ABSTRACT
The gut microbiota has been recognized as an important factor in the development of metabolic diseases such as obesity and is considered an endocrine organ involved in the maintenance of energy homeostasis and host immunity. Dysbiosis can change the functioning of the intestinal barrier and the gut-associated lymphoid tissues (GALT) by allowing the passage of structural components of bacteria, such as lipopolysaccharides (LPS), which activate inflammatory pathways that may contribute to the development of insulin resistance. Furthermore, intestinal dysbiosis can alter the production of gastrointestinal peptides related to satiety, resulting in an increased food intake. In obese people, this dysbiosis seems be related to increases of the phylum Firmicutes, the genus Clostridium, and the species Eubacterium rectale, Clostridium coccoides, Lactobacillus reuteri, Akkermansia muciniphila, Clostridium histolyticum, and Staphylococcus aureus.
Introduction
The gut microbiota has recently been recognized as an important factor for the development of metabolic diseases and is considered an endocrine organ involved in the maintenance of energy homeostasis and host immunity.Citation1 Changes in the composition of the gut microbiota due to environmental factors may result in a change in the relationship between the bacteria and the host. This change can result in a low-grade chronic inflammatory process and in metabolic disorders such as those present in obesity.Citation2
The human gut microbiota consists of up to 100 trillion microbes that exist in a largely symbiotic relationship with their human hosts, carrying at least 150 times more genes (the microbiome) than the human genome.Citation3 Based on 16S rRNA-targeted molecular analyses, most bacteria detected in fecal samples from healthy human volunteers belong to two phyla, Bacteroidetes and Firmicutes. The gram-negative Bacteroidetes phylum includes the genera Bacteroides, Prevotella, Parabacteroides, and Alistipes, while the gram-positive Firmicutes includes species such as Faecalibacterium prausnitzii, Eubacterium rectale, and Eubacterium hallii,Citation4 as well as many other low abundance species.
The metabolism of some bacteria can facilitate the extraction of calories from the diet, increase fat deposition in adipose tissue, exacerbate hepatic inflammatory processes, and provide energy and nutrients for microbial growth and proliferation.Citation5,Citation6 Several microbial genes involved in human metabolism are enriched or depleted in the guts of obese humans.Citation7 Obese people tend to have a higher proportion of genes which encode membrane transport functionsCitation8 and are involved in butyrate production,Citation9 whereas the genes related to cofactor, vitamin, and nucleotide metabolism or transcription are more frequently depleted.Citation8
Considering this influence of the gut microbiome on the onset and progression of obesity as well as its consequences, knowledge about the gut microbiota could contribute to the development of adjuvant treatments that can beneficially modulate obesity. Some studies have already evaluated the gut microbiota composition in obese individuals; however, the characterization of this microbiota is still not well established, and some results are discordant. Here, we present a review of the physiology and composition of the human gut microbiota with a focus on obese individuals. We divided our review into two topics: the physiology of the gut microbiota and the composition of this microbiota in obese patients.
Methods
In order to discuss gut microbiota composition of obese individuals, we undertook a systematized literature search that included observational studies (cross-sectional, cohort, or case-control) and experimental studies. The following exclusion criteria were used to reduce possible relationships observed due to other comorbidities: diabetes, intestinal diseases, cancer, experimental studies, and studies that supplemented gut microbiota modulators. The literature search was performed in the MEDLINE and Scopus databases, and the references of studies obtained were scanned for other relevant articles that may not have been detected by the primary search. Only studies published in English in the last 10 years were considered for review. The following Medical Subject Headings (MeSH) search strategy was used: (obesity[Title/Abstract] AND full text[sb] AND “last 10 years”[PDat]) AND (gut microbiota composition[Title/Abstract]) AND (full text[sb] AND “last 10 years”[PDat] AND (Humans[Mesh])).
Methodological quality was assessed using the STROBE recommendations (Strengthening the Reporting of Observational Studies in Epidemiology Statement) with separate checklists for conference case-control studies, cohort studies, and cross-sectional studies, and CONSORT recommendations (Consolidated Standards of Reporting Trials) using a checklist of items for reporting trials of nonpharmacological treatments. The final system was a combination of STROBE and CONSORT.
We also conducted a narrative review about the subject in the following topics: function of the gut microbiota on the development of lymphoid structures, function of the gut microbiota on the immune system, function of the gut microbiota on nutrient and lipid metabolism, function of the gut microbiota on the hormones involved in food intake, and gut microbiota and obesity: future perspectives. There were no restrictions placed on the year of publication in this section.
Physiology of the gut microbiota
The gut microbiota harbors incredibly large microbial and genetic diversity, with distinct species associated with specific parts of the gastrointestinal tract. The stomach contains about 101 microbial cells per gram of content. The duodenum contains about 103 cells; the jejunum,104 cells; the ileum, 107 cells; and the colon, 1012 microbial cells per gram of contents.Citation10 Therefore, the quantity of bacteria increases from the proximal to the distal portions of the gastrointestinal tract. Notably, the large intestine contains more than 70% of all microorganisms in the body, which are usually associated with the health/disease of the host.Citation11 In addition, the diversity of bacteria is higher in the lumen and lower in the mucus layer.Citation12
High numbers of bacteria in the gastrointestinal tract result in biochemical diversity and metabolic activity that interacts with host physiology. These microorganisms can facilitate the metabolism of non-digestible polysaccharides, produce essential vitamins, and they also play an important role in the development and differentiation of the intestinal epithelium and the host immune system.Citation13
Most species are anaerobic and belong to two phyla: Firmicutes and Bacteroidetes. Bacteria belonging to the phyla Proteobacteria, Verrucomicrobia, Actinobacteria, Fusobacteria, and Cyanobacteria are widely spread in human populations, but at much lesser abundance.Citation14 Although controversial, the ratio of Firmicutes-to-Bacteroidetes has been investigated and associated with the predisposition of diseases.Citation15 Moreover, the low abundance of phylum Proteobacteria associated with a high amount of the genera Bacteroides, Prevotella, and Ruminococcus has been associated with a healthy intestinal microbiota.Citation16 The maintenance of a healthy gut microbiota is important for a symbiosis relationship with the host.
Function of the gut microbiota on the development of lymphoid structures
The lymphatic system consists of a set of lymphatic vessels that interconnect primary to secondary lymphoid organs. Recirculation of the interstitial fluid and the transport of lymphocytes and antigen-presenting cells occur through this system. These immune cells are produced in the primary lymphoid tissues (thymus and bone marrow) and are activated in the secondary lymphoid tissues (spleen, lymph nodes, and mucosa-associated lymphoid tissue (MALT)).Citation17
Among the MALT, the gut-associated lymphoid tissues (GALT) are non-encapsulated tissues composed of Peyer's patches, isolated lymphoid follicles, and crypt plaquesCitation18 that begin to form during embryogenesis, when the environment is sterile. At this stage, the mesenchymal cells are induced by retinoic acid to produce the chemokine (C-X-C motif) ligand 13 (CXCL13) that attracts the human lymphoid tissue inducer (LTi) cells. Mature LTi cells induce differentiation of stromal cells and attract immune cells, which form the GALT.Citation17
The maturation of this tissue depends on microbial colonization after birth.Citation19 The stromal and epithelial cells recognize bacterial peptidoglycan through the signaling pattern recognition receptors (PRR), nucleotide-binding oligomerization domain-containing protein 1 (NOD1), and Toll-like receptors (TLRs). Activation of these receptors by the gut microbiota increases the expression of CC chemokine ligand 20 (CCL20) and β defensin 3 ligand (HBD3), which activate the formation of isolated lymphoid follicles from the binding of chemokine receptor 6 (CCR6) in LTi.Citation20 Changes in the microbial composition, which happens in obese individuals, can further disrupt the integrity of the intestinal barrier promoted by GALT, leading to pathological bacterial translocation and the initiation of an inflammatory response.Citation21
Function of the gut microbiota on the immune system
Besides acting on the maturation of GALT, the commensal bacteria also prevent the intestinal colonization by pathogens. The gut microbiota improves the function of the epithelial barrier, while its absence decreases the production of antimicrobial peptides by Paneth cells. This event causes intestinal barrier dysfunction and increases bacterial translocation.Citation22 Furthermore, bacteria-induced myeloid differentiation factor 88 (MyD88) signaling in the intestine increases epithelial cell IgA secretion. In addition, bacterial flagellin activates Toll-like receptors 5 (TLR5) from dendritic cells, and promotes the differentiation of B lymphocytes into IgA-producing cellsCitation23 IgA binds to the microbial antigens, neutralizes the activity of the pathogens, and prevents infection.Citation24
Commensal bacteria modulate the innate immune response of the host by stimulating the production of homeostatic levels of pro-IL-1β by resident macrophages so that the response of these cells to an enteric infection occurs more rapidly.Citation25 The protective role of IL-1β in intestinal immunity is mediated by the induction of expression of endothelial adhesion molecules, which contribute to neutrophil recruitment and destruction of pathogens in the gut.Citation26
Besides that, modulation of natural killer (NK) T cells is also performed by commensal bacteria. NK T cells are a subset of T cells that simultaneously express both T cell receptor (TCR) and NK cell receptors. These cells promote inflammation from the secretion of cytokines IL-2, IL-4, IL-13, IL-17A, IL-21, tumor necrosis factor (TNF), and interferon-γ (IFN- γ).Citation27 Maintenance of homeostasis of these cells prevents an exaggerated inflammatory reaction.Citation28
Also, an increase in inflammation has been associated with an increase in obesity-associated diseases, such as cardiovascular diseaseCitation29 and type 2 diabetes.Citation30 Intestinal dysbiosis (changes in gut microbiota composition) can be related to the trigger of a persistent low-grade inflammatory response in obese individuals. Lipopolysaccharides (LPS) contain lipid A, which can cross the intestinal mucosa through tight junctions or with the aid of chylomicrons.Citation31,Citation32 Lipoproteins are responsible for the absorption and transport of dietary triglycerides, and could thus initiate an inflammatory process that could result in the insulin resistance often observed in obesity.Citation31,Citation32 In the systemic circulation, LPS causes an innate immune response in liver and adipose tissue. This occurs from the binding of LPS to the LPS binding protein (LBP), which activates the CD14 receptor.Citation32 This complex binds to Toll-like 4 receptors (TLR4) on macrophages and adipose tissue, resulting in a signaling pathway that activates the expression of genes encoding pro-inflammatory proteins, such as factor nuclear kappa B (NF-κB) and activator protein 1 (AP-1).Citation32,Citation33
LPS concentrations are low in healthy people, but may reach high concentrations in obese individuals and cause metabolic endotoxemia.Citation31 This metabolic endotoxemia is related to the development of insulin resistance.Citation34 The molecular mechanisms that relate the activation of TLR4 by LPS with insulin resistance still need to be clarified, but evidence indicates that it involves alteration of insulin receptor signaling by the presence of inflammatory cytokines.Citation35
Function of the gut microbiota on nutrient metabolism and lipid metabolism
The gut microbiota derives its nutrients from the fermentation of carbohydrates ingested by the host. Bacteroides, Roseburia, Bifidobacterium, Fecalibacterium, and Enterobacteria are among the bacterial groups that typically ferment undigested carbohydrates and synthesize short chain fatty acids (SCFA)Citation36 such as acetate, butyrate, and propionate. A significant amount of acetate enters the systemic circulation and reaches the peripheral tissues, while the propionate is mainly used in liver, and the butyrate is used in intestinal epithelium as an energy source.Citation37 The total and relative concentrations of SCFA depend on the fermentation site, the carbohydrate consumed, and the composition of the gut microbiota.Citation38
In addition to synthesizing vitamin K and vitamin B components, several species belonging to the Firmicutes and Actinobacteria phyla are conjugated linoleic acid (CLA) producers.Citation39 CLA is a mixture of positional and geometric isomers of linoleic acid shown by some studies to have anti-obesity properties such as: increase in energy metabolism and expenditure, decrease in adipogenesis, decrease in lipogenesis, and increase in lipolysis and adipocyte apoptosis.Citation39 The biological effects of CLA have been attributed to two possible mechanisms of action: 1) CLA displaces the arachidonic acid from cell membrane phospholipids, which decreases the synthesis of arachidonic acid-derived eicosanoids such as prostaglandins and leukotrienes involved in inflammation,Citation40 and 2) CLA mediates activation of transcription factors such as peroxisome proliferator-activated receptors (PPARs), which impact cell processes such as lipid metabolism, apoptosis, and immune function.Citation40
Short chain fatty acids
The gut microbiota of obese mice had a higher amount of genes that encode enzymes involved in carbohydrate metabolism and greater capacity to extract energy from the diet and to produce SCFA when compared to non-obese mice.Citation41 In addition, germ-free mice were resistant to diet-induced obesity.Citation42
SCFAs bind to G protein-coupled receptors (GPCR41 and GPCR43).Citation36 Acetate binds primarily to GPCR43, the propionate binds to both GPCR41 and GPCR43, and the butyrate binds to GPCR41. GPCR41 and GPCR43 receptors are expressed in the intestinal epitheliumCitation37 and in adipose tissue.Citation36 The presence of GPCRs in adipose tissue suggests that this tissue is an important target for the metabolites produced by the gut microbiota. One study identified that rats fed a high fat diet had higher GPCR43 expression in adipose tissue and in vitro. SCFA increased the expression of PPARs, an important mediator of adipogenesis.Citation43 SCFAs that are bound to GPCR41 stimulate the expression of leptin in adipocytes and those that bind to GPCR43 appear to stimulate adipogenesis.Citation44 Thus, the profile of fatty acids produced may be related to the development of obesity. However, further investigations should be performed to confirm these results in humans.
Lipid metabolism
The endocannabinoid system is expressed in tissues that control energy balance (pancreas, muscle, gut, fat, liver, and hypothalamus) and regulates feeding behavior and metabolism.Citation45 This system is composed of bioactive lipids that bind to cannabinoid receptors, which results in cell signaling. The best characterized of these lipids are anandamide (AEA) and 2-arachidonoylglycerol (2-AG),Citation46 which activate receptors coupled to G, CB1, and CB2 proteins, thus activating the PPARα, GPR55, and GPR119 receptors.Citation47 The modulation of the gut microbiota or the reduction of CB1 activation improves the integrity of the intestinal barrier and reduces metabolic endotoxemia and low-grade inflammation.Citation47 Metabolic endotoxemia increased adipocyte hyperplasia and recruitment of macrophages into adipose tissue in a CD14 dependent pathway and increases the production of activin A, which activated the proliferation of adipocyte precursor cells. In addition, the consumption of a high fat diet caused endotoxemia and favored the development of metabolic diseases, suggesting that components of gut bacteria can remodel adipose tissue.Citation48 The control of this mechanism can prevent the development of obesity and its comorbidities.Citation48
In addition to altering the adiposity process, the microbiota acts at many levels, from lipid processing and absorption to systemic lipid metabolism.Citation49,Citation50 This change can be explained by the assimilation of cholesterol by bacterial cells, binding of cholesterol to bacterial cell walls, inhibition of hepatic cholesterol synthesis, redistribution of cholesterol from the plasma to the liver through the action of SCFA and/or deconjugation of bile acids by hydrolysis.Citation51
Evidence also suggests a link between dysbiosis and pathological changes in the metabolism of deconjugated bile acids in obese patients.Citation52 Bacterial bile salt hydrolase (BSH) enzymes in the gut cleave the amino acid side chain of glyco- or tauro-conjugated bile acids to generate unconjugated bile acids (cholic and chenodeoxycholic acids), which are then amenable to further bacterial modification to yield secondary bile acids (deoxycholic and lithocholic acid).Citation53 Secondary bile acids binded to cellular receptors, such as G protein-coupled receptor TGR5,Citation54 and reduced macrophage inflammation and lipoprotein uptake resulting in less atherosclerotic plaque formation, which decreased the development of atherosclerosis.Citation55
Function of the gut microbiota on the hormones involved in food intake
The gut microbiota has been implicated in the control of food intake and satiety through gut peptide signaling, where bacterial products activate enteroendocrine cells by modulating enterocyte-produced paracrine signaling molecules.Citation56 Gut microbiota may increase production of certain SCFA, which have been shown to be associated with an increase in peptide YY (PYY),Citation57 ghrelin, insulin, and glucagon-like peptide-1 (GLP-1) production.Citation58
Ghrelin was negatively correlated with Bifidobacterium, Lactobacillus, and B. coccoides/Eubacterium rectale, and positively correlated with Bacteroides and Prevotella.Citation59 Ingestion of oligofructose, a prebiotic that promotes the growth of Bifidobacterium and Lactobacillus, decreased the secretion of ghrelin in obese human.Citation60
GLP-1 also is modulated by the gut microbiota and is responsible for controlling food intake and insulin secretion. The concentration of this hormone was lower in obese individuals compared to eutrophic individuals.Citation61,Citation62 Butyrate produced by intestinal bacteria was present in smaller amounts in obese individualsCitation63 and regulated energetic homeostasis by stimulating adipocytes to produce leptin and by inducing GLP-1 secretion by L cells.Citation64 At least in mice, modulation of the gut microbiota by probiotics increased the production of butyrate by commensal bacteria, inducing the production of GLP-1 by intestinal L cells and thus reducing adiposity.Citation65
In addition, the gut microbiota may favor the formation of specific bile acids that activate the TGR5 receptors. Intestinal bacteria dehydrate chenodeoxycholic acidCitation66 and produce lithocholic acid, which binds to TGR5Citation67 and increases energy expenditure in brown adipose tissue and GLP-1 secretion by activation in the intestinal L cells,Citation54 thus preventing obesity and insulin resistance.Citation68
The insulin concentrations also appear to be altered in accordance with the gut microbiota.Citation69 Gut microbiota transplantation from lean subjects to patients with metabolic syndrome increased insulin sensitivity.Citation70 This effect is probably related to the reduction of chronic low-grade inflammation, resulting from LPS translocation and, consequently, to greater activation of the insulin signaling cascade.Citation71 Like GLP-1, PYY is also produced by intestinal L cells in the form of PYY1–36 and PYY3–36, the latter being present in higher concentrations in the postprandial period, causing a sensation of satiety.Citation72 Obese individuals produced less PYY3–36, and no resistance to the hormone was observed. Batterham et al.Citation73 found a 30% reduction in food intake 90 minutes after the infusion of PYY3–36 in obese individuals, a value similar to eutrophic patients. The modulation of the gut microbiota with prebiotic (oligofructose) of healthy subjects resulted in increased bacterial fermentation, glucose tolerance, and reduced appetite from increased concentrations of GLP-1 and PYY,Citation74 probably due to a mechanism associated with the production of propionate by intestinal bacteria.Citation75 Therefore, the gut microbiota is also related to the development of obesity, due to the possible capacity to alter the food intake.
The human gut microbiota composition in obesity
Phyla changes after weight loss
A higher Firmicutes-to-Bacteroidetes ratio related to obesity was observed in obese children when compared to normal weight children,Citation76,Citation77 in overweight/obese women with metabolic syndrome when compared with overweight/obese women with non-metabolic syndrome,Citation78 and in Japanese overweight individuals when compared with non-overweight individuals.Citation79 Furthermore, the Firmicutes phylum has been shown to be negatively correlated with the resting energy expenditure (REE) as well as positively correlated with fat mass percentage.Citation80 A cross-over clinical trial observed that a 20% increase in the Firmicutes phylum abundance was associated with an increase of 150 kcal in energy harvest.Citation81 Finally, one study reported a decrease in the Firmicutes-to-Bacteroidetes ratio after weight loss by obese individuals ().Citation15
Table 1. Main results of studies that evaluated the gut microbiota composition in obesity.
Obese individuals seem to have fewer Bacteroidetes counts than normal weight individuals.Citation79,Citation82,Citation83 On the other hand, two studies associated the Bacteroidetes phylum with weight gain in pregnant women.Citation84,Citation85 A cross-over study with 29 subjects did not find differences in the proportion of Bacteroidetes between obese and non-obese individuals ().Citation86
A decrease of Firmicutes was observed after Roux-en-Y gastric bypass (RYGB)Citation87,Citation88 and after laparoscopic sleeve gastrectomy (LSG).Citation89 In contrast, Bacteroidetes counts increased after RYGB and LSGCitation88,Citation89 but after a very low-calorie diet.Citation89 This phylum decreased with a concomitant increase in the Firmicutes phylum. A decrease in the Firmicutes-to-Bacteroidetes ratio after diet therapy also was observed, and the Bacteroidetes proportion was positively correlated with a percentage of loss of body fat ().Citation15
Obesity related genus changes
The genera StaphylococcusCitation77,Citation84,Citation85,Citation90,Citation91 and ClostridiumCitation84,Citation85,Citation89,Citation92 have been shown to be positively associated with obesity. A decrease in the genus Faecalibacterium was reported after LSG,Citation89 while the same genus increased after RYGB.Citation93 All these genera belong to the Firmicutes phylum (). The Firmicutes phylum contains many butyrate producing species, and an increase in butyrate and acetate synthesis may contribute to an increase in energy harvest in obese people.Citation15,Citation94 Furthermore, acetate can be absorbed and used as a substrate for lipogenesis and gluconeogenesis in the liver.Citation49
The genus Bacteroides, which belongs to the phylum Bacteroidetes, was shown to have an inverse relationship with obesity in overweight/obese women with metabolic disorderCitation78 after RYGBCitation88,Citation93 and LSG ().Citation89 Bifidobacterium, which belongs to the phylum Actinobacteria, was also shown to have an inverse relationship with obesity in pregnant women,Citation84,Citation90 children,Citation91 and infants of normal weight mothersCitation85; however, this genus was decreased in individuals subjected to RYGB.Citation88,Citation93 Bifidobacterium species have been shown to deconjugate bile acids, which may decrease fat absorption.Citation95 In contrast, strains of the same species can have contradictory effects, as it has been shown that different Bifidobacterium strains might increase (strain M13–4) or decrease body weight (strain L66–5).Citation96
Methane-producing archaea (methanogens) have been shown to affect caloric harvest by increasing the capacity of polysaccharide-eating bacteria to digest polyfructose containing glycans, which leads to increased weight gain in mice.Citation41 A study demonstrated that humans with methane detectable via a breath test have a significantly higher body mass index (BMI) than methane-negative controls (). This implies a higher amount of M. smithii in obese individuals, which was not observed in studies assessing gut archaeal populations.Citation97
Gut microbiota and obesity: future perspectives
Although several links have been reported between the gut microbiome and obesity (), the mechanisms are not yet understood that explain how and when the microbiome affects the obese state. Most studies investigating the relationships between obesity and the gut microbiome use very small sample sizes and use a variety of analytical methods to infer the intestinal microbial composition. Such factors are likely responsible for the considerable heterogeneity observed in the results. For instance, different DNA extraction kits have an impact on the assessment of the human gut microbiota, making it difficult to compare data across studies.Citation98
Table 2. Genus and species of bacteria and its relation to obesity.
Probiotics, prebiotics, and antibiotics have been evaluated, and they may become new therapeutic possibilities for the treatment of obesity. Oral supplementation with probiotics seems to reduce the concentrations of low-density lipoproteins (LDL) and total cholesterol; to ameliorate atherogenic indices; to improve glycemic controlCitation99; to reduce body weight, waist circumference, BMI, and abdominal visceral adipose tissueCitation100; to improve body composition,Citation101 and to reduce the concentrations of pro-inflammatory markers such as interleukin 6 (IL-6) and TNF-α[102 Prebiotics also have been shown to contribute to weight loss and improve metabolic parameters including insulin resistance.Citation60 Nevertheless, modulations performed with probiotics show results only for specific strains and for the period evaluated, with little data available regarding long-term benefits. In addition, the different ways in which different hosts can react to supplementation make it impossible to carry out generalizations. In the future, the modulation of the gut microbiota may be a way of assisting in the treatment of obesity, but for this idea to become a reality, there is a need to understand the metabolic interactions between the modulated bacteria and the host.
Conclusions
Although there is a large amount of heterogeneity in the data that is available, the following conclusions can be drawn from the literature review: 1) obesity was characterized by the presence of intestinal dysbiosis, marked by the distinct microbiome profile existing between obese and non-obese individuals; 2) the resulting dysbiosis could change the functioning of the intestinal barrier and the GALT, allowing the passage of structural components of bacteria, such as LPS, and activating inflammatory pathways that may contribute to the development of insulin resistance by alteration of insulin receptor signaling by the presence of inflammatory cytokines; 3) intestinal dysbiosis could alter the production of gastrointestinal peptides related to satiety, resulting in an increased food intake and contributing to a self-sustaining cycle; and 4) lipid metabolism could be altered by the changes observed in the gut microbiome, resulting in a stimulus to increase body adiposity ().
Figure 1. Possible mechanisms that related the obesity and intestinal dysbiosis with the physiological changes that contributed to the maintenance of obesity. GALT: gut-associated lymphoid tissue; IgA: immunoglobulin A; LPS: lipopolysaccharide; NF-κB: nuclear factor kappa B; CLA: conjugated linoleic acids; PPAR: peroxisome proliferator-activated receptor; LPL: lipoprotein lipase; Angptl4: angiopoietin like protein 4; GLP-1: glucagon-like peptide 1; PYY: peptide YY.
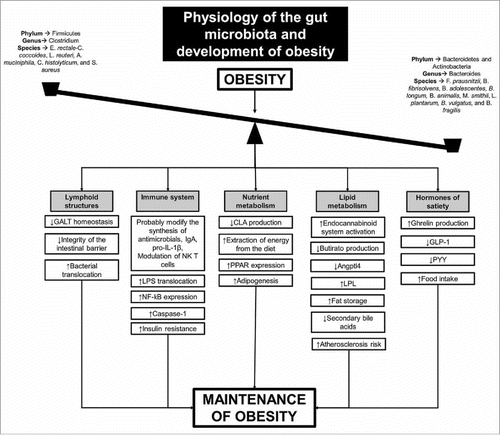
Understanding the changes occurring in the gut microbiome of obese individuals and the physiological consequences of these changes is a necessary step in creating modulation strategies that can be used to help treat this condition.
List of Abbreviations
| 2-AG | = | 2-arachidonoylglycerol |
| AEA | = | anandamide |
| AP-1 | = | activator protein 1 |
| BMI | = | body mass index |
| BSH | = | bile salt hydrolase |
| CCL20 | = | CC chemokine ligand 20 |
| CCR6 | = | chemokine receptor 6 |
| CLA | = | conjugated linoleic acid |
| C-X-C | = | chemokine |
| CXCL13 | = | ligand 13 chemokine |
| GALT | = | gut-associated lymphoid tissues |
| GLP-1 | = | glucagon-like peptide-1 |
| GPCR | = | G protein-coupled receptors |
| HBD3 | = | β defensin 3 ligand |
| IFN- γ | = | interferon-γ |
| IL-6 | = | interleukin 6 |
| LBP | = | LPS binding protein |
| LDL | = | low-density lipoproteins |
| LPS | = | lipopolysaccharides |
| LSG | = | laparoscopic sleeve gastrectomy |
| LTi | = | lymphoid tissue inducer |
| MALT | = | mucosa-associated lymphoid tissue |
| MyD88 | = | myeloid differentiation factor 88 |
| NF-κB | = | factor nuclear kappa B |
| NK | = | natural killer |
| NOD1 | = | nucleotide-binding oligomerization domain-containing protein 1 |
| PRR | = | pattern recognition receptors |
| PYY | = | peptide YY |
| RYGB | = | Roux-en-Y gastric bypass |
| SCFA | = | short chain fatty acids |
| TCR | = | T cell receptor |
| TLR4 | = | Toll-like 4 receptors |
| TLR5 | = | Toll-like receptors 5 |
| TLRs | = | Toll-like receptors |
| TNF | = | tumor necrosis factor. |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors' contributions
ACG: drafted the manuscript and performed the design of the study. CH and JFM: drafted and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Not applicable
References
- Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28:1221–38. doi:10.1210/me.2014-1108. PMID:24892638.
- Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–9. doi:10.1136/gutjnl-2015-309990. PMID:1026338727.
- Ursell L, Haiser HJ, Van Treuren W, Garg N, Reddivari L, Vanamala J, Dorrestein PC, Turnbaugh PJ, Knight R. The intestinal metabolome: an intersection between microbiota and host. Gastroenterol. 2014;146:1470–6. doi:10.1053/j.gastro.2014.03.001.
- Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol. 2010;12:304–14. doi:10.1111/j.1462-2920.2009.02066.x. PMID:19807780.
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host bacterial mutualism in the human intestine. Science. 2005;307:1915–20 doi:10.1126/science.1104816. PMID:15790844.
- Neyrinck AM, Etxeberria U, Taminiau B, Daube G, van Hul M, Everard A, Cani PD, Bindels LB, Delzenne NM. Rhubarb extract prevents hepatic inflammation induced by acute alcohol intake, an effect related to the modulation of the gut microbiota. Mol Nutr Food Res. 2017;61:1500899. doi:10.1002/mnfr.201500899. PMID:26990039
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi:10.1126/scitranslmed.3000322. PMID:20368178.
- Greenblum S, Turnbaugh PJ, Borenstein E. Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proc Natl Acad Sci USA. 2012;1099:594–9. doi:10.1073/pnas.1116053109.
- Ferrer M, Ruiz A, Lanza F, Haange SB, Oberbach A, Till H, Bargiela R, Campoy C, Segura MT, Richter M, et al. Microbiota from the distal guts of lean and obese adolescents exhibit partial functional redundancy besides clear differences in community structure. Environ Microbiol. 2013;15:211–26. doi:10.1111/j.1462-2920.2012.02845.x. PMID:22891823.
- Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi:10.1152/physrev.00045.2009. PMID:20664075.
- Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–803. doi:10.3748/wjg.v21.i29.8787. PMID:26269668.
- Swidsinski A, Loening-Baucke V, Lochs H, Hale LP. Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice. World J. Gastroenterol. 2005;11:1131–40. doi:10.3748/wjg.v11.i8.1131. PMID:15754393.
- Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19:59–69. doi:10.1016/j.smim.2006.10.002. PMID:17118672.
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi:10.1038/nature08821. PMID:20203603.
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi:10.1038/4441022a. PMID:17183309.
- Hollister EB, Gao C, Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterol. 2014;146:1449–58. doi:10.1053/j.gastro.2014.01.052.
- Van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nature Rev Immunol. 2010;10:664–74. doi:10.1038/nri2832.
- Pabst O, Herbrand H, Worbs T, Friedrichsen M, Yan S, Hoffmann MW, Körner H, Bernhardt G, Pabst R, Förster R. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur J Immunol. 2005;35:98–107. doi:10.1002/eji.200425432. PMID:15580658.
- Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nature Rev Immunol. 2012;12:9–23. doi:10.1038/nri3112.
- Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–10. doi:10.1038/nature07450. PMID:18987631.
- Brandl K, Schnab B. Is intestinal inflammation linking dysbiosis to gut barrier dysfunction during liver disease?. Expert Rev Gastroenterol Hepatol. 2015;9:1069–76. doi:10.1586/17474124.2015.1057122. PMID:26088524.
- Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–63. doi:10.1073/pnas.0808723105. PMID:19075245.
- Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nature Immunol. 2008;9:769–76. doi:10.1038/ni.1622.
- Frantz AL, Rogier EW, Weber CR, Shen L, Cohen DA, Fenton LA, Bruno ME, Kaetzel CS. Targeted deletion of MyD88 in intestinal epithelial cells results in compromised antibacterial immunity associated with downregulation of polymeric immunoglobulin receptor, mucin-2, and antibacterial peptides. Mucosal Immunol. 2012;5:501–12. doi:10.1038/mi.2012.23. PMID:22491177.
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi:10.1016/j.cell.2009.09.033. PMID:19836068.
- Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nature Rev Immunol. 2013;13:321–35. doi:10.1038/nri3430.
- Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells: bridging innate and adaptive immunity. Cell Tissue Res. 2011;343:43–55. doi:10.1007/s00441-010-1023-3. PMID:20734065.
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–93. doi:10.1126/science.1219328. PMID:22442383.
- Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–20. doi:10.1056/NEJMoa1107477. PMID:23034020.
- Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–7. doi:10.2337/diabetes.52.3.812. PMID:12606524.
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi:10.2337/db06-1491. PMID:17456850.
- Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176:3070–9. doi:10.4049/jimmunol.176.5.3070. PMID:16493066.
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–31. doi:10.1126/science.1179721. PMID:20203013.
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25. doi:10.1172/JCI28898. PMID:17053832.
- Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem. 2000;275:9047–54. doi:10.1074/jbc.275.12.9047. PMID:10722755.
- Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta. 2010;1801:1175–83. doi:10.1016/j.bbalip.2010.07.007. PMID:20691280.
- Lin HV, Frassetto A, Kowalik EJ, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240. doi:10.1371/journal.pone.0035240. PMID:22506074.
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–19. doi:10.1111/j.1365-2036.2007.03562.x. PMID:17973645.
- Kennedy A, Martinez K, Schmidt S, Mandrup S, Lapoint K, McIntosh MK. Antiobesity mechanisms of action of conjugated linoleic acid. J Nutr BioChem. 2010;21:171–9. doi:10.1016/j.jnutbio.2009.08.003. PMID:19954947.
- Belury MA. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu Rev Nutr. 2002;22:505–31. doi:10.1146/annurev.nutr.22.021302.121842. PMID:12055356.
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi:10.1038/nature05414. PMID:17183312.
- Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–84. doi:10.1073/pnas.0605374104. PMID:17210919.
- Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092–9. doi:10.1210/en.2005-0545. PMID:16123168.
- Macfarlane GT, Macfarlane S. Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J Clin Gastroenterol. 2011;45:S120–S12. doi:10.1097/MCG.0b013e31822fecfe. PMID:21992950.
- Matias I, MarzoV Di. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab. 2007;18:27–37. doi:10.1016/j.tem.2006.11.006. PMID:17141520.
- Lambert DM, Muccioli GG. Endocannabinoids and related N-acylethanolamines in the control of appetite and energy metabolism: emergence of new molecular players. Curr Opin Clin Nutr Metab Care. 2007;10:735–44. doi:10.1097/MCO.0b013e3282f00061. PMID:18089956.
- Muccioli GG, Naslain D, Backhed F, Reigstad CS, Lambert DM, Delzenne NM, Cani PD. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. doi:10.1038/msb.2010.46. PMID:20664638.
- Luche E, Cousin B, Garidou L, Serino M, Waget A, Barreau C, André M, Valet P, Courtney M, Casteilla L, et al. Metabolic endotoxemia directly increases the proliferation of adipocyte precursors at the onset of metabolic diseases through a CD14-dependent mechanism. Mol Metabolis. 2013;2:281–91. doi:10.1016/j.molmet.2013.06.005.
- Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, Felin J, Perkins R, Borén J, Oresic M, et al. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res. 2010;51:1101–12. doi:10.1194/jlr.M002774. PMID:20040631.
- Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–9. doi:10.1038/nature11552. PMID:22972297.
- Sonnenburg JL, Chen CT, Gordon JI. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 2006;4:e413 doi:10.1371/journal.pbio.0040413. PMID:17132046.
- Ma H, Patti ME. Bile acids, obesity, and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28:573–83. doi:10.1016/j.bpg.2014.07.004. PMID:25194176.
- Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–51. doi:10.1016/j.femsre.2004.09.003. PMID:16102595.
- Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–77. doi:10.1016/j.cmet.2009.08.001. PMID:19723493.
- Pols TW, Nomura M, Harach T, Lo Sasso G, Oosterveer MH, Thomas C, Rizzo G, Gioiello A, Adorini L, Pellicciari R, et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011; 14:747–57. doi:10.1016/j.cmet.2011.11.006. PMID:22152303.
- Cox HM, Tough IR, Woolston AM, Zhang L, Nguyen AD, Sainsbury A, Herzog H. Peptide YY is critical for acylethanolamine receptor Gpr119-induced activation of gastrointestinal mucosal responses. Cell Metab. 2010;11:532–42. doi:10.1016/j.cmet.2010.04.014. PMID:20519124.
- Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–1276. doi:10.1152/ajpregu.00442.2002. PMID:12676748.
- Neuman H, Debelius JW, Knight R, Koren O. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol Rev. 2015;39:509–21. doi:10.1093/femsre/fuu010. PMID:25701044.
- Queipo-Ortuno MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, Cardona F, Casanueva F, Tinahones FJ. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One. 2013;8:e65465. doi:10.1371/journal.pone.0065465. PMID:23724144.
- Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. 2009;89:1751–9. doi:10.3945/ajcn.2009.27465. PMID:19386741.
- Ranganath LR, Beety JM, Morgan LM, Wright JW, Howland R, Marks V. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut. 1996;38:916–9, 1996.
- Vrieze A, Holleman F, Zoetendal EG, de Vos WM, Hoekstra JB, Nieuwdorp M. The environment within: how gut microbiota may influence metabolism and body composition. Diabetologia. 2010;53:606–13. doi:10.1007/s00125-010-1662-7. PMID:20101384.
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi:10.1038/nature11450. PMID:23023125.
- Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–72. doi:10.1073/pnas.0808567105. PMID:18931303.
- Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288:25088–97. doi:10.1074/jbc.M113.452516. PMID:23836895.
- Gustafsson BE, Midtvedt T, Norman A. Isolated fecal microorganisms capable of 7-alpha-dehydroxylating bile acids. J Exp Med. 1966;123:413–32. doi:10.1084/jem.123.2.413. PMID:5325994.
- Iguchi Y, Yamaguchi M, Sato H, Kihira K, Nishimaki-Mogami T, Une M. Bile alcohols function as the ligands of membrane-type bile acid-activated G protein-coupled receptor. J. Lipid Res. 2010;51:1432–41. doi:10.1194/jlr.M004051. PMID:20023205.
- Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9. doi:10.1038/nature04330. PMID:16400329.
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62:3341–9. doi:10.2337/db13-0844. PMID:24065795.
- Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterol. 2012;134:913–6.e7. doi:10.1053/j.gastro.2012.06.031.
- Gomes AC, Bueno AA, De Souza RG, Mota JF. Gut microbiota, probiotics and diabetes. Nutr J. 2014;13. doi:10.1186/1475-2891-13-60.
- Grandt D, Schimiczek M, Beglinger C, Layer P, Goebell H, Eysselein VE, Reeve JR Jr. Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1–36 and PYY 3–36. Regul Pept. 1994;51:151–9. doi:10.1016/0167-0115(94)90204-6. PMID:8059011.
- Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–8. doi:10.1056/NEJMoa030204. PMID:12954742.
- Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–103. doi:10.1136/gut.2008.165886. PMID:19240062.
- Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes. 2015;39:424–9. doi:10.1038/ijo.2014.153.
- Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, Berry D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ MicroBiol. 2017;19:95–105. doi:10.1111/1462-2920.13463. PMID:27450202.
- Bervoets L, Hoorenbeeck KV, Kortleven I, Noten CV, Hens N, Vael C, Goossens H, Desager KN, Vankerckhoven V. Differences in gut microbiota composition between obese and lean children: a cross-sectional study. Gut Pathogens. 2013;5:10. doi:10.1186/1757-4749-5-10. PMID:23631345.
- Munukka E, Wiklund P, Pekkala S, Völgyi E, Xu L, Cheng S, Lyytikäinen A, Marjomäki V, Alen M, Vaahtovuo J, et al. Women with and without metabolic disorder differ in their gut microbiota composition. Obesity. 2012;20:1082–7. doi:10.1038/oby.2012.8. PMID:22293842.
- Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, Tameda M, Shiraki K, Ito M, Takei Y, et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015;15:100. doi:10.1186/s12876-015-0330-2. PMID:26261039.
- Kocełak P, Zak-Gołąb A, Zahorska-Markiewicz B, Aptekorz M, Zientara M, Martirosian G, Chudek J, Olszanecka-Glinianowicz M. Resting energy expenditure and gut microbiota in obese and normal weight subjects. Eur Rev Med Pharmacol Sci. 2013;17:2816–21. PMID:24174366.
- Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. doi:10.3945/ajcn.110.010132. PMID:21543530.
- Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and methanogens in anorexic patients. PLoS One. 2009;4(9):e7125. doi:10.1371/journal.pone.0007125. PMID:19774074.
- Million M, Thuny F, Angelakis E, Casalta JP, Giorgi R, Habib G, Raoult D. Lactobacillus reuteri and Escherichia coli in the human gut microbiota may predict weight gain associated with vancomycin treatment. Nutr Diabetes. 2013;3:e87. doi:10.1038/nutd.2013.28. PMID:24018615.
- Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight-women. Am J Clin Nutr. 2008;88:894–9. doi:10.1093/ajcn/88.4.894. PMID:18842773.
- Collado MC, Isolauri E, Laitinen K, Salminen S. Effect of mother's weight on infant's microbiota acquisition, composition, and activity during early infancy: a prospective follow-up study initiated in early pregnancy. Am J Clin Nutr. 2010;92:1023–30. doi:10.3945/ajcn.2010.29877. PMID:20844065.
- Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, Flint HJ. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes. 2008;32:1720–4. doi:10.1038/ijo.2008.155.
- Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, Krajmalnik-Brown R. Human gut microbiota in obesity and after gastric bypass. PNAS. 2009;106:2365–70. doi:10.1073/pnas.0812600106. PMID:19164560.
- Kong L, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot J, Zucker J, Doré J, Clément K. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98:16–24. doi:10.3945/ajcn.113.058743. PMID:23719559.
- Damms-Machado A, Mitra S, Schollenberger AE, Kramer KM, Meile T, Königsrainer A, Huson DH, Bischoff SC. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. BioMed Res Int. 2015;2015:1–12. doi:10.1155/2015/806248.
- Santacruz A, Collado MC, García-Valdés L, Segura MT, Martín-Lagos JA, Anjos T, Martí-Romero M, Lopez RM, Florido J, Campoy C, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104:83–92. doi:10.1017/S0007114510000176. PMID:20205964.
- Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–8. doi:10.1093/ajcn/87.3.534. PMID:18326589.
- Remely M, Tesar I, Hippe B, Gnauer S, Rust P, Haslberger AG. Gut microbiota composition correlates with changes in body fat content due to weight loss. Benef Microbes. 2015;6:431–9. doi:10.3920/BM2014.0104. PMID:25609655.
- Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Doré J, Henegar C, et al. Differential adaptation of human gut microbiota to bariatric surgery–induced weight loss links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–57. doi:10.2337/db10-0253. PMID:20876719.
- Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 2010;18:190–5. doi:10.1038/oby.2009.167. PMID:19498350.
- Begley M, Hill C, Gahan CG. Bile salt hydrolase activity in probiotics. Appl Environ MicroBiol. 2006;72:1729–38 doi:10.1128/AEM.72.3.1729-1738.2006. PMID:16517616.
- Yin Y-N, Yu Q-F, Fu N, Liu X-W, Lu F-G. Effects of four Bifidobacteria on obesity in high-fat diet induced rats. World J Gastroenterol. 2010;16:3394–401. doi:10.3748/wjg.v16.i27.3394. PMID:20632441.
- Basseri RJ, Basseri B, Pimentel M, Chong K, Youdim A, Low K, Hwang L, Soffer E, Chang C, Mathur R. Intestinal methane production in obese individuals is associated with a higher body mass index. Gastroenterol Hepatol. 2012;8:22–8.
- Wesolowska-Andersen A, Bahl MI, Carvalho V, Kristiansen K, Sicheritz-Pontén T, Gupta R, Licht TR. Choice of bacterial DNA extraction method from fecal material influences community structure as evaluated by metagenomic analysis. Microbiome. 2014;2:1–11. doi:10.1186/2049-2618-2-19.
- Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28:539–43. doi:10.1016/j.nut.2011.08.013. PMID:22129852.
- Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010;64:636–43. doi:10.1038/ejcn.2010.19. PMID:20216555.
- Gomes AC, de Sousa RG, Botelho PB, Gomes TL, Prada PO, Mota JF. The additional effects of a probiotic mix on abdominal adiposity and antioxidant status: a double-blind, randomized trial. Obesity. 2017;25:30–38. doi:10.1002/oby.21671. PMID:28008750.
- Zhang Y, Wang L, Zhang J, Li Y, He Q, Li H, Guo X, Guo J, Zhang H. Probiotic Lactobacillus casei Zhang ameliorates high-fructose-induced impaired glucose tolerance in hyperinsulinemia rats. Eur J Nutr. 2014;53:221–32. doi:10.1007/s00394-013-0519-5. PMID:23797890.
- Mai V, McCrary QM, Sinha R, Glei M. Associations between dietary habits and body mass index with gut microbiota composition and fecal water genotoxicity: an observational study in African American and Caucasian American volunteers. Nutr J. 2009;8:49. doi:10.1186/1475-2891-8-49. PMID:19845958.
- Santacruz A, Marcos A, Wärnberg J, Martí A, Martin-Matillas M, Campoy C, Moreno LA, Veiga O, Redondo-Figuero C, Garagorri JM, et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity. 2009;17:1906–15. doi:10.1038/oby.2009.112. PMID:19390523.
- Gauffin Cano P, Santacruz A, Moya Á, Sanz Y. Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS One. 2012;7:e41079. doi:10.1371/journal.pone.0041079. PMID:22844426.
- Rhee KJ, Sethupathi P, Driks A, Lanning DK, Knight KL. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J Immunol. 2004;172:1118–24 doi:10.4049/jimmunol.172.2.1118. PMID:14707086.
- Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3:487–95. doi:10.1038/mi.2010.29. PMID:20531465.
- Collins MD, Lawson PA, Willems A, Cordoba JJ, Fernandez-Garayzabal J, Garcia P, Cai J, Hippe H, Farrow JA. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–26. doi:10.1099/00207713-44-4-812. PMID:7981107.
- De Graaf AA, Venema K. Gaining insight into microbial physiology in the large intestine: a special role for stable isotopes. Adv Microb Physiol. 2008;53:73–168. PMID:17707144.
- Casas IA, Dobrogosz WJ. Validation of the probiotic concept: Lactobacillus reuteri confers broad-spectrum protection against disease in humans and animals. Microb Ecol Health Dis. 2000;12:247–85. doi:10.1080/08910600050216246-1.
- Lee HY, Park JH, Seok SH, Baek MW, Kim DJ, Lee KE, Paek KS, Lee Y, Park JH. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim Biophys Acta. 2006;1761:736–44. doi:10.1016/j.bbalip.2006.05.007. PMID:16807088.
- Le TK, Hosaka T, Le TT, Nguyen TG, Tran QB, Le TH, Pham XD. Oral administration of Bifidobacterium spp. improves insulin resistance, induces adiponectin, and prevents inflammatory adipokine expressions. Biomed Res. 2014;35:303–10. doi:10.2220/biomedres.35.303. PMID:25355437.
- Chen J, Wang R, Li XF, Wang RL. Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br J Nutr. 2012;107:1429–34. doi:10.1017/S0007114511004491. PMID:21914236.
- Fukuda S, Furuya H, Suzuki Y, Asanuma N, Hino T. A new strain of Butyrivibrio fibrisolvens that has high ability to isomerize linoleic acid to conjugated linoleic acid. J Gen Appl MicroBiol. 2005;51:105–13 doi:10.2323/jgam.51.105. PMID:15942871.
- Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8:42. doi:10.1186/s13073-016-0303-2. PMID:27098727.
- Flint HJ, Duncan SH, Scott KP, Louis P. Interactions and competition within the microbial community of the human colon: links between diet and health. Environ MicroBiol. 2007;9:1101–11. doi:10.1111/j.1462-2920.2007.01281.x. PMID:17472627.
