ABSTRACT
Most of the genome is transcribed into RNA but only 2% of the sequence codes for proteins. Non-coding RNA transcripts include a very large number of long noncoding RNAs (lncRNAs). A growing number of identified lncRNAs operate in cellular stress responses, for example in response to hypoxia, genotoxic stress, and oxidative stress. Additionally, lncRNA plays important roles in epigenetic mechanisms operating at chromatin and in maintaining chromatin architecture. Here, we address three lncRNA topics that have had significant recent advances. The first is an emerging role for many lncRNAs in cellular stress responses. The second is the development of high throughput screening assays to develop causal relationships between lncRNAs across the genome with cellular functions. Finally, we turn to recent advances in understanding the role of lncRNAs in regulating chromatin architecture and epigenetics, advances that build on some of the earliest work linking RNA to chromatin architecture.
There have been many excellent and recent reviews of long non-coding RNA (lncRNA) that offer important principles for addressing lncRNA functions and molecular mechanisms [Citation1,Citation2]. There are now large numbers of annotated lncRNAs, often defined as RNAs larger than 200 base pairs with no significant protein/peptide coding function, and many lncRNA correlations with cell state. The molecular mechanisms by which most of these lncRNAs might regulate cell state are unknown but prominently include epigenetic mechanisms operating on the genome. We have recently reviewed the early experimental history leading to the concept that nuclear RNA and its continuing synthesis are necessary for normal chromatin architecture and gene expression, concluding that this RNA is predominantly large and non-coding RNA [Citation3].
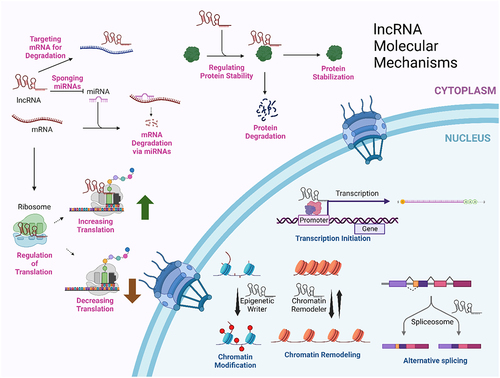
High throughput or massively parallel screening methods increasingly allow the correlation of specific lncRNAs with a limited, but increasing, number of cellular states and processes. Elucidating the molecular mechanisms behind these correlations is a frontier. One crude way to classify mechanisms is whether they act in the nucleus or the cytoplasm (). Among the lncRNA molecular mechanisms acting in the nucleus are the recruitment of chromatin-remodeling factors, interaction with transcription factors, and alteration of RNA splicing events. Cytoplasmic mechanisms that have been identified include: sponging microRNAs, altering the stability of mRNAs by other mechanisms, regulating protein stability, and regulating translation. There are likely to be many other mechanisms, given the enormous number of lncRNAs coded for in the genome. A 2022 compilation of lncRNAs for LncBook 2.0 found 95,243 lncRNA genes and 323,950 lncRNA transcripts [Citation4]
Figure 1. Cytoplasmic and nuclear mechanisms for lncRNAs. This summarizes just a few of the molecular mechanisms by which lncRNAs can function. This figure was created with BioRender.com.
The study and understanding of lncRNAs is advancing in many directions. In this article, we will address just three topics that have recent advances that we predict to be paradigm-setting. The first is an emerging role for many lncRNAs in cellular stress responses. The second is the development of high throughput screening assays to correlate lncRNAs across the genome with cellular functions, emphasizing the critical need for elucidating the molecular mechanisms underlying these correlations. Finally, we turn to an older interest with exciting recent advances and with the promise of providing many of those missing molecular mechanisms- the role of lncRNAs in regulating chromatin architecture and epigenetics.
lncRNAs in cellular stress: mechanisms and implications
Cells exposed to stimuli that cause damage to intracellular molecules or their functions respond to these stresses with mechanisms that restore homeostasis, force cells to an adaptive state, or induce cell death. Among the many stresses encountered by cells and leading to specific cell stress responses are DNA damage by genotoxic agents, oxidative stress through reactive oxygen species, heat shock, and cellular stress to hypoxia as described below and additionally presented in .
Table 1. Function of selected additional lncRNAs in cellular stress.
lncRNAs and genotoxic stress
One frontier in understanding lncRNA function and mechanism is identifying their roles in cellular responses to genotoxic stress, serving as critical factors in the maintenance of genomic stability and cellular homeostasis. Genotoxic stress, caused by factors such as chemical agents, UV radiation, and ionizing radiation, leads to DNA damage, triggering a complex cellular and molecular response. lncRNAs are increasingly recognized as pivotal regulators in this process, influencing DNA damage response (DDR) pathways, cell cycle progression, cell adaptive responses, and cell death pathways. lncRNAs can modulate gene expression and participate in chromatin remodeling, thereby influencing the cellular response to DNA damage. This modulation is crucial in understanding the progression of carcinogenesis, where genotoxic stress plays a pivotal role [Citation24]. In a broader context, the evolutionary and functional aspects of lncRNAs and their roles in various molecular mechanisms, including the regulation of gene expression in response to DNA damage and the activation of DDR pathways is critical for cell survival and genomic integrity under stress conditions, chronic diseases and aging.
Numerous lncRNAs are directly controlled by p53, the guardian of genome stability, binding to their promoter regions, and subsequently, they exert their functions in either apoptosis induction or gene suppression [Citation25]. P53-induced lncRNAs play a pivotal role in promoting apoptosis by interacting with PARP1 (reviewed in [Citation26]). In addition to promoting apoptosis, p53-induced lncRNAs can also inhibit cell proliferation. This can be achieved through the inhibition of MYC, a well-known controller of cell cycle progression and metabolism [Citation27]. For instance, it has been shown that PVT1b, a p53-dependent lncRNA, is a key regulator in suppressing MYC. PVT1b is induced by DNA damage or oncogenic signals and functions to suppress MYC transcription directly, without altering chromatin organization. This suppression of MYC by PVT1b is crucial for reinforcing the anti-proliferative activities of p53, thereby playing a significant role in controlling cellular proliferation and impacting tumor growth [Citation27]. On the other hand, the E2F1 messenger RNA (mRNA) stabilizing factor (EMS) is a direct transcriptional target of c-MYC. EMS acts as an oncogene by inducing cell cycle progression from G1 to S phase. In terms of the molecular mechanism, EMS collaborates with the RNA binding protein RALY to enhance the stability of E2F1 mRNA, leading to an increase in E2F1 expression. Additionally, EMS links MYC to cell cycle regulation and tumor development through its role in regulating the stability of E2F1 mRNA [Citation28].
The lncRNA NEAT1 is essential for the formation of nuclear structures called paraspeckles [Citation29]. Paraspeckles are 0.2 to1 μm structures adjacent to RNA splicing speckled domains. Paraspeckles contain many RNA binding proteins and form by phase separation (reviewed in [Citation30]). Paraspeckles play a crucial role in modulating replication stress response and chemosensitivity [Citation31]. The p53 protein, upon activation by various stress signals including DNA damage, upregulates the expression of NEAT1 lncRNA, stimulating the formation of paraspeckles. Paraspeckles sequester specific RNA-binding proteins and RNAs, thereby influencing gene expression patterns and cellular responses to replication stress and to chemotherapeutic agents. Understanding this pathway and the role of lncRNAs is essential for cancer biology, as it provides insights into cellular response to DNA damage and chemotherapy efficacy.
Severe genotoxic stress could lead to the accumulation of double-strand breaks, which are severe assaults on genome integrity. BRCA2 is essential for the repair of these breaks by homologous recombination (reviewed in [Citation32]). The lncRNA PCAT-1 represses BRCA2 expression in prostate cancer cells by a post-transcriptional mechanism operating in the cytoplasm where it reduces the stability of the BRCA2 mRNA [Citation33]. BRCA2 reduction by PCAT-1 impairs homologous recombination to repair double-strand breaks. In a practical application of these results, PCAT-1 may be a biomarker for tumors that are susceptible to inhibitors of PARP1, an enzyme that detects DNA damage and participates in the double-strand break repair pathways that are alternative to homologous recombination [Citation34,Citation35].
Guo et al. [Citation36] explored the role of lncRNA SOX2-OT in regulating oxidative stress in Parkinson’s disease. The study showed that microRNA-942-5p (miR-942-5p) was a direct target of lncRNA SOX2-OT and nuclear apoptosis-inducing factor 1 (NAIF1) was a direct target of miR-942-5p. This axis is critical in mediating cellular responses to oxidative stress, inflammation, and apoptosis in neurons, thereby playing a significant role in the pathogenesis of Parkinson’s disease [Citation36].
lncRNAs and oxidative stress
Studies have revealed dysregulated lncRNAs in oxidative stress-driven pathogenesis, with their mechanisms often involving the binding and regulation of transcription factors, such as Nrf2. Nrf2, a vital factor in cellular homeostasis, is often differentially expressed in chronic diseases, making it a potential therapeutic target. Specific lncRNAs, including lncRNA ROR, ENSMUST00000125413, lncRNA ODRUL, and Nrf2-lncRNA, have been linked to the Nrf2 signaling pathway in response to various stressors, highlighting their role in modulating Nrf2’s transcriptional activities and their significance in disease contexts (reviewed in [Citation37]). It has been shown that LINC00239 is significantly overexpressed in colorectal cancer (CRC), correlating with poorer survival and prognosis in patients. LINC00239 functions as a tumor-promoting factor in CRC by inhibiting ferroptosis, a novel iron-dependent cell death mechanism. LINC00239 interacts with Keap1, stabilizing Nrf2 protein and potentially offering a therapeutic strategy for CRC patients with low LINC00239 expression to induce ferroptosis and combat recurrence and chemoresistance [Citation38].
Studying the mechanism of lncRNA KCNQ1OT1 in hydrogen peroxide-stimulated human lens epithelial cells found that KCNQ1OT1 modulates the microRNA-124-3p/BCL-2-like 11 (BIM) axis in response to oxidative stress-induced by hydrogen peroxide [Citation39]. KCNQ1OT1 acts as a molecular sponge for microRNA-124-3p, leading to the upregulation of BIM, a pro-apoptotic protein. This modulation helps protect the cells from oxidative stress-induced damage, which is a key factor in the development of age-related cataracts. The findings suggest a potential strategy of targeting lncRNA KCNQ1OT1 for age-related cataracts and provide a deeper understanding of the cellular defense mechanisms against oxidative stress [Citation39].
The lncRNA NEAT1, localized at paraspeckles but also across the genome [Citation29,Citation40], and discussed above for its role in genotoxic stress responses, also acts in other stress responses. For example, Neat1−/− neurons are characterized by hyperexcitability and dysregulated calcium homeostasis, and enhanced stress-induced neuronal activity [Citation41]. NEAT1, an activator of an antioxidant pathway, protects brain microvascular endothelial cells from oxygen–glucose deprivation/reoxygenation [Citation42]. NEAT1 exerts its function by stabilizing Mfn2 mRNA by recruiting Nova, an RNA-binding protein, increasing Mfn2 expression. NEAT1 alleviates the oxidative stress and apoptosis by OGD/R-induced via activating Sirt3. This axis alleviates ischemia-reperfusion induced oxidative stress and apoptosis [Citation42].
Tauopathies are neurodegenerative disorders in which Tau protein aggregates in the brain. Some tauopathies result from mutations in the MAPT gene which codes for Tau. Searching for mechanisms in neurons derived from patient stem cells carrying three different MAPT mutations (p.P301L, IVS10 + 16, or p.R406W) and comparing them to CRISPR-corrected control neurons, transcriptomic changes associated with mutant MAPT alleles were identified. Specifically, 15 lncRNAs were differentially expressed across all three MAPT mutations. These lncRNAs were found to interact with RNA-binding proteins involved in the assembly of stress granules, cytoplasmic condensates containing RNA and protein that have been linked to neurodegeneration. One of these lncRNAs, SNHG8, had reduced levels in a mouse model of tauopathy and in the brains of individuals with frontotemporal lobar degeneration (FTLD)-tau, progressive supranuclear palsy, and Alzheimer’s disease.
The lncRNA SNHG8 interacts with both the tau protein and the stress granule-associated RNA-binding protein TIA1 [Citation43]. Notably, the overexpression of mutant Tau led to decreased SNHG8 expression and increased formation of stress granules. However, restoring SNHG8 expression resulted in reduced stress granule formation and decreased TIA1 levels in cultured cells and MAPT mutant neurons. This suggests that the dysregulation of SNHG8, a non-coding RNA, is a causative factor in promoting stress granule formation through TIA1 in tauopathies.
lncRNAs and the response to hypoxia
Hypoxia arises in various physiological and pathological contexts, including embryonic development, solid tumor growth, chronic inflammation, and ischemic diseases. LncRNAs have emerged as crucial regulators of cellular responses to hypoxic stress. In hypoxic environments, cells undergo extensive transcriptional reprogramming to adapt to the limited oxygen supply.
LncRNAs actively participate in this response, functioning as both sensors and effectors of hypoxia [Citation44,Citation45]. Several lncRNAs, including hypoxia-inducible factor 1-alpha (HIF-1α) anti-sense, H19, HOTAIR, HOTAIRM1, and MALAT1, have been identified as key players in hypoxia-associated pathways. HIF1A-AS2 is induced upon hypoxia and has been reported to stabilize the HIF-1α protein, a master regulator of the hypoxic response [Citation46]. HIF-1α anti-sense functions by recruiting prolyl hydroxylase 3 (PHD3) to pyruvate kinase 2 (PKM2), a critical enzyme in glycolysis. This recruitment leads to the prolyl hydroxylation of PKM2, and subsequently, the HIF-1α anti-sense -PKM2/PHD3 complex is transported into the nucleus with the assistance of heterogeneous nuclear ribonucleoprotein F (hnRNPF). This nuclear translocation enhances HIF-1α transactivation, a crucial factor in cancer cell survival and proliferation [Citation46].
In Glioblastoma multiforme, HIF1A-AS2 interacts with IGF2BP2 and DHX9, maintaining the expression of their target gene, HMGA1, and its deregulation impacts cancer cell growth, self-renewal, and hypoxia-dependent molecular reprogramming [Citation47]. This research demonstrates that HIF1A-AS2 is crucial for GSC speciation and adaptation to hypoxia within the tumor microenvironment, influencing tumor growth and survival through its interactome/targets and by modulating responses to hypoxic stress in a subtype-specific genetic context [Citation47].
The lncRNA HOTAIRM1, carried by exosomes from alveolar epithelial cells under hypoxic conditions, contributes to the progression of interstitial pulmonary fibrosis by disrupting the miR-30d-3p-mediated inhibition of HSF1 and promoting the recruitment of HSF1 by the transcriptional repressor YY1. Meanwhile, H19 operates as a competing endogenous RNA (ceRNA), sequestering microRNAs and indirectly influencing the expression of hypoxia-related genes [Citation48,Citation49]. MALAT1 has been implicated in promoting angiogenesis and metastasis in hypoxic tumor microenvironments [Citation50,Citation51]. The HOTAIR/miR-1277-5p/ZEB1 axis is important in mediating oxaliplatin resistance in colorectal cancer under hypoxic conditions [Citation52]. This study suggests that this axis regulates the epithelial–mesenchymal transition, offering a potential therapeutic target for improving oxaliplatin efficacy in colorectal cancer.
Furthermore, lncRNAs participate in the epigenetic regulation of gene expression under hypoxic conditions by interacting with and regulating chromatin-modifying complexes and by recruiting transcription factors, thus contributing to the shaping of the hypoxic transcriptional landscape [Citation53].
Understanding the intricate interplay between lncRNAs and hypoxia holds great promise for uncovering novel therapeutic targets and diagnostic markers in diseases associated with oxygen deprivation, such as cancer and ischemic disorders. Further research is needed to elucidate the specific mechanisms of individual lncRNAs in hypoxia and to harness this knowledge for the development of innovative treatments and interventions.
High throughput screens for linking lncRNAs with cellular phenotypes
Early confirmed lncRNAs were found one-by-one, by identifying an RNA transcript correlated to a cellular function or location in the genome, sequencing it, and then, surprisingly, failing to find a protein-coding reading frame. For example, a gene was identified from the human X-inactivation center of the X chromosome in human female cells that was not expressed in male cells or from somatic cell hybrids containing only an active X chromosome [Citation54]. Sequencing of the spliced 1.6kb transcript showed multiple stop codons in every reading frame forcing the conclusion that, perhaps, it was the RNA itself that was involved in inactivating its chromosome.
In a similar case, the H19 cDNA was first identified in a screen to find mouse genes coordinately regulated with the α-fetoprotein gene [Citation55]. Sequencing of the RNA, which was transcribed by RNA polymerase II and spliced, surprisingly found abundant stop codons in every reading frame and even the short plausible reading frames were not conserved between mouse and human [Citation56]. This led to the bold conclusion that the H19 gene product was the RNA itself.
The key advance allowing the identification of lncRNAs genome-wide was, of course, whole-genome sequencing [Citation57–59] combined with increasingly sophisticated analysis tools for identifying and annotating lncRNA genes [Citation60–62]. Correlations between annotated lncRNAs that have higher or lower levels after a cellular manipulation or mutation or induction of a disease state, as measured by RNA-seq, are then possible.
In approaches more capable of finding causal relationships, pooled CRISPRa or CRISPRi screens, are being developed and deployed. CRISPRa or CRISPR activation uses catalytically dead Cas9 (dCas9) coupled to a transcriptional activator and targeted to specific genome sequences by single guide RNAs (sgRNAs) in order to upregulate transcription at those sites [Citation63]. CRISPRi or CRISPR inhibition also uses catalytically dead Cas9, but this time coupled to a transcriptional repressor, and targeted to specific genome sites by a sgRNA for decreased transcription at those sites [Citation63]. In a pooled sgRNA assay, a library of sgRNAs targeting lncRNAs is introduced into a population of cells. After a time period or experimental challenge, the sgRNAs for the pool of cells are sequenced to estimate abundance, identifying sgRNAs that are positively or negatively selected for in the population under the experimental conditions. CRISPR-based screens are preferable to siRNA- or shRNA-based screens for lncRNAs, most of which remain nuclear, since the RNAi machinery is primarily in the cytoplasm [Citation64]. While initial CRISPR screens are powerful, there are serious caveats in interpretation requiring strong follow-up experimentation. As shown by [Citation65,Citation66], a large subset of lncRNAs may have effects on neighboring genes just by being transcribed at specific sites and for these, stopping transcription by CRISPRi or increasing it by CRISPRa are likely to affect function independent of the final lncRNA.
One application has identified specfic lncRNAs as drug resistance factors by CRISPRa [Citation67]. Cytarabine, also known as cytosine arabinoside (ara-C), a cytosine anti-metabolite, has been used for decades in chemotherapy for acute myeloid leukemia (AML) [Citation68]. Combined with a DNA-intercalating anthracycline, cytarabine therapy is initially effective but most patients develop drug resistance and relapse [Citation69]. Addressing this problem, a search for lncRNAs that could mediate drug resistance used pooled lncRNA CRISPRa screening targeting 14,701 lncRNA genes with multiple sgRNAs per transcription start site (TSS) and many lncRNAs having multiple TSS, resulting in a library of 88,444 targeting guides [Citation67]. After Cytarabine treatment, sgRNAs for the challenged but surviving cells were sequenced. Among the lncRNAs whose upregulation conferred drug resistance were GAS6-AS, PVT1, HOTAIRM1, and TUG1. We earlier discussed a proposed role for HOTAIRM1 in cellular responses to hypoxia [Citation47].
More mechanistic studies on GAS6-AS showed it to upregulate the neighboring protein coding gene GAS6, an important ligand for the TYRO3-AXL-MERTK (TAM) receptor tyrosine kinase signaling pathway to promote cellular survival.
In a complementary screening approach, a pooled CRISPRi-based genome-scale screen has identified lncRNAs contributing to cell growth and survival in human cells [Citation70]. In this work, a library of 16,401 sgRNAs targeting lncRNA loci in six cancer cell lines and in human induced pluripotent stem cells identified 499 lncRNA loci required for cell growth/survival. Surprisingly, 89% of these lncRNA loci affected growth/survival in only one cell type. This cell-type specificity of lncRNA function, even in something as common to cell lines as proliferation and survival, will be laborious to address as studies go beyond reporting lists generated by clever screens to developing molecular mechanisms.
There are important caveats to these screening approaches. As noted there can be effects of manipulating lncRNA transcription that are independent of the lncRNA itself. Simply transcribing something at a specific genome locus can have important local effects on chromatin and nuclear architecture. For example, PVT1 in humans is 55kB distant from the MYC oncogene at 8q24.21. A CRISPRi screen showed that PVT1 silencing increased proliferation in multiple human cell types [Citation70]. It turned out that silencing PVT1 transcription by CRISPRi promotes MYC transcriptional activation [Citation71]. Normally, the PVT1 promoter contacts four PVT1 enhancers, but when the PVT1 promoter is silenced, these enhancers can move to contacts with the MYC promoter, driving MYC activation and growth. Promoter competition for enhancers may be a normal mechanism for reciprocal regulation of PVT1 and MYC and a more widespread mechanism for the interaction of non-coding and coding genes in cis. Genome-wide screening approaches only produce lists. More extensive and rewarding work is required to move from such a list to a molecular mechanism.
Mechanisms for lncRNAs in chromatin architecture and epigenetics
There are many connections between non-coding RNAs and chromatin and genome organization. For example, as early as 2009, it was clear that a large fraction of lncRNAs were bound to chromatin-modifying complexes, including 20% bound to just one chromatin modifier, polycomb repressive complex 2 (PRC2), a complex with histone methyl transferase activity that targets to specific sites for histone H3K27me3 transcriptional silencing [Citation72]. Subsequent work has shown that PRC2 binds to many RNAs, both lncRNA and pre-mRNA, with high affinity at G-quadruplex structures, with medium affinity through unstructured G-rich sequences, and with low affinity to stem-loops [Citation73].
There is an earlier literature we will discuss, showing that RNAs and their transcription at specific genome sites are necessary for normal chromatin architecture. RNAs, including RNA Polymerase II transcripts that are not precursors for mRNA, are widely distributed in the nucleus. Early observations of the nucleus by light microscopy found a nucleolus and clumps of chromatin surrounded by clearer material initially called ‘nuclear sap’ or ‘karyolymph’ [Citation74,Citation75] but the advent of electron microscopy revealed a much more structured nucleus with many substructures enriched in RNA rather than DNA. Don Fawcett [Citation74] recommended ‘nuclear matrix’ as a better term for the RNA-enriched non-chromatin structures of the nucleus, a term making no claims about molecular composition, functions or dynamics but avoiding the fluid implications of ‘sap’.
RNA-selective staining procedures for electron microscopy revealed an RNA-containing nuclear network in unextracted cells [Citation76,Citation77]. Using EDTA regressive staining to localize RNA, Monneron and Bernhard found a fibrillogranular RNP network throughout the nucleus and characterized some of its components including interchromatin granule clusters, perichromatin fibrils, and perichromatin granules [Citation78]. Steps in nuclear RNA metabolism were later mapped to these structures. Perichromatin fibrils, for example, are at sites of RNA transcription [Citation79], while interchromatin granule clusters organize RNA splicing [Citation80] and organize a transcriptionally-active euchromatic compartment [Citation81]. These structures are not removed or perturbed by detergent removal of the nuclear envelope or by digestion of chromatin as shown by selective staining for RNA and electron microscopy [Citation82] (). The ultrastructure of this RNP network, stained for both protein and nucleic acid after cross-linking and chromatin removal is shown in . Electron microscopy cannot show us whether there is a continuous RNA-enriched network throughout a large region of the nucleus or, alternatively, local structures, and it cannot measure dynamics. The fine structure of this RNP network, visualized after cross-linking and chromatin removal ()
Figure 2. The RNP network of the nucleus is well preserved after chromatin removal. Shown are epon sections of CaSki cervical carcinoma cells before (a) and after (b) the isolation of a crosslink stabilized nuclear matrix and selectively stained for RNA by the EDTA- regressive procedure [Citation76] to visualize the RNP-network. The nuclear lamina (L) forms the periphery of the nucleus (panel A) and is retained in the nuclear matrix (panel B). The removal of chromatin after formaldehyde crosslinking does not substantially alter the structure or spatial distribution of the nuclear RNP network. (c) Higher magnification reveals well-preserved interchromatin granule clusters, enriched in RNA-splicing factors, in the RNP-network of the crosslink-stabilized nuclear matrix. The CaSki nuclear matrix in this panel was counterstained with an antibody recognizing the RNA-splicing factor SRm160 and a colloidal-gold-conjugated second antibody. The bar in panels a and B is 500 nm and in panel C the bar is 200 nm. This is from of Nickerson et al. [Citation82].
![Figure 2. The RNP network of the nucleus is well preserved after chromatin removal. Shown are epon sections of CaSki cervical carcinoma cells before (a) and after (b) the isolation of a crosslink stabilized nuclear matrix and selectively stained for RNA by the EDTA- regressive procedure [Citation76] to visualize the RNP-network. The nuclear lamina (L) forms the periphery of the nucleus (panel A) and is retained in the nuclear matrix (panel B). The removal of chromatin after formaldehyde crosslinking does not substantially alter the structure or spatial distribution of the nuclear RNP network. (c) Higher magnification reveals well-preserved interchromatin granule clusters, enriched in RNA-splicing factors, in the RNP-network of the crosslink-stabilized nuclear matrix. The CaSki nuclear matrix in this panel was counterstained with an antibody recognizing the RNA-splicing factor SRm160 and a colloidal-gold-conjugated second antibody. The bar in panels a and B is 500 nm and in panel C the bar is 200 nm. This is from Figure 4 of Nickerson et al. [Citation82].](/cms/asset/3769b107-ddf0-4afc-8d05-35e1ffad4139/kncl_a_2350180_f0002_b.gif)
Figure 3. The RNP network of a CaSki cell isolated after crosslink-stabilization and chromatin removal. When visualized by resinless section electron microscopy. (a) The RNP network or nuclear matrix consists of two parts, the nuclear lamina (L) and a network of structured fibers connected to the lamina and well distributed through the nuclear volume. The matrices of nucleoli (nu) remain and are connected to the fibers of the internal nuclear matrix. Three remnant nucleoli may be seen in this section. (b) Seen at higher magnification, the highly structured fibers of an internal RNP-netork or nuclear matrix are constructed on an underlying structure of 10-nm filaments, which occasionally branch. These are seen most clearly when, for short stretches, they are free of covering material (arrowheads). The bar shown in panel a represents 1 µM, and in panel B it is 100 nm. This is from of Nickerson et al., 1997 [Citation82].
![Figure 3. The RNP network of a CaSki cell isolated after crosslink-stabilization and chromatin removal. When visualized by resinless section electron microscopy. (a) The RNP network or nuclear matrix consists of two parts, the nuclear lamina (L) and a network of structured fibers connected to the lamina and well distributed through the nuclear volume. The matrices of nucleoli (nu) remain and are connected to the fibers of the internal nuclear matrix. Three remnant nucleoli may be seen in this section. (b) Seen at higher magnification, the highly structured fibers of an internal RNP-netork or nuclear matrix are constructed on an underlying structure of 10-nm filaments, which occasionally branch. These are seen most clearly when, for short stretches, they are free of covering material (arrowheads). The bar shown in panel a represents 1 µM, and in panel B it is 100 nm. This is from Figure 3 of Nickerson et al., 1997 [Citation82].](/cms/asset/ae249606-1623-499c-85f6-deb572fff7db/kncl_a_2350180_f0003_b.gif)
RNA is packaged by proteins in ribonucleoprotein (RNP), often considered to be globular granules or particles like those isolated after the sonication of nuclei. However, as early as 1963 electron microscopy had shown that RNA, packaged in such RNP proteins, is part of a fibrillogranular nuclear network [Citation83].
Early studies on the kinetics of RNA Polymerase II RNA metabolism found – for example – that only a small portion of large RNA in the nucleus, then termed heterogeneous nuclear RNA (hnRNA), is converted into cytoplasmic mRNA and that some of the nuclear-retained species are long-lived [Citation84,Citation85]. While some of these hnRNA molecules were precursors to mRNA [Citation86,Citation87], Penman suggested that a stable class of hnRNA would have a role in nuclear ultrastructure and organization. This was consistent with later work showing that most hnRNA molecules in the nucleus were not precursors to mRNAs [Citation88,Citation89]. Today, with advanced molecular techniques we can confirm that a large part of the human genome outside of annotated protein coding genes is transcribed into long transcripts but protein sequences are only 2% of the genome [Citation90–94].
Removal of the nuclear envelope or even gentle homogenization of the nucleus does not release RNA or RNP ‘particles’ [Citation85]; this requires digestion with ribonucleases [Citation85,Citation95–97] or severe mechanical forces [Citation98,Citation99], showing that the RNP of the nucleus is either integrated into intranuclear structures. Isolated RNP ‘particles’ are released by sonication which makes their biochemical characterization easier but much more attractive for artifactual interpretation.
Isolation of these RNP structures of the nucleus with intact RNA shows that they retain ultrastructural features of the RNP network of the nucleus [Citation100,Citation101]. Proteomic analysis of these preparations shows a high content of lamins, nuclear actin, and RNP proteins [Citation101,Citation102]. As expected, the ultrastructure is destroyed by RNase digestion [Citation101].
RNP networks and genome organization
The Penman lab proposed that the RNA in the RNP network of the nucleus was an architectural organizer of chromatin and included a large content of long non-coding RNAs [Citation103]. The reason for initially proposing that the RNA underlying nuclear architecture was long non-coding RNA (lncRNA) was that there was more of it, it was transcribed across the genome, and most species had longer lifetimes in the nucleus. Today, the identification of so many lncRNAs with unknown function and many with chromatin-association makes this hypothesis seem obvious [Citation4,Citation61,Citation104].
Chromatin has some characteristics of a self-organizing structure [Citation105] but is also organized by contacts with other nuclear structures such as the nuclear lamina [Citation106,Citation107] and by the clustering of rDNA sequences in nucleoli [Citation108]. Coming out of mitosis individual chromosomes decondense into territories and remain as separate territories in nuclei [Citation109,Citation110] with nonrandom positions [Citation111], but the molecular basis of folding within these territories is poorly understood. Chromosome conformation capture methods, such as Hi-C [Citation112]- a proximity ligation method- and the alternative technique SPRITE (Split-Pool Recognition of Interactions by Tag Extension) which is a split-and-pool barcoding method [Citation113], have shown the spatial organization of chromatin into self-interacting Topologically Associating Domains (TADs) and loops, as well as into spatially separated active and inactive compartments [Citation114].
TAD boundaries are only subtly affected by RNase A digestion before crosslinking while transcriptional inhibition with Actinomycin D, a DNA intercalating compound, leads to weaker TAD boundary scores showing differential contributions of RNA and its transcription to TAD formation [Citation115]. This suggests that the mechanisms for large-scale chromatin compaction after RNA digestion or transcriptional inhibition are mediated beyond the TAD level of chromatin organization.
An older literature reports that larger chromatin loops, on the order of 100kb, are formed by periodic attachments of the genome to some nuclear structure. These were first discovered by nuclear sedimentation experiments [Citation116,Citation117] and then by Coffey and colleagues by direct visualization of DNA extending beyond the nuclear lamina after the stripping of histones [Citation118]. These loops remain anchored to some nuclear structure and can be wound back into the nucleus by inducing positive supercoiling. There is clear functional significance to these loops in S-phase cells since pulse labeled DNA moves to increasingly peripheral locations along these loops with time suggesting that DNA Polymerase complexes are fixed at nuclear sites [Citation119] with replicating DNA reeled through these fixed polymerases. Stably anchored sites of DNA replication have been observed in living cells [Citation120]. Time-lapse examination during S-phase shows little movement of replication foci, a result consistent with stable anchoring of replication complexes on a non-chromatin structure. Changes in the distribution of replication sites occurs by disassembly of early sites with new assembly of later sites, not by movement, fusion or fission This arrangement of DNA replication is recapitulated by the transcription factory model of RNA synthesis where active RNA polymerases may be at fixed sites in the nucleus [Citation121,Citation122] which may also be the bases of chromatin loops in cells not undergoing DNA replication.
RNAs with repetitive sequences are important for chromatin architecture
Davidson and Britten, working with sea urchins, proposed that nuclear RNA transcribed from repetitive sequences in the genome contributed to regulation of gene expression [Citation123]. More recently, RNA with highly repetitive sequences has been shown important for keeping chromatin in an open, active state [Citation124].
CoT-1 DNA is a mixture enriched in highly repetitive DNA sequences [Citation125]. Preparations of CoT-1 DNA have been used to block nonspecific binding to repetitive sequences in molecular biology procedures analyzing low-copy number sequences. In a clever use of these Cot-1 preparations, the Lawrence lab used them as in situ hybridization probes to identify and localize RNAs that are transcribed highly repetitive elements (CoT-1 RNA) in cells [Citation126–128]. CoT-1 RNA is abundant and euchromatin-associated, localizes to interphase chromosome territories in cis, and remains after long-term transcriptional inhibition [Citation127].
Sequence analysis of chromosome-associated CoT-1 RNA shows that this pool of RNA is highly enriched in introns (82%) with another 6% being transcribed from intergenic regions [Citation128]. Analysis of splice junction sequences showed that most of these chromosome-associated introns were still in pre-spliced RNAs while read coverage suggested that there were both nascent and completed transcripts. Nascent transcripts could simply bridge interactions between the DNA of transcribing genes and an RNP-interacting network. It should be noted that nuclei also have a pool of mostly processed pre-mRNA with ‘detained’ introns [Citation129,Citation130] but it is not clear where they are located or whether they have a nuclear function. While they may be available for later splicing and export to the cytoplasm in response to cellular signals, stable nuclear introns may also − as in TERT mRNA and TUG1 lncRNA − control spatial compartmentalization of the RNAs in the nucleus [Citation131].
Studies using APEX2-proximity labeling and RNA sequencing to localize proximate RNAs at sites in the nucleus show different RNA species associated with RNA splicing speckled domains, with the nuclear lamina, and with other nuclear domains [Citation132]. APEX2 labeling identifies RNAs within a radius of about 20 nm. While a third of genes coding for speckled domain-associated transcripts are distal to speckled domains, most of are near speckled domains, structures enriched in splicing factors which may organize a euchromatic neighborhood in the nucleus [Citation81]. These RNA sets include retained introns, which may include both transcripts in the process of being spliced and RNAs stably retaining introns, detained introns. In this data, about a third of introns at speckled domains overlap with previously annotated stably detained introns. This compares to about 10% for lamina-associated introns.
Localization of RNAs in the neighborhood of their genes or at distant sites
Several techniques have now been developed that can map the spatial location of specific RNA species at higher resolution relative to genome loci or nuclear compartments. One is a refinement of the SPRITE technique, RNA & DNA SPRITE [Citation133], that comprehensively maps RNA – DNA sequence proximity. This approach has been validated by mapping RNA-DNA proximity in previously characterized nuclear compartments. For example, non-coding RNAs involved in mRNA splicing are spatially concentrated near active RNA Polymerase II-transcribed genes, while non-coding RNAs known to participate in ribosomal RNA processing are adjacent to transcribed ribosomal RNA genes.
Going beyond well characterized non-coding RNAs, the Guttman lab was able to map 650 lncRNAs to DNA loci. About 93% of these lncRNAs are highly enriched at their own transcriptional loci. After treatment with Flavopiridol which allows completion of RNA Polymerase II transcription but blocks further initiation, a tested subset of lncRNAs were stably bound at these sites, unlike nascent pre-mRNAs coding for proteins which complete and leave their sites of transcription.
Some previously described lncRNAs do not remain associated with their transcriptional loci, for example MALAT1 and NEAT1 [Citation29,Citation134] whose genes are adjacent in the human genome but which associate and localize with active chromatin across the genome while being more enriched in RNA Splicing Speckled Domains and in Paraspeckles, respectively [Citation40].
Summarizing, localization defines different classes of nuclear lncRNAs. There are lncRNAs that, like CoT-1 RNA are constrained to regions like chromosomal territories, a lower-resolution in cis localization. We do not know whether most of these are tethered near the sites of their own transcription, like those identified by SPRITE. Perhaps, these different methods [Citation128,Citation133] are taking snapshots of the same nuclear mechanisms at different resolutions?
How are lncRNAs tethered to chromatin?
Nascent transcripts
Simply by being transcribed at specific sites, lncRNAs may affect chromatin structure. Early work showed that stopping transcription causes the condensation of chromatin [Citation103] suggesting that the default state of chromatin is compacted and silent. It may be that the act of transcription opens chromatin for expression. This is supported by the abundance of repeat-rich RNA derived from nascent transcripts that is enriched in chromatin-associated RNA [Citation128].
RNA Polymerase II promoter-proximal pausing was shown to occur in about half of a set of 2482 annotated lncRNAs in mouse ES cells [Citation135]. In the same study, screening for lncRNAs that were activated or suppressed in human HEK293 cells by serum induction from Go to G1 found that 37% had significant pausing changes, increasing or decreasing after serum induction [Citation135]. Paused transcripts may serve different functions, but these may include keeping local chromatin domains in an open state.
DNA-RNA hybridization
In another mechanism, nascent transcripts might affect chromatin and patterns of gene expression by interaction with their DNA template, for example by R-loop formation. R-loops can form during transcription and are three-stranded nucleic acid structures with nascent RNA hybridized with one strand of the DNA template, leaving the other DNA strand single-stranded [Citation136,Citation137]. R-loops can persist because of the higher stability of RNA-DNA hybrids and can have important effects on chromatin epigenetics and gene expression, increasing chromatin accessibility while targeting histone modifications [Citation138,Citation139]. R-loops from eight genes selected for analysis had half-lives of 10 to 20 minutes after DRB treatment, though a significant fraction persisted for 2 hours [Citation140].
Genome-wide analysis found R-loops over 5% of the human genome in a large population of cells [Citation140]. This included the promoters for over 8,000 genes, especially open and active promoters, as well as in gene bodies. Genome-wide, for intron-containing genes, R-loops are often located between the transcription start site and the first exon-intron junction, but intron-less genes, including the lncRNA genes MALAT1 and NEAT1, often have promoter-associated R-loop forming regions [Citation141].
While R-loops form co-transcriptionally in cis, lncRNAs can also form R-loops at complementary sequences in trans as a means for transcriptional and chromatin control [Citation142,Citation143]. For example, in acute myeloid leukemia cells, the lncRNA HOTTIP regulates a subset of CTCF genome binding sites by forming sequence-complementary R-loops at those sites, participating in TAD formation [Citation143]. HOTTIP deletion strongly reduces R-loops at promoters and in intergenic regions, but not at exonic, intronic, and UTR regions. Especially relevant to AML, where Wnt/β-Catenin signaling plays an important role, HOTTIP deletion impaired TAD formation at Wnt/β-Catenin target loci along with the decreased transcription. In complementary experiments, HOTTIP overexpression reinforces CTCF boundaries and enhances Wnt/β-catenin TADs, with increases in transcription. HOTTIP lncRNA is found in complexes with many proteins including the CTCF/cohesion proteins SMC3, CTCF, RAD21, and SMC1A [Citation143] that coordinate TAD formation by loop extrusion [Citation144,Citation145]. HOTTIP is also in complexes with nuclear matrix proteins including SATB1 and RUNX1, with RNA binding proteins hnRNPA2B1 and hnRNPL, and with a subset of R-loop sensors and regulators [Citation143].
hnRNP proteins
RNAs in cells are always packaged by proteins. The classical set of these, with well characterized RNA-binding motifs, have been called heterogeneous nuclear ribonucleproteins (hnRNP proteins) and play critical roles in RNA processing, transport and interaction with nuclear structures [Citation146,Citation147].
hnRNP proteins have interaction domains with other proteins, as well as with RNA. A subset of RNP proteins have DNA-binding domains as well as RNA-binding domains [Citation148]. These are promising candidates for tethering lncRNA and other RNAs to chromatin. The number of these proteins is large but uncertain. One example is an RNP protein that participates in CoT-1 RNA tethering to chromatin. CoT-1 RNA can be released by the C-terminal piece of SAF-A (hnRNP U), which has the RNA-binding domain but not the DNA-binding domain [Citation149]. While SAF-A could link DNA to RNA directly; other proteins can do this indirectly with other DNA- and RNA-binding proteins that may themselves oligomerize. In the presence of DNA, full-length SAF-A forms fiber structures which can be joined head-to-tail in rings [Citation150]. SAF-A binding of ATP and chromatin-associated RNAs can transition it from a monomeric to an oligomeric state which drives the decompaction of chromatin [Citation151].
Besides the hnRNP proteins with well-studied RNA-binding domains, chromatin-associated proteins that lack classically defined RNA binding domains can also bind RNA, both mRNA and lncRNA [Citation152].
U1 snRNP tethering of lncRNAs
Small nuclear RNA (snRNA) U1, first discovered by Penman and Weinberg [Citation153], is a small nuclear RNA packaged in proteins into U1 snRNP. U1 snRNP is necessary to begin spliceosome assembly by binding at the 5’ splice site of a precursor RNA [Citation154–156]. This binding includes hybridization of U1 snRNA to a nine nucleotide partially conserved sequence marking the exon-intron junction [Citation157], though many of these partially conserved sequences in precursor RNAs are not sites of splicing [Citation158]. U1 snRNA has also been found in association with the RNA-binding protein TBP-associated factor 15 (TAF15) in a complex lacking the protein constituents of U1 snRNP [Citation159].
Surprisingly, knockdown of U1 snRNA releases lncRNA from chromatin, as does the degradation of a U1 snRNP protein [Citation160]. The RNA motif that can hybridize to U1 snRNP is essential for the localization of reporter RNAs to chromatin. Genome-wide, chromatin-bound lncRNAs are enriched in 5’ splice site sequences, which could be either authentic- or pseudo-splicing junctions. Chromatin-bound RNAs are also depleted of 3’ splice site sequences, and they exhibit high levels of U1 snRNA binding compared with cytoplasm-localized messenger RNAs. This suggests that U1 snRNP may be part of the tether of lncRNAs to chromatin. This concept needs to be reconciled with the localization of U1snRNP in nuclei. Fluorescence in situ hybridization shows U1 snRNA is enriched in RNA splicing speckled domains [Citation161]. Chromatin – lncRNA tethering may preferentially occur in the euchromatin environment created around RNA splicing specked domains [Citation81].
Building structures from RNA by RNA-RNA hybridization, RNP protein oligomerization, and phase separation
A combinatorial model has proposed [Citation162] that hybridization between complementary RNA sequences, as well as binding between RNP proteins combine to build RNP structures. The relative contribution of these two mechanisms may vary with composition, valence, and concentrations of components.
RNA molecules have the three-dimensional folding versatility of protein but a more predictable mechanism for linking molecules together − intermolecular hybridization. Nina Federoff first showed that mammalian nuclear RNA had a fraction sedimenting too fast to be single hnRNA molecules [Citation163]. Seen by electron microscopy, this RNA was not an aggregate but had multiple long RNAs linked into a branched network by intermolecular hybridization. Intermolecular RNA duplexes were also seen by Eric Davidson in Xenopus cells and oocytes [Citation164]. There was disagreement about whether these duplexes had formed in vivo [Citation163,Citation164]. Today, in vivo intermolecular RNA hybridization is well accepted. It has been argued [Citation162] that RNA–RNA interactions occur readily in cells and that RNA binding proteins block many of these interactions while allowing specific interactions in support of RNP assembly into larger structures.
Interactions between RNP proteins might assemble oligomeric RNA-containing structures. In one simple example of this, Melissa Moore and colleagues [Citation165] showed that Exon Junction Complexes bound 24nt upstream from exon–exon junctions of both coding and non-coding long RNAs can oligomerize, forming megadalton-sized complexes. This is a simple and experimentally established mechanism that could link together RNAs to build a structure. There may be as many as 1500 RNA-binding proteins coded for in the human genome [Citation166].
Many proteins contain domains that are intrinsically disordered; these regions have a sequence intrinsically unlikely to form a specific three-dimensional structure [Citation167]. Intrinsically disordered domains function in many binding interactions − RNA binding, DNA binding, and protein binding − and in many cellular processes [Citation168]. Intrinsically disordered domains of RNP proteins can interact and then phase-separate from the nucleoplasm creating high concentrations of RNP that can transition into more ordered assemblies. The best characterized example is FUS (Fused in Sarcoma). Isolated FUS can form liquid-like droplets that then mature with time to form a hydrogel containing filaments [Citation169,Citation170]. CLIP-Seq identification of FUS-bound RNAs identifies 57% of the sequences as introns in pre-mRNA and 12% as lncRNAs [Citation171]. As already noted, chromosome-associated RNAs are enriched in introns (82%) [Citation128] and include many lncRNAs [Citation133].
RNP protein phase transitions drive the physical chemistry underlying formation of membrane-less organelles and assemblies in cells [Citation172]. In chromatin architecture and transcriptional regulation, structures forming from phase-separated droplets include RNA Polymerase II transcriptional complexes [Citation173,Citation174] and heterochromatin [Citation175]. Complexity is added to these transitions by protein- and RNA-binding and by regulators of these interactions, as for example in the interactions between FUS and nucleoporins [Citation176]. Liquid-in-liquid multiphase condensates can form. Liquid condensates of RNA-binding proteins can transition into less dynamic states with reduced fluidity and molecular dynamics, driving fiber assembly [Citation169,Citation177–179]. Disturbing the equilibrium of these processes can be associated with diseases such as amyotrophic lateral sclerosis for FUS and TDP43. While these are the best characterized RNP proteins with disordered domains, there are many more that have yet to be explored.
The fibers forming out of liquid phase concentrates of RNA-binding proteins in vitro can resemble the 10 nm nuclear matrix core filaments reported by the Penman () and Cook laboratories and proposed to be a core structure of the nuclear interior that also connects to the nuclear lamina [Citation82,Citation181,Citation182]. Similar filaments assemble from in vitro liquid phases of purified FUS [Citation169]. These results suggest a third plausible mechanism for assembly of RNP filament structures in the nucleus.
Figure 4. The RNA-containing nuclear matrix from a HeLa cell when isolated without a cross-linking step before the removal of chromatin. The structure is constructed on a branched network of 10-nm core filaments that resemble filaments that form in phase-separated condensates of RNP proteins [Citation169,Citation177–179]. This high magnification view shows connections between core filaments (arrow) and the nuclear lamina (L). The bar is 100nM [Citation180].
![Figure 4. The RNA-containing nuclear matrix from a HeLa cell when isolated without a cross-linking step before the removal of chromatin. The structure is constructed on a branched network of 10-nm core filaments that resemble filaments that form in phase-separated condensates of RNP proteins [Citation169,Citation177–179]. This high magnification view shows connections between core filaments (arrow) and the nuclear lamina (L). The bar is 100nM [Citation180].](/cms/asset/be897d40-5da6-45da-a0d1-09c45dcf51e7/kncl_a_2350180_f0004_b.gif)
The normal architecture of open, transcriptionally active chromatin requires RNA. Recent work has globally identified sets of RNAs that are associated with chromatin or located at specific DNA loci [Citation128,Citation133]. These new results and technologies give us the exciting opportunity to probe the mechanisms by which specific RNAs associate with chromatin at specific sites to structurally organize the genome. Structures emerging in cis from newly transcribed RNA and RNA-packaging proteins would be expected to be highly dynamic so the development of more tools to observe those dynamics is needed.
Biophysical techniques should also contribute to our study of nuclear RNA. If RNA and RNA-binding proteins are forming dynamic scaffoldings in the nucleus, either in chromatin or in inter-chromatin regions, then interfering with or ablating such architectural molecules should have mechanical consequences measurable in live cells. For example, nuclear shape or the organization and mechanical properties of individual tagged genetic loci, might be measured by cell biological and biophysical methods in high-throughput screens [Citation183].
lncRNA therapeutics
Advances in RNA biology over decades have now generated medical applications with many FDA approved therapies or vaccines, and a much larger number of clinical trials [Citation184,Citation185]. The major approaches are currently designed to 1) target and reduce cellular RNAs by RNA-interference or antisense methods, 2) to deliver mRNAs to cells for generating immune responses or as therapy, or 3) using RNA guides to edit cell genomes.
lncRNAs are attractive therapeutic targets because of their many roles in cellular regulation, for example in that cellular responses to stress that we have discussed. Already lncRNAs are linked to the pathogenesis of an increasing number of diseases [Citation186–188], with novel tools being developed to find many more lncRNA–disease relationships [Citation189,Citation190]. Non-coding RNA therapeutic development has been extensively reviewed [Citation191,Citation192].
Tactics for interfering with lncRNAs may depend on cellular location. It has been shown that nuclear lncRNAs can be more effectively targeted by anti-sense oligonucleotides (ASOs), while cytoplasmic lncRNAs are more effectively targeted by RNA-interference [Citation193]. These are both mature technologies; the first FDA approval for an ASO was in 1998, and for an siRNA-based drug was in 2018. CRISPRi can be a useful screen for lncRNAs, avoiding the cellular location issues, but it less likely to be useful for a therapeutic because of the more difficult delivery problem, the greater problem of off-target genome binding, and the issue that decreasing transcription of a large subset of lncRNAs can have effects on neighboring genes [Citation65,Citation66].
Since there are currently many lncRNAs that are attractive targets for cancer therapy and non-coding RNA therapeutic development has been extensively reviewed [Citation191,Citation192], we will briefly discuss only one target. MALAT 1 (metastasis associated lung adenocarcinoma transcript 1) is an 8.7knt lncRNA expressed from chromosome 11q13 in humans and that is both abundant in human tissues and highly conserved across vertebrate species [Citation194]. MALAT1 is localized to RNA splicing speckled domains [Citation134], although it is not essential for their structure [Citation195,Citation196] and it is not essential for mouse development [Citation195–197]. MALAT1 was first identified as an RNA in non-small cell lung cancer whose levels were prognostic for metastasis and reduced survival [Citation198]. MALAT1 is not polyadenylated but its 3’ end is processed by RNase P cleavage downstream of an oligoA sequence to generate the mature 3’ end and release a tRNA-like small RNA that localizes to the cytoplasm [Citation199]. We have discussed MALAT1 above as a mediator of stress responses to hypoxia.
Since MALAT1 is not essential to cells [Citation195–197] but does have higher expression in many tumors where it is levels correlate with progression and metastasis (references in [Citation194]), and since knockdown or genetic loss of MALAT1 reduces tumor growth or metastasis in various cancer model systems (references in [Citation194]), it seems like a strong candidate for pre-clinical development for a therapy inhibiting cancer progression.
In the case of breast cancer, Arun et al. [Citation200] showed that loss of Malat1 lncRNA in the MMTV-PyMT mouse model for luminal B breast cancer decreased tumor cell proliferation, and induced the differentiation of primary tumors to cystic tumors. Ionis pharmaceuticals was granted patent US11279932B2 in 2022 for an ASO targeting MALAT1 in breast and other cancers. We wait with hope for the rest of the story to unfold.
LncRNA questions outstanding: a perspective for future investigation
We have come a long way since a benighted reviewer could label as crazy the ideas that large non-protein coding RNAs existed and that they were important for chromatin organization. Despite the impressive progress, we are just starting to frame the questions about lncRNA, hoping that some of them are the right questions. While it has been relatively easy to correlate lncRNA expression or loss with functions, the mechanisms supporting those functions are a greater challenge. This should be the greatest focus; elucidating mechanisms to a biochemistry level of understanding.
One set of important questions could be summarized as: what is the code? We assume that there is a code to lncRNA nucleotide sequence or three-dimensional structure that supports mechanism, but we have not been able to decrypt it. For the code used by mRNA, Francis Crick proposed in 1958 that DNA and RNA sequence coded for the amino acid sequence of proteins, and that amino acid sequence also coded for protein three-dimensional structure [Citation201]. For this mRNA code, apparently far simpler than the one which may explain lncRNA mechanisms, it took the development of novel techniques by Nirenberg [Citation202] and Khorana [Citation203] and 8 more years of experiments to develop a complete table of codons, mapping trinucleotides to amino acids.
Even before we had complete genome assemblies, linguistic analysis of DNA sequences according to Zipf’s law and Shannon entropy found that non-coding regions, even more than coding regions, had statistical properties similar to natural and artificial languages [Citation204]. It would be interesting to do a similar analysis today with annotated lncRNA sequences compared to whole genomes and annotated protein-coding sequences. It has been proposed that there is a structural grammar to non-coding RNA, including lncRNA, with features like natural language grammar [Citation205]. In this view, ‘letters’ are non-coding RNA elements, assembled into ‘words’ or larger RNA domains that would then be combined into ‘phrases’ or whole non-coding RNA structures. Sentences could be built from interaction of RNAs by a combinatorial mechanism [Citation162] combining hybridization between complementary RNA sequences and binding between RNP proteins to build higher-order RNP structures as we have proposed [Citation3]. We have also proposed that such large multiple RNA-based structures will be important in the architecture of chromatin [Citation3].
One influential idea about lncRNA mechanisms proposes that lncRNAs are flexible modular scaffolds [Citation206], with discrete domains that each bind specific proteins. In this way, lncRNAs could bring together proteins in unique complexes. Domains in lncRNA would be the code that is read by binding proteins. The sequences between protein-binding modules could influence the geometry of interactions, and so determinants of RNA folding in these regions would be critical [Citation205]. A more expansive version of this idea would include lncRNA modules that, instead of binding proteins, hybridize to other RNAs or DNA or even bind to lipids [Citation207].
Problems with this model as a guide include our inadequate understanding of the determinants of RNA-protein binding, as in the large number of RNA-binding proteins whose RNA-interactions are highly promiscuous [Citation152]. Newer experimental approaches capable of visualizing RNA–protein interactions at specific locations in living cells may help with some mechanisms [Citation132,Citation208].
Summary of key ideas
From all of the work discussed in this article, we would like to draw three main points. 1) The lncRNAs are important modulators of the mechanisms by which cells respond to stresses on their molecules or functions, determining whether stressed cells return to homeostasis, transition to an adaptive state, or die. 2) The lncRNAs are key determinants of chromatin structure and its epigenetic regulation. Mature lncRNAs may be important for this but we must also consider that the act of transcribing lncRNAs at specific genome locations may be equally important. Participating lncRNAs may not act alone in support of chromatin and nuclear architecture, but could combine to form higher-order structures by some combination of inter-RNA hybridization, RNA-binding protein oligomerization, and phase separation. 3) The continuing development of genome-wide lncRNA screens is an important path forward, remembering, as in point 2), that interfering with specific lncRNA transcription may also affect local chromatin structure and the expression of neighboring coding and non-coding genes.
Author contribution statement
J. A. Nickerson and F. Momen-Heravi both contributed to the design and drafting of this review. J. A. Nickerson and F. Momen-Heravi both contributed to the viewpoints and perspectives expressed. J. A. Nickerson and F. Momen-Heravi both will be responsible for revisions and final approval. J. A. Nickerson and F. Momen-Heravi both agree to be responsible for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Mattick JS, Amaral PP, Carninci P, et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat Rev Mol Cell Biol. 2023;24(6):430–25. doi: 10.1038/s41580-022-00566-8
- Rinn JL, Chang HY. Long noncoding RNAs: molecular modalities to organismal functions. Annu Rev Biochem. 2020;89(1):283–308. doi: 10.1146/annurev-biochem-062917-012708
- Nickerson JA. The ribonucleoprotein network of the nucleus: a historical perspective. Curr Opin Genet Dev. 2022;75:101940. doi: 10.1016/j.gde.2022.101940
- Li Z, Liu L, Feng C, et al. LncBook 2.0: integrating human long non-coding RNAs with multi-omics annotations. Nucleic Acids Res. 2023;51(D1):D186–D191. doi: 10.1093/nar/gkac999
- Abe R, Yagi Y, Tani H. Identifying long non-coding RNA as potential indicators of bacterial stressin human cells. BPB Reports. 2023;6(6):226–228. doi: 10.1248/bpbreports.6.6_226
- Tao X, Xue F, Xu J, et al. Platelet-rich plasma-derived extracellular vesicles inhibit NF-kappaB/NLRP3 pathway-mediated pyroptosis in intervertebral disc degeneration via the MALAT1/microRNA-217/SIRT1 axis. Cell Signal. 2024;117:111106. doi: 10.1016/j.cellsig.2024.111106
- Rajabi D, Khanmohammadi S, Rezaei N. The role of long noncoding RNAs in amyotrophic lateral sclerosis. Rev Neurosci. 2024;0(0). doi: 10.1515/revneuro-2023-0155
- Liau WS, Zhao Q, Bademosi A, et al. Fear extinction is regulated by the activity of long noncoding RNAs at the synapse. Nat Commun. 2023;14(1):7616. doi: 10.1038/s41467-023-43535-1
- Corrado C, Costa V, Giavaresi G, et al. Long non coding RNA H19: a new player in hypoxia-induced multiple myeloma cell dissemination. Int J Mol Sci. 2019;20(4):20. doi: 10.3390/ijms20040801
- Hall JR, Messenger ZJ, Tam HW, et al. Long noncoding RNA lincRNA-p21 is the major mediator of UVB-induced and p53-dependent apoptosis in keratinocytes. Cell Death Dis. 2015;6(3):e1700. doi: 10.1038/cddis.2015.67
- Liu L, Chen Y, Huang Y, et al. Long non-coding RNA ANRIL promotes homologous recombination-mediated DNA repair by maintaining ATR protein stability to enhance cancer resistance. Mol Cancer. 2021;20(1):94. doi: 10.1186/s12943-021-01382-y
- Yao L, Peng P, Ding T, et al. m(6)A-Induced lncRNA MEG3 promotes cerebral ischemia-reperfusion injury via modulating oxidative stress and mitochondrial dysfunction by hnRNPa1/sirt2 axis. Mol Neurobiol. 2024. doi: 10.1007/s12035-024-04005-x
- Lee S, Kopp F, Chang TC, et al. Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO proteins. Cell. 2016;164(1–2):69–80. doi: 10.1016/j.cell.2015.12.017
- Xu W, Mo W, Han D, et al. Hepatocyte-derived exosomes deliver the lncRNA CYTOR to hepatic stellate cells and promote liver fibrosis. J Cell Mol Med. 2024;28(8):e18234. doi: 10.1111/jcmm.18234
- Peng C, Hu W, Weng X, et al. Over expression of long non-coding RNA PANDA promotes hepatocellular carcinoma by inhibiting senescence associated inflammatory factor IL8. Sci Rep. 2017;7(1):4186. doi: 10.1038/s41598-017-04045-5
- Han L, Yang L. Multidimensional mechanistic spectrum of long non-coding RNAs in heart development and disease. Front Cardiovasc Med. 2021;8:728746. doi: 10.3389/fcvm.2021.728746
- Chen C, Lin X, Tang Y, et al. LncRNA Fendrr: involvement in the protective role of nucleolin against H(2)O(2) -induced injury in cardiomyocytes. Redox Rep. 2023;28(1):2168626. doi: 10.1080/13510002.2023.2168626
- Atef MM, Shafik NM, Hafez YM, et al. The evolving role of long noncoding RNA HIF1A-AS2 in diabetic retinopathy: a cross-link axis between hypoxia, oxidative stress and angiogenesis via MAPK/VEGF-dependent pathway. Redox Rep. 2022;27(1):70–78. doi: 10.1080/13510002.2022.2050086
- Li T, Xiao Y, Huang T. HIF‑1α‑induced upregulation of lncRNA UCA1 promotes cell growth in osteosarcoma by inactivating the PTEN/AKT signaling pathway. Oncol Rep. 2018;39(3):1072–1080. doi: 10.3892/or.2018.6182
- Guo Z, Wang X, Yang Y, et al. Hypoxic tumor-derived exosomal long noncoding RNA UCA1 promotes angiogenesis via miR-96-5p/AMOTL2 in pancreatic cancer. Mol Ther Nucleic Acids. 2020;22:179–195. doi: 10.1016/j.omtn.2020.08.021
- Chang H, Zou Z. Targeting autophagy to overcome drug resistance: further developments. J Hematol Oncol. 2020;13(1):159. doi: 10.1186/s13045-020-01000-2
- Bettin N, Oss Pegorar C, Cusanelli E. The emerging roles of TERRA in telomere maintenance and genome stability. Cells. 2019;8(3):8. doi: 10.3390/cells8030246
- Marin-Bejar O, Marchese FP, Athie A, et al. Pint lincRNA connects the p53 pathway with epigenetic silencing by the polycomb repressive complex 2. Genome Biol. 2013;14(9):R104. doi: 10.1186/gb-2013-14-9-r104
- Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452–463. doi: 10.1016/j.ccell.2016.03.010
- Mitra S, Muralidharan SV, Di Marco M, et al. Subcellular distribution of p53 by the p53-responsive lncRNA NBAT1 determines chemotherapeutic response in Neuroblastoma. Cancer Res. 2021;81(6):1457–1471. doi: 10.1158/0008-5472.CAN-19-3499
- Statello L, Guo CJ, Chen LL, et al. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22(2):96–118. doi: 10.1038/s41580-020-00315-9
- Olivero CE, Martinez-Terroba E, Zimmer J, et al. p53 activates the long noncoding RNA Pvt1b to inhibit myc and suppress tumorigenesis. J Reine und Angew Math. 2020;77(4):761–774 e768. doi: 10.1016/j.molcel.2019.12.014
- Wang C, Yang Y, Zhang G, et al. Long noncoding RNA EMS connects c-myc to cell cycle control and tumorigenesis. Proc Natl Acad Sci USA. 2019;116(29):14620–14629. doi: 10.1073/pnas.1903432116
- Clemson CM, Hutchinson JN, Sara SA, et al. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell. 2009;33(6):717–726. doi: 10.1016/j.molcel.2009.01.026
- Fox AH, Nakagawa S, Hirose T, et al. Paraspeckles: where long noncoding RNA meets phase separation. Trends Biochem Sci. 2018;43(2):124–135. doi: 10.1016/j.tibs.2017.12.001
- Adriaens C, Standaert L, Barra J, et al. p53 induces formation of NEAT1 lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity. Nat Med. 2016;22(8):861–868. doi: 10.1038/nm.4135
- Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2011;12(1):68–78. doi: 10.1038/nrc3181
- Prensner JR, Chen W, Iyer MK, et al. PCAT-1, a long noncoding RNA, regulates BRCA2 and controls homologous recombination in cancer. Cancer Res. 2014;74(6):1651–1660. doi: 10.1158/0008-5472.CAN-13-3159
- Ray Chaudhuri A, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18(10):610–621. doi: 10.1038/nrm.2017.53
- Huang D, Kraus WL. The expanding universe of PARP1-mediated molecular and therapeutic mechanisms. Mol Cell. 2022;82(12):2315–2334. doi: 10.1016/j.molcel.2022.02.021
- Guo Y, Liu Y, Wang H, et al. Long noncoding RNA SRY-box transcription factor 2 overlapping transcript participates in Parkinson’s disease by regulating the microRNA-942-5p/nuclear apoptosis-inducing factor 1 axis. Bioengineered. 2021;12(1):8570–8582. doi: 10.1080/21655979.2021.1987126
- Jayasuriya R, Ramkumar KM. Role of long non-coding RNAs on the regulation of Nrf2 in chronic diseases. Life Sci. 2021;270:119025. doi: 10.1016/j.lfs.2021.119025
- Han Y, Gao X, Wu N, et al. Long noncoding RNA LINC00239 inhibits ferroptosis in colorectal cancer by binding to Keap1 to stabilize Nrf2. Cell Death Dis. 2022;13(8):742. doi: 10.1038/s41419-022-05192-y
- Xu Y, Zheng Y, Shen P, et al. Role of long noncoding RNA KCNQ1 overlapping transcript 1/microRNA-124-3p/BCL-2-like 11 axis in hydrogen peroxide (H2O2)-stimulated human lens epithelial cells. Bioengineered. 2022;13(3):5035–5045. doi: 10.1080/21655979.2022.2032966
- West JA, Davis CP, Sunwoo H, et al. The long noncoding RNAs NEAT1 and MALAT1 bind active chromatin sites. Mol Cell. 2014;55(5):791–802. doi: 10.1016/j.molcel.2014.07.012
- Kukharsky MS, Ninkina NN, An H, et al. Long non-coding RNA Neat1 regulates adaptive behavioural response to stress in mice. Transl Psychiatry. 2020;10(1):171. doi: 10.1038/s41398-020-0854-2
- Zhou ZW, Ren X, Zheng LJ, et al. LncRNA NEAT1 ameliorate ischemic stroke via promoting Mfn2 expression through binding to nova and activates Sirt3. Metab Brain Dis. 2022;37(3):653–664. doi: 10.1007/s11011-021-00895-1
- Bhagat R, Minaya MA, Renganathan A, et al. Long non-coding RNA SNHG8 drives stress granule formation in tauopathies. Mol Psychiatry. 2023;28(11):4889–4901. doi:10.1038/s41380-023-02237-2
- Tian F, Yi J, Liu Y, et al. Integrating network pharmacology and bioinformatics to explore and experimentally verify the regulatory effect of Buyang Huanwu decoction on glycolysis and angiogenesis after cerebral infarction. J Ethnopharmacol. 2024;319:117218. doi: 10.1016/j.jep.2023.117218
- Chen W, Ye Q, Dong Y. Long term exercise-derived exosomal LncRNA CRNDE mitigates myocardial infarction injury through miR-489-3p/Nrf2 signaling axis. Nanomedicine. 2024;55:102717. doi: 10.1016/j.nano.2023.102717
- Zheng F, Chen J, Zhang X, et al. The HIF-1α antisense long non-coding RNA drives a positive feedback loop of HIF-1α mediated transactivation and glycolysis. Nat Commun. 2021;12(1):1341. doi: 10.1038/s41467-021-21535-3
- Mineo M, Ricklefs F, Rooj AK, et al. The long non-coding RNA HIF1A-AS2 facilitates the maintenance of mesenchymal glioblastoma stem-like cells in hypoxic niches. Cell Rep. 2016;15(11):2500–2509. doi: 10.1016/j.celrep.2016.05.018
- Zhu H, Jin YM, Lyu XM, et al. Retracted Article: long noncoding RNA H19 regulates HIF-1α/AXL signaling through inhibiting miR-20b-5p in endometrial cancer. Cell Cycle. 2019;18(19):2454–2464. doi: 10.1080/15384101.2019.1648958
- Peng PH, Hsu KW, Chieh-Yu Lai J, et al. The role of hypoxia-induced long noncoding RNAs (lncRNAs) in tumorigenesis and metastasis. Biomed J. 2021;44(5):521–533. doi: 10.1016/j.bj.2021.03.005
- Tee AE, Liu B, Song R, et al. The long noncoding RNA MALAT1 promotes tumor-driven angiogenesis by up-regulating pro-angiogenic gene expression. Oncotarget. 2016;7(8):8663–8675. doi: 10.18632/oncotarget.6675
- Michalik KM, You X, Manavski Y, et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res. 2014;114(9):1389–1397. doi: 10.1161/CIRCRESAHA.114.303265
- Weng X, Liu H, Ruan J, et al. HOTAIR/miR-1277-5p/ZEB1 axis mediates hypoxia-induced oxaliplatin resistance via regulating epithelial-mesenchymal transition in colorectal cancer. Cell Death Discov. 2022;8(1):310. doi: 10.1038/s41420-022-01096-0
- Zhang J, Hu C, Jiao X, et al. Potential role of mRnas and LncRNAs in chronic intermittent hypoxia exposure-aggravated atherosclerosis. Front Genet. 2020;11:290. doi: 10.3389/fgene.2020.00290
- Brown CJ, Ballabio A, Rupert JL, et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349(6304):38–44. doi: 10.1038/349038a0
- Pachnis V, Belayew A, Tilghman SM. Locus unlinked to alpha-fetoprotein under the control of the murine raf and Rif genes. Proc Natl Acad Sci USA. 1984;81(17):5523–5527. doi: 10.1073/pnas.81.17.5523
- Brannan CI, Dees EC, Ingram RS, et al. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990;10(1):28–36. doi: 10.1128/MCB.10.1.28
- Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062
- International Human Genome Sequencing C: finishing the euchromatic sequence of the human genome. Nature. 2004;431(7011):931–945. doi: 10.1038/nature03001
- Venter JC, Adams MD, Myers EW, et al. The sequence of the human genome. Science. 2001;291(5507):1304–1351. doi: 10.1126/science.1058040
- Iyer MK, Niknafs YS, Malik R, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47(3):199–208. doi: 10.1038/ng.3192
- Hon CC, Ramilowski JA, Harshbarger J, et al. An atlas of human long non-coding RNAs with accurate 5 ends. Nature. 2017;543(7644):199–204. doi: 10.1038/nature21374
- Frankish A, Carbonell-Sala S, Diekhans M, et al. GENCODE: reference annotation for the human and mouse genomes in 2023. Nucleic Acids Res. 2023;51(D1):D942–D949. doi: 10.1093/nar/gkac1071
- Gilbert LA, Horlbeck MA, Adamson B, et al. Genome-scale CRISPR-Mediated control of gene repression and activation. Cell. 2014;159(3):647–661. doi: 10.1016/j.cell.2014.09.029
- Boettcher M, MT M. Choosing the right tool for the job: RNAi, TALEN, or CRISPR. Mol Cell. 2015;58(4):575–585. doi: 10.1016/j.molcel.2015.04.028
- Kornienko AE, Guenzl PM, Barlow DP, et al. Gene regulation by the act of long non-coding RNA transcription. BMC Biol. 2013;11(1):59. doi: 10.1186/1741-7007-11-59
- Goyal A, Myacheva K, Gross M, et al. Challenges of CRISPR/Cas9 applications for long non-coding RNA genes. Nucleic Acids Res. 2017;45:e12. doi: 10.1093/nar/gkw883
- Bester AC, Lee JD, Chavez A, et al. An integrated genome-wide CRISPRa approach to functionalize lncRNAs in drug resistance. Cell. 2018;173(3):649–664 e620. doi: 10.1016/j.cell.2018.03.052
- Lowenberg B, Downing JR, Burnett A. Burnett A: acute myeloid leukemia. N Engl J Med. 1999;341(14):1051–1062. doi: 10.1056/NEJM199909303411407
- Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29(5):487–494. doi: 10.1200/JCO.2010.30.1820
- Liu SJ, Horlbeck MA, Cho SW, et al. Crispri-based genome-scale identification of functional long noncoding RNA loci in human cells. Science. 2017;355(6320):355. doi: 10.1126/science.aah7111
- Cho SW, Xu J, Sun R, et al. Promoter of lncRNA gene PVT1 is a tumor-suppressor DNA boundary element. Cell. 2018;173:1398–1412 e1322.
- Khalil AM, Guttman M, Huarte M, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106(28):11667–11672. doi: 10.1073/pnas.0904715106
- Wang X, Goodrich KJ, Gooding AR, et al. Targeting of polycomb repressive complex 2 to RNA by short repeats of consecutive guanines. Mol Cell. 2017;65(6):1056–1067 e1055. doi: 10.1016/j.molcel.2017.02.003
- Fawcett DW. An atlas of fine structure: the cell, its organelles and inclusions. Philadelphia: W. B. Saunders Co.; 1966.
- Berezney R. Organization and functions of the nuclear matrix. In: LS H, editor. Chromosomal nonhistone proteins. Vol. IV. CRC Press; 1984. p. 119–180.
- Bernhard W. A new staining procedure for electron microscopical cytology. J Ultrastruct Res. 1969;27(3–4):250–265. doi: 10.1016/S0022-5320(69)80016-X
- Biggiogera M, Fakan S. Fine structural specific visualization of RNA on ultrathin sections. J Histochem Cytochem. 1998;46(3):389–395. doi: 10.1177/002215549804600313
- Monneron A, Bernhard W. Fine structural organization of the interphase nucleus in some mammalian cells. J Ultrastruct Res. 1969;27(3–4):266–288. doi: 10.1016/S0022-5320(69)80017-1
- Bachellerie JP, Puvion E, Zalta JP. Ultrastructural Organization and biochemical characterization of chromatin · RNA · protein complexes isolated from Mammalian Cell Nuclei. Eur J Biochem. 1975;58(2):327–337. doi: 10.1111/j.1432-1033.1975.tb02379.x
- Mintz PJ, Patterson SD, Neuwald AF, et al. Purification and biochemical characterization of interchromatin granule clusters. Embo J. 1999;18(15):4308–4320. doi: 10.1093/emboj/18.15.4308
- Shopland LS, Johnson CV, Byron M, et al. Clustering of multiple specific genes and gene-rich R-bands around SC-35 domains: evidence for local euchromatic neighborhoods. J Cell Bio. 2003;162(6):981–990. doi: 10.1083/jcb.200303131
- Nickerson JA, Krockmalnic G, Wan KM, et al. The nuclear matrix revealed by eluting chromatin from a cross-linked nucleus. Proc Natl Acad Sci USA. 1997;94(9):4446–4450. doi: 10.1073/pnas.94.9.4446
- Smetana K, Steele WJ, Busch H. A nuclear ribonucleoprotein network. Exp Cell Res. 1963;31(1):198–201. doi: 10.1016/0014-4827(63)90169-1
- Herman RC, Penman S. Multiple decay rates of heterogeneous nuclear RNA in HeLa cells. Biochemistry. 1977;16(15):3460–3465. doi: 10.1021/bi00634a026
- Herman R, Weymouth L, Penman S. Heterogeneous nuclear RNA-protein fibers in chromatin-depleted nuclei. J Cell Bio. 1978;78(3):663–674. doi: 10.1083/jcb.78.3.663
- Pagoulatos GN, Darnell JE Jr. Fractionation of heterogeneous nuclear RNA: rates of hybridization and chromosomal distribution of reiterated sequences. J Mol Biol. 1970;54(3):517–535. doi: 10.1016/0022-2836(70)90123-3
- Pagoulatos GN, Darnell JE. A comparison of the heterogeneous nuclear RNA of HeLa cells in different periods of the cell growth cycle. J Cell Bio. 1970;44(3):476–483. doi: 10.1083/jcb.44.3.476
- Salditt-Georgieff M, Harpold MM, Wilson MC, et al. Large heterogeneous nuclear ribonucleic acid has three times as many 5’ caps as polyadenylic acid segments, and most caps do not enter polyribosomes. Mol Cell Biol. 1981;1(2):179–187. doi: 10.1128/MCB.1.2.179
- Salditt-Georgieff M, Darnell JE Jr. Further evidence that the majority of primary nuclear RNA transcripts in mammalian cells do not contribute to mRNA. Mol Cell Biol. 1982;2(6):701–707. doi: 10.1128/mcb.2.6.701-707.1982
- Bertone P, Stolc V, Royce TE, et al. Global identification of human transcribed sequences with genome tiling arrays. Science. 2004;306(5705):2242–2246. doi: 10.1126/science.1103388
- Consortium EP, Birney E, Stamatoyannopoulos JA, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816.
- Kapranov P, Cheng J, Dike S, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316(5830):1484–1488. doi: 10.1126/science.1138341
- Djebali S, Davis CA, Merkel A, et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101–108. doi: 10.1038/nature11233
- Carninci P, Kasukawa T, Katayama S, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309(5740):1559–1563. doi: 10.1126/science.1112014
- Samarina OP, Lukanidin EM, Molnar J, et al. Structural organization of nuclear complexes containing DNA-like RNA. J Mol Biol. 1968;33(1):251–263. doi: 10.1016/0022-2836(68)90292-1
- Walker BW, Lothstein L, Baker CL, et al. The release of 40S hnRNP particles by brief digestion of HeLa nuclei with micrococcal nuclease. Nucleic Acids Res. 1980;8(16):3639–3657. doi: 10.1093/nar/8.16.3639
- Narayan KS, Steele WJ, Smetana K, et al. Ultrastructural aspects of the ribonucleo-protein network in nuclei of walker tumor and rat liver. Exp Cell Res. 1967;46(1):65–77. doi: 10.1016/0014-4827(67)90409-0
- van Venrooij WJ, Janssen DB. HnRNP particles. Mol Biol Rep. 1978;4(1):3–8. doi: 10.1007/BF00775172
- Faiferman I, Pogo AO. Isolation of a nuclear ribonucleoprotein network that contains heterogeneous RNA and is bound to the nuclear envelope. Biochemistry. 1975;14(17):3808–3816. doi: 10.1021/bi00688a013
- Capco DG, Wan KM, Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982;29(3):847–858. doi: 10.1016/0092-8674(82)90446-9
- Fey EG, Krochmalnic G, Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Bio. 1986;102(5):1654–1665. doi: 10.1083/jcb.102.5.1654
- Fey EG, Ornelles DA, Penman S. Association of RNA with the cytoskeleton and the nuclear matrix. J Cell Sci Suppl. 1986;5(Supplement_5):99–119. doi: 10.1242/jcs.1986.Supplement_5.6
- Nickerson JA, Krochmalnic G, Wan KM, et al. Chromatin architecture and nuclear RNA. Proc Natl Acad Sci USA. 1989;86(1):177–181. doi: 10.1073/pnas.86.1.177
- Guttman M, Amit I, Garber M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458(7235):223–227. doi: 10.1038/nature07672
- Misteli T. The self-organizing genome: principles of genome architecture and function. Cell. 2020;183(1):28–45. doi: 10.1016/j.cell.2020.09.014
- van Steensel B, Belmont AS. Lamina-Associated Domains: links with chromosome architecture, Heterochromatin, and gene repression. Cell. 2017;169(5):780–791. doi: 10.1016/j.cell.2017.04.022
- Briand N, Collas P. Lamina-associated domains: peripheral matters and internal affairs. Genome Biol. 2020;21(1):85. doi: 10.1186/s13059-020-02003-5
- Tchurikov NA, Kravatsky YV. The role of rDNA clusters in global epigenetic gene regulation. Front Genet. 2021;12:730633. doi: 10.3389/fgene.2021.730633
- Cremer T, Cremer M. Chromosome territories. Cold Spring Harb Perspect Biol. 2010;2(3):a003889. doi: 10.1101/cshperspect.a003889
- Stack SM, Brown DB, Dewey WC. Visualization of interphase chromosomes. J Cell Sci. 1977;26(1):281–299. doi: 10.1242/jcs.26.1.281
- Fritz AJ, Sehgal N, Pliss A, et al. Chromosome territories and the global regulation of the genome. Genes Chromosomes Cancer. 2019;58(7):407–426. doi: 10.1002/gcc.22732
- Lieberman-Aiden E, van Berkum NL, Williams L, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326(5950):289–293. doi: 10.1126/science.1181369
- Quinodoz SA, Ollikainen N, Tabak B, et al. Higher-order inter-chromosomal hubs shape 3D genome organization in the nucleus. Cell. 2018;174(3):744–757 e724. doi: 10.1016/j.cell.2018.05.024
- Dekker J, Heard E. Structural and functional diversity of topologically associating domains. FEBS Lett. 2015;589(20PartA):2877–2884. doi: 10.1016/j.febslet.2015.08.044
- Barutcu AR, Blencowe BJ, Rinn JL. Differential contribution of steady-state RNA and active transcription in chromatin organization. EMBO Rep. 2019;20(10):e48068. doi: 10.15252/embr.201948068
- Cook PR, Brazell IA, Jost E. Characterization of nuclear structures containing superhelical DNA. J Cell Sci. 1976;22(2):303–324. doi: 10.1242/jcs.22.2.303
- Benyajati C, Worcel A. Isolation, characterization, and structure of the folded interphase genome of drosophila melanogaster. Cell. 1976;9(3):393–407. doi: 10.1016/0092-8674(76)90084-2
- Vogelstein B, Pardoll DM, Coffey DS. Supercoiled loops and eucaryotic DNA replicaton. Cell. 1980;22(1):79–85. doi: 10.1016/0092-8674(80)90156-7
- Pardoll DM, Vogelstein B, Coffey DS. A fixed site of DNA replication in eucaryotic cells. Cell. 1980;19(2):527–536. doi: 10.1016/0092-8674(80)90527-9
- Leonhardt H, Rahn HP, Weinzierl P, et al. Dynamics of DNA replication factories in living cells. J Cell Bio. 2000;149(2):271–280. doi: 10.1083/jcb.149.2.271
- Cook PR. The organization of replication and transcription. Science. 1999;284(5421):1790–1795. doi: 10.1126/science.284.5421.1790
- Jackson DA, Iborra FJ, Manders EM, et al. Numbers and organization of RNA polymerases, nascent transcripts, and transcription units in HeLa nuclei [published erratum appears. Mol Biol Cell. 1998;9(9):1523–1536. doi: 10.1091/mbc.9.6.1523
- Davidson EH, Britten RJ. Regulation of gene expression: possible role of repetitive sequences. Science. 1979;204(4397):1052–1059. doi: 10.1126/science.451548
- Hall LL, Lawrence JB. RNA as a fundamental component of interphase chromosomes: could repeats prove key? Curr Opin Genet Dev. 2016;37:137–147. doi: 10.1016/j.gde.2016.04.005
- Britten RJ, Graham DE, Neufeld BR. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418.
- Clemson CM, Hall LL, Byron M, et al. The X chromosome is organized into a gene-rich outer rim and an internal core containing silenced nongenic sequences. Proc Natl Acad Sci USA. 2006;103(20):7688–7693. doi: 10.1073/pnas.0601069103
- Hall LL, Carone DM, Gomez AV, et al. Stable C0T-1 repeat RNA is abundant and is associated with euchromatic interphase chromosomes. Cell. 2014;156(5):907–919. doi: 10.1016/j.cell.2014.01.042
- Creamer KM, Kolpa HJ, Lawrence JB. Nascent RNA scaffolds contribute to chromosome territory architecture and counter chromatin compaction. Mol Cell. 2021;81(17):3509–3525 e3505. doi: 10.1016/j.molcel.2021.07.004
- Braunschweig U, Barbosa-Morais NL, Pan Q, et al. Widespread intron retention in mammals functionally tunes transcriptomes. Genome Res. 2014;24(11):1774–1786. doi: 10.1101/gr.177790.114
- Boutz PL, Bhutkar A, Sharp PA. Detained introns are a novel, widespread class of post-transcriptionally spliced introns. Genes Dev. 2015;29(1):63–80. doi: 10.1101/gad.247361.114
- Dumbovic G, Braunschweig U, Langner HK, et al. Nuclear compartmentalization of TERT mRNA and TUG1 lncRNA is driven by intron retention. Nat Commun. 2021;12(1):3308. doi: 10.1038/s41467-021-23221-w
- Barutcu AR, Wu M, Braunschweig U, et al. Systematic mapping of nuclear domain-associated transcripts reveals speckles and lamina as hubs of functionally distinct retained introns. Mol Cell. 2022;82(5):1035–1052 e1039. doi: 10.1016/j.molcel.2021.12.010
- Quinodoz SA, Jachowicz JW, Bhat P, et al. RNA promotes the formation of spatial compartments in the nucleus. Cell. 2021;184(23):5775–5790 e5730. doi: 10.1016/j.cell.2021.10.014
- Hutchinson JN, Ensminger AW, Clemson CM, et al. Chess A: a screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics. 2007;8(1):39. doi: 10.1186/1471-2164-8-39
- Bunch H, Lawney BP, Burkholder A, et al. RNA polymerase II promoter-proximal pausing in mammalian long non-coding genes. Genomics. 2016;108(2):64–77. doi: 10.1016/j.ygeno.2016.07.003
- Drolet M, Phoenix P, Menzel R, et al. Overexpression of RNase H partially complements the growth defect of an Escherichia coli delta topA mutant: R-loop formation is a major problem in the absence of DNA topoisomerase I. Proc Natl Acad Sci USA. 1995;92(8):3526–3530. doi: 10.1073/pnas.92.8.3526
- Thomas M, White RL, Davis RW. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc Natl Acad Sci USA. 1976;73(7):2294–2298. doi: 10.1073/pnas.73.7.2294
- Skourti-Stathaki K, Proudfoot NJ. A double-edged sword: R loops as threats to genome integrity and powerful regulators of gene expression. Genes Dev. 2014;28(13):1384–1396. doi: 10.1101/gad.242990.114
- Chedin F. Nascent connections: R-Loops and Chromatin Patterning. Trends Genet. 2016;32(12):828–838. doi: 10.1016/j.tig.2016.10.002
- Sanz LA, Hartono SR, Lim YW, et al. Prevalent, dynamic, and conserved R-Loop structures associate with specific epigenomic signatures in mammals. Mol Cell. 2016;63(1):167–178. doi: 10.1016/j.molcel.2016.05.032
- Dumelie JG, Jaffrey SR. Defining the location of promoter-associated R-loops at near-nucleotide resolution using bisDRIP-seq. Elife. 2017;6:6. doi: 10.7554/eLife.28306
- Ariel F, Lucero L, Christ A, et al. R-Loop mediated trans action of the APOLO long noncoding RNA. Mol Cell. 2020;77(5):1055–1065 e1054. doi: 10.1016/j.molcel.2019.12.015
- Luo H, Zhu G, Eshelman MA, et al. HOTTIP-dependent R-loop formation regulates CTCF boundary activity and TAD integrity in leukemia. Mol Cell. 2022;82(4):833–851 e811. doi: 10.1016/j.molcel.2022.01.014
- Sanborn AL, Rao SS, Huang SC, et al. Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc Natl Acad Sci U S A. 2015;112(47):E6456–6465. doi: 10.1073/pnas.1518552112
- Fudenberg G, Imakaev M, Lu C, et al. Formation of chromosomal domains by loop extrusion. Cell Rep. 2016;15(9):2038–2049. doi: 10.1016/j.celrep.2016.04.085
- Beyer AL, Christensen ME, Walker BW, et al. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977;11(1):127–138. doi: 10.1016/0092-8674(77)90323-3
- Dreyfuss G, Matunis MJ, Pinol-Roma S, et al. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62(1):289–321. doi: 10.1146/annurev.bi.62.070193.001445
- Hudson WH, Ortlund EA. The structure, function and evolution of proteins that bind DNA and RNA. Nat Rev Mol Cell Biol. 2014;15(11):749–760. doi: 10.1038/nrm3884
- Kolpa HJ, Creamer KM, Hall LL, et al. SAF-A mutants disrupt chromatin structure through dominant negative effects on RNAs associated with chromatin. Mamm Genome. 2021;33(2):366–381. doi: 10.1007/s00335-021-09935-8
- Fackelmayer FO, Dahm K, Renz A, et al. Nucleic-acid-binding properties of hnRNP-U/SAF-A, a nuclear-matrix protein which binds DNA and RNA in vivo and in vitro. Eur J Biochem. 1994;221(2):749–757. doi: 10.1111/j.1432-1033.1994.tb18788.x
- Nozawa RS, Boteva L, Soares DC, et al. SAF-A regulates interphase chromosome structure through oligomerization with chromatin-associated RNAs. Cell. 2017;169(7):1214–1227 e1218. doi: 10.1016/j.cell.2017.05.029
- GH D, Kelley DR, Tenen D, et al. Widespread RNA binding by chromatin-associated proteins. Genome Biol. 2016;17(1):28. doi: 10.1186/s13059-016-0878-3
- Weinberg RA, Penman S. Small molecular weight monodisperse nuclear RNA. J Mol Biol. 1968;38(3):289–304. doi: 10.1016/0022-2836(68)90387-2
- Rogers J, Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci USA. 1980;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877
- Mount SM, Pettersson I, Hinterberger M, et al. The U1 small nuclear RNA-protein complex selectively binds a 5 splice site in vitro. Cell. 1983;33(2):509–518. doi: 10.1016/0092-8674(83)90432-4
- Setyono B, Pederson T. Ribonucleoprotein organization of eukaryotic RNA. XXX. Evidence that U1 small nuclear RNA is a ribonucleoprotein when base-paired with pre-messenger RNA in vivo. J Mol Biol. 1984;174(2):285–295. doi: 10.1016/0022-2836(84)90339-5
- Horowitz DS, Krainer AR. Mechanisms for selecting 5 splice sites in mammalian pre-mRNA splicing. Trends Genet. 1994;10(3):100–106. doi: 10.1016/0168-9525(94)90233-X
- Sun H, Chasin LA. Multiple splicing defects in an intronic false exon. Mol Cell Biol. 2000;20(17):6414–6425. doi: 10.1128/MCB.20.17.6414-6425.2000
- Jobert L, Pinzon N, Van Herreweghe E, et al. Human U1 snRNA forms a new chromatin-associated snRNP with TAF15. EMBO Rep. 2009;10(5):494–500. doi: 10.1038/embor.2009.24
- Yin Y, Lu JY, Zhang X, et al. U1 snRNP regulates chromatin retention of noncoding RNAs. Nature. 2020;580(7801):147–150. doi: 10.1038/s41586-020-2105-3
- Huang S, Spector DL. U1 and U2 small nuclear RNAs are present in nuclear speckles. Proc Natl Acad Sci USA. 1992;89(1):305–308. doi: 10.1073/pnas.89.1.305
- Van Treeck B, Parker R. Emerging roles for intermolecular RNA-RNA interactions in RNP assemblies. Cell. 2018;174(4):791–802. doi: 10.1016/j.cell.2018.07.023
- Fedoroff N, Wellauer PK, Wall R. Intermolecular duplexes in heterogeneous nuclear RNA from HeLa cells. Cell. 1977;10(4):597–610. doi: 10.1016/0092-8674(77)90092-7
- Sommerville J, Scheer U. Transcription of complementary repeat sequences in amphibian oocytes. Chromosoma. 1982;86(1):95–113. doi: 10.1007/BF00330732
- Singh G, Kucukural A, Cenik C, et al. The cellular EJC interactome reveals higher-order mRNP structure and an EJC-SR Protein Nexus. Cell. 2012;151(4):750–764. doi: 10.1016/j.cell.2012.10.007
- Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nat Rev Genet. 2014;15(12):829–845. doi: 10.1038/nrg3813
- van der Lee R, Buljan M, Lang B, et al. Classification of intrinsically disordered regions and proteins. Chem Rev. 2014;114(13):6589–6631. doi: 10.1021/cr400525m
- Chong S, Mir M. Towards decoding the Sequence-based grammar governing the functions of intrinsically disordered protein regions. J Mol Biol. 2021;433(12):166724. doi: 10.1016/j.jmb.2020.11.023
- Kato M, Han TW, Xie S, et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149(4):753–767. doi: 10.1016/j.cell.2012.04.017
- Kato M, SL M. The low-complexity domain of the FUS RNA binding protein self-assembles via the mutually exclusive use of two distinct cross-β cores. Proc Natl Acad Sci USA. 2021;118(42):118. doi: 10.1073/pnas.2114412118
- Xiao R, Tang P, Yang B, et al. Nuclear matrix factor hnRNP U/SAF-A exerts a global control of alternative splicing by regulating U2 snRNP maturation. Mol Cell. 2012;45(5):656–668. doi: 10.1016/j.molcel.2012.01.009
- Shin Y, Brangwynne CP. Liquid phase condensation in cell physiology and disease. Science. 2017;357(6357):357. doi: 10.1126/science.aaf4382
- WK C, JH S, Hecht M, et al. Cisse II: Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science. 2018;361(6400):412–415. doi: 10.1126/science.aar4199
- Guo YE, Manteiga JC, Henninger JE, et al. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature. 2019;572(7770):543–548. doi: 10.1038/s41586-019-1464-0
- Larson AG, Elnatan D, Keenen MM, et al. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature. 2017;547(7662):236–240. doi: 10.1038/nature22822
- Lin YC, Kumar MS, Ramesh N, et al. Interactions between ALS-linked FUS and nucleoporins are associated with defects in the nucleocytoplasmic transport pathway. Nat Neurosci. 2021;24(8):1077–1088. doi: 10.1038/s41593-021-00859-9
- Boczek EE, Fursch J, Niedermeier ML, et al. HspB8 prevents aberrant phase transitions of FUS by chaperoning its folded RNA binding domain. Elife. 2021;10:10. doi: 10.7554/eLife.69377
- Molliex A, Temirov J, Lee J, et al. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell. 2015;163(1):123–133. doi: 10.1016/j.cell.2015.09.015
- Bose M, Lampe M, Mahamid J, et al. Liquid-to-solid phase transition of oskar ribonucleoprotein granules is essential for their function in drosophila embryonic development. Cell. 2022;185(8):1308–1324.e23. doi: 10.1016/j.cell.2022.02.022
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412
- He DC, Nickerson JA, Penman S. Core filaments of the nuclear matrix. J Cell Bio. 1990;110(3):569–580. doi: 10.1083/jcb.110.3.569
- Jackson DA, Cook PR. Visualization of a filamentous nucleoskeleton with a 23 nm axial repeat. Embo J. 1988;7(12):3667–3677. doi: 10.1002/j.1460-2075.1988.tb03248.x
- Tamashunas AC, Tocco VJ, Matthews J, et al. High-throughput gene screen reveals modulators of nuclear shape. Mol Biol Cell. 2020;31(13):1392–1402. doi: 10.1091/mbc.E19-09-0520
- Sparmann A, Vogel J. RNA-based medicine: from molecular mechanisms to therapy. Embo J. 2023;42(21):e114760. doi: 10.15252/embj.2023114760
- Zhu Y, Zhu L, Wang X, et al. RNA-based therapeutics: an overview and prospectus. Cell Death Dis. 2022;13(7):644. doi: 10.1038/s41419-022-05075-2
- Sparber P, Filatova A, Khantemirova M, et al. The role of long non-coding RNAs in the pathogenesis of hereditary diseases. BMC Med Genomics. 2019;12(S2):42. doi: 10.1186/s12920-019-0487-6
- DiStefano JK. The emerging role of long noncoding RNAs in human disease. Methods Mol Biol. 2018;1706:91–110.
- Aznaourova M, Schmerer N, Schmeck B, et al. Disease-causing mutations and rearrangements in long non-coding RNA gene loci. Front Genet. 2020;11:527484. doi: 10.3389/fgene.2020.527484
- Wang S, Qiao J, Feng S. Prediction of lncRNA and disease associations based on residual graph convolutional networks with attention mechanism. Sci Rep. 2024;14(1):5185. doi: 10.1038/s41598-024-55957-y
- Lu C, Yang M, Luo F, et al. Prediction of lncRNA-disease associations based on inductive matrix completion. Bioinformatics. 2018;34:3357–3364. doi: 10.1093/bioinformatics/bty327
- Khorkova O, Stahl J, Joji A, et al. Long non-coding RNA-targeting therapeutics: discovery and development update. Expert Opin Drug Discov. 2023;18(9):1011–1029. doi: 10.1080/17460441.2023.2236552
- Winkle M, SM E-D, Fabbri M, et al. Noncoding RNA therapeutics — challenges and potential solutions. Nat Rev Drug Discov. 2021;20(8):629–651. doi: 10.1038/s41573-021-00219-z
- Lennox KA, Behlke MA. Cellular localization of long non-coding RNAs affects silencing by RNAi more than by antisense oligonucleotides. Nucleic Acids Res. 2016;44(2):863–877. doi: 10.1093/nar/gkv1206
- Arun G, Aggarwal D, Spector DL. MALAT1 long non-coding RNA: functional implications. Noncoding RNA. 2020;6(2):6. doi: 10.3390/ncrna6020022
- Zhang B, Arun G, Mao YS, et al. The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult. Cell Rep. 2012;2(1):111–123. doi: 10.1016/j.celrep.2012.06.003
- Nakagawa S, Ip JY, Shioi G, et al. Malat1 is not an essential component of nuclear speckles in mice. RNA. 2012;18(8):1487–1499. doi: 10.1261/rna.033217.112
- Eissmann M, Gutschner T, Hammerle M, et al. Loss of the abundant nuclear non-coding RNA MALAT1 is compatible with life and development. RNA Biol. 2012;9(8):1076–1087. doi: 10.4161/rna.21089
- Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22(39):8031–8041. doi: 10.1038/sj.onc.1206928
- Wilusz JE, Freier SM, Spector DL. 3 end processing of a long nuclear-retained Noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell. 2008;135(5):919–932. doi: 10.1016/j.cell.2008.10.012
- Arun G, Diermeier S, Akerman M, et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016;30(1):34–51. doi: 10.1101/gad.270959.115
- Crick FH. On protein synthesis. Symp Soc Exp Biol. 1958;12:138–163.
- Nirenberg M, Leder P. RNA codewords and protein synthesis. The effect of trinucleotides upon the binding of srna to ribosomes. Science. 1964;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399
- Morgan AR, Wells RD, Khorana HG. Studies on polynucleotides, lix. Further codon assignments from amino acid incorporations directed by ribopolynucleotides containing repeating trinucleotide sequences. Proc Natl Acad Sci USA. 1966;56(6):1899–1906. doi: 10.1073/pnas.56.6.1899
- Havlin S, Buldyrev SV, Goldberger AL, et al. Statistical and linguistic features of DNA sequences. Fractals. 1995;3(2):269–284. doi: 10.1142/S0218348X95000229
- Fabbri M, Girnita L, Varani G, et al. Decrypting noncoding RNA interactions, structures, and functional networks. Genome Res. 2019;29(9):1377–1388. doi: 10.1101/gr.247239.118
- Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482(7385):339–346. doi: 10.1038/nature10887
- Lin A, Hu Q, Li C, et al. The LINK-A lncRNA interacts with PtdIns(3,4,5)P3 to hyperactivate AKT and confer resistance to AKT inhibitors. Nat Cell Biol. 2017;19(3):238–251. doi: 10.1038/ncb3473
- Duan N, Arroyo M, Deng W, et al. Visualization and characterization of RNA–protein interactions in living cells. Nucleic Acids Res. 2021;49(18):e107. doi: 10.1093/nar/gkab614
