medium composition. Walnut press cake and brewer’s grains extracts showed high potential due to their magnesium, calcium, and phosphate content. Brewer’s grains extracts also provided glucose as an additional carbon source. Cultivations in shaking flasks and a photobioreactor (PBR) monitored cell growth through optical density and biomass concentration. The excess of magnesium and reduced phos-phate concentrations positively affected biomass production. A. platensis’s ability to metabolize glucose resulted in 68% higher biomass production. Growth in brewer’s grains extract required no additional phosphate. PBR cultivation showed A. platensis consumed significant magnesium and nitrate, achieving higher biomass concentrations with optimized gas exchange and light reflection systems.
Introduction
Due to nowadays’ challenges like global warming, overpopulation and its resulting resource scarcity and food shortage, it is paramount to establish new technologies to ensure the efficient and sustainable production of food, while minimizing the CO2 emissions in the atmosphere. Furthermore, it is important to recycle or reuse food waste in the foodstuff industry to ensure the full utilization of potential valuable nutrients Krishnan et al. (Citation2020).
Edible cyanobacteria like the genus Arthrospira, which is mostly found in tropical and subtropical regions, have a long tradition, especially in Africa and Mexico. In the 16th century, Spanish conquerors discovered Arthrospira used from the indigenous people as food source in Mexico (Torres-Tiji et al., Citation2020).
Since its rediscovery in the 1950’s, the genus has been of growing interest for various applications (Nowicka-Krawczyk, Mühlensteinová et al., Citation2019, Vonshak, Citation2002), as it even fixates CO2 from the atmosphere by its photosynthesis activity during growth. For instance, the organism Arthrospira platensis (A. platensis), also known under the commercial name “Spirulina”, can uptake CO2 with a carbon dioxide fixation rate up to 413 mg CO2 * L−1 * day−1 (Morais & Costa, Citation2007).
A. platensis is a filamentous, photosynthetic cyanobacterium and is often classified as blue-green microalgae. Despite of its bacterial morphological structure, the photosynthetic system is the same as in algae (Ashby & Houmard, Citation2006). A. platensis has a high nutritional value and is an important source in food, chemical and pharmaceutical industry. Furthermore, it is used as feed for fish in aquaculture and in wastewater treatment for the removal of nutrients and heavy metals (De Oliveira et al., Citation1999; Pelizer et al., Citation2002).
One big project is bringing all these qualities together: For space expeditions (Gòdia et al., Citation2002) Arthrospira creates an artificial ecosystem: recycling waste and forming oxygen by the simultaneous removal of CO2 from atmosphere, water recycling and production (Badri et al., Citation2015; Hendrickx et al., Citation2006).
Nowadays, the species is still commonly used as a food source, but is produced for food supplements and food additives (Koru, Citation2011; Wikfors & Ohno, Citation2001) due to its valuable ingredients:
The biochemical composition of A. platensis contains a high protein ratio with 55%−70% referred to the dry mass, different vitamins (A, B1, B2, B12) and mineral nutrients. Furthermore, it contains polyunsaturated fatty acids (PUFA), phycobiliproteins, carotenoids and exopolysaccharides (Borowitza, Citation2013; Delattre et al., Citation2016; Parvin et al., Citation2008; Richmond & Hu, Citation2013). The high nutritional value allows different therapeutic applications, like regulation of blood pressure and cholesterol synthesis, stimulating the immune system and the treatment of zinc deficiency (Hayashi et al., Citation1996; Huang et al., Citation1982; Iwata et al., Citation1990; Nayaka et al., Citation1988). The main phycobiliprotein in A. platensis is phycocyanin with 6.1%−17.8% referred to the dry mass. Due to the high phycocyanin yields of A. platensis, the cyanobacterium is commonest in industrial phycocyanin production (Borowitza, Citation2013; Das, Citation2015; Eriksen, Citation2008; Richmond & Hu, Citation2013). Besides phycocyanin (λmax ~620 nm), there are two other major classes of phycobiliproteins in cyanobacteria called phycoerythrin (λmax ~565 nm) and allophycocyanin (λmax ~650 nm), which all share similar tertiary and quaternary structures (Schluchter & Glazer, Citation1999). The phycobiliprotein phycocyanin, with its intense blue color, is hydrophilic and comprises a protein and the chromophore phycocyanobilin. There is a wide range of biological activities like antioxidant, antimutative, antiviral and antitumor properties as well as the stimulation of the immune system (Fernández-Rojas et al., Citation2014; Ramos et al., Citation2011; Romay et al., Citation2003). The usage of phycocyanin as a natural pigment for food and cosmetic applications is also increasing (Chethana et al., Citation2015).
A. platensis has its optimal growth conditions at 35–37°C and a pH optimum between 8.7 and 10.0 (Aruna & Ravindran, Citation2008). In 1966, the first synthetic medium for the cultivation of A. platensis, called Zarrouk medium, was formulated and is still used as a standard medium (Zarrouk, Citation1966). Many components are replaceable easily by organic sources. The major bottleneck component is biogenic phosphate.
One billion tons of food waste are produced every year, which must be recycled, treated or disposed in landfills (Gustavsson et al., Citation2011). Food waste is an interesting, highly promising source of nutrients for fermentation processes after hydrolysis (Lau et al., Citation2014). By hydrolysis of carbohydrates, proteins and polyphosphates important nutrients like glucose, free amino nitrogen, phosphate and nitrate can be extracted and used for fermentation processes (Leung et al., Citation2012; Pleissner et al., Citation2014). Consequently, food waste can be used and recycled by using its nutrients to produce microbial biomass and its metabolites (Lau et al., 2014). For instance, the food wastes brewers grains, beetroot peel and walnut press cake have the potential to replace several ingredients of the synthetic Zarrouk medium.
Walnuts have a high content of magnesium, copper, folic acid, protein, potassium and vitamin E (Hu et al., Citation1998). The investigated composition of minerals in 100 g walnuts is 441 mg potassium, 158 mg magnesium, 98 mg calcium, 2.91 mg iron and 2 mg sodium. Furthermore, a high amount of phosphorus with 346 mg per 100 g walnut was detected. Additionally, walnuts include 2.43 g sucrose, 0.08 g glucose and 0.09 g fructose per 100 g walnut (USDA Food Composition Databases Citation2020).
Beetroot has a high content of potassium, iron, folic acid and vitamin B (Egger & Stuppner, Citation1996). 100 g beetroot contains certain minerals, such as 77 mg sodium, 16 mg iron, 38 mg phosphorus, 305 mg potassium and 23 mg magnesium (Yashwant, Citation2015). The average sugar content of different beetroot cultivars is 77.5 g/L. The highest percentage thereof has sucrose with 94.8% followed by glucose with 3.3% and fructose with 1.9% (Wruss et al., Citation2015).
Brewers grain is a potentially valuable resource for industrial utilization, because it is easily available and cost-effective (Robertson & Andersen-Pinstrup, Citation2010). Due to its high nutritive value, possible applications of brewers grains are the utilization in animal feeds, production of value-added compounds (xylitol, lactic acid), raw material for extraction of sugars, proteins or acids and microorganism cultivation (Mussatto, Citation2009). Additionally, high amounts of calcium, magnesium, silicon and phosphorus were detected. Other minerals like cobalt, copper, iron, manganese, selenium, potassium, sodium and sulfur were found in lower concentrations. Brewers grains also include several vitamins and amino acids (Essien & Udotong, Citation2008; Huige, Citation1994; Khidzir et al., Citation2010; Mussatto et al., Citation2006). 36.4 million tonnes of the material are each year available (Nyhan et al., Citation2023) at brewery sites all over the world the independent of saisonal factors and in constant quality, making it the first choise for medium replacement experiments.
The present work focuses on the total as well as natural replacement of phosphate in the cultivation medium. Phosphorous or phosphate is the representative of highest importance in two regards: on the one hand, it is essential for biological pathways and on the other hand as limiting bio-ecological factor. As the ratio of components beyond phosphate is different to Zarrouk ratio it is also important to ensure the limits of tolerance of A. platensis towards limited or highly enriched essential components. To finally choose the extract, which is suitable for cultivation, its components especially its phosphate content should be as similar as possible to the concentration in the Zarrouk medium.
Materials and methods
MilliQ water was prepared by the Millipore system from Billerica (Ma, U.S.A.) and used for all analyses. Hydrochlorid Acid (37% HCl, p. a.), sodium hydroxide plates (NaOH, p. a.), nitric acid (65% HNO3, p. a.) for pH adjustment and ion stabilization were purchased from Carl Roth GmbH & Co. KG (Karlsruhe, Germany).
Standard solutions (1000 mg/L in water of NO3−, SO42-, PO43, Cl−) for anion calibration with ion chromatography were purchased from VWR BDH Chemicals (Leuven, Belgium).
Single element standard solutions (1000 mg/L in 2 wt% nitric acid K+, Na+, Fe2+/3+, Ca2+, Mg2+) for cation calibration with atomic absorption spectroscopy were obtained from Carl Roth GmbH & Co. KG (Karlsruhe, Germany).
All experiments were referred to cultivation in Zarrouk standard medium. It was composed with salts in p. a. (pro analysi) quality as given in .
Table 1. Composition and concentrations of components in stock solutions for and in Zarrouk medium (Zarrouk, Citation1966.).
The Arthrospira platensis strain was obtained from the culture collection of Algae at university of Göttingen – strain number 21.99.
Three food waste sources were under investigations:
The solid walnut press cake is produced as a by-product after the extraction of walnut oil and was received from the Erlenbacher Ölmühle (Erlenbach).
The skin of beetroot (beetroot peel) is a by-product after the industrial utilization of beetroot for the food industry. Beetroot peel in this study was given by the company Hengstenberg (Esslingen).
Brewers grains of the brewery Gold Ochsen (Ulm) were used as received from the industrial process. To check reproducibility of the natural material, two batches of brewers grains were under investigation, which are both from exactly the same kind of brewing process and final product but different dates.
Drying and dry-weight determination
Drying was always performed in the drying oven at 100°C to constant weight but at minimum for 24 h.
The dried material of brewers grains offers various advantages, such as easy handling, storing, space-saving and long durability. Therefore, the extract cooked with dried brewers grains was used for cultivation of A. platensis. Another reason of using dried brewers grains was the up to higher efficiency of ion extraction (e.g. 10.8-fold higher phosphate content).
To determine the dry weight development of A. platensis during cultivation, 10 mL of homogeneously mixed culture medium were added to dry glass tubes of constant weight. The precipitates after centrifugation was resuspended in 10 mL of 0.9% NaCl solution and centrifuged again. The glass tubes were stored in a drying oven at 100°C for 24 h to constant weight.
Centrifugation, sampling and OD measurement
All centifugations steps for separation and washing were performed in reaction tubes at 3500 rpm, 20°C for 15 min in a ThermoFisher Heraeus Multifuge 3SR+. All material was stored at −18°C in the freezer for further analysis.
For ion quantification during cultivation, 5 mL of each experiment were taken, centrifuged and the supernatant as well as cell pellet used seperatly for further analysis.
Optical density was determined on 1 mL liquid sample at 680 nm by photometer ThermoFisher Genesis 10S UV-Vis. An absorption higher than 0.8 forced dilution of the sample.
Resuspended cells were diluted and measured against water, while for cultivation samples, medium was used as blank and for dilution.
Atomic absorption spectroscopy (AAS)
Cation concentration of magnesium, sodium, potassium, calcium and iron were determined on a PerkinElmer PinAccle 900 T in flame technique. Quantification was performed by a five point calibration for each cation using five single element AAS standard within the individual limit of detection. The samples and the standard solutions were diluted with 0.2% nitric acid. For the determination of sodium, 1 wt% of lanthanum chloride/strontium chloride in 2 wt% nitric acid were added to the samples and the standards to stabilize atomization.
Ion chromatography (IC)
To determine the anion concentration of phosphate, sulfate, nitrate and chloride, ion chromatography with conductivity detection was performed on Metrohm 861 Advanced Compact. A standard series of five IC standard solutions between 1 mg/L and 50 mg/L were prepared, containing all anions to be determined. For the dilution of samples and standards, MilliQ water was used. The used eluent was a solution containing 3 mM sodium carbonate and 1 mM sodium bicarbonate with a flow rate of 0.7 mL/min. The injection volume was 20 µL. Because of the high conductivity of the eluents, a suppressor system was used, which is protonating the carbonate, and was regenerated with 50 mM sulfuric acid. The used IC-column’s carrier material consists of polyvinyl alcohol with quarternary ammonium groups, which has a particle size of 5 µm (Metrosep A Supp 5–150/4.0). The column temperature was kept at 25°C and the working pressure at 7.84 MPa.
Microwave digestion of brewers grain (complete pulping)
Samples of brewers grain were treated and analyzed by the University of Graz (Institute of Chemistry) in order to define maximum concentration of ions to expect for optimized extraction. Therefore, samples were grinded with an ultra centrifugal grinder (Retsch ZM 200). Afterwards, an aliquot with 250 mg of brewers grain with 5 mL HNO3 was digested in MLS Ultraclave. The temperature ramp was performed until 250°C and this temperature was kept for 30 min. The samples were analyzed with inductively coupled plasma- massspectrometry (ICPMS, Agilent 7900).
Food waste extracts and acid hydrolysis to maximize ion concentration
Extraction experiments were referred to dried material and performed in triplicate. Each food waste material was kept in the drying cabinet Memmert UN 110 at 100°C for 24 h. 3.0 g aliquots of each material were extracted by 50 mL deionized water in a 250 mL round bottom flask. The suspensions were heated up and kept under reflux by an oil bath for 4 h, continuously stirring with a magnetic stirrer at 250 rpm. After separating the solid by vacuum filtration with a Buchner funnel (filter paper: Whatmann 113, radius: 90 mm) particle free, light yellow extracts were stored for further analysis in the freezer at −18°C.
The different food waste materials were extracted in their origin and dried state at pH 6 and pH 11. For adjustment to pH 11, 1 M aqueous sodium hydroxide was used.
On brewers grains extracts acid hydrolysis of starch was done to achieve a higher glucose concentration. Therefore, 1 mL of 10 M hydrochloric acid solution was given to 49 mL of deionized water of pH 6 and 3.0 g of brewers grains and boiled for 4 h under reflux. After acid hydrolysis, the pH value of the extract was set to 7 with 1 M NaOH solution.
Composition of Zarrouk medium (standard medium as positive reference)
The organism A. platensis was cultivated in shaking flasks and photobioreactors in different Zarrouk-based media (Zarrouk, Citation1966). Growth in Zarrouk medium itself as a standard and a reference is compared to growth in extract based, adjusted medium.
The composition of the Zarrouk medium is given in . Each stock solution was produced with deionized water (dH2O) and autoclaved separately.
Composition of modified synthetic Zarrouk medium for studies on growth limitations
The ratio of magnesium and phosphate in all extracts differs most from their ratio in the Zarrouk medium. Therefore, a prestudy was carried out to observe how and up to which limit the concentrations of phosphate (down to one third) and magnesium (up to the 9-fold) influence the growth of A. platensis. Therefore, cultivation took place in Zarrouk medium (based on ), whose components K2HPO4 (stock solution 3) and MgSO4 *7 H2O (stock solution 1) were added and adjusted as given in .
Table 2. Concentration of MgSO4 * 7 H2O and K2HPO4 in modified Zarrouk medium during cultivation of A. platensis under limitation.
Composition of brewers grains medium to Zarrouk similar composition
For the cultivation of A. platensis on brewers grains extract, 60 g of dried brewers grains were boiled in 1 L of water pH 6. Its ion concentrations were determined () and missing components were added to receive a medium, which is as close as possible to the Zarrouk medium. In brewers grains extract, beside an excess of magnesium, also a higher calcium concentration in comparison with the Zarrouk medium was determined. So no additional magnesium and calcium was added. The phosphate should be completely replaced, so there was no addition of another phosphate source. Sodium bicarbonate and trace elements were not analyzed in this work, so they were added in the same concentration than in Zarrouk medium (see , stock solutions 4, 5 and 6). shows the concentration of components, that were added to the brewers grains extract. The components of stock solution 1 were dissolved in brewers grain extract and sterile filtrated with a 0.2 µm pore sized filter. The other stock solutions were autoclaved separately. The stock solutions were mixed to the final concentration of the medium.
Table 3. Composition and concentrations of stock solution 1 and 2 in brewers grains medium.
Cultivation in shaking flasks
All shaking flask cultivations of A. platensis were performed in the incubator shaker Infors HT Multitron Pro at 30°C, 120 rpm and 24 µmol*m−2*s−1 photon flux.
A pre-culture of A. platensis was cultivated for 10 to 14 days in 300 mL shaking flasks in 150 mL Zarrouk medium (). For inoculation, it was split into 50 mL reaction tubes and centrifuged in a ThermoFisher Heraeus Multifuge 3SR+. The supernatant was removed, and the cell pellets were washed with 20 mL of 0.9% NaCl solution. After centrifugation, the precipitations were resuspended in 10 mL of 0.9% NaCl solution and given into a 10 mL reaction tube. The inoculum volume of the main culture was calculated, so that its starting OD680 was 0.1. The main culture was started in triplicates with 500 mL shake flasks at a culture volume of 250 mL.
In the case of precipitation of media components or use of natural extracts in the medium, two negative controls without A. platensis were used.
Cultivation in photobioreactors (up scale)
The cultivation of A. platensis in Zarrouk medium was performed in duplicates in in-house developed photobioreactors. They consist of a double-walled glass vessel for temperature control. Its flange cover provides four NS 14/23 and three NS 29/32 ports for gasing, probes, filters and sampling.
shows the experimental setup of the photobioreactors during cultivation of A. platensis in Zarrouk medium (front) and brewers grains (background) extract.
Figure 1. Setup of photobioreactors during cultivation of A. platensis in Zarrouk medium (PBR in front) and brewers grains extract (PBR behind).
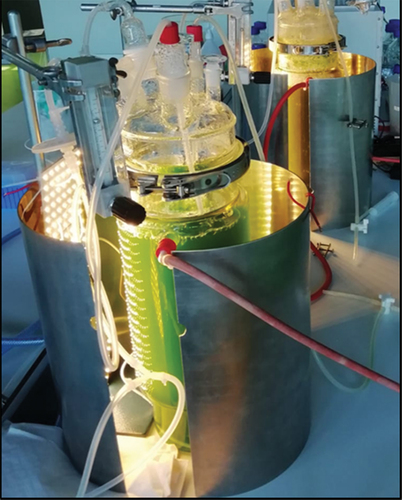
The cultivation was carried out in a 5 L photobioreactor containing 3.5 L of the respective medium and 0.5 L of the inoculum. The photobioreactor was autoclaved with intake air and exhaust air filters (0.2 µm pore size), gas supply system and sampling system. The autoclaved and sterile filtered medium as well as the inoculum were pumped peristaltically in the photobioreactor.
For illumination, four LED plates (warm white light) were plugged together and placed vertically behind the photobioreactor. The distance of the light source was adapted to an incoming photon flux of 70 µmol*m−2*s−1. For better light distribution, two reflector modules per photobioreactor were placed behind the light source and in front of the photobioreactor. The illumination was performed in a photoperiod cycle of 16 h to 8 h light/dark cycle. The temperature of the medium was kept at 25 ± 1°C by a cooling system, which was set to 27°C. The OD680 at the beginning of cultivation was adjusted with inoculum to a value between 0.1 and 0.3.
With a gas flow of 1.0 NL/min, compressed air was blown in the reactor and was divided into fine air bubbles with a gas sparger.
presents the detailed structure of the self-constructed sparger, which was used for gassing in the photobioreactor.
Figure 2. Construction of two similar gas spargers for gassing in photobioreactors during cultivation of A. platensis.
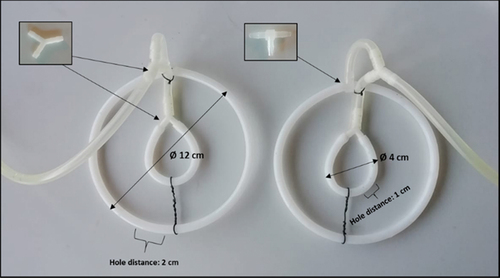
During cultivation of A. platensis in photobioreactors a constant water evaporation has been observed. The loss of water was linear, and an evaporation of 1.8 mL/h was determined. By steady gassing of the photobioreactors, a moisture saturated flow is continuously formed. As there is no cooling system at the reactor lid, the evaporated water cannot condensate effectively, and leaves the reactor fast with the exhaust air.
Results and discussion
Ion concentrations in food waste extracts
In the ion concentrations of all extracts are summarized. For direct comparison, Zarrouk medium composition is given, too. To show the effect of the acid hydrolysis on the brewers grains, the glucose concentration of the different brewers grains extracts is also presented.
Table 4. Overview of ion and glucose concentrations in extracts of wet beetroot peel, walnut press cake and brewers grains under different conditions (wet or dried, at pH 6 and 11, and after acid hydrolysis in 10 M HCl). Not determined compounds marked with “n.d.”. Compounds marked with ‘b.d.l.’ are below the detection limit. Compounds marked with ”[P]” are measured as phosphorus content.
Phosphat as biogene ingriedient is of highest interest in food wast extracts.The extract of dried walnut press cake contains up to 167.7 mg phosphate/L which is still 1.6 times lower than the concentration in Zarrouk medium. Beetroot peel does not contain any detectable phosphate. The highest phosphate concentration has been found in the extract out of the first batch of dried brewers grains with 450.7 mg/L, which is over 1.6 times higher than in the Zarrouk medium. Due to an obviously high deviation between different batches, the second batch, which was used for further cultivations in the photobioreactor, showed a three times lower phosphate concentration of 184.1 mg/L which is 1.5 times lower than in Zarrouk medium. Acid hydrolysis did not increase phosphate release significantly, but forces dissolution of other ions, getting to high for successful cultivation.
The magnesium concentration of the extract out of the second batch of dried brewers grains was with 80.3 mg/L over 4.5 times higher than in the Zarrouk medium. The high concentrations of phosphate, magnesium and calcium in brewers grains extracts and walnut press cake extracts offer the possibility of partial or entire replacement of these ions in the modified Zarrouk medium. Furthermore, potassium could be completely replaced through walnut press cake extract boiled in wet state at pH 11. Thus, walnut press cake extracts are as promising for the cultivation with A. platensis as brewers grains for further investigation.
The glucose concentration in the extract of dried brewers grains after acid hydrolysis was with 0.27 g/L glucose about 6 times higher than the same material without the acid hydrolysis (see ). This means, the hydrolysed extract offers A. platensis sugar as an additional carbon source besides carbon dioxide. Brewers grains are expected to contain 1 to 12 wt% of starch and around 2 wt% of glucose (Jin et al., Citation2022). Regarding these values, for the extract under investigation can contain 5.4 g/L starch and 0.9 g/L glucose maximum. Consequently, extraction conditions do not allow an efficient transition of glucose and hydrolysis is not complete. Most likely, starch and glucose stored in cells of the material and get in a very low percentage accessible for extraction or acid hydrolysis.
The microwave digestion of brewers grain seems to be the most effective extraction method and shows the high potential of brewers grain extracts to supply nutrients for A. platensis.
Cultivation of arthrospira platensis in shaking flasks and (modified) Zarrouk medium for studies on growth limitations
In Zarrouk medium the magnesium concentration is 17.46 mg/L and the phosphate concentration is 273.0 mg/L (). Due to literature, at least in one metabolism pathway both elements interact:
In cyanobacteria, magnesium is a central ion in chlorophylls and the flux of magnesium in chloroplasts across the thylakoid membrane is required for counterbalancing the light-induced generation of a pH gradient across the thylakoid membrane (Pohland & Schneider, Citation2019). The transport occurs via the Mg2+ channels CorA and/or MgtE, which facilitate the transmembrane Mg2+ flux down the electrochemical gradient (Pohland & Schneider, Citation2019).
Photosystem II contains P680, a chlorophyll-protein complex with an absorption maximum of 680 nm. Photosystem II oxidizes water molecules and transports the energized electrons over a series of carriers, whereby adenosine triphosphate (ATP) can be produced (Sadava et al., Citation2019), if enough phosphate is available.
All extracts contain a ratio of magnesium and phosphate deviating from Zarrouk conditions, like for example a magnesium concentration of 80.3 mg/L and a phosphate concentration of 184.1 mg/L in the extract of one batch of dried brewers grains. Three cultivations were performed to investigate the influence of excess of magnesium and the lack of phosphate on the growth behavior of A. platensis:
Cultivation with 80 mg/L Magnesium and 80 mg/L Phosphate in Zarrouk Medium
In this cultivation A. platensis was cultivated in triplicates with 80 mg/L magnesium (red triangles in ) as well as with 80 mg/L phosphate (green crosses in ) in modified Zarrouk medium. Positive control (blue dots in ) has been a cultivation in standard Zarrouk medium ().
Figure 3. OD680 of A. platensis during 15 d of cultivation in Zarrouk medium cultivation 1 (blue circles), Zarrouk medium with 80 mg/L magnesium cultivation 2 (red triangles) and Zarrouk medium with 80 mg/L phosphate cultivation 3 (green crosses). Error bars show the standard deviation of threefold determination.
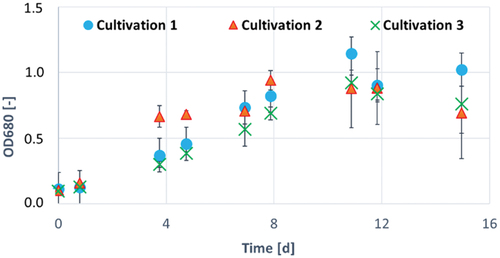
gives the growth of A. platensis by optical density at 680 nm during the cultivation in the three different media. The standard deviation of all cultivations increased strongly at about 7 d caused by clumping of the cells. This forming of cells clumps might be caused by insufficient gas exchange in shaking flask cultivation. It could be possible, that at a certain cell concentration the dissolved CO2 is too low and therefore, the photosynthesis does not proceed optimally. With increasing cell concentration, the level of oxygen rises due to the photosynthesis process. Due to the insufficient gas exchange in shaking flasks, the oxygen cannot evaporate and inhibits the growth of A. platensis or leads to cell death (Das, Citation2015; Richmond & Hu, Citation2013).
shows that the limited phosphate supply flattens and reduces the growth in the second decimal place of OD within the first 90 h. Resulting, the calculated maximal growth rate is the lowest and the doubling time is the longest compared with the other cultivation approaches ().
Table 5. Cell dry concentration, maximal specific growth rate μmax and doubling time td of A. platensis during cultivation in modified and standard Zarrouk medium. The maximal specific growth rate μmax and doubling time td were calculated between 19 h and 90 h.
In contrast, a fourfold excess of magnesium nearly doubles the OD in the same period (). It stands to reason that the high availability of magnesium in this cultivation leads to its higher uptake and a higher production of chlorophyll-a in photosystem II. The result might be a higher optical density at 680 nm and a possible increase of ATP production in the first 90 h of cultivation (Sadava et al., Citation2019). Furthermore, the cultivation with 80 mg/L magnesium has the highest maximal growth rate µmax and the shortest doubling time td within 261 h of cultivation (), confirming the assumption that an excess of magnesium ensures faster growth of A. platensis. However, the cell dry concentration is not significantly higher than in the other cultivation approaches after 114 h and 261 h (compare ), what leads to the assumption that the chlorophyll-a concentration is clearly higher, but its production might not be linear to the cell growth. Due to the maximal growth rate, which is calculated with the absorption values at 680 nm, this possible nonlinearity must be considered.
Table 6. Cell dry concentration, maximal specific growth rate μmax and doubling time td of A. platensis during cultivation in modified and standard Zarrouk medium. The maximal specific growth rate μmax and doubling time td were calculated between 0 h and 69 h.
Table 7. Cell dry concentration, maximal specific growth rate μmax and doubling time td of A. platensis during cultivation in modified and standard Zarrouk medium with 80 mg/L Mg2+ and 160 mg/L PO43-. The maximal specific growth rate μmax and doubling time td were calculated between 74 h and 99 h.
During cultivation, the magnesium concentration of the cultivation with 80 mg/L magnesium decreased about 75% until the end of cultivation (). The negative control also has a decrease of magnesium concentration, which is around 50%. So the theoretical calculated uptake of magnesium of A. platensis is about 20 mg/L. The significantly weakened behaviour represents changes in solubilty.
Figure 4. Magnesium (a) and phosphate (b) concentration during 15 d of cultivation of A. platensis in Zarrouk medium cultivation 1 (blue circles), Zarrouk medium with 80 mg/L magnesium cultivation 2 (red triangles), Zarrouk medium with 80 mg/L phosphate cultivation 3(green crosses) and Zarrouk medium with 80 mg/L magnesium as cultivation 2 negative control (without culture of A. platensis) (purple squares). Error bars show the standard deviation of threefold determination.
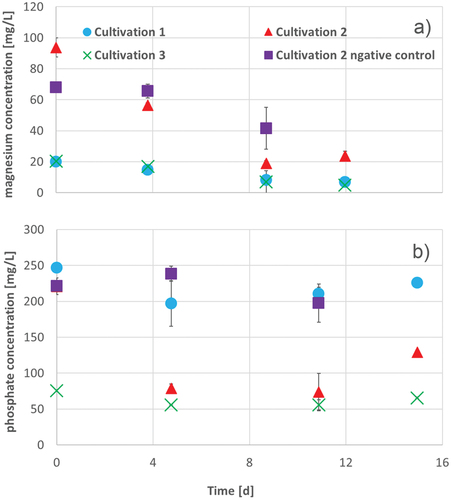
This result shows that A. platensis is able to transport and store a high amount of magnesium in the cell, which is in good agreement to literature suggesting that cyanobacteria can notice nutrient fluctuations for a few hours, which allows them to adjust the growth rate to the phosphate availability (Wagner et al., Citation1995).
Therefore, it can be assumed that the growth rate of A. platensis decreased, because of the lower phosphate concentration in the medium.
The phosphate concentration during cultivation in enriched medium decreased strongly about 150 mg/L as shown in , while the phosphate concentration during standard cultivation decreased about 20 mg/L, which explains the slower cell growth during cultivation. The magnesium concentration decreases similar to the decrease of magnesium in Zarrouk medium (), so there might be no influence of magnesium uptake by a lack of phosphate in the medium.
The corresponding negative control has not shown any significant decrease of phosphate. This means, first, there must be a high phosphate uptake of A. platensis in the cultivation with the excess of magnesium fitting to influence of magnesium described by Pohland and Schneider (Citation2019) on the eventual increase of chlorophyll-a concentration and its resulting ATP production. Secondly, the high uptake of phosphate is at least in part caused by the ability of cyanobacteria to store phosphate predominantly in the form of polyphosphate, which could be broken when phosphate is not available in medium (Kulaev & Vagabov, Citation1983; Stewart & Alexander, Citation1971). At the end of the cultivation, the phosphate concentration in the medium increased, which possibly results in cell death, whereby the stored polyphosphate might be broken, and the phosphate is released in the medium.
Cultivation with 160 mg/L Magnesium and 160 mg/L Phosphate in Zarrouk medium
In this cultivation a tenfold excess of magnesium and 60% of phosphate in Zarrouk medium were investigated. It was cultivated in triplicates, with stardard Zarrouk medium as positive control. As a negative control, two shaking flasks with 160 mg/L magnesium in Zarrouk medium were also performed without culture of A. platensis (purple squares in ).
describes the temporal progession of optical density during the cultivation of A. platensis in the three different media. Again, standard deviation after 171 h increased caused by clumping of the cells, probably caused by insufficient gas exchange in shaking flask cultivation.
Figure 5. OD680 of A. platensis during 17 d of cultivation in Zarrouk medium cultivation 1 (blue circles), Zarrouk medium with 160 mg/L magnesium cultivation 2 (red triangles) and Zarrouk medium with 160 mg/L phosphate cultivation 3 (green crosses). Error bars show the standard deviation of threefold determination.
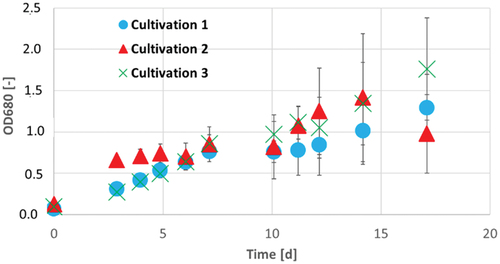
The cultivation of A. platensis with an excess of 160 mg/L magnesium had a notable stronger increase of OD680 in the first 96 h. The increase of OD is comparable with the cultivation with 80 mg/L magnesium (see ). The cultivations of A. platensis with 160 mg/L phosphate and with Zarrouk medium had a quite similar increase of OD in the first 171 h. Therefore, it can be assumed that the limitation of phosphate does not have a strong influence in contrast to the higher phosphate limitation with 80 mg/L. After 171 h of cultivation, the cultivation with 160 mg/L phosphate had a stronger increase of OD than the Zarrouk medium and a higher maximum OD at the end of cultivation (see ). Both, the cultivation with 80 mg/L magnesium and the cultivation with 160 mg/L magnesium had the highest maximal growth rates µmax and doubling times td compared with the other cultivations (see and ). The influence of an oversupply of magnesium regarding the OD is already explained above.
shows the magnesium concentration over the cultivation time. The decrease of the magnesium concentration during cultivation was quite similar to that in Zarrouk medium. The determined magnesium concentration of the cultivation with 160 mg/L magnesium dropped strongly to 27.67 mg/L. However, the concentration of the negative control also decreased but only to a value of 70 mg/L (see ). Therefore, the theoretical calculated uptake of magnesium of A. platensis is about 40 mg/L, which is twice as high as in the cultivation with 80 mg/L magnesium. The magnesium, which was not absorbed by A. platensis but precipitated as the negative control implies.
Figure 6. Magnesium (a) and phosphate (b) concentration during 17 d of cultivation of A. platensis in Zarrouk medium cultivation 1 (blue circles), Zarrouk medium with 160 mg/L magnesium cultivation 2 (red triangles), Zarrouk medium with 160 mg/L phosphate cultivation 3 (green crosses) and Zarrouk medium with 160 mg/L magnesium cultivation 2 negative control (without culture of A. platensis) (purple squares). Error bars show the standard deviation of threefold determination.
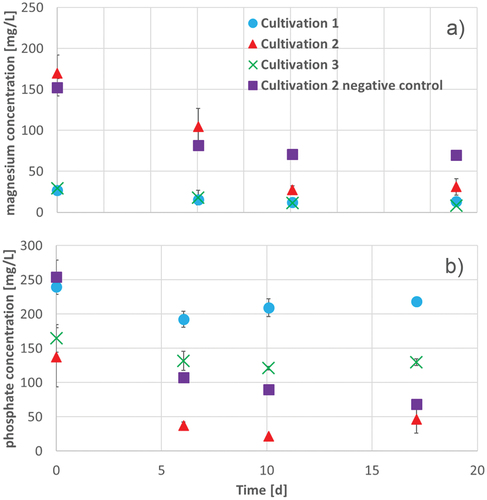
shows a significant decrease of the phosphate concentration within the first 242 h. The concentration at the beginning of the cultivation is about 100 mg/L lower than the expected concentration of 273 mg/L. Nevertheless, the standard deviation of this start value is also very high, so it can be expected that there were inaccuracies in the measurement or sample preparation.
At the end of the cultivation the phosphate concentration in the reduced approach increased to nearly the same concentration than in the negative control. This indicates again, A. platensis stores much phosphate intracellular by the oversupply and in case of cell death, the stress response is the release of phosphate. Also, the decrease of phosphate was quite similar to that in Zarrouk medium with about 30 mg/L. In comparison with Zarrouk medium, the cultivation with 160 mg/L phosphate had no noticeable effect on the cell growth and magnesium and phosphate uptake. Therefore, it is possible to cultivate A. platensis with a lack of phosphate.
Cultivation with the mixture of 80 mg/L magnesium and 160 mg/L phosphate in Zarrouk medium
Dried brewers grains extract boiled at pH 6 contained roughly 80 mg/L magnesium and 160 mg/L phosphate. The cultivation of A. platensis with 80 mg/L magnesium in Zarrouk medium had a higher increase of OD than in standard Zarrouk medium (see ). Furthermore, the cultivation with 160 mg/L phosphate in Zarrouk medium had no influence on the growth of A. platensis (see ). Consequently, the cultivation of A. platensis was performed in an Zarrok medium adapted to this range in triplicates. One shaking flask without cells serves as a negative control (purple squares in ). Again, the stronger clumping of the cells resulted in bigger error bars with enhanced cultivation time.
describes the optical density at 680 nm during the cultivation of A. platensis.
Figure 7. OD680 of A. platensis during 15 d of cultivation in Zarrouk medium with 160 mg/L phosphate and 80 mg/L magnesium (green circles). Error bars show the standard deviation of threefold determination.
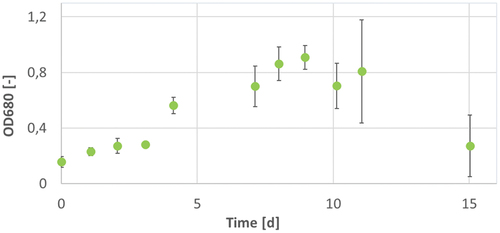
In the first 74 h of cultivation, the OD rose slowly (see ), this is comparable with the OD increase in cultivations with Zarrouk medium. After this, the OD increased significantly faster. The maximal growth rate was the highest of all performed shaking flask cultivations, whereas the doubling time was the shortest (). The course of magnesium and phosphate concentration during the cultivation was similar to the cultivations with 80 mg/L magnesium ().
In , the concentrations of magnesium and phosphate in the medium during the cultivation are described. The difference of magnesium concentration at the end of cultivation between the negative control and the approach with A. platensis is about 48 mg/L.
Figure 8. Magnesium (a) and phosphate (b) concentration during 15 d cultivation of A. platensis in Zarrouk medium with 160 mg/L phosphate and 80 mg/L magnesium cultivation 1 (green circles) and Zarrouk medium with 160 mg/L phosphate and 80 mg/L magnesium (without culture of A. platensis) as cultivation 1 negative control (purple squares) is described. Error bars show the standard deviation of threefold determination.
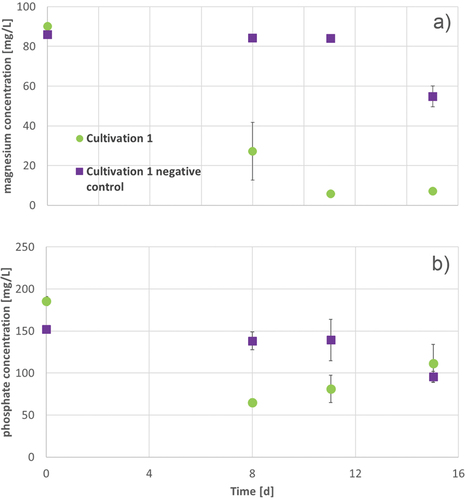
Concluding, it is possible for A. platensis to grow with a lack of phosphate and an excess of magnesium without inhibitory effects, but rather a faster increase of OD680 during cultivation. That arises the opportunity to cultivate A. platensis on brewers grains extract.
Cultivation of arthrospira platensis with brewers grains extract in shaking flasks
To develop a new medium from the food waste extract brewers grains, its 184.1 mg/L phosphate extracted at pH 6 out of dried material, served as only phosphate source. Other components were complemented to the concentration level of the Zarrouk medium () and first cultivation was performed in shaking flasks. To see the effects of temperature and light on the modified Zarrouk medium, three shaking flasks without cyanobacteria were investigated as negative control.
Because the medium was very turbid, the OD680 measurement was imprecise, but during cultivation the bright green color of A. platensis at the beginning turns into a dark green color (). Concluding, there must be cell growth or a noticeable increased concentration of chlorophyll in the cells, respectively. Therefore, a higher amount of chlorophyll might be forced of the turbid medium and the reduced number of photons, which reach the cells. More chlorophyll increases the capacity of light harvesting complexes under low light conditions. The increasing number of cells also could lead to the effect of more chlorophyll production, because of lower light availability. The reduction of light intensity can lead to an increase of 29% of total chlorophyll production, whereas the cellular growth shows the best results at a higher light intensity (Danesi et al., Citation2011). Nevertheless, it can be assumed that there was a substantial increase in cell concentration in the culture. Another indicator of cell growth is the completely consumption of glucose in the first 44 h of cultivation of A. platensis (), which has been sucessfully double checked with negativ control.
Cultivation of arthrospira platensis with Zarrouk medium in photobioreactors
According to the results of shaking flask investigation on growth, it is worthful to continue the cultivation in the same medium mixture, but on bigger scale for more representative sampling. The use of the photobioreactor allows to take more sample volume in higher frequency and simultanously better control and regulation. Especially, the influence of evaporation was calculated, and the determined values were corrected to evaporation. Furthermore, in the photobioreactors the active gassing of the medium provides a better gas exchange, what offers the possibility of longer-term cultivation with higher reachable OD values and cell dry concentrations. Furthermore, clumping only took place in shaking flasks but not in photobioreactors. Three reactors were operated paralelly in two cultivation series for standard deviation assessment.
In , the OD680 (A) and the dry mass concentration (B) during cultivation are presented. The progress of dry mass concentration increased until about 500 h and decreases then slowly (see ). In contrast, the OD680 increased till the end of cultivation and no significant decrease has been determined (see ). Eventually, the increase of OD is due to the higher chlorophyll-a production, caused by the high cell concentrations and the resulting lower light irradiation. Compared to the shaking flasks cultivation of A. platensis with Zarrouk medium, the cultivations in photobioreactors has reached a substantially lower maximum growth rate and a longer doubling time. A reason for the slower growth in photobioreactors could be the 5°C lower cultivation temperature of about 25°C, which was hold to depress water evaporation. The optimal growth temperature of A. platensis lies between 30°C and 35°C (De Oliveira et al., Citation1999). Aruna and Ravindran described an optimal growth temperature of A. platensis between 35°C and 37°C (Aruna & Ravindran, Citation2008).
Figure 11. OD680 (a) and dry mass concentration (b) in two cultivation series of A. platensis during 30 (cultivation 1) and 32 d (cultivation 2) of cultivation in Zarrouk medium. Error bars show the standard deviation between the three reactors operated in parallel.
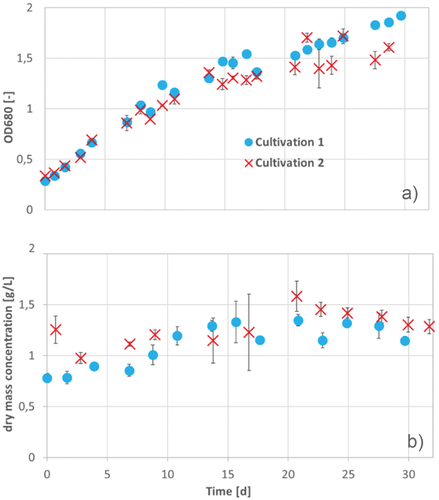
Furthermore, the ion concentrations during cultivations were determined ().
Figure 12. Magnesium (a), iron (b) nitrate (c), and phosphate (d) concentrations during 30 d (cultivation 1) and 32 d (cultivation 2) of two cultivation series of A. platensis in Zarrouk medium.
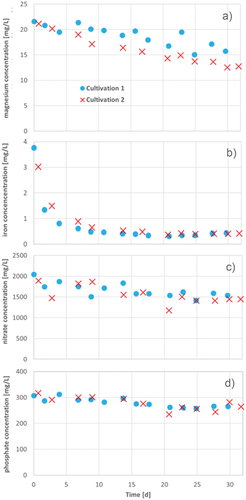
During cultivation, the iron concentration decreased strongly in the first 200 h and almost has been completely consumed at the end of cultivation (see ). Iron is an important factor for the chlorophyll-a production. The deficiency of iron causes chlorosis, which decreases chlorophyll-a and heme function and represses the porphyrin biosynthesis. Furthermore, the structures of nitrogen assimilatory enzymes possess iron as a cofactor (Esen & Oztur, Citation2014). The nitrate concentration decreased about 25% and the consumed iron is an important source for A. platensis to assimilate nitrate with iron containing enzymes.
The magnesium concentration decreased during cultivation between 27 wt% and 40 wt% (see ). The relative high magnesium uptake occurs via the Mg2+ channels CorA and / or MgtE, which facilitate the transmembrane Mg2+ flux down the electrochemical gradient (Pohland & Schneider, Citation2019). Magnesium is a central ion in chlorophylls and its flux in chloroplasts is required for counterbalancing the light-induced generation of a ΔpH across the thylakoid membrane (Pohland & Schneider, Citation2019). Consequently, magnesium is very important for photosynthesis and the resulting production of ATP, so the requirement of up to 40% of magnesium is confirmed.
The nitrate concentration decreased about 25% of its initial concentration. After the uptake of nitrate in the cell, it is reduced by two enzymes to nitrite. The resulting ammonium is catalyzed by glutamine synthetase and secondly by glutamate synthase. Glutamine and glutamate are the starting points for the synthesis of important organic nitrogen compounds like amino acids, nucleotides, chlorophylls, polyamines and alkaloids (Esen & Oztur, Citation2014). The organism A. platensis has a very high protein content of about 55% to 70% (Richmond & Hu, Citation2013) and therefore requires sufficient amounts of nitrate for amino acid and protein synthesis.
Cultivation of arthrospira platensis with brewers grains extract in photobioreactors
The cultivation of A. platensis in brewers grains extract was transferred from shaking flask to photobioreactors. Again, the phosphate source was completely replaced by the extract and other limited components in the extract were added to get the same composition as the Zarrouk medium (). The organism A. platensis was cultivated in triplicates in photobioreactors with brewers grains extract. For the illumination, two reflector modules were used per photobioreactor. There was also performed an approach for the negative control without the organism (see , purple squares).
Figure 13. OD680 (a) and dry mass concentration (b) in three cultivation series of A. platensis during 30–32 d of cultivation 1 (blue circles), cultivation 2 (red triangles), cultivation 3 (green crosses) and the negative control (purple squares) in photobioreactors with brewer’s grains extract. Error bars show the standard deviation of threefold determination.
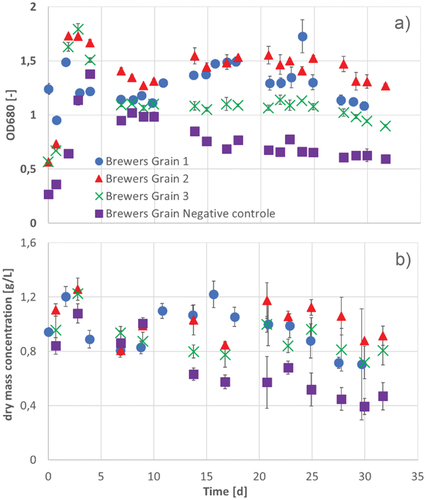
During the cultivation of A. platensis in brewers grains extract, OD680 and the dry mass concentration were determined ().
During cultivation, the turbidity of the medium decreased in the first 95 h. As a result, the OD680 rapidly increased during the first 100 h of cultivation in all approaches. Additionally, the dry mass concentration of all approaches rose in the first 95 h (). In the same period of time, the negative control formed white flakes in the medium, which precipitated and accumulated at the sampling and gassing pipes and formed crystals, so the medium became clearer. So OD and dry mass concentration of the negative control decreased constantly. It could be determined, that a large part of the calcium in the negative control decreased during cultivation (). The calcium concentration in the brewers grains extract is noticeable higher, than in Zarrouk medium and other added components could possibly trigger the precipitation of calcium. In contrast, in all approaches with A. platensis an increase in OD and dry mass concentration was detected after 165 h. Possibly, the organism needs about 165 h to use the brewers grains extract and its components before it starts to grow. Compared to the cultivation of A. platensis in photobioreactor with Zarrouk medium, this cultivation had a lower growth regarding the OD and dry mass concentration. The turbidity of the brewers grains extract medium might inhibit the light transmission and consequently the photosynthesis. Furthermore, foam formation of the brewers grains at the beginning of the cultivation carried the cells to the surface, where they probably dehydrate or have no nutrients. The foam probably is formed by proteins under the gassing in the brewers grains extract (Wilhelmson et al., Citation2009).
Figure 14. Magnesium (a) potassium (b) iron (c) and calcium (d), concentrations during 30 d − 32 d in three cultivation series of A. platensis: cultivation 1 (blue circles), cultivation 2 (red triangles), cultivation 3 (green crosses) and the negative control (purple squares) in Zarrouk medium.
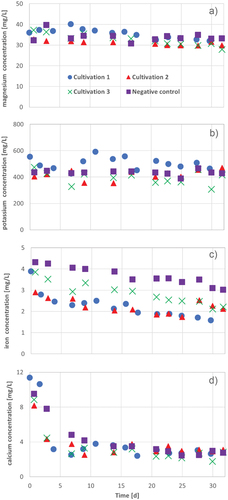
Compared with the negative control, there was a significant decrease of iron, magnesium and potassium in the approaches with A. platensis cultivated in brewers grains extract ().
The uptake of iron and magnesium can be explained by the increase of OD and chlorophyll concentration during the cultivation of A. platensis. Magnesium and iron are very important for the formation of chlorophyll-a in A. platensis (Esen & Oztur, Citation2014; Pohland & Schneider, Citation2019).
presents the determined anion concentrations of the cultivation. The analyzed anions were chloride, nitrate, phosphate and sulfate. The nitrate concentration in the cultivations with A. platensis decreased more than in the negative control. Furthermore, nitrate is essential for the synthesis of important organic nitrogen compounds like amino acids, nucleotides, chlorophylls, polyamines, and alkaloids (Esen & Oztur, Citation2014).
Figure 15. Nitrate (a) and phosphate (b) concentrations during 30 d − 32 d in three cultivation series of A. platensis: cultivation 1 (blue circles), cultivation 2 (red triangles), cultivation 3 (green crosses) and the negative control (purple squares) in Zarrouk medium.
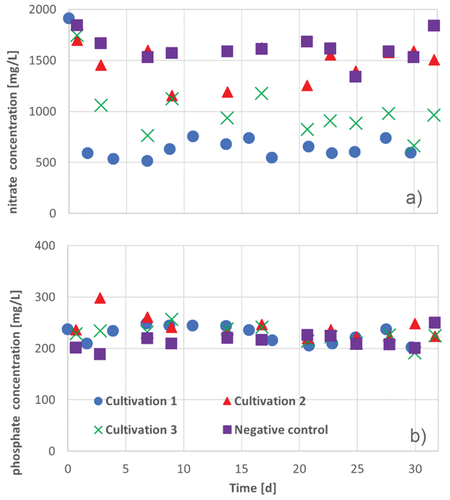
However, in all cultivation approaches, there was no significant decrease in phosphate concentration (see 12 B and ). Due to the low formation of biomass, probably only low amounts of phosphate are required for the synthesis of e.g. ATP, DNA or cell wall formation. It is possible, that the synthesis of ATP was inhibited through the lower photon flux and the consequently ineffective photosynthesis caused by the turbid medium.
The glucose concentration during cultivation decreased in all approaches (see ). The organism was able to take up all the containing glucose in the medium. However, in the negative control a microbial contamination was found, which consumed the glucose. The microbial contamination was detected by plating the negative control on LB (lysogeny broth) agar plates, were three different colony morphologies could be seen after an incubation of 24 h at 30°C.
The increase of the chlorophyll concentration is comparable to the increase of OD during the cultivation. The organism probably has to adapt to the medium and the light availability, so there has been no chlorophyll production at the beginning of the cultivation. After that, the organism adapted to the medium and the chlorophyll concentration increased.
Conclusions
The food waste extracts out of walnut press cake and brewers grains showed high amounts of magnesium, calcium and phosphate and therefore have a good potential for the cultivation of A. platensis. Beetroot peel extract has no identifiable amount of phosphate and nitrate and was insufficient for the cultivation of A. platensis. Dried brewers grains has the best characteristics in terms of its higher ion concentration in extracts, such as magnesium, calcium and phosphate, better handling and durability. In this study, it was possible to totally replace phosphate as well as magnesium and calcium in the cultivation medium by brewers grains extract. Other investigated ions can be partly substituted by brewers grains extract. Because of high magnesium amounts in brewers grains extract and lower phosphate concentrations compared to Zarrouk medium, the regarding growth of A. platensis was investigated. Thereby, A. platensis shows no growth inhibition during cultivation in Zarrouk medium with a lack of 160 mg/L phosphate and an excess of 80 mg/L magnesium. Furthermore, an oversupply of magnesium seems to increase the growth rate of A. platensis and could be related to a higher production of chlorophyll-a (Danesi et al., Citation2011). A. platensis is also able to absorb and metabolize glucose as an additional carbon source and shows a better cell growth with 68% higher biomass production 1.9 g/L vs. 1.3 g/L within 16 days. It was successfully proved, that A. platensis is able to grow on modified brewers grains extract. However, the determined growth is verifiable lower than the growth in well-established Zarrouk medium (1.2 g/L biomass vs. 1.5 g/L biomass in Zarrouk medium).
The growth in photobioreactors provides the opportunity to reach higher cell concentrations without cell clumping (1.5 g/L vs. 1.3 g/L biomass in shaking flasks). Due to that, the investigated gas supply system ensures an optimal mixing and availability of carbon dioxide and outgassing of produced oxygen. Furthermore, the use of light reflectors and therefore the higher light distribution triggers a substantially faster cell growth, by which the biomass yield increased from 1.3 g/L to 1.6 g/L within 22 d.
Acknowledgement
We kindly thank Prof. Dr. Breithaupt from the company Hengstenberg GmbH & Co. KG (Esslingen) for giving us the opportunity to investigate beetroot peel. We further thank Mr. Kerner, owner of the Erlenbacher Ölmühle (Erlenbach) for the walnut press cake sample as well as Mr. Verdi from Gold Ochsen GmbH (Ulm) for provided us two batches of brewers grains. Their support is highly acknowledged.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aruna, S., & Ravindran, A. (2008). Cultivation of spirulina sp. using organic substrates. Journal of Pure & Applied Microbiology, 2(2), 483–14.
- Ashby, M. K., & Houmard, J. (2006). Cyanobacterial two-component proteins: Structure, diversity, distribution, and evolution. Microbiology and Molecular Biology Reviews, 70(2), 472–509. https://doi.org/10.1128/MMBR.00046-05
- Badri, H., Monsieurs, P., Coninx, I., Nauts, R., Wattiez, R., Leys, N., & Battista, J. R. (2015). Temporal gene expression of the cyanobacterium Arthrospira in response to gamma rays. PLoS One, 10(8), e0135565. https://doi.org/10.1371/journal.pone.0135565
- Borowitza, M. (2013). High-value products from microalgae—their development and commercialisation. Journal of Applied Phycology, 25(3), 743–756. https://doi.org/10.1007/s10811-013-9983-9
- Chethana, S., Nayak, C. A., Madhusudhan, M., & Raghavarao, K. S. (2015). Single step aqueous two-phase extraction for downstream processing of C-phycocyanin from Spirulina platensis. Journal of Food Science and Technolology, 52(4), 2415–2421. https://doi.org/10.1007/s13197-014-1287-9
- Danesi, E. D., Rangel-Yagui, C. O., Sato, S., & Monteiro de Carvalho, J. C. (2011). Growth and content of Spirulina platensis biomass chlorophyll cultivated at different values of light intensity and temperature using different nitrogen sources. Brazilian Journal of Mikrobiology, 42(1), 362–373. https://doi.org/10.1590/S1517-83822011000100046
- Das, D. (2015). Algal biorefinery: An integrated approach. Springer.
- De Oliveira, M., Monteiro, M., Robbs, P., & Leite, S. (1999). Growth and chemical composition of Spirulina maxima and Spirulina platensis biomass at different temperatures. Aquaculture International, 7(4), 261–275. https://doi.org/10.1023/A:1009233230706
- Delattre, C., Pierre, G., Laroche, C., & Michaud, P. (2016). Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnology Advances, 34(7), 1159–1179. https://doi.org/10.1016/j.biotechadv.2016.08.001
- Egger, R. and Stuppner H. (1996), Application of capil lary zone electrophoresis to the analysis of betalains from Beta vulgaris. J. Chromatogr. A 735, p. 409–413.
- Eriksen, N. (2008). Production of phycocyanin—A pigment with applications in biology, biotechnology, foods and medicine. Applied Microbiology and Biotechnology, 80(1), 1–14. https://doi.org/10.1007/s00253-008-1542-y
- Esen, M., & Oztur, U. (2014). Ammonium nitrate and iron nutrition effects on some nitrogen assimilation enzymes and metabolites in Spirulina platensis. Biotechnology and Applied Biochemistry, 62(2), 275–286. https://doi.org/10.1002/bab.1268
- Essien, J., & Udotong, I. (2008). Amino acid profile of biodegraded brewers spent grains. Journal of Applied Sciences and Environmental Management, 12(1), 109–111. https://doi.org/10.4314/jasem.v12i1.55582
- Fernández-Rojas, B., Hernández-Juárez, J., & Pedraza-Chaverri, J. (2014). Nutraceutical properties of phycocyanin. Journal of Functional Foods, 11, 375–392. https://doi.org/10.1016/j.jff.2014.10.011
- Gustavsson J., Cederberg C., Sonesson U., van Otterdijk R., Meybeck A. Food and Agriculture Organization of the United Nations (FAO); Rome, Italy: 2011. Global Food Losses and Food Waste– Extent, Causes and Prevention.
- Gòdia, F., Albiol, J., Montesinos, J. L., Pérez, J., Creus, N., Cabello, F., Mengual, X., Montras, A., & Lasseur, C. (2002). MELISSA: A loop of interconnected bioreactors to develop life support in space. Journal of Biotechnology, 99(3), 319–330. https://doi.org/10.1016/S0168-1656(02)00222-5
- Hayashi, T., Hayashi, K., Maeda, M., & Kojima, I. (1996). Calcium Spirulan, an inhibtor of enveloped virus replication, from blue-green alga Spirulina platensis. Journal of Natural Products, 59(1), 83–87. https://doi.org/10.1021/np960017o
- Hendrickx, L., De Wever, H., Hermans, V., Mastroleo, F., Morin, N., Wilmotte, A., Janssen, P., & Mergeay, M. (2006). Microbiol ecology of the closed artificial ecosystem MELiSSA (micro-ecological life support system alternative): Reinverting and compartmentalizing the Earth’s food and oxygen regeneration system for long-haul space exploration missions. Research in Microbiology, 157(1), 77–86. https://doi.org/10.1016/j.resmic.2005.06.014
- Hu, F., Stampfer, M., Manson, J., Rim, E., Colditz, G., Rosner, B., Speizer, F., Hennekens, C., & Wilett, W. (1998). Frequent nut consumption and risk of coronary. BMJ, 317(7169), 1341–1345. https://doi.org/10.1136/bmj.317.7169.1341
- Huang, Y., Cunnane, S., Horrobin, D., & Davignon, J. (1982). Most biological effects of zinc deficiency corrected by γ-linolenic acid (18: 3ω6) but not by linolenic acid (18: 2ω6). Atherosclerosis, 41(2–3), 193–207. https://doi.org/10.1016/0021-9150(82)90185-X
- Huige, N. (1994). Brewery by-products and effluents. In W. Hardwick (Hrsg.), Handbook of Brewing CRC Press. (p. 501–550).
- Iwata, K., Inayama, T., & Kato, T. (1990). Effects of Spirulina platensis on plasma lipoprotein lipase activity in fructose-induced hyperlipidemic rats. Journal of Nutritional Science and Vitaminology, 36(2), 165–171. https://doi.org/10.3177/jnsv.36.165
- Jin, Z., Lan, Y., Ohm, J.-B., Gillespie, J., Schwarz, P., & Chen, B. (2022). Physicochemical composition, fermentable sugars, free amino acids, phenolics, and minerals in brewers’ spent grains obtained from craft brewing operations. Journal of Cereal Science, 104, 103413. https://doi.org/10.1016/j.jcs.2022.103413
- Khidzir, K. M., Noorlidah, A., & Agamuthu, P. (2010). Brewery spent grain: Chemical. Malaysian Journal of Science, 29(1), 41–51. https://doi.org/10.22452/mjs.vol29no1.7
- Koru, E. (2011). Earth food Spirulina (Arthrospira): Production and quality standards. Y. El-Samragy.
- Krishnan, R., Agarwal, R., Bajada, C., & Arshinder, K. (2020). Redesigning a food supply chain for environmental sustainability - an analysis of resource use and recovery. Journal of Cleaner Production, 242, 118374. https://doi.org/10.1016/j.jclepro.2019.118374
- Kulaev, I. S., & Vagabov, V. M. (1983). Polyphosphate metabolism in micro-organisms. Advances in Microbial Physiology, 24, 83–171.
- Lau, Ryan & Pleissner, Daniel & Lin, Carol. (2014). Recycling of food waste as nutrients in Chlorella vulgaris cultivation. Bioresource technology. 170C. 144-151. https://doi.org/10.1016/j.biortech.2014.07.096.
- Leung, C. C., Cheung, A. S., Zhang, A. Y.-Z., Lam, F. K., & Lin, C. S. (2012). Utilisation of waste bread for fermentative succinic acid production. Biochemical Engineering Journal, 65, 10–15. https://doi.org/10.1016/j.bej.2012.03.010
- Morais, M., & Costa, J. (2007). Carbon dioxide fixation by chlorella kessleri, C. vulgaris, scenedesmus obliquus and Spirulina sp. Cultivated in flasks and vertical tubular photobioreactors. Biotechnology Letters, 29(9), 1349–1352. https://doi.org/10.1007/s10529-007-9394-6
- Mussatto, S. I. (2009). Biotechnolology for agro-industrial residue utilitsation (P. S. Nigam, & A. Pandey ed). Springer.
- Mussatto, S. I., Dragone, G., & Roberto, I. C. (2006). Brewers’ spent grain: Generation, characteristics (review). Journal of Cereal Science, 43(1), 1–14. https://doi.org/10.1016/j.jcs.2005.06.001
- Nayaka, N., Homma, Y., & Goto, Y. (1988). Cholesterol lowering effect of Spirulina. Nutrition Reports International, 37, 1329–1337.
- Nowicka-Krawczyk, P., Mühlensteinová, R., & Hauer, T. (2019). Detailed characterization of the Arthrospira type species separating commercially grown taxa into the new genus limnospira (Cyanobacteria). Scientific Reports, 9(649), 1–11. https://doi.org/10.1038/s41598-018-36831-0
- Nyhan, L., Sahin, A. W., Schmitz, H. H., Siegel, J. B., & Arendt, E. K. (2023). Brewers’ spent grain: An unprecedented opportunity to develop sustainable plant-based nutrition ingredients addressing global malnutrition challenges. Journal of Agricultural and Food Chemistry, 71(28), 10543–10564. https://doi.org/10.1021/acs.jafc.3c02489
- Parvin, M., Huntington, T., & Hasan, M. (2008). A Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish. Food and Agriculture Organization of the United Nations. Rome.
- Pelizer, L., Carvalho, J. C. M., Sato, S., & De Oliveira Moraes, I. (2002). Spirulina platensis growth estimation by pH determination at different cultivations conditions. Electronic Journal of Biotechnology, 5(3), 17–18. https://doi.org/10.2225/vol5-issue3-fulltext-8
- Pleissner, D., Kwan, T. H., & Lin, C. S. (2014). Fungal hydrolysis in submerged fermentation for food waste treatment and fermentation feedstock preparation. Bioresource Technology, 158, 48–54. https://doi.org/10.1016/j.biortech.2014.01.139
- Pohland, A.-C., & Schneider, D. (2019). Mg2+ homeostasis and transport in cyanobacteria – at the crossroads of bacterial and chloroplast Mg2+ import. Biological Chemistry, 400(10), 1289–1301. https://doi.org/10.1515/hsz-2018-0476
- Ramos, A., Acién, F. G., Fernández-Sevilla, J. M., Gonzáles, C. V., & Bermejo, R. (2011). Development of a process for large-scale purification of C-phycocyanin from synechocystis aquatilis using expanded bed adsorption chromatography. Journal of Chromatography B, 879(7–8), 511–519. https://doi.org/10.1016/j.jchromb.2011.01.013
- Richmond, A., & Hu, Q. (2013). Handbook of microalgal culture: Phycology and biotechnology. John Wiley & Sons, Inc.
- Robertson, B., & Andersen-Pinstrup, P. (2010). Global land acquisition: neo-colonialism or development opportunity? Food Security, 2(3), 271–283. https://doi.org/10.1007/s12571-010-0068-1
- Romay, C., González, R., Ledón, N., Ramirez, D., & Rimbau, V. (2003). C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Current Protein and Peptide Science, 4(3), 207–216. https://doi.org/10.2174/1389203033487216
- Sadava, D., Hillis, D., Heller, H. C., & Hacker, S. (2019). Photosynthese: Energie aus Sonnenlicht. In J. Markl (Hrsg.), Purves Biology (10 Ausg.). Springer.
- Schluchter WM, Glazer AN. Biosynthesis of phycobiliproteins in cyanobacteria. In: Peschek GA, Löffelhardt W, Schmetterer G, editors. The Phototrophic Prokaryotes. New York: Kluwer/Plenum Press; 1999. pp. 83–95.
- Stewart, W. D., & Alexander, G. (1971). Phosphorus availability and nitrogenase activity in aquatic blue‐green algae. Freshwater Biology, 1(4), 389–401. https://doi.org/10.1111/j.1365-2427.1971.tb01570.x
- Stuppner, R., & Egger, H. (1996). Application of capil lary zone electrophoresis to the analysis of betalains from beta vulgaris. Journal of Chromatography, A, 735(1–2), 409–413. https://doi.org/10.1016/0021-9673(95)00885-3
- Torres-Tiji, Y., Fields, F. J., & Mayfield, S. P. (2020). Microalgae as a future food source. Biotechnology Advances, 41, 107536. https://doi.org/10.1016/j.biotechadv.2020.107536
- USDA Food Composition Databases (2020): https://www.nal.usda.gov/human-nutrition-and-food-safety/food-composition(10.02.2020).
- Vonshak, A. (2002). Spirulina platensis (Arthrospira): Physiology, cell-biology, and biotechnology. Taylor & Francis.
- Wagner, F., Falkner, R., & Falkner, G. (1995). Information about previous phosphate fluctuations is stored via an adaptive response of the high-affinity phosphate uptake system of the cyanobacterium anacystis nidulans. Planta, 197(1), 147–155. https://doi.org/10.1007/BF00239951
- Wikfors, M., & Ohno, G. (2001). Impact of Algal research in aquaculture. Journal of Phycology, 37(6), 968–974. https://doi.org/10.1046/j.1529-8817.2001.01136.x
- Wilhelmson, A., Lehtinen, P., & von Weymarn, N. (2009). Future applications for brewers’ spent grain. New Food, 12, 59–61.
- Wruss, J., Waldenberger, G., Huemer, S., Uygun, P., Lanzerstorfer, P., Müller, U., Höglinger, O., & Weghuber, J. (2015). Compositional characteristics of commercial beetroot products. Journal of Food Composition and Analysis, 42, 46–88. https://doi.org/10.1016/j.jfca.2015.03.005
- Yashwant K., (2015). Beetroot: A super food. International Journal of Engineering Studies and Technical Approach. 1(3), p. 20–26.
- Zarrouk, C. (1966). Contribution a l’etude d’une Cyanophycee. Influence de divers facteurs physiques et chimiques sur la croissance et la photosynthese de Spirulina maxima [ PhD Thesis]. University of Paris.


![Figure 10. Glucose concentration during 424.5 h cultivation of A. platensis in brewer’s grains extract (blue circles). As a negative control the glucose concentration [g/L] of brewers’ grains extract without culture of A. platensis (orange crosses) is also shown.](/cms/asset/b4c2606e-823d-4ccb-9723-3f75a1264b01/tcyt_a_2331067_f0010_oc.jpg)