ABSTRACT
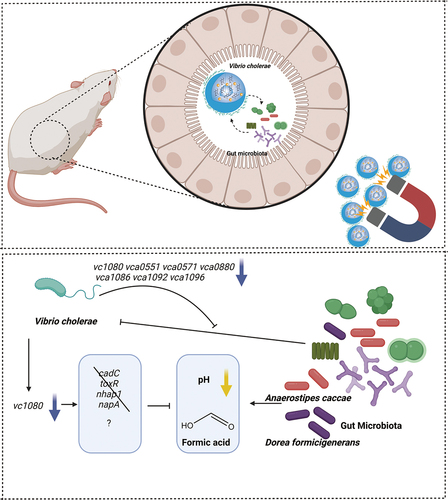
Vibrio cholerae adapts to the host environment by altering gene expression. Because of the complexity of the gut microbiome, current in vivo V. cholerae transcriptome studies have focused on microbiota-undeveloped conditions, neglecting the interaction between the host’s commensal gut microbiota and V. cholerae. In this study, we analyzed the transcriptome of fully colonized adult mice in vivo using V. cholerae coated-magnetic chitin beads (vcMCB). This provides a simple yet powerful method for obtaining high-quality RNA from V. cholerae during colonization in mice. The transcriptome of V. cholerae recovered from adult mice infected with vcMCB shows differential expression of several genes when compared to V. cholerae recovered from the infant mouse and infant rabbit model. Some of these genes were also observed to be differentially expressed in previous studies of V. cholerae recovered from human infection when compared to V. cholerae grown in vitro. In particular, we confirmed that V. cholerae resists the inhibitory effects of low pH and formic acid from gut microbiota, such as Anaerostipes caccae and Dorea formicigenerans, by downregulating vc1080. We propose that the vc1080 product may protect V. cholerae from formic acid stress through a novel acid tolerance response mechanism. Transcriptomic data obtained using the vcMCB system provide new perspectives on the interaction between V. cholerae and the gut microbiota, and this approach can also be applied to studies of other pathogenic bacteria.
Introduction
Vibrio cholerae is the causative agent of cholera, which causes large epidemics.Citation1 In aquatic environments, V. cholerae mainly adheres to the surface of crustacean chitin-rich exoskeletons, where it forms biofilms. V. cholerae colonizes the human small intestinal epithelium villi through oral ingestion of contaminated food and water. Here, toxin co-regulated pili (TCP) are expressed for adhesion and colonization, and cholera toxin (CT) is expressed, which leads to typical watery diarrhea and allows bacteria to escape from the host. V. cholerae exercises its pathogenic ability mainly through CT and TCP.Citation2,Citation3 However, to achieve intestinal colonization, bacteria must first overcome multiple stressors such as reactive oxygen species (ROS) and reactive nitrogen species (RNS),Citation4,Citation5 low pH during stomach passage,Citation6 exposure to bile salts,Citation7 short-chain fatty acid salts (SCFAs),Citation8 and antimicrobial peptidesCitation9 produced by the host and its gut microbiota.
There is increasing evidence that crosstalk between the gut microbiota and V. cholerae plays an important role in its pathogenesis.Citation10–13 Using an artificially defined healthy microbial community in mice, Hsiao et al.Citation14 showed that Blautia obeum (former named Ruminococcus obeum) suppresses several key colonization factors of V. cholerae by upregulating luxS expression and increasing AI-2 production during V. cholerae infection. Zhao et al.Citation15 revealed that V. cholerae could kill intestinal commensal bacteria isolated from infant mice via modulators expressed by the type 6 secretion system (T6SS), while upregulating virulence gene expression and enhancing intestinal colonization. Pauer et al.Citation16 revealed that some intestinal commensal bacteria suppressed the motility of V. cholerae from the transcriptome of V. cholerae in human fecal supernatants. Chen et al.Citation17 revealed the colibactin-dependent killing of V. cholerae by Escherichia coli through the isolation of murine commensal bacteria. However, a rapid and convenient method to study V. cholerae gene expression in a complex microbial context is still lacking.
Transcriptomic sequencing can identify transient changes at the transcriptional level within bacterial cells, and this can be used to explore pathogenic mechanisms. Transcript data of V. cholerae during human colonization were originally obtained using microarrays,Citation18,Citation19 which were complemented with RNA sequencing (RNA-seq) data from bacteria-colonizing infant mice and rabbits.Citation20,Citation21 Thus, a vast amount of information on the gene expression profiles of V. cholerae under these different conditions was obtained. To date, most transcriptomic studies of V. cholerae in hosts have been performed using germ-free or antibiotic-treated animals or animals with underdeveloped gut microbiota, such as suckling mice and rabbits, thereby neglecting the role of the host’s gut microbiota. It is now recognized that a fully developed gut microbiota is involved in many important organismal activities. The natural gut microbiota of certain hosts can result in colonization resistance toward V. cholerae, which is an obstacle to studying intestinal colonization by V. cholerae in natural hosts.
Studies on adult mice have primarily investigated the response of V. cholerae to the host environment and the effects of ROS,Citation22,Citation23 RNS,Citation24 bacterial metabolites,Citation25,Citation26 and the immune systemCitation27 on the pathogen.
Results
Construction and characterization of magnetic chitin beads (MCB)
Magnetic Fe3O4 nanoparticles (MNP) were obtained by high-temperature oil-phase cracking,Citation28,Citation29 and magnetic chitin beads (MCB) were prepared using an electrostatic spray techniqueCitation30 (). The morphology of MCB in a fully swollen state and in a dehydrated state was investigated by light microscopy () and scanning electron microscopy (). The obtained average particle size of 121 μm () was suitable for loading their surface with bacteria and would allow feeding to an animal host by gavage. The produced MCB displayed good magnetic responsiveness and could be fully absorbed by the magnetic frame within ~ 3 s when wrapping MNP above 160 μL/mL (). In the hysteresis curve measurements, the MCB also exhibited a standard magnetization profile (), although its magnetic strength was slightly lower than that of the MNPs. Characterization of the MNPs and MCB by Fourier transform infrared spectroscopy (FTIR) displayed a characteristic peak for the latter near 3480 cm−1, consistent with chitin ().
Figure 1. Construction and characterization of the magnetic chitin beads (MCB). (a) schematic diagram of the MCB synthesis. (b) bright field microscopic image of MCB in 1×PBS solution. Scale bar: 100 μm. (c) scanning electron microscope image of MCB. Scale bar: 100 μm. (d) MCB particle size distribution. (e) magnetic rack adsorption time of MCB encapsulating different concentrations of MNP. (f) hysteresis curves of MCB and MNP. (g) FTIR spectrums of MCB, MNP and pure chitin beads. (h) viable counts of V. cholerae co-cultured with MCB, MNP and chitin beads; glass beads were used as a control. (I) the viability of human cell lines HT29 and LS174 after 24h treatment with the indicated additives. Asterisks indicated statistically significant differences compared to the blank by t-test (**p < .01, ***p < .001, ns not significant).
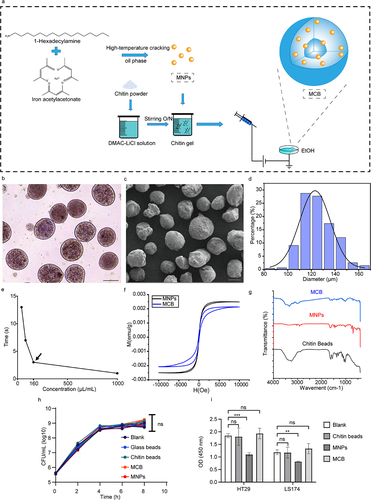
To investigate whether MCB has any unwanted effects on the interactions between V. cholerae and host cells, we tested their biocompatibility with V. cholerae and two enterocyte cell lines. The results showed that MCB did not impair the growth of V. cholerae () or enterocyte cell lines HT29 and LS174, although MNP were toxic to the cell cultures (). To investigate whether the harsh environment of the gastrointestinal system impairs MCB, we tested its stability in vitro in artificial gastric and intestinal fluids. This showed that MCB was highly resistant to low pH and proteases (Figure S1). In Luria-Bertani (LB) medium, MCB was completely stable, and in the presence of the bacteria (which can digest chitin) stability was demonstrated for a period of 8 h (Figure S2a). In summary, the produced MCB exhibited good magnetic responsiveness. They are biocompatible and resistant to low pH conditions and proteases. Their size allowed application by oral gavage, enabling noninvasive introduction into the host, so we considered that stable presence in vivo and targeted recovery from the intestine would be feasible.
V. cholerae biofilm coating to produce vcMCB for in vivo studies
In marine environments, V. cholerae forms biofilms on the chitin of crustaceans, using chitin both as a support and as a carbon and energy source.Citation31 It is therefore not surprising that the bacteria were able to colonize the MCB and form surface biofilms, containing 8.8 × 108 CFU/mL after 8–10 h of inoculation (). We used V. cholerae harboring a plasmid expressing green fluorescent protein (p-GFP) to enable visualization by confocal microscopy. This indicates that the bacteria were attached to the surface of the chitin beads (). The fluorescence of the beads significantly increased as a result of V. cholerae coating (). SEM micrographs confirmed that V. cholerae bacteria were attached to the surface of chitin-covered beads, where they exhibited biofilm-like aggregation (). The attached bacteria could be separated from those loosely present by washes, and after release of the attached bacteria by vortexing, they could be quantified by CFU plating (Figure S2b).
Figure 2. Characterization of the V. cholerae biofilm-coated magnetic chitin beads (vcMCB). (a) viable counts of V. cholerae on vcMCB. (b) confocal fluorescence microscopy showing fluorescent V. cholerae around the surface of vcMCB. Scale bar: 50 μm. (c) quantitative comparison of fluorescence of MCB and vcMCB. Significance by t-test is indicated (***p < .001). (d) SEM images of the surface of MCB (top) and vcMCB (bottom) at two magnifications, (left and right, as indicated). V. cholerae are seen adhering to the surface of vcMCB. (e) viable bacterial counts obtained with two methods for bacterial recovery from the intestine of adult mice dosed with vcMCB. Significant difference of magnetic bead recovery compared to PEG by t-test is indicated (**p < .01.) (f) quantification of live bacteria recovered from the mouse intestine, based on gene expression of bacterial 16S rRNA (total bacteria) and of V. cholerae-specific gyrB. β-actin was used as the internal reference gene. PBS buffer recovery method was used as a control. Statistical significance compared to PEG by t-test is indicated (*p < .05).
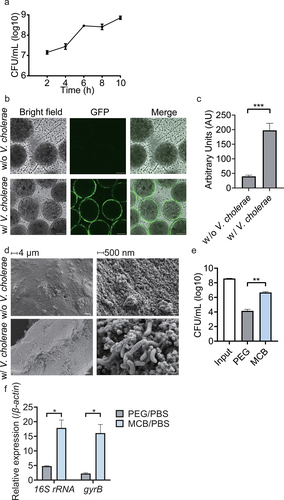
The vcMCB were gavaged to adult CD-1 mice and after 2 h the animals were sacrificed and the beads were recovered from the removed intestine. The most effective extraction of bacterial RNA was compared between magnetic bead recovery and polyethylene glycol (PEG) purification, a common method for isolating microorganisms such as viruses, bacteria from animal and plant tissues based on gradient centrifugation.Citation32–35 The results showed that the number of viable bacterial cells recovered from vcMCB after exposure to the murine intestine was significantly higher than that recovered using PEG ().
Since samples obtained from the host intestine usually contain host cells and a number of other microorganisms, we compared the abundance of total bacteria and V. cholerae within the vcMCB and PEG samples, for which the isolated RNA was assessed for the presence of bacterial 16S rRNA and V. cholerae-specific gyrB by qPCR. The results, shown in , showed that vcMCB-RNA contained both higher fractions of total bacterial RNA (16S rRNA amounts relative to internal standard β-actin) and V. cholerae-specific gyrB, compared to PEG-isolated samples.
Whether vcMCB system causes additional transcriptional interference needs to be assessed using infant rabbits. The animals were dosed with vcMCB and the beads were recovered from the intestines 2 h after gavage. The transcripts of five V. cholerae genes previously shown to be involved in rabbit colonization were assessed. The results showed that tcpA, ctxA, vc0841, vc1042 and vc2231 were all detected (Figure S2c), with trends similar to those reported in the literature.Citation21 This was used to validate the experimental system.
The V. cholerae transcriptome during residence in the intestines of adult mice in presence of their natural microbiota
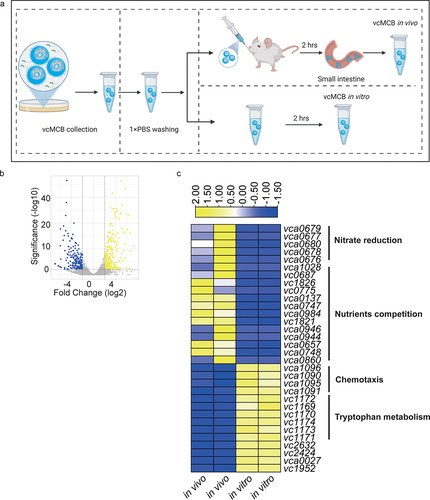
The established vcMCB system was used to investigate gene expression in V. cholerae during residence in a host gastrointestinal environment, for which we used adult mice with an undisturbed intestinal microbiota (Figure S3). The transcripts were compared with the transcriptomes of in vitro vcMCB cultures (). To ensure the reliability of our data, differentially expressed genes were identified using a threshold of | logFC |>2. No differences in log2 (FPKM +1) were identified, although the relative transcription levels varied between in vivo and in vitro treatments (Figure. S3). The log2 fold change in bacterial transcripts was captured in a volcano plot (). A total of 569 differentially expressed genes (DEGs) were identified, of which 291 were upregulated and 278 were downregulated in vivo. Functional enrichment analysis revealed that significantly upregulated genes were related to the nitrate reduction pathway or nutrient competition (). These pathways have been reported to be associated with the intestinal colonization of V. cholerae. Significantly downregulated genes were related to chemotaxis and tryptophan metabolism (). The observed expression patterns of nitrate reduction and chemotaxis pathways were consistent with data from human samples of clinical cholera infections.Citation18
Figure 3. Comparative analysis of the V. cholerae transcriptome in adult mice with a complete intestinal microbiota. (a) schematic figure of samples collection. MCB were cultured in LB medium for 8 hours to form vcMCB. vcMCB were collected using a magnet and transferred to centrifugal tubes. The vcMCB was washed 3 times using 1× PBS buffer to remove planktonic cells. A portion of the obtained vcMCB was gavaged to mice. After incubation in vivo for 2 hours, mice were sacrificed to obtain the entire small intestine. The vcMCB in vivo samples were recovered using a magnet after longitudinal sectioning. The other portion of vcMCB was kept in 1×PBS buffer for 2 hours and subsequently recovered as vcMCB in vitro sample. (b) volcano plots showing fold-change differences in transcripts of vcMCB compared to in vitro vcMCB cultures. The negative log10 (adjusted p-value) is plotted against the log2FC on the x-axis. The colored genes had an FDR < 0.05. (c) expression heatmap of the top 20 up- and down-regulated differentially expressed genes (DEGs), with the annotation of gene function listed to the right of the heatmap.
Because we focused on the interaction between V. cholerae and gut microbiota, we checked our DEGs with data from the literature obtained from microbiota-undeveloped animalsCitation21 and human infection.Citation18 Surprisely, we found that 7 up-regulated DEGs and 39 down-regulated DEGs in our study were also observed the same expression trend in microarray-based transcriptome study of V. cholerae recovered from human infection when compared to V. cholerae grown in vitro, but with a different expression pattern in infant rabbits and infant mice when compared to V. cholerae grown in vitro.
Identification of functional genes in V. cholerae responding to the gut microbiota
Next, we summarized the in vivo DEGs in hosts containing microbiota (vcMCB and human samples) and those with limited microbiota (infant mice and infant rabbits). Eight genes that were consistently expressed in opposite directions under these two conditions were identified (Table S4). We considered that these genes might be associated with the response of V. cholerae to the resident microbiota.
To verify this hypothesis, we constructed V. cholerae mutant strains that overexpress each of the identified genes. These mutants were gavaged to infant mice as bacterial suspensions, together with the wild-type strain, at an equal ratio to allow in vivo competition. After 18 h, the wild-type and mutant bacteria present in the intestinal fragments were quantified. This demonstrated that there were no colonization defects in the overexpressing mutants for any of these genes in the presence of a limited gut microbiota in these infant mice (). Only overexpression of vca0880 resulted in a slightly lower mutant/wild-type ratio.
Figure 4. A microbiota recovery mouse model demonstrates functional genes in V. cholerae that respond to presence of gut microbiota. (a) infant CD1 mice were administrated with vcMCB containing a mixture of wild-type V. cholerae and the indicated overexpressing mutant at a ratio of 1:1. Intestinal samples were collected at 18 h post inoculation and after quantitation of bacterial loads the ratio of mutant to wild-type was calculated to give the competition index (CI). (b) the gut microbiota of four-week-old CD-1 mice was disrupted by streptomycin prior to V. cholerae dosage. Sm+/+ mice continued to receive streptomycin in their drinking water, while Sm+/- mice were allowed to restore their gut microbiota 14 h after dosage of the bacteria. All animals received a mixture of wild-type and the indicated overexpressing mutant. The CI of mutant/wild-type detected in fecal pellets 5 days post gavage is shown, normalized with the input ratio. Significance by t-test is indicated (ns, not significant, *p < .05, ****p < .0001).
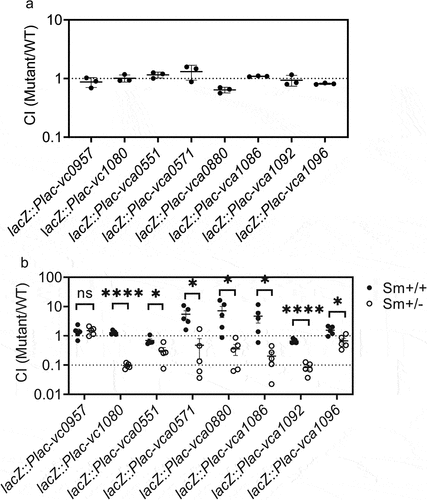
Next, we examined whether overexpression of these genes affected colonization in mice disrupting intestinal microflora. The animals were pretreated with streptomycin, and one group of mice received streptomycin during the complete experiment (Sm+/+ mice), while the second group received normal drinking water 12 h after inoculation with the V. cholerae mixture (Sm+/- mice). The model we used was introduced from previous studies.Citation26,Citation36 Their data reveals that the microbiota load in Sm+/+ mice had a significant decrease, although not completely eliminated, providing an opportunity for V. cholerae to enter the host. And the microbiota load in Sm+/- mice had a significant increase compared to Sm+/+ mice. The mutant to wild-type ratio was determined in fecal pellets on day 5. Most overexpressing mutants showed a significant decrease in colonization ability in the disruption of microbiota (Sm+/+ mice) compared to conditions allowing microbiota recovery (Sm+/- mice), whereby the effect of overexpression of vca1080 and vca1092 was highly significant (). The exception was lacZ::Plac-vc0957, in which no effect was observed. These data indicate that V. cholerae interacts with microbiota by downregulating vc1080, vca0551, vca0571, vca0880, vca1086, vca1092, and vca1096.
We subsequently tested in vitro whether the overexpression of these genes affected the growth, biofilm formation, motility, and ROS sensitivity of the bacteria. No differences were observed in growth or biofilm formation (Figure S4a, c). However, vca0571 overexpression resulted in a significant reduction in motility (Figure S4b), whereas vc0957 and vca0880 overexpression significantly increased H2O2 sensitivity compared with the wild-type (Figure S4d). We also investigated whether there was a difference in the adhesion ability of different mutants on MCB to exclude the effect of MCB on gene expression. The results showed no significant difference between the mutants and the wild-type (Figure S4e). These data suggest that the intestinal microbiota may influence the physiological phenotype of V. cholerae, including motility and ROS resistance, by regulating the expression of the identified genes.
V. cholerae resists low pH and formic acid stress through down-regulation of gene vc1080
To determine whether the interaction was mediated by direct contact with bacteria or by active metabolites produced by microbes, we examined this competitive defect in vitro using Sm+/- mouse fecal supernatants. The results show that the metabolites exhibited an inhibitory state against all seven mutants (). That is, the active metabolites produced by the gut microbiota-mediated interactions are associated with changes in the expression of these seven genes in V. cholerae.
Figure 5. V. cholerae resists low pH and formic acid stress caused by A. caccae and D. formicigenerans through down-regulating vc1080. (a) competition index of mutants in fecal supernatants of Sm+/+ and Sm± mice. The fecal droppings were collected, homogenized and centrifuged. The resultant supernatants were filtered and added to a 1:1 mixture of WT and overexpression mutant. Significance by t-test is indicated (***p-value < .001). (b) LEfSe analysis of Sm+/+ mice vs. Sm+/- mice. LDA score processed by log. (c) viability of V. cholerae wild-type and overexpression mutants during co-culture with the intestinal bacteria under anaerobic conditions for 6h. The ratio indicated by the heat map color mean to indicate relative growth of V. cholerae strain co-cultured with gut bacteria compared to V. cholerae strain cultured alone. (d) SCFAs content in fermentation broth of A. caccae, L. murinus and L. reuteri. Significance by t-test is indicated (****p < .0001). (e) CFU of V. cholerae wild-type and mutant lacZ::Plac-vc1080 after 4 h of anaerobic growth in medium containing HCl, formic acid or acetic acid at a pH of 5.7. Control medium at pH 7 was included. Significance to wild-type by t-test is shown (*p-value < .05). (f) Sm+/+ mice were challenged by a mixture of wild-type and mutant lacZ::Plac-vc1080 while their drinking water contained 0.9 μg/mL formic acid and streptomycin for the whole experiment. A second group of Sm+/+ mice were inoculated with the same mixture but in combination with A. caccae Strr. The control of adult mice treated by Sm was included. CI values are based on fecal droppings collected after 5 days post gavage. Significance by t-test is indicated (****p < .0001). (g) viability of V. cholerae wild-type and overexpression mutants during co-culture with D. formicigenerans under anaerobic conditions for 6 h. Significance to wild-type by t-test is shown (*p-value < .05). (h) Sm+/+ mice were challenged by a mixture of wild-type and mutant lacZ::Plac-vc1080 in combination with D. formicigenerans Strr. The control of adult mice treated by Sm was included. CI values are based on fecal droppings collected after 5 days post gavage. Significance by t-test is indicated (**** p < .0001).
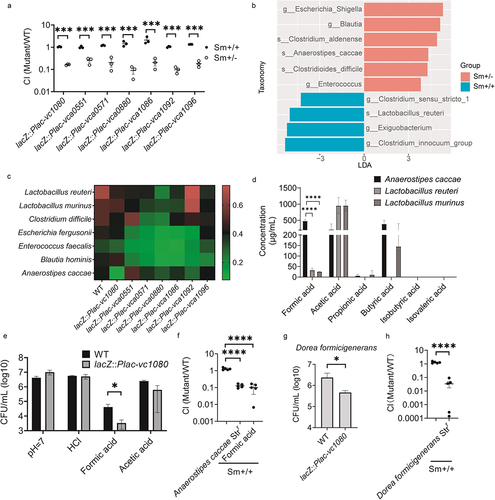
To clarify which gut bacteria are involved in the interaction process, we used 16S rRNA gene amplicon sequencing to identify different microbes in the feces of Sm+/+ and Sm+/- mice based on linear discriminant analysis Effect Size (LEfSe, v.1.1.2) with a LDA score cut off > 3.0Citation37. The results showed that Sm± mice were enriched in Escherichia_Shigella spp., Blautia spp., Enterococcus spp., Clostridium aldenense, Anaerostipes caccae and Clostridioides difficile, whereas Sm+/+ mice were enriched in Clostridium sensu stricto 1, Clostridium innocuum group spp., Exiguobacterium, and Lactobacillus reuteri (). Combined with culturomics, we obtained the above differential species and representative strains from different genera. By co-incubating the gut bacteria with V. cholerae mutants in vitro, we tested whether these bacteria were antagonistic to V. cholerae mutants. Among them, Lactobacillus murinus and L. reuteri, which were enriched in Sm+/+ mice, showed almost no antagonistic effect on the mutants (except for L. reuteri with the vca0880 overexpression strain). In contrast, C. difficile, Escherichia fergusonii, Enterococcus faecalis, Blautia hominis and A. caccae, which were enriched in Sm+/- mice, showed some antagonism to different mutants. Notably, A. caccae exhibited the most significant antagonistic effect on the vc1080 overexpression strain ().
Active metabolites produced by the gut microbiota include SCFAs, bile acids, and bacteriocins, of which SCFAs are common fermentation products of microbes.Citation25 It has also been shown that SCFAs are involved in colonization resistance of V. cholerae.Citation8 Therefore, we examined SCFAs products from L. murinus and L. reuteri, which were not antagonistic to vc1080 overexpression strain, and A. caccae. The results showed that the formate concentration in the fermentation broth of A. caccae was significantly higher than that in L. murinus and L. reuteri ().
Through in vitro acid tolerance assays, we also found that the vc1080 overexpression strain was more sensitive to solutions of formic acid than the wild-type strain, but not to the inorganic acids HCl or acetic acid (). We further validated the phenotype by pre-colonizing A. caccae Strr in the Sm+/+ mouse model, followed by gavage of the wild-type and vc1080 overexpression strains. We found that vc1080 overexpression strains had the same colonization defect as that in Sm+/- mice. Furthermore, vc1080 overexpression strain showed the same colonization defect by gavage with formate added to drinking water (). Thus, A. caccae antagonizes V. cholerae colonization in the mouse gut by producing secreted formic acid, whereas V. cholerae reduces its susceptibility to formic acid by downregulating vc1080.
We examined microbiome function in both animal models and found that the hexanol fermentation pathway, which produces formic acid, lactic acid, acetic acid, and alcohol, was significantly enriched in Sm+/- mice (Figure S5a). Because formate is involved in the TCA cycle, bacteria contain formate at varying concentrations intracellularly, but the secretion of formate requires formate transporter proteins. We examined the relative abundance of focA and focB for formic acid transporter proteins by qPCR and found that the transporter protein genes were significantly higher in Sm+/- mice than in Sm+/+ mice (Figure S5b).
Dysfunctional formate production was also present in the cholera-infected human microbiome dataset.Citation14 At the same time, the formic acid producer, Dorea formicigenerans was significantly enriched in patients recovering from V. cholerae compared to the infection period. Therefore, we co-incubated the vc1080 overexpression strain in vitro with the human gut microbe D. formicigenerans and found that vc1080 overexpression strain was more sensitive to D. formicigenerans than the wild-type strain (). Similarly, D. formicigenerans Strr was pre-colonized in Sm+/+ mice and then gavaged with the wild-type and vc1080 overexpression strain. We found that vc1080 overexpression strain had the same colonization defects (). In other words, V. cholerae interacts with formate producers by downregulating the expression of vc1080 in both humans and mice.
The response to oxidative stress was compared between the wild-type and mutant lacZ::Plac-vca0880 after exposure to H2O2. A number of genes involved in oxidative stress were more strongly expressed in the mutant than in the wild-type following H2O2 treatment; however, the protein activities of peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD) were not significantly different between the wild-type and the mutant in the presence or absence of H2O2. However, there was a trend towards higher CAT activity in the wild-type than in the mutant in the presence of hydrogen peroxide, possibly suggesting that vca0880 is associated with the regulation of hydrogen peroxide enzyme activity (Figure S6). Similarly, no differences in the known acid stress response were identified when tested in the presence of formic acid, and the genes involved in classical acid resistance pathways in V. cholerae, cadC, toxR, napA, and nhap1, did not reveal differences in expression between the wild-type and the mutant overexpressing vc1080 (Figure S7). We conclude that vc1080 may protect V. cholerae from formic acid stress through a novel acid-tolerance response mechanism.
Discussion
In this study, we dissected the transcriptional changes in V. cholerae during infection in a mouse model while reducing host and gut microbiota interference in in vivo transcriptome studies. Chitin beads containing magnetic Fe3O4 nanoparticles coated with bacteria were used. This vcMCB is safe for the host and allows efficient dosage and targeted recovery, with efficient enrichment of V. cholerae for transcriptomic analysis. Thus, it provides a simple and powerful method for studying the interactions between gut microbiota and pathogens. Using the developed vcMCB, we were able to achieve in situ V. cholerae recovery in the gut and simulated the changes in V. cholerae when entering the host as biofilms on a natural support, chitin, of crustaceans. Based on transcriptome changes, we also identified several functional genes whose expression was regulated in response to the intestinal microbiota and revealed that the bacteria resist low pH and formic acid stress by downregulating the gene vc1080.
In the intestinal environment of adult mice, a well-established intestinal microbiota is more complex than that of the infant mouse, and this poses a barrier to the study V. cholerae colonization in adult mice. Previous studies have solved this problem by using human fecal supernatants to simulate the intestinal environment in vitro;Citation16 however, this ignores host factors and the complex dynamics of the intestinal environment. Our work describes, for the first time, the transcriptome of V. cholerae in adult mice with developed microbiota. The chitin-covered beads maintained V. cholerae activity and allowed effective enrichment and recovery from a complex intestinal environment, providing usable RNA-seq data. Compared to the in vivo transcriptome data from infant rabbits and infant mice, our in vivo samples shared some differentially expressed genes with microarray based transcriptomic data of V. cholerae from human stool.
The detected differentially expressed genes included upregulated genes involved in the nitrate reduction pathway and in the nutrient competition pathway, which were similar to human microarray data,Citation18 whereas infant rabbit and infant mouse studies failed to demonstrate the involvement of the nitrate reduction pathway.Citation21 Nitrate reductase is important for the anaerobic respiration of V. cholerae in vivo, and V. cholerae regulates the nitrate reduction pathway in a pH-dependent manner to achieve successful colonization in the intestine.Citation38 In addition, nitrate reductase is important for intestinal colonization and expansion of most gram-negative pathogens, and the NO3− that accumulates under inflammatory conditions can act as a substrate for nitrate reduction, supporting the growth of pathogens while promoting their defensive capacities toward antioxidants, antibiotic resistance, and enhancing bacterial virulence.Citation39
A recent studyCitation16 found that bioactive metabolites of the gut microbiota can inhibit the motility and chemotaxis-related genes of V. cholerae to hinder the penetration of V. cholerae into mucin. We also demonstrated the inhibition of V. cholerae chemotaxis and motility by the active metabolites of the gut microbiota. In addition, we unexpectedly found that tryptophan metabolism was also inhibited in the presence of the gut microbiota, which may be related to the involvement of the tryptophan metabolite indole in regulating the vital activity of V. cholerae. Indole appears to inhibit the expression of V. cholerae virulence factors in a dose-dependent manner.Citation40 Cholera autoinducer-1 (CAI-1), an indole derivative, is an important population-sensing signal of V. cholerae and has been shown to promote hapR expression to inhibit virulence factor expression and biofilm formation.Citation41 We propose that, in the complex intestinal microenvironment, V. cholerae may enhance virulence factor expression by inhibiting tryptophan metabolism; however, this hypothesis has yet to be experimentally tested.
This study focused on functional genes that may respond to the gut microbiota and its metabolites. Transcriptome comparisons identified several candidate genes for further studies: vc0957 codes for a zinc ribbon-containing protein, the product of vc1080 contains a Hpt domain, vca0551 and vca0880 give hypothetical proteins, vca0571 produces a DUF3319 domain-containing protein, vca1086 gives a SpoIIE family protein phosphatase, vca1092 was identified as HAMP domain-containing protein, and vca1096 was identified to code for a response regulator. Finally, vca1092 and vca1096 are chemotaxis-related genes. Mutants overexpressing these genes could colonize infant mice assays; therefore, colonization in the absence of a fully developed gut microbiota was not impaired. This was validated by in vitro competition assays using fecal supernatants and in vivo Sm+/- colonization models (). The results showed that overexpression of all genes, except vc0957 resulted in defective colonization in the presence of gut microbiota. We were interested in which gut bacteria the genes were responding to; therefore, we used microbiome data from two animal models to identify different bacteria. In vitro co-culture experiments were also used to test for gene-bacteria interactions. Interestingly, the seven mutants showed a more sensitive phenotype than the wild-type for several bacteria enriched in microbiota recovery mice. Based on the strength of its killing ability, we selected a combination of A. caccae and vc1080 for further study. After excluding the involvement of classical acid resistance pathways via cadC, toxR, napA, and nhap1 in V. cholerae, we conclude that vc1080 may protect V. cholerae from formic acid stress through a novel acid tolerance response mechanism. Combined with human data, we found that formic acid producers (human or mouse sources) both showed more significant killing and reduced intestinal colonization levels of vc1080 overexpression strains. Therefore, we suggest that V. cholerae may downregulate vc1080 to improve formic acid resistance. We also found that the genes vca0880 and vca0571 may be involved in ROS resistance and motility, respectively, based on a phenotypic screen. We have also identified several gut bacteria with stronger killing phenotypes for these two mutants, and we will next investigate whether the ROS resistance and motility of the two mutants are involved in these interactions.
The product of vc1080 is predicted to be a phosphotransferase of a two-component signal transduction system, which may be involved in multiple stress responses to regulate downstream gene expression against environmental changes. The involvement of vca0880 in ROS resistance was assumed, but no effect was observed in the overexpression mutant in the presence of H2O2. We subsequently examined enzyme activity and found lower levels of catalase production in this mutant than in the wild-type, although not reaching statistical significance (Figure S6); therefore, it remains to be seen whether vca0880 affects ROS resistance in V. cholerae by influencing the level of catalase enzyme activity.
Our approach has some shortcomings, as the transcriptome does not completely mimic in vivo studies of clinical isolates. Moreover, the presence of enterobacterial RNA can lead to a low practical availability of RNA sequencing data. Up to date, studies of V. cholerae interacting with gut microbes are scant. Animal models currently used for this study include Grem-free mice,Citation14 antibiotics cocktail treated mice,Citation42 and streptomycin treated mice.Citation26,Citation36 The streptomycin treated mice model was used in this study. The reliability of experimental results can be ensured by using multiple models for cross-validation. Also, this method used V. cholerae in biofilm state. The study of planktonic cells in a complex intestinal environment cannot be performed using this model for the time being. Nevertheless, we demonstrated that the in situ V. cholerae transcriptome can be studied in a host with fully developed intestinal gut microbiota. This recoverable MCB system provides a new approach for intestinal transcriptome studies and can be extended to other pathogenic bacteria. Our study provides a practical method to increase our understanding of the interaction between V. cholerae and host gut microbiota.
Materials and methods
Ethics statement
Animal experiments were performed using protocols approved by the Ethical Committee of the Huazhong University of Science and Technology (Permit Number: SYXK (E) 2016–0057).
Bacteria strains, plasmids, and growth conditions
The strains and plasmids used in this study are shown in Supplementary Table S1. All the primers used are listed in Supplementary Table S2. V. cholerae EI Tor C6706Citation43 was used as the parental strain and propagated in Luria-Bertani (LB) media with appropriate antibiotics for plasmid selection (100 μg/mL streptomycin or ampicillin) at 37°C unless stated otherwise.
Synthesis and characterization of magnetic chitin bead
Magnetic Fe3O4 nanoparticles (MNP) was prepared by the reaction of ferric acetylacetonate, HDA, and oleic acid, as previously described.Citation29 Chitin was dissolved by overnight stirring in DMAC-LiClCitation44 and the MNP were subsequently dispersed. Chitin beads were produced similarly but in the absence of MNP. Using electrostatic spraying, the solution was dispersed into small droplets and cured in anhydrous ethanol for 24 h to form microspheres. The microspheres were soaked in ultrapure water for 48 h, washed twice with ultrapure water, and sterilized by autoclaving. The morphology of the MCB was observed using scanning electron microscopy (SEM; Sigma300, Zeiss, Germany) and confocal laser scanning microscopy (CLSM; FV3000, Olympus, Japan). Micrographs under bright-field light using an inverted fluorescence microscope were recorded using ImageJ, and the diameter of 100 particles was determined to calculate the size distribution. The functional groups were identified using Fourier transform infrared spectroscopy (FTIR; Nicolet iN10, Thermo Scientific, USA). Magnetic properties were measured using a vibrating sample magnetometer (VSM, MPMS-VSM, and MPMS-XL, Quantum Design, USA). The stability of MCB was first tested in LB medium in the absence of bacteria, and then in their presence. The stability toward gastric secretion was tested in the presence of 10 g/L pepsin and 1.5% HCl (v/v) in phosphate-buffered saline (PBS) at a pH of 2.0, and intestinal fluids were mimicked with 10 g/L trypsin and 6.8 g/L K2HPO4 in PBS (pH 6.8).
Biocompatibility tests
The human colonic cell lines HT29 and LS174 (both kind gifts from Prof. Chenhui Wang’s Laboratory) were cultured in Dulbecco’s modified Eagle’s medium (DMEM GlutaMAX, Gibco, Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin (Gibco) in a 5% CO2-incubator at 37°C. Biocompatibility was tested with 200 µL of cell cultures in 96-well plates grown to 80% confluence, to which 10 µL of MCB beads, MNPs, or chitin beads were added. After 24 and 48 h, cell viability was evaluated using a cell counting kit-8 (CCK-8; Beyotime Biotechnology, C0038). For bacterial biocompatibility tests, V. cholerae was cultured in 10 mL LB medium in the presence of 500 µL of various beads, and samples were taken for viability counts every 2 h.
Coating of MCB with V. cholerae to produce vcMCB
An overnight culture of V. cholerae was diluted 1:100 in 10 mL of fresh LB medium and 500 μL of MCB was added. Following incubation at 37°C and 60–70 rpm in a shaker for 8 h, the vcMCB was collected every 2 h using a magnet. The free bacteria were removed by washing 3 times with 1×PBS. Enumeration of bacteria attached to the beads was performed by vortexing after release. ImageJ quantification was used to confirm a significant increase in fluorescence as a result of coating MCB with V. cholerae.
Recovery of vcMCB from adult mice and infant rabbits
An adult mouse model was used to test two methods of bacterial recovery following animal exposure. Five animals per group (aged 4–5 weeks) were subjected to gastric acid neutralization for 10 min by gavage with 100 μL 10% NaHCO3 followed by gavage of 500 μL vcMCB. The animals were sacrificed 2 h later and their entire small intestines were collected. A longitudinal section was transferred to 15 mL of 1× PBS, and a pipette was used to release vcMCB, followed by recovery of vcMCB using a magnet for quantitation. This was compared with PEG purification.Citation32 The amount of recovered total bacteria and viable V. cholerae was assessed for both samples (vcMCB and PEG) by qPCR targeting the 16S rRNA (16S-RT-F: 5’-CGGTAATACGGAGGGTGCAA-3’, 16S-RT-R: 5’-CACCTGCATGCGCTTTACG-3’)Citation45 and gyrB genes, respectively, using previously described methods. Values are related to the amount of β-actin detected as an internal reference gene.
A previously published infant rabbit modelCitation21 was used to assess whether MCB caused transcriptional interference. Infant rabbits (3 per group) were treated as described for the adult mice, although gastric acid neutralization was performed using ralteonidine, followed by gavage with 500 μL vcMCB. The expression levels of five genes previously described to be involved in rabbit colonization were assessed: tcpA, ctxA, vc0841, vc1042, and vc2231.
RNA sequencing and data analysis
The recovered vcMCB was washed with PBS 3 times and then frozen in liquid nitrogen and ground for RNA extraction. Total RNA was extracted using TRIzol® Reagent according the manufacturer’s instructions (Invitrogen) and genomic DNA was removed using DNase I (TaKara). RNA-seq transcriptome library was prepared following Kapa RNA hyper prep kit using 2 μg of total RNA. Paired-end RNA-seq sequencing library was sequenced with the Illumina Novaseq 6000 (Illumina, USA). Following the run, the raw sequences were aligned to both chromosomes of the V. cholerae N16961 genome (GenBank accession numbers NZ_CP028827.1 and NZ_CP028828.1). Significance analysis (| log 2-fold change | > 2 and p-value <0.05) was used to identify differentially expressed genes (DEGs). Differential expression data is in the supplementary material (Table S3).
V. cholerae mutants overexpressing genes of interest
Mutants of V. cholerae C6706 that overexpressed specific genes were constructed using a previously described method.Citation46 The target gene was amplified and cloned under the regulation of a strong promoter in the lacZ locus using the suicide vector pJL1. All mutants were confirmed using DNA sequencing and real-time PCR. The growth of the mutants was characterized in LB medium by measuring OD600 in a 96-well microplate using a microplate reader (Spark, Tecan). Samples were incubated at 37°C with shaking and readings were taken every 20 min for 20 h. Motility was assayed in soft agar, and colony diameters were recorded. Biofilm formation at the solid-liquid interface of static biphasic cultures was visualized by crystal violet staining after 24 h of incubation at 25°C in glass test tubes and photographed. Biofilms were quantified by OD570 measurements in 96-well microplates. Survival rates toward H2O2 were tested in M9 minimal medium.
Competition assays of overexpressing V. cholerae mutants in vivo
To establish the in vivo effect of the overexpression of specific V. cholerae genes, an infant mouse model was used. Five-day-old CD-1 mice (3 per group) were transferred to a 30°C incubator 2 h before inoculation. Mice were intragastrically administered 50 μL of a 1:1 mixture of wild-type and mutant V. cholerae (approximately 106 CFU for each strain per mouse) and sacrificed 18 h after gavage. Samples of the small intestine were removed and homogenized in 1.5 mL of PBS buffer, serially diluted, and plated on LB agar containing streptomycin and 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) for quantification of bacterial loads. The competition index (CI) was calculated as the ratio of mutant to wild-type colonies, normalized to the input ratio.
To establish whether overexpression of the specific V. cholerae affected colonization competition in the disturbed or stable microbiota, adult mice were used whose gut microbiome was disrupted by streptomycin treatment as previously described.Citation26 Four-week-old CD-1 mice were supplied with drinking water containing 5 g/L streptomycin and 4 g/L aspartame for 24 h. The mice were then subjected to gastric acid neutralization as described above and intragastrically administered 100 μl of a 1:1 mixture of wild-type (wild-type) and an overexpressing V. cholerae mutant (approximately 109 CFU for each strain per mouse). One day after gavage, Sm+/+ mice continued to receive streptomycin in their drinking water, whereas the second group (Sm+/- mice) received normal drinking water from day 1 after gavage. Fecal pellets were collected from each mouse at day 5 post-gavage, homogenized by bead beating, resuspended in PBS buffer, serially diluted, and plated on LB agar containing streptomycin and X-gal for quantification of bacterial loads. The CI was calculated as described above.
16S rRNA sequencing analysis
Fecal samples were freshly collected and DNA was extracted. Total DNA was extracted utilizing the HiPure Stool DNA Mini Kit and amplified with 16S V3-V4 primers to construct a library (341F 5’-CCTACGGGGNGGCWGCAG-3’, 806 R 5’-GGACTACHVGGGGTWTCTAAT-3’), with a sequencing depth of 50,000 reads using an Illumina MiSeq apparatus. Paired-end reads were analyzed and classified into amplicon sequence variants (ASV) using dada2 (1.18.0). Taxonomic assignments were determined using the SILVA 16S rRNA reference file release 138.
In vitro competition and co-cultivation assays
Cultures of intestinal isolates (Table S1) in BGI104 medium or Modified Gifu Anaerobic Medium (mGAM) after 24-36 h incubation in an anaerobic atmosphere were centrifuged, and the spent supernatant was added to growing V. cholerae wild-type cultures to establish any inhibitory effect. The deoxidized LB medium is used as the anaerobic culture medium for Vi. cholerae. To prepare this medium, transfer the LB medium to a round-bottomed flask, add 1 mg/L resazurin indicator, and deoxidize the medium with nitrogen gas while heating and boiling it. Finally, 0.5 g/L L-cysteine hydrochloride is added, and the resulting solution is transferred to an anaerobic culture bottle, sealed, and autoclaved. Anaerobic conditions are maintained using a Coy anaerobic glove box. A starter culture of wild-type V. cholerae (OD600 = 1, approximately 109 CFU/mL) was diluted 1:100 and co-cultured with these strains for 6 h. The overexpressing mutants were also co-incubated with the intestinal strains for 6 h, and relative growth ratios were obtained compared to V. cholerae strain grown alone.
Fecal pellets were collected from each four-week-old CD-1 mouse, homogenized by bead beating, and resuspended in PBS. The samples were centrifuged at 8000 rpm for 5 min, and the supernatant was filtered through a 0.22-µm membrane. A co-culture of a 1:1 mixture of wild-type and mutant V. cholerae (approximately 106 CFU per tube) was inoculated with 1:100 (v/v) fecal supernatant. Following incubation at 37°C for 12 h, bacterial loads were quantified by serial dilution and plating on LB agar containing streptomycin and X-gal, after which the CI was calculated as above.
The effects of formic acid and acetic acid on the growth of V. cholerae wild-type and mutant lacZ::Plac-vc1080 were assessed in non-fermentable M9 medium containing succinic acid. Formic acid or acetic acid was added until a pH of 5.7 was reached. The cultures were incubated under anaerobic conditions for 4 h. A control with HCl was also included.
SCFA measurement
Pre- and post-fermentation samples were centrifuged at 13,000 g, 4°C for 10 min, and supernatants collected for SCFA analysis. The concentrations of acetic acid, propionic acid, isobutyric acid, butyric acid, and isovaleric acid were measured using gas chromatography (SHIMADZU, GC-2010Pro). For formic acid, the concentrations were measured using high-performance liquid chromatography (SHIMADZU, LC-20AT).
In vivo effect of formic acid on V. cholerae colonization
Four-week-old Sm+/+ mice were inoculated with a 1:1 mixture of V. cholerae wild-type and mutant lacZ:Plac-vc1080. The mice were provided drinking water containing 0.9 μg/L formic acid throughout the challenge. Fecal pellets were collected 5 days post-gavage, and bacterial loads and CI were as before. A second group of Sm+/+ mice was treated similarly, but inoculated with the wild-type/mutant mixture and A. caccae Strr or D. formicigenerans Strr.
Expression analysis of targeted enzymes following ROS exposure and acid stress
V. cholerae wild-type and mutant lacZ::Plac-vca0880strains were grown in M9 minimal medium to mid-log phase and exposed to 1 mM H2O2 for 10 min, after which RNA was extracted. Using real-time qPCR, the relative expression of eight key genes encoding oxidative stress-related enzymes was determined using gyrB as the reference. Hieff® qPCR SYBR Green Master Mix (Yeasen Biotechnology Co., Ltd.) was used for qPCR. Superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) activities were determined in crude cell extracts. Similar experiments were performed for treatment with formic acid (pH 5.7, 30 min), after which the activities of cadC, toxR, nhap1, and napA were assessed.
Statistical methods
All calculations were performed using SPSS Statistics 26 (IBM).
Contributions
ZXQ, JH and ZL designed and completed the study. ZXQ and XMY conducted bioinformatics analysis. ZXQ, WB, GZC, YM, XMY, CRY, and ML performed experiments. ZXQ, GZC, YM, XMY, and ML helped with the sample collection. ZXQ drew charts and wrote the manuscript. ZL supervised the project. All the authors approved the final version of the manuscript.
Provenance and peer review
Not commissioned; externally peer reviewed.
Supplemental Material
Download ()Acknowledgments
This work was supported by the National Key R&D Program of China (2019YFA0905600) and National Natural Science Foundation of China (31770132 and 81873969). We are grateful to the mice for their contribution to this study. We thank the cell line support of Dr Chen-Hui Wang from Huazhong University of Science and Technology. We thank Dr. Fan Jin for plasmid support from the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences. We thank the Research Core Facilities for Life Science (RCFLS) at Huazhong University of Science and Technology for their assistance with confocal microscopy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. The datasets presented in this study can be found in the online repositories. The names of the repository/repositories and accession numbers (s) are PRJNA980451 and PRJNA980554 in the SRA database.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2023.2274125
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- WHO. Cholera annual report 2020 weekly epidemiological record 37 September 2021. Sep 2021;96:445–18. https://www.who.int/health-topics/cholera#tab=tab_1.
- Faruque SM, Mekalanos JJ. Pathogenicity islands and phages in vibrio cholerae evolution. Trends Microbiol. 2003;11(11):505–510. doi:10.1016/j.tim.2003.09.003.
- Fan F, Liu Z, Jabeen N, Birdwell LD, Zhu J, Kan B. Enhanced interaction of vibrio cholerae virulence regulators TcpP and ToxR under oxygen-limiting conditions. Infect Immun. 2014;82(4):1676–1682. doi:10.1128/IAI.01377-13.
- Wang H, Xing X, Wang J, Pang B, Liu M, Larios Valencia J, Liu T, Liu G, Xie S, Hao G, et al. Hypermutation-induced in vivo oxidative stress resistance enhances vibrio cholerae host adaptation. PLoS Pathog. 2018;14(10):e1007413. doi:10.1371/journal.ppat.1007413.
- Davies BW, Bogard RW, Dupes NM, Gerstenfeld TAI, Simmons LA, Mekalanos JJ. DNA damage and reactive nitrogen species are barriers to Vibrio cholerae colonization of the infant mouse intestine. PLoS Pathog. 2011;7(2):e1001295. doi:10.1371/journal.ppat.1001295.
- Kovacikova G, Lin W, Skorupski K. The LysR-type virulence activator AphB regulates the expression of genes in vibrio cholerae in response to low pH and anaerobiosis. J Bacteriol. 2010;192(16):4181–4191. doi:10.1128/JB.00193-10.
- Lembke M, Pennetzdorfer N, Tutz S, Koller M, Vorkapic D, Zhu J, Schild S, Reidl J. Proteolysis of ToxR is controlled by cysteine-thiol redox state and bile salts in vibrio cholerae. Mol Microbiol. 2018;110(5):796–810. doi:10.1111/mmi.14125.
- You JS, Yong JH, Kim GH, Moon S, Nam KT, Ryu JH, Yoon MY, Yoon SS. Commensal-derived metabolites govern vibrio cholerae pathogenesis in host intestine. Microbiome. 2019;7(1):132. doi:10.1186/s40168-019-0746-y.
- Duperthuy M, Sjöström AE, Sabharwal D, Damghani F, Uhlin BE, Wai SN. Role of the Vibrio cholerae matrix protein Bap1 in cross-resistance to antimicrobial peptides. PLoS Pathog. 2013;9(10):e1003620. doi:10.1371/journal.ppat.1003620.
- Weil AA, Becker RL, Harris JB, Papasian CJ. Vibrio cholerae at the intersection of immunity and the microbiome. mSphere. 2019;4(6). doi:10.1128/mSphere.00597-19.
- Cho JY, Liu R, Macbeth JC, Hsiao A. The interface of vibrio cholerae and the gut microbiome. Gut Microbes. 2021;13(1):1937015. doi:10.1080/19490976.2021.1937015.
- Hsiao A, Zhu J. Pathogenicity and virulence regulation of vibrio cholerae at the interface of host-gut microbiome interactions. Virulence. 2020;11(1):1582–1599. doi:10.1080/21505594.2020.1845039.
- Qin Z, Yang X, Chen G, Park C, Liu Z. Crosstalks between gut microbiota and vibrio cholerae. Front Cell Infect Microbiol. 2020;10:582554. doi:10.3389/fcimb.2020.582554.
- Hsiao A, Ahmed AMS, Subramanian S, Griffin NW, Drewry LL, Petri WA, Haque R, Ahmed T, Gordon JI. Members of the human gut microbiota involved in recovery from vibrio cholerae infection. Nature. 2014;515(7527):423–426. doi:10.1038/nature13738.
- Zhao W, Caro F, Robins W, Mekalanos JJ. Antagonism toward the intestinal microbiota and its effect on vibrio cholerae virulence. Sci. 2018;359(6372):210–213. doi:10.1126/science.aap8775.
- Pauer H, Teixeira FL, Robinson AV, Parente TE, De Melo MAF, Lobo LA, Domingues RMCP, Allen-Vercoe E, Ferreira RBR, Antunes LCM, et al. Bioactive small molecules produced by the human gut microbiome modulate vibrio cholerae sessile and planktonic lifestyles. Gut Microbes. 2021;13(1):1–19. doi:10.1080/19490976.2021.1918993.
- Chen J, Byun H, Liu R, Jung IJ, Pu Q, Zhu CY, Tanchoco E, Alavi S, Degnan PH, Ma AT, et al. A commensal-encoded genotoxin drives restriction of vibrio cholerae colonization and host gut microbiome remodeling. Proc Natl Acad Sci U S A. 2022;119(11):e2121180119. doi:10.1073/pnas.2121180119.
- Merrell DS, Butler SM, Qadri F, Dolganov NA, Alam A, Cohen MB, Calderwood SB, Schoolnik GK, Camilli A. Host-induced epidemic spread of the cholera bacterium. Nature. 2002;417(6889):642–645. doi:10.1038/nature00778.
- Larocque RC, Harris JB, Dziejman M, Li X, Khan AI, Faruque ASG, Faruque SM, Nair GB, Ryan ET, Qadri F, et al. Transcriptional profiling of vibrio cholerae recovered directly from patient specimens during early and late stages of human infection. Infect Immun. 2005;73(8):4488–4493. doi:10.1128/IAI.73.8.4488-4493.2005.
- Rivera-Chavez F, Mekalanos JJ. Cholera toxin promotes pathogen acquisition of host-derived nutrients. Nature. 2019;572(7768):244–248. doi:10.1038/s41586-019-1453-3.
- Mandlik A, Livny J, Robins W, Ritchie J, Mekalanos J, Waldor M. RNA-Seq-based monitoring of infection-linked changes in vibrio cholerae gene expression. Cell Host & Microbe. 2011;10(2):165–174. doi:10.1016/j.chom.2011.07.007.
- Liu Z, Wang H, Zhou Z, Naseer N, Xiang F, Kan B, Goulian M, Zhu J. Differential thiol-based switches jump-start vibrio cholerae pathogenesis. Cell Rep. 2016;14(2):347–354. doi:10.1016/j.celrep.2015.12.038.
- Liu Z, Wang H, Zhou Z, Sheng Y, Naseer N, Kan B, Zhu J. Thiol-based switch mechanism of virulence regulator AphB modulates oxidative stress response in vibrio cholerae. Mol Microbiol. 2016;102(5):939–949. doi:10.1111/mmi.13524.
- Stern AM, Hay AJ, Liu Z, Desland FA, Zhang J, Zhong Z, Zhu J. The NorR regulon is critical for vibrio cholerae resistance to nitric oxide and sustained colonization of the intestines. mBio. 2012;3(2):e00013–12. doi:10.1128/mBio.00013-12.
- Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–478. doi:10.1038/s41575-019-0157-3.
- Liu M, Hao G, Li Z, Zhou Y, Garcia-Sillas R, Li J, Wang H, Kan B, Zhu J. CitAB two-component system-regulated citrate utilization contributes to vibrio cholerae competitiveness with the gut microbiota. Infect Immun. 2019;87(3). doi:10.1128/IAI.00746-18.
- Vidakovic L, Mikhaleva S, Jeckel H, Nisnevich V, Strenger K, Neuhaus K, Raveendran K, Ben-Moshe NB, Aznaourova M, Nosho K, et al. Biofilm formation on human immune cells is a multicellular predation strategy of vibrio cholerae. Cell. 2023;186(12):2690–2704.e20. doi:10.1016/j.cell.2023.05.008.
- Lin CR, Chiang RK, Wang JS, Sung TW. Magnetic properties of monodisperse iron oxide nanoparticles. J Appl Phys. 2006;99(8):99. doi:10.1063/1.2172891.
- Hu J, Xie M, Wen CY, Zhang ZL, Xie HY, Liu AA, Chen YY, Zhou SM, Pang DW. A multicomponent recognition and separation system established via fluorescent, magnetic, dualencoded multifunctional bioprobes. Biomater. 2011;32(4):1177–1184. doi:10.1016/j.biomaterials.2010.10.015.
- Mutukuri TT, Maa YF, Gikanga B, Sakhnovsky R, Zhou QT. Electrostatic spray drying for monoclonal antibody formulation. Int J Pharm. 2021;607:120942. doi:10.1016/j.ijpharm.2021.120942.
- Meibom KL, Li XB, Nielsen AT, Wu CY, Roseman S, Schoolnik GK. The vibrio cholerae chitin utilization program. Proc Natl Acad Sci U S A. 2004;101(8):2524–2529. doi:10.1073/pnas.0308707101.
- Jones TH, Johns MW. Improved detection of F-specific RNA coliphages in fecal material by extraction and polyethylene glycol precipitation. Appl Environ Microbiol. 2009;75(19):6142–6146. doi:10.1128/AEM.00436-09.
- Lewis GD, Metcalf TG. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis a virus and human rotavirus, from oyster, water, and sediment samples. Appl Environ Microbiol. 1988;54(8):1983–1988. doi:10.1128/aem.54.8.1983-1988.1988.
- Sanchez G, Elizaquivel P, Aznar R. A single method for recovery and concentration of enteric viruses and bacteria from fresh-cut vegetables. Int J Food Microbiol. 2012;152(1–2):9–13. doi:10.1016/j.ijfoodmicro.2011.10.002.
- Luby CJ, Coughlin BP, Mace CR. Enrichment and recovery of mammalian cells from contaminated cultures using aqueous two-phase systems. Anal Chem. 2018;90(3):2103–2110. doi:10.1021/acs.analchem.7b04352.
- Sheng Y, Fan F, Jensen O, Zhong Z, Kan B, Wang H, Zhu J. Dual zinc transporter systems in vibrio cholerae promote competitive advantages over gut microbiome. Infect Immun. 2015;83(10):3902–3908. doi:10.1128/IAI.00447-15.
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi:10.1186/gb-2011-12-6-r60.
- Bueno E, Sit B, Waldor MK, Cava F. Anaerobic nitrate reduction divergently governs population expansion of the enteropathogen vibrio cholerae. Nature Microbiol. 2018;3(12):1346–1353. doi:10.1038/s41564-018-0253-0.
- Vazquez-Torres A, Baumler AJ. Nitrate, nitrite and nitric oxide reductases: from the last universal common ancestor to modern bacterial pathogens. Curr Opin Microbiol. 2016;29:1–8. doi:10.1016/j.mib.2015.09.002.
- Howard MF, Bina XR, Bina JE, Payne SM. Indole inhibits ToxR regulon expression in vibrio cholerae. Infect Immun. 2019;87(3). doi:10.1128/IAI.00776-18.
- Higgins DA, Pomianek ME, Kraml CM, Taylor RK, Semmelhack MF, Bassler BL. The major vibrio cholerae autoinducer and its role in virulence factor production. Nature. 2007;450(7171):883–886. doi:10.1038/nature06284.
- Alavi S, Mitchell JD, Cho JY, Liu R, Macbeth JC, Hsiao A. Interpersonal gut microbiome variation drives susceptibility and resistance to cholera infection. Cell. 2020;181(7):1533–1546.e13. doi:10.1016/j.cell.2020.05.036.
- Joelsson A, Liu Z, Zhu J. Genetic and phenotypic diversity of quorum-sensing systems in clinical and environmental isolates of vibrio cholerae. Infect Immun. 2006;74(2):1141–1147. doi:10.1128/IAI.74.2.1141-1147.2006.
- Yusof NL, Lim LY, Khor E. Preparation and characterization of chitin beads as a wound dressing precursor. J Biomed Mater Res. 2001;54(1):59–68. doi:10.1002/1097-4636(200101)54:1<59:AID-JBM7>3.0.CO;2-U.
- Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41(1):e1. doi:10.1093/nar/gks808.
- Liu Z, Yang M, Peterfreund GL, Tsou AM, Selamoglu N, Daldal F, Zhong Z, Kan B, Zhu J. Vibrio cholerae anaerobic induction of virulence gene expression is controlled by thiol-based switches of virulence regulator AphB. Proc Natl Acad Sci U S A. 2011;108(2):810–815. doi:10.1073/pnas.1014640108.