Abstract
Ssanghwa-tang (SHT) is a traditional Korean herbal medicine widely prescribed to decrease fatigue following an illness. The purpose of this study was to investigate the effects of SHT on osteoclast differentiation in vitro, and on bone loss in ovariectomized (OVX) rats in vivo. SHT significantly reduced the receptor activator for the nuclear factor κB (NF-κB) ligand (RANKL)-induced tartrate-resistant acid phosphatase (TRAP) activity, and multinucleated osteoclast formation in RAW264.7 cells without affecting cell viability. In addition, SHT significantly attenuated RANKL-induced mRNA expression levels of c-Src and cathepsin K. To examine the in vivo effect of SHT on OVX-induced bone loss in OVX rats, we administered SHT (0.6 g/kg BID) orally to OVX rats for 12 weeks. SHT administration significantly blocked OVX-induced decrease of femoral bone mineral density (BMD) and femoral trabeculae in OVX rats. In conclusion, these results suggest that SHT treatment effectively prevents OVX-induced bone loss, and this effect may result from its inhibitory effect on osteoclast differentiation.
Introduction
Bone constantly maintains its homeostasis state through bone resorption by osteoclasts and bone formation by osteoblasts, which is referred to as bone remodeling (Teitelbaum Citation2000). When the rate of bone resorption is increased compared to that of bone formation, it causes an imbalance in bone remodeling, which results in reduction of bone mass and weakness of bone microstructure. It eventually leads to several bone diseases such as osteoporosis (Rodan and Martin Citation2000). Therefore, bone research has focused on the regulation of osteoclast differentiation and/or the bone resorptive activity of osteoclasts, in the quest to develop therapeutic treatment for osteoporosis (Boyle et al. Citation2003).
Traditional herbal medicines based on natural ingredients have been investigated in bone research, particularly for their beneficial effect on bone through inhibition of osteoclast differentiation (Putnam et al. Citation2007). Ssanghwa-tang (SHT), a traditional herbal medication, is known to relieve fatigue, boost energy and help recuperation following an illness (Heo Citation1994). Several pharmacological activities of SHT, including anti-inflammatory, hepatoprotective and analgesic effects have been reported (Kim and Hwang Citation1981; Han et al. Citation1983; Han and Shim Citation1989). Recently, five constituents (cinnamic acid, paeoniflorin, glycyrrhizic acid, 6-gingerol and decursin) of SHT are simultaneously determined by HPLC analysis for the quality analysis of SHT (Won et al. Citation2010). Interestingly, SHT with Cervi cornu parvum increases osteoblast growth, collagen generation and femoral bone mineral density (BMD) in ovariectomized (OVX) rats, suggesting a preventive effect of SHT containing Cervi cornu parvum on OVX-induced bone loss by stimulating bone formation (Lee and Lim Citation2003). However, no research has been conducted until now to study the effects of SHT on osteoclast differentiation and OVX-induced bone loss.
RAW264.7 macrophage cells are derived from a murine monocyte cell line that differentiates into osteoclasts in response to the receptor activator for the nuclear factor κB (NF-κB) ligand (RANKL). Upon RANKL treatment, RAW264.7 cells express osteoclast differentiation-related genes, including tartrate-resistant acid phosphatase (TRAP), c-Src and cathepsin K (Hsu et al. Citation1999). OVX rats are a typical osteoporosis animal model with increased bone resorption, increased bone loss and reduced bone mineral density (BMD) (Kalu Citation1991; Lelovas et al. Citation2008). In this study, we investigated the effects of SHT on osteoclast differentiation using RAW264.7 cells, and on OVX-induced bone loss in OVX rats.
Materials and methods
Preparation of SHT
SHT was prepared by using Paeoniae radix 468.5 g, Rehmanniae radix et rhizoma preparata 187.5 g, Astragali radix 187.5 g, Angelicae gigantis radix 187.5 g, Cnidii rhizoma 187.5 g, Cinnamon bark 140.5 g, Glycyrrhizae radix 140.5 g, Zingiber officinale 74.5 g and Zizyphus jujuba 100 g. All medicinal herbs were purchased from the Korea Medicine Herbs Association (Youngcheon, Kyoungbook, Korea). All voucher specimens were deposited in the herbal bank of the Center for the Herbal Medicine Improvement Research, Korea Institute of Oriental Medicine. The total quantity of medicinal herbs was placed in 16.74 L of distilled water, and then extracted by heating for 3 hr (Gyeongseo Extractor Cosmos-600, Inchon, Korea). After extraction, SHT was filtered out using standard testing sieves (150 µm) (Retsch, Haan, Germany), lyophilized and stored at 4°C before use.
Cell culture and induction of multinucleated osteoclasts
All materials for cell culture were purchased from Gibco (Invitrogen Inc., NY, USA). RAW264.7 cells were purchased from the American Type Culture Collection (ATCC), and maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL of penicillin and 100 mg/mL streptomycin, with a change of medium every 3 days in 5% CO2 at 37°C. For osteoclast differentiation, RAW264.7 cells (1 × 103 cells/well) were plated in a 96-well plate and cultured in α-minimal essential medium (α-MEM), supplemented with 10% FBS and 100 ng/mL RANKL (R&D Systems Inc., Minneapolis, MN, USA). After 3 to 4 days, multinucleated osteoclasts were observed.
Cell viability assay
RAW264.7 cells (1×103 cells/well) were plated in a 96-well plate with α-MEM containing 10% FBS. After 24 hr, serially diluted SHT was treated and incubated for 3 days. Cell viability was then measured by the Cell Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies, Sunnyvale, CA, USA) according to the manufacturer's protocol. Data represented the mean±SD of three independent experiments.
TRAP staining and activity assay
Multinucleated osteoclasts were fixed with 10% formalin for 10 min and ethanol/acetone (1:1) for 1 min, and then stained by the Leukocyte Acid Phosphatase Kit 387-A (Sigma, St. Luis, MO, USA). The images of TRAP-positive multinucleated cells were captured under a microscope with DP Controller (Olympus Optical Co. Ltd., Tokyo, Japan). To measure TRAP activity, multinucleated osteoclasts were fixed with 10% formalin for 10 min and 95% ethanol for 1 min, and then incubated with 100 µL of citrate buffer (50 mM, pH 4.6) containing 10 mM sodium tartrate and 5 mM p-nitrophenylphosphate (Sigma, St. Luis, MO, USA). After incubation for 1 hr, the enzyme reaction mixtures were transferred into a new plate containing an equal volume of 0.1 N NaOH. Absorbance was measured at 410 nm, and TRAP activity was presented as a percentage (%) of control. Data represented the mean±SD of three independent experiments.
Primer design and real-time quantitative PCR (QPCR)
Primers were designed using an online primer design program (Rozen and Skaletsky Citation2000). The following primer sequences were used in this study: TRAP forward, 5′-ACA CAG TGA TGC TGT GTG GCA ACT C-3′; TRAP reverse, 5′-CCA GAG GCT TCC ACA TAT ATG ATG G-3′; c-Src forward, 5′-CCA GGC TGA GGA GTG GTA CT-3′; c-Src reverse, 5′-CAG CTT GCG GAT CTT GTA GT-3′; Cathepsin K forward, 5′-GGC CAA CTC AAG AAG AAA AC-3′; Cathepsin K reverse, 5′-GTG CTT GCT TCC CTT CTG G-3′; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5′-aac ttt ggc att gtg gaa gg-3′; GAPDH reverse, 5′-aca cat tgg ggg tag gaa ca-3′. Total RNA was isolated with TRIzol reagent (Invitrogen Inc., Carlsbad, CA, USA) according to the manufacturer's protocol. Subsequently, first-strand cDNA was synthesized with 2 µg of total RNA, 1 µM of oligo-dT18 primer and 10 units of RNase inhibitor RNasin (Promega, WI, USA) using Omniscript Reverse Transcriptase (Qiagen, Valencia, CA, USA), according to the manufacturer's protocol. Next, SYBR green-based QPCR amplification was performed using the Brilliant SYBR Green Master Mix (Stratagene, Santa Clara, CA, USA) and the Stratagene Mx3000P Real-Time PCR system with first-strand cDNA diluted 1:50 and 20 pmol of primers according to the manufacturer's protocol. The PCR reaction consisted of three segments. The first segment at 95°C for 10 min was conducted to activate the polymerase; the second one corresponded to three-step cycling (40 cycles) at 95°C for 40 sec (denaturation), 60°C for 40 sec (annealing) and 72°C for 1 min (extension). The third segment was conducted to generate PCR product temperature dissociation curves (melting curves) at 95°C for 1 min, 55°C for 30 sec and 95°C for 30 sec. All reactions were run in triplicate, and data were analyzed by the 2-ΔΔCT method as described previously (Livak and Schmittgen Citation2001). GAPDH was used as the internal standard gene.
Animal model of OVX rats
Ten-week-old female Sprague-Dawley rats (Orient Bio Inc., Seoul, Korea) weighing 190–210 g were housed at 22±1°C and 55±10% humidity on a 12 hr light/12 hr dark cycle with free access to food and water. After acclimatization for one week in the laboratory environment, they were either sham-operated (sham, n=9) or underwent surgical OVX (OVX, n=18). One week after surgery, OVX rats were randomly divided into two groups of nine rats: (1) OVX: bilateral OVX; and (2) SHT: bilateral OVX followed by SHT administration. Measurement of the body weight as well as the administration of SHT began one week after surgery. SHT were orally administered at 0.6 g/kg BID based on a daily clinical dose (15 mL/kg). The same amount of saline was orally administered to the sham and OVX groups. Animal experiments were carried out in accordance with the National Institute of Health's Guidelines for the Care and Use of Laboratory Animals. The experiments were approved by the Institutional Animal Care and Use Committee at the Korea Institute of Oriental Medicine.
Biochemical analysis of serum
A biochemical analyzer (Hitachi7080, Tokyo, Japan) was used for measuring the serum concentrations of alkaline phosphatase (ALP), triglyceride, phosphate and calcium.
Measurement of BMD and histological analysis
Rats were sacrificed 12 weeks after SHT administration. Upon autopsy, the right femur of each animal was extracted and fixed in 10% formalin for 7 days. After removing the formalin, BMD was measured by dual-energy X-ray absorption (DEXA) (LUNAR Co. Ltd., Madison, WI, USA). The removed femurs were decalcified in formic acid, and then dehydrated progressively in 70% alcohol, 100% alcohol and acetone. After dehydration, the bones were immersed in xylene and treated for paraffin embedding. Paraffin-embedded bone tissues were dissected into 3-µm slices, and stained with hematoxylin & eosin (H&E). An optical microscope (Nikon ECLIPSE 80i, Nikon, Tokyo, Japan) was used for the analysis of trabeculae bone matrix.
HPLC analysis
All HPLC reagents were purchased from J.T. Baker (Phillipsburg, NJ, USA). The Ultimate-3000 HPLC system (Dionex, Sunnyvale, CA, USA) consisted of a pump (LPG 3X00), auto sampler (ACC-3000), column oven (TCC-3000SD) and diode array UV/VIS detector (DAD-3000(RS)). The column was a C18 column (5 µm, 120 Å, 4.6 mm×150 mm) (Dionex, Sunnyvale, CA, USA), and its temperature was maintained at 25°C. The mobile phase consisted of water and methanol with gradient elution at a flow rate of 1.0 mL/min. The column effluents were simultaneously monitored at 230 nm (paeoniflorin, 6-gingerol, decursin), 254 nm (glycyrrhizic acid) and 280 nm (cinnamic acid). The authentic standards of cinnamic acid, paeoniflorin, glycyrrhizic acid, 6-gingerol and decursin were purchased from NPC Biotechnology (Seoul, Korea). They were prepared for HPLC analysis at concentrations of 250 µg/mL, 50 µg/mL, 100 µg/mL, 100 µg/mL and 400 µg/mL, respectively. They were progressively diluted using 60% methanol. For the analysis of SHT, 20 mg of SHT was mixed in 10 mL of 60% methanol, and filtrated using a 0.45 µm syringe filter. The injection volume of SHT was 20 µL.
Statistical analysis
SPSS software (SPSS Inc., Chicago, IL, USA) was used to perform all statistical analyses. The significance of TRAP activity was determined using the Student t-test. The significance of the mRNA expression levels of osteoclast differentiation-related genes was determined with the Student t-test using GAPDH-normalized 2-ΔΔCT values. For statistical analysis on the results of the animal experiment, a parametric one-way analysis of variance was used to test for differences among the three groups. Duncan multiple comparison tests were used to confirm significant differences in the mean value between the groups. A P value of less than 0.05 was considered statistically significant.
Results
Effect of SHT on RAW264.7 cell proliferation
Before investigating the effect of SHT on osteoclast differentiation, the effect of SHT (25–200 µg/mL) on RAW264.7 cell proliferation was examined. SHT up to a concentration of 200 µg/mL did not adversely affect RAW264.7 cell proliferation (A). Therefore, the following experiment was conducted using 200 µg/mL of SHT as the maximum concentration.
Figure 1. Effects of SHT on (A) cell growth, (B) RANKL-induced TRAP activity and (C) formation of TRAP-positive multinucleated osteoclasts in RAW264.7 cells. RAW264.7 cells were placed in a 96-well plate at a density of 1×103 cells/well with or without RANKL (100 ng/mL). The cells were incubated with different concentrations of SHT (25–200 µg/mL) for 3 days, and multinucleated osteoclasts were observed under a microscope. Cell viability was tested with CCK-8 assay. TRAP activity and staining were performed as described in Materials and methods. * P<0.05; ** P<0.01.
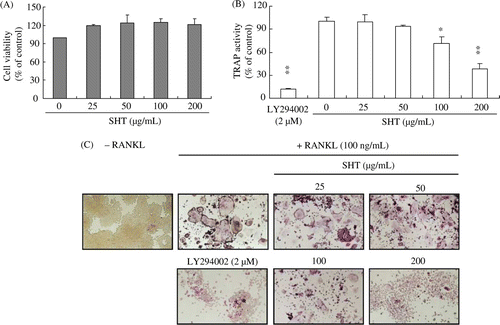
Effect of SHT on osteoclast differentiation in RANKL-induced RAW264.7 cells
TRAP is highly expressed in osteoclasts and widely used as a phenotypic marker of osteoclast differentiation. To investigate the effect of SHT on osteoclast differentiation, we measured TRAP activity, and monitored multinucleated osteoclast formation by TRAP staining. SHT dose-dependently and significantly decreased TRAP activity from 100 µg/mL (P<0.05) (B). In addition, multinucleated osteoclast formation was decreased as the concentration of SHT increased (C). PI3K inhibitor (LY294002), known to inhibit osteoclastogenesis, significantly blocked RANKL-induced increase of TRAP activity and multinucleated osteoclast formation.
Effect of SHT on mRNA expression levels of osteoclast differentiation-related genes in RANKL-induced RAW264.7 cells
TRAP, c-Src and cathepsin K genes are necessary for osteoclast differentiation and bone resorptive activity of osteoclasts. Therefore, the inhibitory effects of SHT on mRNA expression levels of these genes were investigated by real-time QPCR. The mRNA expression levels of these genes were dramatically increased by RANKL treatment, while SHT (200 µg/mL) significantly decreased the mRNA expression levels of c-Src and cathepsin K (P<0.05). In addition, SHT treatment decreased the mRNA expression level of TRAP, although it was not statistically significant ().
Table 1. Effect of SHT on RANKL included in RNA expression differentiation related genes in RAW264.7 cells
Effect of SHT on body weight and organ weight of OVX rats
To investigate the effect of SHT on OVX rats, SHT was administered orally to OVX rats for 12 weeks. When compared to the body weight of the sham group, the body weight of OVX rats was significantly increased one week after administration. However, SHT administration did not alter OVX-induced increase of body weight (). In addition, when compared to the sham group, the uterine weight of OVX rats showed significant reduction of about 84% (P<0.01), but SHT administration did not affect this reduction (). When comparing the weight of the other organs with that of the sham group, no significant differences were found between the groups (data not shown).
Figure 2. Change in body weight of rats during SHT administration for 12 weeks. The body weights of OVX and SHT (0.6 g/kg BID) groups were significantly increased for 12 weeks when compared to the sham group (P<0.01).
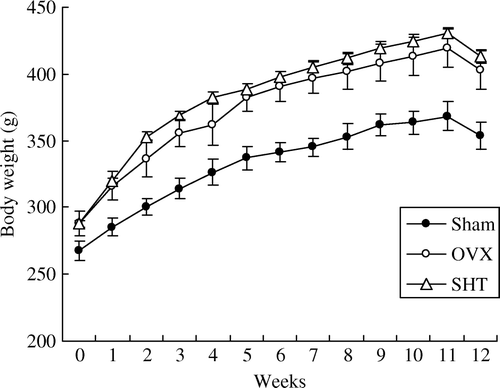
Table 2. Effect of SHT on RANKL uterine weight and blochemical parameters in serum of ovx rats
Biochemical analysis of serum samples
The OVX group showed a significant increase in serum triglyceride levels compared to the sham group (P<0.05) (). SHT administration reduced the increase in serum triglyceride levels, although it was not statistically significant. The SHT group showed no differences in other biochemical parameters, when compared to the OVX group.
Measurement of BMD and histological analysis
To investigate the effect of SHT on bone loss in OVX rats, SHT was administered for 12 weeks, and BMD of the sternum and femur was then measured. The OVX group showed a significant decrease in sternal and femoral BMD compared to the sham group (P<0.01). However, when compared to the OVX group, the SHT group showed prevention of OVX-induced decrease of femoral BMD (). Femoral histological analysis was conducted after SHT administration. The sham group showed normal femoral trabeculae, but the OVX group showed obvious reduction of femoral trabeculae (A). However, compared to the OVX group, the SHT group showed prevention of OVX-induced decrease of femoral trabeculae. Estrogen treatment (50 µg/kg of body weight), known to suppress bone loss, prevented the decrease of femoral BMD in OVX rats (data not shown). Normal uterine histology was observed in the sham group, whereas uterine atrophy was observed in the OVX group. When compared to the OVX group, SHT administration did not affect OVX-induced uterine atrophy in OVX rats (B).
Figure 3. Histomorphological analysis of (A) femur (× 20) and (B) uterus (× 40). After SHT administration (0.6 g/kg BID) for 12 weeks, the femur and uterus of each animal were fixed and stained with H&E as described in Materials and methods. Representative photomicrographs from the sham, OVX and SHT groups. Arrow, growth plate; arrow head, trabeculae.
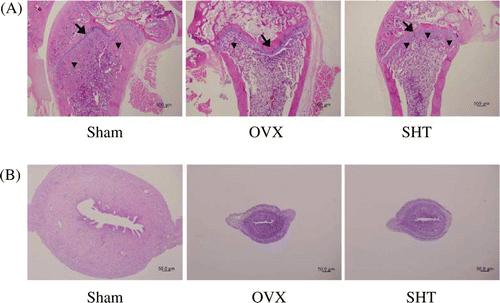
Table 3. Effect of SHT on BMD of OVX rats
HPLC analysis
To analyze the ingredients of SHT associated with the inhibition of osteoclast differentiation and bone loss in OVX rats, HPLC analysis was applied to simultaneously identify five marker components in SHT. The five components in SHT were detected at the same retention times obtained from HPLC analysis of standard components reported previously (data not shown). The major components in SHT were found to be paeoniflorin and glycyrrhizic acid ().
Table 4. Content of marker components in SHT
Discussion
Osteoclast precursor cells are differentiated into mature osteoclasts in the presence of RANKL. Osteoclasts express osteoclast differentiation-related genes and have bone resorptive activity on mineralized bone (Teitelbaum Citation2000). Cathepsin K, the specific cysteine protease frequently found within osteoclasts, is known to play an important role in osteoclast differentiation and bone resorption (Saftig et al. Citation2000). In addition, c-Src knockout mice have a sufficient number of osteoclasts incapable of bone resorptive activity, suggesting that c-Src is necessary for the bone resorptive activity of osteoclasts (Soriano et al. Citation1991; Lowe et al. Citation1993). Expression of these genes is regulated by essential transcription factors, such as AP-1, NF-κB or NFAT, during osteoclast differentiation (Takayanagi et al. Citation2002; Asagiri and Takayanagi Citation2007; Pang et al. Citation2007). Therefore, the inhibitory effect of SHT on osteoclast differentiation may result from inhibiting the expression or function of transcription factors that regulate the expression of osteoclast differentiation-related genes.
Traditional herbal medicines are mixtures of active ingredients affecting bone cells differently. They contain stimulative ingredients on osteoblast differentiation increasing bone formation and inhibitory ingredients on osteoclast differentiation reducing bone loss, which contribute together to the anti-osteoporotic effect of herbal medicine (Qin et al. Citation2008). Extracts of medicinal herbs (Rehmannia glutinosa Libosch and angelicae gigantis radix) of which SHT is composed stimulate osteoblast proliferation and activity while inhibiting osteoclast differentiation or bone loss in OVX rats (Oh et al. Citation2003; Kil et al. Citation2008). The anabolic effect of SHT on osteoblast differentiation (Lee and Lim Citation2003) and the inhibitory effect of SHT on osteoclast differentiation () have been studied. In addition, a major marker component (paeoniflorin, ) in SHT stimulates osteoblast differentiation (Yen et al. Citation2007) although its effect on osteoclast differentiation is still unknown. Therefore, different components affecting osteoclast or osteoblast differentiation within SHT could cooperate to suppress OVX-induced bone loss in OVX rats.
Phytoestrogens have estrogen-like activity and the potential to prevent bone loss in OVX rats (Branca Citation2003). In addition, phytoestrogens are known to have a suppressive effect on osteoclast differentiation, as well as osteoclastic bone resorption. They inhibit RANKL-induced osteoclast differentiation in RAW264.7 cells by inhibiting NF-κB activation (García Palacios et al. Citation2005). Phytoestrogens also inhibit the inward rectifier K+ channel in osteoclasts, leading to membrane depolarization, intracellular influx of Ca2+ and inhibition of osteoclast-mediated bone resorption (Okamoto et al. Citation2001). Thus, some SHT components with estrogenic properties could inhibit osteoclastogenesis and bone resorption activity, which prevent OVX-induced bone loss in OVX rats.
In conclusion, we first demonstrated that SHT inhibits RANKL-induced formation of multinucleated osteoclasts, mRNA expression levels of osteoclast differentiation-related genes, and OVX-induced bone loss. These results suggested that the inhibitory effect of SHT on osteoclast differentiation may consequently contribute to prevent OVX-induced bone loss in OVX rats . Further studies are required to identify the active ingredients preventing bone loss in SHT, and to determine the precise molecular mechanism of these ingredients.
Acknowledgements
This work was supported by a grant (No. K10050) from the Korea Institute of Oriental Medicine funded by the Ministry of Education, Science and Technology (MEST), the republic of Korea.
References
- Asagiri , M and Takayanagi , H. 2007 . The molecular understanding of osteoclast differentiation . Bone. , 40 : 251 – 264 .
- Boyle , WJ , Simonet , WS and Lacey , DL. 2003 . Osteoclast differentiation and activation . Nature. , 423 : 337 – 342 .
- Branca , F. 2003 . Dietary phyto-oestrogens and bone health . Proc Nutr Soc. , 62 : 877 – 887 .
- García Palacios , V , Robinson , LJ , Borysenko , CW , Lehmann , T , Kalla , SE and Blair , HC. 2005 . Negative regulation of RANKL-induced osteoclastic differentiation in RAW264.7 cells by estrogen and phytoestrogens . J Biol Chem. , 280 : 13720 – 13727 .
- Han , DS , Lee , HK and Cho , HJ. 1983 . Analgesic and anticonvulsionary effects of Ssanghwa-Tang . Kor J Pharmacog. , 14 : 60 – 63 .
- Han , YH and Shim , CK. 1989 . Effect of a blended Korean herbal remedy, Ssang Wha Tang, on the liver cytoplasmic protein binding of sulfobromophthalein in rats . Phytother Res. , 3 : 109 – 111 .
- Heo J. 1994 Dongeuibogam Seoul Namsandang Press 670 675
- Hsu , H , Lacey , DL , Dunstan , CR , Solovyev , I , Colombero , A , Timms , E , Tan , HL , Elliott , G , Kelley , MJ Sarosi , I . 1999 . Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand . Proc Natl Acad Sci USA. , 96 : 3540 – 3545 .
- Kalu , DN. 1991 . The ovariectomized rat model of postmenopausal bone loss . Bone Miner. , 15 : 175 – 191 .
- Kil , JS , Kim , MG , Choi , HM , Lim , JP , Boo , Y , Kim , EH , Kim , JB , Kim , HK and Leem , KH. 2008 . Inhibitory effects of Angelicae Gigantis Radix on osteoclast formation . Phytother Res. , 22 : 472 – 476 .
- Kim , IH and Hwang , KJ. 1981 . Studies on the anti-inflammtory activities of Ssangwha-tang . Natural Pro Sci. , 12 : 131 – 136 .
- Lee , H and Lim , HH. 2003 . Effects of Ssangwha-tang added to Cervi Cornu Parvum on bone density and bone biochemical marker in ovariectomized rats . J Ori R Med. , 13 : 45 – 67 .
- Lelovas , PP , Xanthos , TT , Thoma , SE , Lyritis , GP and Dontas , IA. 2008 . The laboratory rat as an animal model for osteoporosis research . Comp Med. , 58 : 424 – 430 .
- Livak , KJ and Schmittgen , TD. 2001 . Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method . Methods. , 25 : 402 – 408 .
- Lowe , C , Yoneda , T , Boyce , BF , Chen , H , Mundy , GR and Soriano , P. 1993 . Osteoporosis in Src-deficient mice is due to an autonomous defect of osteoclasts . Proc Natl Acad Sci USA. , 90 : 4485 – 4489 .
- Oh , KO , Kim , SW , Kim , JY , Ko , SY , Kim , HM , Baek , JH , Ryoo , HM and Kim , JK. 2003 . Effect of Rehmannia glutinosa Libosch extracts on bone metabolism . Clin Chim Acta. , 334 : 185 – 195 .
- Okamoto , F , Okabe , K and Kajiya , H. 2001 . Genistein, a soybean isoflavone, inhibits inward rectifier K(+) channels in rat osteoclasts . Jpn J Physiol. , 51 : 501 – 509 .
- Pang , M , Martinez , AF , Fernandez , I , Balkan , W and Troen , BR. 2007 . AP-1 stimulates the cathepsin K promoter in RAW 264.7 cells . Gene. , 15 : 151 – 158 .
- Putnam , SE , Scutt , AM , Bicknell , K , Priestley , CM and Williamson , EM. 2007 . Natural products as alternative treatments for metabolic bone disorders and for maintenance of bone health . Phytother Res. , 21 : 99 – 112 .
- Qin , L , Han , T , Zhang , Q , Cao , D , Nian , H , Rahman , K and Zheng , H. 2008 . Antiosteoporotic chemical constituents from Er-Xian Decoction, a traditional Chinese herbal formula . J Ethnopharmacol. , 118 : 271 – 279 .
- Rodan , GA and Martin , TJ. 2000 . Therapeutic approaches to bone diseases . Science. , 289 : 1508 – 1514 .
- Rozen , S and Skaletsky , HJ. 2000 . Primer3 on the WWW for general users and for biologist programmers . Methods Mol Biol. , 132 : 365 – 386 .
- Saftig , P , Hunziker , E , Everts , V , Jones , S , Boyde , A , Wehmeyer , O , Suter , A and von Figura , K. 2000 . Functions of cathepsin K in bone resorption. Lessons from cathepsin K deficient mice . Adv Exp Med Biol. , 477 : 293 – 303 .
- Soriano , P , Montgomery , C , Geske , R and Bradley , A. 1991 . Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice . Cell , 64 : 693 – 702 .
- Takayanagi , H , Kim , S , Koga , T , Nishina , H , Isshiki , M , Yoshida , H , Saiura , A , Isobe , M , Yokochi , T Inoue , J . 2002 . Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts . Dev Cell. , 3 : 889 – 901 .
- Teitelbaum , SL. 2000 . Bone resorption by osteoclasts . Science. , 289 : 1504 – 1508 .
- Won , JB , Ma , JY , Um , YR and Ma , CJ. 2010 . Simultaneous determination of five marker constituents in Ssanghwa tang by HPLC/DAD . Pharmacogn Mag , 6 : 111 – 115 .
- Yen , PH , Kiem , PV , Nhiem , NX , Tung , NH , Quang , TH , Minh , CV , Kim , JW , Choi , EM and Kim , YH. 2007 . A new monoterpene glycoside from the roots of Paeonia lactiflora increases the differentiation of osteoblastic MC3T3-E1 cells . Arch Pharm Res. , 30 : 1179 – 1185 .