ABSTRACT
Clostridioides difficile infection (CDI) is a common cause of antimicrobial-associated diarrhea. Probiotics have shown variable results in decreasing its incidence and severity. We examined the efficacy of Lactobacillus reuteri administered using a novel probiotic biofilm delivery system in the treatment and prevention of CDI in a murine model.
For prophylactic therapy, mice received an oral antibiotic cocktail followed by clindamycin injection, followed by probiotic administration (planktonic vs. biofilm state), followed by C. difficile oral gavage. For treatment therapy, mice received antibiotics and C. difficile first, followed by probiotic administration. Clinical sickness scores (CSS) and intestinal histologic injury scores (HIS) were assigned.
In the Prophylactic Therapy model, CSS: 67% of untreated mice exposed to C. difficile demonstrated CSS ≥ 6, which is consistent with C. difficile infection (p< .001 compared to unexposed mice). In mice treated with planktonic Lr, 55% had a CSS ≥ 6, but only 19% of mice treated with Lr in its biofilm state had CSS ≥ 6 (p< .001). Mice receiving Lr + DM-Maltose lost the least amount of weight compared to mice receiving saline (p = .004676) or to mice receiving Lr (p= .003185). HIS: 77% of untreated mice exposed to C. difficile had HIS scores ≥4, which is consistent with C. difficile infection. In mice treated with planktonic Lr, 62% had HIS ≥4, but only 19% of mice treated with Lr in its biofilm state had HIS ≥4. (p< .001). Additionally, mice treated with Lr in its biofilm state had better survival compared to untreated mice and to mice treated with planktonic Lr (p ≤ 0.05). Similar findings for weight loss, CSS, HIS and survival were obtained for Treatment Therapy.
A single dose of Lactobacillus reuteri in its biofilm state reduces the severity and incidence of experimental C. difficile infection when administered as both prophylactic and treatment therapy.
Introduction
Clostridioides difficile (C. difficile) colitis is an antibiotic-associated infection that has led to a significant healthcare crisis in developed countries, affecting over 500,000 individuals annually and causing ~30,000 deaths each year worldwide.Citation1–3 In the United States alone, C. difficile results in an economic burden of 4.8 USD billion in associated health-care costs annually.Citation1,Citation4 The typical pathogenesis of the disease is commonly understood to start when the host is first predisposed by gut microbiome dysbiosis. This is often a result of receiving single – or multiple-dose antibiotic treatment, but can result from other causes. Antibiotics lead to reduced microbial-produced secondary bile acids, and a resultant increase in primary bile acids. Some primary bile acids, such as taurocholate, increase germination of C. difficile spores,Citation5 whereas secondary bile acids, such as ursodeoxycholic acid inhibits germination and vegetative C. difficile growth.Citation6 Disease induction occurs via release of toxins A and B, and intestinal epithelial cell internalization of these toxins.Citation7,Citation8 The toxins cause inflammation and tissue infiltration necrosis in the mucous layer and colonic epithelial cells of the host. This damage manifests in the host as severe diarrhea.Citation7,Citation8 The most common treatments available to patients afflicted with C. difficile colitis involve antibiotic administration.Citation9 However, despite antibiotic treatment, C. difficile colitis often recurs.Citation4,Citation10 An estimated 20–30% of adult patients experience recurrent C. difficile infection.Citation10 This recurrence can be associated with increased morbidity, require additional medication or longer courses of therapy, and can result in increased patient mortality.Citation8,Citation10 In the most severe cases, care may also require fecal transplantation or colonic resection, but these procedures are not completely effective and are invasive.Citation8,Citation10
Given the potential for antibiotic treatment inefficacy,Citation4,Citation10 as well as the need for effective treatment options for recurrent C. difficile infection,Citation4,Citation8,Citation10 further research is required. Probiotics have been previously shown to moderately restore eubiosis and reduce C. difficile infection in high-risk patients receiving antibiotics.Citation11 However, clinicians have been hesitant to adopt probiotic therapies due to the lack of ease of administration and inconsistent study results regarding long-lasting protection against C. difficile colitis.Citation3 Due to the increasing risk of C. difficile colitis disease and recurrence in recent years, a defined methodology to track the progression, diagnosis and treatment options for this disease is not only beneficial but imperative. Our previous work supported this notion by establishing novel clinical and histological scoring systems that allow for efficient and accurate identification of diseased animals in an experimental murine model of C. difficile colitis.Citation12 We now incorporate these scoring systems to validate disease inoculation and to analyze the use of probiotics to prevent and treat C. difficile infection.
Previous studies from our laboratory have demonstrated that Lactobacillus reuteri (Lr) can be induced to form a biofilm by incubation with biocompatible dextranomer microspheres (DM).Citation13 Furthermore, these microspheres can be loaded with beneficial cargo such as sucrose or maltose in order to increase biofilm production. Compared to Lr in its planktonic, free-living state, Lr in its biofilm state has significantly increased efficacy in protecting the intestines from neonatal necrotizing enterocolitis,Citation14,Citation15 another disease associated with intestinal dysbiosis. Based on these findings, we now examine the ability of Lr in its planktonic and biofilm state to protect the intestines from C. difficile colitis.
Materials and methods
All experiments and procedures were reviewed and approved by the Nationwide Children’s Hospital Institutional Animal Care and Use Committee (IACUC Protocol # AR16-00095).
Animals
A combination of mice (conventional C57BL/6 mice) bred in house and commercially obtained (Jackson Laboratories, Bar Harbor, ME) was utilized. Only fully weaned 8- to 10-week old mice were utilized. All mice were housed under identical conditions in groups of no less than 3 and no greater than 5 mice per cage. They were fed an irradiated, soy-free, low-fat rodent diet product # 2920x.10 (Harlan, Indianapolis, IN). UV-sterilized drinking water was provided ad lib. Animals were housed in a room designated only for C. difficile experimentation. All bedding and enrichment toys were autoclaved to avoid the introduction of outside microbes.
Antibiotic administration
Antibiotics were administered over the course of 4 d in the form of a water cocktail as we have previously described.Citation12 The water cocktail contained kanamycin (0.4 mg/mL), gentamicin (0.035 mg/mL), colistin (850 U/mL), metronidazole (0.215 mg/mL), and vancomycin (0.045 mg/mL), in the manner described by Julia et al.Citation16 and by Chen et al.Citation17 Antibiotics were purchased from Sigma-Aldrich (St. Louis, MO), reconstituted in sterile water, and provided to the mice ad libitum in their drinking water. The antibiotic concentrations were calculated based on an average weight of the mice used (20–25 gm), and expected water consumption over 4 d (4–6 mL/mouse/day). Twenty-four hours after antibiotic cocktail completion, an intraperitoneal (IP) injection of clindamycin (10 mg/kg) prepared in sterile water was administered.
Clostridioides difficile
Clostridioides difficile was prepared as we have described previously.Citation12 Vegetative (non-sporulated) C. difficile was prepared from a stock strain of VPI 10463 (ATCC 43255), which was purchased from the American Type Culture Collection (Manassas, VA). C. difficile was grown anaerobically in Modified Reinforced Clostridial Medium (ATCC medium 2107). To remove dissolved oxygen to facilitate C. difficile growth, the medium was degassed by briefly boiling while bubbling with N2 gas and reduced with 4 mM L-Cysteine, followed by pH adjustment to 6.8. C. difficile was grown in an anaerobic chamber in an atmosphere of 5% H2/10% CO2/85% N2 at 37°C for 48 hours. Bacteria were centrifuged for 5 minutes at 8000 x g, the media removed, and the pellet washed twice and resuspended with sterile degassed PBS in an anaerobic atmosphere. The final dosage per animal achieved was 1.5 × 107 CFU of vegetative C. difficile in 150 μl. Individual aliquots were made under anaerobic conditions to minimize oxygen exposure during mouse treatment.
The optimum dosage of vegetative C. difficile needed to establish colitis was determined in preliminary experiments. This was accomplished by gastric gavage of varying colony forming units (CFUs) (106, 107, 108 and 109) of vegetative C. difficile after receipt of the oral antibiotic cocktail and IP clindamycin. In addition to varying CFUs, different incubation time periods were also tested, obtaining vegetative cultures at 24 hours, 36 hours, and 48 hours of growth. The dosage of 1.5 × 107 CFU grown in culture medium for 48 hours prior to administration was chosen based on the findings that this dose led to C. difficile colitis in a substantial number of mice but was not uniformly lethal. Each mouse received 150 μL of C. difficile solution by gastric gavage.
Lr biofilm preparation
Human-feces derived L. reuteri 23272 (American Type Culture Collection; ATCC, Manassas, VA) was grown overnight in de Man, Rogosa, and Sharpe (MRS) broth (Fisher Scientific, Pittsburgh, PA) at 37°C in 5% CO2. For planktonic L. reuteri, 1 × 109 CFU/mL was pelleted and resuspended in sterile 0.9% saline prior to gastric gavage. For L. reuteri administered with unloaded microspheres, sterile dry dextranomer microspheres (Sephadex G-25 Superfine, GE Healthcare Bio-Sciences, Pittsburgh, PA) were hydrated in a sterile saline solution overnight. For L. reuteri administered with maltose-loaded microspheres, the microspheres were hydrated in a 1 M maltose solution in normal saline overnight. All microspheres were removed from the overnight solution via vacuum filter and aseptically transferred into a tube containing the resuspended bacteria. The bacteria were allowed to incubate with the microspheres for 1 hour at room temperature to facilitate binding. Each mouse received 200 μL of the bacterial solution by gastric gavage, for a final dose of 1 × 10Citation8 CFU of Lr.
Experimental model
The experimental scheme of the C. difficile colitis models for prophylactic and therapeutic treatments are illustrated in and respectively. The experimental models span a 15-day time period. In the prophylaxis experiments, following randomization into control or treatment groups, mice to be subjected to the C. difficile protocol were provided an antibiotic cocktail in sterilized drinking water over 4 d (days −8 to −4). Two days after oral antibiotic administration, mice received a single IP injection of clindamycin (day −2). Twenty-four hours after IP clindamycin, mice randomized to treatment groups received one dose of (1) saline (2) planktonic (Lr), or (3) Lr+ DM-maltose. A single gastric gavage dose of C. difficile was administered 24 hours after prophylactic treatment. Control mice received no antibiotics in their drinking water, saline gavage instead of probiotics gavage, and saline injection instead of C. difficile injection. Mice were observed for 6 d post treatment.
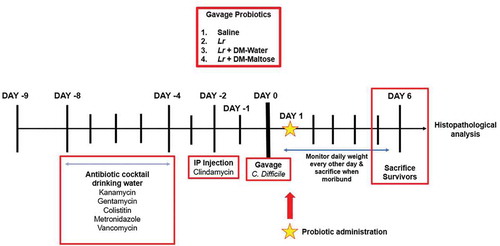
Figure 1. Experimental model for CDI prophylaxis. The experimental model spans 15 d. Following randomization into control or treatment groups, mice to be subjected to the C. difficile protocol were provided an antibiotic cocktail in sterilized drinking water over 4 d (days −8 to −4). Two days after oral antibiotic administration, mice received a single IP injection of clindamycin (day −2). 24 hours after IP clindamycin, mice randomized to treatment groups received one dose of: (1) saline (2) planktonic (Lr), or (3) Lr+ DM-maltose. A single gastric gavage dose of C. difficile was administered 24 hours after prophylactic treatment. Control mice received no antibiotics, no probiotics, and no C. difficile. Mice were observed for 6 d post treatment.
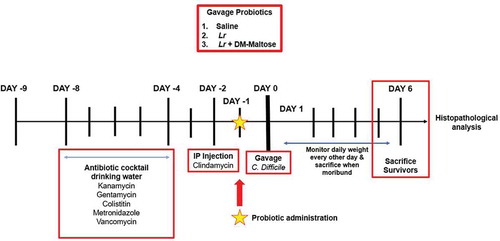
Figure 2. Experimental model for CDI therapy. The experimental model spans 15 d. Following randomization into vehicle control and treatment groups, mice to be subjected to the C. difficile protocol were provided an antibiotic cocktail in sterilized drinking water over 4 d (days −8 to −4). Two days after oral antibiotic administration, mice received a single IP injection of clindamycin (day −2). 24 hours after the IP clindamycin, mice received a single dose of C. difficile. 24 h later, mice randomized to treatment groups received one dose of: (1) saline (2) planktonic Lr, (3) Lr + DM-water, or (4) Lr+ DM-maltose. Control mice received no antibiotics, no C. difficile and no probiotics. Mice were observed for 6 d post C. difficile inoculation.
In the therapeutic experiments, following randomization into vehicle control and treatment groups, mice to be subjected to the C. difficile protocol again received an antibiotic cocktail over 4 d (days −8 to −4) followed 2 d later by a single IP injection of clindamycin (day −2). Twenty-four hours after the IP clindamycin, mice received a single dose of C. difficile. 24 h later, mice blindly randomized to treatment groups received one dose of (1) saline (2) planktonic Lr, (3) Lr + DM-water, or (4) Lr+ DM-maltose. Control mice received no antibiotics in their drinking water, saline injection instead of C. difficile injection, and saline gavage instead of probiotics gavage. Mice were observed for 6 d post C. difficile inoculation. Mice were weighed every other day. Symptoms of disease (stool characteristics, weight loss, and decreased response to stimuli) were recorded and mortality was tracked. Animals judged to be in a moribund state were euthanized. Tissue samples from the cecum and colon were taken for histopathologic analysis.
Changes in weight, clinical sickness scoring and histopathologic analysis
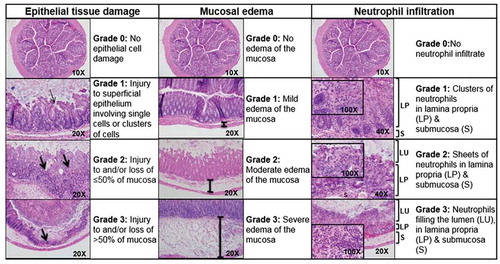
Mice were weighed and assigned clinical sickness scores (CSS) and histologic injury scores (HIS) in a blinded fashion, as we previously described.Citation12 CSS is based on clinical symptoms of stool characteristics, behavioral change, and percent weight loss (). Each category is scored from 0 to 4, and the individual values are added to provide an overall score (). A CSS of 6 or greater is considered consistent with C. difficile colitis, as we have previously reported.Citation12 Animals achieving a CSS of ≥6 were euthanized. Upon sacrifice, the entire colon and cecum were collected for analysis. Histologic injury was graded based on epithelial tissue damage, amount of edema, and neutrophil infiltration (). Each category was scored from 0 to 3 with the individual values added for an overall score. An HIS of ≥4 is indicative of C. difficile colitis, as we have previously reported.Citation12
Figure 3. Clinical sickness scoring (CSS). Following C. difficile gavage, all mice were observed and assigned a daily clinical sickness score. Clinical sickness scoring is based on three categories: stool scoring, behavior and weight loss. Each category is scored in a range, from a score of 0 indicating no signs of sickness to a score of 4 indicating maximum signs of sickness. Scores assigned in each category are added into one cumulative CSS for each mouse. Combined final scores for each mouse range from 0 to 12, with the total score recorded in the final analysis. A CSS of 6 or greater is considered consistent with C. difficile colitis.Citation12

Figure 4. Histologic injury scoring (HIS). Mouse colon histology illustrating tissue damage in mice afflicted with C. difficile colitis. Scores in these categories range from no injury (0) to severe injury (3), and are combined into a cumulative HIS score ranging from 0 to 9. A HIS of ≥4 is indicative of C. difficile colitis.Citation12 LU, lumen; LP, lamina propria, S, submucosa, thick arrow, level of mucosal injury; thin arrow, superficial epithelium injury.
Statistical methods
Differences in CSS and HIS were assessed using analysis of variance (ANOVA) with protected t-tests used as post hoc tests, using each individual score assigned to each individual animal. Survival was assessed by Kaplan–Meier survival analysis and tested using the log-rank test. All analyses were conducted using the SAS 9.4 statistical software program (SAS Institute, Cary, NC) with two-sided p-values <0.05 considered statistically significant.
Results
Lactobacillus reuteri administered prophylactically in its biofilm state decreases the incidence and severity of C. difficile colitis and improves survival
For these experiments, probiotics were administered as prophylaxis prior to C. difficile administration.
Weight loss
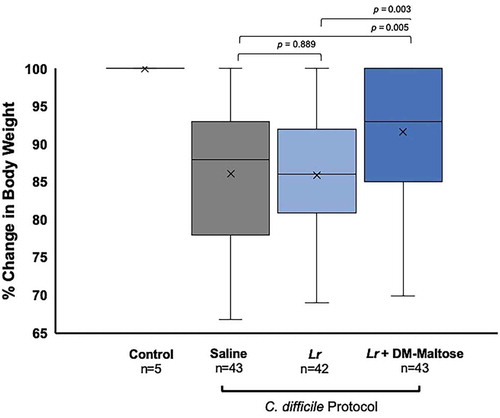
Control mice maintained their original body weight (). Animals receiving Lr + DM-Maltose lost the least amount of weight compared to animals receiving saline (p = .005) and compared to animals receiving Lr (p= .003). There was no difference in the percent of weight loss between animals receiving saline vs. Lr (p= .889).
Figure 5. Percent of original body weight in CDI prophylaxis. Control mice received no antibiotics, no probiotics, and no C. difficile. Mice subjected to the C. difficile protocol were randomized to receive one dose of: (1) saline (2) planktonic Lr, or (3) Lr + DM-maltose. Weight was measured every other day. All 5 Control mice maintained their original body weights, indicated by the mean and bar range at 100%. Animals receiving Lr + DM-Maltose lost the least amount of weight compared to animals receiving saline (p = .004676) or compared to animals receiving Lr (p= .003185). There was no difference in percent of weight loss between animals receiving saline vs. Lr (p= .888959).
CSS
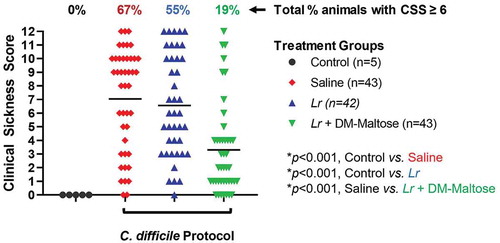
Control mice that were not subjected to the C. difficile protocol did not develop signs of sickness. Compared to control mice, 67% of mice subjected to the C. difficile protocol that received saline only had CSS scores >6, consistent with C. difficile infection (p < .001) (). Fifty-one percent of those animals had severe clinical signs of sickness with CSS ≥9. There was no significant difference in the incidence of clinical sickness in mice subjected to the C. difficile protocol that had prophylaxis with planktonic Lr compared to mice that received saline only (p= .553). However, compared to mice that received saline only, mice that received prophylaxis with a single dose of Lr + DM-maltose (Lr in its biofilm state) had a significantly lower incidence of CSS score ≥6 (p< .001).
Figure 6. Clinical sickness score (CSS) grading with CDI prophylaxis. Control mice received no antibiotics, no probiotics, and no C. difficile. Mice subjected to the C. difficile protocol were randomized to receive one dose of: (1) saline (2) planktonic Lr, or (3) Lr + DM-maltose. Clinical sickness scores were assigned with daily observation. Total CSS for each animal ranges from 0 to 12 as illustrated in . Each colored symbol represents one animal. Animals with scores ≥ 6 have CSS consistent with clinical C. difficile infection. The percentages at the top of each treatment group reflect the percent of animals with CSS ≥6 for each group. All p values are calculated based on individual animal scores.
HIS
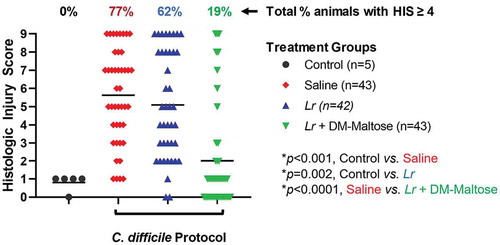
Control mice that were not subjected to the C. difficile protocol did not develop histologic injury. Compared to control mice, 77% of mice subjected to the experimental C. difficile protocol that received saline only had HIS scores ≥4, which is consistent with C. difficile infection (p < .001) (). Forty-seven percent of those animals had a severe histological injury with HIS ≥7. There was no significant difference in the incidence of histologic injury in mice subjected to the C. difficile protocol that had prophylaxis with planktonic Lr compared to mice that received saline only (p= .379). However, compared to mice that received saline only, mice that received prophylaxis with a single dose of Lr + DM-maltose (Lr in its biofilm state) had a significantly lower incidence of HIS ≥ 4 (p < .001).
Figure 7. Histologic injury score (HIS) grading with CDI prophylaxis. Control mice received no antibiotics, no probiotics, and no C. difficile. Mice subjected to the C. difficile protocol were randomized to receive one dose of: (1) saline (2) planktonic Lr, or (3) Lr + DM-maltose. Total HIS for each animal ranges from 0 to 9 (illustrated in ). Each colored symbol represents one animal. Animals with scores ≥ 4 have HIS consistent with C. difficile infection. The percentages at the top of each treatment group reflect the percent of animals with HIS ≥ 4 for each group. All p values are calculated based on individual animal scores.
Survival
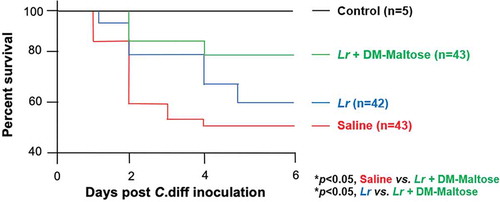
Kaplan–Meier survival analysis revealed a significant difference in animal survival in mice exposed to the prophylactic C. difficile protocol treated with saline only compared to mice that were treated with a single dose of Lr + DM-Maltose at the end of the experiment on Day 6 (48% vs. 78%; p< .05) (). There was no significant difference in the survival of mice treated with saline only compared to planktonic Lr treated mice within this experimental timeframe (48% vs. 59%, p> .05) ().
Figure 8. Survival in CDI prophylaxis. Control mice received no antibiotics, no C. difficile and no probiotics. Mice subjected to the C. difficile protocol were randomized to receive one dose of: (1) Saline (2) Planktonic Lr, (3) Lr + DM-Water or (4) Lr + DM-Maltose. Kaplan–Meier survival curve demonstrating percent death and survival of animals in each treatment group over the time course of the experiment.
Lactobacillus reuteri administered therapeutically in its biofilm state decreases the incidence and severity of C. difficile colitis and improves survival
For these experiments, probiotics were administered as a treatment after C. difficile infection was already established.
Weight loss
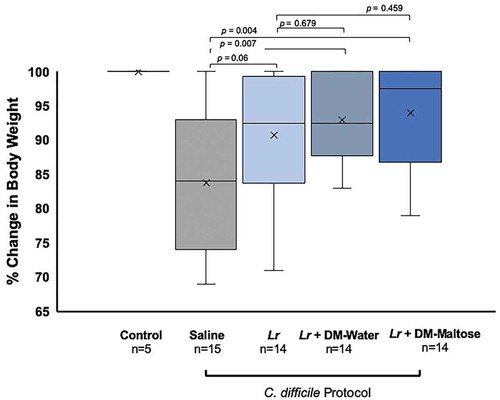
Control mice maintained their original body weight. Animals receiving Lr + DM-Maltose and Lr + DM-Water lost the least amount of weight compared to animals receiving saline (p= .0043 and 0.0067, respectively), whereas there was no difference in percent weight loss between animals receiving Lr vs. saline (p= .0628) (). There was no difference in the percent of weight loss between animals receiving Lr + DM-Maltose or Lr + DM-Water vs. Lr (p= .4591 and p= .6790, respectively).
Figure 9. Percent of original body weight in CDI therapy. Control mice received no antibiotics, no probiotics, and no C. difficile. Mice subjected to the C. difficile protocol were randomized to receive one dose of: (1) saline (2) planktonic Lr, (3) Lr + DM-maltose, or (4) Lr + DM-maltose. Weight loss was measured every other day. All 5 Control mice maintained their original body weights, indicated by the mean and bar range at 100%. Animals receiving Lr + DM-Maltose and Lr + DM-Water lost the least amount of weight compared to animals receiving saline (p= .0043 and 0.0067, respectively). There was no difference in percent of weight loss between animals receiving saline vs. Lr (p= .0.0628). There was no difference in percent of weight loss between animals receiving Lr + DM-Maltose or Lr + DM-Water vs. Lr (p= .4591 and p= .6790, respectively).
CSS
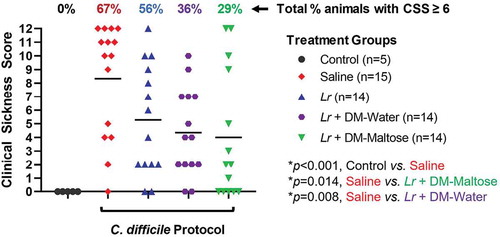
Control mice that were not subjected to the C. difficile protocol did not develop signs of sickness. Compared to control mice, 67% of mice subjected to the experimental C. difficile protocol that received saline only had CSS scores ≥6, consistent with C. difficile infection (p < .001) (). All of these animals had CSS scores consistent with severe signs of sickness (CSS ≥9). There was no significant difference in the incidence of clinical sickness scores in mice subjected to the C. difficile protocol that were treated with planktonic Lr compared to mice that received saline only (p= .056). However, compared to mice that received saline only, mice that received a single dose of Lr + DM-water had a decreased incidence of CSS ≥ 6 (p= .008), and mice treated with Lr + DM-maltose had a further decrease in the incidence of CSS ≥ 6 (p= .014).
Figure 10. Clinical sickness score (CSS) grading with CDI therapy. Control mice received no antibiotics, no C. difficile and no probiotics. Mice subjected to the C. difficile protocol were randomized to receive one dose of: (1) saline (2) planktonic Lr, (3) Lr + DM-water or (4) Lr + DM-maltose. Clinical sickness scores were assigned with daily observation. Total CCS for each animal ranges from 0 to 12 as illustrated in . Each colored symbol represents one animal. Animals with scores ≥ 6 have CSS consistent with clinical C. difficile infection. The percentages at the top of each treatment group reflect the percent of animals with CSS ≥6 for each group. All p values are calculated based on individual animal scores.
HIS
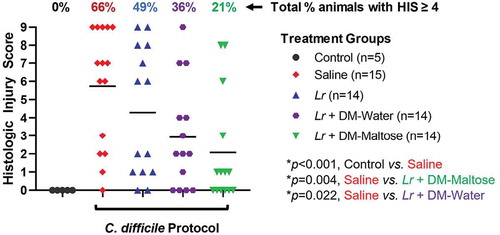
Control mice that were not subjected to the C. difficile protocol did not develop histologic injury. Compared to control mice, 66% of mice subjected to the C. difficile protocol that received saline only had HIS scores ≥4, consistent with C. difficile infection (p = .002) (). Fifty-three percent of those animals had HIS scores consistent with severe histological injury with HIS ≥7. There was no significant difference in the incidence of histologic injury in mice subjected to the C. difficile protocol that were treated with planktonic Lr compared to mice that received saline only (p= .263). However, compared to mice that received saline only, mice that received a single dose of Lr + DM-Water had a decreased incidence of histologic injury ≥4 consistent with C. difficile infection (p= .022), and mice treated with Lr + DM-maltose had a further and significant decrease in histologic injury ≥4 (p = .004).
Figure 11. Histologic injury scores (HIS) grading with CDI therapy. Control mice received no antibiotics, no C. difficile and no probiotics. Mice subjected to the C. difficile protocol were randomized to receive one dose of: (1) saline (2) planktonic Lr, (3) Lr + DM-water or (4) Lr + DM-Maltose. Total HIS for each animal ranges from 0 to 9 (illustrated in Figure 4). Each colored symbol represents one animal. Animals with scores ≥ 4 have HIS consistent with C. difficile infection. The percentages at the top of each treatment group reflect the percent of animals with HIS ≥4 for each group. All p values are calculated based on individual animal scores.
Survival
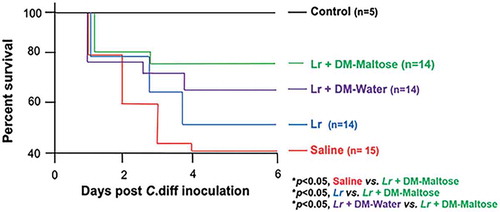
Kaplan–Meier survival analysis revealed a significant difference in animal survival between saline-treated mice and mice that received a single dose of Lr + DM-Maltose therapeutically by the end of the experiment on Day 6 (42% vs. 78%; p< .05) (). There was also a significant difference in survival between animals that received Lr + DM-maltose and those that received planktonic Lr at Day 6 (78% vs. 50%; p< .05). There was no significant difference in survival of mice that received a single dose of Lr + DM-maltose compared to those that received Lr + DM-Water by Day 6 (78% vs. 66%, p > .05).
Figure 12. Survival in CDI treatment. Control mice received no antibiotics, no C. difficile and no probiotics. Mice subjected to the C. difficile protocol were randomized to receive one dose of: (1) Saline (2) Planktonic Lr, (3) Lr + DM-water or (4) Lr + DM-Maltose. Kaplan–Meier survival curve demonstrating percent death and survival of animals in each treatment group over the time course of the experiment.
Discussion
The incidence of C. difficile colitis is rising in both pediatric and adult populations.Citation1,Citation18 Despite this, the management of C. difficile colitis still requires optimization. At present, the most common instigator that leads to the development of C. difficile, antibiotics, is also the first-line treatment option for both initial and recurrent C. difficile colitis.Citation8,Citation11,Citation18 However, in spite of these established therapies, many patients often have a number of recurrent infections that are resistant to antibiotic therapy. Additionally, disease in some patients can rapidly escalate in severity, leaving no other treatment option but surgical intervention.Citation11,Citation19,Citation20
With the continued rise in the incidence of initial and recurrent disease, as well as associated complications, there has been an increased interest in the development of alternative therapies. In particular, probiotics have been of significant interest in the treatment and prevention of C. difficile colitis.Citation3,Citation11,Citation21 Probiotics are theoretically efficacious because of their potential to restore eubiosis to the gastrointestinal microbiota after disruption by antimicrobials.Citation22 There have been several studies recently published supporting the use of probiotics for their protective effect against C. difficile infection.Citation3,Citation23 However, to date, only moderate efficacy of the several probiotics used has been obtained.Citation24–27 It is important to note that in all of these studies and analyses, probiotics were evaluated in the prevention of C. difficile colitis and not in the treatment of the disease. Furthermore, in all of these studies, probiotics were administered in their planktonic state and required repeated doses to demonstrate efficacy.Citation28,Citation29 In the current study, we have demonstrated that the administration of one single dose of Lr in its biofilm state can significantly reduce the incidence and severity of C. difficile colitis when administered either prophylactically or therapeutically.
Lr is present in the healthy human intestinal tract and was originally isolated from human breast milk.Citation30,Citation31 The current studies utilized clade II Lr (ATCC 23272) originally isolated from human stool. This strain of Lr, among others, has antimicrobial activity conferred by its ability to convert glycerol into the antimicrobial compound reuterin, and it has anti-inflammatory properties due to its ability to produce histamine and to modulate cytokine production.Citation32,Citation33 Lr also readily forms a biofilm and has a significant affinity for the cross-linked dextran in DM. Because of these properties, as well as our own experience with this probiotic formulation in the prevention of necrotizing enterocolitis (NEC),Citation14,Citation15 we chose to use Lr for our experimental model.
In the current study, we found no efficacy of a single dose of Lr administered in its planktonic state either prophylactically or therapeutically. On the other hand, we were able to demonstrate efficacy with the administration of just one single dose of Lr administered in its biofilm state. Other strains of planktonic Lr have demonstrated various levels of efficacy with repeated multi-dose delivery in other studies.Citation3,Citation24,Citation34 Britton et al. demonstrated that Lr as ‘precursor-directed antimicrobial therapy’ is effective in targeting CDI. In addition, they argue that antimicrobial resistance can be leveraged in the natural human microbiome response to evade C. diff – demonstrating the efficacy of administering Lr as a prophylactic probiotics.Citation23 This is similar to our prophylactic results; however, in our studies, the best efficacy was noted in our novel formulation of Lr adhered to maltose-loaded DM, where there is the greatest biofilm formation. We have previously shown that Lr in its biofilm state demonstrates prolonged survival in acidic environments and improved adherence to intestinal epithelial cells in vitroCitation13,Citation15 and protects the intestines from NEC in vivo.Citation14,Citation15 In our NEC studies, augmentation of biofilm formation by adherence of Lr to DM loaded with sucrose or maltose leads to increased intestinal protection,Citation14 and conversely, decreasing the ability of Lr to produce a biofilm by using a genetically altered strain of Lr (ΔgtfW) decreases intestinal protection.Citation14
The adherence of Lr to DM to promote biofilm formation is a central component of its improved protective effect seen in this study. Lr relies on a novel extracellular glucosyltransferase (GtfW), rare among bacteria, that does not rely on sugar nucleotide intermediates but uses the energy of existing glycosidic linkages to generate chains of polysaccharides. Indeed, Lr adheres to DM via the glucan-binding domain of the GtfW enzymeCitation14 as well as the ability to directly use maltose to make glucan homopolymers that facilitate binding to DM. Importantly, we have previously shown that Escherichia coli, Salmonella typhimurium, and Clostridioides difficile do not detectably bind to DM,Citation13 allaying any concerns that the administration of DM might provide a platform for increased biofilm formation in pathogenic bacteria.
A limitation of the current work is that an analysis of the gut microbiome after antibiotic administration C. difficile inoculation or probiotic therapy was not performed. However, we have investigated the effect of L. reuteri in its biofilm state on the incidence and severity of necrotizing enterocolitis (NEC), and in those studies we were able to demonstrate a change in the microbiome when L. reuteri was administered in its biofilm compared to its planktonic state.Citation14 Based on this, a change in microbiome may also play a role in our C. difficile studies, and will be addressed in future studies.
The novel formulation presented here represents a potential exciting development toward improving probiotic therapy for a devastating public health problem. Our results showing that Lr in its biofilm state is superior to planktonic Lr provide great insight into future potential therapeutics.
Conclusion
Lr administered in its biofilm state exhibits a protective effect against C. difficile colitis when administered both prophylactically and as a treatment, with just one single dose. These results support the future translation of this treatment to the bedside given the dual efficacy of this probiotic.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Yijie Wang (Nationwide Children’s Hospital, Laboratory of Dr. Gail Besner) who played an integral role in experiment concept and design, obtaining all necessary supplies, animals, and overall function of the experiments.
References
- Lessa FC, Winston LG, McDonald LC, Team EIPCdS. Burden of clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369–13. doi:10.1056/NEJMoa1408913.
- Olsen MA, Young-Xu Y, Stwalley D, Kelly CP, Gerding DN, Saeed MJ, Mahé C, Dubberke ER. The burden of clostridium difficile infection: estimates of the incidence of CDI from U.S. administrative databases. BMC Infect Dis. 2016;16(1):177. doi:10.1186/s12879-016-1501-7.
- Mills JP, Rao K, Young VB. Probiotics for prevention of clostridium difficile infection. Curr Opin Gastroenterol. 2018;34(1):3–10. doi:10.1097/MOG.0000000000000410.
- Dieterle MG, Young VB. Reducing recurrence of C. difficile infection. Cell. 2017;169(3):375. doi:10.1016/j.cell.2017.03.039.
- Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol. 2008;190(7):2505–2512. doi:10.1128/JB.01765-07.
- Weingarden AR, Chen C, Zhang N, Graiziger CT, Dosa PI, Steer CJ, Shaughnessy MK, Johnson JR, Sadowsky MJ, Khoruts A, et al. Ursodeoxycholic acid inhibits clostridium difficile spore germination and vegetative growth, and prevents the recurrence of ileal pouchitis associated with the infection. J Clin Gastroenterol. 2016;50(8):624–630. doi:10.1097/MCG.0000000000000427.
- Abt MC, McKenney PT, Pamer EG. Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol. 2016;14(10):609–620. doi:10.1038/nrmicro.2016.108.
- Evans CT, Safdar N. Current trends in the epidemiology and outcomes of clostridium difficile infection. Clin Infect Dis. 2015;60(suppl_2):S66–S71. doi:10.1093/cid/civ140.
- Ofosu A. Clostridium difficile infection: a review of current and emerging therapies. Ann Gastroenterol. 2016;29(2):147–154. doi:10.20524/aog.2016.0006.
- Nicholson MR, Thomsen IP, Slaughter JC, Creech CB, Edwards KM. Novel risk factors for recurrent clostridium difficile infection in children. J Pediatr Gastroenterol Nutr. 2015;60(1):18–22. doi:10.1097/MPG.0000000000000553.
- Maziade PJ, Pereira P, Goldstein EJ. A decade of experience in primary prevention of clostridium difficile infection at a community hospital using the probiotic combination lactobacillus acidophilus CL1285, lactobacillus casei LBC80R, and lactobacillus rhamnosus CLR2 (Bio-K+). Clin Infect Dis. 2015;60(Suppl 2):S144–147. doi:10.1093/cid/civ178.
- Shelby RD, Tengberg N, Conces M, Olson JK, Navarro JB, Bailey MT, Goodman SD, Besner GE. Development of a standardized scoring system to assess a murine model of clostridium difficile colitis. J Invest Surg. 2019;1–9. doi:10.1080/08941939.2019.1571129.
- Navarro JB, Mashburn-Warren L, Bakaletz LO, Bailey MT, Goodman SD. Enhanced probiotic potential of. Front Microbiol. 2017;8:489.
- Olson JK, Navarro JB, Allen JM, McCulloh CJ, Mashburn-Warren L, Wang Y, Varaljay VA, Bailey MT, Goodman SD, Besner GE, et al. An enhanced lactobacillus reuteri biofilm formulation that increases protection against experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2018;315(3):G408–G419. doi:10.1152/ajpgi.00078.2018.
- Olson JK, Rager TM, Navarro JB, Mashburn-Warren L, Goodman SD, Besner GE. Harvesting the benefits of biofilms: A novel probiotic delivery system for the prevention of necrotizing enterocolitis. J Pediatr Surg. 2016;51(6):936–941. doi:10.1016/j.jpedsurg.2016.02.062.
- Julia V, McSorley SS, Malherbe L, Breittmayer J-P, Girard-Pipau F, Beck A, Glaichenhaus N. Priming by microbial antigens from the intestinal flora determines the ability of CD4+ T cells to rapidly secrete IL-4 in BALB/c mice infected with leishmania major. J Immunol. 2000;165(10):5637–5645. doi:10.4049/jimmunol.165.10.5637.
- Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. A mouse model of clostridium difficile-associated disease. Gastroenterology. 2008;135(6):1984–1992. doi:10.1053/j.gastro.2008.09.002.
- D’Ostroph AR, So TY. Treatment of pediatric. Infect Drug Resist. 2017;10:365–375. doi:10.2147/IDR.S119571.
- Hall BR, Armijo PR, Leinicke JA, Langenfeld SJ, Oleynikov D. Prolonged non-operative management of clostridium difficile colitis is associated with increased mortality, complications, and cost. Am J Surg. 2019;217(6):1042–1046. doi:10.1016/j.amjsurg.2019.01.017.
- Hall JF, Berger D. Outcome of colectomy for clostridium difficile colitis: a plea for early surgical management. Am J Surg. 2008;196(3):384–388. doi:10.1016/j.amjsurg.2007.11.017.
- Valdés-Varela L, Gueimonde M, Ruas-Madiedo P. Probiotics for prevention and treatment of clostridium difficile infection. Adv Exp Med Biol. 2018;1050:161–176.
- Dinleyici M, Vandenplas Y. Clostridium difficile Colitis Prevention and Treatment. Adv Exp Med Biol. 2019;1125:139–146.
- Spinler JK, Auchtung J, Brown A, Boonma P, Oezguen N, Ross CL, Luna RA, Runge J, Versalovic J, Peniche A, et al. Next-generation probiotics targeting clostridium difficile through precursor-directed antimicrobial biosynthesis. Infect Immun. 2017;85(10):10. doi:10.1128/IAI.00303-17.
- Guo Q, Goldenberg JZ, Humphrey C, El Dib R, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4:CD004827.
- Goldenberg JZ, Mertz D, Johnston BC. Probiotics to prevent clostridium difficile infection in patients receiving antibiotics. JAMA. 2018;320(5):499–500. doi:10.1001/jama.2018.9064.
- Johnson S, Maziade PJ, McFarland LV, Trick W, Donskey C, Currie B, Low DE, Goldstein EJC. Is primary prevention of Clostridium difficile infection possible with specific probiotics? Int J Infect Dis. 2012;16(11):e786–792. doi:10.1016/j.ijid.2012.06.005.
- Goldstein EJC, Johnson SJ, Maziade PJ, Evans CT, Sniffen JC, Millette M, McFarland LV. Probiotics and prevention of clostridium difficile infection. Anaerobe. 2017;45:114–119. doi:10.1016/j.anaerobe.2016.12.007.
- Goldenberg JZ, Yap C, Lytvyn L, Lo CK, Beardsley J, Mertz D, Johnston BC. Probiotics for the prevention of clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
- Johnston BC, Lytvyn L, Lo CK, Allen SJ, Wang D, Szajewska H, Miller M, Ehrhardt S, Sampalis J, Duman DG, et al. Microbial preparations (Probiotics) for the prevention of clostridium difficile infection in adults and children: an individual patient data meta-analysis of 6,851 participants. Infect Control Hosp Epidemiol. 2018;39(7):771–781. doi:10.1017/ice.2018.84.
- Reuter G. The Lactobacillus and Bifidobacterium microflora of the human intestine: composition and succession. Curr Issues Intest Microbiol. 2001;2:43–53.
- Urbańska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr. 2014;173(10):1327–1337. doi:10.1007/s00431-014-2328-0.
- Spinler JK, Sontakke A, Hollister EB, Venable SF, Oh PL, Balderas MA, Saulnier DMA, Mistretta T-A, Devaraj S, Walter J, et al. From prediction to function using evolutionary genomics: human-specific ecotypes of Lactobacillus reuteri have diverse probiotic functions. Genome Biol Evol. 2014;6(7):1772–1789. doi:10.1093/gbe/evu137.
- Spinler JK, Taweechotipatr M, Rognerud CL, Ou CN, Tumwasorn S, Versalovic J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe. 2008;14(3):166–171. doi:10.1016/j.anaerobe.2008.02.001.
- Rao K, Young VB. Probiotics for prevention of clostridium difficile infection in hospitalized patients: is the jury still out? Gastroenterology. 2017;152(8):1817–1819. doi:10.1053/j.gastro.2017.04.027.
