?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Roselle flower is an edible flower with health-beneficial bioactive compounds. In this study, red and purple roselle calyces were dried by foam-mat drying with different hydrophilic-lipophilic balance (HLB) of commercial emulsifiers as the foaming agents, including TBM, SP, and SSL. For a comparison is the roselle calyces powder from conventional drying. The mixture of emulsifiers of TBM and SP produces better foam and powder characteristics than a single emulsifier of SSL. HLB value and emulsifier mixture composition affect the foaming properties that influence the powder characteristics. The roselle variety also affected the foaming properties in which purple roselle calyces are better. The antioxidant activity seems to be affected by the foaming stability. The lack of conventional dried roselle calyces powder is the very low solubility indicated by undefined dissolution time. The formation of foam and the use of maltodextrin as a filler contribute to the foam mat-dried powder color.
Introduction
An edible flower is a harmless and non-toxic part of the plant, which improves the aesthetics of food and also provides health benefits for those who consume it (Lara-Cortés et al., Citation2013; Pensamiento-Niño et al., Citation2023). One type of edible flower abundantly found and cultivated is the rosella (Hibiscus sabdariffa L.) flower. Roselle calyces are one of the essential parts of the roselle plant that is widely utilized due to rich in bioactive compounds, such as organic acids, phenolic compounds, anthocyanins, and minerals (Izquierdo-Vega et al., Citation2020). Moreover, the presence of bioactive compounds causes roselle calyces to be known to have pharmacological properties that are useful for health and therapeutic potential agents (Sapian et al., Citation2023).
Roselle calyces are traditionally consumed by brewing with hot water to produce roselle drinks. Additionally, the abundant anthocyanin content in roselle calyces is also used as a natural colorant in various food products (Tan & Sulaiman, Citation2020). However, the fresh roselle calyces have the drawback of being perishable due to their high water content and quick wilting. As a result, it is not appropriate to transport them fresh over a considerable distance and time (Tan & Sulaiman, Citation2020).
Conventional drying using solar drying or heating in a dryer at a specific temperature is frequently used for drying fresh roselle calyces and then further ground into a powder. The drawback of this process is that the final roselle calyces powder is brown, not exceptionally soluble in water, and degrades essential compositions like phenolics and vitamin C (Tajudin et al., Citation2019). Spray drying, and freeze drying of roselle calyces extract are two other often used drying techniques (Khan et al., Citation2022; Nguyen et al., Citation2022). However, only soluble components can be dried using spray and freeze-drying techniques, and the equipment is relatively expensive. As a result, these methods are less cost-effective when used on a larger scale, such as in an industrial or pilot plant scale (Minh, Citation2020; Rezvankhah et al., Citation2020). Foam mat drying is another technique often utilized and offers several benefits. The advantages of this method are that it is cheap, does not require immense energy, produces a stable powder, and the quality deterioration is less than ordinary air drying, spray drying, and drum drying (Franco et al., Citation2016; Hardy & Jideani, Citation2017).
Previously, researchers have used foam-mat drying to dry viscous fluid, such as fruit juice and other food ingredients (Fauziyah et al., Citation2023; Kumar et al., Citation2022; Li et al., Citation2021). Typically, these techniques make use of foaming agents, which serve as surfactants to reduce surface tension and encourage foam production, while foam stabilizers like maltodextrin are also preferred to increase foam stability (Fauziyah et al., Citation2023; Kumar et al., Citation2022; Sangamithra et al., Citation2015). Then, the foamed product with stable viscoelastic gas bubbles should be dried and crushed into powder (Kumar et al., Citation2022). Earlier research on foam-mat drying of rosella powder uses calcium caseinate, protein isolates, ovalbumin, and methylcellulose as foaming agents (Djaeni et al., Citation2018; Fauziyah et al., Citation2023). The lack of use of these foaming agents is costly and affects the product characteristics due to the biomacromolecule used, such as solubility. However, the research foam-mat drying on roselle calyces typically only focuses on the red roselle and is still limited to exploring the other roselle calyces, like the purple roselle calyces. Alternatively to costly foaming agents are commercial emulsifiers easily often found in the market, such as sodium stearoyl lactylate (SSL), the mixture of monoglyceride, diglyceride, and polyglycerol ester of fatty acid (SP), and the mixture of monoglyceride, polyglycerol ester, and sorbitan monostearate (TBM). These emulsifiers might have different hydrophilic-lipophilic balance (HLB) values, which is interesting to study. Therefore, this study aims to evaluate roselle calyces types (red and purple calyces) and the uses of various foaming agents with different hydrophilic-lipophilic balance (HLB) values on the foam-mat dried roselle calyces powder properties and also compare their characteristics with conventional dried roselle calyces powder.
Materials and methods
Materials
The fresh red and purple calyces () were obtained from local farmers at Kediri, East Java, Indonesia. Maltodextrin with DE (dextrose-equivalent) of 10–12 was from Xingmao (China). Commercial emulsifiers as foaming agents, including commercial sodium stearoyl lactylate (SSL), the mixture of monoglyceride, diglyceride, and polyglycerol ester of fatty acid (SP), and the mixture of monoglyceride, polyglycerol ester, and sorbitan monostearate (TBM), were purchased from the local market. The composition and HLB of these emulsifiers are shown in . All chemical reagents were obtained in analytical grades from Sigma Aldrich (St. Louis, MO, U.S.A.) and Merck (Darmstadt, Germany).
Table 1. The composition and HLB of commercial emulsifier.
HLB value determination
The hydrophilic-lipophilic balance (HLB) of commercial emulsifiers, including SSL, SP, and TBM, was determined according to Low and Ng (Citation1987) method, which is divided into two steps: the determination of saponification value and an acid value. The HLB of each commercial emulsifier was calculated using EquationEquation (1)(1)
(1) as follows:
Where: SV = saponification value, and AV = acid value
Preparation of conventional dried roselle calyces powder
The production of dried roselle calyces powder follows methods described by Tajudin et al. (Citation2019). The fresh red and purple calyces were dried at the local cabinet dryer at 60°C until constant weight (±10–12 h). Then, the dried roselle calyces were ground using a blender and sieve at 40 mesh sieves to obtain conventional dried roselle calyces powder. Then, the conventional dried roselle calyces powder was stored in aluminum bags for further analysis.
Preparation of foam-mat dried roselle calyces powder
The preparation of foam-mat dried roselle calyces powder is a simple method. Briefly, the fresh roselle calyces were weighed in a certain mass and combined with the water in a ratio of 4:10 w/v, then ground using a blender for 3 min, followed by a filtration to obtain roselle calyx water extract. Roselle water extract was added by 10% w/v maltodextrin as a foam stabilizer and homogenized using a homogenizer (Ultraturrax IKA T18 Basic) for 1 min at 6000 rpm, followed by the addition of foaming agent as much as 1% w/v and homogenized again at 14,000 rpm for 7 minutes. Then, the foam was layered thinly (about 1 mm) in the trays and dried for six hours at 60°C in a cabinet dryer. The dried foam sheet was ground to obtain foam-mat dried roselle calyces powder and ready for further analysis. The appearance of foam mat dried roselle calyces powder is shown in .
Foaming properties analysis
The foam expansion (FE) indicated the capacity of the foaming agent to introduce air into roselle calyces solution for foam production. FE was measured according to the method explained by Kumar et al. (Citation2022) and computed with EquationEquation (2)(2)
(2) .
Where: Vi = Initial volume of roselle calyces solution (cm3), and Vf = Final volume of foamed of roselle calyces solution (cm3).
The foam density (FD) of the roselle calyces solution was measured using the methods described by Kumar et al. (Citation2022) and then calculated using EquationEquation (3)(3)
(3) .
Where: Vi = Initial volume of roselle calyces solution (cm3), and m = mass of foamed of roselle calyces solution (g).
The foam stability was assessed using Kumar et al. (Citation2022) methods. Briefly, a transparent graduated cylinder containing 50 mL of foamed roselle calyces was left at room temperature (28 ± 2°C) for four hours to test the foam’s stability. The foam volume reduction was calculated as an index for the foam stability at intervals of every 30 min using EquationEquation (4)(4)
(4) .
Where: Vi = Initial volume foam of roselle calyces solution (cm3), and Vi = Volume foam of roselle calyces solution at 30 min interval time (cm3).
Chemical and antioxidant activity analysis
Total anthocyanin content
The pH differential approach was used to calculate total anthocyanin content (TAC) (Samadi & Fard, Citation2020). Briefly, 1 gram conventional or foam-mat dried roselle calyces powder was macerated with hot water in a ratio of 1:15 w/v. Then, the solution was combined with 25 mmol/L of potassium chloride buffer (pH 1), and 0.4 mol/L of sodium acetate buffer (pH 4.5) were subsequently incubated at room temperature (25 ± 3°C) for 15 minutes. Then, the samples were measured at 510 and 700 nm. The formula for calculating the absorbance variation (A) was (A = [A510 -A700] pH 1.0 - [A510-A700] pH 4.5), and total anthocyanin content was computed using EquationEquation (5)(5)
(5) .
Where: TAC is the total anthocyanins content (mg/g), MW is the molecular weight of cyanidin-3-glucoside (449.2 g/mole), V is the total volume of extract (mL), L is the cell width (1 cm), Ɛ is the coefficient of molar extinction for cyanidin-3-glucoside (26,900 L/mole-cm), A is the absorbance difference and DF is the dilution factor.
Total phenolics content
The Folin-Ciocalteau method, as described by Ifie et al. (Citation2016) with minor modifications, was used to determine the total phenolic content of conventional or foam-mat dried roselle calyces powder. Briefly, 1 gram of conventional or foam-mat dried roselle calyces powder was macerated with an 80% methanol solution in a ratio of 1:15 b/v. Then, 1 ml of the extract was combined with 5 mL of diluted Folin-Ciocalteu’s phenol reagent (10%) and incubated for 5 minutes in dark conditions. After 5 min, 4 mL of sodium carbonate solution (75 g/L) was added and incubated for 30 minutes again in dark conditions, followed by measuring the absorbance reading at 752 nm and the gallic acid used as a standard for estimating total phenolic content.
The pH values
The pH values of conventional and foam-mat dried roselle calyces powder solution was analyzed as follows: 1 g of foam-mat dried roselle calyces powder was dissolved in 100 ml of hot water and mixed until all powder dissolved completely. The pH of the solution was measured using a stand pH meter probe until the stable conditions.
Antioxidant activity assay using DPPH
The DPPH assay was used to estimate the antioxidant activity of conventional and foam-mat dried roselle calyces powder expressed in inhibition percentage (%). According to the method of Baliyan et al. (Citation2022), with slight modifications. Briefly, the 1 g foam-mat dried roselle calyces powder was macerated with an 80% methanol solution in a ratio of 1:15 b/v. Then, 1 gram of the solution samples was diluted with methanol in a 25 ml volumetric flask and shaken again for 15 min. Then, 0.5 ml of samples were combined with the 3.5 ml methanol and 1 ml of 0.2 mM of DPPH in methanol and incubated for 30 min. After being incubated in dark conditions, the absorbance was measured at the wavelength of 517 nm (As). The control without a sample was prepared similarly (AC). Then, the inhibition percentage was computed using EquationEquation (6)(6)
(6) . The antioxidant activity (mg TE/g) of conventional and foam-mat dried roselle calyces powder was estimated using the Trolox standard curve and expressed as mg Trolox equivalent (TE)/g, as Thaipong et al. (Citation2006) described. For the DPPH assay, the Trolox standard curve was built at 0 to 125 mM concentrations.
Where: AC is the absorbance of the control (blank) solution, and AS is the absorbance of conventional or foam-mat dried roselle calyces powder
Antioxidant activity assay using ABTS
The method by Thaipong et al. (Citation2006) with a slight modification was used to measure the ABTS radical scavenging activity. ABTS reagent was freshly made by combining ABTS solution and potassium persulfate solution in a 1:1 (v/v) ratio and incubated in a dark room for 12–16 hours before use, and after incubation, 1 ml of the mixture solution was diluted with 50 ml methanol, and reagent ready to use. For samples, 0.1 gram of conventional or foam-mat dried roselle calyces powder was diluted with 10 mL of methanol in a volumetric flask. Then, 0.15 mL of prepared samples was combined with 2.85 mL of the ABTS reagent and then incubated for 30 minutes in dark conditions, following read absorbance at a wavelength of 745 nm (As). The controls were prepared as sample treatment without adding samples (Ac). The % inhibition percentage was computed using EquationEquation (7)(7)
(7) . The antioxidant activity (in mg TE/g) of foam-mat dried roselle calyces powder was estimated using the Trolox standard curve, and the antioxidant expressed as mg Trolox equivalent (TE)/g. The Trolox standard curve was built at 0 to 300 mM concentrations for the ABTS assay.
Where: AC is the absorbance of the control (blank) solution, and AS is the absorbance of foam-mat dried roselle calyces powder
Functional and physical properties analysis
Dissolution time
The dissolution time calculation was adopted using the method described by . Briefly, 1 g of conventional or foam-mat dried roselle calyces powder was combined with 10 mL of distilled water and mixed continuously using a magnetic stirrer probe (M-SH-Pro GSA) at 500 rpm. The duration of the power to dissolve well was calculated and expressed as dissolution time.
Bulk density
The bulk density was ascertained in compliance with the investigations of Keyata et al. (Citation2023). A 10 mL test tube containing one gram of conventional or foam-mat dried roselle calyces powder was compacted by tapping it repeatedly on the lab bench. The final bulk volume was noted, and EquationEquation (8)(8)
(8) was used to estimate the bulk density.
Water absorption capacity (WAC) and oil absorption capacity (OAC)
The water absorption capacity (WAC) was measured using a method by Keyata et al. (Citation2023). Briefly, 10 mL of distilled water and 1 gram (M0) of conventional or foam-mat dried roselle calyces powder were added to a preweighed 50 mL centrifuge tube. A mechanical shaker (Orbital Genie, Scientific Industries, Inc., U.S.A.) was used to shake the sample for an hour. After that, it was centrifuged for 30 minutes at 5000 rpm (EBA 200, Germany). The separated water was removed with a pipette, and the residues containing the remaining water were weighed again (M0). The WAC was calculated using EquationEquation (9)(9)
(9) .
The oil absorption capacity (OAC) was measured using methods expressed by Manupriya et al. (Citation2020). In short, five milliliters of refined palm oil were used to disperse 1 gram (M0) of conventional or foam-mat dried roselle calyces powder, and the resulting slurry was centrifuged for 30 min at 5000 rpm. After the supernatant was poured out and the centrifuge tube was inverted on a paper towel for five minutes, the residue was weighed (M1). EquationEquation (10)(10)
(10) was used to compute the OAC.
Color analysis
The L*, a*, and b* values of conventional or foam-mat dried roselle calyces powder were directly read using a chromameter (Minolta Chroma Meter CR-100, Japan). The color quality, including color intensity (CI), violet index (VI), and browning index (BI), was measured according to the methods explained by Marpaung and Paramaputri (Citation2023) and computed using EquationEquation (11)(11)
(11) –(Equation13
(13)
(13) ).
Notes: Aλmax is the absorbance at the wavelength of maximum absorbance; A580 is the absorbance at 580 nm; A520 is the absorbance at 520 nm; A420 is the absorbance at 420 nm; and A700 is the absorbance at 700 nm.
Particle size analysis
The particle size distribution of foam-mat dried roselle calyces powder was measured using the particle size analyzer Shimadzu SALD-7500nano (Shimadzu, Japan), and the results were processed using the Wing SALD II, Ver. 3.4.11 software
Data analysis
Data from three replications was analyzed by ANOVA, followed by Tukey for the significant (p < .05) treatments.
Results and discussions
HLB value
shows that all emulsifiers have high HLB values above 12. The mixture of monoglyceride, sorbitan monostearate, and polyglycerol ester in TBM reveals the highest HLB value, and the lowest is SSL. SSL is a single emulsifier reported to have an HLB value of 21 (Gómez et al., Citation2012). However, Liu et al. (Citation2023) and Liu et al. (Citation2023) reported the HLB value of SSL was 8.0. Monoglyceride has lower HLB than SSL, depending on the fatty acid in its structure, such as 3.8 for monostearate and 5.2 for monolaurate (Liu et al., Citation2023). Meanwhile, polyglycerol ester has HLB values ranging from 6 to 11 (Norn, Citation2014), this HLB value depends on the degree of polymerization (Vyakhaya & Parvez, Citation2020). The HLB value of sorbitan monostarate is 4.7 (Trujillo-Ramírez et al., Citation2019).
TBM and SP are cake emulsifiers in the gel form. Both comprise several emulsifiers with different HLB values. However, the emulsifier mixture has a much higher HLB value than the individual emulsifiers such as SSL. Combining different emulsifiers seems to have a synergistic effect on HLB value. A cake emulsifier is used mainly to incorporate air into the batter or to produce a sponge. Vyakhaya and Parvez (Citation2020) indicated that the hydrated monoglyceride helped provide air incorporation or aeration, emulsification, and a crystalline alpha form. Commercial emulsifiers are usually available in several emulsifier blends and are often provided as cake emulsifiers. The selection of emulsifiers is based on complementary and functional compatibility and the preservation of the alpha stability of monoglyceride. Propylene glycol, as the ingredient for SP and TBM, also reveals foaming ability with the HLB value ranging from 5.8 to 10.4 depending on the molecular weight (Tan et al., Citation2005). Therefore, the HLB values of emulsifier mixtures such as TBM and SP are higher than those of a single emulsifier SSL.
Foaming properties of roselle calyces extract
The amphiphilic surfactant molecules will absorb into the air bubble interface during foam formation (Pugh, Citation2016), and this process requires a high HLB value (Hollenbach et al., Citation2021) and high emulsifier concentration (Pugh, Citation2016). Hollenbach et al. (Citation2021) reported that high HLB values correlated well with better foam stability and foamability. The foaming properties of roselle calyces water extract, both red and purple calyces with various foaming agents, are summarized in . All emulsifiers have high HLB values that are suitable for incorporating air into the extract solution. SP produces the lowest foam expansion for both red and purple roselle calyces, meanwhile, TBM and SSL are better in foam formation. The highest ingredient concentration in SP is sorbitol syrup, meanwhile, monoglycerides dominate TBM. Although SSL has the lowest HLB value, it exhibits a better foam expansion than SP, which might be related to the higher purity emulsifier. All emulsifiers produced good foam expansion ranging from 33.61 to 42.30% (). Kumar et al. (Citation2022) reported foam expansion of 19.82% by using gum derivatives as foaming agent. The minimum requirement of foam expansion is 35%.
Table 2. The foaming properties of roselle calyces extract with different foaming agents.
Foam expansion is well correlated with foam density. The higher the foam expansion, the lower the density, which means more air bubbles were incorporated into the roselle extract solution. A similar result was reported by Sifat et al. (Citation2021), that the lower the density, the more the foam expands. All commercial emulsifiers exhibited low foam density which meant that they produced good foaming expansion. The foam density was ranging from 0.13 to 0.22 g/cm3 (). Kumar et al. (Citation2022) reported the initial density of banana puree by foam mat drying preparation varied from 0.93 to 0.97 g/cm3, and addition of gum derivatives as foaming agents decreased foam density into 0.44–0.56 g/cm3.
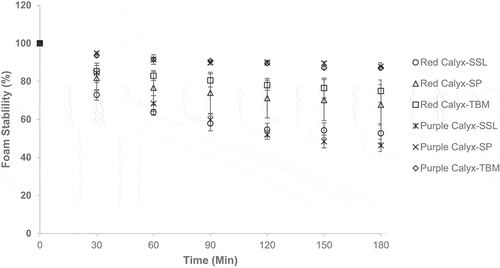
shows the foam stability is affected by the emulsifier. The foam gradually collapsed over time, indicating the foam instability. Among the three emulsifiers, SP shows the best foam stability both for purple and red roselle calyces extract solution, and the least stable is the foam stabilized by SSL. All the water extract uses maltodextrin as a filler, which also contributes to stabilizing the foam by increasing viscosity. This study used the low dextrose equivalent (DE) of maltodextrin. Siemons et al. (Citation2020) reported that low DE-value maltodextrins exhibited higher viscosity. The highest amount of ingredients in SP is monoglyceride sorbitol syrup, which might contribute to the foam viscosity and result in the highest foam stability for both red and purple roselle calyces extract. Meanwhile, due to the single emulsifier, SSL exhibits the lowest foam stability. TBM and SP are cake emulsifiers that are prepared in gel form and hydrated monoglyceride (Vyakhaya & Parvez, Citation2020) that aim to stabilize the foam during mixing and baking.
The roselle variety also affects the foam expansion, density, and stability. Purple roselle calyces show better foam expansion and stability than those of the red. The composition of the water extract might influence the foam. Fresh purple roselle calyces have lower pectin (data not shown) than the red ones, which pectin might contribute to the viscosity of the roselle water extract. However, pectin produces better viscosity at lower pH and depends on the degree of methoxylation. Fresh purple roselle calyces have higher methoxy content than the red ones (data not shown). Chen et al. (Citation2021) found that the gelation rate of pectin decreased by increasing the pH. The higher methoxyl pectin formed the gel at a low pH of less than 3.5 and a high sugar concentration (Gawkowska et al., Citation2018). Fresh purple and red roselle calyces have a pH of 3.54 and 3.70, respectively. Therefore, the pectin of purple roselle has a better contribution to the foaming properties than that of the red one.
Chemical properties and antioxidant activity
The red and purple foam-mat dried roselle calyces powder has lower total phenolics content and anthocyanins than the corresponding conventional dried powder (). Anthocyanins are part of phenolic classes, and their anthocyanin level is much lower than the total phenolic content. This means there are much higher quantities of other phenolic compounds besides anthocyanin. The level of anthocyanin is higher in the purple roselle calyces powder for both conventional and foam-mat dried. A similar finding was also reported by Aryanti et al. (Citation2019). The anthocyanin of red roselle calyces comprised of delphinidin-3-glucoside, delphinidin-3-sambubioside, cyanidin-3-glucoside, and cyanidin-3-sambubioside (Nguyen et al., Citation2022). To the best of our knowledge, no studies reported the anthocyanin types of purple roselle calyces.
Table 3. Chemical properties of conventional and foam-mat dried roselle calyces powder with different foaming agents.
Foam-mat dried powder has lower phenolics and anthocyanins, because of the water extraction of roselle calyces before foaming and drying. The detected phenolics are only water-soluble compounds thus the concentration is lower. Anthocyanins are water-soluble, and their solubility increases with the increase in pH value (Khoo et al., Citation2017). The pH value of the roselle powder is relatively low, mainly the foam-mat dried powder. Thus, they should be well soluble in the water. Usually, acidic pH is required to extract anthocyanin from the roselle calyces matrix to facilitate solvent penetration and remove anthocyanin from the vacuole (Idham et al., Citation2022). In this study, the solvent for extraction is neutral pH water thus the anthocyanin extractability becomes low.
The anthocyanin level of red roselle calyces powder with different emulsifiers is not significantly different from that of foam-mat dried powder with different emulsifiers. However, the type of emulsifiers affects the anthocyanin level. The red roselle powder has a much lower anthocyanin content than the purple one.
The highest anthocyanin level is found in the powder foamed by TBM or SSL depending on the roselle variety, and the lowest is SP-foamed powder. SP produced the most stable foam, preserving the interface between air and water, thus facilitating anthocyanins to contact with oxygen. The presence of oxygen will favour faster degradation of anthocyanins (Enaru et al., Citation2021).
The red and purple roselle calyces powder by conventional drying (cabinet drying) had different total phenolic compounds than those reported by Akther et al. (Citation2023) which revealed total phenolic compounds of 64.70, 32.23, and 25.84 mg GAE/g by freeze, cabinet, and sun drying, respectively. Foam mat drying produced much lower total phenolic compounds (). A similar phenomenon with anthocyanin was found for total phenolic content. The types of emulsifiers affect the total phenolic content of both the red and purple roselle calyces powder. The purple roselle calyces powder has a higher phenol content than the red one. Similar to anthocyanin content, the powder stabilized by TBM shows the highest total phenolic content. Phenolic compounds, as the parent of anthocyanins, have similar properties, such as they are easily degraded by oxygen. Therefore, the phenomena of both compounds are also identical.
Anthocyanins are a good antioxidant thus, anthocyanin-rich roselle extract has good antioxidant capacity (Wu et al., Citation2018). Data in shows that the antioxidant activity of conventional dried roselle calyces powder is higher than that of foam-mat dried one for both DPPH and ABTS radical scavenging activities. The higher phenolics and anthocyanin content contribute to the antioxidant activity, and the antioxidant activity of ABTS is higher than that of DPPH. This is also confirmed by previous study from Floegel et al. (Citation2011), and the DPPH method had lower sensitivity than ABTS (Martysiak-Zurowska & Wenta, Citation2012). The aim of comparing DPPH and ABTS methods in this study is to evaluate the different compounds that are responsible for antioxidant activity in the powder. Wu et al. (Citation2018) and Platzer et al. (Citation2021) explained that DPPH is hydrophobic and its reactions are via hydrogen atom transfer. On the contrary, ABTS is water-soluble, which reflects its antioxidant activity in an aqueous environment. The higher antioxidant activity of ABTS indicates that most of the antioxidant compounds are water-soluble. Data in shows that the antioxidant activity in equivalence to Trolox (mg TE/g) is well correlated to the percentage of DPPH inhibition for foam mat dried powders. Meanwhile, there is no consistent correlation between ABTS antioxidant activity (mg TE/g) with the percentage of ABTS inhibition. Trolox is a vitamin E with oil-soluble properties and is not soluble well in water. The ABTS analysis is suitable for aqueous environments in which vitamin E is not well-soluble.
The antioxidant activity of conventional dried roselle calyces is higher than that of foam-mat dried powder for both red and purple roselle calyces. This data correlates well with the total number of phenolic compounds and anthocyanins in both powders. The highest antioxidant activity in the percentage of radical scavenging inhibition is found in SSL-foamed dried powder for all samples, except the purple roselle calyces powder, in which the highest activity is TBM foamed powder. It seems that the antioxidant activity is affected by the foam stability. SSL stabilized foam before drying reveals the lowest stability and foam expansion thus preventing the oxidation of antioxidative compounds severely during drying. The antioxidant activity seems not to be in line with the total phenolics and anthocyanins content. The analysis of anthocyanins is based on the differential color changes regardless the anthocyanins have been oxidized and lost their antioxidant activity. Total phenolic content is determined based on the reduction of the Follin Ciocalteu reagent by phenols, and in this study, it might not be correlated well with the ability to scavenge free radicals.
Functional and physical properties
Conventional dried red and purple roselle calyces powder do not dissolve well in the water, which prevented the dissolution time from being defined. Meanwhile, the foam mat-dried powders dissolve well, with the dissolution time ranging from 72 to 128 s. The emulsifier types affect the dissolution time, the longest is found in powder foamed by SSL for both red and purple roselle calyces powder. SSL has the lowest HLB among emulsifiers, meaning it has a more dominant hydrophobic group than SP and TBM, thus making slow dissolution time.
The bulk density of the conventional dried purple roselle calyces powder is lower than that of the foam-mat dried powder, except for the red roselle calyces. The bulk density is affected by many factors, such as the density of the solids, the amount of entrapped air, and interstitial air (Kalyankar et al., Citation2016). The foam mat-dried powders tend to have higher bulk density than conventional dried. Conventional dried powders have a more complex mixture with different bulk densities. Although some air is incorporated during foaming and facilitates rapid water evaporation during foam mat drying, the bulk density is higher. Maltodextrin is used as a filler and has a high bulk density of 0.33–0.49 g/ml depending on the dextrose equivalent (Takeiti et al., Citation2010), thus contributing to the bulk density of foam mat-dried powders. Other compounds such as emulsifiers and solid extracts are also responsible for the foam mat dried powder bulk density.
Powder foamed by SSL has the lowest bulk density for both red and purple foam mat dried roselle calyces powders. This emulsifier exhibits the least stable foam resulting in less air entrapped and producing bigger particle size () than SP and TBM for both roselle calyces. The volume of air void between bigger particle sizes is higher than the smaller ones, resulting in lower bulk density (Qiu et al., Citation2015). The types of emulsifiers affect the particle size distribution (). The biggest mean particle diameter is SSL foamed powder and the smallest is TBM. Foam stability might influence particle size. The more stable the foam during drying, the more air entrapped is preserved and produces a more fragile film and ease to produce smaller particles.
Figure 4. Particle size distribution of foam mat-dried red (a) and purple (b) roselle calyces powder.
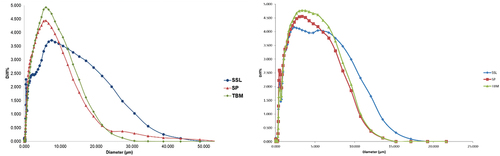
Table 4. Functional properties and pH values of conventional and foam-mat dried roselle calyces powder with different foaming agents.
Water and oil absorption capacities are important parameters for the application of roselle calyces powders in food processing. Conventional dried powder exhibits higher WAC than foam mat dried ones (). The foam mat dried powders comprise maltodextrin, emulsifier, and water-soluble roselle extract, meanwhile, the conventional dried powder is a complex mixture of protein, carbohydrate, fat, minerals, dietary fiber, and phytochemicals. Some components such as soluble dietary fiber and other hydrophilic substances are responsible for absorbing water. A similar phenomenon is also found for OAC, in which the presence of hydrophobic compounds such as fat contributed to higher OAC in conventional dried powder.
WAC and OAC are affected by the emulsifier types. A higher HLB value produces higher WAC because of the more dominant hydrophilic groups within the emulsifier, resulting in a higher ability for interaction with water. The HLB values seem not to be consistent with OAC, but the foam mat dried red and purple roselle calyces powders exhibit a similar pattern. The SP stabilized foam has the lowest OAC and the highest was found in the SSL. SSL has the lowest HLB value and the most dominant hydrophobic groups thus enabling it to absorb oil more. TBM has a higher HLB value than SP but exhibits higher OAC. The composition of the commercial emulsifiers used in this study might affect the ability to absorb oil. Sorbitol is the most dominant ingredient in SP and has hydrophilic properties thus preventing oil absorption, meanwhile, the most dominant ingredient of TBM is amphiphilic monoglycerides.
Color values and index
Color is one of the essential qualities of powdered and other dried food products. Conventional dried roselle calyces powder has lower lightness (L*) than the format dried powder both for red and purple roselle calyces (). Conventional dried powder has a higher total phenolic content and a longer drying time of 10–12 h, resulting in a brown color from the oxidized phenols. The purple roselle calyces powder has lower lightness than the red ones due to the higher anthocyanin and phenolic content. The formation of foam and the use of maltodextrin as a filler in the foam mat dried powder also contributed to the higher lightness than conventional dried powder. Based on the value of a* and b*, all samples have the positive a* and b* values which means the color between redness and yellowness. The yellowness values are very low and the redness is predominant. The purple roselle calyces powder has lower a* and b* values because the color tends to be purple.
Table 5. Color value and index of conventional and foam-mat dried roselle calyces powder with different foaming agents.
The dried powder foamed by SSL showed the lowest lightness both for red and purple roselle calyces, meanwhile, SP and TBM show no different lightness values. SSL exhibits the lowest foam expansion and foam stability and has the lowest bulk density. The SSL foamed powder is the most compact with the biggest particle size causing the lowest L * . The L* of foam-mat dried roselle calyces powders is higher than those produced with different foaming agents, such as egg albumin, of 25.27 to 30.40 (Tan & Sulaiman, Citation2020), also higher than spray-dried and freeze-dried calyces powder with L* of 39.3 and 29.7, respectively (Idham et al., Citation2012).
All color indices (CI, VI, and BI) of conventional dried powder are higher than those of foam-mat dried ones. CI and VI are closely related to the anthocyanin content of the powders, meanwhile, the BI indicates the browning degree during powder preparation and is used to examine the color degradation (Gao et al., Citation2022). The conventional dried powder has a higher phenolic content and longer drying time thus is more susceptible to oxidation and produces a brown color. The higher anthocyanin content contributes to the higher CI and VI values of conventional dried powder. The purple roselle calyces powders have a lower VI and BI, but a higher CI, than the red ones. A higher level of anthocyanin in purple roselle resulted in a higher CI. According to Marpaung and Paramaputri (Citation2023), a lower VI value means the color is becoming redder; thus, the VI value of the purple roselle calyces powder is lower because the color of this roselle calyces is more purplish. The increasing BI value indicates the brown compound formation from the degradation of anthocyanins (Cisse et al., Citation2012). A higher phenolic content of purple roselle calyces powder than the red ones, reveals the degradation is not more pronounced. Powders foamed by SSL had the lowest CI and the highest is found in TBM-stabilized powders for both red and purple roselle calyces, but inconsistent values are found for VI and BI.
The foam mat dried-roselle calyces powder had better lightness than the foam mat dried blueberry powder foamed by egg white powder, maltodextrin, and carboxymethyl cellulose (Gao et al., Citation2022). The foam mat drying formula with maltodextrin as stabilizer and all commercial emulsifiers exhibits better color preservation than those reported by Gao et al. (Citation2022) for blueberry extract powder indicating by lower BI. BI value indicates the browning degree, and the higher BI value means less reaction degree (Gao et al., Citation2022) or degradation reaction.
Conclusions
The characteristic of roselle calyces powder with various commercial emulsifiers as foaming agents (SSL, SP, and TBM) produced by foam-mat drying was successfully investigated. Foam mat-dried roselle calyces powder can be produced by using high HLB value emulsifiers. An HLB value less than 12 is not recommended for use as the foaming agent in foam mat-dried powder preparation. The composition of commercial emulsifiers affects the emulsifier performance during foam mat-dried powder preparation and characteristics. The roselle calyces type and various foaming agents used in producing foam-mat dried roselle calyces powder have different properties. The foam-mat dried roselle calyces powders have antioxidant activity both evaluated using DPPH and ABTS assay. In the future, foam-mat dried roselle calyces powders have the potentials as a food ingredient with high antioxidants. The foam mat dried roselle calyces is suitable to use as an ingredient for food industry. The better dissolution time than conventional dried powder makes this powder is suitable to use as an instant beverage. The contribution of color makes this foam mat dried roselles calyces is suitable as a natural food colorant and other purposes. Further studies are required to establish the effect of foam mat dried roselle calyces on the food product characteristics as well as the sensory properties and consumer acceptance.
Acknowledgment
The authors would like to thank Universitas Brawijaya for funding this study through Indonesian Collaborative Research (RKI, Riset Kolaborasi Indonesia) Year 2023 and the Ministry of Education, Culture, Research, and Technology, the Republic of Indonesia for RKI program.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Akther, M. F., Alim, A., Nasrin, N. A., Khan, M., Gomes, D. N., Suhan, M., Islam, M., & Begum, R. (2023). Effects of different drying methods on the proximate composition, antioxidant activity, and phytochemical content of Hibiscus sabdariffa L. Calyx. Food Chemistry Advances, 3, 100553. https://doi.org/10.1016/j.focha.2023.100553
- Aryanti, N., Nafiunisa, A., & Wardhani, D. H. (2019). Conventional and ultrasound-assisted extraction of anthocyanin from red and purple roselle (Hibiscus sabdariffa L.) calyces and characterisation of its anthocyanin powder. International Food Research Journal, 26(2), 529–11.
- Baliyan, S., Mukherjee, R., Priyadarshini, A., Vibhuti, A., Gupta, A., Pandey, R. P., & Chang, C. M. (2022). Determination of antioxidants byDPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules, 27(4), 1326. https://doi.org/10.3390/molecules27041326
- Chen, R., Ratcliffe, I., Williams, P. A., Luo, S., Chen, J., & Liu, C. (2021). The influence of pH and monovalent ions on the gelation of pectin from the fruit seeds of the creeping fig plant. Food Hydrocolloids, 111, 106219. https://doi.org/10.1016/j.foodhyd.2020.106219
- Cisse, M., Vaillant, F., Kane, A., Ndiaye, O., & Dornier, M. (2012). Impact of the extraction procedure on the kinetics of anthocyanin and color degradation of roselle extracts during storage. Journal of the Science of Food and Agriculture, 92(6), 1214–1221. https://doi.org/10.1002/jsfa.4685
- Djaeni, M., Kumoro, A. C., Sasongko, S. B., & Utari, F. D. (2018). Drying rate and product quality evaluation of roselle (Hibiscus sabdariffa L.) calyces extract dried with foaming agent under different temperatures. International Journal of Food Science, 2018, 1–8. https://doi.org/10.1155/2018/9243549
- Enaru, B., Dret Canu, G., Pop, T. D., Stanila, A., & Diaconeasa, Z. (2021). Anthocyanins: Factors affecting their stability and degradation. Antioxidants, 10. https://doi.org/10.3390/antiox10121967
- Fauziyah, N., Ifie, I., Syarief, O., & Darniadi, S. (2023). Impact of hydrocolloid and foaming agent on the physicochemical, microstructural and bioactive characteristics of foam-mat freeze-dried tapai (fermented black glutinous rice) powder. Food Science and Nutrition, 11(1), 578–589. https://doi.org/10.1002/fsn3.3098
- Floegel, A., Kim, D. O., Chung, S. J., Koo, S. I., & Chun, O. K. (2011). Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. Journal of Food Composition and Analysis, 24(7), 1043–1048. https://doi.org/10.1016/j.jfca.2011.01.008
- Franco, T. S., Perussello, C. A., Ellendersen, L. N., & Masson, M. L. (2016). Effects of foam mat drying on physicochemical and microstructural properties of yacon juice powder. LWT - Food Science and Technology, 66, 503–513. https://doi.org/10.1016/j.lwt.2015.11.009
- Gao, R., Xue, L., Zhang, Y., Liu, Y., Shen, L., & Zheng, X. (2022). Production of blueberry pulp powder by microwave-assisted foam-mat drying: Effects of formulations of foaming agents on drying characteristics and physicochemical properties. LWT, 154, 112811. https://doi.org/10.1016/j.lwt.2021.112811
- Gawkowska, D., Cybulska, J., & Zdunek, A. (2018). Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers, 10(7), 762. https://doi.org/10.3390/polym10070762
- Gómez, A. V., Ferrer, E., Añón, M. C., & Puppo, M. C. (2012). Analysis of soluble proteins/aggregates derived from gluten-emulsifiers systems. Food Research International, 46(1), 62–68. https://doi.org/10.1016/j.foodres.2011.12.007
- Hardy, Z., & Jideani, V. A. (2017). Foam-mat drying technology: A review. Critical Reviews in Food Science and Nutrition, 57(12), 2560–2572. https://doi.org/10.1080/10408398.2015.1020359
- Hollenbach, R., Oeppling, S., Delavault, A., Völp, A. R., Willenbacher, N., Rudat, J., Ochsenreither, K., & Syldatk, C. (2021). Comparative study on interfacial and foaming properties of glycolipids in relation to the gas applied for foam generation. RSC Advances, 11(54), 34235–34244. https://doi.org/10.1039/D1RA06190A
- Idham, Z., Muhamad, I. I., & Sarmidi, M. R. (2012). Degradation kinetics and color stability of spray-dried encapsulated anthocyanins from hibiscus sabdariffa L. Journal of Food Process Engineering, 35(4), 522–542. https://doi.org/10.1111/j.1745-4530.2010.00605.x
- Idham, Z., Putra, N. R., Aziz, A. H. A., Zaini, A. S., Rasidek, N. A. M., Mili, N., & Yunus, M. A. C. (2022). Improvement of extraction and stability of anthocyanins, the natural red pigment from roselle calyces using supercritical carbon dioxide extraction. Journal of CO2 Utilization, 56, 101839. https://doi.org/10.1016/j.jcou.2021.101839
- Ifie, I., Marshall, L. J., Ho, P., & Williamson, G. (2016). Hibiscus sabdariffa (roselle) extracts and wine: Phytochemical profile, physicochemical properties, and carbohydrase inhibition. Journal of Agricultural and Food Chemistry, 64(24), 4921–4931. https://doi.org/10.1021/acs.jafc.6b01246
- Izquierdo-Vega, J. A., Arteaga-Badillo, D. A., Sánchez-Gutiérrez, M., Morales-González, J. A., Vargas-Mendoza, N., Gómez-Aldapa, C. A., Castro-Rosas, J., Delgado-Olivares, L., Madrigal-Bujaidar, E., & Madrigal-Santillán, E. (2020). Organic acids from roselle (Hibiscus sabdariffa L.)-A brief review of its pharmacological effects. Biomedicines, 8(5), 1–16. https://doi.org/10.3390/BIOMEDICINES8050100
- Kalyankar, S. D., Deshmukh, M. A., Chopde, S. S., Khedkar, C. D., Lule, V. K., & Deosarkar, S. S. (2016). Milk powder. In B. Caballero, P. Finglas, & F. Toldra (Eds.), Encyclopedia of food and health (pp. 724–728). Elsevier.
- Keyata, E. O., Tola, Y. B., Bultosa, G., & Forsido, S. F. (2023). Bioactive compounds, antioxidant capacity, functional and sensory properties of optimized complementary weaning flour processed from sorghum, soybean, and karkade (Hibiscus sabdariffa L.) seeds. Scientific African, 19, e01457. https://doi.org/10.1016/j.sciaf.2022.e01457
- Khan, N. H., Abdulbaqi, I. M., Darwis, Y., Aminu, N., & Chan, S. Y. (2022). A stability-indicating HPLC-UV method for the quantification of anthocyanin in roselle (Hibiscus Sabdariffa L.) spray-dried extract, oral powder, and lozenges. Heliyon, 8(3), e09177. https://doi.org/10.1016/j.heliyon.2022.e09177
- Khoo, H. E., Azlan, A., Tang, S. T., & Lim, S. M. (2017). Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food and Nutrition Research, 61(1), 1361779. https://doi.org/10.1080/16546628.2017.1361779
- Kumar, P. S., Keran, D. A., Pushpavalli, S., Shiva, K. N., & Uma, S. (2022). Effect of cellulose and gum derivatives on physicochemical, microstructural and prebiotic properties of foam-mat dried red banana powder. International Journal of Biological Macromolecules, 218, 44–56. https://doi.org/10.1016/j.ijbiomac.2022.07.071
- Lara-Cortés, E., Osorio-Díaz, P., Jiménez-Aparicio, A., & Bautista-Bañios, S. (2013). Nutritional content, functional properties and conservation of edible flowers- review. Archivos Latinoamericanos de Nutricion, 63(3), 197–208.
- Li, T. S., Sulaiman, R., Rukayadi, Y., & Ramli, S. (2021). Effect of gum Arabic concentrations on foam properties, drying kinetics and physicochemical properties of foam mat drying of cantaloupe. Food Hydrocolloids, 116, 106492. https://doi.org/10.1016/j.foodhyd.2020.106492
- Liu, C., Li, Y., Liang, R., Sun, H., Wu, L., Yang, C., & Liu, Y. (2023). Development and characterization of ultrastable emulsion gels based on synergistic interactions of xanthan and sodium stearoyl lactylate. Food Chemistry, 400, 133957. https://doi.org/10.1016/j.foodchem.2022.133957
- Liu, Z., Zhao, M., Shehzad, Q., Wang, J., & Sun, B. (2023). Whippable emulsions co-stabilized by protein particles and emulsifiers: The effect of emulsifier type. Food Hydrocolloids, 137, 108379. https://doi.org/10.1016/j.foodhyd.2022.108379
- Low, L. K., & Ng, C. S. (1987). Determination of saponification value. In H. Hasegawa (Ed.), Laboratory manual on analytical methods and procedures. Singapore: Marine Fisheries Research Department, Southeast Asian Fisheries Development Center.
- Manupriya, B. R., Lathika, Somashekarappa, H. M., Patil, S. L., & Shenoy, K. B. (2020). Study of gamma irradiation effects on the physico-chemical properties of wheat flour (Triticum aestivum, L.), Radiation Physics & Chemistry, 172, 108693. https://doi.org/10.1016/j.radphyschem.2020.108693
- Marpaung, A. M., & Paramaputri, A. (2023). UV-visible light spectra of Clitoria ternatea L. flower extract during aqueous extraction and storage. International Food Research Journal, 30(3), 764–773. https://doi.org/10.47836/ifrj.30.3.18
- Martysiak-Zurowska, D., & Wenta, W. (2012). A comparison of ABTS and DPPH methods for assessing the total antioxidant capacity of human milk. Acta Scientiarum Polonorum, Technologia Alimentaria, 11(1), 83–89.
- Minh, N. P. (2020). Technical parameters affecting the spray drying of roselle (Hibiscus Sabdariffa) powder. Journal of Pure & Applied Microbiology, 14(4), 2407–2416. https://doi.org/10.22207/JPAM.14.4.18
- Nguyen, Q. D., Dang, T. T., Nguyen, T. V. L., Nguyen, T. T. D., & Nguyen, N. N. (2022). Microencapsulation of roselle (Hibiscus sabdariffa L.) anthocyanins: Effects of different carriers on selected physicochemical properties and antioxidant activities of spray-dried and freeze-dried powder. International Journal of Food Properties, 25(1), 359–374. https://doi.org/10.1080/10942912.2022.2044846
- Norn, V. (2014). Emulsifiers in food technology. John Wiley & Sons.
- Pensamiento-Niño, C. A., Castañeda-Ovando, A., Añorve-Morga, J., Hernández-Fuentes, A. D., Aguilar-Arteaga, K., & Ojeda-Ramírez, D. (2023). Edible flowers and their relationship with human health: Biological activities. Food Reviews International, 40(1), 620–639. https://doi.org/10.1080/87559129.2023.2182885
- Platzer, M., Kiese, S., Herfellner, T., Schweiggert-Weisz, U., Miesbauer, O., & Eisner, P. (2021). Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays. Molecules, 26(5), 1244. https://doi.org/10.3390/molecules26051244
- Pugh, R. J. (2016). Bubble and foam chemistry. Cambridge University Press.
- Qiu, J., Khalloufi, S., Martynenko, A., Van Dalen, G., Schutyser, M., & Almeida-Rivera, C. (2015). porosity, bulk density, and volume reduction during drying: Review of measurement methods and coefficient determinations. Drying Technology, 33(14), 1681–1699. https://doi.org/10.1080/07373937.2015.1036289
- Rezvankhah, A., Emam-Djomeh, Z., & Askari, G. (2020). Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: A review. Drying Technology, 38(1–2), 235–258. https://doi.org/10.1080/07373937.2019.1653906
- Samadi, S., & Fard, F. R. (2020). Phytochemical properties, antioxidant activity and mineral content (Fe, Zn and Cu) in Iranian produced black tea, green tea and roselle calyces. Biocatalysis and Agricultural Biotechnology, 23, 101472. https://doi.org/10.1016/j.bcab.2019.101472
- Sangamithra, A., Venkatachalam, S., John, S. G., & Kuppuswamy, K. (2015). Foam mat drying of food materials: A review. Journal of Food Processing and Preservation, 39(6), 3165–3174. https://doi.org/10.1111/jfpp.12421
- Sapian, S., Ibrahim Mze, A. A., Jubaidi, F. F., Mohd nor, N. A., Taib, I. S., Abd Hamid, Z., Zainalabidin, S., Mohamad Anuar, N. N., Katas, H., Latip, J., Jalil, J., Abu Bakar, N. F., & Budin, S. B. (2023). Therapeutic potential of Hibiscus sabdariffa linn. In attenuating cardiovascular risk factors. Pharmaceuticals, 16(6), 1–25. https://doi.org/10.3390/ph16060807
- Siemons, I., Politiek, R. G. A., Boom, R. M., van der Sman, R. G. M., & Schutyser, M. A. I. (2020). Dextrose equivalence of maltodextrins determines particle morphology development during single sessile droplet drying. Food Research International, 131, 108988. https://doi.org/10.1016/j.foodres.2020.108988
- Sifat, S. A. D., Trisha, A. T., Huda, N., Zzaman, W., & Julmohammad, N. (2021). Response surface approach to optimize the conditions of foam mat drying of plum in relation to the physical-chemical and antioxidant properties of plum powder. International Journal of Food Science, 2021, 3681807. https://doi.org/10.1155/2021/3681807
- Tajudin, N. H. A., Tasirin, S. M., Ang, W. L., Rosli, M. I., & Lim, L. C. (2019). Comparison of drying kinetics and product quality from convective heat pump and solar drying of roselle calyx. Food and Bioproducts Processing, 118, 40–49. https://doi.org/10.1016/j.fbp.2019.08.012
- Takeiti, C. Y., Kieckbusch, T. G., & Collares-Queiroz, F. P. (2010). Morphological and physicochemical characterization of commercial maltodextrins with different degrees of dextrose-equivalent. International Journal of Food Properties, 13(2), 411–425. https://doi.org/10.1080/10942910802181024
- Tan, S. N., Pugh, R. J., Fornasiero, D., Sedev, R., & Ralston, J. (2005). Foaming of polypropylene glycols and glycol/MIBC mixtures. Minerals Engineering, 18(2), 179–188. https://doi.org/10.1016/j.mineng.2004.08.017
- Tan, S. L., & Sulaiman, R. (2020). Color and rehydration characteristics of natural red colorant of foam mat dried hibiscus sabdariffa L. powder. International Journal of Fruit Science, 20(1), 89–105. https://doi.org/10.1080/15538362.2019.1605557
- Thaipong, K., Boonprakob, U., Crosby, K., Cisneros-Zevallos, L., & Hawkins, D. B. (2006). Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. Journal of Food Composition and Analysis, 19(6–7), 669–675. https://doi.org/10.1016/j.jfca.2006.01.003
- Trujillo-Ramírez, D., Lobato-Calleros, C., Jaime Vernon-Carter, E., & Alvarez-Ramirez, J. (2019). Cooling rate, sorbitan and glyceryl monostearate gelators elicit different microstructural, viscoelastic and textural properties in chia seed oleogels. Food Research International, 119, 829–838. https://doi.org/10.1016/j.foodres.2018.10.066
- Vyakhaya, J. D., & Parvez, R. (2020). Emulsifier gel as a cake improver: A review. Pramana Research Journal, 10(4), 21–26.
- Wu, H. Y., Yang, K. M., & Chiang, P. Y. (2018). Roselle anthocyanins: Antioxidant properties and stability to heat and pH. Molecules, 23(6), 1357. https://doi.org/10.3390/molecules23061357