ABSTRACT
Background
. Oral administration of bovine antibodies active against enterotoxigenic Escherichia coli (ETEC) have demonstrated safety and efficacy against diarrhea in human challenge trials. The efficacy of bovine serum immunoglobulins (BSIgG) against recombinant colonization factor CS6 or whole cell ETEC strain B7A was assessed against challenge with the CS6-expressing B7A.
Methods
. This was a randomized, double-blind, placebo-controlled trial in which healthy adults received oral hyperimmune BSIgG anti-CS6, anti-B7A whole cell killed or non-hyperimmune BSIgG (placebo) in a 1:1:1 ratio then challenged with ETEC B7A. Two days pre-challenge, volunteers began a thrice daily, seven day course of immunoprophylaxis. On day 3, subjects received 1 × 1010 CFUs of B7A. Subjects were observed for safety and the primary endpoint of moderate-severe diarrhea (MSD).
Results
. A total of 59 volunteers received product and underwent ETEC challenge. The BSIgG products were well-tolerated across all subjects. Upon challenge, 14/20 (70%) placebo recipients developed MSD, compared to 12/19 (63%; p = .74) receiving anti-CS6 BSIgG and 7/20 (35%; p = .06) receiving anti-B7A BSIgG. Immune responses to the ETEC infection were modest across all groups.
Conclusions
. Bovine-derived serum antibodies appear safe and well tolerated. Antibodies derived from cattle immunized with whole cell B7A provided 50% protection against MSD following B7A challenge; however, no protection was observed in subjects receiving serum antibodies targeting CS6. The lack of observed efficacy in this group may be due to low CS6 surface expression on B7A, the high dose challenge inoculum and/or the use of serum derived antibodies versus colostrum-derived antibodies.
Introduction
Enterotoxigenic Escherichia coli (ETEC), one of several pathotypes of diarrheagenic E.coli, causes a secretory diarrhea that can range in presentation from mild discomfort to cholera-like purging. ETEC-mediated diarrhea involves colonization factors (CFs) promoting bacterial adherence to and colonization of the small intestine. This is followed by secretion of one or both of the two enterotoxins (heat-stable enterotoxin (ST) and heat-labile enterotoxin (LT)) that induce fluid and electrolyte secretion resulting in watery diarrhea.Citation1-Citation4
Evidence substantiating CFs as protective antigens comes from a number of epidemiological studies as well as controlled human infection models (CHIM).Citation4-Citation8 This is further substantiated by studies demonstrating passive oral administration of hyperimmune bovine immunoglobulin (BIgG) generated against inactivated whole cell ETEC and against purified CFs protects against moderate to severe diarrhea (MSD) in CHIM using ETEC strains expressing homologous CFs.Citation9-Citation12
CS6 is an atypical polymeric antigen that is highly prevalent among ETEC disease isolates globally.Citation4,Citation13,Citation14 Individuals naturally infected with CS6-expressing ETEC strains exhibit mucosal and serologic immune responses against CS6;Citation15-Citation17 however, despite data supporting the role of CS6 as a key CF in ETEC-mediated disease, immunological correlates of protection are lacking. We sought to assess if CS6 is a protective antigen against CS6-expressing ETEC mediated-diarrhea. Using recombinant CS6, hyperimmune bovine serum IgG (BSIgG) targeting CS6 was derived and assessed for efficacy using previously established models for immunoprophylaxis against ETEC in a CHIM.Citation11,Citation18
Methods
Passive vaccination-challenge trial design
Healthy non-pregnant adult subjects aged 18–50 years were recruited from the Mid-Atlantic area. Consented subjects were evaluated to assure good health and eligibility through medical history, physical examination, and screening laboratory tests. Eligible subjects were admitted in two cohorts to the inpatient research unit at the Johns Hopkins University (JHU) Center for Immunization Research Bayview facility. On admission, subjects were block randomized 1:1:1 (20 subjects per group; block size = 6) to receive anti-CS6 BSIgG, anti-B7A whole cell killed BSIgG, or a placebo control (non-hyperimmune BSIgG) in a double-blinded fashion. All investigators, data collectors, and subjects were blinded to treatment allocation.
Study oversight
This clinical trial was approved by the Johns Hopkins Bloomberg School of Public Health IRB in compliance with all federal regulations governing the protection of human volunteers. Clinicaltrials.gov registration NCT03040687.
Study dosing
Two days prior to challenge, subjects began their assigned treatment, thrice daily, 15 minutes after each meal. Each unit dose consisted of approximately 1 gram of total protein of anti-CS6 BSIgG, anti-B7A whole cell BSIgG or the non-hyperimmune BSIgG placebo dose dissolved in 150 mL of sodium bicarbonate buffer (13.35 gm of sodium bicarbonate in 1000 mL of sterile water).
On the day of the challenge, subjects followed the prescribed routine through breakfast and then fasted for 90 minutes. One minute pre-challenge, subjects drank 120 mL sodium bicarbonate buffer, then received approximately 1 × 1010 CFUs of ETEC strain B7A diluted in 30 mL sodium bicarbonate buffer. Subjects received a second dose of BSIgG 15 minutes after challenge, but otherwise fasted for 90 minutes following the challenge. Subjects then received their assigned treatment on the evening of the challenge and then three times daily for an additional four days or until receiving antibiotic treatment.
Subjects were actively monitored for adverse events including signs and symptoms of gastrointestinal illness, rehydrated as needed, and all stools were collected and assessed as previously described.Citation19-Citation21 Stool (or rectal swab) was plated directly on MacConkey (Mac) agar or Mac agar supplemented with 25ug/ml chloramphenicol (Mac+CM) as strain B7A is resistant to chloramphenicol. Lactose positive colonies were screened for agglutination in anti-B7A whole cell antiserum. Based on the percentage of positive reactions, the CFUs of B7A per gram of stool were estimated. If no B7A colonies were found on the Mac+CM places, additional colonies from the Mac plates were screened.
All subjects initiated a three day course of antibiotics (ciprofloxacin 500 mg orally twice daily) five days after challenge or earlier as indicated per protocol. Subjects were discharged from the unit when clinical symptoms were resolved or resolving and upon culture confirmation of ETEC challenge strain clearance.
Definitions and trial endpoints
The primary endpoint for this study was MSD defined as the following: ≥4 loose/liquid stools or ≥401 grams of loose/liquid stool in any 24 hour period post challenge. Secondary objectives included immune responses to key antigens and a variety of clinical endpoints to include duration and burden of ETEC colonization, total number and volume of stools, maximum 24 hour stool output, and ETEC attributable associated symptoms (to include nausea, vomiting, abdominal pain/cramps). An ETEC disease severity score was calculated using the algorithm described by Porter et alCitation22.
Antigens for bovine immunization
CS6
Recombinant CS6 (Lot 0840) was manufactured under cGMP conditions in Jan 2001 at the Walter Reed Army Institute of Research (WRAIR) Pilot Bioproduction Facility (PBF). The CS6 was derived from ETEC strain E8775 as previously describedCitation23 and as per the Supplemental materials. It is stored in a phosphate buffer, at a concentration of 2.56 mg/ml with an endotoxin content of 60 EU/ml.
Inactivated whole-cell B7A ETEC
Whole cell ETEC strain B7A cells were grown in colonization factor antigen (CFA) brothCitation24 CS6 expression was confirmed using a CS6-specific inhibition ELISA.Citation25 The cells were harvested, inactivated, washed and quantified as described in the Supplemental Methods.
Production of bovine serum immunoglobulin products
Bovine immunization
Anti-CS6 BSIgG, anti-whole cell killed B7A BSIgG, and non-hyperimmune BSIgG, were manufactured at SAB Biotherapeutics Inc, Sioux Falls, SD. Cattle were prescreened, randomized and vaccinated five times at four week intervals with either 2 mg of recombinant CS6 (Lot 0840) formulated with SAB proprietary adjuvant SAB-adj-1 or 3 × 1010 CFU killed whole cell B7A formulated with SAB proprietary adjuvant SAB-adj-2. Additional details are included in the supplemental materials. All animal work was conducted under Institutional Animal Care and Use Committee (IACUC) approved protocol 68–000143.
Hyperimmune plasma collection
In dedicated rooms, plasma was collected and separated from four cattle per vaccination group at days 8, 11, and 14 following the fourth and fifth vaccinations under sterile conditions by using an automated plasmapheresis system (Baxter Healthcare, Autopheresis C Model 200). Additional details are included in the supplemental materials. Briefly, plasma collected from the individual animals were measured by ELISA for antibody titers against the B7A ETEC strain, CS6, and O148 lipopolysaccharide (LPS).
Negative control plasma (Non-hyperimmune)
Bovine plasma was collected from four non-immunized cattle using the same method described for the immunized animals. Up to 2.1% of body weight of nonimmune plasma per animal was collected every three to four weeks. The final liquid product was assessed for quality and stored at −20°C ± 5ºC. For the negative control, the plasma from the three animals with the lowest background IgG titers against B7A and CS6 were pooled.
Bovine IgG enrichment
The three independent pooled plasma products (anti-B7A, anti-CS6, non-immunized negative control) then underwent a bovine IgG enrichment process in a cGMP clinical manufacturing facility. Each pooled plasma product was thawed at 18-30°C and the pH was adjusted to pH 4.5–4.9 with 20% acetic acid (USP grade). Then the pH adjusted plasma was fractionated with caprylic (octanoic) acid (CA), which selectively precipitates non-Ig bovine plasma proteins (BSA and blood coagulation proteins). After fractionation, the plasma was passed through a depth filtration system to remove non-Ig proteins. The CA-fractionated filtrate was collected into a sterile container. The resulting bulk solution was formulated in USP-grade PBS (1.42 g/L of Na2HPO4, 0.24 g/L KH2PO4, 7 g/L KCl, pH 7.3–7.5) by diafiltration and addition of PBS and was then concentrated to the desired final protein concentration, 65–80 mg/mL. The final bulk solution was filtered into sterile bioprocess bags (10–20 L) using a 0.22 µm filter. The final Bovine serum IgG bulk product was then filled into 250 mL sterile Nalgene™ multi-dose bottles and stored at −20°C ± 5ºC.
Characterization of bovine serum IgG products (BSIgG)
A panel of quality control tests were performed on the final liquid products. The protein content was measured, aggregation assessed, IgG bands in the products were identified, bacterial contamination and endotoxin contents measured, and IgG titers to CS6 and whole-cell B7A quantified. Additional details are found in the Supplemental Materials.
Bacterial challenge strain preparation
The B7A strain, Lot 0481 was manufactured under cGMP at the WRAIR PBF in October 1997. Each vial contains ~9 x 108 colony forming units (CFUs) of live B7A (O148:H28- CS6+ LT+ST+) in Luria Broth (LB) with 15% glycerol as cryopreservative. Approximately 48 hours prior to the scheduled inoculation, the contents of a single vial were plated for isolation onto CFA agar plates (without bile salts). The plates were incubated for 22–24 hours at 37°C ± 1°C and single colonies were selected and suspended in 3 mL sterile saline (0.9%). The suspension was re-plated on CFA agar plates which were then incubated at 37°C. Cells were harvested in sterile saline after 18–20 hours. Cell density was determined by optical density and the suspension was adjusted to correspond to approximately 1 × 1010 CFUs/ml. Actual CFUs/ml were determined by pre- and post-challenge dilution plating of a sample of the challenge inoculum and averaging the two. The percentage of colonies expressing CS6 was estimated by colony blots developed with rabbit anti-CS6 antisera (provided by NMRC) and goat anti-rabbit IgG conjugated to horse radish peroxidase (Pierce catalog no. 31460). CS6 expression was observed ≥90% of colonies tested in all preparations (data not shown).
Serum antibody responses
Serum was collected three days before challenge and on days 7 and 28 after challenge and stored at −20°C until use. Serum IgA and IgG antibody titers against CS6, LT, and LPS were determined by ELISA as previously described.Citation26 Seroconversion was defined as a ≥ 4 fold increase over baseline.
Antigen in lymphocyte supernatant (ALS)
Fresh isolated peripheral blood mononuclear cells were resuspended in complete RPMI (10% heat-inactivated fetal calf serum, 1% Penicillin-Streptomycin (Life Technologies) and 1% GlutaMAX (Life Technologies) and incubated in duplicate in 24-well plates (Corning Inc., Corning, NY) at 5 × 106 cells/mL, 1 mL/well, at 37°C and 5% CO2 for 72 h. Following incubation, culture supernatants were collected and stored at −80°C until tested by ELISA. Abn ALS response was defined as a ≥ 4 fold increase over baseline.
Statistical analysis
The prevalence of adverse events was compared between each active group and the placebo group using Fisher’s exact test. Continuous (and ordinal) variables were similarly compared using the Kruskal-Wallis test. Protective efficacy was calculated as (1 − relative risk). The null hypotheses were that there would be no difference in the post-challenge moderate-severe diarrhea rates between subjects receiving non-hyperimmune BSIgG and either group receiving hyperimmune BSIgG. Based on a Fisher’s exact test with a 1-sided alpha = 0.05, a sample size of 20 subjects per group yielded an 88% power to detect a 60% reduction in moderate-severe diarrhea rates presuming at least an 80% moderate-severe diarrhea rate in subjects receiving non-hyperimmune BSIgG. All other comparisons were made using a 2-sided alpha = 0.05. There were no adjustments for multiple comparisons.
Results
One hundred and eighteen subjects were recruited and screened for participation, of whom 68 were eligible. A total of 60 subjects were admitted to the inpatient research ward, randomized, and started on anti-CS6 (n = 20), anti-B7A whole cell killed (n = 20) or non-hyperimmune (n = 20) BSIgG (Supplemental ). No baseline differences were noted across groups in terms of age, sex, or ethnicity (). Prior to challenge, one subject from the anti-CS6 group was discharged from the inpatient facility due to health events unrelated to the investigational products. All other subjects were compliant with oral immunoprophylaxis and all doses were taken within fifteen minutes after each meal. Adverse events (AEs) determined to be at least possibly related to the BsIgG were infrequent and mild with the exception of one case of moderate severity abdominal cramping. Reported adverse events were predominantly gastrointestinal in nature with flatulence (15.3%) the most common across groups ().
Table 1. Demographic characteristics of subjects completing the passive prophylaxis series and receiving the ETEC B7A challenge.
Table 2. Adverse events determined by the clinical investigator to be at least possibly related to the BsIgG products.
Figure 1. Quantitative shedding of ETEC strain B7A on two days after oral challenge.
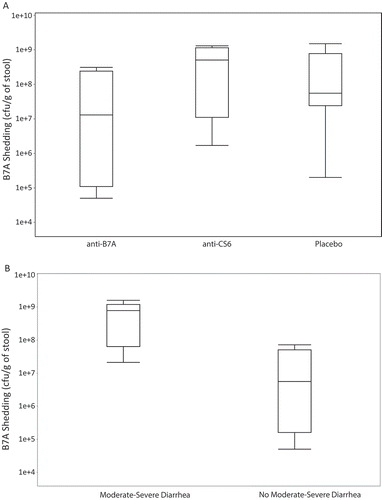
After challenge with 1.5 to 1.7 × 1010 CFUs of strain B7A, 70.0% (14/20) of subjects receiving non-hyperimmune BSIgG met the primary endpoint of moderate-severe diarrhea (). In contrast, 35.0% (7/20) of subjects receiving anti-B7A BSIgG had moderate to severe diarrhea yielding a 50% (95% confidence intervals (CI): 3.0%, 74.2%) protective efficacy (Fisher’s exact 1-sided p = .03). A total of 12/19 (63.2%) subjects receiving anti-CS6 BSIgG had moderate to severe diarrhea yielding a 9.8% (95% CI: −41.2%, 42.3%) protective efficacy (Fisher’s exact 1-sided p = .45). Additionally, subjects receiving anti-B7A BSIgG had lower rates of several other signs/symptoms of ETEC infection as well as a lower ETEC disease severity score compared to non-hyperimmune BSIgG recipients (). There was also a non-significant reduction in the frequency and volume of loose stool output in subjects receiving anti-B7A BSIgG compared to the other study groups.
Table 3. Signs and symptoms of ETEC following challenge with 1.7 to 1.5 × 1010 colony forming units of strain B7A.
All subjects shed the B7A in their stool with no significant differences in quantity of shedding at Day 2 when comparing treatment groups to placebo (anti-CS6 p = .55; anti-B7A p = .13); however, there appeared to be a trend toward reduced shedding in subjects receiving anti-B7A BSIgG compared to non-hyperimmune BSIgG recipients (). Among all subjects, those who had moderate-severe diarrhea shed significantly (p < .0001) more B7A on Day 2 than subjects with mild or no diarrhea ().
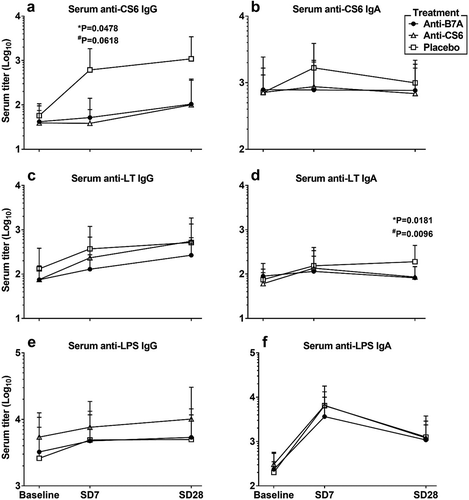
Anti-CS6 serologic and ALS response rates were low (≤30%) across all study groups (). Interestingly, prophylaxis with anti-CS6 (p = .05) and anti-B7A (p = .06) BSIgG appeared to blunt anti-CS6 serum IgG responses by day 7 compared to subjects receiving non-hyperimmune serum (). Anti-CS6 serum IgA was less affected by the oral prophylaxis () but appeared somewhat blunted as well. Post-challenge anti-LT responses as assessed by serology and ALS ranged from 21.1% to 63.2% in subjects receiving anti-CS6 BSIgG, 5.0% to 35.0% in subjects receiving anti-B7A BSIgG, and 25.0% to 65.0% in subjects receiving the placebo BSIgG (, ). Additionally, as shown in , by Day 28, lower anti-LT IgA titers in were observed subjects treated with anti-CS6 or anti-B7A BSIgG compared to the subjects receiving non-hyperimmune serum (p = .02 and p = .01, respectively). There was no apparent difference in anti-LT IgG titers. The most frequent immune response was to LPS. Specifically, ALS IgA response to LPS was noted in 100.0% of anti-CS6 BSIgG recipients, 80.0% of anti-B7A BSIgG recipients, and 90.0% of placebo recipients. The levels of anti-LPS IgG and IgA antibodies were unaffected by either treatment compared to the levels observed in the placebo group (,).
Table 4. Number and proportion of subjects developing a ≥ 4-fold rise in serologic or ALS responses following controlled human infection with ETEC strain B7A.
Figure 2. Serologic responses to oral challenge with ETEC strain B7A stratified by treatment group.
Discussion
Travelers’ diarrhea remains a significant threat with ETEC a primary bacterial pathogen cause.Citation27,Citation28 Vaccines or therapeutics against ETEC will need to target different strains expressing various CFs to ensure a sufficient breadth of coverage. This study was designed to evaluate whether orally administered bovine serum antibodies targeting various ETEC antigens (a CF and the whole cell) would be able to prevent or reduce moderate to severe diarrhea following challenge with the CS6-expressing ETEC strain B7A. The products were safe and well-tolerated, similar to other studies with orally administered bovine antibodies.Citation9-Citation12,Citation18 Additionally, antibodies targeting the B7A whole cell yielded a 50% reduction in MSD and a reduced disease severity score; however, anti-CS6 yielded no significant protection against MSD.
There are several potential reasons for the lack of observed efficacy. First, it is possible that despite epidemiologic associations between CS6-expressing strains and human disease,Citation29-Citation31 CS6 does not play a critical role in disease in the ETEC CHIM. However, preclinical studies, including a non-human primate model, demonstrate vaccination with CS6-derived antigens co-administered with an LT-based adjuvant confers protection against diarrhea following challenge with B7A.Citation32 While beyond the scope of this work, it would be of interest to compare moderate to severe diarrhea in the CHIM between a wildtype B7A strain and an isogenic mutant that fails to express CS6 to better understand the role of CS6 in a controlled infection.
If CS6 is an important virulence factor, there are other possibilities for the lack of observed protection in subjects receiving anti-CS6 antibody. The CS6 clone used to generate the antigen delivered to the cattle was from ETEC strain E8775Citation33 whereas the challenge strain in the CHIM was strain B7A. The CssA and CssB subunits between these two strains share 92% and 96% identity, respectively, at the protein level (GenBank accession numbers as follows: E8775 CssA: AAB51361.1; E8775 CssB: AAB51362.1; B7A CssA: EDV60821.1; B7A CssB: EDV60824.1). Sabui et al reported that specific alleles of the CS6 subunits were strongly associated with children presenting with diarrhea or non-diarrheagenic controls, raising the possibility that CS6 variation may affect host binding and subsequent disease.Citation34 It is possible that a critical, protective residue involved in B7A pathogenesis may not be expressed by E8775, and thus the anti-CS6 antibody product may have not had the capacity to prevent B7A disease.
It is also possible that serum derived antibodies are incapable of providing robust protection compared to the colostral antibodies we have utilized previously.Citation11,Citation18 To our knowledge, this is the first attempt to utilize orally delivered bovine serum antibodies to protect against an enteric infection. It may be that colostrum-derived antibodies, due to their intrinsic nature, are able to survive the orogastric delivery route better, thereby providing more active antibody to the gut.Citation35 While not addressed in this study, it would be of value to determine the amount of functional antibody available in the gut following ingestion.
Consistent with the data seen here, we previously observed a low number of anti-CS6 responders after oral challenge with CS6+ ETEC B7A strain (K. Talaat et al, submitted for publication). Interestingly, the proportion of responders and the magnitude of responses were higher among the volunteers in the placebo group, compared to volunteers treated with anti-CS6 or B7A BSIgG, possibly suggesting that both products partially blunted the anti-CS6 response. It seems plausible to speculate that, contrary to CS6 or LT, blunting of the anti-LPS response by oral prophylaxis is rather difficult, given LPS represents a major component of the bacterial cell.
These data build on prior findings that passive immunoprophylaxis targeting ETEC, including products directed against the colonization factors, can reduce ETEC-mediated disease.Citation9-Citation12 Interestingly, in this study, we did not see significant protection with a product designed against the major colonization factor of B7A, CS6, whereas the anti-whole cell product did provide protection. The lack of protection provided by the anti-CS6 product may be specific to the B7A challenge strain, or it may reflect a different role or function of this CF as compared to some of the others (e.g. CFA/I) on the surface of the bacteria that complicates passive protection in this setting. Alternatively, there may be a role for other antigens, such as CS21 which is also expressed on B7A, in combination with CS6 which may be important and points to the potential value in immune proteomics in identifying a protective immunologic profile.Citation36 This may need to be explored further as multivalent vaccines and therapeutics are developed for ETEC.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Copyright Statement
TKL, CAD, CKP, RLG, MSR, and MGP are military service members or federal employees of the United States government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that `copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. 101 defines a U.S. Government work as work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Disclaimer
The views expressed in this article reflect the results of research conducted by the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.” The study protocol was approved by the Institutional Review Boards at the Naval Medical Research Center and Johns Hopkins Bloomberg School of Public Health in compliance with all applicable federal regulations governing the protection of human subjects. There were no financial conflicts of interests among any of the authors.
Supplemental Material
Download Zip (50.5 KB)Acknowledgments
We would like to thank Dr. Nils Carlin for the manufacturing the killed whole cell ETEC strain B7A cells, and the subjects who volunteered for this study and participated in the clinical trial.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Chakraborty S, Harro C, DeNearing B, Ram M, Feller A, Cage A, Bauers N, Bourgeois AL, Walker R, Sack DA, et al. Characterization of mucosal immune responses to Enterotoxigenic Escherichia coli vaccine antigens in a human challenge model: response profiles after primary infection and homologous rechallenge with strain H10407. Clin Vaccine Immunol. 2016;23(1):55–11. doi:10.1128/CVI.00617-15.
- DuPont HL, Formal SB, Hornick RB, Snyder MJ, Libonati JP, Sheahan DG, LaBrec EH, Kalas JP. Pathogenesis of Escherichia coli diarrhea. N Engl J Med. 1971;285(1):1–9. doi:10.1056/NEJM197107012850101.
- Gaastra W, Svennerholm AM. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 1996;4(11):444–452. doi:10.1016/0966-842X(96)10068-8.
- Qadri F, Das SK, Faruque AS, Fuchs GJ, Albert MJ, Sack RB, Svennerholm AM. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J Clin Microbiol. 2000;38:27–31.
- Clemens JD, Sack DA, Harris JR, Chakraborty J, Neogy PK, Stanton B, Huda N, Khan MU, Kay BA, Khan MR, et al. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J Infect Dis. 1988;158(2):372–377. doi:10.1093/infdis/158.2.372.
- Cravioto A, Reyes RE, Ortega R, Fernández G, Hernández R, López D. Prospective study of diarrhoeal disease in a cohort of rural Mexican children: incidence and isolated pathogens during the first two years of life. Epidemiol Infect. 1988;101(1):123–134. doi:10.1017/S0950268800029289.
- Levine MM, Nalin, DR, Hoover, DL, Bergquist, EJ, Hornick, RB and Young, CR. Immunity to enterotoxigenic Escherichia coli. Infect Immun. 1979;23(3):729–736. doi:10.1128/IAI.23.3.729-736.1979.
- Lopez-Vidal Y, Calva JJ, Trujillo A, de Leon AP, Ramos A, Svennerholm A-M, Ruiz-Palacios GM. Enterotoxins and adhesins of enterotoxigenic Escherichia coli: are they risk factors for acute diarrhea in the community? J Infect Dis. 1990;162(2):442–447. doi:10.1093/infdis/162.2.442.
- Freedman DJ, Tacket C, Delehanty A, Maneval D, Nataro J, Crabb J. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J Infect Dis. 1998;177(3):662–667. doi:10.1086/jid.1998.177.issue-3.
- Otto W, Najnigier B, Stelmasiak T, Robins-Browne RM. Randomized control trials using a tablet formulation of hyperimmune bovine colostrum to prevent diarrhea caused by enterotoxigenic Escherichia coli in volunteers. Scand J Gastroenterol. 2011;46(7–8):862–868. doi:10.3109/00365521.2011.574726.
- Savarino SJ, McKenzie R, Tribble DR, Porter CK, O’Dowd A, Cantrell JA, Sincock SA, Poole ST, DeNearing B, Woods CM, et al. Prophylactic efficacy of hyperimmune bovine colostral antiadhesin antibodies against Enterotoxigenic Escherichia coli diarrhea: a randomized, double-blind, placebo-controlled, phase 1 trial. J Infect Dis. 2017;216(1):7–13. doi:10.1093/infdis/jix144.
- Tacket CO, Losonsky G, Link H, Hoang Y, Guesry P, Hilpert H, Levine MM. Protection by milk immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. N Engl J Med. 1988;318(19):1240–1243. doi:10.1056/NEJM198805123181904.
- Jiang ZD, Lowe B, Verenkar M, Ashley D, Steffen R, Tornieporth N, von Sonnenburg F, Waiyaki P, DuPont H. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay). J Infect Dis. 2002;185(4):497–502. doi:10.1086/jid.2002.185.issue-4.
- Porter CK, Riddle MS, Tribble DR, Putnam SD, Rockabrand DM, Frenck RW, Rozmajzl P, Kilbane E, Fox A, Ruck R, et al. The epidemiology of travelers’ diarrhea in Incirlik, Turkey: a region with a predominance of heat-stabile toxin producing enterotoxigenic Escherichia coli. Diagn Microbiol Infect Dis. 2010;66(3):241–247. doi:10.1016/j.diagmicrobio.2009.10.002.
- Alam MM, Aktar A, Afrin S, Rahman MA, Aktar S, Uddin T, Rahman MA, Mahbuba DA, Chowdhury F, Khan AI, et al. Antigen-specific memory B-cell responses to enterotoxigenic Escherichia coli infection in Bangladeshi adults. PLoS Negl Trop Dis. 2014;8(4):e2822. doi:10.1371/journal.pntd.0002822.
- Qadri F, Saha, A, Ahmed, T, Al Tarique, A, Begum, YA and Svennerholm, AM. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect Immun. 2007;75(8):3961–3968. doi:10.1128/IAI.00459-07.
- Rao MR, Wierzba, TF, Savarino, SJ, Abu-Elyazeed, R, El-Ghoreb, N, Hall, ER, Naficy, A, Abdel-Messih, I, Frenck Jr, RW, Svennerholm, AM and Clemens, JD, et al. Serologic correlates of protection against enterotoxigenic Escherichia coli diarrhea. J Infect Dis. 2005;191(4):562–570. doi:10.1086/427662.
- Savarino SJ, McKenzie R, Tribble DR, Porter CK, O’Dowd A, Sincock SA, Poole ST, DeNearing B, Woods CM, Kim H, et al. Hyperimmune bovine colostral anti-CS17 antibodies protect against Enterotoxigenic Escherichia coli diarrhea in a randomized, doubled-blind, placebo-controlled human infection model. J Infect Dis. 2019;220(3):505–513. doi:10.1093/infdis/jiz135.
- Savarino SJ, McKenzie R, Tribble DR, Porter CK, O’Dowd A, Cantrell JA, Sincock SA, Poole ST, DeNearing B, Woods CM, et al. Prophylactic efficacy of hyperimmune bovine colostral antiadhesin antibodies against Enterotoxigenic Escherichia coli diarrhea: a randomized, double-blind, placebo-controlled, phase 1 trial. J Infect Dis. 2017;216(1):7–13. doi:10.1093/infdis/jix144.
- Savarino SJ, McKenzie, R, Tribble, DR, Porter, CK, O’Dowd, A, Sincock, SA, Poole, ST, DeNearing, B, Woods, CM, Kim, H and Grahek, SL, et al. Hyperimmune bovine colostral anti-CS17 antibodies protect against enterotoxigenic Escherichia coli diarrhea in a randomized, doubled-blind, placebo-controlled human infection model. J Infect Dis. 2019;21(5418505):505–513.
- Harro C, Chakraborty S, Feller A, DeNearing B, Cage A, Ram M, Lundgren A, Svennerholm A-M, Bourgeois AL, Walker RI, et al. Refinement of a human challenge model for evaluation of enterotoxigenic Escherichia coli vaccines. Clin Vaccine Immunol. 2011;18(10):1719–1727. doi:10.1128/CVI.05194-11.
- Porter CK, Riddle MS, Alcala AN, Sack DA, Harro C, Chakraborty S, Gutierrez RL, Savarino SJ, Darsley M, McKenzie R, et al. An evidenced-based scale of disease severity following human challenge with Enteroxigenic Escherichia coli. PLoS One. 2016;11(3):e0149358. doi:10.1371/journal.pone.0149358.
- Lapa JA, Sincock SA, Ananthakrishnan M, Porter CK, Cassels FJ, Brinkley C, Hall ER, van Hamont J, Gramling JD, Carpenter CM, et al. Randomized clinical trial assessing the safety and immunogenicity of oral microencapsulated enterotoxigenic Escherichia coli surface antigen 6 with or without heat-labile enterotoxin with mutation R192G. Clin Vaccine Immunol. 2008;15(8):1222–1228. doi:10.1128/CVI.00491-07.
- Halvorsen T, VALVATNE H, GREWAL HMS, GAASTRA W, SOMMERFELT H. Expression of colonization factor antigen I fimbriae by enterotoxigenic Escherichia coli; influence of growth conditions and a recombinant positive regulatory gene. APMIS. 1997;105(3):247–254. doi:10.1111/apm.1997.105.issue-1-6.
- Tobias J, Svennerholm A-M, Carlin NIA, Lebens M, Holmgren J. Construction of a non-toxigenic Escherichia coli oral vaccine strain expressing large amounts of CS6 and inducing strong intestinal and serum anti-CS6 antibody responses in mice. Vaccine. 2011;29(48):8863–8869. doi:10.1016/j.vaccine.2011.09.096.
- Coster TS, Wolf MK, Hall ER, Cassels FJ, Taylor DN, Liu CT, Trespalacios FC, DeLorimier A, Angleberger DR, McQueen CE, et al. Immune response, ciprofloxacin activity, and gender differences after human experimental challenge by two strains of enterotoxigenic Escherichia coli. Infect Immun. 2007;75(1):252–259. doi:10.1128/IAI.01131-06.
- Olson S, Hall, A, Riddle, MS and Porter, CK. Travelers’ diarrhea: update on the incidence, etiology and risk in military and similar populations - 1990-2005 versus 2005-2015, does a decade make a difference? Trop Dis Travel Med Vaccines. 2019;5:1. doi:10.1186/s40794-018-0077-1.
- Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg. 2009;80(4):609–614. doi:10.4269/ajtmh.2009.80.609.
- Kharat VB, Ahmed M, Jiang Z-D, Riddle MS, DuPont HL. Colonization factors in Enterotoxigenic Escherichia coli strains in travelers to Mexico, Guatemala, and India compared with children in Houston, Texas. Am J Trop Med Hyg. 2017;96(1):83–87. doi:10.4269/ajtmh.16-0405.
- Vidal RM, Muhsen K, Tennant SM, Svennerholm A-M, Sow SO, Sur D, Zaidi AKM, Faruque ASG, Saha D, Adegbola R, et al. Colonization factors among enterotoxigenic Escherichia coli isolates from children with moderate-to-severe diarrhea and from matched controls in the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis. 2019;13(1):e0007037. doi:10.1371/journal.pntd.0007037.
- Isidean SD, Riddle MS, Savarino SJ, Porter CK. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine. 2011;29(37):6167–6178. doi:10.1016/j.vaccine.2011.06.084.
- Poole S, Maciel Jr M, Ramakrishnan A, Liu Y, Dori K, Prouty MG, et al. Development, characterization, and immunological evaluation of parenterally delivered CS6-subunit vaccine candidates against enterotoxigenic E. coli (ETEC). in E. coli and the mucosal immune system. Ghent (Belgium); 2019.
- Thomas LV, Cravioto A, Scotland SM, Rowe B. New fimbrial antigenic type (E8775) that may represent a colonization factor in enterotoxigenic Escherichia coli in humans. Infect Immun. 1982;35(3):1119–1124. doi:10.1128/IAI.35.3.1119-1124.1982.
- Sabui S, Ghosal A, Dutta S, Ghosh A, Ramamurthy T, Nataro JP, Hamabata T, Chatterjee NS. Allelic variation in colonization factor CS6 of enterotoxigenic Escherichia coli isolated from patients with acute diarrhoea and controls. J Med Microbiol. 2010;59(Pt 7):770–779. doi:10.1099/jmm.0.017582-0.
- Sears KT, Tennant SM, Reymann MK, Simon R, Konstantopoulos N, Blackwelder WC, Barry EM, Pasetti MF. Bioactive immune components of anti-diarrheagenic Enterotoxigenic Escherichia coli hyperimmune bovine colostrum products. Clin Vaccine Immunol. 2017;24(8). doi:10.1128/CVI.00186-16.
- Chakraborty S, Randall A, Vickers TJ, Molina D, Harro CD, DeNearing B, Brubaker J, Sack DA, Bourgeois AL, Felgner PL, et al. Interrogation of a live-attenuated enterotoxigenic Escherichia coli vaccine highlights features unique to wild-type infection. NPJ Vaccines. 2019;4:37. doi:10.1038/s41541-019-0131-7.
