ABSTRACT
Introduction
Rats are experimental animals, frequently used as model organisms in the biomedical studies, and increasingly used to study the gut microbiota. Specifically, the aim of latter studies is either the elucidation of relationship between intestinal dysbiosis and diseases or the determination of nutrients or pharmaceutical agents which can cause the modulation in the presence or abundance of gut microbiota.
Aim
Herein, the research studies conducted on the gut microbiota of healthy rats are presented in a summarized and concise overview. The focus is on studies aimed to reveal the shifts in microbial composition and functional changes after exposure to various types of nutritional supplements.
Methods
We performed the search of PubMed database using the term “rat gut microbiome microbiota” and examined studies aimed to assess the composition of gut microbiota in physiological homeostasis as well as the effect of various nutritional supplements on the gut microbiota of healthy rats.
Introduction
In recent years microbiota studies increased in number and relevance, and different study models have been employed to better elucidate the occurrence and role of certain microbiota in health and disease. This subject is complex and researchers in the field might benefit from comprehensively presented data available for specific study models, such as for the animal models. The presented review, therefore, brings a systematic overview of the literature covering the topic of microbiota study in rat models, used widely in different in vivo studies. Rats are indeed, often used as model organisms to study the gut microbiota, to elucidate the relationship between intestinal dysbiosis and diseases and/or to analyze the effects of pharmaceutical agents or food supplements/nutrients on the microbiota status. The latter studies strongly rely on data and knowledge of the microbiota status in physiological conditions. The presented review summarizes relevant information through the sections covering specific subtopics. First, the general role of gut microbiota in mammals’ health is explained along with data on the formation and dynamics of the rat gut microbiota in the early life cycle. Second, the available rat models for the study of microbiota dysbiosis are presented, followed by the description of the physiology of microbiota in the gastrointestinal tract and feces of healthy rats. A paragraph explaining the use of rat models for human microbiota research in comparison with the mouse models is given, followed by two sections explaining known data on modulation of the gut microbiota in healthy rats by use of food supplements.
Gut microbiota role in mammals’ health
It is assumed that the number of microbial cells in human microbiota is ten times greater (1014) than the total number of human cells (1013).Citation1 Moreover, the gut microbiota in mammals is an extremely complex ecosystem, ranging from bacteria, viruses, and archaea to unicellular eukaryotes, such as fungi and yeast.Citation1 Besides diversity of microbial composition, fluctuations in microbial relative abundance, and the variety of secreted functional molecules, microbial metabolites, also play an important role in the host health status.Citation2 Due to such complexity and significance, the gut microbiota is often referred to as “the forgotten organ” or “the second genome”.Citation3,Citation4
Firstly, gut microbiota secrete enzymes, crucial for digestion of complex carbohydrates, such as resistant starches, plant cell wall polysaccharides, and non-digestible oligosaccharides.Citation5 Secondly, gut microbiota perform vitamin synthesis, i.e. synthesis of cobalamin (vitamin B12), which is synthesized exclusively by anaerobic gut microorganisms. In addition, gut bacteria take part in the synthesis of vitamin K, biotin, folate, nicotinic acid, pantothenic acid, pyridoxine, riboflavin, and thiamine.Citation6 Thirdly, the gut microbiota is essential for development and function of the host immune system. Indeed, bacterial colonization of the gut is crucial for normal development of the immunity, which was proven in the studies conducted on germ-free animals.Citation7 Precisely, commensal and mutualistic bacteria protect the host against pathogenic species by: (1) competing for the same nutrients; (2) forming microenvironment unsuitable for the growth of parasitic species and (3) producing antimicrobial peptides or promoting T and B cell responses.Citation8 Moreover, it was shown that the intestinal angiogenesis is also regulated by gut microbiota, for instance, the study by Stappenbeck et al. showed an arrested capillary network formation in adult germ-free mice. The latter state was successfully restarted and capillary formation was completed 10 d after the transplantation of gut microbiota was performed, by use of conventionally raised mice as donors or after the inoculation of single culture Bacteroides thetaiotaomicron.Citation9 Finally, the gut microbiota is part of a complex communication system known as “gut-brain axis”. Microbiota interacts locally with enterocytes and the enteric nervous system, which also has a direct influence on the central nervous system through neuroendocrine and metabolic pathways, modulating behavior, motivation, and higher cognitive functions.Citation10
It can be concluded that gut microbiota directly and indirectly influences the host health status through secreted functional molecules (proteins, peptides, and the molecules of low molecular weight) and influence pathogens as well.
As in humans, the formation of intestinal microbiota in rats occurs during and after birth, where neonatal rats are more exposed to fecal and environmental bacteria than humans.Citation11 According to Yajima et al., during the first few weeks after birth, Gram-negative Escherichia Coli and Gram-positive Lactobacillus and Streptococcus genus dominate in the rat gut, while the anaerobic bacteria, Bacterioidaceae, and facultative anaerobic or microaerophilic Lactobacilli take over after weaning.Citation12 Inoue and Ushida reported a clear change in the diversification of rat intestinal microbiota from suckling to maturity.Citation11 Precisely, the first observed changes occur at 21–22 d after birth, and are due to weaning, diet change, and the simultaneous decrease in the maternal IgA levels. The second wave of changes occurs from d 24 to 27 after birth, probably attributed to the morphological and the immunological maturation of the gut.Citation12 After the formative period is finished, the delicate equilibrium of gut microbiota is continuously perturbed by diet and environmental factors.
Rat models for studies of microbiota dysbiosis
A link between gut dysbiosis and certain human diseases has been established so far, pointing to gut microbiota as an important topic in preventive medicine. Dysbiosis is often defined as an “imbalance” in the gut microbial community that is associated with disease. This imbalance could be due to the gain or loss of community members as well as changes in relative abundance of microbes.Citation13 Dysbiosis or a definitive change of the normal gut microbiota with a breakdown of host-microbial mutualism is probably the defining event in the development of inflammatory bowel diseases.Citation14 Also, changes in the gut microbiota are associated with specific metabolic states, such as obesity, diabetes, and metabolic syndrome.Citation15–Citation17 For instance, low fecal bacterial diversity is associated with marked overall adiposity and obese individuals have a higher abundance of Firmicutes, and nearly 90% lower abundance of Bacteroidetes in comparison with lean subjects.Citation18 Changes in gut microbiota likely precede food allergies as well.Citation19 Neuropsychiatric conditions, including autism, Parkinson’s disease, and depression are also states accompanied by changes in the gut microbiota.Citation20 Recently, the topic of microbiota role in bone health, such as in osteoporosis has also been discussed in the scientific literature. The latter is based on the knowledge that microbiota has an effect on the bone.Citation21,Citation22 Acknowledging the importance of microbiota in some of the major medical issues of the modern world, studies are performed with the aim to establish scientifically based evidence on the correlation of the microbiota status with specific pathological states. However, this is an extremely complex research topic that requires a broad interdisciplinary and sophisticated methodological approach. Also, enormous complexity and a huge number of factors influencing the microbiota status in real time should be taken into account while performing such studies.
Animal models are accepted as an important research tool as they can be used to reduce and control parameters influencing the fluctuations and changes of the microbiota. In particular, rat models are a valuable tool for determining intestinal dysbiosis and the previously discussed human diseases relationship. Moreover, these models may help in discovery of nutrients or pharmaceutical agents which can prevent or reduce the microbiota alterations and gut microbiota dysbiosis.
The dextran sodium sulfate (DSS) colitis murine model has advantages over other various chemically induced experimental models due to its simplicity, reproducibility, and controllability. It may be particularly useful in the research of inflammatory bowel disease (IBD).Citation23 Furthermore, according to Ghattamaneni et al. chronic administration of 0.5% DSS produces selective and reversible gastrointestinal changes in Wistar rats; increase of Firmicutes and decrease of Bacteroidetes and Actinobacteria, providing an improved chronic model in rats.Citation24 Furthermore, metabolic syndrome as a combination of disorders that increases the risk of diabetes and cardiovascular diseases may be induced experimentally in rats fed with a fructose-rich diet.Citation25,Citation26 Previously, Srinivasan et al. determined that the combination of high-fructose diet and low-dose injections of streptozotocin in rats can serve as an alternative animal model for type 2 diabetes, simulating the human metabolic syndrome also suitable for testing anti-diabetic agents.Citation27
One of the best rodent models for the study of autism and autism spectrum disorder (ASD) is the valproic acid-induced rat model. Using the latter experimental animal model, Liu et al. proved that valproic acid stimulates alterations in the microbiota features seen in autism, in addition to behavioral and anatomical changes characteristic for autistic brain.Citation28 Rodent models, including rats, are extensively used in the discovery of novel treatments for Parkinson’s disease. Particularly, reserpine- and haloperidol-treated rats, 6-hydroxydopamine, and less frequently, rotenone and paraquat models, have proven as very useful.Citation29 While some of the symptoms of depression are found exclusively in humans (guilt, suicidality, and sad mood), part of the depression symptoms can be replicated in laboratory rats (measures of helplessness, anhedonia, behavioral despair and other neurovegetative changes such as sleep alterations and appetite patterns) and moderated with antidepressant treatment.Citation30 Differences in the gut microbiota composition between the depressive rat models and control animals were found, once more emphasizing the importance of gut-brain axis.Citation31 The microbiota of depressed animals have similarities with those of depressive patients; for example, the richness of Bacteroidetes increases with a concomitant decrease of Firmicutes and abundance of Lactobacillus.Citation31 In addition, Yu et al. found that relative abundances of the bacterial genera Marvinbryantia, Corynebacterium, Psychrobacter, Christensenella, Lactobacillus, Peptostreptococcaceae incertae sedis, Anaerovorax, Clostridiales incertae sedis, and Coprococcus were significantly decreased, whereas Candidatus Arthromitus and Oscillibacter were markedly increased in rats with chronic variable stress (CVS)-induced depression, compared with normal controls.Citation32 Recently, different depression rat models were used as well, such as the olfactory bulbectomized rat, maternal separation, chronic variable stress-induced depression, and chronic restraint stress.Citation31
Physiology of microbiota in the gastrointestinal tract and feces of healthy rats
An important baseline for study of microbiota changes and dysbiosis is knowledge and information on the physiology of microbiota in the gastrointestinal tract and feces of healthy animals. This is why a number of studies were focused on the investigation of the microbial composition in the gastrointestinal tract and feces of healthy rats. Data from these studies represent a first baseline for microbiota research in rat models as well. Particularly, the contribution of recent studies is in comprehensive characterization of the so-called “normal” rat microbiota, which provides a basis for understanding and predicting disease-related alterations.Citation33
The fecal flora of BioBreeding rats was, for example, analyzed by Brooks and coworkers by the use of two methods, namely (1) the randomly cloned 16 S rDNA comparative sequence analysis and (2) the bacterial cultures in different anaerobic media.Citation34 The culture-independent approach provided deeper insights; however, only 20% of bacterial species, which were estimated to be present, were also successfully identified. For instance, the most dominant species of Gram-positive bacteria were Lactobacilli, representing 7% of in total 69 operational taxonomic units (OTUs). In addition, 16 S rDNA clones aligned with the Clostridium coccoides group (9%), the Clostridium leptum subgroup (18%), and Gram-negative Bacteroides–Cytophaga phylum. However, the majority of clone sequences were aligned with previously cultured, but still unknown bacterial species.Citation34
Subsequently, a long-term consequence of cecal microbiota transplantation from Sprague-Dawley and Wistar rat strains on the intestinal microbiota of recipients Lewis strain rats was assessed by analyzing fecal samples in several rat model systems.Citation35 In the control Lewis rat strain the authors identified 926 phylotypes with dominant phyla Firmicutes at 74% and Bacteroidetes at 23%. Obtained data allowed examining how different the rat and human intestinal microbiota are. The number of species in a fecal sample of control rat was two to three times higher than in fecal samples of two healthy human individuals. Finally, Manichanh et al. concluded that, at the phylum level, rat and human microbiota are similar, while specificity can be observed at the genus level.
In a detailed study, Li et al. performed the characterization of microbiota and microbial metabolites along the longitudinal axis of rat gastrointestinal (GI) tract, including feces.Citation33 Results unambiguously revealed that the microbial biogeography of six male, pathogen-free Sprague-Dawley rats, which were held on a chow diet, is distinct from other murine animals, such as mouse or woodrat. Furthermore, the species richness and phylogenetic diversity increased from the upper to the lower GI segments, while the samples extracted from the colon mucus layer were of the highest richness and diversity. In mice, gastric, duodenal, and large-intestinal samples show similar diversity levels.Citation33 Moreover, at the phylum level, 21 taxonomic groups were identified, but only Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria were identified in all parts of the GI tract. Inter-individual microbiota variability is much higher in humans than in rats, and the authors attribute this to the similarity in genetic composition of laboratory rats, the uniform diet, the controlled environmental factors, and the coprophagy. Finally, in the gastrointestinal tract of healthy rats, the lactate-producing bacteria, such as Lactobacillus and Turicibacter, were dominant in the stomach and small intestine. In contrast, the core microbiota of the large intestine were anaerobic Lachnospiraceae and Ruminococcaceae.Citation33
Furthermore, Flemer et al. showed that gut microbiota profiles may separate rats into three different clusters according to their age (1) before weaning, (2) first year of life (12- to 26-week-old animals) and (3) second year of life (52- to 104-week-old).Citation36 A core of 46 bacterial species was present in all rats but relative abundance decreased progressively with age. This was accompanied by an increase of microbiota α-diversity (or number of different species in a sample), likely due to the acquisition of environmental microorganisms during the lifespan. In a study by Ferrario et al., the effect of three different dietary fibers on rat fecal microbiota was examined.Citation37 A basal rat fecal microbiota content at the end of acclimatization week showed that Bacteroidetes (53.9%) represent the dominant phylum, outnumbering the Firmicutes (39.8%) and Proteobacteria (4%) phyla.Citation37 Actinobacteria, unclassified members of Saccharibacteria phylum, Cyanobacteria, and Tenericutes together represent about 2% of the microbiota, while Verrucomicrobia, Spirochaetae, Fusobacteria, and Elusimicrobia phyla were determined at a low-level presence (≤0.1%).
As part of a study conducted by Nagpal et al., fecal microbiota composition of widely used animal models mice, rats, and non-human primates (NHPs) was analyzed using data generated on a single platform and with the same protocols.Citation38 Data acquired was, subsequently, compared with those obtained for female (18 samples) and male (7 samples) human subjects confirming higher inter-individual variation in human gut microbiota.Citation38 This comprehensive study revealed a more complex community of microbes present in rat feces, when compared to above-discussed data. In this study, the dominant phyla were Bacteroidetes, Firmicutes and Proteobacteria, followed by the Spirochetes, Verrucomicrobia, Tenericutes and hardly detectible Actinobacteria.Citation38 In addition, relative abundance at the family level showed that the rat microbiota profile is distinct from other evaluated subjects, with the Prevotellaceae as the most dominant family. Also, Bacterioidaceae, Clostridiales, Ruminococcaceae, Helicobacteraceae, Paraprevotellaceae, and other less abundant families were detected.Citation38 Species-specific unique bacterial profiles were also presented at the class- and order-level. However, the general patterns of gut microbiota abundance species were similarCitation38 and the most abundant genera are presented in . When comparing the results with Li et al. (), we cannot make a straightforward conclusion. Since Li et al. found Lactobacillus and Turicibacter genera most abundant in the rat’s gastrointestinal tract, while it was not the case in rat feces from the study of Nagpal et al., it could be concluded that they confirm the results of Li et al. and that indeed fecal samples cannot represent the whole microbiota in the gastrointestinal tract. However, since rats in the study of Nagpal et al. were fed low fat and high-fat diets we cannot rule out the possibility that the differences in the results are not attributed to differences in rat’s diets as well as the influence of other confounding factors like rat’s age or housing environment. In line with the findings of the study conducted by Brooks et al.,Citation34 the Lactobacillus genus was confirmed as the most abundant in rat feces. However, the same findings were not confirmed in mice, non-human primates, and human samples. The latter study, therefore, demonstrated that the microbiota profile of the rat is distinguishable from other evaluated species. Moreover, the authors showed that the microbiota profile of humans is more similar to that of non-human primates, when compared to rodents. On the other hand, the mice microbiota profile is more similar to human than to rat.Citation38
Table 1. Microbiota of the rat gastrointestinal tract according to Li et al.Citation33 and Nagpal et al.Citation38.
A first catalog of microbial genes in fecal samples of Sprague-Dawley (SD) rat was established recently.Citation39 The study included analyses of 98 fecal samples, sampled at two time points, from 49 SD rats divided into 7 experimental groups. Intervention was the application of probiotic supplementation (Lactobacillus casei), methotrexate, and two Chinese experimental herb formulas to adjuvant-induced arthritis rat model. From 64.6% genes that were annotated to the phylum level, most of them belonged to Firmicutes (75.9%), Bacteroidetes (10.83%), and Proteobacteria (6.77%). From 26.7% genes that were annotated to the genus level most of them belonged to Clostridium (8.74%), Bacteroides (6.25%), Roseburia (4.75%), Ruminococcus (4.44%), and Lachnoclostridium (2.58%).Citation39
Rat models in human microbiota research and their comparison with mouse models
According to Hugenholtz and de Vos, rodent models used in human microbiota research, enable a rather easy collection of many samples from different sites of the gastrointestinal tract, allow multiple comparisons at a large scale, and offer a wide range of different genotypic backgrounds.Citation40 A variety of information can be obtained from rodent models due to shared anatomical, histological, and physiological features of the gastrointestinal tract. At the same time, we have to keep in mind the differences, for instance, the morphological differences or dietary habits. Therefore, Hillman et al. provided an anatomical comparison of the gastrointestinal tract in humans and animal models,Citation41 presented in .
Table 2. Comparison of the anatomy of the rat, mice, and human intestinal tract.40–42.
Vdoviaková et al. presented the morphology of the stomach and intestine of adult Wistar rats of both genders and gave a comparison with the human gastrointestinal tract.Citation42 The authors found that the anatomy of the rat stomach is greatly influenced by adaptation, nature of food, body size, and shape.Citation42 Morphologically, they describe rat stomach as semilunar shaped sac weighing 1.8% of the total body weight while in humans stomach is pear-shaped sac weighing 6.2% of the total body weight.Citation42 More importantly, unlike in humans, the rat stomach is divided into the forestomach (pars proventricularis) and glandular stomach (corpus or pars glandularis) comprising fundus and pylorus, with forestomach occupying about three-fifths of the stomach area and functionally serving as a storage organ.Citation42 Another difference that the authors point out, is that humans have a poorly defined cecum, which is only continuous with the colon while rat cecum is as large as rat stomach.Citation42 Also, colon in humans consists of the ascending, transverse, descending, and sigmoid sections with all parts of colon in human being sacculated on the other hand, the rat colon is simple and not sacculated.Citation42 Regarding the dietary habits of laboratory rodents, Nguyen et al. noted that mice are fed with standardized chow diet throughout the experiment, which is composed mainly of plant materials and thus differs considerably to the usual composition and variation in a human daily diet.Citation43 The same can be applied to rats. Nagpal et al. point out that mice and rats are herbivores with present coprophagy, while humans can be herbivores, carnivores, and omnivores based on their ethnicity, geography culture, and traditions.Citation38
According to Franklin and Ericsson, rats are better suited for studies of microbiota as they provide a biological system similar to mice that is, however, large enough to better accommodate certain experimental techniques, i.e. colonoscopy and surgical manipulation.Citation44 In addition, rats possess certain physiological parameters more closely related to those of humans.Citation44 Moreover, Fritz et al. provided a comparison of the advantages and disadvantages of different animal models, including rat and mouse, commonly used for studying host–microbe interactions.Citation45 The advantages of the rat models according to Fritz et al., are the availability of a number of rat-specific disease models or genetically altered rats with the completely sequenced genome. Also, they are relatively small in size and can be maintained easily.Citation45 Further on, their reproduction is rather quick so that several generations can be observed in a relatively short period of time as they generally live 2 to 3 y.Citation45 The disadvantage that Fritz et al. emphasized is expectedly, a diet and a living environment that differs substantially from those of humans.Citation45 Mouse models, according to Fritz et al., have basically the same advantages as rat models, while disadvantages include again a living environment that differs substantially from those of humans and marked differences in the immune system and microbiota composition from those observed in humans.Citation45
Generally, the rat, mouse, and human intestinal microbiota are similar at the phylum level but different at the genus level.Citation38,Citation45 The rat dominant phyla are Firmicutes (74%) and Bacteroidetes (23%).Citation45 In humans, the dominant phyla are again the Firmicutes and Bacteroidetes as approximately 90% of bacterial species in the adult are members of these two phyla.Citation46 Human microbiota also includes Actinobacteria, Proteobacteria, and Verrucomicrobia at the phylum level, and at lower proportions, Fusobacteria, Tenericutes, Spirochetes, Cyanobacteria, and TM7.Citation41 At the lower levels of taxonomic classification, microbiome compositions vary with each individual.Citation41 The mouse intestinal bacterial composition is also dominated by Firmicutes and Bacteroidetes phyla.Citation43 According to Nguyen et al. genera with higher abundance in human gut microbiota in comparison with the mouse gut microbiota, include Prevotella, Faecalibacterium, and Ruminococcus, while Lactobacillus, Alistipes, and Turicibacter are more abundant in the mouse gut microbiota.Citation43 In the study of Pan et al. where a catalog of microbial genes in fecal samples of Sprague-Dawley (SD) rat was established, the authors compared the obtained catalog with those of mouse and integrated human gut microbial gene catalogs, and found that only a low percentage of genes were shared by all three species, 1.29% in the rat, 0.58% in the human, and 2.72% in the mouse gut microbiota.Citation39 They concluded that a comparison of the rat gut metagenome catalog with a human or a mouse revealed a higher pairwise overlap between rats and humans (2.47%) than between mouse and humans (1.19%).Citation39 Additionally, Pan et al. noted that the potential of rats for biomedical research high because 97% of the functional pathways in the human catalog were present in the rat catalog as well.Citation39 In the previously mentioned study by Nagpal et al., in which fecal microbiota composition of mice, rats, and non-human primates was compared to human subjects, the results showed that the gut microbiota, based on β-diversity (measure of diversity between communities) in humans seems to be closer to NHPs than to mice and rats, while mice microbiota appears to be closer to humans than rats.Citation38 In the rat samples from Nagpal et al. study, genera represented with the highest frequency were Prevotella (29.4%), S24-7 (14.3%) and Clostridiales (13.1%), in mouse samples these were S24-7 (44.7%), Clostridiales (25.3%) and Oscillospira (5.0%), while in the human samples these were Bacterioides (27.5%), Ruminococcaceae (10.2%), and Clostridiales (9.7%).Citation38 Additionally, according to Nguyen et al. who assessed the capability of mouse models to recapitulate the gut microbiota shifts associated with human diseases, rats are proposed to be more representative of the human gut microbiota than mice because the gut bacterial communities of humanized rats (germ-free rats as recipients of a human microbial community) reflect more closely the gut microbiota of human donors.Citation43
Taking into account all the above-mentioned differences, rat models may be considered as a useful tool in the microbiome research due to the minimization of confounding experimental factors such as genetics, age, environment, and diet, which are all controlled in laboratory conditions. Nguyen et al. recognized clear differences which were observed at the level of specific genus/species abundances between the mouse and human gut microbiota, but still considered that although absolute comparison might be difficult, these models are relevant for studying microbiota variation and shifts upon disturbance.Citation43 Mice are indeed, frequently used for evaluation of modulatory effect of different types of diets and nutrients on gut intestinal microbiota composition.Citation47–Citation51 We consider that the same relevance for studying microbiota shifts upon disturbance applies for rat models and present herein data on gut microbiota shifts in healthy rats after exposure to nutritional supplements. The observations of microbiota shifts in rats may be used as a ground for design of similar human microbiota research as well.
Gut microbiota shifts in healthy rat models by different nutritional supplements
An interesting field of research are studies on the gut microbiota alterations in healthy rat models after dietary interventions. For this purpose, rat strains whose properties are presented in , such as Sprague-Dawley, Wistar, and Fischer 344 (F-334) rats, are the most commonly used, while Lewis, wild-type Groningen, and BioBreeding rats are not that commonly employed in these studies. As presented in , animals used within the same study are usually of the same sex, while male animals are more frequently utilized. The gender of animals, along with species, genetics, age, and factors such as diet, antimicrobials, and microenvironment, should be considered as potential confounding variables in the microbiota modulation studies.Citation11,Citation87,Citation88 As dealing with confounding variables often relies on matching,Citation89 scientists usually choose animals of the same gender in the experimental design. When effects of the gender on the microbiota composition were evaluated, the results proved inconsistent. Indeed, the gender as a variable has not been investigated in details as other factors, both in humans and in animals.Citation90 A study by Org et al. showed that dietary effects on the composition and diversity of gut microbiota are partially dependent on sex-specific interactions. The authors examined sex differences related to the gut microbiota composition in a population of 89 common inbred mouse strains.Citation91 Another study that showed the influence of gender on the microbiota composition was a study by Bernbom et al. where fecal suspension from a 32-y-old woman was administered to male and female GF rats. The afterward collected microbiota clustered according to the gender of the host animal.Citation87 These findings should be taken into account in sex-comparative studies aimed to investigate potential health effects of diet as, for example, emphasized by Shastri et al. In their study administration of oligofructose increased the abundance of Bacteroidetes in female BioBreeding rats, but did not affect microbiota composition in males.Citation92 At last, 1–2 months old rats are mainly used in experimental set-ups even though some studies rely on older rats as well (6-month-old).
Table 3. Properties of different rat strains.
Table 4. Overview of the studies of modulation of rat gut microbiota by different nutritional supplements.
The experimental design in research of microbiota initiates often with animals undergoing an acclimatization period, usually for 1 week. During this period rats retain their habits and are kept on a normal chow diet. These rats represent a negative control of themselves, acting as the baseline for further microbiota evaluation.Citation37
A typical microbiota experiment is designed in such a way that microbiota diversity and species richness between different experimental groups of rats are compared. One group of animals has not been treated with a nutritive supplement under evaluation, this is the control group, and one or more groups of animals are exposed to the assessed nutrient (treated groups), often at different doses. The diet of treated rats has the same composition as the diet of control rats but supplemented with the assessed nutrient. In majority of the studies presented in the assessed nutrient was mixed with the feed. In fewer occasions, the supplement was added to drinking water as in (1) the study conducted by Wang et al. where treatment groups were given daily freshly prepared distilled water mixed with green tea polyphenols, (2) in the study by Chacar et al. where treatment groups were given different concentrations of phenolic compounds in the drinking water and (3) in Boudreau et al. study where aloin was administered to rats at different doses in drinking water.Citation68,Citation70,Citation85 In the study conducted by Jin et al. the rats in treatment group received the polysaccharide Ganoderma lucidum in aqueous suspension daily by oral administration.Citation83 Also, the assessed nutrient can be given to treatment groups by gavage as it was the case in the study of Casanova-Martí et al. where treatment groups were given grape seed proanthocyanidins or gallic acid 1 h prior to chow replacement by gavage, using tap water as vehicle.Citation69 Gastric administration of the assessed supplement was also performed in the study of Ou et al. where the effects of feruloylated oligosaccharides from maize bran on the microbial diversity and profiles were investigated in rat feces and in the study of Lee et al. where camelia oil, olive oil, and soybean oil were administered to rats by gastric gavage.Citation77,Citation84 Also, in the study of Pauer et al., rats received violacein directly in the mouth, twice a day, by gavage for a month.Citation86
Typically, six to 12 animals are assigned to each group although variations have been seen. Therefore, the smallest number of animals in a group was three, while the largest groups comprised 18 animals. Moore and Stanley highlighted factors that need to be taken into consideration when designing an animal trial aimed to investigate the gastrointestinal tract microbiota in the context of inflammation studies. They noted that unlike in traditional studies of immune mechanisms and inflammatory diseases in mouse models where the group sizes usually include 3 to 12 animals, larger treatment groups are necessary in microbiota experiments to achieve sufficient statistical power to draw valid conclusions.Citation93 This is mainly due to the inherent variability in microbiota between animals as well as to the temporal variation and strong responsiveness to diverse environmental stimuli.
The effect of nutritional supplementation on the composition of gut microbiota in an experiment is evaluated after a certain period of time. Most treatments presented in the literature continued for 3, 4, or 6 weeks; however, there were several studies focused on short-term and long-term nutritive modulation.Citation37,Citation65,Citation68-Citation70 For example, Casanova-Martí and coworkers evaluated the effect of grape seed proanthocyanidins on the rat microbiota during an 8-d trail.Citation69 In addition, in a study by Ferrario et al. where the effects of three different dietary fibers on rat fecal microbiota were evaluated, the grouping of the samples after a period of intervention of 1 week was indicative for different effects induced by dietary ingredients.Citation37 The study conducted by Liu and coworkers evaluated the effect of dietary broccoli with a multiple-sampling points (after 1, 2, 4, 7, and 14 d of treatment). The latter study demonstrated that changes in microbiota were present already after 4 d of treatment.Citation65,Citation69 In contrast, Wang et al. studied the effects of green-tea polyphenols over a period of 6 months while the study of Chacar et al. who investigated a long-term intake of phenolic compounds had an even longer study period of 14 months.Citation68,Citation70 The study of Ferrario et al. had a wash-out period where all animals returned to the standard chow diet without added substances, for 1 week after the end of the experiment in which dietary fibers were supplemented and it showed a reversion back to baseline microbiota.Citation37 Longitudinal studies that incorporate samples from the same habitat over time might provide more accurate conclusions rather than simple cross-sectional studies that compare ‘snapshots’ of two sample sets.Citation94
At the end of the defined experimental period, sampling is conducted and fecal or intestinal material is collected followed by microbiota analyses. Fecal material, freshly voided or collected from bedding is sampled in sterile tubes while intestinal content is collected at the time of sacrifice, dissected, and also sampled in sterile containers. In the study of Ferrario et al. fresh fecal samples were collected manually from a clean sawdust bedding for each animal, at most 1 h after deposition.Citation37 In the study of Pauer et al. whole intestinal content was collected, while Han et al. sampled ileal, cecal, and colonic content separately.Citation66,Citation86 The samples are then stored at −80°C (in some cases −40°C and −20°C) until further analysis.
Insight into rat intestinal microbiota modulation by food supplements
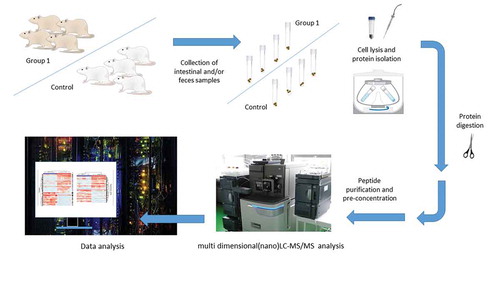
The usage of food supplements has increased in recent years even though scientific data on their effects in vivo, including effects on the microbiota, have been often vague.Citation95 This is why studies on animals may provide a good rationale for further, translational studies in human subjects. Rat models have been also used in studies that rely on genomics, proteomics, and metabolomics methods for the identification of microbiota members, mainly bacterial population, and the elucidation of functional changes. Main genomics methods used for this purpose are polymerase chain reaction (PCR) used in conjunction with high-throughput 16 S ribosomal RNA (rRNA) sequence analysis, that is, the second generation of sequencing technologies ().Citation95 Recently, the third generation of sequencing approaches is also applied, however, less frequently (), while real-time quantitative PCR (RT-qPCR) is rarely employed in the (targeted) estimation of bacterial loads, particularly for selected bacterial species.Citation95 These approaches are known as metagenomics methods preformed according to the widely agreed standards.Citation63 Genomics methods for the analysis of microbiota have reached a satisfactory level of technological maturity,Citation96–Citation98 as well as metaproteomics and metabolomics approaches for a comprehensive assessment of taxonomic composition and, particularly, its corresponding functionality. However, the usual metaproteomics experimental workflow includes multiple steps that require additional standardization (). These steps include isolation of bacteria from feces or intestinal content, bacterial protein isolation, tryptic digestion, and the identification/quantification of proteins by liquid chromatography coupled with tandem mass-spectrometry (LC-MS/MS).Citation99 Latter approach aimed to characterize the metaproteome is based on the comparison of experimentally obtained protein data with genomics information. This often implies an experimental design comprising both metagenomics and metaproteomics analyses from the same samples.Citation33,Citation100 Such – omics-based methods are crucial for the microbiota identification and have been exploited to analyze the effects of food, nutrients, and food supplements on the microbiota status of the rats.
The key effects on the rat gut microbiota after consummation of different nutritional supplements are summarized from the literature in . The results presented in are denoting changes that were statistically significant or otherwise emphasized by the authors as noteworthy.
Different foods, for example, fruits, vegetables, nuts, pulses, and cereal grains exerted their beneficial effect in part due to their polyphenolic or dietary fiber content. The consumption of walnuts, as the consumption of broccoli, increased the overall species diversity in the gut microbial communities.Citation63,Citation65 For instance, walnut diet increased the abundance of Firmicutes and in parallel decreased the abundance of Bacteriodetes, making the Firmicutes predominant microbe phyla in the descending colon. Moreover, probiotic-type bacteria including Lactobacillus, Ruminococcaceae, and Roseburia were enriched, while some others, like Bacteroides and Anaerotruncus were significantly reduced.Citation63 Interestingly, the latter changes in microbial composition may not be followed by significant changes in rat body weight and food intake, between the control and treated groups. Nevertheless, as the authors emphasize the addition of walnuts to the diet may shift the relative abundance of the functional capacities of the microbial communities. In total, 12 KEGG (Kyoto Encyclopedia of Genes and Genomes) metabolic pathways may be affected; the most of them involved in the amino acid and omega −3 and −6 fatty acid metabolism.Citation63
Several supplements, for example, lentil, unpolished rice, and whole wheat reduced the Firmicutes/Bacteroidetes ratio.Citation62,Citation66 In human and in animal studies, higher Firmicutes/Bacteroidetes ratio is linked to higher body mass index (BMI).Citation101,Citation102 In line, body weight gain reported for the rats fed with unpolished rice and whole wheat was lower compared to other groups fed by polished rice and refined wheat. For the same beneficial diets, an increased content of total short-chain fatty acids (SCFA), like acetate and butyrate in cecal and colonic digesta were reported.Citation66 The latter observation is normal for bacteria that ferment fibers and is required for optimal health, frequently attributed to the wide-ranging impacts of SCFA on the host physiology.Citation103–Citation106 In addition, Han et al. found beneficial bacteria like Lactobacillus and Akkermansia significantly increased in microbiota community of wheat-fed rats compared to in rice-fed rats.Citation66 The both species of the latter human intestinal bacteria are known to facilitate fermentation of indigestible carbohydrates, originating from dietary fibers, resistant starches, and non-starch polysaccharides.
Ounnas et al. reported microbiota changes in feces after consumption of whole rye, while no changes were evident in cecum microbiota.Citation67 Nevertheless, as author indicate this was one of the first studies in which the consumption of whole rye and its beneficial health effects were investigated, and results showed major biological modifications. Precisely, next to gut modifications like decreased Firmicutes/Bacteroidetes ratio, rats with whole rye diet had significantly increased n-3 long-chain fatty acids (LCFA) in their plasma and liver. The specific diet particularly influenced the metabolism of eicosapentanoic and docosahexanoic acids, while the content of SCFA was decreased, both in cecum and feces.Citation67
Polyphenolic compounds, such as tannins and hesperidin or hesperetin and polyphenolic mixtures, like green tea polyphenols (GTP), grape seed proanthocyanidins extract (GSPE), grape pomace extract have been studied for their effect on the rat gut microbiota. For example, the Firmicutes/Bacteroidetes ratio decreased after consumption of GSPE and persimmon tannin, while the abundance of Bacteroidetes phylum increased after supplementation with green tea polyphenols.Citation68,Citation69,Citation72 Indeed, the long-term treatment (6 months) with GTP significantly decreased the biodiversity in a dose-dependent manner at Sprague-Dawley rats. Moreover, similar patterns were observed at both sampling times, at the end of month 3 and 6.Citation68 Along with increased Bacteroidetes phylum, Wang et al. reported enrichment for Oscillospira. The effect of GTP intake may be further evaluated on obese gut microbiome as discussed changes in the gut microbiome were previously associated with leanness in humans and animals.Citation107 Another beneficial effect was the decrease of Peptostreptococcaceae which was linked to colorectal cancer phenotype in a study of Ahn et al.Citation108
Interestingly, similar results were obtained after only 8-d (short-term) treatment with GSPE, however, the latter study in a systematic approach evaluated also the effect of polyphenolic compounds on enteroendocrine secretions in female rats. Consequently, when polyphenolic mixtures are used as supplements it is difficult to differentiate which compounds are immediately absorbed, and which remain in the lumen, potentially causing gut modulation and, subsequently, indirectly changing the host’s health status. Due to present limitations of applied analytical approaches and techniques and the complexity of human/animal organisms, it is still not possible to observe the impact separately. In their study of proanthocyanidins, Casanova-Martí et al. defined several new target taxonomic groups that are modulated by proanthocyanidins intake, these are Sutterella, Pharscolarctobacterium, Parabacteroides, Bilophila, and Ruminococcus.Citation69 The increase in S24-7 family in the latter study is in accordance with the results of the previous study involving apple procyanidins in mice.Citation109 Next to microbial shifts, Casanova-Martí et al. hypothesized that observed gut modulation may correlate with metabolic and morphometric variables. Indeed, their study confirmed correlation between the gut modulation and systems effect, specifically, the reduction in cecal butyrate amount as well as the increased level of plasma glucagon-like-peptide-1.Citation69 In other word, the authors suggest that specific changes in microbiota caused by GSPE treatment may be linked to the modulation of plasma triacylglycerol, adiposity, and enterohormone secretion. Noteworthy posttreatment effects on the gut rat microbiota composition after long‐term (14 months) intake of grape pomace extracts rich with phenolic compounds were reported. Precisely, quantitative analysis of intestinal microbiota by qPCR revealed selective modulation, for example the growth inhibition of Clostridium (cluster I) 14 months posttreatment and the enhanced growth of probiotic Bifidobacterium 6 and 14 months posttreatment, compared to control and young groups.Citation70 The authors of the latter study emphasized that the second presented microbiota modulation was dose-specific, that is, the concentrations of phenolic compounds above 5 mg/kg/d did not result in such beneficial modulations. In general, the abundance reduction of Bifidobacterium was reported with age-related changes in the gut microbiota; therefore, phenolic compounds might have a protective effect on gut bacterial population, and even modulate outcomes of aging. Decrease in Clostridium was noticed also after supplementation with hesperidin and hesperetin, major flavonoids in citrus fruits, that significantly decreased the ratio of Clostridium subcluster XIVa.Citation71 The long-term intake of polyphenolic components potentially inhibits age-related increase of Clostridium, but only after 14 months posttreatment, and the effect seems to be independent of the administrated dose.Citation70 Finally, the study of Zhu et al., besides changes in the Firmicutes/Bacteroidetes ratio, showed that persimmon tannin when ingested at low doses may modulate the microbiota by increasing Bifidobacterium sp. and Lactobacillus sp., while decreasing E. coli and Enterococcus.Citation72 As persimmon tannin is highly polymerized and, therefore, non-absorbable in the intestine, its effect after ingestion is local. However, previous studies on animal models showed anti-hyperlipidemic and cholesterol-lowering effects, which in the latter study were somewhat attributed to the changes in bacterial structure and SCFA metabolism.
When comparing the results of probiotic and prebiotic supplementation studies, it can be observed that microbial shifts share common patterns. For instance, the increase of the Actinobacteria phylum was evident in at least three studies, these were the studies in which rats were fed (1) with Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 in combination with polysaccharides,Citation73 (2) food supplemented with galactooligosaccharide-fish peptide conjugatesCitation75 and (3) feruloylated oligosaccharides from maize bran.Citation77 As one can expected, the latter type of feed supplementation may in general significantly modulate bacterial richness and diversity, and particularly increase the amount of probiotic bacteria. Specifically, Wang et al. reported an increase of Bifidobacterium pseudolongum, Lactobacillus salivarius, and Lactobacillus reuteri and in parallel a decrease of Anaerostipes, Enterococcus, and Parabacteroides.Citation73 The authors unambiguously proved the importance of both probiotics and prebiotics in the maturation of healthy gut microbiota biological function. Moreover, the modulation of bacterial community resulted in elevated activities of digestive enzymes and several metabolism pathways (amino acid, energy, and SCFA-related), finally resulting in healthy progress of the weaning rats. On the other side, Ou et al. showed that feruloylated oligosaccharides from maize bran may exert a beneficial effect through multiple ways, that is by decreasing the ratio of Firmicutes to Bacteroidetes, increasing Lactobacillus and Ruminococcus and decreasing Clostridia.Citation77 It was previously reported, that feruloylated oligosaccharides release ferulic acid after fermentation by gut microorganisms, which was recognized as doubled physiological function, as ferulic acid may exhibit antimicrobial activity versus different microorganisms.Citation110,Citation111 All recently enumerated modulations of the gut microbiota have been recognized as contributing to protection against diabetes.
After receiving fructans from Agave salmiana in the study of Jasso-Padilla et al., rats had an increase in Lactobacillus spp. and Bifidobacterium spp.Citation76 Shifts of colonic microbiota composition induced by pectins were not as prominent as shifts of cecal microbiota in the study conducted by Tian et al. 105 In the cecal microbiota the rise in Lactobacillus was also present, specifically in groups receiving low-methyl esterified citrus pectin and sugar beet pectin.
Upon supplementation of marine mineral blend, rich in bioactive calcium, magnesium, and 70 other trace minerals, bacterial species diversity increased, specifically increased levels of Proteobacteria were also noticed.Citation80 Therefore, seaweed and seawater-derived functional food may be considered as a reasonable supplement next to the high fat/high sugar “Western diet”. In another study, phylum TM7, associated with IBD and Chron’s disease, decreased in the group receiving lower concentration of the supplement, while Ruminococcaceae family associated with gut health increased for a group given a higher concentration of supplement. Families Christensenellaceae, associated with lean BMI, and Porphyromonadaceae were increased as well. In parallel, phylum Proteobacteria level increased and the latter observation was suggested as a potential diagnostic signature for dysbiosis and illness as it is known that Proteobacteria have a low abundance in the gut of healthy humans.Citation112
After administration of theobromine, a methylxantine from cocoa powder, several changes that were exclusive for the rats fed with theobromine were noticed. For instance, Candidatus Arthromitus belonging to Firmicutes phylum, Clostridia class, known for inducing adaptive immune responses in the gut, was found only in the theobromine group, while Ruminicoccus flavefaciens disappeared.Citation81 Besides, significantly lower counts of Bifidobacterium spp., Streptococcus spp., Clostridium histolyticum C. perfringens and Escherichia coli were seen in comparison with controls. All enumerated changes were reflected in enhanced generation of SCFA, mainly the butyric acid. Finally, the authors hypothesize that theobromine, both on its own and as part of a cocoa diet, may contribute to the lower proportion of IgA-coated bacteria.
Furthermore, Ganoderma lingzhi mushroom, used in traditional Chinese medicine, was evaluated for its effect on gut microbiota in rats.109 The treatment significantly reduced the numbers of Clostridium coccoides and Clostridium leptum per gram of digesta, while Akkermancia muciniphila and Enterobacteriaceae increased. In addition, the supplementation of polysaccharide from mycelia of Ganoderma lucidum decreased Firmicutes/Bacteroidetes ratio, Proteobacteria phylum and caused a significant change in 37 OTUs among which were S24-7, SMB53, Rikenellaceae, Allobaculum, Rc4-4, and Ruminococcaceae.Citation83 Previously, increased diversity of Clostridium coccoides and Clostridium leptum was seen in microbiota profiles of patients with colon cancer and adenomatous polyposis.Citation113 The both presented studies, emphasize the potential anti-colon cancer effect of mushroom extracts through firstly, modulating intestinal microflora, and secondly, a wide net of action, such as modulation of secondary bile acids, mucins, propionate, and regulation the intestinal barrier functions.
Rat gut microbiota changes after consummation of camellia olive and soybean oil were also reported.Citation84 Moreover, the intake of camellia oil showed improved results in comparison to soybean and olive oil. Camellia oil modifies the composition of gut microbiota and alleviates acetic acid-induced colitis in rats. In more detail, the increased ratio of Firmicutes/Bacteroidetes, the species diversity, and the relative abundance of the Bifidobacterium, while reducing Prevotella was shown. Therefore, camellia oil is preferable treatment/preventive measure as it is able to reduce damage caused by antioxidant system induced by acetic acid, and finally may prevent the development of chronic inflammatory bowel disease. A component of Aloe vera plant leaf, aloin, may induce dose-related changes, for example, increased Bacteroidetes (mostly Prevotellaceae and S24-7) and Verrucomicrobia phylums and decreased Firmicutes (specially members Ruminococcaceae and Lachnispiraceae).Citation85 Moreover, the similarities in effects were observed for aloin and the Aloe vera whole leaf extract,Citation114 including serious pathological changes leading to the increased incidences of adenomas and carcinomas of the rat cecum and large intestine. The latter findings suggest at caution when using Aloe vera latex laxative properties in humans and animals. Indeed, to achieve its purpose food/feed supplements must be further studied in order to hamper severe damage and increase resistance of pathogenic strains. For instance, violacein is a natural violet pigment produced by Chromobacterium violaceum with broad antibacterial, antiviral, antifungal, and antioxidant properties, however, its effect on rat gut microbiota of Wistar rats has been explored recently.Citation86
Conclusions
A link between gut dysbiosis and various diseases in humans has been observed, and the modulation of gut microbiota may be used for studies on prevention measure for various pathological states. The use of rats as disease models to study specific pathological states may be a valuable tool for determination of the relationship between intestinal dysbiosis and disease. In the presented review we gathered data from studies conducted on healthy rats where the influence of different nutritional supplements on gut microbiota was assessed. When comparing the observed modulatory effects of tested supplements, one has to keep in mind that scientist used different techniques of microbiota analysis and different strains, gender, and age of rats. A need for standardization of experimental procedures and guidelines that would enhance reproducibility and comparability across microbiome studies in animal models, can be indeed, deduced from the rat studies included in this review as well. No commonly acknowledged standards for the choice of adequate rat strain, gender, sample size, diet, housing environment, or techniques for microbiome analysis are currently in place. All these factors remain therefore possible confounders and vary among different research according to the specific needs of the experiment, availability of resources, and research design. In studies included in this review, microbiota responded very differently to different supplements, but some microbial shifts were seen more frequently and some were common for different groups of supplements. For instance, a change in Firmicutes/Bacteroidetes ratio is observed frequently, whereas the increase of Lactobacillus is often observed upon prebiotic and probiotic supplementation. The scientists have provided evidence on beneficial effects of different nutritional supplements on the microbiota composition and function in rats that suggests a beneficial role of these supplements in human as well. The modulation of microbiota members whose compositional shifts and functional changes may be an important line of defense against diseases, may be an important research field in the years to come.
Disclosure of interest
The authors report no conflict of interest.
Acknowledgments
We want to thank the Croatian Government and the European Union (European Regional Development Fund—the Competitiveness and Cohesion Operational Programme—KK.01.1.1.01) for support through project Bioprospecting of the Adriatic Sea (KK.01.1.1.01.0002) granted to The Scientific Centre of Excellence for Marine Bioprospecting—BioProCro. The study was also supported by the University of Rijeka research grant uniri-biomed-18-133 given to S.K.P. We acknowledge the project “Research Infrastructure for Campus-based Laboratories at the University of Rijeka,” co-financed by European Regional Development Fund (ERDF).
Additional information
Funding
References
- D’Argenio V, Salvatore F. The role of the gut microbiome in the healthy adult status. Clin Chim Acta. 2015;451:97–102. doi:10.1016/j.cca.2015.01.003. PMID: 25584460.
- Belizário JE, Faintuch J. Microbiome and gut dysbiosis. Exp Suppl 2012. 2018;109:459–476. doi:10.1007/978-3-319-74932-7_13. PMID: 30535609.
- O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Reports. 2006;7(7):688–693. doi:10.1038/sj.embor.7400731. PMID: 16819463.
- Grice EA, Segre JA. The human microbiome: our second genome. Annual Review of Genomics and Human Genetics. 2012;13(1):151–170. doi:10.1146/annurev-genom-090711-163814. PMID: 22703178.
- Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3(4):289–306. doi:10.4161/gmic.19897. PMID: 22572875.
- Hill MJ. Intestinal flora and endogenous vitamin synthesis. European Journal of Cancer Prevention. 1997;6(Suppl 1):S43–45. doi:10.1097/00008469-199703001-00009. PMID: 9167138.
- Purchiaroni F, Tortora A, Gabrielli M, Bertucci F, Gigante G, Ianiro G, Ojetti V, Scarpellini E, Gasbarrini A. The role of intestinal microbiota and the immune system. Eur Rev Med Pharmacol Sci. 2013;17:323–333. PMID: 23426535.
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi:10.1016/j.cell.2014.03.011. PMID: 24679531.
- Stappenbeck TS, Hooper LV, Gordon JI. Nonlinear partial differential equations and applications: developmental regulation of intestinal angiogenesis by indigenous microbes via paneth cells. Proceedings of the National Academy of Sciences. 2002;99(24):15451–15455. doi:10.1073/pnas.202604299. PMID: 12432102.
- Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol Q Publ Hell Soc Gastroenterol. 2015;28:203–209. PMID: 25830558.
- Inoue R, Ushida K. Development of the intestinal microbiota in rats and its possible interactions with the evolution of the luminal IgA in the intestine. FEMS Microbiol Ecol. 2003;45:147–153. doi:10.1016/S0168-6496(03)00134-X. PMID: 19719625.
- Yajima M, Nakayama M, Hatano S, Yamazaki K, Aoyama Y, Yajima T, Kuwata T. Bacterial translocation in neonatal rats: the relation between intestinal flora, translocated bacteria, and influence of milk. J Pediatr Gastroenterol Nutr. 2001;33:592–601. doi:10.1097/00005176-200111000-00015. PMID: 11740235.
- Said HM,editor. Physiology of the gastrointestinal tract. 6th. Cambridge (MA): Elsevier; 2018. doi:10.1016/C2015-1-04889-X.
- Hold GL. Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World Journal of Gastroenterology. 2014;20(5):1192–1210. doi:10.3748/wjg.v20.i5.1192. PMID: 24574795.
- Federico A, Dallio M, DI Sarno R, Giorgio V, Miele L. Gut microbiota, obesity and metabolic disorders. Minerva Gastroenterol Dietol. 2017;63(4):337–344. doi:10.23736/S1121-421X.17.02376-5. PMID: 28927249.
- Baothman OA, Zamzami MA, Taher I, Abubaker J, Abu-Farha M. The role of gut microbiota in the development of obesity and diabetes. Lipids in Health and Disease. 2016;15(1):108. doi:10.1186/s12944-016-0278-4. PMID: 27317359.
- Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. Role of gut microbiota in the aetiology of obesity: proposed mechanisms and review of the literature. J Obes 2016. 2016;7353642. doi:10.1155/2016/7353642. PMID: 27703805.
- Davis CD. The gut microbiome and its role in obesity. Nutr Today. 2016;51(4):167–174. doi:10.1097/NT.0000000000000167. PMID: 27795585.
- Zhao W, Ho H-E H-E, Bunyavanich S. The gut microbiome in food allergy. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2019;122(3):276–282. doi:10.1016/j.anai.2018.12.012. PMID: 30578857.
- Cenit MC, Sanz Y, Codoñer-Franch P. Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol. 2017;23(30):5486–5498. doi:10.3748/wjg.v23.i30.5486. PMID: 28852308.
- Li L, Rao S, Cheng Y, Zhuo X, Deng C, Xu N, Zhang H, Yang L. Microbial osteoporosis: the interplay between the gut microbiota and bones via host metabolism and immunity. Microbiol Open. 2019;e00810. doi:10.1002/mbo3.810. PMID: 31001921.
- Xu X, Jia X, Mo L, Liu C, Zheng L, Yuan Q, Zhou X. Intestinal microbiota: a potential target for the treatment of postmenopausal osteoporosis. Bone Res. 2017;5(1):17046. doi:10.1038/boneres.2017.46. PMID: 28983411.
- Eichele DD, Kharbanda KK. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol. 2017;23(33):6016–6029. doi:10.3748/wjg.v23.i33.6016. PMID: 28970718.
- Ghattamaneni NKR, Panchal SK, Brown L. An improved rat model for chronic inflammatory bowel disease. Pharmacol Rep PR. 2019;71(1):149–155. doi:10.1016/j.pharep.2018.10.006. PMID: 30550995.
- Di Luccia B, Crescenzo R, Mazzoli A, Cigliano L, Venditti P, Walser J-C, Widmer A, Baccigalupi L, Ricca E, Iossa S. Rescue of fructose-induced metabolic syndrome by antibiotics or faecal transplantation in a rat model of obesity. PLoS One. 2015;10(8):e0134893. doi:10.1371/journal.pone.0134893. PMID: 26244577.
- Barrière DA, Noll C, Roussy G, Lizotte F, Kessai A, Kirby K, Belleville K, Beaudet N, Longpré J-M, Carpentier AC, et al. Combination of high-fat/high-fructose diet and low-dose streptozotocin to model long-term type-2 diabetes complications. Scientific Reports. 2018;8(1):424. doi:10.1038/s41598-017-18896-5. PMID: 29323186.
- Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacological Res. 2005;52(4):313–320. doi:10.1016/j.phrs.2005.05.004. PMID: 15979893.
- Liu F, Horton-Sparks K, Hull V, Li RW, Martínez-Cerdeño V. The valproic acid rat model of autism presents with gut bacterial dysbiosis similar to that in human autism. Molecular Autism. 2018;9(1):61. doi:10.1186/s13229-018-0251-3. PMID: 30555669.
- Duty S, Jenner P. Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011;164:1357–1391. doi:10.1111/j.1476-5381.2011.01426.x. PMID: 21486284.
- Krishnan V, Nestler EJ. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci. 2011;7:121–147. doi:10.1007/7854_2010_108. PMID: 21225412.
- Liang S, Wu X, Hu X, Wang T, Jin F. Recognizing depression from the microbiota–gut–brain axis. Int J Mol Sci 2008;10:E1592. doi:10.1111/j.1462-2920.2007.01503.x. PMID: 29843470.
- Yu M, Jia H, Zhou C, Yang Y, Zhao Y, Yang M, Zou Z. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J Pharm Biomed Anal. 2015;34:231–239. doi:10.1016/j.jpba.2017.02.008. PMID: 28219800.
- Li D, Chen H, Mao B, Yang Q, Zhao J, Gu Z, Zhang H, Chen YQ, Chen W. Microbial biogeography and core microbiota of the rat digestive tract. Scientific Reports. 2017;7(1):45840. doi:10.1038/srep45840. PMID: 28374781.
- Brooks SPJ, McAllister M, Sandoz M, Kalmokoff ML. Culture-independent phylogenetic analysis of the faecal flora of the rat. Canadian J Microbiol. 2003;49(10):589–601. doi:10.1139/w03-075. PMID: 14663493.
- Manichanh C, Reeder J, Gibert P, Varela E, Llopis M, Antolin M, Guigo R, Knight R, Guarner F. Reshaping the gut microbiome with bacterial transplantation and antibiotic intake. Genome Res. 2010;20(10):1411–1419. doi:10.1101/gr.107987.110. PMID: 20736229.
- Flemer B, Gaci N, Borrel G, Sanderson IR, Chaudhary PP, Tottey W, O’Toole PW, Brugère J-F. Fecal microbiota variation across the lifespan of the healthy laboratory rat. Gut Microbes. 2017;8(5):428–439. doi:10.1080/19490976.2017.1334033. PMID: 28586297.
- Ferrario C, Statello R, Carnevali L, Mancabelli L, Milani C, Mangifesta M, Duranti S, Lugli GA, Jimenez B, Lodge S, et al. How to feed the mammalian gut microbiota: bacterial and metabolic modulation by dietary fibers. Front Microbiol. 2017;8:1749. doi:10.3389/fmicb.2017.01749. PMID: 28955319.
- Nagpal R, Wang S, Solberg Woods LC, Seshie O, Chung ST, Shively CA, Register TC, Craft S, McClain DA, Yadav H. Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front Microbiol. 2018;9:2897. doi:10.3389/fmicb.2018.02897. PMID: 30555441.
- Pan H, Guo R, Zhu J, Wang Q, Ju Y, Xie Y, Zheng Y, Wang Z, Li T, Liu Z, et al. A gene catalogue of the sprague-dawley rat gut metagenome. GigaScience. 2018;7(5). doi:10.1093/gigascience/giy055. PMID: 29762673.
- Hugenholtz F, de Vos WM. Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci. 2018;75(1):149–160. doi:10.1007/s00018-017-2693-8. PMID: 29124307.
- Hillman ET, Lu H, Yao T, Nakatsu CH. Microbial ecology along the gastrointestinal tract. Microbes Environ. 2017;32(4):300–313. doi:10.1264/jsme2.ME17017. PMID: 29129876.
- Vdoviaková K, Petrovová E, Maloveská M, Krešáková L, Teleky J, Elias MZJ, Petrášová D, Maloveská M. Surgical anatomy of the gastrointestinal tract and its vasculature in the laboratory rat. Gastroenterol Res Pract. 2016;2016:2632368. doi:10.1155/2016/2632368. PMID: 26819602.
- Nguyen TL, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Models Mech. 2015;8(1):1–16. doi:10.1242/dmm.017400. PMID: 25561744.
- Franklin CL, Ericsson AC. Microbiota and reproducibility of rodent models. Lab Animal. 2017;46(4):114–122. doi:10.1038/laban.1222. PMID: 28328896.
- Fritz JV, Desai MS, Shah P, Schneider JG, Wilmes P. From meta-omics to causality: experimental models for human microbiome research. Microbiome. 2013;1(1):14. doi:10.1186/2049-2618-1-14. PMID: 24450613.
- Brahe LK, Astrup A, Larsen LH. Can we prevent obesity-related metabolic diseases by dietary modulation of the gut microbiota? Adv Nutr Bethesda Md. 2016;7(1):90–101. doi:10.3945/an.115.010587. PMID: 26773017.
- Wang L, Zeng B, Zhang X, Liao Z, Gu L, Liu Z, Zhong Q, Wei H, Fang X. The effect of green tea polyphenols on gut microbial diversity and fat deposition in C57BL/6J HFA mice. Food & Function. 2016;7(12):4956–4966. doi:10.1039/c6fo01150k. PMID: 27845787.
- Kim E, Kim D-B, Park J-Y. Changes of mouse gut microbiota diversity and composition by modulating dietary protein and carbohydrate contents: a pilot study. Preventive Nutr Food Sci. 2016;21(1):57–61. doi:10.3746/pnf.2016.21.1.57. PMID: 27069907.
- Espley RV, Butts CA, Laing WA, Martell S, Smith H, McGhie TK, Zhang J, Paturi G, Hedderley D, Bovy A, et al. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J Nutr. 2014;144(2):146–154. doi:10.3945/jn.113.182659. PMID: 24353343.
- Uyeno Y, Katayama S, Nakamura S. Changes in mouse gastrointestinal microbial ecology with ingestion of kale. Benef Microbes. 2014;5(3):345–349. doi:10.3920/BM2013.0073. PMID: 24736315.
- Jiang X, Ding H, Liu Q, Wei Y, Zhang Y, Wang Y, Lu Y, Ma A, Li Z, Hu Y. Effects of peanut meal extracts fermented by Bacillus natto on the growth performance, learning and memory skills and gut microbiota modulation in mice. British J Nutr. 2020;123(4):383–393. doi:10.1017/S0007114519002988. PMID: 31769373.
- Brower M, Grace M, Kotz CM, Koya V. Comparative analysis of growth characteristics of Sprague Dawley rats obtained from different sources. Laboratory Animal Research. 2015;31(4):166–173. doi:10.5625/lar.2015.31.4.166. PMID: 26755919.
- Prodinger PM, Bürklein D, Foehr P, Kreutzer K, Pilge H, Schmitt A, Eisenhart-Rothe R, Burgkart R, Bissinger O, Tischer T. Improving results in rat fracture models: enhancing the efficacy of biomechanical testing by a modification of the experimental setup. BMC Musculoskelet Disord. 2018;19(1):243. doi:10.1186/s12891-018-2155-y. PMID: 30025531.
- Singhal A, Aliouat EM, Hervé M, Mathys V, Kiass M, Creusy C, Delaire B, Tsenova L, Fleurisse L, Bertout J, et al. Experimental tuberculosis in the wistar rat: a model for protective immunity and control of infection. PLoS One. 2011;6(4):e18632. doi:10.1371/journal.pone.0018632. PMID: 21533270.
- Alvarado JC, Fuentes-SantamarÃ-a V, Gabaldón-Ull MC, Blanco JL, Juiz JM. Wistar rats: a forgotten model of age-related hearing loss. Front Aging Neurosci. 2014;6:29. doi:10.3389/fnagi.2014.00029. PMID: 24634657.
- McCormick DL. Chapter 17 - preclinical evaluation of carcinogenicity using the rodent two-year bioassay. In: Faqi AS, editor. A comprehensive guide to toxicology in preclinical drug development. Cambridge (MA): Elsevier; 2013. p. 423–436. doi:10.1016/B978-0-12-387815-1.00017-4.
- Maronpot RR, Nyska A, Foreman JE, Ramot Y. The legacy of the F344 rat as a cancer bioassay model (a retrospective summary of three common F344 rat neoplasms). Crit Rev Toxicol. 2016;46:641–675. doi:10.1080/10408444.2016.1174669. PMID: 27278595.
- Cadoni C. Fischer 344 and lewis rat strains as a model of genetic vulnerability to drug addiction. Front Neurosci. 2016;10:13. doi:10.3389/fnins.2016.00013. PMID: 26903787.
- Coppens CM, de Boer SF, Buwalda B, Koolhaas JM. Aggression and aspects of impulsivity in wild-type rats. Aggress Behav. 2014;40:300–308. doi:10.1002/ab.21527. PMID: 24464354.
- Vidal J, Buwalda B, Koolhaas JM. Male Wistar rats are more susceptible to lasting social anxiety than wild-type groningen rats following social defeat stress during adolescence. Behav Processes. 2011;88:76–80. doi:10.1016/j.beproc.2011.08.005. PMID: 21854839.
- van den Brandt J, Fischer HJ, Walter L, Hünig T, Klöting I, Reichardt HM. Type 1 diabetes in biobreeding rats is critically linked to an imbalance between Th17 and regulatory T cells and an altered TCR repertoire. J Immunol Baltim Md 1950. 2010;185:2285–2294. doi:10.4049/jimmunol.1000462. PMID: 20644174.
- Siva N, Johnson CR, Richard V, Jesch ED, Whiteside W, Abood AA, Thavarajah P, Duckett S, Thavarajah D. Lentil (Lens culinaris Medikus) diet affects the gut microbiome and obesity markers in rat. J Agric Food Chem. 2018;66:8805–8813. doi:10.1021/acs.jafc.8b03254. PMID: 0102041.
- Byerley LO, Samuelson D, Blanchard E, Luo M, Lorenzen BN, Banks S, Ponder MA, Welsh DA, Taylor CM. Changes in the gut microbial communities following addition of walnuts to the diet. J Nutr Biochem. 2017;48:94–102. doi:10.1016/j.jnutbio.2017.07.001. PMID: 8797931.
- Pan P, Lam V, Salzman N, Huang Y-W, Yu J, Zhang J, L-S W. Black raspberries and their anthocyanin and fiber fractions alter the composition and diversity of gut microbiota in F-344 rats. Nutr Cancer. 2017;69:943–951. doi:10.1080/01635581.2017.1340491. PMID: 28718724.
- Liu X, Wang Y, Hoeflinger JL, Neme BP, Jeffery EH, Miller MJ. Dietary broccoli alters rat cecal microbiota to improve glucoraphanin hydrolysis to bioactive isothiocyanates. Nutrients 2017;9:E262. doi:10.3390/nu9030262. PMID: 28287418.
- Han F, Wang Y, Han Y, Zhao J, Han F, Song G, Jiang P, Miao H. Effects of whole-grain rice and wheat on composition of gut microbiota and short-chain fatty acids in rats. J Agric Food Chem. 2018;66:6326–6335. doi:10.1021/acs.jafc.8b01891. PMID: 29766722.
- Ounnas F, Privé F, Salen P, Gaci N, Tottey W, Calani L, Bresciani L, López-Gutiérrez N, Hazane-Puch F, Laporte F, et al. Whole rye consumption improves blood and liver n-3 fatty acid profile and gut microbiota composition in rats. PLoS One. 2016;11(2):e0148118. doi:10.1371/journal.pone.0148118. PMID: 26862900.
- Wang J, Tang L, Zhou H, Zhou J, Glenn TC, Shen C-L, Wang J-S. Long-term treatment with green tea polyphenols modifies the gut microbiome of female sprague-dawley rats. J Nutr Biochem. 2018;56:55–64. doi:10.1016/j.jnutbio.2018.01.005. PMID: 29454999.
- À À, Serrano J, Portune KJ, Sanz Y, Blay MT, Terra X, Ardévol A, Pinent M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female rats. Food Function. 2018;9(3):1672–1682. doi:10.1039/c7fo02028g. PMID: 29473070.
- Chacar S, Itani T, Hajal J, Saliba Y, Louka N, Faivre J-F, Maroun R, Fares N. the impact of long-term intake of phenolic compounds-rich grape pomace on rat gut microbiota. J Food Sci. 2018;83:246–251. doi:10.1111/1750-3841.14006. PMID: 29227528.
- Unno T, Hisada T, Takahashi S. Hesperetin modifies the composition of fecal microbiota and increases cecal levels of short-chain fatty acids in rats. J Agri Food Chem. 2015;63(36):7952–7957. doi:10.1021/acs.jafc.5b02649. PMID: 26306898.
- Zhu W, Lin K, Li K, Deng X, Li C. Reshaped fecal gut microbiota composition by the intake of high molecular weight persimmon tannin in normal and high-cholesterol diet-fed rats. Food Funct. 2018;9(1):541–551. doi:10.1039/c7fo00995j. PMID: 29260181.
- Wang M, Chen Y, Wang Y, Li Y, Zheng H, Ma F, Ma C, Zhang X, Lu B, Xie Z, et al. The effect of probiotics and polysaccharides on the gut microbiota composition and function of weaned rats. Food Funct. 2018;9:1864–1877. doi:10.1039/c7fo01507k. PMID: 29521393.
- Ahrén IL, Xu J, Önning G, Olsson C, Ahrné S, Molin G. Antihypertensive activity of blueberries fermented by lactobacillus plantarum DSM 15313 and effects on the gut microbiota in healthy rats. Clin Nutr Edinb Scotl. 2015;34:719–726. doi:10.1016/j.clnu.2014.08.009. PMID: 25194632.
- Jin W, Han K, Dong S, Yang Y, Mao Z, Su M, Zeng M. Modifications in gut microbiota and fermentation metabolites in the hindgut of rats after the consumption of galactooligosaccharide glycated with a fish peptide. Food Funct. 2018;9:2853–2864. doi:10.1039/c7fo02002c. PMID: 29700505.
- Jasso-Padilla I, Juárez-Flores B, Alvarez-Fuentes G, De la Cruz-martínez A, González-Ramírez J, Moscosa-Santillán M, González-Chávez M, Oros-Ovalle C, Prell F, Czermak P, et al. Effect of prebiotics of agave salmiana fed to healthy wistar rats. J Sci Food Agric. 2017;97:556–563. doi:10.1002/jsfa.7764. PMID: 27097820.
- Ou J-Y, Huang J-Q, Song Y, Yao S-W, Peng X-C, Wang M-F, Ou S-Y. Feruloylated oligosaccharides from maize bran modulated the gut microbiota in rats. Plant Foods Hum Nutr Dordr Neth. 2016;71:123–128. doi:10.1007/s11130-016-0547-4. PMID: 27165128.
- Tian L, Scholte J, Borewicz K, van den Bogert B, Smidt H, Scheurink AJW, Gruppen H, Schols HA. Effects of pectin supplementation on the fermentation patterns of different structural carbohydrates in rats. Mol Nutr Food Res. 2016;60:2256–2266. doi:10.1002/mnfr.201600149. PMID: 27174558.
- Kalmokoff M, Franklin J, Petronella N, Green J, Brooks SPJ. Phylum level change in the cecal and fecal gut communities of rats fed diets containing different fermentable substrates supports a role for nitrogen as a factor contributing to community structure. Nutrients 2015;7:3279–3299. doi:10.3390/nu7053279. PMID: 25954902.
- Crowley EK, Long-Smith CM, Murphy A, Patterson E, Murphy K, O’Gorman DM, Stanton C, Nolan YM. Dietary supplementation with a magnesium-rich marine mineral blend enhances the diversity of gastrointestinal microbiota. Mar Drugs. 2018;16:E216. doi:10.3390/md16060216. PMID: 29925774.
- Martín-Peláez S, Camps-Bossacoma M, Massot-Cladera M, Rigo-Adrover M, À F, Pérez-Cano FJ, Castell M. Effect of cocoa’s theobromine on intestinal microbiota of rats. Mol Nutr Food Res. 2017;61:1700238. doi:10.1002/mnfr.201700238. PMID: 28605130.
- Yang Y, Nirmagustina DE, Kumrungsee T, Okazaki Y, Tomotake H, Kato N. Feeding of the water extract from Ganoderma lingzhi to rats modulates secondary bile acids, intestinal microflora, mucins, and propionate important to colon cancer. Biosci Biotechnol Biochem. 2017;81:1796–1804. doi:10.1080/09168451.2017.1343117. PMID: 28661219.
- Jin M, Zhu Y, Shao D, Zhao K, Xu C, Li Q, Yang H, Huang Q, Shi J. Effects of polysaccharide from mycelia of Ganoderma lucidum on intestinal barrier functions of rats. Int J Biol Macromol. 2017;94:1–9. doi:10.1016/j.ijbiomac.2016.09.099. PMID: 27693834.
- Lee W-T, Tung Y-T, Wu -C-C, Tu P-S, Yen G-C, Oil C. (Camellia oleifera Abel.) modifies the composition of gut microbiota and alleviates acetic acid-induced colitis in rats. J Agric Food Chem. 2018;66:7384–7392. doi:10.1021/acs.jafc.8b02166. PMID: 29895146.
- Boudreau MD, Olson GR, Tryndyak VP, Bryant MS, Felton RP, Beland FA. From the cover: aloin, a component of the aloe vera plant leaf, induces pathological changes and modulates the composition of microbiota in the large intestines of F344/N male rats. Toxicol Sci. 2017;158:302–318. doi:10.1093/toxsci/kfx105. PMID: 28525602.
- Pauer H, Hardoim CCP, Teixeira FL, Miranda KR, Barbirato D da S, Carvalho DP de, Antunes LCM, Leitão ÁA da C, Lobo LA, Domingues RMCP. Impact of violacein from chromobacterium violaceum on the mammalian gut microbiome. PLoS One. 2018;13:e0203748. doi:10.1371/journal.pone.0203748. PMID: 30212521.
- Bernbom N, Nørrung B, Saadbye P, Mølbak L, Vogensen FK, Licht TR. Comparison of methods and animal models commonly used for investigation of fecal microbiota: effects of time, host and gender. J Microbiol Methods. 2006;66(1):87–95. doi:10.1016/j.mimet.2005.10.014. PMID: 16289391.
- Tomas J, Langella P, Cherbuy C. The intestinal microbiota in the rat model: major breakthroughs from new technologies. Animal Health Research Reviews. 2012;13(1):54–63. doi:10.1017/S1466252312000072. PMID: 22853927.
- McDonald JH. Handbook of biological statistics. Baltimore (MD): Sparky House Publishing; 2009.
- Kim YS, Unno T, Kim B-Y, Park M-S. Sex differences in gut microbiota. World J Mens Health. 2020;38(1):48–60. doi:10.5534/wjmh.190009. PMID: 30929328.
- Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, Lusis AJ, Org E. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7(4):313–322. doi:10.1080/19490976.2016.1203502. PMID: 27355107.
- Shastri P, McCarville J, Kalmokoff M, Brooks SPJ, Green-Johnson JM. Sex differences in gut fermentation and immune parameters in rats fed an oligofructose-supplemented diet. Biology of Sex Differences. 2015;6(1):13. doi:10.1186/s13293-015-0031-0. PMID: 26251695.
- Moore RJ, Stanley D. Experimental design considerations in microbiota/inflammation studies. Clin Transl Immunol. 2016;5:e92. doi:10.1038/cti.2016.41. PMID: 27525065.
- Quince C, Walker AW, Simpson JT, Loman NJ, Segata N. Shotgun metagenomics, from sampling to analysis. Nat Biotechnol. 2017;35:833–844. doi:10.1038/nbt.3935. PMID: 28898207.
- Dwyer JT, Coates PM, Smith MJ. Dietary supplements: regulatory challenges and research resources. Nutrients. 2018;10:E41. doi:10.3390/nu10010041. PMID: 29300341.
- Hiergeist A, Gläsner J, Reischl U, Gessner A. Analyses of intestinal microbiota: culture versus sequencing. ILAR J. 2015;56:228–240. doi:10.1093/ilar/ilv017. PMID: 26323632.
- O’Sullivan DJ. Methods for analysis of the intestinal microflora. Curr Issues Intest Microbiol. 2000;1:39–50. PMID: 11709868.
- Sommer MOA. Advancing gut microbiome research using cultivation. Curr Opin Microbiol. 2015;27:127–132. doi:10.1016/j.mib.2015.08.004. PMID: 26401902.
- Hettich RL, Pan C, Chourey K, Giannone RJ. Metaproteomics : harnessing the power of high performance mass spectrometry to identify the suite of proteins that control metabolic activities in microbial communities. Anal Chem. 2013;85:4203–4214. doi:10.1021/ac303053e. PMID: 23469896.
- Guirro M, Costa A, Gual-Grau A, Herrero P, Torrell H, Canela N, Arola L. Effects from diet-induced gut microbiota dysbiosis and obesity can be ameliorated by fecal microbiota transplantation: A multiomics approach. PLoS One. 2019;14:e0218143. doi:10.1371/journal.pone.0218143. PMID: 31545802.
- Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, Gavalko Y, Dorofeyev A, Romanenko M, Tkach S, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17:120. doi:10.1186/s12866-017-1027-1. PMID: 28532414.
- Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences. 2005;102(31):11070–11075. doi:10.1073/pnas.0504978102. PMID: 16033867.
- Chambers ES, Preston T, Frost G, Morrison DJ. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Current Nutrition Reports. 2018;7(4):198–206. doi:10.1007/s13668-018-0248-8. PMID: 30264354.
- Yusuf F, Adewiah S, Syam AF, Fatchiyah F. Altered profile of gut microbiota and the level short chain fatty acids in colorectal cancer patients. J Phys Conf Ser. 2019;1146:012037. doi:10.1088/1742-6596/1146/1/012037.
- Gill PA, van, Muir JG, Gibson PR, van Zelm MC. Review article: short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther. 2018;48(1):15–34. doi:10.1111/apt.14689. PMID: 29722430.
- Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Frontiers in Immunology. 2019;10:277. doi:10.3389/fimmu.2019.00277. PMID: 30915065.
- Konikoff T, Gophna U. Oscillospira : a central, enigmatic component of the human gut microbiota. Trends in Microbiology. 2016;24(7):523–524. doi:10.1016/j.tim.2016.02.015. PMID: 26996766.
- Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, Goedert JJ, Hayes RB, Yang L. Human gut microbiome and risk for colorectal cancer. JNCI: Journal of the National Cancer Institute. 2013;105(24):1907–1911. doi:10.1093/jnci/djt300. PMID: 24316595.
- Masumoto S, Terao A, Yamamoto Y, Mukai T, Miura T, Shoji T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Scientific Reports. 2016;6(1):31208. doi:10.1038/srep31208. PMID: 27506289.
- Zhao Z, Egashira Y, Sanada H. Digestion and absorption of ferulic acid sugar esters in rat gastrointestinal tract. J Agric Food Chem. 2003;51:5534–5539. doi:10.1021/jf034455u. PMID: 12926910.
- Ou S, Kwok K-C. Ferulic acid: pharmaceutical functions, preparation and applications in foods. J Sci Food Agric. 2004;84:1261–1269. doi:10.1002/jsfa.1873.
- Shin N-R, Whon TW, Bae J-W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi:10.1016/j.tibtech.2015.06.011. PMID: 26210164.
- Scanlan PD, Shanahan F, Clune Y, Collins JK, O’Sullivan GC, O’Riordan M, Holmes E, Wang Y, Marchesi JR. Culture-independent analysis of the gut microbiota in colorectal cancer and polyposis. Environ Microbiol. 2008;10:789–798. doi:10.1111/j.1462-2920.2007.01503.x. PMID: 18237311.
- Boudreau MD, Mellick PW, Olson GR, Felton RP, Thorn BT, Beland FA. Clear evidence of carcinogenic activity by a whole-leaf extract of Aloe barbadensis miller (aloe vera) in F344/N rats. Toxicol Sci Off J Soc Toxicol. 2013;131:26–39. doi:10.1093/toxsci/kfs275. PMID: 22968693.