ABSTRACT
Fritillariae Cirrhosae Bulbus (FCB) is a Chinese medicine that has been used to treat lung diseases for thousands of years. This study aimed to investigate the protective effect and mechanism of four alkaloids (peimisine, ebeiedinone, imperialine and chuanbeinone) from FCB on cigarette smoke extract (CSE)-induced oxidative injury in A549 cells. The results showed that the four alkaloids could increase the levels of glutathione/oxidized glutathione (GSH/GSSG), superoxide dismutase (SOD) and heme oxygenase 1 (HO-1), decrease the levels of malondialdehyde (MDA) and reactive oxygen species (ROS), thereby alleviating oxidative injury in CSE-induced A549 cells. Furthermore, after treated with four alkaloids, the mRNA expression of HO-1, NQO-1, Nrf2 and SIRT1 were increased, while MST1 and FOXO3 were decreased in CSE-induced A549 cells, which was consistent with the results of western blotting. These results suggest that the mechanism of the four alkaloids may be related to the regulation of Nrf2/Keap1 and SIRT1/FOXO3a signaling pathways.
Introduction
Fritillaria is a perennial herb from the Liliaceae family, there are about 80 species in China and mainly distributed in Sichuan, Xinjiang, Gansu, Hubei, Anhui and other locations (Chen et al., Citation2019). Fritillariae Cirrhosae Bulbus (FCB) is a precious species of Fritillaria and famous genuine medicinal material in Sichuan Province. As a valuable traditional Chinese medicine, FCB has been used to treat lung diseases for thousands of years. Meanwhile, FCB is also widely used in the field of healthcare products and food because of its unique healthcare effect. Many studies have shown that FCB has a good effect on various lung diseases, such as chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, lung injury and asthma, etc (C. Liu et al., Citation2022; D. Wang et al., Citation2016; Yeum et al., Citation2007; Yuan et al., Citation2022). However, the specific active components and mechanism of FCB in treating lung diseases are still unclear. FCB contains alkaloids, terpenes, polysaccharides and steroids, etc. chemical components, among which alkaloids are the main active components (Xu et al., Citation2022). Previous studies have shown that four alkaloids (imperialine, chuanbeinone, verticinone and verticine) from FCB have antitussive, expectorant and anti-inflammatory effect (D. Wang et al., Citation2011), the six alkaloids (verticinone, verticine, imperialine-3-β-D-glucoside, delavine, imperialine and peimisine) from FCB could attenuate oxidative stress and inflammatory response in RAW 264.7 cells (S. Liu et al., Citation2020a, Citation2020b). The activity of ebeiedinone is less reported, with only reported anti-acetylcholinesterase activity in vitro (Lin et al., Citation2006). Overall, the activity of alkaloids from FCB has been less studied and still needs further investigation.
Oxidative stress is a pathological condition characterized by the disturbance of prooxidant-antioxidant balance and is considered as important indicator of disease and aging (Ornatowski et al., Citation2020; Zhuang et al., Citation2018). Meanwhile, oxidative stress is an important causative factor in various chronic lung diseases such as COPD, lung injury and asthma (Barnes, Citation2020; Chanda et al., Citation2019; Mishra et al., Citation2018; Saunders et al., Citation2022; Wang et al., Citation2022; Zhang et al., Citation2022). Smoking-induced oxidative stress is an important factor in the development of COPD, and ROS are important signaling molecules in redox homeostasis (Yi et al., Citation2022). When the organism is stimulated by various unfavorable factors in vivo and in vitro, ROS will be largely produced, leading to the oxidation of proteins, lipids and DNA in the lungs, which will result in lung tissue damage and an imbalance in the redox system (Ryter et al., Citation2019). Therefore, reducing the damage of oxidative stress is essential for the treatment of lung diseases.
Inflammation and oxidative stress are the main damage factors in lung disease, and previous studies by our research group have shown that four alkaloids (peimisine, ebeiedinone, imperialine and chuanbeinone) from FCB showed good anti-inflammatory activity in vitro. However, the antioxidant activity of alkaloids from FCB has been little studied and the mechanism is unclear, which still needs further investigation. Therefore, this study established the oxidative stress model by cigarette smoke extract (CSE)-induced A549 lung epithelial cells to investigate the antioxidant activity and mechanism of four different chemically structured alkaloids (peimisine, ebeiedinone, imperialine and chuanbeinone) from FCB. This study will provide the basis for the study of chemical composition characteristics, pharmacodynamic material basis and applications of FCB. Meanwhile, this study will also provide data support for the development of foods with healthcare effect and new drugs for the treatment of lung diseases.
Materials and methods
Chemicals and reagents
Peimisine, ebeiedinone, imperialine and chuanbeinone were purchased from Chengdu Must Bio-technology (Chengdu, China). The purity of all these substances was above 98%. Dimethylsulfoxide (DMSO), phenylmethylsulfonyl fluoride (PMSF) and phosphatase inhibitor were purchased from Solaribo Life Sciences (Beijing, China). RIPA buffer, reactive oxygen species (ROS) assay kit, glutathione (GSH) and oxidized glutathione (GSSG) assay kit, total superoxide dismutase (SOD) detection kit, malondialdehyde (MDA) detection kit, bicinchoninic acid (BCA) protein kit and DEPC water were purchased from Beyotime Biotechnology (Shanghai, China). Enzyme-linked immunosorbent assay kit of Heme oxygenase 1 (HO-1) was purchased from Wuhan ColorfulGene Biological Technology (Wuhan, China). TRIzol™ Reagent was purchased from Thermo Scientific (Waltham, U.S.A.). The anti-GAPDH and horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Affinity Biosciences (OH, U.S.A.). Anti-SIRT1, anti-Nrf2, anti-MST1, anti-FOXO3a and anti-Keap1 were purchased from Cell Signaling Technology (Boston, U.S.A.).
Cell culture
A549 cells were purchased from American Type Culture Collection (Manassas, VA, U.S.A.) and cultured in RPMI 1640 medium (Gibco, NY, U.S.A.) supplemented with 10% FBS (Gibco, NY, U.S.A.) and 1% penicillin-streptomycin (Grand Island, U.S.A.) at 37°C with 5% CO2.
CSE preparation
One Tianxiaxiu cigarette (Sichuan Zhongyan, China) was continuously smoked with a 50 mL syringe, and all the smoke was injected into 10 mL RPMI 1640 medium. After thorough shaking and mixing, the CSE solution was filtered with a sterile 0.22 μm needle filter, and the concentration of the CSE solution was set to 100% and used within 1 h.
Cell viability assay
A549 cells in the logarithmic growth phase were inoculated in 96-well plates at a density of 5 × 103 cells/well and cultured for 24 h. Then, the alkaloids-treated group cells were incubated with chuanbeinone, ebeiedinone, imperialine and peimisine at the indicated concentrations (1, 5, 10, 20, 40, 70, 100 μM) for 24 h. The control group cells were cultured with equal volumes of the RPMI 1640 medium and no less than four replicate wells for each group. After 24 h of incubation, 10 μL CCK8 (ApexBio, Houston, U.S.A.) was added to each well and incubated for 4 h. Then, the absorbance of each well was measured at 450 nm using a microplate reader (Bio-rad, Richmond, VA, U.S.A.). The cell viability was calculated according to the following formula: cell viability (%) = (A450 nm of treated cells/A450 nm of control cells) × 100. The effect of different concentrations of CSE (0%, 0.2%, 0.5%, 1%, 2%, 5%, 10%, 20%, 50%, 100%) on the viability of A549 cells was detected by the same method.
ROS detection
A549 cells were inoculated in 24-well plates at a density of 3 × 104 cells/well and cultured for 24 h to 70% confluence. Then the cells were incubated with different concentrations of CSE solution (0%, 0.2%, 0.5%, 1%, 2%, 5%, 10%) and three replicate wells for each concentration. After 24 h of incubation, the old culture medium was removed, and 250 μL of diluted 2’, 7’-dichlorofluorescein diacetate (DCFH-DA) (10 μM, diluted in RPMI 1640 medium) was added to each well and incubated for 20 min at 37°C in the dark. Then the cells were washed twice with PBS and collected, and fluorescence intensity was detected by flow cytometry (BD Biosciences, CA, U.S.A.) at an excitation wavelength of 488 nm and an emission wavelength of 525 nm. The average fluorescence intensity of 1 × 104 cells was analyzed. After 2% CSE-induced A549 cells were incubated with different concentrations of four alkaloids (5 μM, 10 μM, 20 μM) for 24 h, the fluorescence intensity of different treated groups was detected by the same method.
Determination of GSH, SOD, MDA and HO-1 levels
A549 cells in the logarithmic growth phase were inoculated in 6-well plates at a density of 3 × 105 cells/well and incubated for 24 h to 70% confluence. Then different concentrations of four alkaloids (5 μM, 10 μM, 20 μM) were incubated with 2% CSE-induced A549 cells for 24 h. The GSH, SOD and MDA levels were detected according to the manufacturer’s instructions of kits. The level of HO-1 in different treatment groups were detected by enzyme-linked immunosorbent assay kit.
Quantitative real-time PCR (qRT-PCR)
Different concentrations of four alkaloids (5 μM, 10 μM, 20 μM) were incubated with 2% CSE-induced A549 cells for 24 h, then the total RNA from A549 cells was extracted according to the instruction of the TRIzol reagent. The total RNA in each sample was quantified by the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, U.S.A.), and the A260/280 ratio of all samples was in the range of 1.8–2.0. The cDNA was obtained by reverse transcription from RNA samples with the RevertAid First Strand cDNA Synthesis kit (Thermo Scientific, Waltham, U.S.A.). And qRT-PCR was performed using the NovoStart SYBR qPCR SuperMix Plus kit (Novoprotein, Shanghai, China) on the CFX Connect™ RealTime PCR Detection System (BIO-RAD, CA, US). The PCR cycle was as follows: 95°C for 2 min, then 40 cycles of 95°C for 5 s and 60°C for 30 s, and 95°C for 5 s. The 2−ΔΔCt method was used to calculate the relative gene expression, and GAPDH was the reference gene. GAPDH (order NO. B662104) and other primers were purchased from Sangon Biotech (Shanghai, China), and the primer sequences are shown in .
Table 1. Primers for qRT-PCR.
Western blotting analysis
Total proteins from A549 cells were extracted using RIPA lysis buffer (RIPA: PMSF: phosphatase inhibitor = 100:1:1), and the total protein of each sample was quantified by the BCA protein kit. After adding the sample loading buffer, the protein samples were heated at 100°C for 10 min. Then equal amount of protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to the polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, U.S.A.). Subsequently, the membranes were blocked with 5% skim milk for 1 h and incubated with the primary antibodies (1:1000) at 4°C overnight. After being washed three times with TBST, the membrane was incubated with HRP-conjugated secondary antibodies (1:5000) for 1 h at room temperature. Afterward, the membrane was washed with TBST, and protein bands were detected by the BeyoECL Plus (Millipore, Billerica, MA, U.S.A.) on the GelView 5000Plus Gel Imaging System (Biolight, Guangzhou, China) and analyzed with ImageJ software.
Statistical analysis
The data were expressed as mean ± SEM. Multiple comparisons were performed using one-way ANOVA followed by the Bonferroni posttest. All analyses were performed using GraphPad Prism 7 (San Diego, CA, U.S.A.), and p < .05 was considered statistically significant.
Results
Chemical structure and cytotoxicity of four alkaloids
The four isosteroid alkaloids from FCB are classified into cevanine and jervine types according to their chemical structures (). The chemical structure of four alkaloids is shown in , namely, chuanbeinone (T), ebeiedinone (Q), imperialine (J) and peimisine (X). As shown in , after different concentrations of four alkaloids were incubated with A549 cells for 24 h, all four alkaloids had no significant effect on cell viability within 20 μM. Based on these results, all four alkaloids were administered at doses of 5 μM, 10 μM and 20 μM in subsequent experiments.
Figure 1. The skeleton types of alkaloids. (a) The different skeletons of alkaloids. (b) The chemical structures of four isosteroid alkaloids.
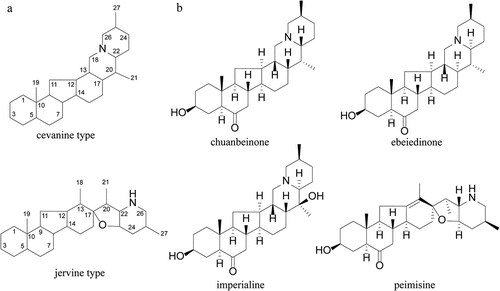
Figure 2. Effect of different concentrations of alkaloids and CSE on viability and ROS production in A549 cells. (A) The cell viability was detected by the CCK8 assay after incubation with 0–100 μM of alkaloids for 24 h. (B) The cell viability was detected by the CCK8 assay after incubation with 0–100% CSE for 24 h. (C) The level of ROS in A549 cells was detected by flow cytometry after incubation with 0–10% CSE for 24 h. T: chuanbeinone; Q: ebeiedinone; J: imperialine; X: peimisine. n = 3, *p < .05, **p < .01, ***p < .001 vs. The control group.
Determination of the intervention concentration of CSE
When the organism is stimulated by cigarette smoke, the prooxidant-antioxidant balance will be disturbed and lung epithelial cells are the primary target cells. Therefore, this study established the CSE-induced oxidative stress model in A549 lung epithelial cells. As shown in , CSE showed no significant effect on cell viability within 10% concentration and showed a dose-dependent increase in cytotoxicity at 20% and above. Therefore, subsequent experiments will further determine the optimal intervention concentration of CSE within the safe dose range. ROS are important signaling molecules in oxidative stress, different concentrations of CSE-induced groups all significantly increased the ROS level in A549 cells (p < .001) (). Based on the above results, the A549 cell oxidative stress model was induced by 2% CSE in this experiment for further study.
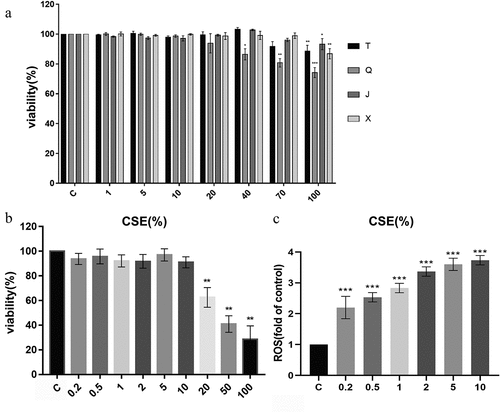
Effect of alkaloids on ROS production
As shown in , the average fluorescence intensity of the CSE group significantly increased compared with the control group (p < .01), indicating that the oxidative stress model was successfully established. Compared with the CSE group, the level of ROS was significantly decreased after the administration of all four alkaloids at 20 μM (p < .05, p < .01, p < .01, p < .01). The above results suggested that four alkaloids could alleviate CSE-induced oxidative stress in A549 cells by reducing intracellular ROS production. Overall, the results showed that the effect of decreasing the ROS production in A549 cells of the four alkaloids is shown as follows: peimisine > ebeiedinone > chuanbeinone > imperialine.
Figure 3. Effect of alkaloids on ROS production in 2% CSE-induced A549 cells. (a-b) the level of ROS in each group of cells was detected by flow cytometry after incubation with four different concentrations of alkaloids for 24 h. C: control; CSE: 2% CSE; T1-T3: 5, 10, 20 μM chuanbeinone; Q1-Q3: 5, 10, 20 μM ebeiedinone; J1-J3: 5, 10, 20 μM imperialine; X1-X3: 5, 10, 20 μM peimisine. n = 3, ##p < .01 vs. The control group, *p < .05, **p < .01, ***p < .001 vs. The CSE group.
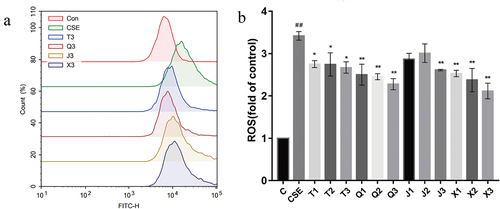
Effect of the four alkaloids on GSH/GSSG, SOD, MDA and HO-1 production in A549 cells
GSH is the main intracellular endogenous antioxidant and is a non-enzymatic antioxidant. As shown in , the GSH/GSSG level of the CSE group was significantly decreased compared with the control group (p < .05), indicating the intracellular antioxidant capacity decreased. All four alkaloid administration groups could effectively up-regulate the GSH/GSSG level compared with the CSE group. Overall, the results showed that the effect of increasing the GSH/GSSG level in A549 cells of the four alkaloids is shown as follows: chuanbeinone > peimisine > imperialine > ebeiedinone.
Figure 4. Effect of the four alkaloids on GSH/GSSG, SOD, MDA and HO-1 production in 2% CSE-induced A549 cells. (a-d) the levels of GSH/GSSG, SOD, MDA and HO-1 in 2% CSE-induced A549 cells were detected after incubation with four different concentrations of alkaloids for 24 h. C: control; CSE: 2% CSE; T1-T3: 5, 10, 20 μM chuanbeinone; Q1-Q3: 5, 10, 20 μM ebeiedinone; J1-J3: 5, 10, 20 μM imperialine; X1-X3: 5, 10, 20 μM peimisine. n = 3, #p < .05, ##p < .01 vs. The control group, *p < .05, **p < .01 vs. The CSE group.
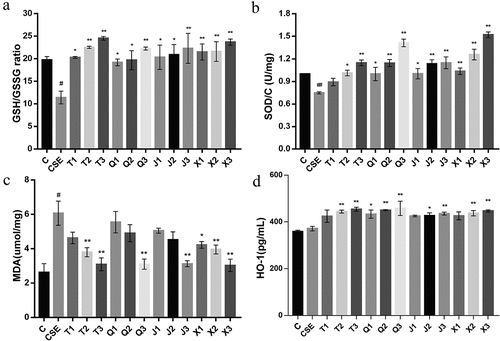
SOD is an essential part of antioxidant enzymes in biological systems. As shown in , the SOD level of CSE-induced A549 cells was significantly decreased (p < .01), and all administration groups could increase the SOD level in A549 cells except for the 5 μM chuanbeinone group. Overall, the results showed that the effect of increasing the SOD level in A549 cells of the four alkaloids is shown as follows: peimisine > ebeiedinone > imperialine > chuanbeinone.
The end product of the peroxidation reaction between oxygen radicals and polyunsaturated fatty acids is MDA, which will exacerbate cellular damage. As shown in , the level of MDA in CSE-induced A549 cells was significantly increased compared with the control group (p < .05), which will cause oxidative damage to the cells. And the MDA level was significantly decreased after the treatment of four alkaloids at 20 μM (p < .01). Overall, the results showed that the effect of decreasing the MDA level in A549 cells of the four alkaloids is shown as follows: peimisine > chuanbeinone > imperialine > ebeiedinone.
HO-1 is an important intracellular antioxidant enzyme that plays an antioxidant role by catalyzing heme decomposition. As shown in , the HO-1 level in CSE-induced A549 cells was significantly increased after the treatment of alkaloids in different concentrations, except for the 5 μM dose group of chuanbeinone, imperialine and peimisine. Overall, the results showed that the effect of increasing the HO-1 level in A549 cells of the four alkaloids is shown as follows: ebeiedinone > chuanbeinone > peimisine > imperialine.
Effect of alkaloids on the Nrf2/Keap1 signaling pathway
Effect of alkaloids on NQO1, HO-1 and Nrf2 gene transcription levels
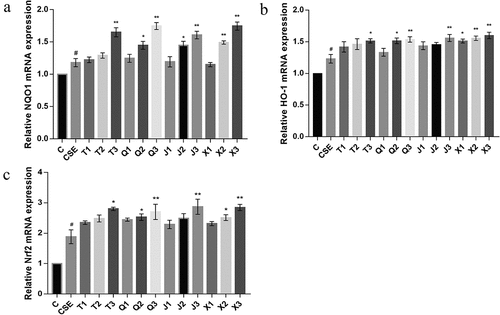
The effect of the four alkaloids on the expression of NAD(P)H quinone dehydrogenase 1 (NQO1), HO-1 and nuclear factor erythroid 2-related factor 2 (Nrf2) genes in CSE-induced A549 cells was detected by qRT-PCR. As shown in , the mRNA expression of NQO1 and HO-1 in the CSE group were significantly increased compared with the control group (p < .05). Compared with the CSE group, all four alkaloids at 20 μM could effectively increase the expression level of NQO1 and HO-1, among which peimisine showed the best effect. As shown in , the mRNA expression level of Nrf2 was significantly increased in CSE-induced A549 cells (p < .05), which was consistent with the mRNA expression of the antioxidant cytokines NQO1 and HO-1. And the expression of Nrf2 was significantly further increased after treatment with the four alkaloids at 20 μM (p < .05, p < .01, p < .01, p < .01).
Figure 5. Effect of four alkaloids on the expression of Keap1/Nrf2 signaling pathway-related genes in 2% CSE-induced A549 cells. (a-c) the mRNA expression levels of NQO1, HO-1 and Nrf2 in 2% CSE-induced A549 cells after incubation with four different concentrations of alkaloids for 24 h. C: control; CSE: 2% CSE; T1-T3: 5, 10, 20 μM chuanbeinone; Q1-Q3: 5, 10, 20 μM ebeiedinone; J1-J3: 5, 10, 20 μM imperialine; X1-X3: 5, 10, 20 μM peimisine. n = 3, #p < .05 vs. The control group, *p < .05, **p < .01 vs. The CSE group.
Effect of alkaloids on Nrf2 and Keap1 protein expression levels
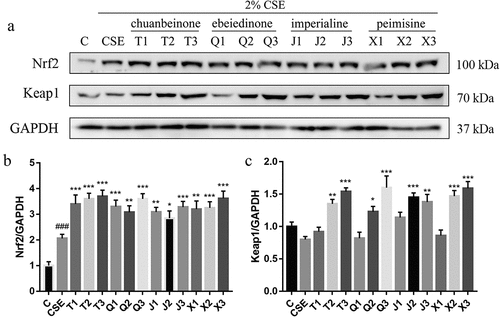
The effect of alkaloids on the expression of Nrf2/Keap1 signaling pathway-related protein in CSE-induced A549 cells was analyzed by western blotting. As shown in , the protein level of Nrf2 in the CSE group was significantly increased compared with the control group (p < .001). After the treatment with different concentrations of four alkaloids, the protein level of Nrf2 was further increased compared with the CSE group. Compared with the CSE group, the protein level of kelch like ECH associated protein 1 (Keap1) in CSE-induced A549 cells was increased after treated with the four alkaloids at 10 μM and 20 μM (). These results suggest that the mechanism of antioxidant activity of the four alkaloids may be related to the regulation of the Nrf2/Keap1 signaling pathway.
Figure 6. Effect of four alkaloids on the expression of Nrf2/Keap1 signaling pathway-related proteins in 2% CSE-induced A549 cells. (a-c) the expression of Nrf2 and Keap1 proteins in 2% CSE-induced A549 cells was analyzed by western blotting after incubation with four different concentrations of alkaloids for 24 h, and GAPDH was the reference protein. C: control; CSE: 2% CSE; T1-T3: 5, 10, 20 μM chuanbeinone; Q1-Q3: 5, 10, 20 μM ebeiedinone; J1-J3: 5, 10, 20 μM imperialine; X1-X3: 5, 10, 20 μM peimisine. n = 3, ###p < .001 vs. The control group, *p < .05, **p < .01, ***p < .001 vs. The CSE group.
Effect of alkaloids on SIRT1/FOXO3a signaling pathway
Effect of alkaloids on FOXO3, MST1 and SIRT1 gene transcription levels
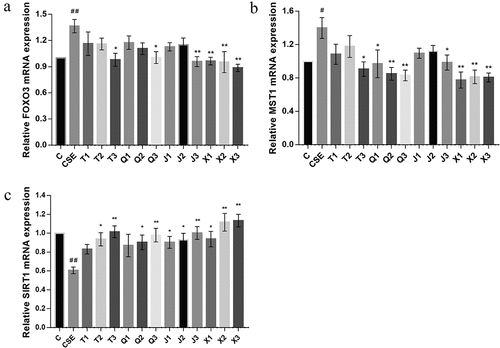
As shown in , compared with the control group, the mRNA expression of Forkhead box transcription factor O3 (FOXO3) and Mammalian sterile line 20-like kinase 1 (MST1) were significantly increased in CSE-induced A549 cells (p < .01, p < .05). After treated with 20 μM of four alkaloids, the mRNA expression of FOXO3 was significantly decreased (p < .05, p < .05, p < .01, p < .01), among which peimisine showed the best effect. The mRNA expression of MST1 was significantly decreased (p < .05, p < .01, p < .05, p < .01), among which ebeiedinone and peimisine showed better effect. As shown in , the mRNA expression of silent information regulator 1 (SIRT1) was significantly decreased in CSE-induced A549 cells (p < .01). Except for chuanbeinone and ebeiedinone at 5 μM, different concentrations of four alkaloids all effectively increased the mRNA expression of SIRT1, among which peimisine showed the best effect.
Figure 7. Effect of four alkaloids on the expression of SIRT1/FOXO3a signaling pathway-related genes in 2% CSE-induced A549 cells. (a-c) the mRNA expression levels of FOXO3, MST1 and SIRT1 in 2% CSE-induced A549 cells after incubation with four different concentrations of alkaloids for 24 h. C: control; CSE: 2% CSE; T1-T3: 5, 10, 20 μM chuanbeinone; Q1-Q3: 5, 10, 20 μM ebeiedinone; J1-J3: 5, 10, 20 μM imperialine; X1-X3: 5, 10, 20 μM peimisine. n = 3, #p < .05, ##p < .01 vs. The control group, *p < .05, **p < .01 vs. The CSE group.
Effect of alkaloids on SIRT1, FOXO3a and MST1 protein expression levels
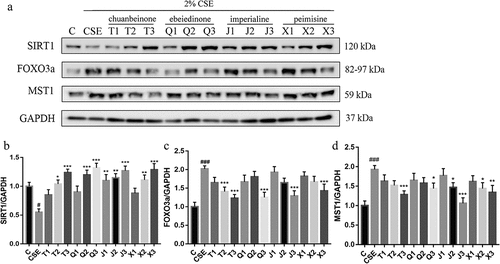
As shown in , the protein level of SIRT1 was significantly decreased in CSE-induced A549 cells (p < .05), and 10 μM and 20 μM of alkaloids administration reversed the decreased SIRT1 protein level. As shown in , the protein levels of FOXO3a and MST1 were significantly increased in CSE-induced A549 cells (p < .001), and 20 μM of alkaloids administration reversed the increased FOXO3a and MST1 protein levels. The western blotting results were consistent with the qRT-PCR results, suggesting that the mechanism of antioxidant activity of the four alkaloids may be related to the regulation of the SIRT1/FOXO3a signaling pathway.
Figure 8. Effect of four alkaloids on the expression of SIRT1/FOXO3a signaling pathway-related proteins in 2% CSE-induced A549 cells. (a-d) the expression of SIRT1, FOXO3a and MST1 proteins in 2% CSE-induced A549 cells was analyzed by western blotting after incubation with four different concentrations of alkaloids for 24 h, and GAPDH was the reference protein. C: control; CSE: 2% CSE; T1-T3: 5, 10, 20 μM chuanbeinone; Q1-Q3: 5, 10, 20 μM ebeiedinone; J1-J3: 5, 10, 20 μM imperialine; X1-X3: 5, 10, 20 μM peimisine. n = 3, #p < .05, ###p < .001 vs. The control group, *p < .05, **p < .01, ***p < .001 vs. The CSE group.
Discussion
In this study, we first demonstrated that the four isosteroid alkaloids (peimisine, ebeiedinone, imperialine and chuanbeinone) could attenuate CSE-induced oxidative stress damage in A549 cells by elevating the levels of GSH/GSSG, SOD and HO-1, and reducing the levels of SOD and MDA. Smoking is the most important causative factor for COPD, and cigarette smoke is a major source of oxidants, which cause significant oxidative stress in the small airways and lead to abnormal apoptosis of respiratory epithelial cells (Dang et al., Citation2020; Zhou et al., Citation2019). Therefore, the 2% CSE-induced A549 lung epithelial cell injury model was used to mimic the oxidative stress microenvironment of lung in vitro. ROS are produced in vivo and cleared by the antioxidant system to maintain a dynamic balance in normal conditions. However, ROS will be largely produced and cause damage to the body in oxidative stress conditions (Liao et al., Citation2021). Meanwhile, a high level of ROS is also one of the major causes of oxidative stress (Zheng et al., Citation2021). Consistent with previous studies, we found that the ROS level was significantly increased in 2% CSE-induced A549 cells in this study, indicating that the A549 cells were damaged by CSE-induced oxidative stress. However, the treatment of the four alkaloids inhibited the elevation of intracellular ROS levels, thereby protecting the cells from CSE-induced oxidative damage.
Furthermore, GSH and SOD are important antioxidant enzymes in the body. GSH usually reacts with reactive GSH molecules to produce GSSG, so the detection of the GSH/GSSG ratio can be used to evaluate the redox state of cells (Bjorklund et al., Citation2020). SOD and other endogenous antioxidants can jointly effectively scavenge intracellular ROS and play an important role in preventing cellular damage caused by oxidative stress (Liao et al., Citation2021). HO-1 is an important antioxidant enzyme in vivo and is considered as important indicator of oxidative stress (Tang et al., Citation2021). In this study, the levels of GSH/GSSG ratio and SOD were significantly decreased in 2% CSE-induced A549 cells, indicating the intracellular antioxidant capacity was decreased. After the treatment of the four alkaloids, the levels of GSH/GSSG, SOD and HO-1 were significantly increased, indicating the four alkaloids could alleviate CSE-induced oxidative damage by increasing the level of intracellular antioxidant enzymes. When the antioxidant system is out of balance, intracellular MDA will be overproduced and exacerbate cellular damage (Zhang et al., Citation2021). In this study, the level of MDA was significantly decreased in 2% CSE-induced A549 cells after treated with the four alkaloids, indicating the four alkaloids could alleviate CSE-induced oxidative damage by decreasing the production of lipid peroxidation products.
Subsequently, this study showed that the potential mechanism of anti-oxidative stress in the four alkaloids may be related to the regulation of Nrf2/Keap1 and SIRT1/FOXO3a signaling pathways. The Nrf2/Keap1 signaling pathway is a redox-sensitive signaling system that plays an important role in the regulation of redox states (H. Li et al., Citation2021). Nrf2 is an important transcription factor that regulates and maintains homeostasis in organisms (Lan et al., Citation2022), which normally binds to the cytoplasmic protein chaperone Keap1 in the cytoplasm and is maintained at low level. When the cells are damaged by oxidative stress, Nrf2 will separate from Keap1 and translocate to the nucleus, which will bind with antioxidant response elements, activate the antioxidant defense system, and produce antioxidant enzymes (HO-1 and NQO1) to exert regulatory ability (Ke et al., Citation2022; Xiao et al., Citation2022; Yang et al., Citation2022; Yao et al., Citation2022). In this study, the western blotting results showed that the protein levels of Nrf2 and Keap1 were significantly increased in 2% CSE-induced A549 cells after treated with the four alkaloids, suggesting that the four alkaloids may attenuate CSE-induced oxidant stress injury in A549 cells by promoting the dissociation of Nrf2 and Keap1. HO-1 and NQO1 are important antioxidant enzymes regulated by the Nrf2 gene. HO-1 can catalyze the decomposition of heme, which has the activity of antioxidant, and the by-products of the decomposition reaction also have the ROS-scavenging activity (Wang et al., Citation2022). NQO1 plays an important role in maintaining redox homeostasis by using NAD(P)H as a receptor for reduction reactions, and the catalytic product protects cells from various oxidative stress damage (Okumura et al., Citation2021; Tsai et al., Citation2021; Wachter et al., Citation2021). We found that the mRNA expression of Nrf2, HO-1 and NQO1 were significantly increased in 2% CSE-induced A549 cells after treated with the four alkaloids, suggesting that the four alkaloids may attenuate CSE-induced oxidative stress injury in A549 cells by increasing the transcription of Nrf2 and the antioxidant enzyme genes HO-1 and NQO-1. The above results suggest that the mechanism of antioxidant activity of the four alkaloids may be related to the regulation of the Nrf2/Keap1 signaling pathway.
Consistent with previous studies (Y. Li et al., Citation2016; Wilk et al., Citation2010; Wu et al., Citation2019), we found that excessive ROS would activate MST1, downregulate the expression and activity of SIRT1, and increase the expression of FOXO3a. SIRT1 is a class III deacetylase closely related to oxidative stress and inflammatory responses, and plays an important role in the pathogenesis of COPD (Hwang et al., Citation2013; Ma et al., Citation2018; Y. Zhao et al., Citation2021). Many studies have shown that SIRT1 acts on the FOXO family and transforms the cell death process into anti-oxidative stress. In this study, the results of western blotting showed that the protein expression of SIRT1 was significantly increased in 2% CSE-induced A549 cells after treated with the four alkaloids, which was consistent with the qRT-PCR results, suggesting that the four alkaloids may regulate CSE-induced oxidative stress by promoting the expression and activity of SIRT1. Previous studies have shown that MST1 promotes oxidative stress by regulating multiple signaling pathways (D. Li et al., Citation2018; Z. Zhao et al., Citation2022). FOXO3a is the center of the FOXO family, which could regulate various adversities such as oxidative stress (Hu et al., Citation2018; Kenyon, Citation2018). Furthermore, studies have shown that oxidative stress activates intracellular MST1, which mediates oxidative stress-induced primary mammalian neuron cell death by directly activating downstream FOXO transcription factors (Lehtinen et al., Citation2006). In the study, the results of western blotting showed that the protein expression of MST1 and FOXO3a was significantly decreased in 2% CSE-induced A549 cells after treated with the four alkaloids, which was consistent with the qRT-PCR results, suggesting that the four alkaloids may regulate CSE-induced oxidative stress by inhibiting the expression and activity of MST1 and FOXO3a. These results suggest that the mechanism of antioxidant activity of the four alkaloids may be related to the SIRT1/FOXO3a signaling pathway.
Conclusion
This study first investigated the protective effect and molecular mechanism of four isosteroid alkaloids (peimisine, ebeiedinone, imperialine and chuanbeinone) from FCB on CSE-induced oxidative stress injury in A549 cells. Furthermore, this study showed that the four alkaloids from FCB have potential protective activity against oxidative stress damage in the lung, and provided the basis for the development of food products with healthcare effect and new drugs for the treatment of lung diseases. However, the specific molecular mechanism and signal transduction process of four alkaloids in anti-oxidative stress still need further investigation, and we will consider it seriously in our future work.
Authors contribution
All authors contributed to the conception and design of the study. XW, MZ and BY designed the study. XW and MZ acquired and analyzed the data. XW and MZ drafted the manuscript. EA, WMT, KWGT and BY revised the manuscript critically for important intellectual content. All authors commented on previous versions of the manuscript. All authors read and approved the version of the manuscript to be published.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used and analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Barnes, P. (2020). Oxidative stress-based therapeutics in COPD. Redox Biology, 33, 101544. https://doi.org/10.1016/j.redox.2020.101544
- Bjorklund, G., Tinkov, A., Hosnedlova, B., Kizek, R., Ajsuvakova, O., Chirumbolo, S., Skalnaya, M. G., Peana, M., Dadar, M., El-Ansary, A., Qasem, H., Adams, J. B., Aaseth, J., & Skalny, A. V. (2020). The role of glutathione redox imbalance in autism spectrum disorder: A review. Free Radical Biology and Medicine, 160, 149–11. https://doi.org/10.1016/j.freeradbiomed.2020.07.017
- Chanda, D., Otoupalova, E., Smith, S., Volckaert, T., De Langhe, S., & Thannickal, V. (2019). Developmental pathways in the pathogenesis of lung fibrosis. Molecular Aspects of Medicine, 65, 56–69. https://doi.org/10.1016/j.mam.2018.08.004
- Chen, Y., Guo, S., Guan, Y., Li, M., An, Y., & Liu, H. (2019). The research progress of medicinal plants fritillaria. Molecular Plant Breeding, 17(18), 6198–6206. https://doi.org/10.13271/j.mpb.017.006198
- Dang, X., He, B., Ning, Q., Liu, Y., Guo, J., Niu, G., & Chen, M. (2020). Alantolactone suppresses inflammation, apoptosis and oxidative stress in cigarette smoke-induced human bronchial epithelial cells through activation of Nrf2/HO-1 and inhibition of the NF-kappaB pathways. Respiratory Research, 21(1), 95–106. https://doi.org/10.1186/s12931-020-01358-4
- Hu, Z., Wang, F., Wu, Z., Gu, H., Dong, N., Jiang, X., Xu, J., Wu, Z., Wechsler, D. S., & Zheng, D. (2018). FOXO3a-dependent up-regulation of Mxi1-0 promotes hypoxia-induced apoptosis in endothelial cells. Cellular Signalling, 51, 233–242. https://doi.org/10.1016/j.cellsig.2018.08.009
- Hwang, J., Yao, H., Caito, S., Sundar, I., & Rahman, I. (2013). Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radical Biology and Medicine, 61, 95–110. https://doi.org/10.1016/j.freeradbiomed.2013.03.015
- Ke, Z., Tan, S., Li, H., Jiang, S., Li, Y., Chen, R., & Li, M. (2022). Tangeretin improves hepatic steatosis and oxidative stress through the Nrf2 pathway in high fat diet-induced nonalcoholic fatty liver disease mice. Food & Function, 13(5), 2782–2790. https://doi.org/10.1039/d1fo02989d
- Kenyon, C. (2018). The first long-lived mutants: Discovery of the insulin/IGF-1 pathway for ageing. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1561), 9–16. https://doi.org/10.1098/rstb.2010.0276
- Lan, C., Zhu, Y., Zhou, J., Wu, R., Yang, N., Bao, Q., Xu, X., & Yin, G. (2022). Luteolin alleviates epithelial-mesenchymal transformation induced by oxidative injury in ARPE-19 cell via Nrf2 and AKT/GSK-3beta pathway. Oxidative Medicine and Cellular Longevity, 2022, 1–12. https://doi.org/10.1155/2022/2265725
- Lehtinen, M., Yuan, Z., Boag, P., Yang, Y., Villen, J., Becker, E., DiBacco, S., de la Iglesia, N., Gygi, S., Blackwell, T. K., & Bonni, A. (2006). A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell, 125(5), 987–1001. https://doi.org/10.1016/j.cell.2006.03.046
- Li, D., Ni, H., Rui, Q., Gao, R., & Chen, G. (2018). MST1: Function and mechanism in brain and myocardial ischemia reperfusion injury. Current Neuropharmacology, 16(9), 1358–1364. https://doi.org/10.2174/1570159X16666180516095949
- Li, H., Shang, Z., Liu, X., Qiao, Y., Wang, K., & Qiao, J. (2021). Clostridium butyricum alleviates Enterotoxigenic Escherichia coli K88-Induced oxidative damage through regulating the p62-Keap1-Nrf2 Signaling Pathway and remodeling the cecal microbial community. Frontiers in Immunology, 12, 1–15. https://doi.org/10.3389/fimmu.2021.771826
- Li, Y., Xing, Y., Li, X., Zhang, J., & Guo, H. (2016). Redox regulation of FOXO3a transcription factor. Chinese Pharmacological Bulletin, 32(9), 1203–1207. https://doi.org/10.3969/j.issn.1001-1978.2016.09.005
- Liao, L., Gong, L., Zhou, M., Xue, X., Li, Y., Peng, C., & Lopez Malo, D. (2021). Leonurine ameliorates oxidative stress and insufficient angiogenesis by regulating the PI3K/Akt-eNOS signaling pathway in H2O2-induced HUVECs. Oxidative Medicine and Cellular Longevity, 2021, 1–12. https://doi.org/10.1155/2021/9919466
- Lin, B., Ji, H., Li, P., Fang, W., & Jiang, Y. (2006). Inhibitors of acetylcholine esterase in vitro - Screening of steroidal alkaloids from fritillaria species. Planta Medica, 72(9), 814–818. https://doi.org/10.1055/s-2006-947168
- Liu, C., Zhen, D., Du, H., Gong, G., Wu, Y., Ma, Q., & Quan, Z. S. (2022). Synergistic anti-inflammatory effect of peimine, peiminine, and forsythoside a combination on LPS-induced acute lung injury by inhibition of the IL-17-NF-kappaB/MAPK pathway activation. Journal of Ethnopharmacology, 295, 115354. https://doi.org/10.1016/j.jep.2022.115343
- Liu, S., Yang, T., Tse, W. M., Tse, K. W. G., Zhou, T., Wang, S., & Ye, B. (2020a). Isosteroid alkaloids from Fritillaria cirrhosa bulbus as inhibitors of cigarette smoke-induced oxidative stress. Fitoterapia, 140, 104434. https://doi.org/10.1016/j.fitote.2019.104434
- Liu, S., Yang, T., Tse, W. M., Tse, K. W. G., Zhou, T., Wang, S., & Ye, B. (2020b). Isosteroid alkaloids with different chemical structures from fritillariae cirrhosae bulbus alleviate LPS-induced inflammatory response in RAW 264.7 cells by MAPK signaling pathway. International Immunopharmacology, 78, 106047. https://doi.org/10.1016/j.intimp.2019.106047
- Ma, Y., Ruan, Y., Wang, Y., & Wu, S. (2018). Polydatin inhibits cell proliferation and expressions of inflammatory cytokines in THP-1 cells induced by ox-LDL via up-regulating SIRT1. Chinese Journal of Cellular and Molecular Immunology, 34(3), 193–198. https://doi.org/10.13423/j.cnki.cjcmi.008557
- Mishra, V., Banga, J., & Silveyra, P. (2018). Oxidative stress and cellular pathways of asthma and inflammation: Therapeutic strategies and pharmacological targets. Pharmacology & Therapapeutics, 181, 169–182. https://doi.org/10.1016/j.pharmthera.2017.08.011
- Okumura, N., Ito, T., Degawa, T., Moriyama, M., & Moriyama, H. (2021). Royal jelly protects against epidermal stress through upregulation of the NQO1 expression. International Journal of Molecular Sciences, 22(23), 12973. https://doi.org/10.3390/ijms222312973
- Ornatowski, W., Lu, Q., Yegambaram, M., Garcia, A., Zemskov, E., Maltepe, E., Fineman, J. R., Wang, T., & Black, S. M. (2020). Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biology, 36, 101679. https://doi.org/10.1016/j.redox.2020.101679
- Ryter, S., Bhatia, D., & Choi, M. (2019). Autophagy: A lysosome-dependent process with implications in cellular redox homeostasis and human disease. Antioxidants & Redox Signaling, 30(1), 138–159. https://doi.org/10.1089/ars.2018.7518
- Saunders, R. M., Biddle, M., Amrani, Y., & Brightling, C. (2022). Stressed out - the role of oxidative stress in airway smooth muscle dysfunction in asthma and COPD. Free Radical Biology and Medicine, 185, 97–119. https://doi.org/10.1016/j.freeradbiomed.2022.04.011
- Tang, Z., Ju, Y., Dai, X., Ni, N., Liu, Y., Zhang, D., Gao, H., Sun, H., Zhang, J., & Gu, P. (2021). HO-1-mediated ferroptosis as a target for protection against retinal pigment epithelium degeneration. Redox Biology, 43, 101971. https://doi.org/10.1016/j.redox.2021.101971
- Tsai, C., Chen, G., Chen, Y., Shen, C., Lu, D., Yang, L., Yeh, W., & Yeh, W.-L. (2021). Regulatory effect of quercetin on M1/M2 macrophage polarization and oxidative/antioxidative balance. Nutrients, 14(1), 67–88. https://doi.org/10.3390/nu14010067
- Wachter, K., Navarrete Santos, A., Grosskopf, A., Baldensperger, T., Glomb, M. A., Szabo, G., & Simm, A. (2021). AGE-Rich bread crust extract boosts oxidative stress interception via stimulation of the NRF2 pathway. Nutrients, 13(11), 3874. https://doi.org/10.3390/nu13113874
- Wang, D., Du, Q., Li, H., & Wang, S. (2016). The isosteroid alkaloid imperialine from bulbs of Fritillaria cirrhosa mitigates pulmonary functional and structural impairment and suppresses inflammatory response in a COPD-Like rat model. Mediators of Inflammation, 2016, 1–17. https://doi.org/10.1155/2016/8369704
- Wang, D., Zhu, J., Wang, S., Wang, X., Ou, Y., Wei, D., & Li, X. (2011). Antitussive, expectorant and anti-inflammatory alkaloids from Bulbus Fritillariae Cirrhosae. Fitoterapia, 82(8), 1290–1294. https://doi.org/10.1016/j.fitote.2011.09.006
- Wang, G., Wang, Y., Yang, Q., Xu, C., Zheng, Y., Wang, L., Wu, J., Zeng, M., & Luo, M. (2022). Metformin prevents methylglyoxal-induced apoptosis by suppressing oxidative stress in vitro and in vivo. Cell Death & Disease, 13(1), 29–40. https://doi.org/10.1038/s41419-021-04478-x
- Wang, M., Huang, W., Chen, L., Yeh, K., Lin, C., & Liou, C. (2022). Sophoraflavanone G from Sophora flavescens ameliorates allergic airway inflammation by suppressing Th2 response and oxidative stress in a murine asthma model. International Journal of Molecular Sciences, 23(11), 6104. https://doi.org/10.3390/ijms23116104
- Wilk, A., Urbanska, K., Yang, S., Wang, J., Amini, S., Valle, L., Reiss, K., Meggs, L., & Reiss, K. (2010). Insulin-like growth factor-I–forkhead box O transcription factor 3a counteracts high glucose/tumor necrosis factor-α-mediated neuronal damage: Implications for human immunodeficiency virus encephalitis. Journal of Neuroscience Research, 89(2), 183–198. https://doi.org/10.1002/jnr.22542
- Wu, F., Li, Z., Dong, C., Wang, L., Yang, Q., Yin, Z., & Li, L. (2019). Qibai Pingfei capsule alleviates inflammation and oxidative stress in a chronic obstructive pulmonary disease rat model with syndromes of Qi deficiency and phlegm and blood stasis by regulating the SIRT1/FoxO3a pathway. Chinese Journal of Cellular and Molecular Immunology, 35(2), 115–120. https://doi.org/10.13423/j.cnki.cjcmi.008771
- Xiao, X., Tong, Z., Zhang, Y., Zhou, H., Luo, M., Hu, T., Hu, L., Kong, L., Liu, Z., Yu, C., Huang, Z., & Hu, L. (2022). Novel prenylated indole alkaloids with neuroprotection on SH-SY5Y cells against oxidative stress targeting Keap1–Nrf2. Marine Drugs, 20(3), 191–206. https://doi.org/10.3390/md20030191
- Xu, L., Fan, L., Jiang, S., Yang, X., Wang, X., & Yang, C. (2022). Recent progress of chemical constituents and pharmacological effect of Fritillaria. Chinese Journal of Medicinal Chemistry, 32(1), 61–73. https://doi.org/10.14142/j.cnki.cn21-1313/r.2022.01.010
- Yang, J., Tang, X., Ke, X., Dai, Y., & Shi, J. (2022). Triptolide suppresses NF-kappaB-mediated inflammatory responses and activates expression of Nrf2-mediated antioxidant genes to alleviate caerulein-induced acute pancreatitis. International Journal of Molecular Sciences, 23(3), 1252–1265. https://doi.org/10.3390/ijms23031252
- Yao, H., Xie, Q., He, Q., Zeng, L., Long, J., Gong, Y., & Gao, Y. (2022). Pretreatment with panaxatriol saponin attenuates mitochondrial apoptosis and oxidative stress to facilitate treatment of myocardial ischemia-reperfusion injury via the regulation of Keap1/Nrf2 activity. Oxidative Medicine and Cellular Longevity, 2022, 1–20. https://doi.org/10.1155/2022/9235358
- Yeum, H., Lee, Y., Kim, S., Roh, S., Lee, J., & Seo, Y. (2007). Fritillaria cirrhosa, anemarrhena asphodeloides, Lee-Mo-Tang and cyclosporine a inhibit ovalbumin-induced eosinophil accumulation and Th2-mediated bronchial hyperresponsiveness in a murine model of asthma. Basic & Clinical Pharmacology & Toxicology, 100(3), 205–213. https://doi.org/10.1111/j.1742-7843.2007.00043.x
- Yi, X., Li, T., Wei, X., & He, Z. (2022). Erythromycin attenuates oxidative stress-induced cellular senescence via the PI3K-mTOR signaling pathway in chronic obstructive pulmonary disease. Frontiers in Pharmacology, 13, 1043474. https://doi.org/10.3389/fphar.2022.1043474
- Yuan, S., Tse, W. M., Tse, K. W. G., Xu, F. C., Xie, H. J., Aga, E. B., Xiong, H., & Ye, B. G. (2022). Ethanol extracts of bulbus of Fritillaria cirrhosa protects against pulmonary fibrosis in rats induced by bleomycin. Journal of Functional Foods, 97, 105239. https://doi.org/10.1016/j.jff.2022.105239
- Zhang, Y., Han, Y., He, J., Ouyang, K., Zhao, M., Cai, L., Wang, W., Meng, W., Chen, L., & Wang, W. (2021). Digestive properties and effect of Chimonanthus nitens Oliv polysaccharides on antioxidant effect in vitro and in immunocompromised mice. International Journal of Biological Macromolecules, 185, 306–316. https://doi.org/10.1016/j.ijbiomac.2021.06.114
- Zhang, Y., Li, T., Pan, M., Wang, W., Huang, W., Yuan, Y., Xie, Z., Chen, Y., Peng, J., Li, X., & Meng, Y. (2022). SIRT1 prevents cigarette smoking-induced lung fibroblasts activation by regulating mitochondrial oxidative stress and lipid metabolism. Journal of Translational Medicine, 20(1), 222. https://doi.org/10.1186/s12967-022-03408-5
- Zhao, Y., Jiang, Q., Zhang, X., Zhu, X., Dong, X., Shen, L., Zhu, L., Niu, L., Chen, L., Zhang, M., Jiang, J., Chen, D., & Zhu, L. (2021). L-Arginine alleviates LPS-Induced oxidative stress and apoptosis via activating SIRT1-AKT-Nrf2 and SIRT1-FOXO3a signaling pathways in C2C12 myotube cells. Antioxidants, 10(12), 1–18. https://doi.org/10.3390/antiox10121957
- Zhao, Z., Wang, Z., Fang, X., Wang, T., Luo, Z., & Xie, T. (2022). Effect of sodium arsenite on oxidative stress, apoptosis, and the expression of MST1, MST2 in mouse hepatocytes AML12. Journal of Guizhou Medical University, 47(1), 7–12. https://doi.org/10.19367/j.cnki.2096-8388.2022.01.002
- Zheng, W., Song, Z., Li, S., Hu, M., Shaukat, H., & Qin, H. (2021). Protective effect of sesamol against liver oxidative stress and inflammation in high-fat diet-induced hepatic steatosis. Nutrients, 13(12), 4484. https://doi.org/10.3390/nu13124484
- Zhou, T., Hu, Y., Wang, Y., Sun, C., Zhong, Y., Liao, J., & Wang, G. (2019). Fine particulate matter (PM2.5) aggravates apoptosis of cigarette-inflamed bronchial epithelium in vivo and vitro. Environmental Pollution, 248, 1–9. https://doi.org/10.1016/j.envpol.2018.11.054
- Zhuang, C., Wang, Y., Zhang, Y., & Xu, N. (2018). Oxidative stress in osteoarthritis and antioxidant effect of polysaccharide from angelica sinensis. International Journal of Biological Macromolecules, 115, 281–286. https://doi.org/10.1016/j.ijbiomac.2018.04.083
