ABSTRACT
Diarrhea caused by enterotoxigenic Escherichia coli (ETEC) has a continuing impact on residents and travelers in underdeveloped countries. Both heat-labile (LT) and heat-stable (ST) enterotoxins contribute to pathophysiology via induction of cyclic nucleotide synthesis, and previous investigations focused on intracellular signal transduction rather than possible intercellular second messenger signaling. We modeled ETEC infection in human jejunal enteroid/organoid monolayers (HEM) and evaluated cyclic nucleotide pools, finding that intracellular cAMP was significantly increased but also underwent apical export, whereas cGMP was minimally retained intracellularly and predominantly effluxed into the basolateral space. LT and virulence factors including EatA, EtpA, and CfaE promoted ST release and enhanced ST-stimulated cGMP production. Intracellular cGMP was regulated by MK-571-sensitive export in addition to degradation by phosphodiesterase 5. HEMs had limited ST-induced intracellular cGMP accumulation compared to T84 or Caco-2 models. Cyclic nucleotide export/degradation demonstrates additional complexity in the mechanism of ETEC infection and may redirect understanding of diarrheal onset.
Introduction
The Global Enteric Multi-Center Study (GEMS) identified enterotoxigenic Escherichia coli (ETEC) infection as one of the four leading causes of acute diarrhea and associated mortalities in developing countries.Citation1 As the leading cause of traveler’s diarrhea, ETEC is also a recognized burden on deployed U.S. military personnel.Citation2 The host diarrheal response is initiated by the secreted peptide heat-stable enterotoxin (ST) and/or the multi-subunit protein heat-labile enterotoxin (LT) via induction of second messenger cGMP and cAMP synthesis, respectively. ETEC strain H10407, originally isolated from a patient in Bangladesh with severe cholera-like diarrhea,Citation3 employs additional factors to facilitate host interaction, including but not limited to the secreted mucinase EatACitation4 and surface adhesins EtpACitation5,Citation6 and CfaE.Citation7 Strains expressing ST, alone or in combination with LT, were more highly associated with diarrhea than LT-only expressing strains,Citation8 confirming the importance of ST in disease pathogenesis. As such, recent renewed efforts to employ ST antigens for vaccine development are under way.Citation9,Citation10
The mechanism of ST is characterized by intracellular accumulation of cGMP and activation of cGMP-dependent protein kinase 2 (cGKII) to explain the disruption of intestinal fluid and ion homeostasis.Citation11 However, the anti-constipation therapeutic linaclotide, a functional analog of ST, has been additionally described to decrease nociception via exported cGMP in rodent models and has demonstrated clinical efficacy in alleviating visceral pain in irritable bowel syndrome with constipation (IBS-C).Citation12,Citation13 This suggests that the effects of ST are similarly more complex than currently appreciated, and likely include activities linked to extracellular cGMP. In the case of both ST and linaclotide, the intracellular and extracellular cGMP pools have not been dissected to deduce their relative magnitudes, nor have their respective contributions to altered intestinal signaling been analyzed. The potential for effluxed cGMP to impact immune response has also not been evaluated. Preventive and therapeutic strategies will be better informed by a more complete understanding of how ST affects normal human intestinal epithelia.
derived epithelial cultures, or enteroids, are an untransformed, non-tumorigenic model that retains segmental identity, epithelial differentiation, and transcriptional profiles similar to native intestinal epithelium.Citation14,Citation15 We and others have shown that enteroids functionally mimic intestinal ion transport regulation driven by elevated cAMP signaling.Citation16-Citation18 However, the enclosed luminal surface of three-dimensional enteroids is a distinct caveat when considering their utility in modeling infection by ingested pathogens. While microinjection of microbes into the lumen of enteroids/organoids has been described,Citation19 only a rough quantitation of delivered material in relation to luminal volume can be estimated. Discrete sampling of apical, basolateral, and intracellular spaces in the 3-D model is not easily accessible. Adoption of the two-dimensional human enteroid monolayer (HEM) culture on permeable supports has facilitated the modeling of numerous bacterial and viral-mediated gastrointestinal diseases,Citation20-Citation23 including the specific affinity of ETEC H10407 for the blood group A antigen that enhances colonization and diarrheal susceptibility.Citation24 These findings highlight the importance of evaluating human biology using enteroids to better understand infectious diarrheal diseases.
In this study, we describe the application of HEMs to model ETEC H10407 infection by assessing the cyclic nucleotide pools induced by ST and LT. The importance of several virulence factors (LT, EatA, EtpA, CfaE) in promoting ST delivery by H10407 and subsequent cGMP production in HEMs was also evaluated. Finally, we examined the relative significance of host epithelial proteins for cGMP export (multi-drug resistance protein 5, MRP5) or degradation (phosphodiesterase 5, PDE5) in regulating each cGMP pool. The HEM model was found to be functionally responsive to ETEC infection and demonstrated a behavioral distinction from the cancer-derived intestinal epithelial cell lines (Caco-2 and T84) routinely used in mechanistic host–pathogen studies.
Results
ETEC/ST induces cGMP synthesis and efflux in human enteroid monolayers (HEMs)
To establish the minimum bacterial concentration and timing required for measurable effects related to ST-stimulated cGMP synthesis, HEMs were subjected to incubation periods of 4, 6, and 8 h at a high starting concentration (108 cfu) applied to the apical epithelial surface, and cGMP was measured in the apical, intracellular, and basolateral pools by ELISA (). The broad-spectrum PDE inhibitor IBMX (1 mM) was present in all experiments, which yielded a slightly increased cyclic nucleotide baseline measurement in uninfected HEM cultures. cGMP was below the limit of detection in the absence of IBMX. Although 6 h exposure yielded a significant increase in total cGMP over controls, the 8 h incubation gave a significantly higher increase in cGMP. Strikingly, most cGMP was detected in the basolateral pool rather than the expected intracellular pool. The 8 h infection period was used to evaluate bacterial concentrations of 106, 10,Citation7 and 108 cfu for relative efficiency in cGMP stimulation (), but only the highest concentration was sufficient to induce measurable cGMP synthesis. Trans-epithelial electrical resistance (TER) was also measured at the initiation and conclusion of the infection period to establish epithelial barrier integrity, and no changes in TER due to the presence of bacteria were noted.
Figure 1. A HEM infection model for ETEC H10407 exhibits polarized efflux of cyclic nucleotides. (a) Exposure period required for detectable cGMP readout. (b) Bacterial load necessary for detectable cGMP readout with 8 h exposure. (c) ETEC strain H10407-stimulated (108 cfu, 8 h) cGMP synthesis compared to incubation with a range of synthetic ST applied to the apical epithelial surface. Basolateral application of ST did not stimulate cGMP production. (d) Induction of cAMP synthesis after exposure to recombinant LT (100 ng/mL), inactive LT mutant E112K (100 ng/mL) or forskolin (1 μM, 10 μM), each in the absence of PDE inhibitors, 8 h. Data points are technical replicates from 3 to 4 independent experiments and graph bars are mean ± SEM. Statistical significance was determined by ANOVA relative to the control/untreated condition. *p < .05; **p < .01; ***p < .001.
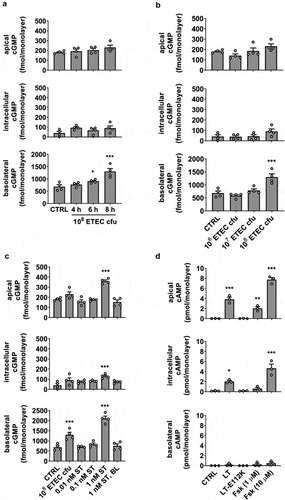
To determine the equivalent synthetic ST dose that would elevate cGMP levels similar to 108 cfu infection for 8 h, HEMs were exposed to a range of ST concentrations (0.01 nM, 0.1 nM, 1 nM) applied to the apical surface for 8 h (). Infection with WT H10407 was found to be within the range of 0.1–1 nM ST exposure. Basolateral administration of ST (1 nM) did not stimulate cGMP production above baseline. Significant apical cGMP efflux occurred only at ST concentrations ≥1 nM.
LT-induced cAMP synthesis and efflux demonstrate the polar selectivity of cyclic nucleotide secretion
We next tested the ability of recombinant LT, LT mutant E112K (LT-E112K), or forskolin to stimulate cAMP synthesis and efflux (), with the aim to compare the results to ST-induced cGMP synthesis and secretion. LT and forskolin are agonists of adenylate cyclase, but LT-E112K lacks ADP-ribosylation activity necessary to elevate cAMP. LT or forskolin exposure induced intracellular cAMP elevation and selective export from the apical side of HEM cultures, whereas the basolateral secreted cAMP pool was not statistically significant. This contrasts with the selective basolateral efflux and minor apical transport observed when cGMP content was elevated. For both cyclic nucleotides, agonist-induced intracellular accumulation was regulated in part by efflux.
ST delivery by ETEC H10407 is dependent on the expression of other virulence factors
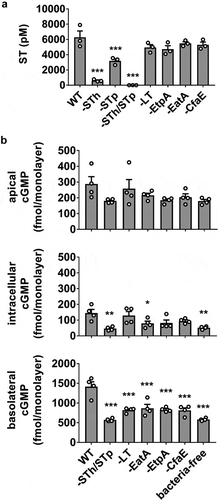
The H10407 strain produces two ST variants, STh and STp, initially distinguished by detection in human or porcine isolates, respectively. Both ST subtypes contribute to disease symptoms,Citation25 although the relative amounts produced by a particular ETEC strain may vary.Citation26 In the next assessment of the infection model, we evaluated total ST secretion (STh and STp) and subsequent induction of cGMP production in HEMs following exposure to WT H10407 or mutant strains deficient in STh, STp, LT, EatA, EtpA, or CfaE expression (). Each of the mutants (other than ST-targeted mutations) secreted approximately equivalent amounts of ST compared to WT, suggesting no impairment in ST synthesis or release mechanisms. The STh and STp mutant strains exhibited decreased ST release, and ST was completely abolished in the STh/STp double mutant. In the HEM ETEC infection model, as expected, the STh/STp mutant did not induce cGMP production above the baseline content stabilized by IBMX in the bacteria-free control (). However, the LT, EatA, EtpA, and CfaE mutants each induced significantly less cGMP compared to WT. The reduction in cGMP was approximately equivalent in each mutant and was most evident in the basolateral pool.
Figure 2. H10407 virulence factor mutants produce equivalent amounts of ST but induce less cGMP production than WT. (a) Quantitation of secreted ST from WT H10407 and mutant strains deficient in STh (-STh), STp (-STp), both STh and STp (-STh/STp), LT (-LT), EatA (-EatA), EtpA (-EtpA), or CfaE (-CfaE). Each strain was grown in LB broth at 37°C with aeration/agitation to stationary phase, 16 h. (b) cGMP measurements in HEM cultures exposed to WT, ST, LT, EtpA, EatA, or CfaE mutants (108 cfu, 8 h). Data points are technical replicates from 3 to 4 independent experiments and graph bars are mean ± SEM. Statistical significance was determined by ANOVA relative to the WT condition. *p < .05; **p < .01; ***p < .001.
cGMP efflux is mediated by MK-571-sensitive transporters
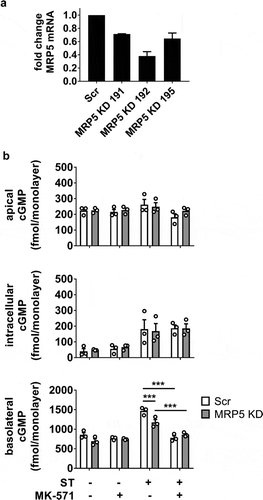
Efflux of cGMP from ST-exposed HEMs was evaluated by targeting MRP5, a cGMP-selective multi-drug resistance protein. A stable shRNA-mediated MRP5 knockdown (KD) was identified by qRT-PCR () and assayed alongside the pan-MRP inhibitor MK-571 (50 μM). MRP5 KD (60% efficiency) afforded only partial inhibition of basolateral cGMP efflux during ST exposure (1.3-fold decrease), whereas MK-571 was sufficient to return basolateral cGMP export to levels similar to the absence of ST stimulation (). The incomplete effect observed in the KD may be due to the remaining MRP5 activity or the participation of another MK-571-sensitive transporter. Surprisingly, the blockade of cGMP export was not accompanied by increased intracellular or apically effluxed pools, suggesting that an additional mechanism contributed to limiting intracellular cGMP.
Figure 3. Role of MRP5 in the regulation of ST-stimulated cGMP compartmentalization in HEMs. (a) MRP5 KD efficiency screening by quantitative RT-PCR in three different shRNA clones. Clone 192 was used for all subsequent experiments. (b) cGMP quantitation in apical, intracellular, and basolateral pools from ST-exposed HEMs (1 nM ST, 1 h) in the presence of the general PDE inhibitor IBMX (1 mM). Scr, scrambled shRNA enteroid line; MRP5 KD, MRP5 shRNA-expressing enteroid line. MK-571 (50 μM) was included in designated samples. Data points represent technical replicates from three independent experiments and graph bars are mean ± SEM. Statistical significance was determined by ANOVA. *p < .05; ***p < .001.
Intracellular cGMP accumulation is limited by PDE5
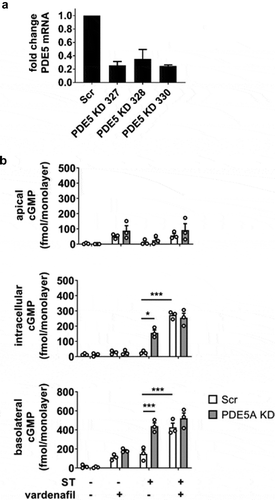
The previous experiment with inhibition or KD of MRP5 suggested that intracellular cGMP accumulation is regulated not only by export but also through efficient degradation by intracellular PDEs. cGMP-specific PDE5 was targeted by the addition of the selective inhibitor vardenafil and compared to a PDE5 KD (efficiency 80%, ) generated with shRNA in a manner similar to that carried out for MRP5 KD. Upon ST stimulation, intracellular cGMP was approximately 3-fold greater in the PDE5 KD than in the scrambled shRNA control, and approximately 5-fold greater in the presence of vardenafil (). The basolateral secretion of cGMP was also enhanced by the PDE5 KD or vardenafil (approximately 3-fold increase under each condition). Combining vardenafil and the PDE5 KD did not enhance intracellular or basolateral cGMP pools beyond their respective independent contributions.
Figure 4. Role of PDE5 in regulating the accumulation of ST-stimulated cGMP in HEMs. (a) PDE5 KD efficiency screening by quantitative RT-PCR in three different shRNA clones. Clone 330 was used in all subsequent experiments. (b) cGMP quantitation in apical, intracellular, and basolateral pools from ST-exposed HEMs (1 nM ST, 1 h). IBMX was not present in these experiments. Scr, scrambled shRNA enteroid line; PDE5 KD, PDE5 shRNA-expressing enteroid line. Vardenafil (10 μM) was included in designated samples. Data points represent technical replicates from three independent experiments and graph bars are mean ± SEM. Statistical significance was determined by ANOVA. *p < .05; ***p < .001.
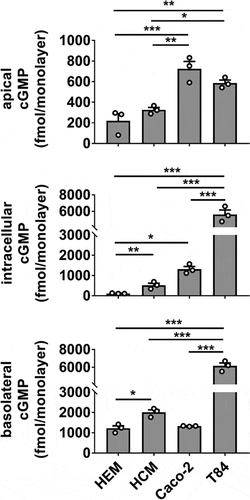
Different human intestinal epithelial models demonstrate varying magnitude of ST-induced cGMP production and distribution
To orient the HEM model in context with extensively characterized human cell culture models of ST intoxication, we compared cGMP production in HEMs, human colonoid monolayers (HCMs), Caco-2, and T84 cell monolayers exposed to ST (). HCMs derived from normal ascending colon were included to determine if the cGMP distribution in Caco-2 or T84 cells could be attributed to a specific colonic phenotype not present in cells of small intestinal origin. HEMs retained significantly less intracellular cGMP than the other models (HEM < HCM < Caco-2 ≪ T84), but demonstrated basolateral cGMP secretion that was similar to Caco-2 and only slightly less than HCMs (HEM ≈ Caco-2 < HCM ≪ T84). Caco-2 monolayers have a unique propensity toward apical cGMP secretion given their total cGMP output evaluated across all three pools. T84 cells produced and secreted substantially more total cGMP than each of the other models. Overall, both HEMs and HCMs retained less total cGMP than Caco-2 or T84 cells.
Figure 5. cGMP production and distribution differ in ST-stimulated human cancer-derived and non-cancerous primary intestinal epithelial cell lines. Measurement of cGMP in apical, intracellular, and basolateral pools obtained from human enteroid (HEM), human colonoid (HCM), Caco-2, and T84 monolayers exposed to ST (1 nM, 1 h) in the presence of IBMX (1 mM). Data points are technical replicates from three independent experiments and graph bars represent the mean ± SEM. Statistical significance was determined by ANOVA. *p < .05; **p < .01; ***p < .001.
Discussion
In the normal human intestinal epithelial model, we found that ST-induced elevation of intracellular cGMP is limited by two factors: Export by MK-571-sensitive cyclic nucleotide transporters, one of which is MRP5 (also known as ABCC5), and degradation via phosphodiesterase (PDE5). Notably, membrane transporter selectivity promoted basolateral/serosal secretion of ST-induced cGMP, whereas LT-stimulated cAMP was released to the apical/luminal side. Total cGMP production was diminished when stimulated by ST-expressing H10407 strains that lack expression of LT or the virulence factors EtpA, EatA, or CfaE. When compared to cancer-derived human intestinal cell culture models T84 and Caco-2, HEMs exhibited much less relative intracellular cGMP content after ST exposure. Together, these results suggest that intracellular cGMP within normal human intestinal epithelia is carefully restricted.
Measurement of intracellular cAMP and cGMP in HEMs colonized by H10407 was recently reported.Citation24,Citation26 However, the characterization of infection-associated cyclic nucleotide efflux has not been explored in the HEM model. Additionally, evaluation of an approximate pathophysiological ST concentration for experimental reference has not been described. In the present study, 108 cfu of log-phase H10407 were required to produce between 0.1 and 1 nM ST over an 8 h period, eliciting a modest cGMP response that is near the lower limits of detection by ELISA. Notably, earlier studies demonstrated that ST production is sensitive to catabolite repression by glucoseCitation27 and that ST production increases as the bacteria enter a stationary phase of growth.Citation28 Thus, it is likely that the use of log-phase bacteria contributed to the inoculum required to elicit measurable cGMP response in the target epithelia. We verified that ST is unable to stimulate cGMP synthesis if administered basolaterally, consistent with apical localization of the receptor (guanylate cyclase C) and demonstrates that ST is unable to breach the mucosa from the serosal side. This necessitates the use of HEMs to conduct ST experiments and limits studies in 3-D enteroid culture due to limited apical access.
After establishing initial conditions for ST-mediated cGMP stimulation, we investigated the relative efficacy of cGMP stimulation by several mutant H10407 strains. cGMP output was reduced by approximately equivalent amounts in each mutant. The adhesin protein EtpA and colonizing factor CFA/I tip subunit CfaE contribute to host cell attachment during infection. EatA is a secreted Serine Protease Autotransporter of Enterobacteriaceae (SPATE) with myriad functions including EtpA degradationCitation29 and mucinase activity.Citation4 Previously, H10407 mutants deficient in EtpACitation6 or CfaECitation7 were demonstrated to exhibit decreased adherence to human intestinal epithelial cells (Caco-2 and small intestinal biopsy, respectively), whereas EatA loss enhanced colonization on Caco-2 monolayers.Citation29 Taken together, these results suggest that adherence ability alone does not correlate with the ST delivery and stimulated cGMP production observed in the current study. The diminished cGMP response elicited by the LT-deficient mutant, which otherwise retains the full complement of colonization factors, is also unclear. Prior observations found that LT-lacking mutants are adherence-impaired.Citation30 The role of LT in enhancing ST promotion of cGMP synthesis in T84 monolayers using purified toxins was previously noted,Citation31 but the underlying mechanism is not fully understood. It is possible that concomitant cAMP and cGMP synthesis results in cross-talk for PDE inhibition to protect cGMP from degradation and will be a topic of future study.
To understand the mechanism restricting intracellular cGMP accumulation, cyclic nucleotide selective transporter MRP5 and cGMP-specific phosphodiesterase PDE5 were each targeted by both small molecule inhibitors and shRNA-mediated KD. MRP5 has a low-affinity preference for the export of cGMP over cAMP.Citation32-Citation34 MRP5 KD or MK-571 exposure was sufficient to block ST-induced basolateral cGMP efflux but did not account for diminished intracellular cGMP. PDE5 KD or inhibition with vardenafil demonstrated that cGMP is degraded in the absence of expedient export. We did not test alternative pools of cGMP for their relative lifetimes, e.g., cGMP derived from stimulation of soluble guanylate cyclase rather than ST-stimulation of membrane-bound GCC. Such information would be interesting but was outside the context of the infection study at hand.
To place our observations from HEMs in context with well-studied models of human intestinal epithelia, we performed ST stimulation in Caco-2 and T84 monolayers, and also included HCMs to account for potential differences in small intestinal and colonic epithelial responses. Total cGMP in HEM and HCM was less than Caco-2 output and only a fraction of T84 production. Caco-2 has been a preferred model for studying Na+/H+ exchanger 3 (NHE3) regulation, whereas T84 cells are standard for evaluation of activated fluid/anion secretion via the Cl−/HCO3− conductance channel CFTR. Both cell lines are derived from colon cancers, known to acquire abnormal chromosome duplications/deletions and transcriptional profiles that can affect downstream protein expression levels and signal transduction. This set of experiments demonstrates that each human intestinal epithelial cell culture model is not necessarily equivalent nor interchangeable, and critical evaluation of model features and limitations is of importance for subsequent conclusions. In light of these findings, the functional implication of the ST-induced cGMP profile in HEMs, specifically whether ion transport through NHE3 and CFTR is mechanistically regulated by the same process defined in T84 or Caco-2, should be evaluated in future studies.
The function of cGMP as an extracellular second messenger cannot be fully interrogated in epithelial-only cultures and remains to be explored in models involving additional human primary non-epithelial cell types normally present in the submucosa, such as immune cells, nerves, or other stromal populations. Purkinje neurons respond to extracellular cGMP through reduced activation of glycine receptors, yielding voltage-gated calcium channel activation and an increase in intracellular Ca2+.Citation35 Whether a similar mechanism or outcome exists in the enteric nervous system remains to be tested. Effluxed cAMP may function in regulating ETEC behaviors, as LT production or exogenous cAMP application was seen to augment host adherenceCitation30,Citation36 in animal infection studies. Additionally, the expression of enterotoxins is controlled by a bacterial cAMP receptorCitation37 that may sense the extracellular host-provided pool. Thus, cognizance of the roles cGMP and cAMP play outside of intestinal epithelia should contribute to mechanistic and therapeutic considerations for controlling ETEC infection.
Materials and methods
Human specimens
De-identified human jejunal and colonic tissues were obtained from healthy subjects provided informed consent. All experimental methods were approved by the Johns Hopkins University Institutional Review Board (IRB# NA_00038329).
Reagents
Synthetic heat-stable enterotoxin (BA Chem, Bubendorf, Switzerland) was resuspended in 100 mM acetic acid, 0.1% BSA, and frozen in single-use aliquots. LT and LT-E112K were reconstituted in water and stored at 4°C. IBMX, MK-571, vardenafil, forskolin, and buffer components were acquired from Sigma (St. Louis, Missouri). Tissue culture media was obtained from ThermoFisher Scientific (Waltham, Massachusetts)
Human enteroid culture
Crypt isolation and enteroid propagation were described previously.Citation17 One jejunal enteroid line of blood group A genotype was used to evaluate the bacterial infection parameters and to generate the shRNA KD lines. Three jejunal lines were evaluated in synthetic ST experiments to establish effects were generalizable; results from one line are shown. One jejunal and one colonoid line was used in the comparative study with T84 and Caco-2. The expansion medium (EM) and differentiation medium (DM) have been detailed previously.Citation20 HEMs were established using a published procedure.Citation20 Transwells (catalog number 3470, Corning Life Sciences, Tewksbury, Massachusetts) were coated with human collagen IV (Sigma) at 10 µg/cm2, 4°C overnight, then were washed with ADF, plated with isolated enteroid fragments, and fed with Undifferentiated Monolayer medium (UDM, consists of EM without A83-01 or SB202190) until confluent. The medium was then replaced with DM for 5 d prior to experiments. Transepithelial electrical resistance measurements (EVOM2, World Precision Instruments, Sarasota, Florida) were used to monitor confluency.
ETEC strains
ETEC H10407 and derivative mutant strains were described previously (). Cultures were inoculated from glycerol stocks stored at −80°C and grown in LB Miller broth at 37°C with agitation overnight, including 10 µg/mL kanamycin and/or 25 µg/mL chloramphenicol as required for mutant positive selection. For infection experiments, an overnight LB culture was diluted 1:50 into fresh LB and incubated at 37°C with agitation for 2 h to achieve a log-phase culture.
Table 1. Bacterial strains.
Human intestinal epithelial cell lines
Caco-2/BBe1 cells (CRL-2102, ATCC, Manassas, Virginia) were cultured in DMEM, 10% v/v FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C, 5% CO2. T84 cells (CCL-248, ATCC) were maintained in DMEM/F12, 5% v/v FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C, 5% CO2. Cells were plated on 24-well plate Transwells (3470, Corning Life Sciences) and cultured in their respective growth media to confluency (T84) or for 12 d post-confluency (Caco-2) prior to use in ST stimulation experiments.
Cyclic nucleotide ELISA
Incubations were conducted in Buffer A (138 mM NaCl, 5.4 mM KCl, 1.3 mM CaCl2, 0.8 mM MgSO4, 0.3 mM Na2HPO4, 0.4 mM KH2PO4, 25 mM NaHCO3, 10 mM HEPES, 25 mM glucose, pH 7.4, 1 mM IBMX unless otherwise noted) at 37°C, 5% CO2. Bacteria from log-phase cultures in LB broth were pelleted at 13,000 g for 5 min, washed once in Buffer A, and then resuspended in Buffer A at the designated cfu/mL. Bacteria or toxins were added to the upper Transwell compartment unless otherwise noted. Inhibitors were present in both upper and lower compartments. At the end of each incubation, medium was collected, centrifuged (13,000 g, 5 min) to pellet cell debris, and then acidified with concentrated HCl (1:100) to inactivate phosphodiesterase activity. Cells were washed with cold PBS, lysed in 0.1 M HCl (300 μL), and centrifuged (13,000 g, 5 min) to pellet insoluble material. cGMP was measured in duplicate using the Direct cGMP ELISA Kit (Enzo Life Sciences, Farmingdale, New York). Lysate from each monolayer was divided between two ELISA wells to achieve measurements within the dynamic range of substrate detection. As such, there was no additional lysate available from each sample to measure total protein content and normalizations were calculated based on the uniform surface area of the Transwell. This approach was validated by comparing total protein isolated from several Transwell monolayers to represent each cell line being tested. A 1.8% coefficient of variation in total protein content was calculated, suggesting that normalization by monolayer did not introduce major error in cyclic nucleotide measurements. To further maximize sensitivity, samples were acetylated and incubated on ELISA plates at 4°C overnight. Well absorbance at 405 nm was recorded on an EnVision plate reader (PerkinElmer, Waltham, Massachusetts). The manufacturer-specified limit of detection for acetylated cGMP is 0.021 pmol/mL. cGMP concentrations were converted to absolute quantities based on the fixed volumes in the upper (150 μL) and lower (800 μL) Transwell chambers or the volume of 0.1 M HCl used to prepare cell lysates. Experiments were evaluated for cAMP by a similar procedure using the Direct cAMP ELISA Kit (Enzo Life Sciences), with the exception that ELISA plates containing acetylated samples were incubated room temperature for 2 h with shaking at 300 rpm.
ST ELISA
ST competitive ELISA was based on the procedure reported by Taxt and coworkers.Citation39 Synthetic ST (BA Chem) was conjugated to ovalbumin using glutaraldehyde, dialyzed against PBS, diluted to 50 ng/mL, and used at 100 μL/well to coat a Maxi-Sorp 96-well plate (catalog number 442404, Thermo Scientific, Rochester, New York) at 4°C overnight. Plates were blocked with 1% ovalbumin prior to adding samples or standards mixed with ST antibody (0.1 μg/mL, mouse monoclonal M120529, Fitzgerald Industries International, Acton, Massachusetts) at 1:1. Detection was carried out by incubation with goat anti-mouse IgG-alkaline phosphatase conjugate (ThermoFisher Scientific), followed by p-NPP solution (ThermoFisher Scientific). Well absorbance was measured on an EnVision plate reader at 405 nm.
shRNA knockdown
Three-dimensional enteroids were treated with 10 μM CHIR99021 for 2 d prior to transduction. Enteroids were harvested from Matrigel and briefly digested (~3 min) with TrypLE (ThermoFisher Scientific) at 37°C. Cells were washed with ADF, pelleted, and resuspended in EM containing polybrene (10 μg/mL) and lentivirus (GIPZ lentiviral shRNA, GE Dharmacon, Lafayette, Colorado) listed in . Cells were incubated at 32°C with shaking for 1 h, then transferred to a tissue culture incubator at 37°C, 5% CO2, for an additional 4–5 h. Cells were pelleted briefly at 300 g, plated in Matrigel, and fed with EM following standard culture methods. At 2–3 d post-transduction, puromycin selection (2.5 μg/mL) was initiated. After at least 2 weekly passages, puromycin was increased to 10 μg/mL.
Table 2. shRNA target sequences.
Quantitative real-time PCR
Knockdown efficiency was assayed by quantitative real-time PCR (qRT-PCR). After propagation under puromycin selection (10 μg/mL) for at least 3 passages, 3-D enteroids were isolated from Matrigel with Cultrex Organoid Harvesting Solution (R&D Systems, Minneapolis, Minnesota) and total RNA was extracted using a PureLink RNA Mini Kit (ThermoFisher Scientific). RNA was transcribed to cDNA with SuperScript VILO Master Mix (ThermoFisher Scientific). qRT-PCR was performed on a QuantStudio 12 K Flex Real-Time PCR instrument (Applied Biosystems, Foster City, California) using Power SYBR Green Master Mix (Applied Biosystems) and oligonucleotide primers specific for the targeted exon (). Each sample was run in triplicate with 5 ng RNA-equivalent cDNA per reaction. Relative fold-changes in mRNA levels were determined using 18 S rRNA for internal normalization and the 2−ΔΔCT method.
Table 3. shRNA knockdown qRT-PCR screening oligonucleotide sequences.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Eileen Barry (University of Maryland, Baltimore, Maryland) for sharing the ETEC H10407 CfaE mutant strain and Elizabeth Norton (Tulane University, New Orleans, Louisiana) for providing purified LT protein. Instrumentation and tissue culture support were provided by the Hopkins Digestive Diseases Basic & Translational Research Core Center.
Additional information
Funding
References
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. The Lancet. 2013;382(9888):209–12. doi:10.1016/S0140-6736(13)60844-2.
- Olson S, Hall A, Riddle MS, Porter CK. Travelers’ diarrhea: update on the incidence, etiology and risk in military and similar populations – 1990-2005 versus 2005–2015, does a decade make a difference? Trop Dis Travel Med Vaccines. 2019;5:1. doi:10.1186/s40794-018-0077-1.
- Evans DJ Jr., Evans DG. Three characteristics associated with enterotoxigenic Escherichia coli isolated from man. Infect Immun. 1973;8:322–328. doi:10.1128/IAI.8.3.322-328.1973.
- Kumar P, Luo Q, Vickers TJ, Sheikh A, Lewis WG, Fleckenstein JM. EatA, an immunogenic protective antigen of enterotoxigenic escherichia coli, degrades intestinal mucin. Infect Immun. 2014;82:500–508. doi:10.1128/IAI.01078-13.
- Fleckenstein JM, Roy K, Fischer JF, Burkitt M. Identification of a two-partner secretion locus of enterotoxigenic Escherichia coli. Infect Immun. 2006;74:2245–2258. doi:10.1128/IAI.74.4.2245-2258.2006.
- Roy K, Hilliard GM, Hamilton DJ, Luo J, Ostmann MM, Fleckenstein JM. Enterotoxigenic Escherichia coli EtpA mediates adhesion between flagella and host cells. Nature. 2009;457:594–598. doi:10.1038/nature07568.
- Baker KK, Levine MM, Morison J, Phillips A, Barry EM. CfaE tip mutations in enterotoxigenic Escherichia coli CFA/I fimbriae define critical human intestinal binding sites. Cell Microbiol. 2009;11:742–754. doi:10.1111/j.1462-5822.2009.01287.x.
- Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388(10051):1291–1301. doi:10.1016/S0140-6736(16)31529-X.
- Taxt A, Aasland R, Sommerfelt H, Nataro J, Puntervoll P. Heat-stable enterotoxin of enterotoxigenic Escherichia coli as a vaccine target. Infect Immun. 2010;78:1824–1831. doi:10.1128/IAI.01397-09.
- Taxt AM, Diaz Y, Aasland R, Clements JD, Nataro JP, Sommerfelt H, Puntervoll P. Towards rational design of a toxoid vaccine against the heat-stable toxin of Escherichia coli. Infect Immun. 2016;84(4):1239–1249. doi:10.1128/IAI.01225-15.
- Markert T, Vaandrager AB, Gambaryan S, Pöhler D, Häusler C, Walter U, De Jonge HR, Jarchau T, Lohmann SM. Endogenous expression of type II cGMP-dependent protein kinase mRNA and protein in rat intestine. Implications for cystic fibrosis transmembrane conductance regulator. J Clin Invest. 1995;96(2):822–830. doi:10.1172/JCI118128.
- Eutamene H, Bradesi S, Larauche M, Theodorou V, Beaufrand C, Ohning G, Fioramonti J, Cohen M, Bryant AP, Kurtz C, et al. Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain. Neurogastroenterol Motil. 2010;22:312e84.
- Castro J, Harrington AM, Hughes PA, Martin CM, Ge P, Shea CM, Jin H, Jacobson S, Hannig G, Mann E, et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3′,5′-monophosphate. Gastroenterology. 2013;145(6):1334–46.e11. doi:10.1053/j.gastro.2013.08.017.
- Middendorp S, Schneeberger K, Wiegerinck CL, Mokry M, Akkerman RDL, van Wijngaarden S, Clevers H, Nieuwenhuis EES. Adult stem cells in the small intestine are intrinsically programmed with their location-specific function. Stem Cells. 2014;32(5):1083–1091. doi:10.1002/stem.1655.
- Kraiczy J, Nayak KM, Howell KJ, Ross A, Forbester J, Salvestrini C, Mustata R, Perkins S, Andersson-Rolf A, Leenen E, et al. DNA methylation defines regional identity of human intestinal epithelial organoids and undergoes dynamic changes during development. Gut. 2019;68(1):49–61. doi:10.1136/gutjnl-2017-314817.
- Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NWM, Bijvelds MJC, Scholte BJ, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19(7):939–945. doi:10.1038/nm.3201.
- Foulke-Abel J, In J, Yin J, Zachos NC, Kovbasnjuk O, Estes MK, de Jonge H, Donowitz M. Human enteroids as a model of upper small intestinal ion transport physiology and pathophysiology. Gastroenterology. 2016;150(3):638–649.e8. doi:10.1053/j.gastro.2015.11.047.
- Yin J, Tse C-M, Avula LR, Singh V, Foulke-Abel J, de Jonge HR, Donowitz M. Molecular basis and differentiation-associated alterations of anion secretion in human duodenal enteroid monolayers. Cell Mol Gastroenterol Hepatol. 2018;5(4):591–609. doi:10.1016/j.jcmgh.2018.02.002.
- Williamson IA, Arnold JW, Samsa LA, Gaynor L, DiSalvo M, Cocchiaro JL, Carroll I, Azcarate-Peril MA, Rawls JF, Allbritton NL, et al. A high-throughput organoid microinjection platform to study gastrointestinal microbiota and luminal physiology. Cell Mol Gastroenterol Hepatol. 2018;6(3):301–319. doi:10.1016/j.jcmgh.2018.05.004.
- In JG, Foulke-Abel J, Clarke E, Kovbasnjuk O. Human colonoid monolayers to study interactions between pathogens, commensals, and host intestinal epithelium. J Vis Exp. 2019;146:e59357. doi:10.3791/59357
- In J, Foulke-Abel J, Zachos NC, Hansen A-M, Kaper JB, Bernstein HD, Halushka M, Blutt S, Estes MK, Donowitz M, et al. Enterohemorrhagic escherichia coli reduces mucus and intermicrovillar bridges in human stem cell-derived colonoids. Cell Mol Gastroenterol Hepatol. 2016;2:4862.e3. doi:10.1016/j.jcmgh.2015.10.001.
- Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, Neill FH, Blutt SE, Zeng X-L, Qu L, et al. Replication of human noroviruses in stem cell-derived human enteroids. Science. 2016;353(6306):1387–1393. doi:10.1126/science.aaf5211.
- Noel G, Baetz NW, Staab JF, Donowitz M, Kovbasnjuk O, Pasetti MF, Zachos NC. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci Rep. 2017;7(1):45270. doi:10.1038/srep45270.
- Kumar P, Kuhlmann FM, Chakraborty S, Bourgeois AL, Foulke-Abel J, Tumala B, Vickers TJ, Sack DA, DeNearing B, Harro CD, et al. Enterotoxigenic Escherichia coli–blood group A interactions intensify diarrheal severity. J Clin Invest. 2018;128(8):3298–3311. doi:10.1172/JCI97659.
- Bölin I, Wiklund G, Qadri F, Torres O, Bourgeois AL, Savarino S, Svennerholm A-M. Enterotoxigenic Escherichia coli with STh and STp genotypes is associated with diarrhea both in children in areas of endemicity and in travelers. J Clin Microbiol. 2006;44(11):3872–3877. doi:10.1128/JCM.00790-06.
- Zhu Y, Luo Q, Davis SM, Westra C, Vickers TJ, Fleckenstein JM. Molecular determinants of enterotoxigenic escherichia coli heat-stable toxin secretion and delivery. Infect Immun. 2018;86:e00526–18. doi:10.1128/IAI.00526-18.
- Martinez-Cadena MG, Guzman-Verduzco LM, Stieglitz H, Kupersztoch-Portnoy YM. Catabolite repression of Escherichia coli heat-stable enterotoxin activity. J Bacteriol. 1981;145:722–728. doi:10.1128/JB.145.2.722-728.1981.
- Johnson WM, Lior H, Johnson KG. Heat-stable enterotoxin from Escherichia coli: factors involved in growth and toxin production. Infect Immun. 1978;20:352–359. doi:10.1128/IAI.20.2.352-359.1978.
- Roy K, Kansal R, Bartels SR, Hamilton DJ, Shaaban S, Fleckenstein JM. Adhesin degradation accelerates delivery of heat-labile toxin by enterotoxigenic escherichia coli. J Biol Chem. 2011;286:29771–29779. doi:10.1074/jbc.M111.251546.
- Allen KP, Randolph MM, Fleckenstein JM. Importance of heat-labile enterotoxin in colonization of the adult mouse small intestine by human enterotoxigenic Escherichia coli strains. Infect Immun. 2006;74:869–875. doi:10.1128/IAI.74.2.869-875.2006.
- Read LT, Hahn RW, Thompson CC, Bauer DL, Norton EB, Clements JD. Simultaneous exposure to escherichia coli heat-labile and heat-stable enterotoxins increases fluid secretion and alters cyclic nucleotide and cytokine production by intestinal epithelial cells. Infect Immun. 2014;82:5308–5316. doi:10.1128/IAI.02496-14.
- Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem. 2000;275:30069–30074. doi:10.1074/jbc.M005463200.
- Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem. 2003;278:17664–17671. doi:10.1074/jbc.M212723200.
- Wijnholds J, Mol CAAM, van Deemter L, de Haas M, Scheffer GL, Baas F, Beijnen JH, Scheper RJ, Hatse S, De Clercq E, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci. 2000;97(13):7476–7481. doi:10.1073/pnas.120159197.
- Cabrera-Pastor A, Malaguarnera M, Taoro-Gonzalez L, Llansola M, Felipo V. Extracellular cGMP modulates learning biphasically by modulating glycine receptors, CaMKII and glutamate-nitric oxide-cGMP pathway. Sci Rep. 2016;6:33124. doi:10.1038/srep33124.
- Johnson AM, Kaushik RS, Francis DH, Fleckenstein JM, Hardwidge PR. Heat-labile enterotoxin promotes escherichia coli adherence to intestinal epithelial cells. J Bacteriol. 2009;191:178–186. doi:10.1128/JB.00822-08.
- Bodero MD, Munson GP. Cyclic AMP receptor protein-dependent repression of heat-labile enterotoxin. Infect Immun. 2009;77:791–798. doi:10.1128/IAI.00928-08.
- Dorsey FC, Fischer JF, Fleckenstein JM. Directed delivery of heat-labile enterotoxin by enterotoxigenic Escherichia coli. Cell Microbiol. 2006;8:1516–1527. doi:10.1111/j.1462-5822.2006.00736.x.
- Taxt AM, Diaz Y, Bacle A, Grauffel C, Reuter N, Aasland R, Sommerfelt H, Puntervoll P. Characterization of immunological cross-reactivity between enterotoxigenic escherichia coli heat-stable toxin and human guanylin and uroguanylin. Infect Immun. 2014;82:2913–2922. doi:10.1128/IAI.01749-14.
