ABSTRACT
Enteric bacteria need to adapt to endure the antibacterial activities of bile salts in the gut. Phospholipase A (PldA) is a key enzyme in the maintenance of bacterial membrane homeostasis. Bacteria respond to stress by modulating their membrane composition. Campylobacter jejuni is the most common cause of human worldwide. However, the mechanism by which C. jejuni adapts and survives in the gut environment is not fully understood. In this study, we investigated the roles of PldA, bile salt sodium deoxycholate (DOC), and oxygen availability in C. jejuni biology, mimicking an in vivo situation. Growth curves were used to determine the adaptation of C. jejuni to bile salts. RNA-seq and functional assays were employed to investigate the PldA-dependent and DOC-induced changes in gene expression that influence bacterial physiology. Survival studies were performed to address oxidative stress defense in C. jejuni. Here, we discovered that PldA of C. jejuni is required for optimal growth in the presence of bile salt DOC. Under high oxygen conditions, DOC is toxic to C. jejuni, but under low oxygen conditions, as is present in the lumen of the gut, C. jejuni benefits from DOC. C. jejuni PldA seems to enable the use of iron needed for optimal growth in the presence of DOC but makes the bacterium more vulnerable to oxidative stress. In conclusion, DOC stimulates C. jejuni growth under low oxygen conditions and alters colony morphology in a PldA-dependent manner. C. jejuni benefits from DOC by upregulating iron metabolism in a PldA-dependent manner.
Introduction
Successful bacteria can adapt their physiology to changing environmental conditions.Citation1 The climate in the mammalian intestine varies from relative high oxygen availability near the epithelial border to almost zero in the lumenCitation2 while bile salts range from 0.2 to 2% (wt/vol).Citation3 Bile salts play an important role in food digestion but also act as effective natural antimicrobials.Citation4 To survive and colonize the human gastrointestinal tract, commensal and pathogenic bacteria must deal with high concentration of bile salts.Citation5,Citation6 Bile salts are bile acids conjugated with taurine or glycine residues.Citation7 Bile acids can be formed in two ways: primary bile acids are intermediate products of cholesterol degradation and are synthesized by the liver, while secondary bile acids such as deoxycholate and litocholate result from the resident microbiota in the gut.Citation8 Many intestinal bacteria can transform primary bile salts into secondary bile salts by removing the hydroxyl group at C7.Citation9 This significantly increases the bile salt pool diversity.Citation10
The release of bile salts into the intestine is one of the factors that the host utilizes to induce gut microbiome alterations.Citation11 Bile salts such as sodium deoxycholate (DOC) in the intestine can be used by bacteria as carbon source or electron acceptorCitation9,Citation12,Citation13 and can be an environmental signal to switch on virulence factors such as in Shigella, Salmonella and Vibrio species.Citation13–17 Bile salts can however also act as antibacterial compounds as they are able to disrupt bacterial membranes, denature proteins, chelate iron and calcium and induce an SOS response, resulting in DNA damage.Citation13 The well-known mechanisms by which enteric bacteria cope with bile salts are bacterial cell envelope modification (such as LPS O-antigen length);Citation18 CmeABC multidrug efflux pumps;Citation19 DNA repairCitation20,Citation21 and the RpoS-dependent stress response.Citation22 Bile exposure also causes significant alterations in bacterial phospholipid profiles.Citation23
The Gram-negative bacterium C. jejuni is the leading cause of foodborne enteritis in humans worldwide.Citation24 Contaminated chicken meat is believed to be the main source of infections.Citation24 C. jejuni is microaerophilic and requires reduced oxygen concentration to grow as exist in the intestine. In the human gut, C. jejuni penetrates the intestinal mucus layer, colonizes crypts, and disrupts the epithelial barrier. However, the molecular basis of C. jejuni infection remains poorly understood.Citation25 Recently, we determined the phospholipidome of C. jejuni grown under different conditions.Citation26 C. jejuni appears to make hundreds of different phospholipids, which display a high variation dependent on the environmental oxygen concentration and the age and of the Campylobacter culture. Very high amounts (30–50%) of the phospholipids of C. jejuni are lysophospholipids (LPLs).Citation26 The LPLs results for the most part, from the activity of the phospholipase A (PldA) enzyme that cleaves fatty acid tails from phospholipids. We and others have shown that micromolar concentrations of distinct bacterial LPLs are toxic to erythrocytes and epithelial cells.Citation26–28 Furthermore, a functional pldA gene is required to allow C. jejuni to colonize the cecum of chickens.Citation29 In the gut C. jejuni is exposed to bile salts which leads to the production of reactive oxygen species (ROS) which have been shown to cause DNA lesions in C. jejuni Citation30.
Considering the important role of oxygen availability, bile salts, and PldA in C. jejuni infection, we investigated the potential impact of these factors on C. jejuni using transcriptome analysis and assessment of bacterial growth. Wild-type and PldA-defective bacteria were cultured in the absence and presence of DOC under microaerobic (10% O2) or low-oxygen conditions (0.3%) mimicking intestinal oxygen concentrations near the epithelial cell border versus in the lumen, respectively.
Results
Effect of PldA mutation on C. jejuni colony morphology
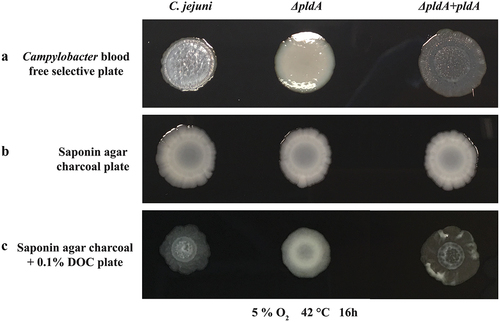
Independent of the oxygen concentration we noticed that the colony morphology of PldA mutant grown on blood free Campylobacter selectivity agar base, differs from that of the wild-type ( and S1A). The pldA mutant had a wet/glossy colony appearance in contrast to dry/dull colonies of the C. jejuni wild-type (). The aberrant morphology of the mutant was restored by the introduction of a pldA complementation plasmid, yielding C. jejuni ΔpldA + pldA. Interestingly, all three strains formed wet or glossy colonies when grown on saponin agar charcoal plates (). One of the major differences between the two media is the presence of bile salts (0.1%) in the blood-free Campylobacter selectivity agar base. Indeed, the addition of DOC (0.1%) to saponin agar charcoal plate resulted in dry/dull colonies of the wild-type C. jejuni and pldA complemented strain, whereas the colonies of the pldA mutant retained their wet/glossy appearance (), all consistent with the colony phenotypes observed on blood-free Campylobacter selectivity agar. These data show that bile salts can alter C. jejuni morphology in a PldA-dependent manner. Transmission electron microscopy (TEM) and lipid oligosaccharide (LOS), of C. jejuni grown on blood-free Campylobacter selectivity agar base did not reveal differences between the wild-type and pldA mutant (Fig S1B, C & D).
Figure 1. DOC modified C. jejuni wild-type colony morphology on Campylobacter blood free selective plate.
Effects of PldA, DOC and oxygen on C. jejuni planktonic growth
To investigate whether exposure to bile salts influenced C. jejuni growth kinetics, the planktonic growth of C. jejuni wild-type 81,116, C. jejuni ΔpldA, and the complemented strain C. jejuni ΔpldA + pldA has been followed by measuring the culture optical density and by CFU counting. Growth was monitored in the presence or absence of 0.1% DOC and at 0.3% and 10% O2. These experiments revealed similar growth kinetics for the three strains when grown in the absence of DOC, irrespective of oxygen concentration ( and S2A & B). In the presence of 0.1% DOC, a clear inhibition of growth was observed for all three C. jejuni strains when grown under 10% O2 concentration ( & S2A). However, unexpectedly, under oxygen-limited conditions (0.3% O2), the addition of DOC stimulated the growth of the C. jejuni strains ( & S2B). The maximum doubling times of the wild type increased from 5.5 h to 2 h and of the ΔpldA strain from 7,5 h to 7 h () and the number of CFU/ml of the wild-type and complemented PldA strain was almost 3 times higher in the presence of DOC then without DOC (Fig S2B). These results suggest that oxygen concentration could influence whether DOC promotes or inhibits the growth of C. jejuni.
Figure 2. C. jejuni PldA is needed for optimal growth in the presence of DOC, but the oxygen concentration determines whether DOC improves or inhibits C. jejuni growth.
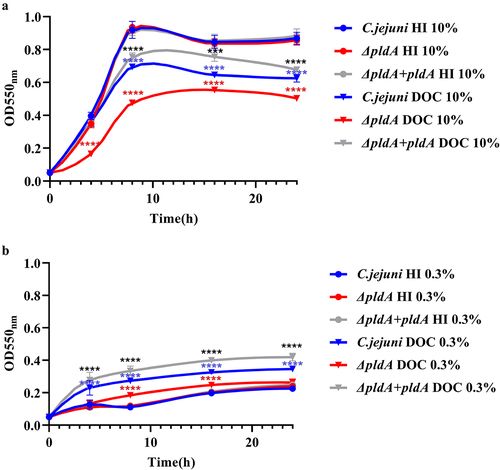
It was also noted that in the presence of 0.1% DOC, the growth rate and CFU/ml of C. jejuni ΔpldA was clearly reduced compared to the wild-type at both 0.3% and 10% O2 (, S2A &B). The maximum doubling time at 0.3% O2 was 2 h for the wild type versus 7 h for ΔpldA mutant (). After 8 h, the number of CFU/ml of the wild-type strain was more than 2 times higher than ΔpldA strain (Fig S2B). At 10% O2, the doubling time dropped to 2 h for the wild type strain and 2.2 h for the ΔpldA mutant (). The growth defect was restored after complementation of the mutant with an intact copy of the pldA gene (, S2A &B). Comparison of the LOS, capsule patterns, biofilm formation, and protein composition after growth in Heart-Infusion (HI) or HI plus 0.1% DOC, showed again no differences between the wild-type and pldA mutant (Fig S3). Overall, these results indicate that the PldA protein of C. jejuni is needed for optimal bacterial growth in the presence of DOC, irrespective of oxygen availability.
Effect of DOC on the membrane integrity
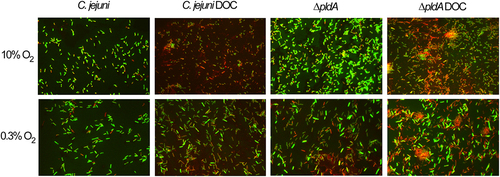
As it is known that DOC reduces the membrane integrity,Citation31 we stained equal amount of bacteria grown with or without DOC at 10 or 0.3% O2 with a 1:1 mixture of the nucleic acid staining dyes SYTO-9 (Green) and propidium iodide (red) (). SYTO-9 labels all bacteria, while propidium iodide penetrates only bacteria with a damaged membrane, causing a reduction in the SYTO-9 stain fluorescent. Most wild-type and pldA mutant bacteria grown at 10% O2 had an intact membrane as they were labeled well with CYTO-9. When DOC was present a large number of bacteria were stained red and no bright green bacteria were observed, indicating that they all possess a damaged membrane. No difference could be observed between the strains cultured with or without DOC at 0.3% O2, indicating that DOC does not affect the membrane integrity of these bacteria under oxygen-limited conditions.
Figure 3. Influence of DOC on the membrane integrity of the C. jejuni wild-type and PldA mutant.
Effects of DOC on C. jejuni gene expression
To better understand how DOC and PldA can promote C. jejuni growth at low O2, we performed RNA-seq on the wild-type and pldA mutant grown for 6 h in HI broth or HI broth plus 0.1% DOC. First, we searched for DOC regulated genes in wild-type C. jejuni (Table S1A & B). Here, the RNA-seq results has been analyzed using the formula (C. jejuni wild-type - C. jejuni wild-type DOC), with a positive value indicating that DOC increases the transcription of the gene (upregulation), whereas a negative value indicates a reduction in transcription (downregulation). DOC induced a > 3-fold change in the transcription of 66 genes compared to bacteria grown without DOC (Table S1C). Real-time PCR of 13 highly regulated and previously reported DOC dependent genesCitation19,Citation30,Citation32 yielded no gross differences in results between the real-time PCR and RNA-seq data (Table S2A), verifying the RNA-seq results. The majority of the identified genes (44 of 66) were upregulated. These genes encode proteins involved in DNA repair (RecO), iron transport (C8J_0168-C8J 0169, C8J_1563, C8J_1564, p19),Citation33 tryptophan catabolism (TrpABF), multidrug efflux pump (CmeBC), RNA polymerase (RpoC), purine biosynthesis (PurH), LIV amino acid transport system (LivFGM), porphyrin metabolism (HemD), peptide translocation (C8J_1479), protein transport (SecY), and the leucine biosynthesis pathway (LeuBCD) (, Table S1A & S1C). The products of the 22 downregulated genes were involved in electron transport (C8J_0040, NapA, NrfA, NrfH), chemotaxis (CheA), non-heme iron metabolism (C8J_1167), motility (C8J_1245), selenocysteine biosynthesis pathway (SelAB) and arsenic resistance (ArsC and ArsR). These results suggest that the exposure of C. jejuni to DOC at low O2 levels induces major alterations in bacterial physiology which may favor bacterial growth.
Figure 4. Genes affected by the addition of DOC in C. jejuni wild-type and its isogenic ΔpldA mutant.
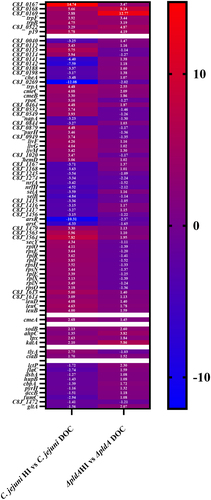
Comparison of the transcript levels in the pldA mutant grown in the absence or presence of DOC revealed seven genes that were also highly upregulated in C. jejuni wild-type (Table S1C). These were iron transport genes (C8J_0167-C8J_0169, p19), tryptophan catabolism genes (trpB and trpF) and the hypothetical gene C8J_0822. Surprisingly, transcript levels of the other 59 genes that changed upon exposure to DOC in the wild type were barely affected by DOC in the pldA mutant. This finding suggests that the expression of many DOC-sensitive genes is PldA-dependent.
Effects of PldA on C. jejuni gene expression
Comparison of the gene transcripts levels in C. jejuni wild-type and C. jejuni ΔpldA grown in the absence and presence of 0.1% DOC (Fig S5; Table S1D and S1E), yielded 19 genes that showed a > 3-fold difference, irrespective of the presence of DOC (Table S1F). In addition to the inactivated pldA gene, 11 of the remaining 18 transcripts were downregulated in the pldA mutant. These genes encode the hypothetical proteins C8J_0029, C8J_0030, C8J_0241 and C8J_0703; a putative cytochrome C-type heme-binding periplasmic protein C8J_0242; an aconitase AcnB;Citation34,Citation35 two disulfide bridge introduction proteins DsbAB;Citation36 arylsulfate sulfotransferase C8J_0813; a sodium/proline symporter PutP and PutA, a proline dehydrogenase/delta-1-pyrroline-5-carboxylate dehydrogenase.Citation37 The six genes that were upregulated in the pldA mutant independent of DOC were the three hypothetical genes C8J_0877, C8J_1306 and C8J_1307; a putative iron-binding gene C8J_0219; an enterochelin ABC transporter substrate-binding protein encoded by ceuB; and an outer membrane hemin and hemoglobin receptor encoded by chuA. The upregulation of iron acquisition gene transcripts in the pldA mutant could be a sign of a shortage of iron availability, but this did not affect bacterial growth ().
Combined effects of PldA and DOC on C. jejuni gene expression
In HI medium, there was no growth rate difference between C. jejuni wild-type and the ΔpldA mutant (). We assumed that the identified pldA-dependent but DOC-insensitive genes were not responsible for the reduced growth of the pldA mutant compared to the wild-type in medium with DOC. To identify the possible genes responsible for the growth defect of the pldA mutant in the presence of DOC, we analyzed our RNA-seq results using the formula [(C. jejuni wild-type DOC - C. jejuni ΔpldA DOC)- (C. jejuni wild-type HI - C. jejuni ΔpldA HI)]. Because differences in gene expression between the two strains cultured in HI medium (C. jejuni wild-type HI - C. jejuni ΔpldA HI) did not directly affect bacterial growth rates, this difference was removed as a background. A positive value indicated that DOC in a PldA-dependent manner increased the transcription of the gene (upregulation), whereas a negative value indicated a reduction in transcription (downregulation).
This analysis revealed several strongly (>5-fold) downregulated genes. These include C8J_0113, C8J_0532, C8J_0879, C8J_0949, C8J_1310, C8J_1563, C8J_1591, C8J_1593, C8J_1595, C8J_1596 and C8J_1620 (; Table S1H). The gene C8J_0113 encodes a putative recombination protein RecO, which is utilized by C. jejuni to defend against bile in the intestinal environment by repairing DNA gaps (single strand breaks).Citation38 The genes C8J_0532, C8J_0879, C8J_0949, C8J_1591 and C8J_1620 encode hypothetical proteins. Genes C8J_1593 C8J_1595 and C8J_1596, encoded three ribosomal proteins. The gene C8J_1310 encodes an MmgE/PrpD family protein which is involved in propionate catabolism.Citation39 C8J_1563 encodes an iron ABC transporter permease. Besides C8J_1563, three iron metabolism genes (C8J_0167, C8J_1564, C8J_1565) (Table S1H) were also downregulated and another three genes (C8J_1548, C8J_1549, C8J_1550) (Table S1H) were not directly involved in iron metabolism but were proposed to be downregulated by iron limitation.Citation40,Citation41 Together, these data indicate that in the presence of DOC, C. jejuni PldA might be especially important for the optimal expression of genes involved in iron metabolism.
Figure 5. Transcription of genes dependent on the availability of DOC and a functional pldA gene.
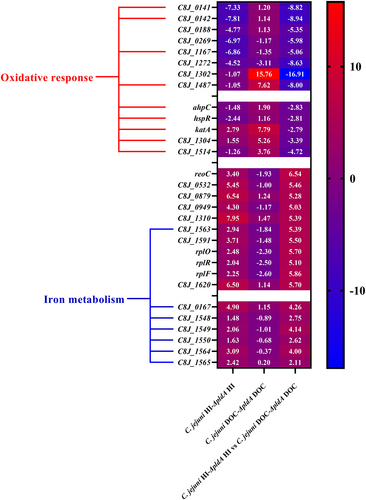
The analysis also identified several numbers of genes that were upregulated by DOC (>5-fold). These include C8J_0141, C8J_0142, C8J_0188, C8J_0269, C8J_1167, C8J_1272, C8J_1302 and C8J_1487 (; Table S1H). C8J_0141, C8J_0142, C8J_0188, C8J_0269 and C8J_1302 encoded four hypothetical proteins. C8J_1167 is a hemerythrin-like non-heme protein.Citation42 C8J_1272 encodes an MGC82361 protein, C8J_1487 encodes a flavohemoprotein which is important for reducing oxidative stress. Based on previous studies, C8J_0141 and C8J_0142 play a role in peroxide stress defense.Citation43,Citation44 C8J_1167 is involved in oxygen storage and transportCitation42 and gene C8J_1487 encodes a single-domain hemoglobin involved in oxidative stress defense. In addition to the highly upregulated genes, ahpC, hspR, katA-C8J_1304 and C8J_1514 which are involved in the oxidative/aerobic stress response are upregulated as well.Citation45–49 Other well-studied oxidative stress resistance genes including ahpC, hspR, katA, C8J_1304 and C8J_1514 are also upregulated (Table S1H). The putative functions of the upregulated genes suggests that DOC exposure elicits an oxidative response, particularly in the absence of PldA.
DOC reduces efficient iron utilization by the PldA mutant
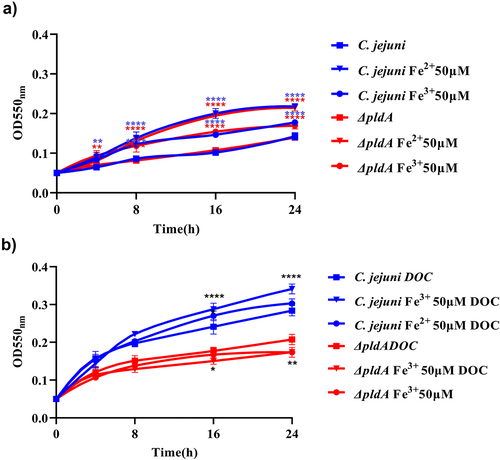
Based on our RNA-seq analysis, PldA appears to play a crucial role in iron metabolism; therefore, we investigated whether the reduced growth of the pldA mutant in the presence of DOC could be rescued by providing additional ferric or ferrous iron sources. Hereto growth curves were generated for the wild-type and pldA mutant strain in HI with or without DOC and with or without 50 μM Fe2+ or Fe3+ at 0.3% O2. In the absence of DOC, no clear growth differences were observed between the strains grown in HI or HI with 50 μM Fe2+ or Fe3+ (). In the presence of DOC, the growth of wild-type bacteria improved when 50 μM Fe2+ or Fe3+ was added to the media. In contrast DOC together with the addition of iron lowered the optical density of the pldA mutant (). This is consistent with the observed down-regulation of iron-regulated genes in the DOC-exposed pldA mutant, indicating that iron uptake in the pldA mutant is highly restricted in the presence of DOC, thereby limiting the bacterial growth of the pldA mutant.
Figure 6. Growth curves of wild-type and pldA mutant with or without DOC and the addition of ferric or ferrous iron.
PldA reduces C. jejuni resistance to oxidative stress in the presence of DOC
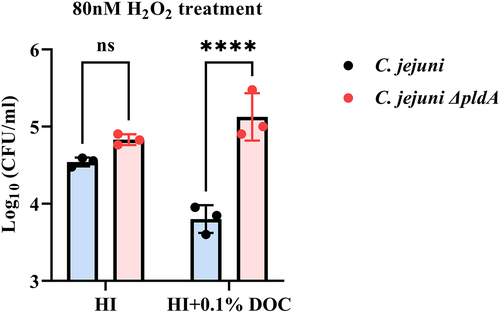
The apparent upregulation of oxygen stress defense systems by DOC in C. jejuni ΔpldA, led us to speculate that C. jejuni ΔpldA may have a stronger capacity to resist oxygen stress. To verify this, the C. jejuni wild-type and pldA mutant were exposed to 80 nM hydrogen peroxide (H2O2) in HI and HI plus 0.1% DOC under microaerobic (5% O2) conditions. Following 30 min exposure to H2O2 in 0.1% DOC, C. jejuni ΔpldA exhibited a significantly greater resistance to oxidative stress killing compared to C. jejuni wild type, as determined by CFU counting (). Without H2O2 treatment, in HI medium with or without 0.1% DOC no difference in CFU between C. jejuni wild-type and C. jejuni ΔpldA strains was observed (data not shown). These results are consistent with the RNA-seq data, suggesting that in the presence of DOC the PldA mutant upregulates oxygen stress systems much better than wild-type C. jejuni.
Figure 7. Survival assay to evaluate the susceptibility of C. jejuni wild-type and C. jejuni ΔpldA to oxidative stress.
Discussion
Bile salts play an important role in the digestion of fat, but are also important as antimicrobial molecules to control different bacterial species in the gut.Citation13 Bacteria have evolved various mechanisms to avoid the toxicity of bile salts.Citation13 The Gram-negative bacterium C. jejuni inhabits the intestine of many mammals where it is exposed to bile salts in an oxygen-limited environment.Citation50,Citation51 The adaptation of C. jejuni to bile has previously been investigated under the microaerobic (5% O2) conditions as present close to the epithelial cell layer, however in the lumen the oxygen concentration is between 0.1–1%.Citation19,Citation30,Citation32,Citation52–54 In this study we provide evidence that DOC stimulates C. jejuni growth under low-oxygen conditions and alters colony morphology in a PldA-dependent manner. Transcriptomic and functional assays indicate that PldA-dependent and DOC-induced changes in gene expression influence bacterial physiology. More specifically, under limited oxygen conditions C. jejuni PldA seems to enable the use of iron needed for optimal growth in the presence of DOC but makes the bacterium more vulnerable to oxidative stress.
We have previously shown that PldA activity results in high levels of lysophospholipids in C. jejuni. Citation26 This may influence the colony phenotype in the presence of bile salts. It can be inferred that exposure to DOC influences bacterial viability depending on the presence of LPLs, resulting in a change in colony morphology. Alternatively, at high percentage of LPLs may cause changes in the surface characteristics that become apparent in the presence of DOC. Outer membrane proteins are known to be important for bacterial colony morphology, as they facilitate the transport of intracellular components to the cell surface.Citation55 We previously showed that PldA influences biological functions such as flagella-driven motility, even without apparent changes in the capsule, LOS, or protein profiles.Citation26 This suggests that this effect may be related to alterations in C. jejuni metabolism.
To further investigate the influence of DOC on PldA activity, the growth of the wild-type and pldA mutant during planktonic growth was monitored. DOC improved the growth of C. jejuni under oxygen limited conditions (0.3% O2) ( & S2B), whereas it reduced the growth in an environment with 10% oxygen ( & S2A). At 10% O2 the membrane integrity was strongly reduced by DOC, but at 0.3% O2 DOC has no influence on membrane integrity (). Bile salts are toxic to many bacteria owing to their amphiphilic character.Citation9 Under microaerobic conditions DOC has been shown as we also have observed to reduce the growth of C. jejuni probably due to the accumulation of reactive oxygen species (ROS) and the occurrence of DNA lesions.Citation30 It might be that bile salts are less toxic for certain gut bacteria under anaerobic conditions as has been observed for Listeria monocytogenes.Citation9,Citation56 C. jejuni is considered to be bile resistant, as it can be isolated from the gallbladder and even directly from bile.Citation57–59 Many aerobic bacteria are known to degrade bile salts, but only a few are known to use bile salts as electron acceptors or carbon sources.Citation9,Citation12,Citation60 For these bacteria bile salts are useful compounds and stress factors at the same time. To the best of our knowledge, this is the first bacterium to benefit from the presence of DOC in an oxygen-dependent manner.
Transcriptomics were performed to better understand how C. jejuni benefits from DOC and the role of PldA in this process. The addition of bile salts has previously been shown to induce transcriptional alterations in C. jejuni grown under microaerophilic (rather than low O2) growth conditions.Citation19,Citation30,Citation32 Results in this study indicate that a number of these genes were also upregulated under the low oxygen conditions that were tested (Table S1C). A general finding was that at low O2, DOC exposure increased the transcript levels of a number of iron-regulated genes. This is likely due to the well-known iron-chelating effect of bile saltsCitation31 which may cause iron starvation, resulting in increased transcription of iron-regulated genes to restore bacterial growth. In Escherichia coli, bile salts have also been reported to induce the expression of genes involved in iron acquisition and metabolism, and to promote bacterial growth under iron-deficient conditions.Citation61
In the presence of DOC, the transcript levels of several iron transport genes have been found were significantly downregulated in C. jejuni ΔpldA. This indicated that in the presence of DOC, C. jejuni ΔpldA (at low O2) suffered from iron starvation and PldA activity might aid the efficient absorption of iron by C. jejuni. This hypothesis is consistent with the previous findings that bacterial pathogens can gain access to additional intracellular iron pools through the upregulation of phospholipase expression.Citation62 The involvement of PldA in the iron acquisition has recently also been observed in Pseudomonas aeruginosa where phospholipase PlaF regulates the iron uptake.Citation63 Growth curve experiments results showed that the addition of extra ferrous or ferric iron did not rescue the growth defect of C. jejuni ΔpldA () but significantly improved the growth performance of C. jejuni wild-type, indicating that iron metabolism in the pldA mutant (at low O2) is strongly limited. Thus, the ability to acquire iron may also contribute to the PldA-dependent increase in C. jejuni growth promotion in the presence of DOC.
RNA-seq analysis also showed alterations in the transcript levels of genes involved in the bacterial oxidative stress response. The results suggest that, in the presence of DOC, the C. jejuni wild-type was more susceptible to ROS than pldA mutant (). This hypothesis is supported by the results of the oxidative stress survival assay (). It should be noted that all of the highly regulated oxidative response genes are known to be directly regulated by Fur family proteins. These proteins are known to play an essential role in the regulation of C. jejuni oxidative stress defense and iron transport systems.Citation43,Citation64 Oxidative stress induced by iron deficiency is a common feature in bacteria.Citation65–67 The results suggest iron starvation in C. jejuni ΔpldA leads to the activation of Fur-regulated oxidative stress-defending genes, which causes the pldA mutant to be less vulnerable to oxidative stress than the wild-type. Previous studies by both Palyada et al.Citation44 and Holmes et al.Citation43 also identified that when C. jejuni are starved with iron, the expression of oxidative stress defense genes including ahpC, katA, C8J_1304 are upregulated. Consistent with our data, but also confirmed that in the presence of DOC, C. jejuni ΔpldA suffers from intracellular iron deficiency. Fur independent iron transporters like the FeoAB,Citation68 may be more active at microaerophilic conditions as we did not observe growth difference at 10% O2 levels () but this needs to be further explored.
A third physiological system in C. jejuni that may be influenced by exposure to DOC is tryptophan and branched-chain amino acids (leucine, isoleucine and valine) metabolism. It is currently unknown how C. jejuni benefits from the upregulation of tryptophan and high-affinity branched-chain amino acids gene pathways during exposure to bile salts. The bacterial tryptophan catabolite indole together with bile acids can regulate epithelial inflammation and gut immunity, and branched-chain amino acids support evasion of host defenses.Citation69,Citation70 In eukaryotic cells, elevated bile acid levels have been implied to be relevant to abnormal tryptophan metabolism, and bacteria that produce branched-chain amino acids such as leucine are known to affect bile hemostasis by conjugating bile acids.Citation71,Citation72 Thus, it can be inferred that an increase in these amino acids is important for the pathogenesis of C. jejuni.
PldA has long been considered an essential factor in the intestinal colonization of C. jejuni as well as other enteric pathogens.Citation29,Citation73 However, the underlying mechanisms have not been completely elucidated. Previously we reported that, in the absence of PldA, C. jejuni is less motile under limited oxygen conditions.Citation26 RNA-seq analysis revealed decreased transcripts of the thiol-disulfide oxidoreductase forming gene dsbA in the pldA mutant (Table S1F). DsbA has been reported to be regulated by iron in a Fur-dependent manner and to play a crucial role in C. jejuni motility, as it influences the activity of the paralyzed flagella gene pflA.Citation36,Citation74,Citation75 Thus, reduced DsbA expression might explain the motility defect in C. jejuni ΔpldA at low O2. The bacterial respiratory electron transfer chain participates in the formation of DsbACitation76 however whether the transcription of dsbA is regulated by oxygen availability awaits future study.
Conclusions
The present study demonstrates that the processes of bile resistance, oxidative stress defense, and iron acquisition of C. jejuni are tightly linked and that bile salts in conjunction with PldA promote C. jejuni growth and survival in a low oxygen environment. Other enteric bacteria might utilize similar mechanisms to defend against the toxic components of bile and optimally adapt to the intestinal niche.
Limitations
In this study, we utilized the RNA-seq assay to investigate how C. jejuni benefits from the DOC under low-oxygen condition in a PldA dependent manner and concluded that bile resistance, oxidative stress defense, and iron acquisition might played essential roles in this process. However, the mutant strains of key genes which were highly regulated by DOC in a PldA dependent manner were not constructed to validate the inference. In the further studies, it is important to verify the hypothesizes in this study by determine the planktonic growth character of those mutant strains.
Material and methods
Bacteria culture
C. jejuni wild-type strain 81,116, is a human isolate originally isolated from a waterborne outbreak,Citation77 its isogenic pldA mutant (C. jejuni ΔpldA), and the complemented pldA mutant (C. jejuni ΔpldA +pldA)Citation26 was routinely grown on saponin agar plates containing 4% lysed horse blood or in Hearth Infusion (HI) medium (Biotrading, Mijdrecht, The Netherlands) under microaerophilic conditions (5% O2, 10% CO2, 75% N2, 10% H2) at 42°C. When appropriate the medium was were supplemented with DOC (0.1%), chloramphenicol (15 μg/ml), or kanamycin (25 μg/ml).
C. jejuni morphology detection
C. jejuni, C. jejuni ΔpldA and C. jejuni ΔpldA +pldA were routinely grown under microaerophilic conditions at 42°C in HI medium for 24 h. Bacteria were collected by centrifugation (10 min, 3,000×g) and resuspended in HI medium to a final OD550 of 1. Five microliter of bacterial suspension was loaded on the surface of 1) Campylobacter blood-free selective agar base plates (Thermo Fisher Scientific), 2) saponin agar charcoal plates containing 4% lysed horse blood and 4% bacteriological charcoal, or 3) saponin agar charcoal plates containing 4% lysed horse blood, 4% bacteriological charcoal and 0.1% DOC (Merck). Plates were incubated for 16 h under microaerophilic conditions at 42°C. Morphology of bacteria colonies were visual examined by size, shape, color, opacity, and consistency and imaged using a were obtained with a 10-megapixel Nikon D200 camera.
Electron microscopy
Electron Microscopy was performed as described before.Citation78 In brief, C. jejuni wild-type and C. jejuni ΔpldA were grown in HI broth with 0.1% DOC under microaerophilic conditions (5% O2, 10% CO2, 75%N2, 10% H2) for 16 h at 42°C. Bacteria were collected by centrifugation (10 min, 3,000×g), washed three times with Dulbecco’s phosphate-buffered saline (DPBS, Thermo Fisher Scientific) and resuspended in DPBS to a final OD550 of 1. Carbon activated copper grids were incubated with 10 μL of the bacterial culture resuspended for 10–30 min and washed three times with DPBS. The bacteria were fixed on the grids using 1% glutaraldehyde (Sigma-Aldrich) in DPBS for 10 min, washed two times with DPBS and, subsequently, four times with Milli-Q water. The grids were then briefly rinsed with methylcellulose/uranyl acetate (pH 4) and incubated for 5 min with methylcellulose/uranyl acetate (pH 4) on ice. The grids were looped out of the solution and air dried. Samples were imaged using a Tecnai-12 electron microscope (FEI, Hillsboro, Oregon, USA).
LOS detection
LOS isolation and staining were performed as described before.Citation79 In brief, C. jejuni strains were grown under oxygen-limited conditions at 42°C on Campylobacter blood-free selective agar base plates or in 5 ml HI or HI plus 0.1% DOC medium for 24 h. Bacteria colonies were taken up in 1 ml DPBS. Bacteria were collected by centrifugation (10 min, 3,000×g) and resuspended in DPBS to a final OD550 of 1. Samples were boiled for 5 min and then treated with 10 μL Proteinase K (20 mg/mL) overnight at 55°C. Three times Laemmli buffer was added, and the samples were loaded onto a 16% Tris-Tricine gel. After electrophoresis the gel was fixed for 30 min with 40% ethanol and 5% acetic acid, oxidized 5 min with 0.7% sodium periodic acid with 40% ethanol and 5% acetic acid, washed 5 min for 3 times with distilled water, stained with distilled water containing 19% 0.1 M NaOH, 1.3% ammonium hydroxide (˃28%), and 3.3% 20% w/v silver nitrate, washed, and developed with distilled water containing 0.1% formaldehyde (37%) and 0.1% citric acid (100 mg/mL) until bands appeared. The reaction was stopped by washing with distilled water containing 7% acetic acid.
Capsule detection
The polysaccharide capsule of C. jejuni was visualized using the cationic dye Alcian Blue, as previously reported.Citation80 In short, C. jejuni strains were grown under oxygen-limited conditions condition at 42°C in 5 ml HI or HI plus 0.1% DOC medium for 24 h. Bacteria (1×108 were collected by centrifugation (10 min, 3,000×g) and resuspended in 100 μL lysis buffer (3.2% 1 M Tris-HCl pH 6.8, 0.14 M SDS, 0.37 mM bromophenol blue, 20% glycerol, and 76.8% distilled water), samples were boiled for 10 min at 100°C and then treated with 10 μL protease K (20 mg/mL) overnight at 55°C. After heating for 10 min at 100°C, 20 μL of sample was loaded onto 10% SDS-PAGE gel. The gel was stained with Alcian blue solution (2% acetic acid, 40% methanol, and 0.5% Alcian blue 8GX (Sigma-Aldrich)) for 30–60 min and destained with 2% acetic acid and 40% methanol until bands appeared.
Biofilm formation
C. jejuni biofilms were detected using a crystal violet staining assay, as reportedCitation81. In summary, C. jejuni strains were grown in 15 ml polypropylene tubes containing 5 ml HI broth or HI broth plus 0.1% DOC under oxygen-limited conditions condition at 42°C. After 24 h, the culture media were removed from 15 ml tubes and 10 ml of fixing solution (0.05% w/v crystal violet, 1% formaldehyde (37%), 10% DPBS and 1% methanol) was added to the tube. The biofilms were stained for 20 min at room temperature, washed with H2O, and air dried. Stained biofilms were imaged on a white paper using a Sony Alpha 6700 camera.
SDS-PAGE
The C. jejuni wild-type and C. jejuni ΔpldA were grown in HI medium with or without DOC for 16 h at 42°C under microaerophilic conditions and then diluted to an OD550 of 1 with DPBS. Bacterial samples were mixed with 3× Laemmli Sample Buffer, lysed, and denatured at 95°C for 15 minutes. Samples were loaded into equal volumes (10 μL) onto a 12% acrylamide gel. Gels were run for 30 min at 50 V and then for another 60 min at 150 V. Gels were stained with 20 ml PageBlue Protein Staining Solution (Thermo Fisher Scientific) for 1 h and destained overnight in 80:10:10 MQ: methanol: acetic acid. Gels were imaged using Universal Hood III (Bio-Rad).
Bacterial growth assay
C. jejuni wild-type, C. jejuni ΔpldA and C. jejuni ΔpldA +pldA starter cultures were grown in HI medium for 24 h at 42°C under microaerophilic conditions and then diluted to an OD550 of 0.05 in T25 flasks containing 5 ml of HI broth or HI broth plus 0.1% DOC. Cultures were shaken (160 rpm) under high-oxygen (10% O2, 10% CO2, 70% N2, 10% H2) or under oxygen-limited conditions (0.3% O2, 10% CO2, 79.7%N2, 10% H2) at 42°C. Ferrous sulfate or ferric sulfate (50 μM) was added to the medium when appropriate. The optical density (OD550) as well as viable counts (CFU/ml) of the cultures was measured after 0, 4, 8, 16 and 24 h of growth. The values are the means of three independent experiments performed in duplicate.
Membrane integrity
C. jejuni wild-type,and the ΔpldA mutant were grown in HI medium with or without 0.1% DOC for 16 h at 42°C under high or oxygen-limited conditions. Equal amount of bacteria were three times washed with physiological salt (0.9% NaCl) and bacteria were taken up in 300 µl 0,9% NaCl. Bacteria were incubated for 15 minutes with 1 µl 1:1 mixture SYTO-9 dye (2,34 mM) and propidium iodide (20 mM) (Live/dead Baclight Bacterial viability kits, Thermo fisher Scientific). Slides with the stained bacteria were captured using EVOS fluorescent microscope (Thermo Scientific) at 100X magnification and equal light intensity between the slides quantified using EVOS software.
RNA-seq
C. jejuni wild-type and C. jejuni ΔpldA start cultures were diluted to an OD550 of 0.05 in HI broth or HI broth plus 0.1% DOC, and then grown under oxygen-limited conditions (0.3% O2, 10% CO2, 79.7%N2, 10% H2) for 6 h at 42°C. RNA was extracted from C. jejuni and C. jejuni ΔpldA using an RNA-Bee kit (Tel-Test). RNA samples were treated with RNAse-free DNase I (Invitrogen) according to the manufacturer’s instructions. RNA-Seq was performed as previously describedCitation82. Briefly, the total RNA for each sample was rRNA depleted by utilizing the Ribo-zero Magnetic Kit for Gram-negative bacterial (Illumina) following instructions of option 1 manufacturer. Libraries for the Illumina MiSeq were processed using the llumina® cDNA synthesis & the illumina® RNA Prep Ligation kit (Illumina) and the IDT for Illumina RNA UD indexes Set A, following instructions of manufacture. Pooled libraries were sequenced on a HiSeq4000, read length 150bp by Macrogen Europe (Amsterdam, The Netherlands).
The fastq sequences were trimmed to remove inferior bases and assembled into reference genome CP000814 using Bowtie within Geneious 11.1. Geneious software was used to calculate the normalized transcripts per million (TPM) and DEseq2 method to compare expression levels between control growth conditions.
Quantitative real-time RT-PCR (RT-PCR) analyses
Real-time RT-PCR was performed as previously described.Citation83 Primers used in this study are listed in Table S2B. The calculated threshold cycle (Ct) for each detected gene amplification was normalized to the Ct value for the housekeeping gene rpoD amplified from the corresponding sample, before calculating the fold change using the arithmetic formula (2−Δ ΔCt).Citation84 Each sample was subjected to three independent preparations RNA.
Oxidative stress resistance assay
Bacteria oxidative stress resistance was measured as described before.Citation85 C. jejuni wild-type and C. jejuni ΔpldA mutant starter cultures were grown in HI medium for 24 h at 42°C under microaerophilic conditions and then diluted to an OD550 of 0.05 in T25 flasks containing 5 ml of HI medium or HI medium plus 0.1% DOC. Cultures were shaken (160 rpm) under oxygen-limited conditions (0.3% O2, 10% CO2, 79.7%N2, 10% H2) at 42°C for 24 h. Bacteria were collected by centrifugation (10 min, 3,000×g) and resuspended in DPBS to a final OD550 of 0.1. Bacterial suspensions were incubated with or without 80 nM H2O2 for 30 min at 42°C under microaerobic conditions. Serial dilutions were prepared and plated onto saponin agar plates. Plates were incubated at 42°C under microaerophilic conditions and colonies were counted after 24 h.
Statistical analysis
Statistical significance was determined using two-way ANOVA using Prism software (GraphPad, San Diego,CA). Results are shown as mean ± SEM.
Supplemental Material
Download Zip (26.2 MB)Acknowledgments
This work was supported by a China Scholarship Council grant 201706910078 to Xuefeng Cao.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2023.2262592
Additional information
Funding
References
- Wani AK, Akhtar N, Sher F, Navarrete AA, Américo-Pinheiro JHP. Microbial adaptation to different environmental conditions: molecular perspective of evolved genetic and cellular systems. Arch Microbiol. 2022;204(2):144. doi:10.1007/s00203-022-02757-5.
- Duque-Correa M, Codron D, Meloro C, McGrosky A, Schiffmann C, Edwards MS, Clauss M. Mammalian intestinal allometry, phylogeny, trophic level and climate. Proc Biol Sci. 2021;288:20202888. doi:10.1098/rspb.2020.2888.
- Dawson PA. Bile formation and the enterohepatic circulation. Physiol Gastrointestinal Track, 6th Ed. 2018;41:931–18. doi:10.1016/C2015-1-04889-X.
- Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi:10.1194/jlr.R500013-JLR200.
- Cremers CM, Knoefler D, Vitvitsky V, Banerjee R, Jakob U. Bile salts act as effective protein-unfolding agents and instigators of disulfide stress in vivo. Proc Natl Acad Sci USA. 2014;111(16):E1610–E1619. doi:10.1073/pnas.1401941111.
- Kristoffersen SM, Ravnum S, Tourasse NJ, Økstad OA, Kolstø A, Davies W. Low concentrations of bile salts induce stress responses and reduce motility in Bacillus cereus ATCC 14570. J Bacteriol. 2007;189(14):5302–5313. doi:10.1128/JB.00239-07.
- Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi:10.1194/jlr.R900010-JLR200.
- Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi:10.1146/annurev.biochem.72.121801.161712.
- Philipp B. Bacterial degradation of bile salts. Appl Microbiol Biotechnol. 2011;89:903–915. doi:10.1007/s00253-010-2998-0.
- Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, Nicholson JK, Holmes E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natil Acad Sci U S A. 2011;Suppl 108(supplement_1):4523–4530. doi:10.1073/pnas.1006734107.
- Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332–338. doi:10.1097/MOG.0000000000000057.
- Birkenmaier A, Holert J, Erdbrink H, Moeller HM, Friemel A, Schoenenberger R, Suter MJ, Klebensberger J, Philipp B. Biochemical and genetic investigation of initial reactions in aerobic degradation of the bile acid cholate in Pseudomonas sp. strain Chol1. J Bacteriol. 2007;189(20):7165–7173. doi:10.1128/JB.00665-07.
- Urdaneta V, Casadesus J. Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts. Front Med. 2017;4:163. doi:10.3389/fmed.2017.00163.
- Faherty CS, Redman JC, Rasko DA, Barry EM, Nataro JP. Shigella flexneri effectors OspE1 and OspE2 mediate induced adherence to the colonic epithelium following bile salts exposure. Mol Microbiol. 2012;85:107–121. doi:10.1111/j.1365-2958.2012.08092.x.
- Pope LM, Reed KE, Payne SM. Increased protein secretion and adherence to HeLa cells by Shigella spp. following growth in the presence of bile salts. Infect Immun. 1995;63(9):3642–3648. doi:10.1128/iai.63.9.3642-3648.1995.
- Hung DT, Zhu J, Sturtevant D, Mekalanos JJ. Bile acids stimulate biofilm formation in Vibrio cholerae. Mol Microbiol. 2006;59(1):193–201. doi:10.1111/j.1365-2958.2005.04846.x.
- Gupta S, Chowdhury R. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect Immun. 1997;65(3):1131–1134. doi:10.1128/IAI.65.3.1131-1134.1997.
- Gunn JS. Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2000;2:907–913. doi:10.1016/s1286-4579(00)00392-0.
- Lin J, Cagliero C, Guo B, Barton YW, Maurel MC, Payot S, Zhang Q. Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni. J Bacteriol. 2005;187(21):7417–7424. doi:10.1128/JB.187.21.7417-7424.2005.
- Heithoff DM, Enioutina EY, Daynes RA, Sinsheimer RL, Low DA, Mahan MJ, Burns DL. Salmonella DNA adenine methylase mutants confer cross-protective immunity. Infect Immun. 2001;69(11):6725–6730. doi:10.1128/IAI.69.11.6725-6730.2001.
- Prieto AI, Ramos-Morales F, Casadesus J. Repair of DNA damage induced by bile salts in Salmonella enterica. Genetics. 2006;174(2):575–584. doi:10.1534/genetics.106.060889.
- Hernandez SB, Cota I, Ducret A, Aussel L, Casadesus J. Adaptation and preadaptation of Salmonella enterica to bile. PLoS Genet. 2012;8(1):e1002459. doi:10.1371/journal.pgen.1002459.
- Giles DK, Hankins JV, Guan Z, Trent MS. Remodelling of the Vibrio cholerae membrane by incorporation of exogenous fatty acids from host and aquatic environments. Mol Microbiol. 2011;79:716–728. doi:10.1111/j.1365-2958.2010.07476.x.
- Hermans D, Van Deun K, Martel A, Van Immerseel F, Messens W, Heyndrickx M, Haesebrouck F, Pasmans F. Colonization factors of Campylobacter jejuni in the chicken gut. Vet Res. 2011;42(1):82–82. doi:10.1186/1297-9716-42-82.
- Lobo de Sa FD, Schulzke JD, Bucker R. Diarrheal mechanisms and the role of intestinal barrier dysfunction in Campylobacter infections. Curr Top Microbiol Immunol. 2021;431:203–231. doi:10.1007/978-3-030-65481-8_8.
- Cao X, Brouwers J, van Dijk L, van de Lest C, Parker C, Huynh S, van Putten JP, Kelly DJ, Wösten MM. The unique phospholipidome of the enteric pathogen Campylobacter jejuni: lysophosholipids are required for motility at low oxygen availability. J Mol Biol. 2020;432(19):5244–5258. doi:10.1016/j.jmb.2020.07.012.
- Cao X, van de Lest CHA, Huang LZX, van Putten JPM, Wösten M. Campylobacter jejuni permeabilizes the host cell membrane by short chain lysophosphatidylethanolamines. Gut Microbes. 2022;14(1):2091371. doi:10.1080/19490976.2022.2091371.
- Colles SM, Chisolm GM. Lysophosphatidylcholine-induced cellular injury in cultured fibroblasts involves oxidative events. J Lipid Res. 2000;41(8):1188–1198. doi:10.1016/S0022-2275(20)33425-8.
- Ziprin RL, Young CR, Byrd JA, Stanker LH, Hume ME, Gray SA, Kim BJ, Konkel ME. Role of Campylobacter jejuni potential virulence genes in cecal colonization. Avian Dis. 2001;45:549–557. doi:10.2307/1592894.
- Talukdar PK, Crockett TM, Gloss LM, Huynh S, Roberts SA, Turner KL, Lewis STE, Herup-Wheeler TL, Parker CT, Konkel ME. The bile salt deoxycholate induces Campylobacter jejuni genetic point mutations that promote increased antibiotic resistance and fitness. Front Microbiol. 2022;13:1062464. doi:10.3389/fmicb.2022.1062464.
- Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–651. doi:10.1016/j.femsre.2004.09.003.
- Malik-Kale P, Parker CT, Konkel ME. Culture of Campylobacter jejuni with sodium deoxycholate induces virulence gene expression. J Bacteriol. 2008;190(7):2286–2297. doi:10.1128/JB.01736-07.
- Palyada K, Sun YQ, Flint A, Butcher J, Naikare H, Stintzi A. Characterization of the oxidative stress stimulon and PerR regulon of Campylobacter jejuni. Bmc Genom. 2009;10(1):481–481. doi:10.1186/1471-2164-10-481.
- Reid AN, Pandey R, Palyada K, Naikare H, Stintzi A. Identification of Campylobacter jejuni genes involved in the response to acidic pH and stomach transit. Appl Environ Microbiol. 2008;74(5):1583–1597. doi:10.1128/AEM.01507-07.
- de Vries SP, Stefan PW, Linn A, Macleod K, MacCallum A, Hardy SP, Douce G, Watson E, Dagleish MP, Thompson H, et al. Analysis of Campylobacter jejuni infection in the gnotobiotic piglet and genome-wide identification of bacterial factors required for infection. Sci Rep. 2017;7(1):44283. doi:10.1038/srep44283.
- Grabowska AD, Wandel MP, Łasica AM, Nesteruk M, Roszczenko P, Wyszyńska A, Godlewska R, Jagusztyn-Krynicka EK. Campylobacter jejuni dsb gene expression is regulated by iron in a Fur-dependent manner and by a translational coupling mechanism. BMC Microbiol. 2011;11(1):166. doi:10.1186/1471-2180-11-166.
- Stahl M, Butcher J, Stintzi A. Nutrient acquisition and metabolism by Campylobacter jejuni. Front Cell Infect Microbiol. 2012;2:5. doi:10.3389/fcimb.2012.00005.
- Gourley CR, Negretti NM, Konkel ME. The food-borne pathogen Campylobacter jejuni depends on the AddAB DNA repair system to defend against bile in the intestinal environment. Sci Rep. 2017;7(1):14777. doi:10.1038/s41598-017-14646-9.
- Mazumder L, Hasan M, Rus’d AA, Islam MA. In-silico characterization and structure-based functional annotation of a hypothetical protein from Campylobacter jejuni involved in propionate catabolism. Genomics Inform. 2021;19(4):e43. doi:10.5808/gi.21043.
- Davies C, Taylor AJ, Elmi A, Winter J, Liaw J, Grabowska AD, Gundogdu O, Wren BW, Kelly DJ, Dorrell N. Sodium taurocholate stimulates Campylobacter jejuni outer membrane vesicle production via down-regulation of the maintenance of lipid asymmetry pathway. Front Cell Infect Microbiol. 2019;9:177. doi:10.3389/fcimb.2019.00177.
- Roier S, Zingl FG, Cakar F, Durakovic S, Kohl P, Eichmann TO, Klug L, Gadermaier B, Weinzerl K, Prassl R, et al. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat Commun. 2016;7(1):10515. doi:10.1038/ncomms10515.
- Li X, Li J, Hu X, Huang L, Xiao J, Chan J, Mi K. Differential roles of the hemerythrin-like proteins of mycobacterium smegmatis in hydrogen peroxide and erythromycin susceptibility. Sci Rep. 2015;5(1):16130. doi:10.1038/srep16130.
- Holmes K, Mulholland F, Pearson BM, Pin C, McNicholl-Kennedy J, Ketley JM, Wells JM. Campylobacter jejuni gene expression in response to iron limitation and the role of Fur. Microbiol. 2005;151(1):243–257. doi:10.1099/mic.0.27412-0.
- Palyada K, Threadgill D, Stintzi A. Iron acquisition and regulation in Campylobacter jejuni. J Bacteriol. 2004;186(14):4714–4729. doi:10.1128/JB.186.14.4714-4729.2004.
- Gaynor EC, Wells DH, MacKichan JK, Falkow S. The Campylobacter jejuni stringent response controls specific stress survival and virulence-associated phenotypes. Mol Microbiol. 2005;56:8–27. doi:10.1111/j.1365-2958.2005.04525.x.
- Gaynor EC, Cawthraw S, Manning G, MacKichan JK, Falkow S, Newell DG. The genome-sequenced variant of Campylobacter jejuni NCTC 11168 and the original clonal clinical isolate differ markedly in colonization, gene expression, and virulence-associated phenotypes. J Bacteriol. 2004;186(2):503–517. doi:10.1128/JB.186.2.503-517.2004.
- Andersen MT, Brondsted L, Pearson BM, Mulholland F, Parker M, Pin C, Wells JM, Ingmer H. Diverse roles for HspR in Campylobacter jejuni revealed by the proteome, transcriptome and phenotypic characterization of an hspR mutant. Microbiol. 2005;151(3):905–915. doi:10.1099/mic.0.27513-0.
- Pesci EC, Cottle DL, Pickett CL. Genetic, enzymatic, and pathogenic studies of the iron superoxide dismutase of Campylobacter jejuni. Infect Immun. 1994;62(7):2687–2694. doi:10.1128/iai.62.7.2687-2694.1994.
- Kim JC, Oh E, Kim J, Jeon B. Regulation of oxidative stress resistance in Campylobacter jejuni, a microaerophilic foodborne pathogen. Front Microbiol. 2015;6:751. doi:10.3389/fmicb.2015.00751.
- D’Aldebert E, Biyeyeme BMJ, Mergey M, Wendum D, Firrincieli D, Coilly A, Fouassier L, Corpechot C, Poupon R, Housset C, et al. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology. 2009;136(4):1435–1443. doi:10.1053/j.gastro.2008.12.040.
- Silva J, Leite D, Fernandes M, Mena C, Gibbs PA, Teixeira P. Campylobacter spp. As a foodborne pathogen: a review. Front Microbiol. 2011;2:200. doi:10.3389/fmicb.2011.00200.
- Man L, Dale AL, Klare WP, Cain JA, Sumer-Bayraktar Z, Niewold P, Solis N, Cordwell SJ. Proteomics of Campylobacter jejuni growth in deoxycholate reveals Cj0025c as a cystine transport protein required for wild-type human infection phenotypes. Molecular & Cellular Proteomics: MCP. 2020;19(8):1263–1280. doi:10.1074/mcp.RA120.002029.
- Raphael BH, Pereira S, Flom GA, Zhang Q, Ketley JM, Konkel ME. The Campylobacter jejuni response regulator, CbrR, modulates sodium deoxycholate resistance and chicken colonization. J Bacteriol. 2005;187(11):3662–3670. doi:10.1128/JB.187.11.3662-3670.2005.
- Singhal R, Shah YM. Oxygen battle in the gut: hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J Biol Chem. 2020;295(30):10493–10505. doi:10.1074/jbc.REV120.011188.
- Bos MP, Tefsen B, Geurtsen J, Tommassen J. Identification of an outer membrane protein required for the transport of lipopolysaccharide to the bacterial cell surface. Proc Natl Acad Sci U S A. 2004;101:9417–9422. doi:10.1073/pnas.0402340101.
- White SJ, McClung DM, Wilson JG, Roberts BN, Donaldson JR. Influence of pH on bile sensitivity amongst various strains of listeria monocytogenes under aerobic and anaerobic conditions. J Med Microbiol. 2015;64:1287–1296. doi:10.1099/jmm.0.000160.
- van der Hoop G, Veringa EM. Cholecystitis caused by Campylobacter jejuni. Clin Infect Dis. 1993;17:133. doi:10.1093/clinids/17.1.133.
- Drion S, Wahlen C, Taziaux P. Isolation of Campylobacter jejuni from the bile of a cholecystic patient. J Clin Microbiol. 1988;26:2193–2194. doi:10.1128/jcm.26.10.2193-2194.1988.
- Darling WM, Peel RN, Skirrow MB, Mulira AE. Campylobacter cholecystitis. Lancet. 1979;1(8129):1302. doi:10.1016/s0140-6736(79)92269-4.
- Philipp B, Erdbrink H, Suter MJ, Schink B. Degradation of and sensitivity to cholate in Pseudomonas sp. strain Chol1. Arch Microbiol. 2006;185(3):192–201. doi:10.1007/s00203-006-0085-9.
- Hamner S, McInnerney K, Williamson K, Franklin MJ, Ford TE. Bile salts affect expression of Escherichia coli O157: H7 genes for virulence and iron acquisition, and promote growth under iron limiting conditions. PloS One. 2013;8(9):e74647. doi:10.1371/journal.pone.0074647.
- Fiester SE, Arivett BA, Schmidt RE, Beckett AC, Ticak T, Carrier MV, Ghosh R, Ohneck EJ, Metz ML, Jeffries MKS, et al. Iron-regulated phospholipase C activity contributes to the cytolytic activity and virulence of Acinetobacter baumannii. PloS One. 2016;11(11):e0167068. doi:10.1371/journal.pone.0167068.
- Caliskan M, Poschmann G, Gudzuhn M, Waldera-Lupa D, Molitor R, Strunk CH, Streit WR, Jaeger KE, Stühler K, Kovacic F. Pseudomonas aeruginosa responds to altered membrane phospholipid composition by adjusting the production of two-component systems, proteases and iron uptake proteins. Biochimica et biophysica acta Mol Cell Biol Lipids. 2023;1868(6):159317. doi:10.1016/j.bbalip.2023.159317.
- Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27(2–3):215–237. doi:10.1016/S0168-6445(03)00055-X.
- Latifi A, Jeanjean R, Lemeille S, Havaux M, Zhang C. Iron starvation leads to oxidative stress in Anabaena sp. strain PCC 7120. J Bacteriol. 2005;187(18):6596–6598. doi:10.1128/JB.187.18.6596-6598.2005.
- Yingping F, Lemeille S, Talla E, Janicki A, Denis Y, Zhang C, Latifi A. Unravelling the cross-talk between iron starvation and oxidative stress responses highlights the key role of PerR (alr0957) in peroxide signalling in the cyanobacterium nostoc PCC 7120. Environ Microbiol Rep. 2014;6(5):468–475. doi:10.1111/1758-2229.12157.
- Leaden L, Silva LG, Ribeiro RA, Dos Santos NM, Lorenzetti APR, Alegria TGP, Schulz ML, Medeiros MHG, Koide T, Marques MV. Iron deficiency generates oxidative stress and activation of the SOS response in Caulobacter crescentus. Front Microbiol. 2018;9:2014. doi:10.3389/fmicb.2018.02014.
- Naikare H, Palyada K, Panciera R, Marlow D, Stintzi A. Major role for FeoB in Campylobacter jejuni ferrous iron acquisition, gut colonization, and intracellular survival. Infect Immun. 2006;74(10):5433–5444. doi:10.1128/IAI.00052-06.
- Gasaly N, de Vos P, Hermoso MA. Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Front Immunol. 2021;12:658354. doi:10.3389/fimmu.2021.658354.
- Kaiser JC, Heinrichs DE, Garsin DA. Branching out: alterations in bacterial physiology and virulence due to branched-chain amino acid deprivation. mBio. 2018;9(5):e01188–18. doi:10.1128/mBio.01188-18.
- Garcia CJ, Kosek V, Beltrán D, Tomás-Barberán FA, Hajslova J. Production of new microbially conjugated bile acids by human gut microbiota. Biomolecules. 2022;12(5):687. doi:10.3390/biom12050687.
- Liu W, Wang Q, Chang J, Bhetuwal A, Bhattarai N, Ni X. Circulatory metabolomics reveals the association of the metabolites with clinical features in the patients with intrahepatic cholestasis of pregnancy. Front Physiol. 2022;13:1295. doi:10.3389/fphys.2022.848508.
- Dorrell N, Martino MC, Stabler RA, Ward SJ, Zhang ZW, McColm AA, Farthing MJ, Wren BW. Characterization of Helicobacter pylori PldA, a phospholipase with a role in colonization of the gastric mucosa. Gastroenterology. 1999;117(5):1098–1104. doi:10.1016/s0016-5085(99)70394-x.
- Banaś AM, Bocian-Ostrzycka KM, Dunin-Horkawicz S, Ludwiczak J, Wilk P, Orlikowska M, Wyszyńska A, Dąbrowska M, Plichta M, Spodzieja M, et al. Interplay between DsbA1, DsbA2 and C8J_1298 periplasmic oxidoreductases of Campylobacter jejuni and their impact on bacterial physiology and pathogenesis. Int J Mol Sci. 2021;22(24):13451. doi:10.3390/ijms222413451.
- Grabowska AD, Wywiał E, Dunin-Horkawicz S, Łasica AM, Wösten MM, Nagy-Staroń A, Godlewska R, Bocian-Ostrzycka K, Pieńkowska K, Łaniewski P, et al. Functional and bioinformatics analysis of two Campylobacter jejuni homologs of the thiol-disulfide oxidoreductase, DsbA. PloS One. 2014;9(9):e106247. doi:10.1371/journal.pone.0106247.
- Kobayashi T, Kishigami S, Sone M, Inokuchi H, Mogi T, Ito K. Respiratory chain is required to maintain oxidized states of the DsbA-DsbB disulfide bond formation system in aerobically growing Escherichia coli cells. Proc Natl Acad Sci U S A. 1997;94(22):11857–11862. doi:10.1073/pnas.94.22.11857.
- Palmer SR, Gully PR, White JM, Pearson AD, Suckling WG, Jones DM, Rawes JC, Penner JL. Water-borne outbreak of Campylobacter gastroenteritis. Lancet. 1983;321(8319):287–290. doi:10.1016/s0140-6736(83)91698-7.
- de Jonge EF, Balhuizen MD, van Boxtel R, Jianjun W, Haagsman HP, Tommassen J. Heat shock enhances outer-membrane vesicle release in bordetella spp. Curr Res Microb Sci. 2020;2:100009. doi:10.1016/j.crmicr.2020.100009.
- Tsai CM, Frasch CE. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi:10.1016/0003-2697(82)90673-x.
- Karlyshev AV, Wren BW. Detection and initial characterization of novel capsular polysaccharide among diverse Campylobacter jejuni strains using alcian blue dye. J Clin Microbiol. 2001;39(1):279–284. doi:10.1128/JCM.39.1.279-284.2001.
- Genovese C, D’Angeli F, Bellia F, Distefano A, Spampinato M, Attanasio F, Nicolosi D, Di Salvatore V, Tempera G, Lo Furno D, et al. In vitro antibacterial, anti-adhesive and anti-biofilm activities of Krameria lappacea (Dombey) burdet & B.B. Simpson root extract against methicillin-resistant Staphylococcus aureus strains. Antibiot (Basel). 2021;10(4):428. doi:10.3390/antibiotics10040428.
- van der Stel AX, van de Lest CH, Huynh S, Parker CT, van Putten JP, Wösten MM. Catabolite repression in Campylobacter jejuni correlates with intracellular succinate levels. Environ Microbiol. 2018;20(4):1374–1388. doi:10.1111/1462-2920.14042.
- van der Stel AX, van Mourik A, van Dijk L, Parker CT, Kelly DJ, van de Lest CH, van Putten JP, Wösten MM. The Campylobacter jejuni RacRS system regulates fumarate utilization in a low oxygen environment. Environ Microbiol. 2015;17(4):1049–1064. doi:10.1111/1462-2920.12476.
- Schmittgen TD. Real-time quantitative PCR. Methods. 2001;25(4):383–385. doi:10.1006/meth.2001.1260.
- Possik E, Pause A. Measuring oxidative stress resistance of Caenorhabditis elegans in 96-well microtiter plates. JoVe. 2015;9(99):e52746. doi:10.3791/52746.
