ABSTRACT
Bama Xiang pig (BM) and Tibetan mini-pig (TM) are used as experimental animals in China; however, the dwarf molecular mechanisms of these Chinese local pig breeds are unknown. IGFBP-3 affects animal growth, carcass and meat quality. The aim of this study was to identify the polymorphisms in the promoter of the IGFBP-3 and analyse their effect on the IGFBP-3 mRNA expression level in liver and muscle tissues. High-density single-nucleotide polymorphisms (SNPs) (31) and InDels (5) were detected in the promoter of the IGFBP-3 in the BM, TM and Junmu No. 1 White (JM, control) pig breeds from 114 individuals by re-sequencing. A perfect Linkage disequilibrium consisted of 13 SNPs was observed in the promoter region and 2 main haplotypes were identified, of which the h1 genotype (GCA-ATGTACATAT) was more prevalent in JM breed than in TM or BM breeds (P < .0001), h2 (ATGTGCACG--CGC) was the dominant haplotype in TM and BM breeds (P < .0001). Expression analysis showed that haplotype of the promoter region is highly associated with the IGFBP-3 mRNA expression level in liver and muscle tissues of pigs. The IGFBP-3 mRNA expression level was determined higher in the liver and muscle tissues of pigs with h2 genotype as compared to that in pigs with h1 genotype (P < .05). The results suggest that the SNPs and haplotypes in the promoter of the IGFBP-3 gene may serve as useful molecular markers for the body growth traits and the breeding in swine.
Introduction
Bama Xiang pig (BM) and the Tibetan mini-pig (TM) are two indigenous Chinese breeds of miniature pig. BM is produced naturally as a result of geographic features and cultivation methods (Tian et al. Citation2014). Due to conventional artificial selection, BM is a highly inbred breed with stable genetic properties (Li et al. Citation2006; Ye et al. Citation2015). TM is a unique miniature pig breed which resides in regions of extremely high elevation (2900–4100 m above sea level), the hostile environment, free-range methods and limited feeding conditions lengthen the fattening period of such pigs and slows live weight gain relative to commercial pig breeds (Hao et al. Citation2011). The mean body weights of adult BM and TM are approximately 41.0 and 45.0 kg, respectively; their corresponding mean body lengths are roughly 81.0 and 87.5 cm, respectively. The miniature pig is characterized by ‘proportional dwarfism’ (Simianer & Koehn Citation2010) in comparison with commercial pig such as Junmu No. 1 White pig (JM; a control group; mean body weight: 250.0 kg, mean body length: 188.0 cm), which is a hybridization of Sanjiang white sows and Seghers boars cultivated in the College of Animal Husbandry and Veterinary Studies at Jilin University (Gao et al. Citation2011), and showed similar growth characteristics and body size with large pig breed (Zhang et al. Citation2013; Chen et al. Citation2014). Nonetheless, the dwarf signatures of selection in the mini-pig genomes have not been identified. In addition, the dwarf molecular mechanisms remain unknown, and little available genomic information has been analysed in relation to these two local breeds of Chinese miniature pig (Shan et al. Citation2014).
The dwarfism of miniature pigs is known as ‘pituitary dwarfism’ and is often caused by growth hormone (GH) deficiency, especially that of insulin-like growth factor 1 (IGF-1) (Simianer & Koehn Citation2010). The GH/IGF-1 axis is regarded as the major regulator of postnatal body growth (Locatelli & Bianchi Citation2014). Furthermore, the over-expression of IGF-1 is associated with increased body weight in transgenic mice (Mathews et al. Citation1988). IGF-binding proteins (IGFBPs) bind to IGF-1 with high affinity, thereby influencing the half-life of the circulating IGF-1 and modulating IGF-1 actions. The most abundant of these proteins is IGFBP-3, which binds the majority of the circulating IGF-1 in a 150-kDa ternary form with an acid-labile subunit. IGFBP-3 transports IGF-1 to the IGF-1 receptor, enhances IGF-1 actions. Or IGFBP-3 sequesters IGF-1 from its receptor, in this process, IGF-1 action is inhibited (Moses et al. Citation1980; Clemmons Citation1997; Hjortebjerg & Frystyk Citation2013). IGFBP-3 also performs important IGF-independent functions. This protein plays a negative role in the growth process because the transgenic over-expression of IGFBP-3 is associated with modest intrauterine and postnatal growth retardation in mice (Modric et al. Citation2001). In pigs, serum IGFBP-3 is negatively associated with average daily gain (Clutter et al. Citation1995). This protein may also contribute to muscle development because its levels are higher in the skeletal muscle cells of children than in adults (Grohmann et al. Citation2005). The quadriceps muscles of IGFBP-3, -4 and -5 triple-knockout mice shrunk significantly (Ning et al. Citation2006). In Meishan and Landrace pigs, IGFBP-3 expression levels reached the maximum peak values one and two months after birth, respectively (Gu et al. Citation2009). Hence, the label IGFBP-3 does not fully describe the roles of this protein, and its functions are complicated.
Genetic variations in the promoter region were investigated to show the influence on gene transcription (Juszczuk-Kubiak et al. Citation2012; Fang et al. Citation2015). The swine IGFBP-3 gene is located on chromosome 18q24 and contains three exons. According to the Database of single-nucleotide polymorphisms (dbSNPs), high-density SNPs and InDels are detected in the promoter of the IGFBP-3 gene and may influence the mRNA expression of this gene. The SNPs in the promoter of the IGFBP-3 gene were detected through re-sequencing in the three pig breeds (JMs, TM and BM). The current work also analysed the Linkage disequilibrium (LD) relationships and identified the haplotypes in this region, in addition, the promoter variant effect on IGFBP-3 mRNA expression level also evaluated in the birth, weaning and adult periods in the liver and muscle tissues of the three pig breeds.
Materials and methods
Animal resources
The use of animals in this study was approved by the Animal Care and Use Committee of Jilin University. Ear notch samples were collected from 36 BM pigs (Yunfu Swine Farm, Guangdong), 38 TM pigs (Tongheshengtai Institute of Comparative Medicine, Beijing) and 40 JMs (swine farm of Jilin University, Changchun). All individuals were selected randomly, equal numbers of males and females were obtained.
DNA preparation and SNP detection of the IGFBP-3 promoter region
Genomic DNA was extracted from the ear notches using genomic DNA extraction kits according to the manufacturer’s instructions (Tiangen Biotech, China). Briefly, the frozen ear notch tissue was pulverized in liquid nitrogen using a mortar and a pestle, then the pulverized tissue was transferred into a centrifuge tube (1.5 mL) with 200 μL buffer GA and 20 μL proteinase K added. The centrifuge tube was shaked and incubated in 56°C until the tissue solubilized. The solution was added with 200 μL buffer GB and incubated in 70°C for 10 min before adding 200 μL anhydrous alcohol. Then, the mixture was transferred to an adsorption tube CB3 and centrifuged for 1 min at 12,000 g. The adsorption tube was added with 500 μL buffer GD and centrifuged for 1 min at 12,000 g. Subsequently, the adsorption tube was added with 600 μL buffer PW and centrifuged for 1 min at 12,000 g. Finally, the adsorption tube CB3 was placed into a new collection tube and added with 50 μL ddH2O to dissolve DNA by centrifuging at 12,000 g for 1 min. The DNA was stored at −80°C deep freezer.
Two pairs of primers (: Prime 1,2) designed by Primer Premier 5 (Premier Biosoft, Canada) were obtained to produce the overlapping fragments (2196 bp) of the IGFBP-3 promoter region (Gene ID: 448812). These primers were synthesized by Genewiz (Beijing, China).
Table 1. Primer pairs used to amplify the IGFBP-3 promoter region.
A DNA pool was prepared to detect the SNPs located in the promoter region; this pool contained a mixture of equal amounts of the individual genomic DNA for each pig breed. The PCR mixtures (50 μL) consisted of 50 ng template DNA, 1 μL of each primer (10 μM), 5 μL of 10× Ex Taq buffer (Mg2+ plus), 4 μL dNTP mixture and 0.25 μL Ex Taq (Takara). A PCR was then conducted under the following conditions: pre-denaturation at 95°C for 5 min, 30 cycles of denaturation at 94°C for 30 s, annealing at an optimized temperature () for 30 s, an extension at 72°C for 1 min and a final extension at 72°C for 10 min. The PCR products amplified from the DNA pool and from each individual were sequenced by Genewiz (Beijing, China).
SNP genotyping and chi-square test
The sequencing data were analysed according to sequence alignment and by screening overlapping peaks to determine the SNPs and genotypes via DNASTAR (USA) and Unipro UGENE (Russia). The genotype frequencies and allele frequencies were counted directly based on the genotyping results. Polymorphism information content (PIC), gene heterozygosity (He) and gene homozygosity (Ho) were calculated using Botstein’s (Botstein et al. Citation1980) and Nei’s (Nei Citation1973).
Haplotype analysis
To ascertain the relation between genetic SNP markers in the IGFBP-3 gene 5′ flanking region and pig breeds, the corresponding haplotype levels were analysed in Haploview v.4.2 (Broad Institute) to obtain additional information (Barrett et al. Citation2005).
RNA isolation, cDNA preparation and real-time PCR analysis
Liver and muscle tissues in birth, weaning and adult periods containing either h1 or h2 haplotype were collected from the three pig breeds respectively, of which six samples (three for h1 and three for h2 haplotypes) were collected from the JM and TM breeds, while three samples of h2 haplotype were collected from the BM breed due to no h1 haplotype was found in BM breed in this study. The tissues were frozen in liquid nitrogen immediately, then stored at −80°C until used. RNA extraction was performed using Eastep® Super Total RNA Extraction Kit (Promega).
The RNA extracted from the liver and muscle tissues in different pig breeds was quantified and qualified by using the NanoDrop™ 2000 (Thermo™ Scientific). The amount of 2.5 μg of RNA was reverse transcribed into cDNA using RevertAid First Strand cDNA Synthesis Kit (ThermoFisher). Real-time PCR was performed in triplicate using Power SYBR® Green PCR Master Mix (ThermoFisher) and ABI PRISM 7900HT thermocycler (Applied Biosystems). The qRT-PCR primers used for produce the part sequences of IGFBP-3 mRNA (Sus scrofa, NM_001005156.1; : Prime 3) and β-actin mRNA (Sus scrofa, XM_003124280.4; : Prime 4) were designed by Primer Premier 5 (Premier Biosoft, Canada). Each reaction mixture contained 2.5 μL of cDNA, 12.5 μL of SYBR Green PCR Master Mix and 50 nM PCR primers in 25 μL reactions. The following amplification conditions were used: 95°C for 3 min, 40 cycles of 95°C for 30 s, 58°C for 30 s and 72°C for 20 s. The experiment was repeated at least three times. The relative expression of IGFBP-3 mRNA in each tissue was analysed by this formula: 2−ΔΔCt (ΔCt = CtIGFBP-3 – Ctβ-actin) method.
Statistical analysis
The genotype frequency of each SNP and haplotype distribution frequency of each breed were calculated by a chi-square test (χ2). Data are represented as mean ± SEM. The statistical analysis between the relative quantification of IGFBP-3 mRNA and IGFBP-3 promoter haplotypes was analysed using multiple T tests (GraphPad Prism 6, USA). When P < .05, the results were regarded as statistically significant and marked with an asterisk.
Identification of putative regulatory SNPs in the IGFBP-3 promoter region
To identify the SNPs which putatively affect promoter elements, the 5′ flanking sequence of IGFBP-3 was screened for transcription factor binding sites (TFBSs) in silico by employing prediction tools such as MatInspector (version 3.4) (Cartharius et al. Citation2005) and JASPAR (version 5.0_ALPHA) (Mathelier et al. Citation2014). To determine only the most likely sites, stringent thresholds were applied: a core similarity of 1.00, a minimum matrix similarity of 0.95 for MatInspector in the vertebrate setting and a 90% relative profile score threshold for JASPAR under the setting ‘CORE Vertebrata’.
Results
Detected polymorphisms
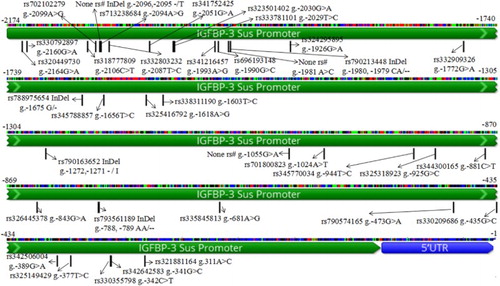
As per the sequencing data for each DNA pool associated with the BM, TM and JM pig breeds, 31 SNPs and 5 InDels were detected within a promoter region of the IGFBP-3 gene (). High-density SNPs were clustered in the promoter of this gene. Thereinto, three SNPs have none RefSNP ID (g.-2096, -2095 -/T; g.-1981 A>C and g.−1055 G>A), the others have been previously deposited in the SNPs databases.
Figure 1. SNPs in the IGFBP-3 promoter region.
SNP genotyping and diversity analysis
Given the high-density SNP loci in the region ranging from −2285 to −1056 bp, the genotype of each individual was detected, and the genotype frequency of each breed was counted directly based on individual re-sequencing data (). The statistical data’s indicated that the genotype frequencies of 13 SNPs exhibited similar variation rules among the three breeds (denoted by ‘f’ and detailed in ). The minimum and maximum PIC values were 0.231 and 0.375, respectively. Therefore, this result indicates the moderately polymorphic sites in the IGFBP-3 promoter region among the three pig breeds.
Table 2. Genetic diversity parameters of the IGFBP-3 promoter regiona in pig breeds.
LD and haplotype distribution
The 21 SNP genotypes among the 114 pig samples were loaded into Haploview v.4.1 to calculate their LD relationships. A perfect LD consisted of 13 SNPs was existed in the IGFBP-3 promoter region (; the 13 SNPs were g.-2164G>A; g.-2106C>T; g.-2099A>G; g.-2096,-2095->T; g.-2094A>G; g.-2087T>C; g.-2030G>A; g.-2029T>C; g.-1993A>G; g.-1980,-1979CA>--; g.-1656T>C; g.-1618A>G; g.-1603T>C). According to , these 13 notable SNPs (annotated by ‘f’) following the same variation rules in terms of genotype frequencies, haplotype analysis shows that between each pairwise of these 13 SNPs, the values of r2 and D′ are all equal to 1, thus indicating a perfect LD among them (). Subsequently, the haplotypes distribution frequencies at block 1 () were evaluated statistically (). Two main haplotypes were identified in the three Chinese pig breeds, h1 was the dominant haplotype in large pig breeds (JM) (72.2%; P < .0001). For mini-pig breeds, h1 was not existed in BM, h2 was the dominant haplotype in mini-pig breeds (TM: 84.2%; BM: 100%; P < .0001).
Figure 2. Perfect LD structure and haplotypes for the 13 SNPs (r2 = 1) located in the IGFBP-3 promoter region.
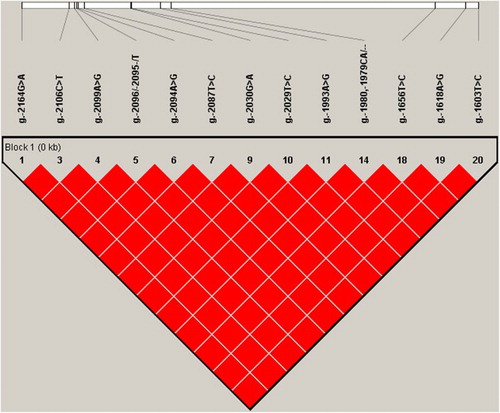
Table 3. Haplotypes of block 1 () and the frequency distributions in the pig breeds.
The haplotypes in promoter region of IGFBP-3 effecting its mRNA expression level in liver and muscle tissue
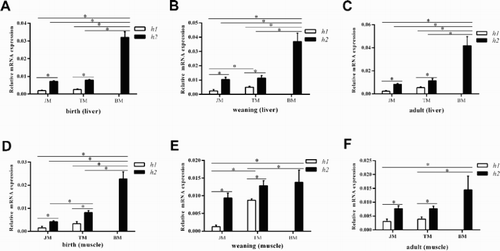
In order to finding out if h1 or h2 is associated with the expression of IGFBP-3 mRNA, thus, the Liver and muscle tissues from birth, weaning and adult containing either h1 or h2 haplotype were used to detect this gene mRNA expression by real-time PCR (). In birth, weaning and adult period of the liver and muscle tissues, the mRNA expression of IGFBP-3 was higher in the JM or TM with h2 than in pigs with h1, respectively (P < .05; (a–f)); in addition, the expression of TM with h1 was also higher than JM with h1 in the weaning period of liver and muscle, respectively (P < .05; (b,e)), the expression of TM with h2 was also higher than JM with h2 in the birth period of muscle (P < .05; (d)). Moreover, the mRNA expression in BM with h2 haplotype is also increased than JM or TM with h2 haplotype in the birth, weaning or adult period of liver, as well as the birth period of muscle, respectively (P < .05; (a–d)).
Figure 3. The haplotypes (h1, h2) of the promoter region are associated with the IGFBP-3 mRNA expression level in liver and muscle tissues of three pig breeds (JM, TM and BM).
Putative regulatory SNPs in the IGFBP-3 promoter region
An in silico analysis was conducted of the putative TFBSs for the SNP loci in IGFBP-3 5′ flanking with the MatInspector and JASPAR prediction tools simultaneously (). Four mutations in the 5′ flanking region were related to mutations in the TFBSs. The AT-rich interactive domain 3A (ARID3A) or paired-related homeobox 2 (PRRX2) binding sites encompass the g.-2094A>G site at allele ‘A’. The growth factor independent 1 (GFI1) transcription repressor, NK2 homeobox 5 (NKX2-5) and myeloid zinc finger 1 (MZF1) binding sites were identified when the g.-1055G>A site was located in allele ‘A’, when the g.-944T>C site was situated in allele ‘C’ and when the g.-389G>A site was located in allele ‘A’, respectively. The aforementioned TFBSs were abolished upon substitution with another allele. The other TFBSs exhibited in were unaffected by the prediction of alternating alleles.
Table 4. In silico predict of TFBSsa at SNPs in the IGFBP-3 promoter region.
Discussion
Our study has demonstrated that a perfect LD consisted of 13 SNPs was observed in the promoter region of IGFBP-3 gene and two main haplotypes were identified in the three Chinese pig breeds, of which h1 or h2 was the dominant haplotype of large pigs (JM) or mini-pigs (TM and BM), respectively. These haplotypes were associated with the expression level of IGFBP-3 mRNA. Via the analysis between the expression rules of IGFBP-3 mRNA and the haplotypes, the h2 haplotype is considered associated with the higher level of IGFBP-3 mRNA expression compared with h1 haplotype.
Recently, some published studies have clearly shown that IGFBP-3 was involved in the growth retardation (Modric et al. Citation2001), in addition, the serum IGFBP-3 was negatively associated with the average daily gain of pigs (Clutter et al. Citation1995). What is more, the research of Nawathe et al. (Citation2016) also showed that the mRNA expression and protein levels of IGFBP-3 were higher in the small for gestational age (SGA) neonates compared to the appropriately grown neonates and the pathogenesis of SGA neonates, which may be caused by the differential methylation of IGFBP-3 as well as other related gene. In the current study, the IGFBP-3 mRNA expression level in birth, weaning and adult period of the liver and muscle tissues in the mini-pigs is generally higher than the large pigs, which showed that the IGFBP-3 may have negative influence in the growth of mini-pigs.
In the research conducted by Lahbib-Mansais et al. (Citation1996) found that the porcine IGFBP-3 gene was mapped on SSC18q24. Furthermore, the quantitative trait locus (QTL) for meat quality and dorsal fat thickness has been identified in these regions (SSC18/S0120/S0062, respectively) (de Koning et al. Citation2001; Lee et al. Citation2003). Several studies have investigated the effect of SNPs in the IGFBP-3 gene on porcine performance. Wang et al. (Citation2009) determined that one SNP located in the IGFBP-3 gene (the combined mutation of G897T-G903A-C911T in intron 2) in 17 pig breeds was connected to dorsal fat thickness and meat colour. Liu et al. (Citation2007) detected 27 SNPs in the IGFBP-3 gene (AY422045) and concluded that these SNPs are potential markers associated with growth-related traits in pigs.
High-density SNPs clustered in the promoter region of IGFBP-3 (31 SNPs and 5 InDels were identified in the 2196 bp region with an average SNP density of 1 SNP per 61 bp), and a few SNP sites may act as eQTL which affect the formation or abolishment of TFBSs. These elements may alter mRNA expression levels through their effects on specific TFBSs which influence mRNA stability (Bregman et al. Citation2011). The in silico of TFBSs prediction analysis conducted in the present study indicated that from the 13 SNP loci which constituted of the haplotypes, only the g.-2094 A>G site caused TFBSs change when it was substituted to another allele. The ARID3A or PRRX2 binding sites encompass the g.-2094A>G site at allele ‘A’.
There may be an A-T rich sequence around the SNP g.-2094A>G when it is allele ‘A’, causing ARID3A or PRRX2 binding (). ARID3A is important for normal embryogenesis, which binds directly to the Oct4, Sox2, Nanog and IgH promoter/enhancer region to activate or repress gene transcription (Webb et al. Citation2011; Popowski et al. Citation2014). PRRX2, also known as S8, is a mesenchymal DNA-binding transcription factor essential for foetal development (Gervais et al. Citation2005), and plays important roles in limb morphogenesis and skeletogenesis (Yoshida et al. Citation2014). Research shows that the highly A-T rich sequence of porcine Fshb promoter region that regulated by PRRX2 and PRRX2 may play a role in suppressing the expression of the Fshb gene (Susa et al. Citation2009). These hint that the transcription factors might be involved in the regulation of the IGFBP-3 transcription which needs further confirmation.
LD is also known as allelic association. Given the differences in the body size traits of miniature (BM and TM) and large (JM) pig breeds, LD analyses were conducted and haplotype distributions were determined to locate SNP markers in the promoter region of IGFBP-3. The h1 (GCA-ATGTACATAT) was the predominant haplotype for the large pig breeds. The high frequency of h2 (ATGTGCACG–CGC) in miniature pigs (0.842 and 1.000 for TM and BM, respectively) may indicate a genomic selection tendency for h2 in the evolutionary determination of the two local Chinese miniature pig breeds, but the haplotype frequency deviation may be caused by the limited number of samples collected. Therefore, large samples must be obtained in future experiments to identify more credible and major haplotypes.
In addition, r2 = 1 is regarded as perfect LD (Gaut & Long Citation2003). In the current study, exactly two out of the four possible two-locus haplotypes were observed in the paired SNPs (), this finding may indicate that the markers have not been separated by recombination and display with the same allele frequencies. In this case, any one of the 13 SNPs in the block () may be a ‘haplotype tag SNP’, because the findings for one SNP marker reflect the information regarding the others. This pattern is convenient for genotyping purposes.
In summary, this study detected a perfect LD in the promoter region of IGFBP-3 in the three pig breeds as well as identified the dominant haplotype in the large or miniature pig breeds, respectively, the haplotypes were also associated with the IGFBP-3 mRNA expression level. With regard to the relationship of IGFBP-3 with animal growth and development, the perfect LD and haplotypes analysis may serve as molecular markers which can be applied in the breeding of pig varieties.
Acknowledgements
This study was conducted under the approval of Animal Care and Use Committee of Jilin University.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Barrett JC, Fry B, Maller J, Daly MJ. 2005. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 21:263–265. doi: 10.1093/bioinformatics/bth457
- Botstein D, White RL, Skolnick M, Davis RW. 1980. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 32:314–331.
- Bregman A, Avraham-Kelbert M, Barkai O, Duek L, Guterman A, Choder M. 2011. Promoter elements regulate cytoplasmic mRNA decay. Cell. 147:1473–1483. doi: 10.1016/j.cell.2011.12.005
- Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T. 2005. Matlnspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics. 21:2933–2942. doi: 10.1093/bioinformatics/bti473
- Chen A, Hao LL, Fang XB, Lu K, Liu SC, Zhang YL. 2014. Polymorphism analysis of IGFBP-5 gene exon 1 in Tibet Mini-pig and Junmu No. 1 White pig. Genet Mol Res. 13:1643–1649. doi: 10.4238/2014.March.12.17
- Clemmons DR. 1997. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev. 8:45–62. doi: 10.1016/S1359-6101(96)00053-6
- Clutter AC, Spicer LJ, Woltmann MD, Grimes RW, Hammond JM, Buchanan DS. 1995. Plasma growth hormone, insulin-like growth factor I, and insulin-like growth factor binding proteins in pigs with divergent genetic merit for postweaning average daily gain. J Anim Sci. 73:1776–1783. doi: 10.2527/1995.7361776x
- Fang X, Liu S, Cheng Y, Li S, Wu Q, Su D, Lu C, Yu H, Hao L. 2015. SNPs in the 5′ terminal-region of IGFBP6 gene and its linkage with pig body size. Anim Cell Syst. 19:417–424. doi: 10.1080/19768354.2015.1108227
- Gao Y, Zhang Y, Zhang S, Li F, Wang S, Dai L, Jiang H, Xiao S, Liu D, Sun B, et al. 2011. Association of A-FABP gene polymorphism in intron 1 with meat quality traits in Junmu No. 1 white swine. Gene. 487:170–173. doi: 10.1016/j.gene.2011.07.005
- Gaut BS, Long AD. 2003. The lowdown on linkage disequilibrium. Plant Cell. 15:1502–1506. doi: 10.1105/tpc.150730
- Gervais C, Mauvieux L, Perrusson N, Helias C, Struski S, Leymarie V, Lioure B, Lessard M. 2005. A new translocation t(9;11)(q34;p15) fuses NUP98 to a novel homeobox partner gene, PRRX2, in a therapy-related acute myeloid leukemia. Leukemia. 19:145–148.
- Grohmann M, Foulstone E, Welsh G, Holly J, Shield J, Crowne E, Stewart C. 2005. Isolation and validation of human prepubertal skeletal muscle cells: maturation and metabolic effects of IGF-I, IGFBP-3 and TNF alpha. J Physiol. 568:229–242. doi: 10.1113/jphysiol.2005.093906
- Gu Y-R, Zhang K, Li M-Z, Li X-W, Zhu L, Wang JY, Chen L. 2009. Developmental expression changes of Insulin-Like Growth Factors (IGFs) system genes in longissimus dorsi muscle of two pig breeds. Yi chuan = Hereditas/Zhongguo yi chuan xue hui bian ji. 31:837–843. doi: 10.3724/SP.J.1005.2009.00837
- Hao LL, Yu H, Zhang Y, Sun SC, Liu SC, Zeng YZ, Ai YX, Jiang HZ. 2011. Single nucleotide polymorphism analysis of exons 3 and 4 of IGF-1 gene in pigs. Genet Mol Res. 10:1689–1695. doi: 10.4238/vol10-3gmr1328
- Hjortebjerg R, Frystyk J. 2013. Determination of IGFs and their binding proteins. Best Pract Res Clin Endocrinol Metab. 27:771–781. doi: 10.1016/j.beem.2013.08.010
- Juszczuk-Kubiak E, Starzynski RR, Wicinska K, Flisikowski K. 2012. Promoter variant-dependent mRNA expression of the MEF2A in longissimus dorsi muscle in cattle. DNA Cell Biol. 31:1131–1135. doi: 10.1089/dna.2011.1533
- de Koning DJ, Harlizius B, Rattink AP, Groenen MAM, Brascamp EW, van Arendonk JAM. 2001. Detection and characterization of quantitative trait loci for meat quality traits in pigs. J Anim Sci. 79:2812–2819. doi: 10.2527/2001.79112812x
- Lahbib-Mansais Y, Yerle M, Pinton P, Gellin J. 1996. Chromosomal localization of homeobox genes and associated markers on porcine chromosomes 3, 5, 12, 15, 16 and 18: comparative mapping study with human and mouse. Mamm Genome. 7:174–179. doi: 10.1007/s003359900049
- Lee C, Chung Y, Kimi JH. 2003. Quantitative trait loci mapping for fatty acid contents in the backfat on porcine chromosomes 1, 13, and 18. Mol Cells. 15:62–67.
- Li J, Liu Y, Zhang JW, Wei H, Yang L. 2006. Characterization of hepatic drug-metabolizing activities of Bama miniature pigs (Sus scrofa domestica): Comparison with human enzyme analogs. Comp Med. 56:286–290.
- Liu D, Zhang Y, Du Y, Yang G, Zhang X. 2007. Identification and characterization of single nucleotide polymorphisms in 6 growth-correlated genes in porcine by denaturing high performance liquid chromatography. DNA Seq. 18:220–227. doi: 10.1080/10425170601150839
- Locatelli V, Bianchi VE. 2014. Effect of GH/IGF-1 on bone metabolism and osteoporsosis. Int J Endocrinol. 2014:235060. Available from: https://www.hindawi.com/journals/ije/2014/235060/ doi: 10.1155/2014/235060
- Mathelier A, Zhao X, Zhang AW, Parcy F, Worsley-Hunt R, Arenillas DJ, Buchman S, Chen CY, Chou A, Ienasescu H, et al. 2014. JASPAR 2014: an extensively expanded and updated open-access database of transcription factor binding profiles. Nucleic Acids Res. 42:D142–D147. doi: 10.1093/nar/gkt997
- Mathews LS, Hammer RE, Behringer RR, D’Ercole AJ, Bell GI, Brinster RL, Palmiter RD. 1988. Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology. 123:2827–2833. doi: 10.1210/endo-123-6-2827
- Modric T, Silha JV, Shi ZD, Gui YT, Suwanichkul A, Durham SK, Powell DR, Murphy LJ. 2001. Phenotypic manifestations of insulin-like growth factor-binding protein-3 overexpression in transgenic mice. Endocrinology. 142:1958–1967.
- Moses AC, Nissley SP, Short PA, Rechler MM, White RM, Knight AB, Higa OZ. 1980. Increased levels of multiplication-stimulating activity, an insulin-like growth factor, in fetal rat serum. Proc Natl Acad Sci USA. 77:3649–3653. doi: 10.1073/pnas.77.6.3649
- Nawathe AR, Christian M, Kim SH, Johnson M, Savvidou MD, Terzidou V. 2016. Insulin-like growth factor axis in pregnancies affected by fetal growth disorders. Clin Epigenet. 8:11. Available from: http://clinicalepigeneticsjournal.biomedcentral.com/articles/10.1186/s13148-016-0178-5 doi: 10.1186/s13148-016-0178-5
- Nei M. 1973. Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA. 70:3321–3323. doi: 10.1073/pnas.70.12.3321
- Ning Y, Schuller AGP, Bradshaw S, Rotwein P, Ludwig T, Frystyk J, Pintar JE. 2006. Diminished growth and enhanced glucose metabolism in triple knockout mice containing mutations of insulin-like growth factor binding protein-3,-4, and-5. Mol Endocrinol. 20:2173–2186. doi: 10.1210/me.2005-0196
- Popowski M, Templeton TD, Lee BK, Rhee C, Li H, Miner C, Dekker JD, Orlanski S, Bergman Y, Iyer VR, et al. 2014. Bright/Arid3A acts as a barrier to somatic cell reprogramming through direct regulation of Oct4, Sox2, and Nanog. Stem Cell Reports. 2:26–35.
- Shan L, Wu Q, Li Y, Shang H, Guo K, Wu J, Wei H, Zhao J, Yu J, Li MH. 2014. Transcriptome profiling identifies differentially expressed genes in postnatal developing pituitary gland of miniature pig. DNA Res. 21:207–216. doi: 10.1093/dnares/dst051
- Simianer H, Koehn F. 2010. Genetic management of the Gottingen Minipig population. J Pharmacol Toxicol Methods. 62:221–226. doi: 10.1016/j.vascn.2010.05.004
- Susa T, Ishikawa A, Kato T, Nakayama M, Kato Y. 2009. Molecular cloning of paired related homeobox 2 (Prx2) as a novel pituitary transcription factor. J Reprod Dev. 55:502–511. doi: 10.1262/jrd.20256
- Tian YG, Yue M, Gu Y, Gu WW, Wang YJ. 2014. Single-nucleotide polymorphism analysis of GH, GHR, and IGF-1 genes in minipigs. Braz J Med Biol Res. 47:753–758. doi: 10.1590/1414-431X20143945
- Wang W, Meng Q, Hu X, Li N. 2009. Genetic variation and association of insulin-like growth factor binding protein-3 with performance in swine. Biochem Genet. 47:315–321. doi: 10.1007/s10528-009-9230-x
- Webb CF, Bryant J, Popowski M, Allred L, Kim D, Harriss J, Schmidt C, Miner CA, Rose K, Cheng HL, et al. 2011. The ARID family transcription factor bright is required for both hematopoietic stem cell and B lineage development. Mol Cell Biol. 31:1041–1053. doi: 10.1128/MCB.01448-10
- Ye RS, Li M, Qi QE, Cheng X, Chen T, Li CY, Wang SB, Shu G, Wang LN, Zhu XT, et al. 2015. Comparative anterior pituitary miRNA and mRNA expression profiles of Bama minipigs and landrace pigs reveal potential molecular network involved in animal postnatal growth. PLoS ONE. 10:e0131987.
- Yoshida S, Ueharu H, Higuchi M, Horiguchi K, Nishimura N, Shibuya S, Mitsuishi H, Kato T, Kato Y. 2014. Molecular cloning of rat and porcine retina-derived POU domain factor 1 (POU6F2) from a pituitary cDNA library. J Reprod Dev. 60:288–294. doi: 10.1262/jrd.2014-023
- Zhang YH, Dai LS, Ma TH, Wang SZ, Guo J, Li FJ, Zhang SM, Sun BX, Liu DF, Gao Y, Zhang JB. 2013. Association of T1740C polymorphism of L-FABP with meat quality traits in Junmu No. 1 white swine. Genet Mol Res. 12:235–241. doi: 10.4238/2013.January.30.9
