Abstract
The dried roots of Saussurea lappa are used in traditional medicine for the treatment of dysentery, nausea, and vomiting, as well as for their antiulcer, anti-inflammatory, and antitumor properties. In this study, the cytotoxic activities of the ethanol extract of Saussurea lappa root extract (SLRE) on neuroblastoma cells were investigated, wherein the effect of the plant extracts on cancer cells has not been fully clarified. Here, cytotoxicity was examined by cell viability assay, fragmented DNA by DAPI, caspase-3 activity by immunofluorescence staining, and activation of proteins was detected by western blotting. SLRE was demonstrated to reduce human SH-SY5Y and rat B103 neuroblastoma cell viability more effectively than mouse and rat fibroblast cells. However, the SLRE-treated cells exhibited morphological changes including cell rounding, cell shrinkage, neurite retraction, and membrane blebbing, which indicated the initiation of apoptosis in the SH-SY5Y cells. Importantly, SLRE-induced cells displayed upregulation of the pro-apoptotic protein Bax and downregulation of the anti-apoptotic proteins Bcl-2, Bcl-xl, and Mcl-1, which suggest the mitochondia-mediated apoptotic pathway. In addition, SLRE activated cleaved caspases-9 and -3, also indicating the intrinsic caspase-mediated apoptosis pathway. Consequently, phosphatidylinositide-3-kinase/protein kinase B (AKT) and glycogen synthase kinase-3β (GSK-3) protein expression and activity were downregulated by SLRE treatment, suggesting that the growth inhibitory activity of this plant extracts is associated with these pathways. Therefore, the findings from this study demonstrated that the growth inhibitory and cytotoxic effects of SLRE on SH-SY5Y cells are accomplished through the mitochondria-mediated pathway, and that this natural extract might be useful as an anticancer agent for neuroblastoma treatment.
Introduction
Naturally occurring plant products have gained increasing attention for potential use against malignant invasive progression of late-stage neoplastic diseases (Shankar et al. Citation2008; Ravindranath et al. Citation2009). Certain foods, including many vegetables, fruits, and grains, as well as many phytochemicals with diverse pharmacological efficacy, offer significant protection against various cancers (Chen et al. Citation2005; Vieira et al. Citation2007; Huang et al. Citation2008). There has been increasing focus on providing a scientific basis for the use of such agents as a preventive strategy for people at high risk of cancer. There are 300 known Saussurea species. Among them, Saussurea lappa (S. lappa) is a representative perennial herb, globally distributed across Himalaya region. S. lappa has been traditionally used in medicines without obvious adverse effects (Zahara et al. Citation2014), where it is utilized in medicine, and its roots have traditionally been used for alleviating pain from abdominal distention and tenesmus, indigestion with anorexia, dysentery, nausea, and vomiting (Sun et al. Citation2003). Previous in vitro cell culture studies have shown that S. lappa has antiulcer (Yoshikawa et al. Citation1993), anti-inflammatory (Cho et al. Citation2000), antiviral (Chen et al. Citation1995), and antitumor properties (Ko et al. Citation2005). However, the effects of S. lappa on neuroblastoma and its mechanisms of action have yet to be elucidated.
Neuroblastoma, one of the few human malignancies to demonstrate spontaneous regression from an undifferentiated state to a completely benign cellular appearance, originates from the sympathetic nervous system, and is the most frequently occurring extracranial malignancy in childhood (Maris et al. Citation2007; Bénard et al. Citation2008). A better understanding of the mechanisms of spontaneous regression might help to identify optimal therapeutic approaches for patients with these tumors (Brodeur & Bagatell Citation2014). However, despite many advances in the diagnostic procedures and standard interventions in the past three decades, neuroblastoma has remained a formidable challenge to clinical and basic scientists (Castel et al. Citation2007). Even with the use of multimodal therapeutic approaches including chemotherapy, radiotherapy, and immunotherapy the survival rate for patients with malignant neuroblastoma remains poor. Therefore, the development of novel agents is needed to improve the treatment outcomes in this high-risk group. In particular, natural products have attracted interest for the development of new approaches to chemotherapy.
The precise molecular mechanism(s) of Saussurea lappa root extract (SLRE)-induced apoptosis in human neuroblastoma cells and its anti-cancer effects have not yet been elucidated. A new dimension in the management of neoplasia is increased awareness of chemoprevention, which refers to the administration of chemical agents to prevent events associated with carcinogenesis (Hong & Sporn Citation1997). A large number of chemopreventive and chemotherapeutic agents have been discovered from natural products, and they provide a promising strategy to fight cancer by inducing apoptosis in malignant cells (Kelloff et al. Citation2000; Sporn & Suh Citation2000). In the present study, the effects of SLRE root extracts on the cytotoxicity and apoptosis of cultured SH-SY5Y cells were investigated, along with the possible underlying mechanisms. The results indicated that SLRE inhibited the viability of neuroblastoma cells by disrupting the mitochondrial apoptotic cell death pathway, downregulating Bcl-2 family proteins, and activating caspases.
Materials and methods
Materials
Roots of Saussurea lappa Clarke were purchased from DeaGuang in Chuncheon, South Korea. A voucher specimen (HRIC-1034) was deposited at the Regional Innovation Center, Hallym University, Chuncheon, South Korea. Roots (1000 g) were chopped and blended using a Waring blender and then boiled with 2 L of 80% ethanol at 80°C for 2 h. The insoluble materials were removed through centrifugation at 10,000 × g for 30 min, and the resulting supernatant was concentrated and freeze-dried to yield a dark brown residue (yield: 23.5%). The chemicals were dissolved in dimethyl sulfoxide at a stock solution of 10 mg/ml and diluted with medium to obtain working concentrations. Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco/BRL (Grand Island, NY, USA). Antibodies against Mcl-1, Bcl-2, and Bcl-xl were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), while the others were obtained from Cell Signaling Technology (Beverly, MA). All other reagents were of analytical grade or of the highest purity available.
Cell culture
Human SH-SY5Y neuroblastoma, Rat B103 neuroblastoma, Rat-2 fibroblast, and NIH 3T3 mouse embryonic fibroblast cells were grown at 37°C under a humidified atmosphere of 5% CO2. The cells were cultured in DMEM plus 10% FBS, 50 U/ml penicillin and 50 µg/ml streptomycin.
Cell viability assay
Cell viability was determined using the Cell Counting Kit-8 (CCK-8) (Dojindo Lab, Japan) according to the manufacturer’s protocol. The cells were plated into 96-wells to a density of 50–60% confluence and then treated with various concentrations of SLRE. After treatment for 24 h, CCK-8 (10 μl) was added to each well of the plates and incubated for 3 h. A 96-well microtitre plate reader (Molecular Devices) was used to determine the absorbance of the CCK-8 at 450 nm. The mean concentrations in each set of three wells were measured.
Cell morphology
The cells were added to 24-well plates at 37°C under a humidified atmosphere of 5% CO2 and treated with various concentrations of SLRE. The culture plates were examined at the Korea Basic Science Institute Chuncheon Center for technical assistance with microscopy and photographed for examination of cell morphology.
Nuclear fragmentation assay–DAPI staining
SH-SY5Y cells were grown on round cover slides in 24-well dishes until the cells reached 80% confluence. The cells were then treated with 0 and 15 μg/ml of SLRE. After 24 h, the media was removed and the cells were washed with 1X phosphate buffered saline (PBS), after which 500 µl 4% paraformaldehyde (PFA) was applied for 15 min. The cells were then washed with PBS and permeablized with 0.1% phosphate buffered saline Tween-20 (PBST) for 10 min. After washing with PBS again, the nuclei of the cells were denatured with 2 N HCl (300 µl) for 10 min, washed three more times, and then treated with 0.1 ug/ml DAPI (1:1000) in PBST for 1 h. After staining, the cells were washed twice with PBS and visualized using Fluorescent Mounting Medium (Dako North America, Inc.) and dried at room temperature.
Immunocytochemical staining for cleaved caspase-3
SH-SY5Y cells were grown on round cover slides in 24-well dishes until the cells reached 80% confluence. The cells were then treated with 0 and 15 μg/ml of SLRE. After 24 h, the media was removed and cells were washed with 1X PBS, after which 500 µl 3–4% PFA was applied for 15 min at room temperature. The cells were then washed with PBS and permeablized with PBS containg 0.25% Triton X-100 for 10 min. After washing three times for 5 min with PBS and incubating with 1% bovine serum albumin (BSA) in PBST for 30 min to block unspecific binding of the antibodies, the cells were incubated in diluted caspase-3 antibody (1:400) in 1% BSA in PBST over night at 4°C. The cells were then washed three times for 5 min with PBS and incubated with the secondary antibody (1:1000) and DAPI (0.1 µg/ml) in 1% BSA for 1 h at room temperature in the dark. After washing three times for 5 min each in the dark the coverslip was mounted using Fluorescent Mounting Medium (Dako North America, Inc.) and dried at room temperature.
Western blot analysis
SH-SY5Y cells were starved in 60-mm culture dishes in DMEM with 0.5% FBS for 24 h. Cells were pretreated with various concentration of SLRE and then washed twice with ice-cold PBS. The cells were then lysed in lysis buffer (2% sodium dodecyl sulfate [SDS], Na3VO4 and protease inhibitor cocktail). After 10 min incubation on ice, the cells were sonicated for 10 sec at 10% amplitude, and the lysates were centrifuged (13,000 × g, 20 min). The supernatants were collected, and protein concentrations were determined by the Bradford assay (Bio-Rad, Richmond, CA, USA). Equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis (PAGE) (8–15% reducing gels), transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA), and blocked with 5% nonfat milk. The membranes were incubated in primary antibody overnight at 4°C and washed with Tris-Buffered Saline and Tween 20 (TBST) (10 mM Tris, 140 mM NaCl, 0.1% Tween-20, pH 7.6). The membranes were then incubated with appropriate secondary antibody and washed again with TBST. The bands were visualized by enhanced chemiluminescence and exposed to X-ray film.
Statistical analysis
Results are expressed as mean ± standard error. Statistical significance was assessed by one-way analysis of variance followed by Dunnett’s test or the paired t-test using Prism 4 (GradPad Software, La Jolla, CA, USA). Values of p < 0.05 were considered significant.
Results
Antiproliferative and cytotoxic effects of SLRE in neuroblastoma and fibroblast cells
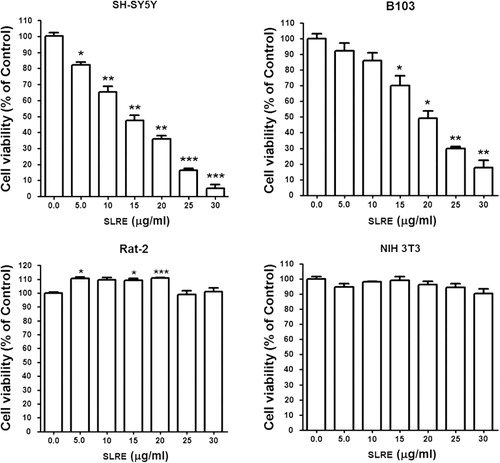
To check whether SLRE exerts antitumor effects, the effects of SLRE on the cell viability of malignant neuroblastoma tumor cells and normal fibroblast cells were screened by cell viability assay. The results showed that the percentage of viable cells of both human SH-SY5Y and rat B103 neuroblastoma was reduced by treatment with SLRE for 24 h (). On the other hand, fibroblast cells such as Rat-2 and Mouse embryonic NIH 3T3 cells did not show cytotoxic effects compared to the malignant neuroblastoma cells after 24 h (). Therefore, this observation clearly emphasizes that neuroblastoma cancer cells showed relatively higher toxicity than normal fibroblast cells when treated with SLRE for 24 h (), which suggests that SLRE may be an effective and safe anticancer agent. However, the mechanisms by which SLRE exerts its anticancer effects are still not fully understood. To date, no studies have described the anticancer effects of SLRE on cancer cells. Therefore, the purpose of this study was to investigate whether SLRE affects the apoptosis of SH-SY5Y cells through the activation of caspases, which might explain the mechanisms underlying the antiproliferative and cytotoxic effects on cancer cells. Based on the observations, further investigation was carried out on the human SH-SY5Y neuroblastoma cells.
SLRE-induced cytotoxicity of human SH-SY5Y neuroblastoma cells by the process of apoptosis
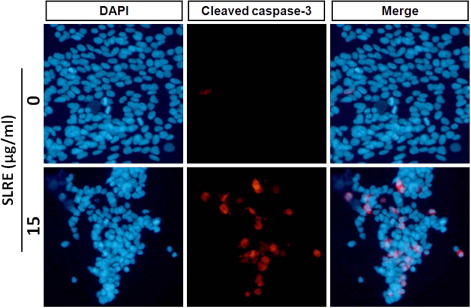
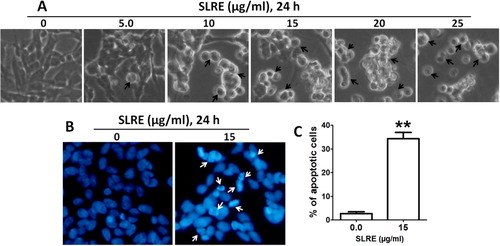
To assess the morphological changes associated with SLRE treatment, confluent monolayers of cells were treated with or without SLRE for 24 h. When observed under phase contrast microscope, control cells exhibited normal morphology (well spread) with a normal rate of proliferation. Cells treated with SLRE showed cell shrinkage, membrane blebbing, and apoptotic vacuole formation, indicating the initiation of apoptosis (). The later stages of apoptosis involve changes in the nuclear morphology of cells such as chromatin condensation and DNA fragmentation. Therefore, DAPI staining was employed to analyze the nuclear morphological changes associated with SLRE-induced apoptosis. DAPI can bind to the minor groove of the adenine–thymine regions of DNA and emit light, observed as blue fluorescence. The results clearly indicated that significant nuclear morphological changes such as chromatin condensation and nuclear fragmentation were associated with the apoptosis observed in the cell lines (). These morphological changes indicate that treatment with SLRE resulted in significant increase of the number of apoptotic cells and cells possessing fragmented DNA when compared with untreated controls (). These results clearly indicate that the morphological changes of SH-SY5Y by SLRE were due to apoptosis, which resulted in fragmented nuclear DNA.
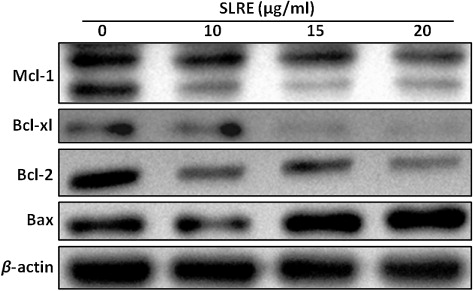
SLRE treatment affects Bcl-2 family member proteins in neuroblastoma cells
To understand the molecular mechanisms involved in the activation of cell death induced by SLRE, it was first evaluated whether this natural compound regulates the expression of the anti-apoptotic and pro-apoptotic Bcl-2 family proteins in SH-SY5Y cells using western blotting. The results showed that the expression of Bax was significantly upregulated in the in SH-SY5Y cells by SLRE treatment in a dose-dependent manner, thus providing a possible explanation for the observed apoptosis. Western blotting analysis also demonstrated that treatment of SH-SY5Y cells with SLRE also caused dose-dependent reduction in the levels of the anti-apoptotic proteins Bcl-2, Bcl-xl and Mcl-1 compared to the control (). This observation suggests that SLRE treatment can alter the protein levels of key members of the Bcl-2 family, which may contribute to the susceptibility of the cancer cells to mitochondrial dysfunction.
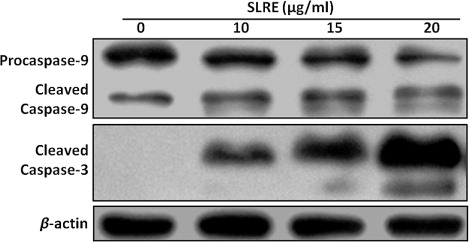
Activation of caspases is essential for SLRE-induced apoptosis in human neuroblastoma cells
To further determine whether SLRE activates the caspase pathway, SH-SY5Y cells were incubated in the presence or absence of SLRE and then harvested for western blot analysis. As caspase appears to be involved in the induction of apoptosis, the levels of cleaved caspases-9 and -3 were measured. Incubation of SH-SY5Y cells with SLRE caused dose-dependent upregulation of the levels of the biologically active cleaved caspases-9 and -3, thereby activating the apoptotic cascade pathway (). Immunofluorescence analysis revealed that SLRE (15 µg/ml, 24 h) enhanced the levels of cleaved caspase-3 within the nucleus compared to the control (). Therefore, the results shown here demonstrate that SLRE increases caspase expression, which suggests that apoptosis is mediated by the activation of an intrinsic pathway.
Effects of SLRE on phosphotidylinositide-3-kinase (PI3K)/AKT/glycogen synthase kinase-3β (GSK-3β) expression and activity
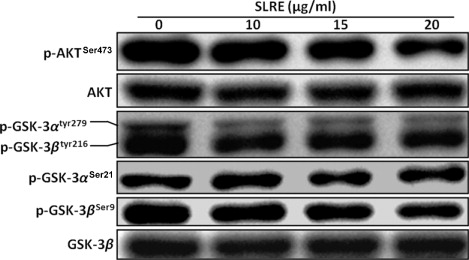
Protein kinase B (Akt) is a known anti-apoptotic factor which was found to be overexpressed in various tumors. Accordingly, the effects of SLRE on the AKT/GSK-3β signaling pathway were examined herein. Akt is activated by phosphorylation at Thr308 and Ser473 sites. Therefore, the activity of Akt was examined by western blotting using phosphospecific antibody against Ser473. A decrease in the levels of phospho-Akt was observed in the SH-SY5Y cells treated with SLRE (). It is known that active Akt phosphorylates GSK-3β at Ser9 site, making it inactive. A decrease in the level of phospho GSK-3αSer21, GSK-3βSer9, and GSK-3αtyr279/βtyr216 protein espression was slightly observed in the treated SH-SY5Y cells (), which suggests SLRE inhibits the growth and proliferation of human neuroblastoma cells by affecting the AKT/GSK-3β signaling pathway.
Discussion
The present study was designed to define the mechanism(s) of the cytotoxic effects of SLRE root extract. The results showed a dose-dependent reduction in the viability of human SH-SY5Y neuroblastoma cells due to apoptosis, which may help in the design of novel approaches for managing the cancer cells. However, the restraining effects and mechanisms by which SLRE activity is involved in cancer metastasis have not yet been elucidated. In addition, no studies have described the anti-cancer effects of SLRE on neuroblastoma cells. The purpose of this study was to investigate whether the ethanol fraction of SLRE extract affected the apoptosis of SH-SY5Y cells through the activation of caspases, which might explain the underlying mechanisms of cytotoxicity to the cancer cells.
Bcl-2 family proteins are key regulators of the mitochondria-mediated apoptosis pathways. They direct the permeabilization of the mitochondrial outer membrane to release cytochrome c into the cytosol, resulting in the activation of caspase molecules to stimulate apoptosis further (García-Sáez Citation2012). However, Bcl-2 proteins are critical for tissue homeostasis like embryo development, maturation of cells, and cell division (Strasser et al. Citation2011). Significantly, deregulation of the Bcl-2 family proteins induces tumor formation. Moreover, Bcl-2 family is also involved in other different diseases formation, such as autoimmune disorder, infectious and neurodegenerative disorders (Martinou & Youle Citation2011). As a consequence of their biological importance and their potential as therapeutic targets, the Bcl-2 proteins have been investigated intensively during to their role in apoptosis signaling (Leber et al. Citation2010). A number of studies have shown that the mitochondrial apoptotic pathway is controlled by the balance between pro-apoptotic and anti-apoptotic members of the Bcl-2 protein family (Strasser Citation2005; Adams & Cory Citation2007; Youle & Strasser Citation2008). Permeability of the mitochondrial membrane is precisely regulated by Bcl-2 family proteins (Cory & Adams Citation2002; Breckenridge & Xue Citation2004). The Bcl-2 family plays an important regulatory role in apoptosis, either as an inhibitor or activator (Adams & Cory Citation2007). In particular, Bcl-2 directly inhibits members of the caspase family, including caspases-3 and -9 (Ekert et al. Citation1999). Thus, the balance among Bcl-2 family members is crucial to the apoptotic trigger. In the present study, SLRE caused dose-dependent downregulation of the anti-apoptotic proteins Bcl-2, Bcl-xl, and Mcl-1, as well as upregulation of the pro-apoptotic protein Bax (). Therefore, dysregulation of the Bax and Bcl-2 ratio may indicate that SLRE-induced apoptosis was strongly correlated with imbalance of the mitochondrial apoptotic pathway. The pathways and mechanisms of induction by SLRE in SH-SY5Y neuroblastoma cells warrant further research.
Apoptosis, or programmed cell death, occurs during normal embryonic development and is involved in cell turnover throughout life. It is now evident that many cancer cells circumvent normal apoptotic mechanisms to prevent self-destruction. Apoptotic cells are characterized by a reduced mitochondrial transmembrane potential, intracellular acidification, production of reactive oxygen species, externalization of phosphatidylserine residues in membrane bilayers, selective proteolysis of cellular proteins, and degradation of DNA into internucleosomal fragments (Chen et al. Citation2003; Bouchier-Hayes et al. Citation2005). Caspases are key executioners of apoptosis, and their activity is triggered via endoplasmic reticulum stress, extracellular stimuli, or mitochondrial damage (Cecconi & Gruss Citation2001; Wang Citation2001). Caspase-3 in particular plays a pivotal role in the terminal and execution phases of apoptosis induced by diverse stimuli (Thornberry & Lazebnik Citation1998). Upon activation, the initiator caspase-9 triggers proteolytic activation of the executioner caspases-3/7 and caspase-8 in a process which results in the cleavage of poly(ADP-ribose) polymerase, subsequent DNA degradation, and apoptotic death (Kuida Citation2000; Cain et al. Citation2002). In the present study, exposure of SH-SY5Y cells to SLRE resulted in the proteolytic activation of caspases-3 and -9, the main executioners of apoptosis, in a concentration-dependent manner (). It was also examined whether caspase-3, a substrate of caspase-9, was cleaved in the cells exposed to SLRE (). As expected, the cleaved form of caspase-3 was clearly expressed, correlating with the caspase signaling pathway and apoptosis. Therefore, SLRE might be useful as a potential apoptosis-inducing agent in neuroblastoma cells.
Further, GSK-3β is one of the direct downstream targets of AKT. Phosphorylation of GSK-3β by AKT inhibits its enzymatic activity, as a serine/threonine kinase (Brady et al. Citation1998; van Weeren et al. Citation1998). GSK-3β activity is attenuated by the activated AKT as a consequence of PI3K signaling. When active, GSK-3β phosphorylates a number of protein targets. One of the primary targets for GSK-3β is β-catenin, which undergoes proteosome degradation after phosphorylated by GSK-3β (Takahashi-Yanaga & Sasaguri Citation2009). Similarly, the anti-apoptotic factor Mcl-1, one of the Bcl-2 family members, is phosphorylated by GSK-3β and subsequently degraded (Maurer et al. Citation2006). AKT activity blocks the ability of GSK-3β to phosphorylate Mcl-1, causing its degradation. AKT also indirectly affects the balance of the Bcl-2 family of pro- and anti-apoptotic factors. Herein, SLRE was found to downregulate AKT phosphorylation (), which is closely linked to cell survival. Thus, the cytotoxicity and induction of apoptosis by SLRE did not occur through activation of the PI3K/AKT/GSK-3β pathway. Therefore, the overall results suggest that SLRE might possess potential anti-cancer activity in SH-SY5Y cells by reducing the expression of anti-apoptotic proteins, as well as inducing the caspase signaling pathway.
This study was conducted to describe the molecular mechanisms of the cellular growth inhibitory and cytotoxic properties of natural plant extracts on human SH-SY5Y neuroblastoma. As far as we know, this is the first report to demonstrate the SLRE dose-dependent activation of the intrinsic caspase signaling, triggered by the modulation of different proteins. These findings suggest that SLRE may serve as a potent chemosensitizer in the treatment of human cancers. Therefore, further investigation of SLRE is warranted as a source of pharmacologically active agents, along with assessment of its antitumor therapeutic efficacy in vivo in experimental brain tumor models.
Acknowledgments
This research was supported by Hallym University Research Fund (HRF-201408-018) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2013M3C7A1056565 and NRF-2010-0013043), the Republic of Korea.
Additional information
Funding
References
- Adams JM, Cory S. 2007. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 19:488–496. 10.1016/j.coi.2007.05.004
- Bénard J, Raguénez G, Kauffmann A, Valent A, Ripoche H, Joulin V, Job B, Danglot G, Cantais S, Robert T, et al. 2008. MYCN-non-amplified metastatic neuroblastoma with good prognosis and spontaneous regression: a molecular portrait of stage 4S. Mol Oncol. 2:261–271.
- Bouchier-Hayes L, Lartigue L, Newmeyer DD. 2005. Mitochondria: pharmacological manipulation of cell death. J Clin Invest. 115:2640–2647. 10.1172/JCI26274DS1
- Brady MJ, Bourbonais FJ, Saltiel AR. 1998. The activation of glycogen synthase by insulin switches from kinase inhibition to phosphatase activation during adipogenesis in 3T3-L1 cells. J Biol Chem. 273:14063–14066. 10.1074/jbc.273.23.14063
- Breckenridge DG, Xue D. 2004. Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Curr Opin Cell Biol. 16:647–652. 10.1016/j.ceb.2004.09.009
- Brodeur GM, Bagatell R. 2014. Mechanisms of neuroblastoma regression. Nat Rev Clin Oncol. [Internet]. [cited 2014 Oct 21];168. Available from: http://www.nature.com/nrclinonc/journal/vaop/ncurrent/abs/nrclinonc.2014.168.html
- Cain K, Bratton SB, Cohen GM. 2002. The Apaf-1 apoptosome: a large caspase activating complex. Biochimie. 84:203–214.
- Castel V, Grau E, Noguera R, Martínez F. 2007. Molecular biology of neuroblastoma. Clin Transl Oncol. 9:478–483. 10.1007/s12094-007-0091-7
- Cecconi F, Gruss P. 2001. Apaf1 in developmental apoptosis and cancer: how many ways to die? Cell Mol Life Sci. 58:1688–1697. 10.1007/PL00000806
- Chen HC, Chou CK, Lee SD, Wang JC, Yeh SF. 1995. Active compounds from Saussurea lappa Clarks that suppress hepatitis B virus surface antigen gene expression in human hepatoma cells. Antiviral Res. 27:99–109. 10.1016/0166-3542(94)00083-K
- Chen Q, Crosby M, Almasan A. 2003. Redox regulation of apoptosis before and after cytochrome c release. Korean J Biol Sci. 7:1–9. 10.1080/12265071.2003.9647675
- Chen PN, Hsieh YS, Chiou HL, Chu SC. 2005. Silibinin inhibits cell invasion through inactivation of both PI3K-Akt and MAPK signaling pathways. Chem Biol Interact. 156:141–150. 10.1016/j.cbi.2005.08.005
- Cho JY, Baik KU, Jung JH, Park MH. 2000. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur. J. Pharmacol. 398:399–407.
- Cory S, Adams JM. 2002. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2:647–656. 10.1038/nrc883
- Ekert PG, Silke J, Vaux DL. 1999. Caspase inhibitors. Cell Death Differ. 6:1081–1086. 10.1038/sj.cdd.4400594
- García-Sáez AJ. 2012. The secrets of the Bcl-2 family. Cell Death Differ. 19:1733–1740.
- Hong WK, Sporn MB. 1997. Recent advances in chemoprevention of cancer. Science. 278:1073–1077. 10.1126/science.278.5340.1073
- Huang HP, Shih YW, Chang YC, Hung CN, Wang CJ. 2008. Chemoinhibitory effect of mulberry anthocyanins on melanoma metastasis involved in the Ras/PI3K pathway. J Agric Food Chem. 56:9286–9293. 10.1021/jf8013102
- Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R, Lawrence JA, et al. 2000. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr. 130:467S–471S.
- Ko SG, Kim HP, Jin DH, Bae HS, Kim SH, Park CH, Lee JW. 2005. Saussurea lappa induces G2-growth arrest and apoptosis in AGS gastric cancer cells. Cancer Lett. 220:11–19. 10.1016/j.canlet.2004.06.026
- Kuida, K. 2000. Caspase-9. Int J Biochem Cell Biol. 32:121–124. 10.1016/S1357-2725(99)00024-2
- Leber B, Lin J, Andrews DW. 2010. Still embedded together binding to membranes regulates Bcl-2 protein interactions. Oncogene. 29:5221–5230. 10.1038/onc.2010.283
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. 2007. Neuroblastoma. Lancet. 369:2106–2120. 10.1016/S0140-6736(07)60983-0
- Martinou JC, Youle RJ. 2011. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 21:92–101. 10.1016/j.devcel.2011.06.017
- Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. 2006. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 21:749–760. 10.1016/j.molcel.2006.02.009
- Ravindranath MH, Ramasamy V, Moon S, Ruiz C, Muthugounder S. 2009. Differential growth suppression of human melanoma cells by tea (Camellia sinensis) epicatechins (ECG, EGC and EGCG). Evid Based Complement Alternat Med. 6:523–530. 10.1158/1055-9965.EPI-03-0040
- Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. 2008. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. 13:440–452. 10.2741/2691
- Sporn MB, Suh N. 2000. Chemoprevention of cancer. Carcinogenesis. 21:525–530. 10.1093/carcin/21.3.525
- Strasser A. 2005. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 5:189–200. 10.1038/nri1568
- Strasser A, Cory S, Adams JM. 2011. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 30:3667–3683. 10.1038/emboj.2011.307
- Sun CM, Syu WJ, Don MJ, Lu JJ, Lee GH. 2003. Cytotoxic sesquiterpene lactones from the root of Saussurea lappa. J Nat Prod. 66:1175–1180. 10.1021/np030147e
- Takahashi-Yanaga F, Sasaguri T. 2009. Drug development targeting the glycogen synthase kinase-3beta (GSK-3beta)-mediated signal transduction pathway: inhibitors of the Wnt/beta-catenin signaling pathway as novel anticancer drugs. J Pharmacol Sci. 109:179–183. 10.1254/jphs.08R28FM
- Thornberry NA, Lazebnik Y. 1998. Caspases: enemies within. Science. 281:1312–1316. 10.1126/science.281.5381.1312
- van Weeren PC, de Bruyn KM, de Vries-Smits AM, van Lint J, Burgering BM. 1998. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation characterization of dominant-negative mutant of PKB. J Biol Chem. 273:13150–13156. 10.1074/jbc.273.21.13150
- Vieira JR, de Souza IA, do Nascimento SC, Leite SP. 2007. Indigofera suffruticosa: an alternative anticancer therapy. Evid Based Complement Alternat Med. 4:355–359. 10.1093/ecam/nel102
- Wang X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 15:2922–2933.
- Yoshikawa M, Hatakeyama S, Inoue Y, Yamahara J. 1993. Saussureamines A, B, C, D, and E, new anti-ulcer principles from Chinese Saussureae radix. Chem Pharm Bull (Tokyo). 41:214–216. 10.1248/cpb.41.214
- Youle RJ, Strasser A. 2008. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 9:47–59. 10.1038/nrm2308
- Zahara K, Tabassum S, Sabir S, Arshad M, Qureshi R, Amjad MS, Chaudhari SK. 2014. A review of therapeutic potential of Saussurea lappa – an endangered plant from Himalaya. Asian Pac J Trop Med. S1:S60–S69. 10.1016/S1995-7645(14)60204-2