ABSTRACT
Azithromycin has recently gained popularity for the treatment of periodontal disease, despite sparse literature supporting efficiency in treating periodontal bacterial biofilms. The aim of this study was to evaluate the effect of azithromycin on biofilms comprised of Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia in comparison to an amoxicillin and metronidazole combination. P. gingivalis W50, T. denticola ATCC35405, and T. forsythia ATCC43037 grown under anaerobic conditions at 37°C were aliquoted into 96-well flat-bottom plates in different combinations with addition of azithromycin or amoxicillin + metronidazole at various concentrations. For the biofilm assay, the plates were incubated at 37°C anaerobically for 48 h, after which the biofilms were stained with crystal violet and measured for absorbance at AU620. In this model, polymicrobial biofilms of P. gingivalis + T. denticola, P. gingivalis + T. forsythia, and T. denticola + T. forsythia were cultured. Combination of all three bacteria enhanced biofilm biomass. Azithromycin demonstrated a minimal biofilm inhibitory concentration (MBIC) of 10.6 mg/L, while the amoxicillin + metronidazole combination was more effective in inhibiting biofilm formation with a MBIC of 1.63 mg/L. Polymicrobial biofilm formation was demonstrated by combination of all three red complex bacteria. Azithromycin was ineffective in preventing biofilm formation within a clinically achievable concentration, whereas the combination of amoxicillin and metronidazole was more effective for this purpose.
Introduction
Control of subgingival plaque is an essential component of periodontal treatment. While mechanical debridement remains the first line of periodontal treatment [Citation1], not all patients respond favourably [Citation2,Citation3]. Therefore, supplementary antibiotic therapy is recommended in specific cases to improve the treatment outcome [Citation4]. Current knowledge of the susceptibility of oral bacterial biofilms to antimicrobial agents is limited. Although results of studies investigating the effects of antimicrobial agents on oral bacteria have revealed significant differences in bacterial growth in planktonic form compared with biofilm [Citation5–Citation8], most of the research evaluating the effect of antibiotics on oral bacteria have been conducted using planktonic growth [Citation9–Citation14]. As late colonisers in dental biofilm formation [Citation15], the red complex bacteria (Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia) express synergistic virulence and pathogenicity [Citation16,Citation17]. Among the antibiotics used in treating periodontitis, amoxicillin in combination with metronidazole has been shown to display a strong effect in reducing numbers of non-periodontal bacteria, as well as P. gingivalis and Fusobacterium nucleatum monomicrobial biofilms in vitro [Citation18], and it is currently proposed to be the most clinically and microbiologically advantageous adjunctive antibiotic regime in treating periodontitis [Citation19,Citation20]. Azithromycin, a macrolide antibiotic [Citation21], has also gained popularity for the treatment of periodontitis [Citation22–Citation25]. It is suggested that azithromycin’s pharmacological benefits [Citation26], broad antibacterial spectrum [Citation27], and host modulatory functions [Citation28] make it a viable alternative to the amoxicillin and metronidazole combination. Despite its popularity, there is no literature supporting the efficiency of azithromycin in treating periodontal bacterial biofilms. Therefore, the objective of this study was to evaluate the in vitro effect of azithromycin on mono- and polymicrobial biofilm formation comprised of the red complex pathogens P. gingivalis, T. denticola, and T. forsythia in comparison to the amoxicillin and metronidazole combination.
Materials and methods
Bacterial strains, growth medium, and culture conditions
P. gingivalis W50, T. denticola ATCC® 35405™, and T. forsythia ATCC® 43037™ were obtained from the culture collection of the Oral Health Cooperative Research Centre, Melbourne Dental School, The University of Melbourne. Planktonic bacterial cultures of P. gingivalis, T. denticola, and T. forsythia were routinely grown in oral bacteria growth medium (chemicals supplied by Sigma–Aldrich, and growth media by Oxoid Australia), a modified and adapted version of new oral spirochete medium [Citation29], and GM-1 [Citation30,Citation31], which had been pre-reduced under anaerobic conditions. The cultures were maintained in an anaerobic workstation (MG500; Don Whitley Scientific) at 37°C. Growth was monitored by measuring absorbance at a wavelength of 650 nm (AU650), and P. gingivalis and T. forsythia were harvested during the mid-exponential phase at an AU650 of 0.6, which equates to a cell density of ~1.5 × 109 cells/mL [Citation32]. T. denticola was grown to an AU650 of 0.15, which equates to a cell density of ~1.0 × 108 cells/mL. Culture purity was routinely monitored by Gram staining and colony morphology examination under light microscope.
Effects of antibiotics on planktonic polymicrobial culture
Exponentially growing P. gingivalis Pg) and T. forsythia Tf) cells diluted to an AU650 of 0.15 and undiluted T. denticola at the same AU650 were used as inoculum. Two hundred microliters of P. gingivalis, T. denticola, or T. forsythia as a monospecies inoculum and the combination of each two bacterial species at equal volumes (100 µL each), as well as all three species (67 µL each) as a polymicrobial inoculum, were aliquoted into 96-well flat-bottom plates (Nunc; Thermo Scientific) to provide the same total number of bacterial cells per inoculum. Azithromycin, amoxicillin, and metronidazole (Thermo Multiskan Ascent; Pathtech) were dissolved in deionized water (MQ). Dissolved metronidazole and amoxicillin were mixed in a 1:1 ratio. Stock solutions at 100 mg/L of azithromycin and amoxicillin + metronidazole (1:1 ratio) were diluted in MQ of different volumes to achieve final antibiotic concentrations in the range of 0.01–100 mg/L.Twenty microliters of each antibiotic concentration was added into the wells of a 96-well plate containing the bacterial cultures. Native bacterial growth with no antibiotic added, as well as uncultured growth medium, served as controls. The plate was sealed with microtiter plate film to maintain the anaerobic condition and was incubated at 37°C, with periodic shaking to prevent bacterial cell precipitation. Growth was monitored for 48 h by measuring absorbance at a wavelength of 620 nm (AU620) using a microplate reader (Thermo Multiskan Ascent; Pathtech). The minimal inhibitory concentration (MIC) for the antibiotics was calculated by linear regression.
Effects of antibiotics on mono- and polymicrobial biofilm formation
Exponentially growing P. gingivalis and T. forsythia cells diluted to an AU650 of 0.15 and undiluted T. denticola at the same AU650 were used as inoculum. Two hundred microliters of P. gingivalis, T. denticola, T. forsythia, P. gingivalis + T. denticola, P. gingivalis + T. forsythia, T. denticola + T. forsythia, and P. gingivalis + T. denticola + T. forsythiabacterial cultures in equal volumes were aliquoted into 96-well flat-bottom plates. Antibiotic dilution, concentrations, and volume used were similar to the planktonic assay. Plates were sealed and incubated at 37°C anaerobically for 48 h.
Crystal violet staining for biofilm assay
Crystal violet staining assay was adapted and modified from Dashper et al. [Citation33]. The adherent biofilms were rinsed with 200 μL of MQ and incubated with 0.1% crystal violet. The crystal violet stained biofilms were then dissolved in 80% ethanol + 20% acetone through repeated pipetting before transfer to a new 96-well plate. Quantification of the biofilms was carried out by measuring AU620 using a plate reader (Perkin Elmer Wallac VICTOR1420 Multilabel Counter; PerkinElmer, Inc.). The minimal biofilm inhibitory concentration (MBIC) for the polymicrobial biofilm was calculated by linear regression.
Statistical analyses
For each bacterial combination and antibiotics concentration, biofilm formation in the presence of azithromycin was compared to that in the presence of amoxicillin and metronidazole using Student’s t-test. The significance level was set at 5%.
Results
Susceptibility of planktonic polymicrobial culture to antibiotics
Azithromycin and the combination of amoxicillin + metronidazole were evaluated to determine the MIC of the planktonic polymicrobial culture. Azithromycin and the combination of amoxicillin + metronidazole had a MIC of 1.52 mg/L and 0.17 mg/L, respectively ().
Table 1. The MIC and MBIC of azithromycin and amoxicillin + metronidazole (1:1 ratio) against polymicrobial planktonic cells and biofilms determined using the 96-well plate model
Biofilm formation
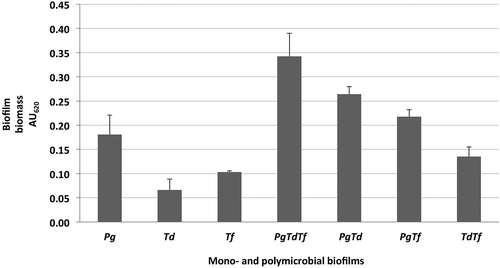
In this model, polymicrobial biofilm formation between P. gingivalis + T. denticola, P. gingivalis + T. forsythia, as well as T. denticola + T. forsythia was demonstrated (). The P. gingivalis and T. forsythia combination enhanced biofilm formation but not as much as that of P. gingivalis and T. denticola. The combination of P. gingivalis, T. denticola, and T. forsythia formed the most biofilm (AU620 = 0.34 ± 0.05; ). Whenever P. gingivalis was involved, the biofilm had a tendency to establish better. T. denticola (AU620 = 0.07 ± 0.02) and T. forsythia (AU620 = 0.10 ± 0.00) formed minimal biofilm when cultured on their own, particularly T. denticola.
Figure 1. Formation of mono- and polymicrobial biofilms in a 96-well plate model after 48 h of incubation at 37°C under anaerobic condition. Native bacterial growth with addition of uncultured growth medium and no antibiotic served as controls. Adherent biofilms were stained with 0.1% crystal violet and the optical density at AU620 was measured. Data represent the mean AU620 value of a minimum of three biological replicates.
Susceptibility of mono- and polymicrobial biofilms to antibiotics
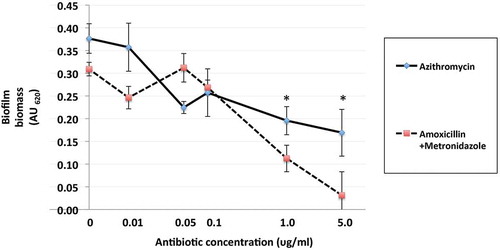
The effect of azithromycin and amoxicillin + metronidazole on mono- and polymicrobial biofilm formation varied, with amoxicillin + metronidazole being more efficacious than azithromycin ( and ). The combination of amoxicillin + metronidazole at a concentration of 1.0 mg/L reduced the biomass of P. gingivalis monomicrobial biofilms by 78%. Concentrations of amoxicillin + metronidazole at 1.0 mg/L and 5.0 mg/L reduced the polymicrobial biofilm biomass by 64 and 89%, respectively(), which was significantly better than azithromycin’s effect of 48 and 55% reduction for those concentrations (p < 0.05). The amoxicillin + metronidazole combination effect was most pronounced in cultures involving P. gingivalis. Of the antibiotics tested, amoxicillin + metronidazole was the most efficacious, with a MBIC against the polymicrobial biofilm of 1.63 mg/L, while azithromycin was much less effective, with a MBIC of 10.6 mg/L ().
Figure 2. Effect of azithromycin up to 5.0 mg/L on the red complex mono- and polymicrobial biofilms in a 96-well plate model. Azithromycin at concentrations 0–100 mg/L was incubated with bacterial cultures for 48 h under anaerobic conditions. Data points represent the mean AU620 value of a minimum of three biological replicates. Note the categorical scale.
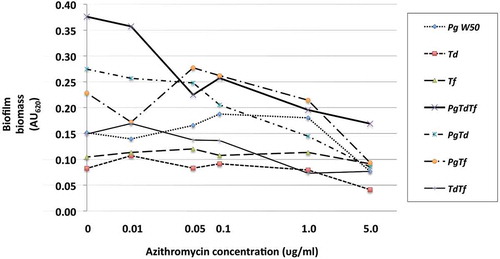
Figure 3. Effect of amoxicillin + metronidazole up to 5.0 mg/L on the red complex mono- and polymicrobial biofilms in a 96-well plate model. Amoxicillin + metronidazole in a 1:1 ratio at concentrations 0–100 mg/L was incubated with bacterial cultures for 48 h at 37°C anaerobically. Data points represent the mean AU620 value of a minimum of three biological replicates. Note the categorical scale.
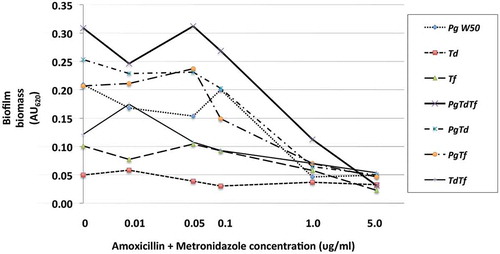
Figure 4. Effects of azithromycin and amoxicillin + metronidazole (1:1 ratio) up to 5.0 mg/L on formation polymicrobial biofilms after 48 h of anaerobic incubation at 37°C in a 96-well plate model. Azithromycin and amoxicillin + metronidazole (1:1 ratio) at concentrations 0–100 mg/L were incubated with bacterial cultures. Data points represent the mean AU620 value of a minimum of three biological replicates and the standard deviation. *p < 0.05, Student’s paired t-test.
Discussion
In this in vitro study, three-species polymicrobial biofilms of the red complex bacteria yielded more biofilm biomass compared to monospecies or two-species biofilms. P. gingivalis, in particular, seemed to increase the biofilm biomass. The red complex bacteria appear later in biofilm development [Citation15] and are repeatedly found together in high levels in the subgingival biofilms of subjects with periodontitis [Citation34]. Although these three species do not fully represent the complexity of the polymicrobial biofilms associated with a pathogenic subgingival plaque, they do form an interdependent bacterial community near the gingival epithelium, and the emergence of this community is associated with disease severity and progression [Citation16,Citation35,Citation36]. Consistent with the current findings, T. denticola strains are known to form insignificant amounts of biofilm when incubated on inert surfaces in vitro [Citation37], while P. gingivalis is able to form substantial biofilms in vitro [Citation38]. Also consistent with the current findings, a positive cooperativity between T. denticola and P. gingivalis in biofilm formation has been demonstrated [Citation39]. The two species co-aggregate [Citation40] and exhibit a mutualistic enhancement of growth in vitro, with each producing nutrients that stimulate the growth of the other [Citation41]. Similarly, T. forsythia also accumulates better in dual species biofilms involving T. denticola [Citation42] or F. nucleatum [Citation43]. Cell extracts of T. forsythia have been shown to stimulate the growth of P. gingivalis [Citation44]. Furthermore, T. forsythia has been detected more frequently and in greater numbers in deep periodontal pockets containing P. gingivalis [Citation45]. In an earlier study using the same methodology with real-time polymerase chain reaction enumeration of the individual bacterial species, it was demonstrated that all three species were present in the 48 h model biofilms, with T. denticola representing 66% of the total cells present and P. gingivalis and T. forsythia contributing18% and 16% of the total biofilm cells, respectively [Citation46].
Of the antibiotics examined in this study, the amoxicillin and metronidazole combination produced the best results, inhibiting both in vitro planktonic and biofilm growth of the polymicrobial combination at relatively low concentrations compared to azithromycin. There are few other studies examining the effects of antibiotics on polymicrobial oral bacterial biofilms. Belibasakis and Thurnheer [Citation18] recently reported that amoxicillin + metronidazole (1:1 ratio) at a concentration of 15 mg/L caused reductions to total cell numbers of established 10-species polymicrobial biofilms, significantly reducing P. gingivalis numbers in these biofilms after 24 h of exposure. Soares et al. have recently reported that the amoxicillin + metronidazole combination significantly reduced metabolic activity of 35 subgingival bacterial species residing in complex biofilms [Citation47]. The present study determined that the MBIC of the amoxicillin + metronidazole combination was 1.63 mg/L for the prevention of polymicrobial biofilm establishment, which should be clinically achievable. Amoxicillin concentrations in gingival crevicular fluid have been shown to reach up to 13–14 mg/L [Citation48] and 13 mg/L for metronidazole [Citation49] following a 500 mg per oral dose. The total bacterial load of P. gingivalis, however, might be considerably lower in in vivo biofilms compared to those reported here, thereby underestimating the clinical efficacy.
Very few studies involving red complex bacteria and azithromycin have been conducted, despite the increasingly widespread clinical use of the antibiotic [Citation50,Citation51]. Macrolides have been found to reduce bacterial adhesion, resulting in reduced biofilm formation, even at very low concentrations in a dose-dependent relationship [Citation52]. In vitro model studies have reported that azithromycin decreased metabolic activity, biofilm viability, and density of P. gingivalis at sub-MIC levels of approximately 0.1 mg/L [Citation53,Citation54]. To date, there are no studies reporting the MBIC for azithromycin against polymicrobial biofilms involving the red complex bacteria. Azithromycin concentrations in gingival crevicular fluid have been shown to reach up to 7–8 mg/L [Citation55,Citation56] following a 500 mg oral dose, and even lower values in periodontal tissues [Citation22]. The azithromycin MBIC of 10.6 mg/L against the polymicrobial biofilm formation obtained in this study is almost 10-fold higher than the MBIC of the amoxicillin + metronidazole combination and is likely to be clinically unachievable. Furthermore, it has been suggested that P. gingivalis and T. forsythia may be developing resistance to azithromycin [Citation57], and resistant T. forsythia has been isolated from patients with untreated periodontitis [Citation58].
Mechanical debridement of the subgingival plaque biofilm is the first line of treatment for periodontitis [Citation59–Citation61], and antibiotic supplementation is warranted in certain cases [Citation62,Citation63]. The emergence of high bacterial resistance [Citation64,Citation65] and tolerance [Citation66] to antimicrobials has led to the recommendation that these agents should only be used in conjunction with mechanical debridement in cases where there is a need to improve the treatment outcome. The biofilm assay model described in this study involved a short exposure (48 h) of the red complex bacteria to the antimicrobial agent during biofilm formation compared to previous studies where the antimicrobial agents were tested on established biofilms [Citation67,Citation68]. This was done to mimic the clinical situation following mechanical debridement before the bacteria have had time to reform an established mature biofilm. In this model, azithromycin was shown to be ineffective, whereas the amoxicillin and metronidazole combination was far more effective in preventing polymicrobial biofilm formation. Mechanical removal of the subgingival plaque biofilm in conjunction with the amoxicillin and metronidazole combination protocol may therefore enhance treatment outcomes.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Hwei Sze Ong
Hwei Sze Ong is a specialist periodontist who completed this research as part of her Doctor of Clinical Dentistry postgraduate degree.
Orit Oettinger-Barak
Orit Oettinger-Barak is a Senior Lecturer in Periodontics at the Melbourne Dental School. Her research interests are the microbiology and treatment of periodontal diseases.
Stuart G. Dashper
Stuart G Dashper is the Professor of Oral Microbiology at the Melbourne Dental School and a Project Leader in the Oral Health Cooperative Research Centre. His main research focus is the polymicrobial nature of oral diseases.
Ivan B. Darby
Ivan B Darby is Professor and Head of Periodontics at the Melbourne Dental School. His research interests are the clinical, microbiological and immunological aspects of the aetiology and pathogenesis of periodontal diseases.
Kheng H. Tan
Kheng H Tan is a postdoctoral researcher at the Melbourne Dental School who works on bacterial interactions.
Eric C. Reynolds
Eric C Reynolds is a Laureate Professor of The University of Melbourne and CEO and Director of Research of the Oral Health Cooperative Research Centre.
References
- Petersilka GJ, Ehmke B, Flemmig TF. Antimicrobial effects of mechanical debridement. Periodontol 2000. 2002;28:1–7.
- Gordon J, Walker C, Lamster I, et al. Efficacy of clindamycin hydrochloride in refractory periodontitis. 12-month results. J Periodontol. 1985;56:75–80.
- van Winkelhoff AJ, Tijhof CJ, de Graaff J. Microbiological and clinical results of metronidazole plus amoxicillin therapy in Actinobacillus actinomycetemcomitans-associated periodontitis. J Periodontol. 1992;63:52–57.
- Slots J. Low-cost periodontal therapy. Periodontol 2000. 2012;60:110–137.
- Addy M, Wright R. Comparison of the in vivo and in vitro antibacterial properties of providone iodine and chlorhexidine gluconate mouthrinses. J Clin Periodontol. 1978;5:198–205.
- Fine DH, Furgang D, Barnett ML. Comparative antimicrobial activities of antiseptic mouthrinses against isogenic planktonic and biofilm forms of Actinobacillus actinomycetemcomitans. J Clin Periodontol. 2001;28:697–700.
- Kara D, Luppens SB, Cate JM. Differences between single- and dual-species biofilms of Streptococcus mutans and Veillonella parvula in growth, acidogenicity and susceptibility to chlorhexidine. Eur J Oral Sci. 2006;114:58–63.
- Noiri Y, Okami Y, Narimatsu M, et al. Effects of chlorhexidine, minocycline, and metronidazole on Porphyromonas gingivalis strain 381 in biofilms. J Periodontol. 2003;74:1647–1651.
- Appelbaum PC, Chatterton SA. Susceptibility of anaerobic bacteria to ten antimicrobial agents. Antimicrob Agents Chemother. 1978;14:371–376.
- Baker PJ, Slots J, Genco RJ, et al. Minimal inhibitory concentrations of various antimicrobial agents for human oral anaerobic bacteria. Antimicrob Agents Chemother. 1983;24:420–424.
- Newman MG, Hulem C, Colgate J, et al. Antibacterial susceptibility of plaque bacteria. J Dent Res. 1979;58:1722–1732.
- Poulet PP, Duffaut D, Lodter JP. Metronidazole susceptibility testing of anaerobic bacteria associated with periodontal disease. J Clin Periodontol. 1999;26:261–263.
- Sbordone L, Barone A, Ramaglia L, et al. Antimicrobial susceptibility of periodontopathic bacteria associated with failing implants. J Periodontol. 1995;66:69–74.
- Slots J, Evans RT, Lobbins PM, et al. In vitro antimicrobial susceptibility of Actinobacillus actinomycetemcomitans. Antimicrob Agents Chemother. 1980;18:9–12.
- Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144.
- Byrne SJ, Dashper SG, Darby IB, et al. Progression of chronic periodontitis can be predicted by the levels of Porphyromonas gingivalis and Treponema denticola in subgingival plaque. Oral Microbiol Immunol. 2009;24:469–477.
- Kesavalu L, Sathishkumar S, Bakthavatchalu V, et al. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect Immun. 2007;75:1704–1712.
- Belibasakis GN, Thurnheer T. Validation of antibiotic efficacy on in vitro subgingival biofilms. J Periodontol. 2014;85:343–348.
- Feres M, Figueiredo LC, Soares GM, et al. Systemic antibiotics in the treatment of periodontitis. Periodontol 2000. 2015;67:131–186.
- Mombelli A, Cionca N, Almaghlouth A, et al. Are there specific benefits of amoxicillin plus metronidazole in Aggregatibacter actinomycetemcomitans-associated periodontitis? Double-masked, randomized clinical trial of efficacy and safety. J Periodontol. 2013;84:715–724.
- Amsden GW. Erythromycin, clarithromycin, and azithromycin: are the differences real? Clin Ther. 1996;18:56–72; discussion 55.
- Gomi K, Yashima A, Nagano T, et al. Effects of full-mouth scaling and root planing in conjunction with systemically administered azithromycin. J Periodontol. 2007;78:422–429.
- Haas AN, de Castro GD, Moreno T, et al. Azithromycin as an adjunctive treatment of aggressive periodontitis: 12-months randomized clinical trial. J Clin Periodontol. 2008;35:696–704.
- Hirsch R, Deng H, Laohachai MN. Azithromycin in periodontal treatment: more than an antibiotic. J Periodontal Res. 2012;47:137–148.
- Sefton AM, Maskell JP, Beighton D, et al. Azithromycin in the treatment of periodontal disease. Effect on Microbial Flora. J Clin Periodontol. 1996;23:998–1003.
- Yashima A, Gomi K, Maeda N, et al. One-stage full-mouth versus partial-mouth scaling and root planing during the effective half-life of systemically administered azithromycin. J Periodontol. 2009;80:1406–1413.
- Retsema J, Girard A, Schelkly W, et al. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother. 1987;31:1939–1947.
- Bartold PM, du Bois AH, Gannon S, et al. Antibacterial and immunomodulatory properties of azithromycin treatment implications for periodontitis. Inflammopharmacology. 2013;21:321–338.
- Leschine SB, Canale-Parola E. Rifampin as a selective agent for isolation of oral spirochetes. J Clin Microbiol. 1980;12:792–795.
- Kesavalu L, Walker SG, Holt SC, et al. Virulence characteristics of oral treponemes in a murine model. Infect Immun. 1997;65:5096–5102.
- Orth R, O’Brien-Simpson N, Dashper S, et al. An efficient method for enumerating oral spirochetes using flow cytometry. J Microbiol Methods. 2010;80:123–128.
- Dashper S, O’Brien-Simpson N, Liu SW, et al. Oxantel disrupts polymicrobial biofilm development of periodontal pathogens. Antimicrob Agents Chemother. 2014;58:378–385.
- Dashper SG, Pan Y, Veith PD, et al. Lactoferrin inhibits Porphyromonas gingivalis proteinases and has sustained biofilm inhibitory activity. Antimicrob Agents Chemother. 2012;56:1548–1556.
- Colombo AP, Boches SK, Cotton SL, et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80:1421–1432.
- Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Microbial composition of supra- and subgingival plaque in subjects with adult periodontitis. J Clin Periodontol. 2000;27:722–732.
- Zijnge V, van Leeuwen MB, Degener JE, et al. Oral biofilm architecture on natural teeth. PLoS One. 2010;5:e9321.
- Vesey PM, Kuramitsu HK. Genetic analysis of Treponema denticola ATCC 35405 biofilm formation. Microbiology. 2004;150:2401–2407.
- Davey ME, Duncan MJ. Enhanced biofilm formation and loss of capsule synthesis: deletion of a putative glycosyltransferase in Porphyromonas gingivalis. J Bacteriol. 2006;188:5510–5523.
- Yamada M, Ikegami A, Kuramitsu HK. Synergistic biofilm formation by Treponema denticola and Porphyromonas gingivalis. FEMS Microbiol Lett. 2005;250:271–277.
- Onagawa M, Ishihara K, Okuda K. Coaggregation between Porphyromonas gingivalis and Treponema denticola. Bull Tokyo Dent Coll. 1994;35:171–181.
- Grenier D. Nutritional interactions between two suspected periodontopathogens, Treponema denticola and Porphyromonas gingivalis. Infect Immun. 1992;60:5298–5301.
- Ikegami A, Honma K, Sharma A, et al. Multiple functions of the leucine-rich repeat protein LrrA of Treponema denticola. Infect Immun. 2004;72:4619–4627.
- Sharma A, Inagaki S, Sigurdson W, et al. Synergy between Tannerella forsythia and Fusobacterium nucleatum in biofilm formation. Oral Microbiol Immunol. 2005;20:39–42.
- Yoneda M, Yoshikane T, Motooka N, et al. Stimulation of growth of Porphyromonas gingivalis by cell extracts from Tannerella forsythia. J Periodontal Res. 2005;40:105–109.
- Kuramitsu HK, Chen W, Ikegami A. Biofilm formation by the periodontopathic bacteria Treponema denticola and Porphyromonas gingivalis. J Periodontol. 2005;76:2047–2051.
- Zhu Y, Dashper SG, Chen YY, et al. Porphyromonas gingivalis and Treponema denticola synergistic polymicrobial biofilm development. PLoS One. 2013;8:e71727.
- Soares GM, Teles F, Starr JR, et al. Effects of azithromycin, metronidazole, amoxicillin, and metronidazole plus amoxicillin on an in vitro polymicrobial subgingival biofilm model. Antimicrob Agents Chemother. 2015;59:2791–2798.
- Tenenbaum H, Jehl F, Gallion C, et al. Amoxicillin and clavulanic acid concentrations in gingival crevicular fluid. J Clin Periodontol. 1997;24:804–807.
- Pahkla ER, Koppel T, Saag M, et al. Metronidazole concentrations in plasma, saliva and periodontal pockets in patients with periodontitis. J Clin Periodontol. 2005;32:163–166.
- Muniz FW, de Oliveira CC, de Sousa Carvalho R, et al. Azithromycin: a new concept in adjuvant treatment of periodontitis. Eur J Pharmacol. 2013;705:135–139.
- Sampaio E, Rocha M, Figueiredo LC, et al. Clinical and microbiological effects of azithromycin in the treatment of generalized chronic periodontitis: a randomized placebo-controlled clinical trial. J Clin Periodontol. 2011;38:838–846.
- Schreiber F, Szewzyk U. Environmentally relevant concentrations of pharmaceuticals influence the initial adhesion of bacteria. Aquat Toxicol. 2008;87:227–233.
- Maezono H, Noiri Y, Asahi Y, et al. Antibiofilm effects of azithromycin and erythromycin on Porphyromonas gingivalis. Antimicrob Agents Chemother. 2011;55:5887–5892.
- Tamura A, Ara T, Imamura Y, et al. The effects of antibiotics on in vitro biofilm model of periodontal disease. Eur J Med Res. 2008;13:439–445.
- Jain N, Lai PC, Walters JD. Effect of gingivitis on azithromycin concentrations in gingival crevicular fluid. J Periodontol. 2012;83:1122–1128.
- Lai PC, Ho W, Jain N, et al. Azithromycin concentrations in blood and gingival crevicular fluid after systemic administration. J Periodontol. 2011;82:1582–1586.
- Haffajee AD, Patel M, Socransky SS. Microbiological changes associated with four different periodontal therapies for the treatment of chronic periodontitis. Oral Microbiol Immunol. 2008;23:148–157.
- van Winkelhoff AJ, Herrera D, Oteo A, et al. Antimicrobial profiles of periodontal pathogens isolated from periodontitis patients in The Netherlands and Spain. J Clin Periodontol. 2005;32:893–898.
- Haffajee AD, Torresyap G, Socransky SS. Clinical changes following four different periodontal therapies for the treatment of chronic periodontitis: 1-year results. J Clin Periodontol. 2007;34:243–253.
- Lindhe J, Liljenberg B, Adielson B, et al. Use of metronidazole as a probe in the study of human periodontal disease. J Clin Periodontol. 1983;10:100–112.
- Listgarten MA, Lindhe J, Hellden L. Effect of tetracycline and/or scaling on human periodontal disease. Clinical, microbiological, and histological observations. J Clin Periodontol. 1978;5:246–2471.
- Dastoor SF, Travan S, Neiva RF, et al. Effect of adjunctive systemic azithromycin with periodontal surgery in the treatment of chronic periodontitis in smokers: a pilot study. J Periodontol. 2007;78:1887–1896.
- Mombelli A, Gusberti FA, Lang NP. Treatment of recurrent periodontal disease by root planing and Ornidazole (Tiberal). Clinical and microbiological findings. J Clin Periodontol. 1989;16:38–45.
- Soares GM, Figueiredo LC, Faveri M, et al. Mechanisms of action of systemic antibiotics used in periodontal treatment and mechanisms of bacterial resistance to these drugs. J Appl Oral Sci. 2012;20:295–309.
- Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138.
- Teles R, Teles F, Frias-Lopez J, et al. Lessons learned and unlearned in periodontal microbiology. Periodontol 2000. 2013;62:95–162.
- Larsen T. Susceptibility of Porphyromonas gingivalis in biofilms to amoxicillin, doxycycline and metronidazole. Oral Microbiol Immunol. 2002;17:267–271.
- Wright TL, Ellen RP, Lacroix JM, et al. Effects of metronidazole on Porphyromonas gingivalis biofilms. J Periodontal Res. 1997;32:473–477.
