ABSTRACT
Background: The flowering parts of Gentiana olivieri, known as ‘Afat’ in the southeastern Anatolia region of Turkey, are used as a tonic, an appetizer, and for the treatment of several mental disorders, including depression. The purpose of this study is to investigate the antidepressant effect of G. olivieri ethanol extract (GOEE) in a chronic mild stress-induced rat model, which was used to mimic a depressive state in humans, and to compare the effect with that of imipramine.
Methods: Male Sprague-Dawley rats were randomly divided into six groups: control, stress, treated with imipramine (positive control) and treated with GOEE at three different (200, 500, 1000 mg/kg) doses groups. The rats in all groups, except the control group, were exposed to chronic mild stress. At the end of the 3-week experimental period, biochemical and behavioral parameters were examined.
Results: The results showed that treatment with GOEE or imipramine significantly improved rats’ sucrose consumption which was diminished by chronic mild stress, restored serum levels of corticosterone and proinflammatory cytokines (interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α)), prevented the increase of liver index of rats. Moreover, in the hippocampus tissue, decreased serotonin and noradrenaline levels were significantly increased by treatment with GOEE or imipramine, and antioxidant parameters (thiobarbituric acid reactive substances (TBARS), superoxide dismutase (SOD), and glutathione (GSH)) were significantly improved by treatment with GOEE though not with imipramine.
Conclusion: The data demonstrate that G. olivieri may exert its antidepressant activity by improving monoaminergic system disorders, and by favorably affecting the antioxidant, inflammatory and the endocrine mechanisms.
1. Introduction
Depression is a psychiatric disorder that manifests itself in symptoms such as sadness, despair, self-depreciation, disturbance of bodily functions (e.g., sleep, appetite, sexual drive), decreased will to live, and suicidal tendency [Citation1]. With the global prevalence of 4.4%, depression represents an ever-growing problem [Citation2]. Animal models of depression are widely used by researchers for the discovery of new drugs, or development of new treatment methods. Among these models, the chronic stress model is important in that it is an experimental animal model that mimics the stress to which humans are exposed during daily living and is widely used in probing pathogenesis of depressive disorders, particularly for evaluating chronic treatments [Citation3].
Based on the biogenic amine hypothesis, the most accentuated hypothesis suggested for the etiology of depression, decreased levels of amines, such as serotonin, noradrenaline, and dopamine, due to a disturbance in the neurotransmitter system in the central nervous system (CNS), may lead to depression [Citation4]. The fact that many antidepressants exert their effects by elevating the levels of these amines in the CNS, and that monoamine oxidase inhibitors are used to treat depression, supports this hypothesis [Citation5,Citation6].
The depressive-like endocrine phenotypes are common in individuals with major depression and manifest with increased serum corticotropin-releasing hormone and cortisol levels [Citation7]. In rodents, increased serum corticosterone levels indicate depressive-like endocrine phenotypes [Citation8]. Because antidepressants favorably affect the depressive-like endocrine phenotypes, studies targeting this mechanism are also underway [Citation9].
Pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), secreted in response to tissue damage, pathogens, or psychosocial stressors, and the increase of these cytokines is balanced by anti–inflammatory mechanisms [Citation10]. In the case of chronic stress exposure, this balance is disrupted and cytokines can lead to depression, anxiety, neuropsychiatric, and other chronic disorders by various mechanisms [Citation11]. Cytokines have been suggested to play a role in the pathogenesis of depressive disorders, as they suppress the negative feedback mechanism of the hypothalamic-pituitary-adrenal axis by causing desensitization of glucocorticoid receptors. Disruption of antioxidant systems and neurotransmitter mechanisms by cytokines also contributes to pathogenesis [Citation12,Citation13].
Regarding the link between depression and oxidative stress, oxidative stress was shown to be increased in depressed patients by demonstrating a diminished plasma antioxidant pool and increased lipid peroxidation [Citation14]. It was also reported that antioxidants may have antidepressant activities, and it has been suggested that compounds with potent antioxidant properties, including ascorbic acid, rosmarinic acid, and caffeic acid, may have a comparable antidepressant effect, with fewer side effects, compared with commonly used agents, such as fluoxetine and imipramine [Citation15–Citation17]. Dhingra et al. proved that chronic stress increased oxidative stress in animals, and that the potent antioxidant, beta-carotene, at the dose of 100 mg/kg, had a similar antidepressant effect as 15 mg/kg of imipramine [Citation18].
Reduced patient compliance to therapy with antidepressants, due to their side effects, is one of the leading causes of treatment failure in clinical practice [Citation5,Citation19]. The newly developed synthetic antidepressants, such as vilazodone, levomilnacipran, and vortioxetine, have not provided a significant advance with regard to side effects [Citation20–Citation22]. Intolerance to, or rejection of, a drug due to its side effects, discontinuation of treatment, absence of response, patient refusal to take drugs for various reasons, and the economic burden of antidepressants are frequently encountered problems in treatment [Citation23]. All these raise the importance of complementary and alternative treatments with fewer side effects. Thus, there is now a need for antidepressant trials to investigate medicinal plants that are expected to have fewer side effects.
The flowering parts of Gentiana olivieri, known as ‘Afat’ in the southeastern Anatolia region of Turkey, are used in folk medicine as an antidiabetic, stomachic, tranquillizer, wound healer, antiemetic, appetizer, and antipyretic and for the treatment of several mental disorders, including depression [Citation24,Citation25]. It is used in Uzbekistan for the treatment of the common cold and gastrointestinal disorders and also as a wound healer [Citation26,Citation27]. In addition, Gentians are used as food and beverage flavoring [Citation28]. Previous studies have examined the hypoglycemic [Citation25], antihyperlipidemic [Citation25], immunomodulatory [Citation29], antihypertensive [Citation30], antioxidant [Citation31], hepatoprotective [Citation31], and antiepileptic [Citation32] effects of the plant, however, the antidepressant effect of G. olivieri has not been investigated. The present study aims to investigate the antidepressant effect of G. olivieri ethanol extract (GOEE) at different doses, compare the effect with that of imipramine.
2. Materials and methods
2.1. Animals
Sprague-Dawley rats obtained from the Experimental Animal Reproducing and Investigational Center of Inonu University were used for this study. The principles of the Ethics Board of Inonu University, Faculty of Medicine, were followed throughout the study (Ethics Board Protocol No: 2015/A-44). Male rats weighing 250–300 g were maintained in standard sheltering cages until the day of the experiment. The rats were kept in rooms equipped with 12 h, dark-light lighting cycles, housed four per cage, under appropriate humidity and ventilation conditions at room temperatures between 24 and 27ºC. Only male rats were selected, to avoid the possible effects on the results of hormonal changes in female rats.
The rats were divided into six groups with eight rats in each, as follows:
Group: Control unstressed group (C); Vehicle
Group: Negative control stressed group (S); Chronic mild stress + Vehicle
Group: Imipramine group (IM); Chronic mild stress + 10 mg/kg/day imipramine
Group: 1000 mg/kg G. olivieri group (G1000); Chronic mild stress + 1000 mg/kg/day GOEE
Group: 500 mg/kg G. olivieri group (G500); Chronic mild stress + 500 mg/kg/day GOEE
Group: 200 mg/kg G. olivieri group (G200); Chronic mild stress + 200 mg/kg/day GOEE
2.2. Chronic mild stress model and sucrose consumption test
A chronic mild stress procedure was carried out according to the method of Muscat et al. [Citation33]. Briefly, rats trained for palatable weak (1%) sucrose solution consumption and grouped such that they would not differ in the amount of sucrose solution consumed were exposed to different stressors in a randomized order. In the 3-week chronic mild stress model, the animals were exposed to the following stressors: 17 h isolation in the cage, exposure to stroboscopic light for 7, 9 and 17 h, nightlong light exposure, keeping in a 45º sloped cage for 7 and 17 h, deprivation of water and food for 20 h, exposure to 85 dB noise for 3 and 5 h, keeping in the cage with an empty bottle for 1 h, and limiting water and food access at various times. At the end of each week, rats were deprived of food and water for 12 h, and were subjected to 1 h sucrose consumption test. The amount of sucrose solution consumption was measured for each rat and averaged across groups by weighing the bottles prior to and after the test. At the baseline (week 0) the consumption of the groups was accepted as 100%, and weekly percentage change in the consumed amount of sucrose solution of groups was calculated. All experiments were performed between 08:00 a.m. and 12:00 p.m.
2.3. Plant material
Considering the flowering window of the plant, samples were collected in May in the Oğuzeli district of Gaziantep (Turkey) and were botanically identified. The voucher specimens (No: AB15-01) and the shadow-dried plant samples were maintained in the Herbarium of Inonu University, Faculty of Pharmacy, at 14–17°C and a relative humidity of about 45–55%, until the time they were used.
2.4. Preparation of the ethanolic extract
The dried and powdered plant parts were subjected to maceration by constant stirring in ethanol 80% (30 g of plant to 100 ml of solution) at room temperature for 3.5 h. This method has been carried out considering the data in the previous studies [Citation32]. Ethanol was vaporized under reduced pressure with Rotavapor to produce the extract. To improve the yield, the herbal extracts were pooled by repeating this extraction twice. Freshly prepared plant extracts and imipramine (Sigma-Aldrich chemicals Co., Germany) were dissolved in a 10% Tween 80 (Sigma-Aldrich chemicals Co., Germany) and were made ready in volumes of 1 ml, to be given by oral gavage at corresponding doses.
2.5. Phytochemical screening
The phytochemical screening of the GOEE was performed as follows: the presence of alkaloid and magnesium with Dragendorff’s reactive; flavonoid with HCI; flavone-derivative glycosides with iron (III) chloride; coumarins with the UV-366 test with sodium hydroxide; reducing sugars with Fehling-A and Fehling-B solutions; anthocyanin-derivative glycosides with lead acetate; and tannin with ferric chloride reagent [Citation34].
2.6. Blood and tissue collection
At the end of the experiment, the animals were sacrificed under anesthesia, using 50 mg/kg of ketamine and 5 mg/kg of xylazine, intraperitoneally. Blood samples taken from the animals’ hearts were centrifuged with a cooled centrifuge at 3000 rpm for 15 min, to obtain serum. The brain region of hippocampus and liver of the rats was rapidly isolated, weighed, washed with cold saline solution (0.9% NaCl), and stored at −80°C until homogenization. All samples were immediately transferred to −80°C refrigerators, and were maintained there until the time of measurement.
2.7. Liver index
Liver index was determined by calculating the percentage of liver/body weight of rats.
2.8. Protein assay
All protein assays of the hippocampus were performed according to the Bradford (1976) method [Citation35]. This method utilizes the protein-coloring property of a reactive solution containing an organic colorant. The reactive used is a dye that binds to protein and causes a shift in the absorption maximum of the dye, from 465 to 595 nm. The dye forms a blue color upon binding to proteins. The color, which appears in 2 min, remains stable for 1 h, and its absorbance values at 595 nm are recorded, to be used to find protein concentrations from a standard curve.
2.9. Elisa assay
The hippocampus was homogenized using a Teflon-glass homogenizer in a cold sodium-phosphate buffer (10mM, pH 7.4). The serum and the supernatant obtained by centrifugation of hippocampus homogenate for 15 min at 3500 rpm were used for Eliza measurements in order to measure IL-6, TNF-α, serotonin, noradrenaline, dopamine, corticosterone, thiobarbituric acid reactive substances (TBARS), superoxide dismutase (SOD), and glutathione (GSH) levels. We utilized the sandwich Elisa technique, as per the protocol of the Elisa kits (Shanghai LZ Biotech Co., China). This technique based on biotin double antibody sandwich technology which enhances the detectability level of the measurement. Briefly, the antigen-containing sample, streptavidin-HRP, and biotin-marked antibody were added to the microtiter plate of 96 wells, after which it is left to incubate. Antigen–antibody complex formed, and unbound proteins and enzymes were removed by washing. Reagents were added for coloring, followed by re-incubation. The developed blue color turned to yellow after adding the acidic stopping solution. Absorbances were read at the 450 nm wavelength, to find concentrations from a standard curve.
2.10. Statistical analysis
The data were expressed as mean ± standard error. The Shapiro–Wilks test confirmed that the data were normally distributed. The significance between the groups was analyzed using one-way analysis of variance (ANOVA). Comparisons of the groups with significant differences were made using the Tukey’s test. Consumed amount of sucrose solution was analyzed using repeated ANOVA, with time as repeated factor, and data between groups for each week were analyzed using one-way ANOVA. The level of significance was set at p < 0.05.
3. Results
The phytochemical screening of GOEE revealed the presence of flavonoids, reducing sugars, coumarins, and tannins ().
Table 1. Qualitative analysis of ethanol extract of G. olivieri.
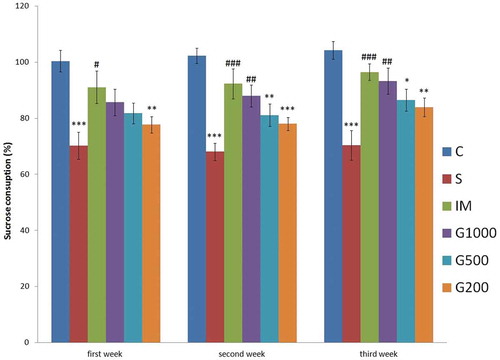
shows the change in the consumed amount of sucrose solution. Repeated ANOVA showed significant effects of time, and time × treatment interaction on consumed amount of sucrose solution. Significant reduction in sucrose consumption was observed in the rats exposed to chronic mild stress as seen by the significant change between C and S groups. Significant increase in sucrose consumption was observed in IM group at the end of the first week (F(5,42) = 5,667, P < 0.001), in G1000 and IM groups at the end of the second (F(5,42) = 10,397, P < 0.001) and third (F(5,42) = 8,723, P < 0.001) weeks as compared to S group.
Figure 1. Change in the consumed amount of sucrose solution in time following chronic administration of GOEE and imipramine in the chronic mild stress model
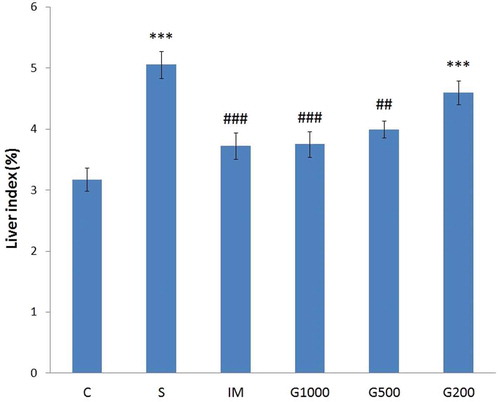
shows animals’ liver index changes in the chronic mild stress model. Chronic mild stress significantly increased the liver index of the rats (F(5,42) = 11,750, P < 0.001). Treatment with GOEE (1000 and 500 mg/kg) or imipramine resulted in a significant reduction in liver index.
Figure 2. Liver index changes following chronic administration of GOEE and imipramine for 3 weeks in the chronic mild stress model
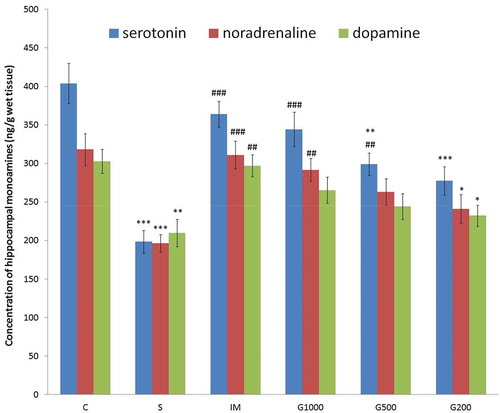
represents monoamine levels in the hippocampus. Significant decrease in hippocampal serotonin, noradrenaline, and dopamine level was found in S group compared to C group. Significant increase in hippocampal serotonin level recorded in IM, G1000 and G500 groups (F(5,42) = 14,314, P < 0.001); significant increase in hippocampal noradrenaline level recorded in IM and G1000 groups (F(5,42) = 7,442, P < 0.001); significant increase in hippocampal dopamine level recorded in IM group (F(5,42) = 5,406, P < 0.01) as compared to S group.
Figure 3. Monoamine levels in the hippocampus following chronic administration of GOEE and imipramine for 3 weeks
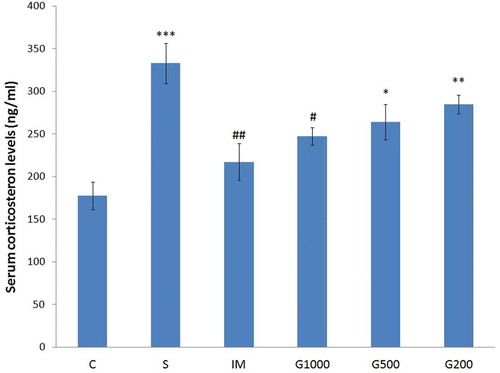
summarizes the results of serum corticosterone level of rats. Significant increase in serum corticosterone level was seen in the group S compared to group C (F(5,42) = 8,981, P < 0.001). Significant decrease in serum corticosterone level was observed in IM and G1000 groups as compared to S group.
Figure 4. Serum corticosterone levels following chronic administration of GOEE and imipramine for 3 weeks
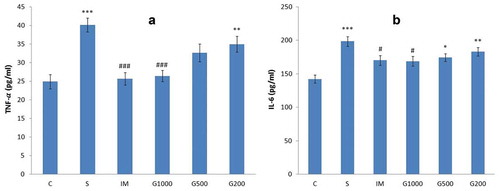
shows serum levels of proinflammatory cytokines: TNF-α and IL-6 of rats. Chronic mild stress led to an increase in serum levels of TNF-α (F(5,42) = 10,035, P < 0.001) and IL-6 (F(5,42) = 7,771, P < 0.001). The increased levels of TNF-α and IL-6 were significantly attenuated by GOEE (1000 mg/kg) or imipramine.
Figure 5. Serum levels of proinflammatory cytokines (a: TNF-α, b: IL-6) following chronic administration of GOEE and imipramine for 3 weeks
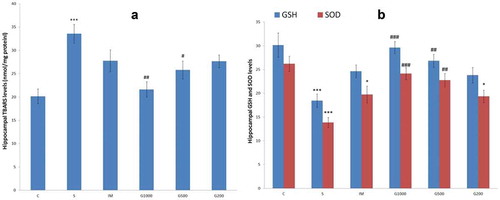
shows TBARS, GSH, and SOD levels in the hippocampus. Significant increase was seen in hippocampal TBARS (F(5,42) = 6,902, P < 0.001) level of group S, significant decrease was seen in hippocampal GSH (F(5,42) = 7,861, P < 0.001) and SOD (F(5,42) = 9,523, P < 0.001) levels of group S compared to C group. Significant decrease in hippocampal TBARS level recorded in G1000 and G500 groups; significant increase in hippocampal GSH level recorded in G1000, G500 groups; significant increase in hippocampal SOD level recorded in G1000 and G500 groups as compared to S group.
Figure 6. TBARS (a), GSH, and SOD (b) levels in the hippocampus following chronic administration of GOEE and imipramine for 3 weeks
4. Discussion
A review of the studies on plants in depression research demonstrates that medicinal plants exert antidepressant activity via their polyphenols (flavonoids, lignans, phenolic acids, coumarins), saponins, sapogenins, alkaloids, terpenes, terpenoids, amines, and carbohydrate content [Citation36]. A qualitative analysis by Aslan et al. showed the presence of flavonoids, terpenes, coumarins, and reducing sugars in ethanolic extract of G. olivieri [Citation32]. Our qualitative analysis also yielded the presence of flavonoids of flavone structure, reducing sugars, coumarins, and tannins. The structure of gentianine and gentianidine was described by another study with the ethanolic extract [Citation30]. This phytochemical content of G. olivieri suggested that the plant may have antidepressant activity. The fact that the plant is being used as folk medicine for the treatment of mental disorders, including depression, reinforced this hypothesis [Citation24,Citation26]. In this study, we preferred ethanol extraction because it is known to be a method for obtaining broad spectrum fractions, including phenolic compounds [Citation37]. Imipramine, which we used as a reference, was found to be effective at a dose of 10 mg/kg in other studies [Citation38].
In our study, the chronic mild stress model induced depressive signs by altering the amount of sucrose solution consumed, liver index, levels of monoamines – including serotonin, dopamine, and noradrenaline – and antioxidant parameters in the hippocampus, serum levels of proinflammatory cytokines and corticosterone. These findings suggest that the depression model was used effectively and successfully. The chronic stress model demonstrated similar results in other studies [Citation39].
Anhedonia, which is the core symptom of depression in humans, is associated with sucrose consumption of rodents in the chronic stress model, and the decrease in consumption indicates depressive symptoms [Citation40]. Our study indicated that the chronic mild stress model reduces animals’ sucrose consumption amount from the first week until the end of the study. Treatment with imipramine or 1000 mg/kg GOEE significantly reversed the reduced consumption of sucrose. Other studies have also reported that imipramine increased sucrose consumption that was reduced as a result of chronic stress [Citation39]. GOEE at the dose of 1000 mg/kg had an effect that was comparable to that of imipramine at week 2, suggesting that the plant may have a delayed onset of antidepressant effect, compared with the synthetic antidepressant imipramine. Concerning the liver index measurement, the data were consistent with sucrose consumption amounts, and the effect anticipated from chronic stress was also present in the animals’ liver index changes. It is known that liver index increases in animals under restraint stress. In our study, chronic stress-induced liver index increase was normalized with imipramine or GOEE (at the dose of 500 and 1000 mg/kg) treatment [Citation41].
We chose the hippocampus region of the brain for biochemical measurements, because behavioral functions such as memory, learning, and emotions that may be associated with depression are important in relation to this region of the brain [Citation42]. Monoamines in the CNS are known to play a key role in the pathophysiology of depression, and of these monoamines, serotonin, noradrenaline, and dopamine are suggested to be closely linked with depressive disorders [Citation43]. Most antidepressant drugs exert their effects over monoamine mechanisms, especially the 5-HT system plays a critical role in these effects [Citation44]. There are also a number of studies indicating the involvement of the dopaminergic system in the pathophysiology of depression [Citation45]. In our study, analysis of hippocampal monoamine levels indicated that chronic stress decreases the levels of these monoamines, which play an important role in the pathophysiology of depression. Since imipramine is a tricyclic antidepressant drug, as expected, imipramine led to an increase in hippocampal monoamine levels that were decreased in chronic stress. Compared with the S group, G. olivieri significantly increased serotonin and noradrenaline levels, at the dose of 1000 mg/kg, and significantly increased serotonin levels, at the dose of 500 mg/kg, which demonstrates that the G. olivieri resulted in an improvement in the hippocampal serotonin and noradrenaline levels that were decreased with chronic stress. The data suggest that G. olivieri may have mediated an antidepressant effect by enhancing neurotransmission of serotonin and noradrenaline, two key monoamines involved in the pathophysiology of depression, in the CNS. Similar to the mechanism of most antidepressants, inhibition of reuptake of serotonin or noradrenaline, inhibition of monoamine oxidase, or antagonism of presynaptic inhibitory serotonin or noradrenaline receptors may have mediated this effect, further investigations are required for clarification [Citation44–Citation46]. However, the data demonstrate that G. olivieri may have a limited effect on the dopaminergic system.
Many antidepressants restore the increased serum corticosterone levels to the normal state, which is considered a good indicator of antidepressant activity [Citation46,Citation47]. In our study, analysis of serum corticosterone levels demonstrated that chronic stress leads to increased serum corticosterone levels, consistent with findings in depressed patients [Citation9]. When GOEE was given at the dose of 1000 mg/kg, it significantly decreased serum corticosterone levels induced by chronic mild stress. Likewise, imipramine-reduced serum corticosterone levels that were increased with chronic mild stress, as noted in other studies [Citation48]. Consistent with studies showing increased proinflammatory cytokines in depressed patients [Citation49], our study demonstrated that treatment with imipramine or GOEE reduced serum levels, which were elevated by chronic stress. These results show that GOEE may regulate chronic stress-induced proinflammatory pathways. Given that these pathways are associated with serum corticosterone levels, monoamines, and antioxidant systems, it can be concluded that other biochemical findings in our study support these results.
Many studies have demonstrated the link between depression and oxidative stress. Increased levels of oxidative stress can be demonstrated by reduced plasma antioxidant pool and increased lipid peroxidation in depressive patients [Citation14]. In addition, preclinical studies have shown that antioxidants may have antidepressant activity, reporting that substances with potent antioxidant properties [Citation15–Citation17]. In our study, analysis of hippocampus demonstrated that hippocampal TBARS levels in the S group were significantly increased, while GSH and SOD levels were significantly decreased, compared with the C group, indicating that chronic stress increased lipid peroxidation and weakened antioxidant mechanisms, as expected. While imipramine failed to improve antioxidant parameters that were adversely affected in chronic stress, G. olivieri decreased TBARS levels significantly – and increased GSH and SOD levels significantly – at the dose of 1000 mg/kg. At the dose of 500 mg/kg, it similarly decreased TBARS levels significantly, and increased GSH and SOD levels significantly, compared with the S group. This indicates that G. olivieri can improve hippocampal antioxidant parameters that were disturbed with chronic stress. Disturbances in antioxidant parameters have been shown in depressive patients, and some clinical studies have demonstrated the positive effects of antioxidants on depression symptoms. Although it is not known through which mechanism antioxidants show antidepressant activity, the fact that they elevate serotonin and noradrenaline levels in the synaptic cleft, in a similar extent to synthetic antidepressants, is believed to be possibly involved in this activity [Citation15]. G. olivieri is known to have antioxidant activity, and the antioxidant effect of the plant has been shown against hepatic damage induced by carbon tetrachloride in rats [Citation31]. Moreover, it has been suggested that isoorientin, a flavonoid, played an important role in this effect, and it was reported that potent flavonoids, such as isoorientin, had antioxidant and antidepressant properties in the chronic stress model [Citation31,Citation50]. When considered from this point of view, the antioxidant effect of G. olivieri may have contributed to the antidepressant activity.
The oral route was chosen for our study because the oral administration of antidepressant drugs is preferred for improved patient compliance. Different routes of administration may lead to different results by altering bioavailability and metabolism. Standardization is one of the most frequent problems in studies with plants. Numerous factors, including the geographic region where the plant grows, the season it is harvested, properties of the soil in which it grows, the part of the plant (e.g., flower, leaf, root) used in the study, drying technique, humidity, and temperature may cause variations in the composition of the active substance or substances responsible for the pharmacological effect of the plant. Studies following standardization of isolation of the active ingredients of G. olivieri will help us better understand the action of this plant.
5. Conclusions
The present study investigated the antidepressant effect of G. olivieri, which is used as a folk medicine, and it concluded that the use of the plant as a folk medicine was consistent with the scientific data. The results also indicate that the possible mechanism of antidepressant action of the G. olivieri is the increasing of the levels of monoamines, such as serotonin and noradrenaline, in the hippocampus as well as restoring the serum corticosterone levels. The biochemical parameters of IL-6 and TNF-α in serum; TBARS, SOD, and GSH levels in the hippocampus indicate that G. olivieri may prevent the oxidative damage and elevation of proinflammatory cytokines caused by chronic mild stress.
Acknowledgments
We would like to thank İnönü University, Scientific Research Project (BAP) for their support to our study. The current data of the project is presented in this article, in the future studies, this topic will be evaluated from different perspectives with other parameters.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet. 1997;349(9064):1498–10.
- WHO. Depression and other common mental disorders: global health estimates. Geneva: World Health Organization. 2017.
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl). 1997;134(4):319–329.
- Stahl SM. Essential psychopharmacology. 2nd ed. Cambridge: Cambridge University Press; 2000.
- Brunello N, Mendlewicz J, Kasper S, et al. The role of noradrenaline and selective noradrenaline reuptake inhibition in depression. Eur Neuropsychopharmacol. 2002;12(5):461–475.
- Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855–870.
- Vrshek-Schallhorn S, Doane LD, Mineka S, et al. The cortisol awakening response predicts major depression: predictive stability over a 4-year follow-up and effect of depression history. Psychol Med. 2013;43(3):483–493.
- Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc Assoc Am Physicians. 1999;111(1):22–34.
- Maric NP, Adzic M. Pharmacological modulation of HPA axis in depression - new avenues for potential therapeutic benefits. Psychiatr Danub. 2013;25(3):299–305.
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):201–217.
- Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83(5):495–502.
- Klings ES, Farber HW. Role of free radicals in the pathogenesis of acute chest syndrome in sickle cell disease. Respir Res. 2001;2(5):280–285.
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24(6):519–525.
- Maes M, De Vos N, Pioli R, et al. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J Affect Disord. 2000;58(3):241–246.
- Bouayed J, Bohn T. Exogenous antioxidants–Double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 2010;3(4):228–237.
- Takeda H, Tsuji M, Inazu M, et al. Rosmarinic acid and caffeic acid produce antidepressive-like effect in the forced swimming test in mice. Eur J Pharmacol. 2002;449(3):261–267.
- Zafir A, Ara A, Banu N. Invivo antioxidant status: a putative target of antidepressant action. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):220–228.
- Dhingra D, Bansal Y. Antidepressant-like activity of beta-carotene in unstressed and chronic unpredictable mild stressed mice. J Funct Foods. 2014;7:425–434.
- MacGillivray S, Arroll B, Hatcher S, et al. Efficacy and tolerability of selective serotonin reuptake inhibitors compared with tricyclic antidepressants in depression treated in primary care: systematic review and meta-analysis. Bmj. 2003;326(7397):1014.
- Zhang XF, Wu L, Wan DJ, et al. Evaluation of the efficacy and safety of vilazodone for treating major depressive disorder. Neuropsychiatr Dis Treat. 2015;11:1957–1965.
- Meeker AS, Herink MC, Haxby DG, et al. The safety and efficacy of vortioxetine for acute treatment of major depressive disorder: a systematic review and meta-analysis. Syst Rev. 2015;4:21.
- Asnis GM, Henderson MA. Levomilnacipran for the treatment of major depressive disorder: a review. Neuropsychiatr Dis Treat. 2015;11:125–135.
- Barnes PM, Powell-Griner E, McFann K, et al. Complementary and alternative medicine use among adults. Adv Data. 2004;343:1–19.
- Baytop T. Türkiyede Bitkiler ile Tedavi. İstanbul Üniversitesi Yayınları. 1984;3255:194–195.
- Sezik E, Aslan M, Yesilada E, et al. Hypoglycaemic activity of Gentiana olivieri and isolation of the active constituent through bioassay-directed fractionation techniques. Life Sci. 2005;76(11):1223–1238.
- Takeda Y, Masuda T, Honda G, et al. Secoiridoid glycosides from Gentiana olivieri. Chem Pharm Bull. 1999;47(9):1338–1340.
- Honda G. A report on traditional medicine of Turkish people (1997, 1998). Kyoto University. 1999. Vol. 43. p. 53,74.
- Orhan N, Hocbac S, Orhan DD, et al. Enzyme inhibitory and radical scavenging effects of some antidiabetic plants of Turkey. Iran J Basic Med Sci. 2014;17(6):426–432.
- Singh S, Yadav CPS, Noolvi MN. Immunomodulatory activity of butanol fraction of Gentiana olivieri Griseb. on Balb/C mice. Asian Pac J Tropical Biomedicine. 2012;2(6):433–437.
- Mansoor A, Zaidi MI, Hyder M, et al. Antihypertensive effect of Gentiana olivieri. J Med Sci. 2004;4:176–178.
- Orhan DD, Aslan M, Aktay G, et al. Evaluation of hepatoprotective effect of Gentiana olivieri herbs on subacute administration and isolation of active principle. Life Sci. 2003;72(20):2273–2283.
- Aslan M, Orhan DD, Orhan N. Effect of Gentiana olivieri on experimental epilepsy models. Pharmacogn Mag. 2011;7(28):344–349.
- Muscat R, Sampson D, Willner P. Dopaminergic mechanism of imipramine action in an animal model of depression. Biol Psychiatry. 1990;28(3):223–230.
- Sakar M, Taner M Fitokimyasal Analizler. Ankara Üniversitesi Eczacılık Fakültesi Yayınları. 1991. p. 67.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.
- Bahramsoltani R, Farzaei MH, Farahani MS, et al. Phytochemical constituents as future antidepressants: a comprehensive review. Rev Neurosci. 2015;26(6):699–719.
- Wen TN, Prasad KN, Yang B. et al. Bioactive substance contents and antioxidant capacity of raw and blanched vegetables. Innov Food Sci Emerg Technol. 2010;11(3):464–469.
- Tomic M, Tovilovic G, Butorovic B, et al. Neuropharmacological evaluation of diethylether extract and xanthones of Gentiana kochiana. Pharmacol Biochem Behav. 2005;81(3):535–542.
- Wainwright SR, Workman JL, Tehrani A, et al. Testosterone has antidepressant-like efficacy and facilitates imipramine-induced neuroplasticity in male rats exposed to chronic unpredictable stress. Horm Behav. 2016;79:58–69.
- Farooq RK, Isingrini E, Tanti A, et al. Is unpredictable chronic mild stress (UCMS) a reliable model to study depression-induced neuroinflammation? Behav Brain Res. 2012;231(1):130–137.
- Laconi E, Tomasi C, Curreli F, et al. Early exposure to restraint stress enhances chemical carcinogenesis in rat liver. Cancer Lett. 2000;161(2):215–220.
- Butterweck V, Böckers T, Korte B, et al. Long-term effects of St. John’s wort and hypericin on monoamine levels in rat hypothalamus and hippocampus. Brain Res. 2002;930(1):21–29.
- Elhwuegi AS. Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):435–451.
- Jans LA, Riedel WJ, Markus CR, et al. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2007;12(6):522–543.
- Nemeroff CB. Fostering foster care outcomes: quality of intervention matters in overcoming early adversity. Arch Gen Psychiatry. 2008;65(6):623–624.
- Mello AF, Juruena MF, Pariante CM, et al. Depression and stress: is there an endophenotype? Rev Bras Psiquiatr. 2007;29(Suppl 1):13–18.
- Tyrka AR, Mello AF, Mello MF, et al. Temperament and hypothalamic-pituitary-adrenal axis function in healthy adults. Psychoneuroendocrinology. 2006;31(9):1036–1045.
- Habib M, Shaker S, El-Gayar N, et al. The effects of antidepressants “fluoxetine and imipramine” on vascular abnormalities and Toll like receptor-4 expression in diabetic and non-diabetic rats exposed to chronic stress. PloS One. 2015;10(3):e0120559.
- O’Brien SM, Scully P, Fitzgerald P, et al. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res. 2007;41(3–4):326–331.
- Ortmann CF, Reus GZ, Ignacio ZM, et al. Enriched flavonoid fraction from cecropia pachystachya trecul leaves exerts antidepressant-like behavior and protects brain against oxidative stress in rats subjected to chronic mild stress. Neurotox Res. 2016;29(4):469–483.
