ABSTRACT
The capacity of bacterial pathogens to infect their hosts depends on the tight spatiotemporal regulation of virulence genes. The Listeria monocytogenes (Lm) metal efflux pump repressor CadC is highly expressed during late infection stages, modulating lipoprotein processing and host immune response. Here we investigate the potential of CadC as broad repressor of virulence genes. We show that CadC represses the expression of the bile salt hydrolase impairing Lm resistance to bile. During late infection, in absence of CadC-dependent repression, the constitutive bile salt hydrolase expression induces the overexpression of the cholic acid efflux pump MdrT that is unfavorable to Lm virulence. We establish the CadC regulon and show that CadC represses additional virulence factors activated by σB during colonization of the intestinal lumen. CadC is thus a general repressor that promotes Lm virulence by down-regulating, at late infection stages, genes required for survival in the gastrointestinal tract. This demonstrates for the first time how bacterial pathogens can repurpose regulators to spatiotemporally repress virulence genes and optimize their infectious capacity.
Introduction
Listeria monocytogenes (Lm) is a major intracellular foodborne bacterial pathogen which causes listeriosis, a human systemic infection.Citation1 Among zoonotic diseases under EU-surveillance, listeriosis is the most severe.Citation2 Lm has the capacity to colonize various niches, from inert and organic matrices to the intestinal lumen where it competes with resident microbiota, translocates across the epithelium, multiplies in phagocytic and non-phagocytic cells and disseminates via the blood.Citation1,Citation3 Lm can grow at temperatures ranging from 0 to 45ºC, under high osmolarity and acidic conditions, which it may encounter in the environment, in the food chain, as well as in the host. During infection, Lm faces proteolytic enzymes, acidic environment, high osmolarity and bile salts.Citation4
Bile salts are amphipathic molecules synthesized from cholesterol in the liver and secreted into the small intestine from the gall bladder.Citation5 They are major bile components able to degrade lipid-containing membranes and represent a key challenge to bacterial survival in the gastrointestinal tract.Citation6 Bacterial tolerance to bile salts is closely related to the activity of bile salt hydrolases (BSH) that catalyze the hydrolysis of glycodeoxycholate- (GDCA) and taurodeoxycholate- (TDCA) conjugated bile salts, thereby impairing bile toxicity toward bacteria. BSH is required for Lm resistance to bile and virulence.Citation7 Deconjugation of bile acids by BSH induces the release of free cholic acids (CA), that are exported by Lm through the MdrT efflux pump. mdrT is controlled by BrtA, a bile sensor which loses the ability to repress mdrT in the presence of CA.Citation8
To adapt and resist to the host environment, Lm evolved an arsenal of mechanisms that must be spatially and timely regulated.Citation9 PrfA, the major Lm virulence regulator, and σB, a general stress responsive sigma factor, were shown to control bsh expression and Lm tolerance to bile.Citation7,Citation10,Citation11 CadC is the transcriptional regulator of CadA, an efflux pump conferring cadmium resistance.Citation12 We previously showed that during in vivo infection, Lm uses CadC to directly repress the expression of LspB (lipoprotein signal peptidase B), thus avoiding the exposure of the lipoprotein LpeA to the host immune system, impairing inflammatory cytokine expression and ultimately promoting intramacrophage survival and virulence.Citation12
Results
CadC controls BSH activity and Lm resistance to bile salts
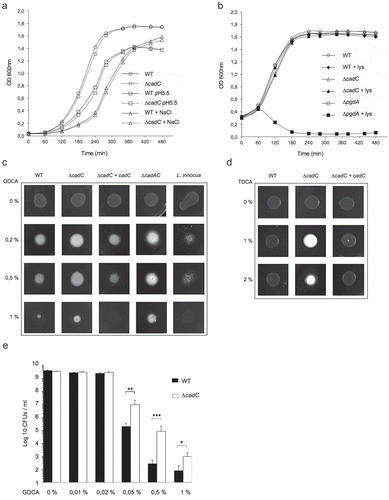
We hypothesized that CadC could be a broader regulator required for Lm survival and virulence. We investigated the potential involvement of CadC in the Lm resistance to different stresses that it could encounter in environmental and host conditions. In particular, we assessed the growth of the ΔcadC mutantCitation12 at pH 5.5 or in the presence of high concentrations of either NaCl or lysozyme. As shown in , no significant difference was observed between the growth of the wild type and the mutant strains in BHI broth at pH 5.5 or containing 5% NaCl. Similarly, no growth defect was detected in BHI broth containing 50 μg/ml of lysozyme (). As expected, we observed a significant decrease in the survival of the lysozyme-hypersensitive ΔpgdA mutant used as positive control ().Citation13 These data demonstrate that CadC has no role in the resistance to these stresses.
During infection, Lm has to resist to host bile.Citation6 This resistance is mainly promoted by the BSH that catalyzes the deconjugation of glyco- (GDCA) and tauro- (TDCA) conjugated bile salts under low oxygen levels.Citation7,Citation10 We analyzed the impact of cadC deletion on BSH activity and resistance to bile acids. The BSH activity of the WT, ∆cadC and ∆cadC+cadC strains was evaluated by patch inoculation onto Man-Rogosa-Sharpe (MRS) medium supplemented with increasing concentrations of GDCA or TDCA and grown under microaerophilic conditions. The WT strain exhibited the formation of a classical white area corresponding to precipitated bile acids,Citation14 confirming the presence of BSH activity (,d), whereas the nonpathogenic L. innocua, that lacks bsh,Citation7 was nearly incapable of precipitating GDCA (). As compared to the WT strain, the ∆cadC mutant displayed a more pronounced precipitate, a phenotype that was reverted in the ∆cadC+cadC strain. In addition, the ∆cadC strain was able to precipitate bile acids at concentrations where the WT was unable to deconjugate them (,d), indicating that CadC plays a role in Lm BSH activity. The ∆cadAC double mutant behaved as the ∆cadC single mutant (), indicating that the role of CadC in Lm BSH activity is independent of CadA. These results show that in absence of CadC, Lm exhibits a higher BSH activity.
To assess the correlation between increased BSH activity and resistance to bile toxicity in the ∆cadC mutant, the survival of WT and ∆cadC strains was compared in BHI broth supplemented with increasing concentrations of GDCA. As previously observed,Citation10 GDCA inhibited the growth of WT cells in a dose-dependent manner, starting from 0.05% (), whereas the ∆cadC mutant appeared significantly more resistant to GDCA than the WT strain.
Altogether these results demonstrate that CadC has a negative impacts on Lm BSH activity and resistance to bile salts.
CadC indirectly represses BSH expression
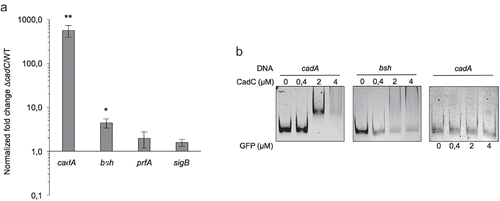
CadC is a transcriptional repressor previously shown to control cadAC and lspB expression.Citation12 CadC-dependent bsh transcription was thus assessed by qRT-PCR on RNAs extracted from WT and ΔcadC strains grown in BHI. CadA was used as control gene whose expression is directly repressed by CadC.Citation12 As expected, cadA was highly expressed in the ΔcadC mutant as compared to the WT strain (). Similarly, bsh expression was significantly increased in the absence of CadC (), indicating that bsh transcription is repressed by CadC.
bsh expression was previously shown to be dependent from PrfA and σB regulation.Citation7,Citation10,Citation11 In addition, we previously showed that PrfA does not control cadC expression.Citation12 To determine if the observed CadC-dependent expression of bsh could be due to an indirect regulation through PrfA and σB, the expression of these two regulators was assessed by qRT-PCR on RNAs extracted from WT and ΔcadC strains. Both prfA and sigB appeared to be expressed independently of CadC (), indicating that the CadC-dependent repression of bsh is not achieved through PrfA or σB.
CadC was shown to repress the expression of target genes by direct binding to conserved CadC boxes present in their promoter.Citation12 Despite the absence of a CadC box in the promoter region of bsh, we analyzed whether bsh expression could be controlled by the direct binding of CadC to its promoter. Increasing amounts of purified CadC were used in EMSA with a DNA fragment containing the bsh promoter region (). The promoter region of cadA was used as a positive control.Citation12 At the CadC concentration sufficient to delay the cadA promoter mobility, no shift was observed for the promoter regions of bsh, this being also observed using higher CadC concentrations (). An unrelated protein (GFP) was used to verify the specificity of the cadA-CadC box delayed migration. This indicates that CadC do not bind directly to the bsh promoter region.
Altogether, these data suggest that CadC indirectly represses bsh expression.
CadC plays no role in Lm survival in the gastrointestinal tract
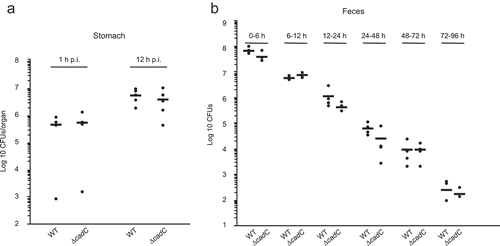
BSH was previously shown to play an important role in Lm persistence within the gastrointestinal tract, and an overexpression of bsh could generate an increased Lm intestinal multiplication.Citation7 As bsh is repressed by CadC, we thus analyzed the possible role of CadC in the gastrointestinal phase of listeriosis, i.e. in the earliest stage of the infectious process. We first assessed the survival/multiplication over 12-h post-inoculation of the WT and ∆cadC strains in the stomach of mice intragastrically inoculated with a sublethal bacterial dose (2x109 CFUs). For the WT strain, the number of bacteria in mouse stomachs was around 106 1-h postinoculation (p.i.), and increased to reach 107 CFUs at 12 h, demonstrating the survival and multiplication of Lm in the mouse stomach environment (). The ∆cadC mutant behaved as the WT strain, excluding a role for CadC in Lm survival in the stomach. In addition, the persistence of the ∆cadC mutant was studied and compared with its parental strain in stools of mice after intragastric inoculation (2x109 CFUs), over 4 d p.i. Both strains showed a regular and similar decrease in the number of viable Lm in mouse stools over the time ().
Altogether, these results demonstrate that CadC plays no critical role in Lm persistence within the gastrointestinal tract.
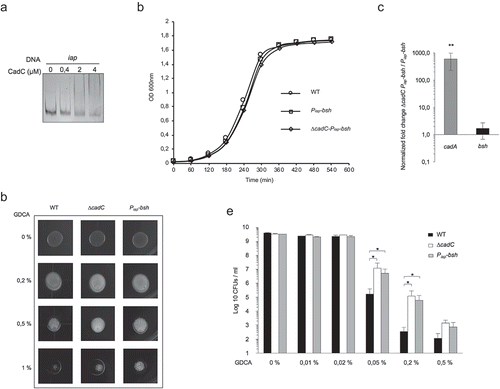
Optimal cadC-dependent regulation of BSH expression is required to confer full Lm virulence
We next evaluated the importance of the fine regulation of bsh expression during infection and addressed the role of CadC in this process. For this purpose, we constructed a strain in which bsh expression would escape the control by CadC. We replaced the bsh promoter by the promoter of the iap gene on the Lm chromosome (Piap-bsh). iap was previously shown to have an expression pattern similar to cadC and inverse to bsh, i.e. highly expressed in infected mouse spleens as compared to growth in the intestinal lumen or in rich medium (BHI).Citation15,Citation16 We first verified by EMSA that CadC does not bind to a DNA fragment corresponding to the iap promoter (). We confirmed that, in vitro, the growth of the Piap-bsh and ∆cadC-Piap-bsh strains was comparable to that of the WT (). CadC-independent bsh expression in the Piap-bsh strain was validated by the analysis of bsh expression, BSH activity and resistance to bile toxicity. Whereas, as expected, cadA transcript levels increased in the ∆cadC-Piap-bsh as compared to the Piap-bsh strain, bsh expression was not significantly different in both strains (), confirming the CadC-independent bsh expression in the Piap-bsh strain. In addition, whereas the ∆cadC generated a more pronounced bile acid precipitate than the WT, no differences were detectable between the ∆cadC and Piap-bsh strains (). In accordance, as compared to Lm WT, both strains revealed similar phenotypes regarding resistance to bile toxicity (). Altogether, these results confirm that, in the Piap-bsh strain, bsh is highly expressed and escapes CadC regulation.
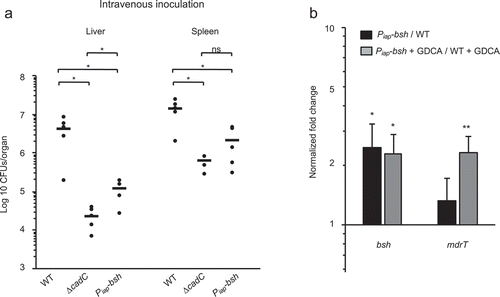
We then tested the effect of bsh mis-regulation on virulence in vivo by intravenously challenging of mice with WT or Piap-bsh strains (105 CFUs). Three days p.i., bacterial counts for Piap-bsh were significantly lower than those for the WT in both livers and spleens, mimicking the phenotype observed for the ∆cadC mutant (). These results strongly suggest that the CadC-independent expression of bsh is detrimental for Lm throughout infection and highlight the importance of the fine-tuning of bsh expression for Lm pathogenicity.
Uncontrolled BSH expression induces MdrT overexpression in presence of bile salts
BSH is able to hydrolyze conjugated glycodeoxycholic and taurodeoxycholic acids, leading to the deconjugation of glyco- and tauro-bile acids, and the release of free cholic acids.Citation7,Citation17 Host cholic acids were shown to be exported by Lm through the MdrT efflux pump.Citation8 mdrT is controlled by BrtA, a bile sensor which loses the ability to bind to and repress the mdrT promoter in the presence of cholic acid.Citation8,Citation18 Interestingly, the overexpression of MdrT was shown to significantly restrict Lm virulence in vivo.Citation19,Citation20 We thus hypothesized that an uncontrolled expression of bsh in vivo could induce an overproduction of cholic acid, in turn promoting a stronger mdrT expression and thus restricting Lm virulence.
To test this hypothesis, we analyzed by qRT-PCR the expression of bsh and mdrT in the WT and Piap-bsh strains, in absence or presence of bile salts (GDCA). We first observed that bsh is more expressed in the Piap-bsh strain as compared to the WT, independently of the presence of GDCA (). Whereas in absence of bile salts the uncontrolled bsh expression in the Piap-bsh strain had no effect on the mdrT expression, the presence of GDCA induced an increased mdrT expression in the Piap-bsh strain as compared to WT (). In vivo, the lack of CadC repression and the subsequent bsh overexpression could thus promote mdrT overexpression limiting Lm virulence.
CadC regulates additional Lm genes
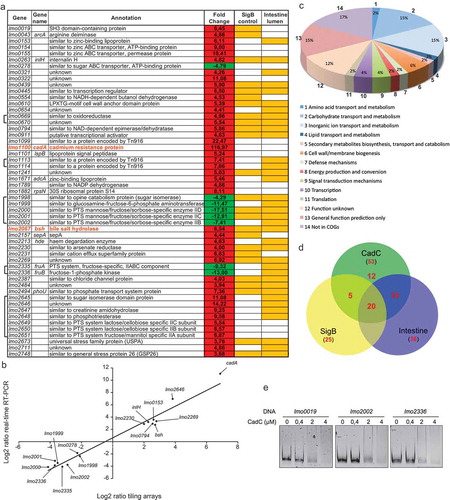
CadC appears thus as a crucial repressor for Lm infection. To assess if CadC could be a broad virulence regulator, we searched for other CadC-regulated genes. The expression profile of ∆cadC was compared to that of WT during exponential growth in BHI at 37ºC, using Lm tiling arrays.Citation16 Genes showing at least a two-fold change in their level of expression are listed in and Table S3. We found 53 genes differentially regulated in the ∆cadC mutant as compared to the WT strain, 45 of which were more expressed in absence of cadC. We further confirmed our results by qRT-PCR. We selected a subset of down- and up-regulated genes and performed qPCR on cDNA from bacteria grown to exponential phase. qRT-PCR results and array data exhibited a very strong correlation coefficient (R2 = 0.96) (), validating the differential expression levels detected by transcriptomics.
A large number of differentially expressed genes appeared to encode nutrient transport systems ( and ). In particular, 8 genes are implicated in inorganic ion transport and metabolism and are up-regulated in ∆cadC. This group includes cadA and lspB previously shown to be directly repressed by CadC,Citation12 and bsh. Remarkably, CadC also negatively controls the expression of the LPXTG surface protein-encoding genes inlH and lmo0610, both previously implicated in Lm virulence.Citation21,Citation22 In addition, CadC also negatively controls the expression of lmo2673 that encodes an universal stress protein A shown to be required for virulence.Citation23 Interestingly, 29 of the 45 genes more expressed in absence of CadC were previously shown to be activated during survival in the mouse intestinal lumen,Citation16 and 25 were shown to be activated by σB,Citation24–Citation26 the master regulator of class II stress genes particularly important for regulating transcription during the gastrointestinal stages of Lm infection ().Citation27 Remarkably, 19 genes are simultaneously controlled by CadC and σB, and activated during the intestinal phase of the infection.
To unravel a potential mechanism of CadC transcriptional regulation, we searched for a conserved motif in the promoter region of the 53 genes. Despite an exhaustive bioinformatic analysis, we were unable to detect any common regulatory sequence. To determine whether the expression of some of these genes could be controlled by the direct binding of CadC to their promoter region, as previously described for cadA and lspB,Citation12 or via other regulatory elements, increasing amounts of purified CadC were used in EMSA with DNA fragments containing the promoter region of genes up- (lmo0019) or down- (lmo2002 and lmo2336) regulated in the ∆cadC mutant. At the CadC concentration that is sufficient to delay the cadA promoter mobility (), and even using higher CadC concentrations, no shift was observed for the promoter regions of the genes tested ().
Together our data suggest that CadC acts as a virulence gene repressor promoting bacterial dissemination during Lm infection.
Discussion
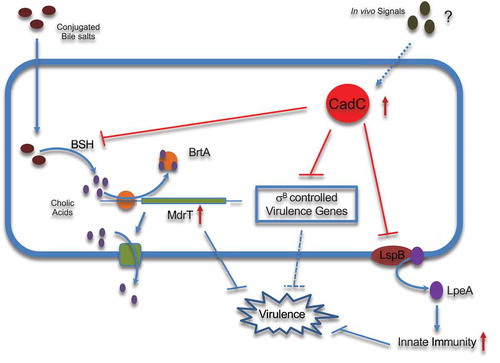
Bacterial adaptation to life inside the host depends on a coordinated network of activators and repressors. The presence of unrequired factors at inappropriate time frames during bacterial colonization can be detrimental to successful survival.Citation28 We previously showed that, during in vivo infection, Lm uses CadC to repress lspB expression and decrease the host inflammatory response by reducing exposure and immune recognition of the lipoprotein LpeA.Citation12 Here, we show that Lm also uses CadC to control BSH activity by repressing bsh expression. In accordance with its low expression in the intestinal lumen,Citation15,Citation16 CadC appears dispensable for Lm survival in the gastrointestinal tract. Inversely, CadC is highly expressed during infection of host organs,Citation15,Citation16 and CadC-dependent repression of bsh expression is required after crossing of the intestinal barrier to confer Lm full virulence. We reveal that, in presence of bile salts, an overexpression of bsh induces an upregulation of mdrT, most probably through the cholic acids (CA)-sensor BrtA. Whereas MdrT protects Lm from the bactericidal effects of bile,Citation8 its unregulated expression was shown to significantly restricts virulence in vivo ().Citation20 This strongly suggests that the uncontrolled expression of bsh in vivo induces CA overproduction, in turn promoting higher mdrT expression and restricting Lm virulence. We thus propose that Lm uses CadC to repress bsh expression specifically during infection stages where its overexpression would be deleterious for virulence. MdrT also transports c-di-AMP which is sensed by the host cytosolic innate immune receptor STING, activating a strong type I interferon (IFN-β) response.Citation29 Paradoxically, IFN-β production was shown to enhance susceptibility to Lm infection.Citation30,Citation31 The diversity of physiologically relevant substrates transported by MdrT reinforces the need for its tight regulation in vivo, in particular through indirect CadC repression.
We identified 53 CadC-regulated genes. Despite an exhaustive bioinformatic analysis, we were unable to identify any common regulatory motif, and no CadC binding was detected on the promoter of regulated genes other than cadAC and lspB, suggesting that CadC acts indirectly to control the expression of the remaining genes. CadC appears mostly as a repressor (85% of repressed genes), ion transport and metabolism genes constituting the largest target group. lmo0153-lmo0155 and lmo1671 encode a zinc ABC transporter and a zinc-binding protein, respectively, lmo2494 a regulatory protein of phosphate transport, lmo2230 an arsenate reductase and lmo2231 a cation efflux protein. This suggests a peripheral function for CadC in controlling arsenic, zinc and phosphate homeostasis. Most importantly, three genes known to be required for Lm virulence, inlH, lmo0610 and lmo2673 are also repressed by CadC, suggesting that the in vivo regulation of these genes by CadC is crucial at specific infection stages. Among the few genes that appear activated by CadC, lmo2000-lmo2002 encode components of a mannose/fructose/sorbose PTS system, and lmo1998-lmo1999 two sugar isomerases implicated in hexosamine metabolism, converting fructose-6-phosphate into glucosamine-6-phosphate. The end product of this pathway is N-acetylglucosamine, a peptidoglycan component also used for teichoic acids decoration,Citation32 suggesting the involvement of CadC in the regulation of the composition and/or structure of the bacterial cell wall. However, we were unable to detect by HPLC analysis any defect in the ∆cadC cell wall. lmo2335-lmo2336 (fruA-fruB) operon encodes components that participate in the transport and conversion of fructose to fructose-1-6-bisphosphate, and lmo0278 is a maltose-maltodextrin ABC transporter.Citation33 Fructose-1-6-bisphosphate and maltose are central metabolism intermediates, being used for glycolysis or deviated to other metabolic pathways. CadC regulation of these carbon source transporters might be important for the Lm growth and adaptability to the host environment, in particular the intestine.
During infection, Lm up-regulates the expression of major virulence regulators (PrfA, VirR), and of the master regulator of class-II stress genes, σB. In particular, the fine regulation of the PrfA regulon through complex PrfA-σB interactions appears essential during infection.Citation27 Indeed, bacteria need to ensure a rapid increased expression of virulence genes and their subsequent down-regulation to avoid irreversible host cell damages.Citation34 cadC is highly expressed during late infection stages,Citation15 and we observed here that a high proportion of CadC repressed genes were previously shown to be activated during survival in the mouse intestinal lumen,Citation16 and controlled by σB, the master transcriptional regulator of the gastrointestinal Lm infection stage. We propose that Lm CadC represses at late infection stages σB-controlled genes otherwise important in the gastrointestinal tract (), revealing CadC as a crucial new player of the complex network of transcriptional regulators that contributes to fine-tune virulence gene expression over the Listeria infectious process. Whereas in the environment CadC is regulated by cadmium,Citation12 in vivo, during infection, it most likely responds to other signals that remain to be identified. Our study also emphasizes the importance for a pathogen of not only activating virulence genes when they are needed but also suppressing them when they are detrimental, pointing new potential therapeutic targets.
Materials and methods
Bacterial strains and growth conditions
Bacterial strains used in this study are listed in Table S1. Listeria monocytogenes (Lm) and Escherichia coli (E. coli) strains were routinely cultured aerobically at 37°C in brain heart infusion (BHI, Difco) and Lysogeny Broth (LB, Difco), respectively, with shaking. When appropriate, the following antibiotics were included in culture media as selective agents: ampicilin (Amp), 100 μg/ml; chloramphenicol (Cm), 7 μg/ml (Lm) or 20 μg/ml (E. coli); erythromycin (Ery), 5 μg/ml.
Construction of mutant strains
Construction of the Piap-bsh strain was performed using the splicing-by-overlap-extension (SOE) procedure. Two pairs of primers were used (lmo2068MA-MB and catMA-MB) (Table S2) to amplify a 556-bp fragment from the upstream region of bsh and the 684-bp cat gene, respectively, using Accuzyme DNA Polymerase (Bioline). Resulting products were mixed in a 1:1 ratio and reamplified using primers lmo2068MA and catMB. The final product was digested and cloned into pMAD.Citation35 Two other primer pairs were used (iapMA-MB and bshMC-MD) (Table S2) in PCR to amplify, respectively, the 226-bp promoter region of iap and the first 583-bp of bsh. Resulting products were mixed and reamplified using primers iapMA and bshMD. The final product was digested and cloned into pMAD already containing the first fragment. The resulting plasmid was then electroporated in Lm EGD-e and transformants selected at 30°C in BHI-Ery. Positive clones were re-isolated in the same medium and grown overnight at 43°C. Integrant clones were inoculated in BHI broth and grown overnight at 30°C, after which the cultures were serially diluted, plated in BHI agar and incubated overnight at 37°C. Individual colonies were tested for growth in BHI-Ery at 30°C and antibiotic-sensitive clones were screened by PCR. Plasmid constructions and mutants were confirmed by PCR and DNA sequencing.
Resistance to ph 5.5, salt stress and lysozyme
Growth under stressful stimuli was monitored as described.Citation36 Lm cultures grown overnight were appropriately diluted in BHI broth and their growth under the presence of stressful stimuli was monitored by optical density measurement at 600 nm (OD600). For comparative analysis of Lm resistance to pH 5.5 and salt stress, bacterial cultures were diluted 100-fold in BHI alone (control) or BHI pH 5.5 or containing 5% NaCl. To assess the Lm resistance to lysozyme, overnight bacterial cultures were diluted 10-fold in BHI alone (control) or BHI containing 50 μg/ml of chicken egg white lysozyme (Sigma). An Lm mutant strain hypersensitive to lysozyme (ΔpgdA)Citation13 was used as a positive control for susceptibility.
BSH activity assays
Stationary cultures were dropped (10 μl) in MRS (Man, Rogosa and Sharpe) agar plates supplemented with increasing concentrations (0.2%, 0.5%, 1% and 2%) of purified glycochenodeoxycholic acid (GDCA, Merck Millipore) or taurochenodeoxycholic acid (TDCA, Santa Cruz Biotechnologies). Plates were incubated anaerobically for 72 h at 37°C (GENbox, Biomérieux).
Sensitivity to bile salts
Lm strains were grown to log phase in BHI broth at 37ºC. Cultures were diluted in BHI and 5 × 103 bacteria/ml were challenged with increasing concentrations (0.01%, 0.02%, 0.05%, 0.5% and 1%) of GDCA in a 24-well plate. Plates were then incubated with agitation at 37ºC in aerobic conditions. After 16 h, CFUs were assessed by bacterial enumeration of serial dilutions on BHI agar.
RNA techniques
Lm cultures were grown in BHI to exponential phase (OD600 nm = 0.8) and total RNA isolated by the phenol-chloroform method described elsewhere,Citation37 with modifications as described next. After lysis, RNA purification were performed using the TripleXtractor reagent (Grisp) following the manufacturer’s recommendations. DNA was eliminated by DNase treatment (Turbo DNA-free, Ambion) and RNA purity and integrity was verified by 1% (w/v) agarose gel electrophoresis and Experion Automated Electrophoresis System (Bio-Rad Laboratories). One μg of RNA was reverse-transcribed into cDNA using a random hexamer cocktail-based kit (iScript Kit, Bio-Rad Laboratories). qPCR was performed on one μg of cDNA in a 20-μl reaction volume using the iTaq™ Universal SYBR® Green Supermix (Bio-Rad Laboratories) and a real-time PCR detection system (iQ5, Bio-Rad Laboratories) with the following cycling protocol: 1 cycle at 95°C (3 min); 40 cycles at 95°C (10 s), 56°C (20 s) and 72°C (20 s). Primers are listed in Table S2. Each group comprises three biological replicates each with three technical replicates. Data were normalized to that of a reference housekeeping gene (16S rRNA) and analyzed by the comparative threshold (ΔΔCt) method.
Electrophoretic mobility shift assays (EMSAs)
Protein-DNA binding was set up in 20 μl reactions containing 100 ng of DNA synthesized with primers described in Table S2, binding buffer (50 mM Tris-HCl pH7.4, 6 mM MgCl2, 100 mM NaCl, 50 mM KCl) and increasing amounts of purified proteins (CadC/GFP) as previously described.Citation12 DNA was first incubated in binding buffer for 5 min followed by gentle mixing of the protein and 20 min incubation at room temperature. The total reaction was loaded into a 10% acrylamide native gel and ran in TAE buffer. The gel was stained for 10 min in a 0.01% GreenSafe Premium (NZYTech) TAE buffer solution and imaged in a GelDoc XR+ System (Bio-Rad Laboratories).
In vivo infection studies
Animal infections were performed on 6- to 9-week-old specific pathogen-free female C57BL/6 mice (Charles River Laboratories) maintained at the IBMC animal facilities, in high-efficiency particulate air (HEPA) filter-bearing cages under 12-h light cycles and in an ad libitum regiment of sterile chow and autoclaved water. Intravenous infections were performed by inoculation of 105 CFUs through tail vein injection as described.Citation38,Citation39 For oral infections mice were starved for 8–12 h before the procedure and inoculated with 2 × 109 CFUs (in PBS with 150 mg/ml CaCO3) by gavage. Mice were sacrificed by general anesthesia at indicated time points. Stomach, spleen and liver of each animal were aseptically removed, homogenized in PBS and homogenates were serially diluted and plated on BHI-agar plates. For analysis of Lm fecal carriage, total feces produced by each infected animal (n = 5 per strain) up to a given time-point were collected, homogenized in PBS and serial dilutions were plated in Listeria selective agar media (Oxoid) for bacterial enumeration. Animal procedures followed the guidelines of the European Commission for the handling of laboratory animals (directive 2010/63/EU), the Portuguese legislation for the use of animals for scientific purposes (Decreto-Lei 113/2013), and were approved by the IBMC Animal Ethics Committee as well as by the Direcção Geral de Alimentação e Veterinária, the Portuguese authority for animal protection, under license 015302.
Expression tiling arrays
ListIP Tiling Arrays were used.Citation16 RNAs were reverse-transcribed using SuperScript II reverse transcriptase (Life Technologies). cDNA was digested by DNase I (Turbo DNA-free, Ambion) and the size of digestion products was analyzed in the Agilent Bioanalyser 2100. Sample preparation for each chip was then processed following the Affymetrix GeneChip Expression Analysis Technical Manual (P/N 702232 Rev. 2) as previously described.Citation16 Scanning of the arrays was then performed using the GeneChip scanner 3000. Intensity signals of each probe cells were computed by the GeneChip operating software (GCOS). Data analysis of the tiling sub-array was performed using the Bioconductor software (http://www.bioconductor.org) based on R package as described in.Citation16
Statistics
Statistics were performed with Prism (GraphPad), using unpaired two-tailed Student’s t-test to compare means of two groups, and one-way ANOVA with Tukey’s post-hoc test for pairwise comparison of means from more than two groups, or with Dunnett’s post-hoc test for comparison of means relative to the mean of a control group.
Author contributions
Conceptualization (Design), S.S. and D.C.; Investigation (Experimental work), A.V., R.P., A.C. and C.A. Writing – Original Draft, D.C.; Writing – Review & Editing, A.V., R.P., A.C., C.A., P.C., S.S. and D.C.; Resources and Funding, P.C., S.S., and D.C.
Disclosure of potential conflict of interest
No potential conflict of interest were disclosed.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper or in the supplementary information. Expression tiling array results are available at the ArrayExpress database (https://www.ebi.ac.uk/arrayexpress/) under the accession number E-MTAB-7857.
Supplemental Material
Download MS Word (26.5 KB)Acknowledgments
We thank Rui Appelberg (IBMC-ICBAS) for PhD co-supervision of R.P. and A.C., S. Lamas (Animal Facility) and P. Magalhães (CCGEN) from IBMC, and G. Soubigou (Génopole Institut Pasteur) for technical support.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Cossart P. Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. Proc Natl Acad Sci U S A. 2011;108:19484–19491. doi:10.1073/pnas.1112371108.
- EFSA., European Food Safety Authority ECfDPaC. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. Efsa J 2017;15:5077.
- Carvalho F, Sousa S, Cabanes D. How Listeria monocytogenes organizes its surface for virulence. Front Cell Infect Microbiol. 2014;4:48. doi:10.3389/fcimb.2014.00048.
- NicAogain K, O’Byrne CP. The role of stress and stress adaptations in determining the fate of the bacterial pathogen Listeria monocytogenes in the food chain. Front Microbiol. 2016;7:1865. doi:10.3389/fmicb.2016.01865.
- Chand D, Avinash VS, Yadav Y, Pundle AV, Suresh CG, Ramasamy S. Molecular features of bile salt hydrolases and relevance in human health. Biochim Biophys Acta. 2017;1861:2981–2991. doi:10.1016/j.bbagen.2016.09.024.
- Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–651. doi:10.1016/j.femsre.2004.09.003.
- Dussurget O, Cabanes D, Dehoux P, Lecuit M, Buchrieser C, Glaser P, Cossart P. Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol Microbiol. 2002;45:1095–1106. doi:10.1046/j.1365-2958.2002.03080.x.
- Quillin SJ, Schwartz KT, Leber JH. The novel Listeria monocytogenes bile sensor BrtA controls expression of the cholic acid efflux pump MdrT. Mol Microbiol. 2011;81:129–142. doi:10.1111/mmi.2011.81.issue-1.
- Camejo A, Carvalho F, Reis O, Leitao E, Sousa S, Cabanes D. The arsenal of virulence factors deployed by Listeria monocytogenes to promote its cell infection cycle. Virulence. 2011;2:379–394. doi:10.4161/viru.2.5.17703.
- Begley M, Sleator RD, Gahan CG, Hill C. Contribution of three bile-associated loci, bsh, pva, and btlB, to gastrointestinal persistence and bile tolerance of Listeria monocytogenes. Infect Immun. 2005;73:894–904. doi:10.1128/IAI.73.2.894-904.2005.
- Zhang Q, Feng Y, Deng L, Feng F, Wang L, Zhou Q, Luo Q. SigB plays a major role in Listeria monocytogenes tolerance to bile stress. Int J Food Microbiol. 2011;145:238–243. doi:10.1016/j.ijfoodmicro.2010.12.028.
- Pombinho R, Camejo A, Vieira A, Reis O, Carvalho F, Almeida MT, Pinheiro JC, Sousa S, Cabanes D. Listeria monocytogenes CadC regulates cadmium efflux and fine-tunes lipoprotein localization to escape the host immune response and promote infection. J Infect Dis. 2017;215:1468–1479. doi:10.1093/infdis/jix118.
- Boneca IG, Dussurget O, Cabanes D, Nahori M-A, Sousa S, Lecuit M, Psylinakis E, Bouriotis V, Hugot J-P, Giovannini M, et al. A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc Natl Acad Sci U S A. 2007;104:997–1002. doi:10.1073/pnas.0609672104.
- Dashkevicz MP, Feighner SD. Development of a differential medium for bile salt hydrolase-active Lactobacillus spp. Appl Environ Microbiol. 1989;55:11–16. doi:10.1128/AEM.55.1.11-16.1989.
- Camejo A, Buchrieser C, Couve E, Carvalho F, Reis O, Ferreira P, Sousa S, Cossart P, Cabanes D, et al. In vivo transcriptional profiling of Listeria monocytogenes and mutagenesis identify new virulence factors involved in infection. PLoS Pathog. 2009;5:e1000449. doi:10.1371/journal.ppat.1000449.
- Toledo-Arana A, Dussurget O, Nikitas G, Sesto N, Guet-Revillet H, Balestrino D, Loh E, Gripenland J, Tiensuu T, Vaitkevicius K, et al. The Listeria transcriptional landscape from saprophytism to virulence. Nature. 2009;459:950–956. doi:10.1038/nature08080.
- Ooi LG, Liong MT. Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci. 2010;11:2499–2522. doi:10.3390/ijms11062499.
- Yamamoto T, Hara H, Tsuchiya K, Sakai S, Fang R, Matsuura M, Nomura T, Sato F, Mitsuyama M, Kawamura I, et al. Listeria monocytogenes strain-specific impairment of the TetR regulator underlies the drastic increase in cyclic di-AMP secretion and beta interferon-inducing ability. Infect Immun. 2012;80:2323–2332. doi:10.1128/IAI.06162-11.
- Crimmins GT, Herskovits AA, Rehder K, Sivick KE, Lauer P, Dubensky TW Jr., Portnoy DA. Listeria monocytogenes multidrug resistance transporters activate a cytosolic surveillance pathway of innate immunity. Proc Natl Acad Sci U S A. 2008;105:10191–10196. doi:10.1073/pnas.0804170105.
- Schwartz KT, Carleton JD, Quillin SJ, Rollins SD, Portnoy DA, Leber JH, Camilli A. Hyperinduction of host beta interferon by a Listeria monocytogenes strain naturally overexpressing the multidrug efflux pump MdrT. Infect Immun. 2012;80:1537–1545. doi:10.1128/IAI.06286-11.
- Personnic N, Bruck S, Nahori MA, Toledo-Arana A, Nikitas G, Lecuit M, Dussurget O, Cossart P, Bierne H. The stress-induced virulence protein InlH controls interleukin-6 production during murine listeriosis. Infect Immun. 2010;78:1979–1989. doi:10.1128/IAI.01096-09.
- Cummins J, Casey PG, Joyce SA, Gahan CG, Bereswill S. A mariner transposon-based signature-tagged mutagenesis system for the analysis of oral infection by Listeria monocytogenes. PLoS One. 2013;8:e75437. doi:10.1371/journal.pone.0075437.
- Seifart Gomes C, Izar B, Pazan F, Mohamed W, Mraheil MA, Mukherjee K, Billion A, Aharonowitz Y, Chakraborty T, Hain T, et al. Universal stress proteins are important for oxidative and acid stress resistance and growth of Listeria monocytogenes EGD-e in vitro and in vivo. PLoS One. 2011;6:e24965. doi:10.1371/journal.pone.0024965.
- Hain T, Hossain H, Chatterjee SS, Machata S, Volk U, Wagner S, et al. Temporal transcriptomic analysis of the Listeria monocytogenes EGD-e sigmaB regulon. BMC Microbiol. 2008;8:20.
- Sue D, Fink D, Wiedmann M, Boor KJ. sigmaB-dependent gene induction and expression in Listeria monocytogenes during osmotic and acid stress conditions simulating the intestinal environment. Microbiology. 2004;150:3843–3855. doi:10.1099/mic.0.27257-0.
- Oliver HF, Orsi RH, Wiedmann M, Boor KJ. Listeria monocytogenes sigmaB has a small core regulon and a conserved role in virulence but makes differential contributions to stress tolerance across a diverse collection of strains. Appl Environ Microbiol. 2010;76:4216–4232. doi:10.1128/AEM.00031-10.
- Kazmierczak MJ, Wiedmann M, Boor KJ. Contributions of Listeria monocytogenes sigmaB and PrfA to expression of virulence and stress response genes during extra- and intracellular growth. Microbiology. 2006;152:1827–1838.
- Gray MJ, Freitag NE, Boor KJ. How the bacterial pathogen Listeria monocytogenes mediates the switch from environmental Dr. Jekyll to pathogenic Mr. Hyde. Infect Immun. 2006;74:2505–2512. doi:10.1128/IAI.74.5.2505-2512.2006.
- Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, et al. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect Immun. 2011;79:688–694.
- Auerbuch V, Brockstedt DG, Meyer-Morse N, O’Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med. 2004;200:527–533. doi:10.1084/jem.20040976.
- O’Connell RM, Saha SK, Vaidya SA, Bruhn KW, Miranda GA, Zarnegar B, Perry AK, Nguyen BO, Lane TF, Taniguchi T, et al. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med. 2004;200:437–445. doi:10.1084/jem.20040712.
- Kamisango K, Fujii H, Okumura H, Saiki I, Araki Y, Yamamura Y, Azuma I. Structural and immunochemical studies of teichoic acid of Listeria monocytogenes. J Biochem. 1983;93:1401–1409. doi:10.1093/oxfordjournals.jbchem.a134275.
- Gopal S, Berg D, Hagen N, Schriefer E-M, Stoll R, Goebel W, Kreft J. Maltose and maltodextrin utilization by Listeria monocytogenes depend on an inducible ABC transporter which is repressed by glucose. PLoS One. 2010;5:e10349. doi:10.1371/journal.pone.0010349.
- Schnupf P, Hofmann J, Norseen J, Glomski IJ, Schwartzstein H, Decatur AL. Regulated translation of listeriolysin O controls virulence of Listeria monocytogenes. Mol Microbiol. 2006;61:999–1012. doi:10.1111/mmi.2006.61.issue-4.
- Arnaud M, Chastanet A, Debarbouille M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl Environ Microbiol. 2004;70:6887–6891. doi:10.1128/AEM.70.11.6887-6891.2004.
- Carvalho F, Atilano ML, Pombinho R, Covas G, Gallo RL, Filipe SR, Sousa S, Cabanes D. L-rhamnosylation of Listeria monocytogenes wall teichoic acids promotes resistance to antimicrobial peptides by delaying interaction with the membrane. PLoS Pathog. 2015;11:e1004919. doi:10.1371/journal.ppat.1004919.
- Pinheiro J, Lisboa J, Pombinho R, Carvalho F, Carreaux A, Brito C, Pöntinen A, Korkeala H, Dos Santos NMS, Morais-Cabral JH, et al. MouR controls the expression of the Listeria monocytogenes Agr system and mediates virulence. Nucleic Acids Res. 2018;46:9338–9352. doi:10.1093/nar/gky624.
- Cabanes D, Lecuit M, Cossart P. Animal models of Listeria infection. Curr Protoc Microbiol. 2008;Chapter 9:Unit9B1.
- Glaser P, Frangeul L, Buchrieser C, Rusniok C, Amend A, Baquero F, Berche P, Bloecker H, Brandt P, Chakraborty T, et al. Comparative genomics of Listeria species. Science. 2001;294:849–852. doi:10.1126/science.1063447.