ABSTRACT
Background:Targeting Streptococcus mutans, the principle cariogenic bacterium, could prove to be an effective means of preventing dental caries. A de novo designed antimicrobial peptide, GH12, has shown bactericidal effects on S. mutans and inhibitory effects on virulence and regulatory systems of S. mutans. However, the effects of GH12 on caries remain to be elucidated.Objectives: The objectives of this study were to evaluate the direct effects of GH12 on caries and numbers of S. mutansin vivo.Design: The Keyes score was evaluated in a rodent model and in vivo S. mutans was quantified by qPCR. To further interpret the in vivo anticaries efficacy, an in vitro biofilm model using short-term treatments with GH12 mimicked treatments in vivo was adopted. Results: In vivo data showed that GH12 at 8 mg/L reduced the incidence and severity of caries. Furthermore, GH12-treated animals had less S. mutans infection. In vitro data revealed that GH12 reduced the number of S. mutans within the biofilm, as well as reducing lactic acid and water-insoluble EPS synthesis of S. mutans biofilms. Afterwards, all rats showed no significant difference in weight gain, and no sign of harm to the mucosa, indicating that GH12 possessed good biocompatibility in vivo.Conclusion: Due to the combined inhibitory effects of GH12 on the biomass and cariogenic properties of S. mutans biofilms, the population of S. mutans in vivo was reduced, residual S. mutans biofilms were less acidic and compact, and thereby the onset and development of caries were arrested. Such findings collectively certified the clinical prospects for GH12.
Introduction
Dental caries is a multifactorial infectious disease that affects millions of people worldwide, causing a large burden on the public economy [Citation1]. Recent opinions on caries aetiology have shifted from a focus on a single cariogenic bacterium (Streptococcus mutans) to an emphasis on the ecological balance and complexity within the entirety of the plaque microbiome [Citation2]. Nevertheless, bacterial factors are still considered important for dental caries. Related microorganisms include acidogenic/aciduric bacteria such as S. mutans and lactobacilli, fungi such as Candida albicans, and bacteria facilitating caries onset and development, such as Veillonella parvula [Citation3,Citation4]. However, S. mutans is still believed to be the principle cariogenic bacterium [Citation5]. S. mutans possesses primary acidogenic and aciduric abilities causing unbalanced pH fluctuations even under salivary buffering, and it can produce large amounts of exopolysaccharides (EPS), which contribute greatly to biofilm formation [Citation6,Citation7]. Thus, targeting S. mutans could prove to be an effective means of preventing or arresting dental caries.
As a traditional anticaries agent, fluoride is a highly effective and economical agent for dental caries prevention and will remain the mainstay of any caries-preventive programme, typically water fluoridation [Citation8]. It has also been found that toothbrushing with fluoride-containing toothpaste is effective in preventing caries mainly because it brings fluoride into the oral cavity at regular intervals [Citation9]. Apart from its major tooth protective property of arresting the demineralization of dental hard tissue and enhancing the remineralization process, fluoride can also affect bacterial growth and microbial metabolic activity by the inhibition of enolase and F-ATPase at high concentrations [Citation10]. However, there is debate concerning the fluoride levels in drinking water because of fluorosis and other health risks [Citation11,Citation12]. Given all these facts, other anticaries agents should be adequately studied.
Over the last decade, a significant advancement in studies on control of oral pathogens by antimicrobial peptides (AMPs) has been made [Citation13]. To date, natural AMPs and synthetic analogous have shown potent efficacy in inhibiting S. mutans, such as Pleurocidin, human beta-defensin-3 C15 peptide and synthetic octadecapeptide derived from α-amylase [Citation14–Citation17]. In addition, due to AMPs’ preferential attack on cell membranes and the ability of individual AMPs to interact with multiple targets [Citation18], bacterial resistance to AMPs is relatively less prone to develop. Given these facts, AMPs are promising alternative solutions for dental caries.
To combat dental caries, a cationic amphipathic α-helical AMP named GH12 was designed and synthesized de novo and confirmed to show negligible effects on human gingival fibroblasts, good stability in human saliva and excellent antimicrobial properties [Citation19–Citation21]. GH12 has rapid bactericidal action with a minimal inhibitory concentration (MIC) and minimal bactericidal inhibitory concentration (MBC) of 8 mg/L against S. mutans. In addition, GH12 is able to inhibit biofilm formation by S. mutans and to kill S. mutans in a preformed biofilm [Citation19]. Moreover, GH12 at 4 mg/L restrained the cariogenic properties of S. mutans by reducing bacterial acidogenicity, compromising the aciduricity, inhibiting EPS synthesis to prevent biofilm formation and attenuating the bacterial response to environmental stresses at the enzymatic and transcriptional levels [Citation21].
Nevertheless, to date, there is little literature describing the direct effects of AMPs on dental caries. Prior to clinical trials, the well-established rat model is widely used for its favourable susceptibility to caries akin to that of humans, with the small body size of rats optimizing operations and a high reproducibility [Citation22,Citation23]. In addition, the physicochemical properties and macromolecular composition of saliva could influence microbial colonization [Citation24,Citation25] and the maintenance of antibacterial agents [Citation26] both in vitro and in vivo. Since AMPs have shown the potential to combat dental caries, an animal model that provides a real biological environment should be investigated with urgency.
The objectives of this study were to 1) evaluate the direct effects of GH12 on caries and numbers of S. mutans in vivo, 2) further interpret the in vivo anticaries efficacy through in vitro S. mutans biofilm assays and 3) to assess the in vivo biocompatibility of GH12. This study is a continuation of previous studies of GH12 and to the best of our knowledge, it is the first attempt to evaluate the effects of an AMP on the onset and development of caries in vivo.
Materials and methods
Peptides, bacterial strains and growth conditions
Peptide GH12 (Gly-Leu-Leu-Trp-His-Leu-Leu-His-His-Leu-Leu-His-NH2) was synthesized, identified, and purified to 98% by GL Biochem (Shanghai, China) as described previously [Citation19]. The peptide was dissolved in sterile deionized water and stored at −20°C. All chemicals and assay kits were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. S. mutans UA159 was obtained from the State Key Laboratory of Oral Diseases at the Sichuan University (Chengdu, China) and grown in brain-heart infusion broth (BHI; Oxoid, Basingstoke, Hampshire, UK) anaerobically (85% N2, 10% H2 and 5% CO2) at 37°C [Citation27]. For lactic acid measurement, buffered peptone water (BPW, Nissui, Tokyo, Japan) was used [Citation28].
In vivo efficacy of GH12
Rat dental assay
All animal experiments were conducted with the approval (approval no. WCHSIRB-D-2017-133) given by whom? Animal experiments were performed in a well-established rodent model of dental caries with some modification [Citation29]. Forty-eight male specific-pathogen-free (SPF) Sprague-Dawley rats aged 17 days and weighing 55 ± 5 g were purchased from the Animal Experimental Center of the Sichuan University and fed a diet supplemented with 0.1% ampicillin, 0.1% chloramphenicol, 0.1% carbenicillin, and water containing 4,000 U/mL penicillin for 3 days [Citation30]. Starting on the fourth day, the animals were infected orally using an actively growing (mid-logarithmic) culture of S. mutans UA159, and their infection was checked via oral swabbing. Thus, the infected animals were randomly placed into four groups (12 rats/group), and their teeth were treated topically using a camel hair brush for 5 min three times daily as follows: (1) positive controls: 250 ppm NaF; 0.12% chlorhexidine (CHX); (2) negative control: sterilized distilled deionized water (DDW); and (3) treatment group: 8 mg/L GH12. The experiment proceeded for 3 weeks. The body weight of each rat was recorded weekly. The animals were fed a cariogenic Keyes 2000 diet (56% sucrose; Trophic, Nantong, China) and 5% sucrose water ad libitum. According to ISO 10993 [Citation31], oral mucosa gross observation was performed at the end of the experimental period. First, the animals were euthanized by CO2 asphyxiation. The jaws, gingiva and palatal tissue were surgically removed and aseptically dissected. All animal experiments followed the ARRIVE guidelines [Citation32].
Keyes scoring
All jaws (48/group) were fixed in 4% paraformaldehyde solution (Solarbio, Beijing, China) and stained with 0.4% murexide for 12 h. The main sulcus of each tooth was exposed by a mesiodistal sagittal hemisection. The sulcus and smooth surfaces of all jaws were imaged by stereo microscopy (EZ4W, Leica, Wetzlar, Germany) for caries scoring using the Keyes system [Citation33]. The determination of the caries score was blinded by codification of the jaws and was done by one experienced examiner.
DNA isolation and quantitative real-time PCR
The viable cell number within the oral cavity of each rat was determined by pipetting saliva, swabbing dental plaque and sonicating jaws. The swabs and jaws were placed in 5 mL PBS and sonicated for 1 min to collect attached viable cells before fixation. After centrifugation (8,000 g, 5 min, 4°C), the precipitates of 12 rats in each group were mixed together and stored at −80°C. S. mutans infection in all groups was confirmed with qPCR methods (primers: Forward GCCTACAGCTCAGAGATGCTATTCT; Reverse GCCATACACCACTCATGAATTGA) [Citation34]. Briefly, total DNA of the precipitate was isolated and purified using a QIAamp DNA mini-kit (51304, Qiagen, Germany) following the instructions of the manufacturer. The purity and concentration of DNA were detected using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA). The quantitative real-time PCR was performed using a CFX96 Real-Time System (C1000™ Thermal Cycler; Bio-Rad, Hercules, CA). In brief, 25 µL of the mixture containing 12.5 µL of SYBR® Premix Ex Taq ™ II (RR820A; Takara Bio, Shiga, Japan), 1 µL of specific forward primer (10 µM), 1 µL of specific reverse primer (10 µM), 1 µL of the template, and 9.5 µL of double distilled water were placed in each well with the same cycle conditions as used in a previous study [Citation21]. The bacterial counts were determined from standard curves and the results were displayed as lg (cells/mL). The qPCR tests were performed in quadruplicate and reproduced three times.
Hematoxylin and eosin staining
Mucosal tissues were harvested for histopathological assessment. The samples were fixed in 4% paraformaldehyde solution (Solarbio) for 24 h. After sectioning and dehydration with ethanol, the samples were stained with hematoxylin for 5 min and differentiated in 1% hydrochloric acid alcohol for 2 s. Then, the samples were incubated in 0.2% ammonia water for 2 min and subsequently stained with eosin for 1 min. After gradual dehydration, the samples were cleared and mounted with neutral resin. The slides were scanned with a Digital Slide Scanning System (PRECICE, Beijing, China). CaseViewer 2.1 (3DHISTECH, Budapest, Hungary) was used for image analysis.
Biofilm assays
Biofilm preparation and short-term treatments
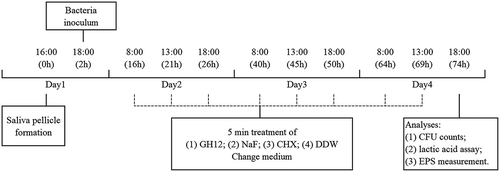
Biofilms of S. mutans UA159 were formed on a saliva-coated glass coverslip in a 24-well cell culture plate in batch cultures for 74 h. The whole mixed saliva used was collected from three volunteers (2 male and 1 female) aged 20–23 years old (mean 21 years old) with the approval (approval no. WCHSIRB-D-2015-103). The whole procedure was conducted on ice, and saliva samples were pooled after clarification by centrifugation (8,500 g, 4°C, 10 min) and filter-sterilization (0.22 μm; ultralow binding protein filter; Millipore, Billerica, MA). The coverslips were incubated with clarified human whole saliva for 2 h at 37°C. Then, the coverslips were placed in 2.0 mL of BHI culture medium with 1% (w/v) sucrose containing 106 CFU of actively growing S. mutans cells per mL and incubated at 37°C anaerobically for 14 h. The biofilms were exposed to 2.0 mL of one of the following: (1) 8 mg/L GH12; (2) 250 ppm NaF; (3) 0.12% CHX; and (4) DDW three times daily until the end of the experimental period (74 h). Each biofilm was exposed to the respective 5 min treatment a total of eight times (). Biofilm assays were performed in quadruplicate and reproduced three times.
Biofilm colony-forming unit counts
The colony-forming unit (CFU) counts were used to calculate the total number of viable bacteria for each coverslip [Citation28]. The coverslip samples with biofilms were transferred into tubes containing 1 mL PBS. The biofilms were harvested via a 3 min sonication and a 20 s vortexing at maximum speed using a vortex mixer, thereby removing and dispersing the biofilms from each sample. The bacterial suspensions were spread onto BHI agar plates after a serial dilution and incubated anaerobically at 37°C for 24 h. The total CFUs were calculated, with the dilution factor taken into consideration, based on the number of colonies.
Lactic acid measurement
For lactic acid measurement, the coverslips were placed in a new 24-well plate with 2.0 mL BPW supplemented with 0.2% sucrose. The plate was further incubated for 120 min at 37°C anaerobically. After removing planktonic cells by centrifugation (8,000 g, 5 min, 4°C), supernatants were decanted to measure the lactate concentrations according to the manufacturer’s instructions for the Lactate Assay Kit (MAK064). The absorbance at 570 nm (A570) was collected using a microplate spectrophotometer, and the lactate concentrations were calculated using standard curves.
Water-insoluble EPS measurement
The water-insoluble EPS of biofilms was determined by the anthrone method [Citation35]. Briefly, biofilms were collected by sonication and vortexing in PBS. Then, the precipitate was obtained by centrifugation, washed twice with sterile water and resuspended in 4 mL of 0.4 M NaOH. After centrifugation, 200 μL of supernatant was mixed with 600 μL of anthrone reagent at 95°C for 6 min. The absorbance at 625 nm (A625) was monitored and the concentrations of water-insoluble EPS were calculated using standard curves.
Statistical analysis
Differences between groups were compared using one-way ANOVA and Tukey HSD tests. Statistical analyses were performed using SPSS 20.0 (IBM, Chicago, IL) at a significance level of 0.05.
Results
GH12 reduced the incidence of caries of different severity and corresponding S. mutans populations in rats
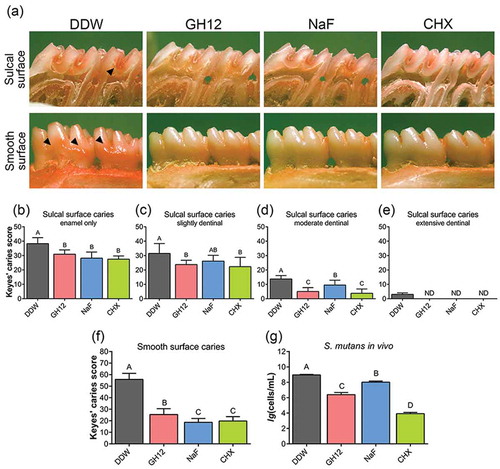
Whether topically applying GH12 could suppress the onset and severity of dental caries in vivo was tested using a well-established rodent model. As shown in ), in this rodent model, teeth progressively develop carious lesions (stained red by murexide). As noted with arrows, the control group had significantly more extensive sulcal-surface and smooth-surface lesions. As shown in , the incidence and severity of sulcal-surface caries was reduced by topical use of 8 mg/LGH12, 250 ppm fluoride and 0.12% CHX, and extensive dentinal sulcal lesions were detected only in the control group. Specifically, when treated with GH12 and CHX, the incidence of moderate dentinal sulcal lesions was significantly lower than in the fluoride treatment group (P < 0.05) ()). Similarly, as shown in ), the incidence of smooth-surface caries was also reduced by topical application of GH12, fluoride and CHX. The fluoride treatment group had the lowest score of smooth-surface lesions among all these treatments. The counts of S. mutans cells recovered from the oral cavity of the rats were confirmed by qPCR. The number of S. mutans in vivo was significantly decreased by GH12 compared to DDW and fluoride (P < 0.05) ()).
Figure 2. Protection of GH12 against the development of carious lesions and S. mutans in vivo. (a) Images of teeth from rats treated as noted. Arrows indicate typical lesions. Keyes caries scores are recorded as the type and extent of carious lesion severity according to Keyes scoring system. (b-e) Keyes caries scores of sulcal surface caries with different severity in rats treated as noted, (b) enamel only; (c) slightly dentinal, within 1/4 of the dentin affected; (d) moderate dentinal, 1/4 ~ 3/4 of the dentin affected; (e) extensive dentinal, beyond 3/4 of the dentin affected. (f) Keyes caries scores for smooth surface caries. Values denote means with SD (n = 12); ND indicates not detectable. (g) the bacterial count of S. mutans in rats treated as noted, as determined by real-time qPCR. Values denote means with SD (n = 4) and different letters indicate significantly different from each other (P < 0.05) (ANOVA; comparison for pairs using the Tukey HSD test).
Short-term treatment with GH12 reduced biomass and suppressed the cariogenic properties of S. mutans biofilms
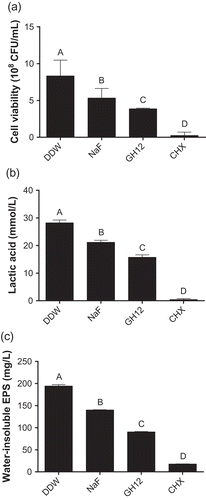
We then determined the in vitro effects of a short-term treatment of GH12 as observed in vivo on S. mutans biofilms by measuring the biomass, lactic acid production and water-insoluble EPS synthesis. As shown in ), compared with DDW and fluoride, the viable S. mutans within biofilms was significantly decreased by 8 mg/L GH12 (P < 0.05), which was consistent with the results in vivo. When treated with 8 mg/L GH12, the lactic acid production ()) and water-insoluble EPS synthesis ()) were also significantly suppressed (P < 0.05), consistent with the results of bacterial quantification by PCR in vivo ()). Not surprisingly, 0.12% CHX had the strongest bactericidal effect.
Figure 3. Biofilm disruption by short-term treatment with GH12. (a) viability of S. mutans within treated biofilms measured by CFU counts. (b) lactic acid production of treated S. mutans biofilms measured by the colorimetric method. (c) the water-insoluble EPS amount of treated S. mutans biofilms measured by the anthrone method. Data are shown as the mean with SD (n = 12); different letters indicate significantly different from each other (P < 0.05) (ANOVA; comparison for pairs using the Tukey HSD test).
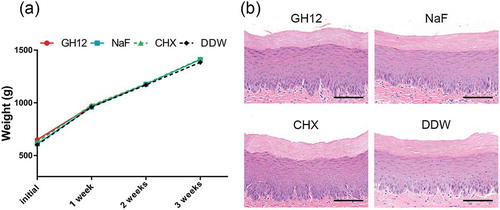
GH12 had negligible effects on weight gain and oral mucosa in rats
We further evaluated the biocompatibility of GH12 in vivo. After a 3-week topical treatment, oral mucosa treated with GH12 or other chemicals showed no hyperemia, hydrops, erosion or ulcer formation. As shown in ), during the 3-week experiment, all rats remained in apparent good health with no unexpected deaths and with no significant differences in weight gain between the control and experimental groups (P < 0.05). Furthermore, histopathological analysis of the oral mucosa showed that the epithelial layer in the GH12 group was complete, the cells were in order, the epithelial process stretched to the basal layer, and there were no signs of harmful effects, such as proliferative changes, inflammatory responses and/or necrosis, when compared to DDW-treated animals ()).
Discussion
In our previous studies, the antimicrobial peptide GH12 showed rapidly acting antibacterial efficacy against S. mutans by inducing lysis and pore formation in the cytomembrane [Citation20]. In addition, the virulence factors of this principle caries pathogen were also suppressed [Citation21]. In the current article, aimed at promoting the clinical effect of GH12, its anticaries efficacy was assessed in a well-established rat model, and the observed effect was further supported through in vitro S. mutans biofilm assays. This research proved the potential of GH12 in arresting caries and its biocompatibility in rats, and certified its clinical prospects. To our knowledge, this is the first study utilizing a rat model to verify the effects of an AMP on the onset and development of caries.
For the caries scoring, the rat model and the Keyes system provide the most detailed and comprehensive information, including the caries locations and the severity of the lesions [Citation36]. Fluoride is still recognized as the mainstay of caries protection through rebalancing the demineralization and remineralization homeostasis and influencing bacterial bioactivity [Citation37]. In this study, 250 ppm fluoride gave the lowest score for smooth-surface lesions among all these treatments, which is consistent with previous studies by other groups [Citation38,Citation39]. The GH12 group had a significantly lower score for both smooth-surface and sulcal-surface lesions than the control group (P < 0.05), showing a promising anticaries efficacy. Impressively, after treatment with GH12, similar to the CHX group, the incidence of moderate dentinal sulcal lesions was significantly lower than in the fluoride group (P < 0.05). With the special anatomic structure of occlusal surfaces and the existence of dentinal canaliculi, the pits and fissures may be out of the reach for fluoride, so fluoride arrests caries better on smooth surfaces than in pits and fissures [Citation40]. However, bacteria and their products may affect additional structures through dentinal canaliculi. Based on these results, it is speculated that the effect of GH12 on carious lesions may be due to its inhibitory effects on the growth and virulence of S. mutans. Thus, qPCR was used to count the numbers of S. mutans within the collected saliva and scraped plaque in each group. qPCR analysis has been widely accepted for quantifying S. mutans populations in vivo owing to its improved rapidity, sensitivity, reproducibility and lower risk of contamination [Citation41]. The number of S. mutans in vivo was significantly decreased by GH12, which validated that GH12 attenuated caries through inhibiting the growth of S. mutans. Additionally, GH12 showed a relatively stronger antimicrobial activity than fluoride, which might partly account for the interesting findings in the Keyes scoring. These results imply that GH12 may be more effective than fluoride when used in certain individuals with a higher risk of emerging sulcal caries. Moreover, since the mechanism of action of GH12 is thought to be distinct from that of fluoride, it is also worthwhile to study the potential of a GH12/fluoride combination in oral hygiene.
Apart from the reduction in the numbers of S. mutans in vivo, biofilm assays in vitro could provide more information about the anticaries mechanisms of GH12. To mimic GH12’s interference with the S. mutans biofilms in vivo, the biofilms were preformed, the saliva was included and short-term treatments three times per day were adopted. The treatment time of 5 min was adopted both in vivo and in vitro, because GH12 at 8 mg/L killed 90% of S. mutans within 5 min in previous studies [Citation19]. In the present study, when treated with 8.0 mg/L GH12 three times a day for three consecutive days, the biomass, lactic acid production and water-insoluble EPS synthesis of the 16-hour-old S. mutans biofilms were significantly and comprehensively suppressed (P < 0.05), which suggested that the biofilms might be easier to obliterate and cause less demineralization. Compared to a long-term treatment using higher concentrations of GH12 previously (over 32.0 mg/L, 24 h) [Citation19], the repeated short-term treatments with lower doses of GH12 were more effective at reducing the biomass of preformed S. mutans biofilms. This might be because GH12 could not maintain the initial concentration throughout the whole period of long-term treatment, suggesting that multiple lower-dose repeated treatments might be more conducive to the maximum effect of GH12. In addition, we determined previously that GH12 at 4.0 mg/L could reduce acidogenicity, aciduricity and EPS synthesis of planktonic S. mutans at enzymatic and transcriptional levels [Citation21], which were also believed to contribute to the reduced biomass and cariogenicity of S. mutans biofilms in this study, when GH12 was used at subinhibitory levels.
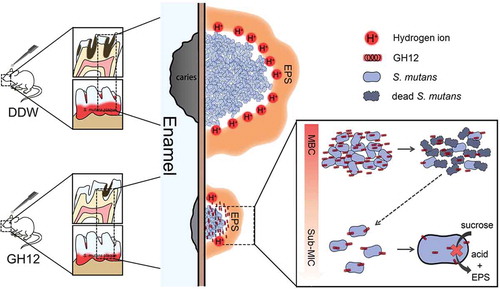
Collectively, as shows, it was concluded that suprathreshold lysis and pores on the cytomembrane of S. mutans were first caused by abundant GH12, which resulted in the death of S. mutans cells and reduced the biomass of S. mutans biofilms. Using GH12 at subinhibitory levels downregulated the expression of virulence-related genes and inhibited the activity of related enzymes of S. mutans, which led to attenuated acidogenicity and EPS synthesis, and thereby the residual S. mutans biofilms were less acidic and compact. The combined effect of GH12 reduces the exposure of enamel to acid, thus reducing the demineralization of enamel, so that S. mutans was inhibited by GH12, and the onset and development of caries in rats were arrested.
Figure 5. Model illustration of the mechanisms of GH12 targeting S. mutans to arrest caries development in rats.
Consistent with many previous studies [Citation42,Citation43], 0.12% CHX performed outstandingly in the biofilm assays, which also supported our decision of choosing it as the positive control group for antibacterial efficacy. It was the most effective agent inhibiting the growth of S. mutans in vivo and almost suppressed all tested parameters of the S. mutans biofilm to an undetectable level in vitro. However, 0.12% (1,200 mg/L), the clinically applied concentration of CHX, is 480–500 times its MBC (2–2.5 mg/L) against S. mutans [Citation19,Citation44]. Herrera et al. asked subjects to rinse with 0.12% CHX for 1 min; however, the mean percentages of survival of aerobic and anaerobic salivary bacteria were still under 30% after 7 h [Citation45]. Consuelo Cousido et al. [Citation46] drew similar conclusions using different methods, suggesting that CHX, with its broad-spectrum antibacterial activity, could subversively damage the oral ecology at high concentrations. Meanwhile, it was reported that sub-MICs of CHX favoured S. mutans biofilm formation, which might encourage dental caries progression [Citation44]. Thus, as suggested previously, although CHX has substantial antimicrobial efficacy against cariogenic bacteria, using it as routine anticaries agent remains controversial [Citation42].
In our animal model, rats were infected with S. mutans, and only S. mutans was quantified. In following studies, more complicated biofilms that include multiple species of bacteria should be included. In addition, not only the amount but also the ratio of S. mutans in the microbiota should be calculated. Last but not least, more bionic substrate materials for biofilm growth, such as hydroxyapatite or enamel, need to be tested.
In conclusion, relying on the combined inhibitory effects of GH12, the biomass and cariogenic properties of S. mutans were reduced, and thereby the onset and development of caries were arrested in vivo. GH12 possessed good biocompatibility in vivo. Such findings collectively demonstrate the clinical prospects of GH12 for caries prevention.
Geolocation information
This work was performed at No.14, Section 3 of Renmin Road South, Chengdu, China.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90(3):294–9.
- Kilian M. The oral microbiome - friend or foe? Eur J Oral Sci. 2018;126(Suppl 1):5–12.
- Koo H, Bowen WH. Candida albicans and Streptococcus mutans: a potential synergistic alliance to cause virulent tooth decay in children. Future Microbiol. 2014;9(12):1295–1297.
- Simon-Soro A, Guillen-Navarro M, Mira A. Metatranscriptomics reveals overall active bacterial composition in caries lesions. J Oral Microbiol. 2014;6:25443.
- Gao X, Jiang S, Koh D, et al. Salivary biomarkers for dental caries. Periodontol 2000. 2016;70(1):128–141.
- Chokshi A, Mahesh P, Sharada P, et al. A correlative study of the levels of salivary Streptococcus mutans, lactobacilli and Actinomyces with dental caries experience in subjects with mixed and permanent dentition. J Oral Maxill Pathol. 2016;20(1):25–28.
- Huo LJ, Huang XY, Ling JQ, et al. Selective activities of STAMPs against Streptococcus mutans. Exp Ther Med. 2018;15(2):1886–1893.
- McDonagh MS, Whiting PF, Wilson PM, et al. Systematic review of water fluoridation. BMJ. 2000;321(7265):855–859.
- ten Cate JM, Zaura E. The numerous microbial species in oral biofilms: how could antibacterial therapy be effective? Adv Dent Res. 2012;24(2):108–111.
- Oh HJ, Oh HW, Lee DW, et al. Chronologic trends in studies on fluoride mechanisms of action. J Dent Res. 2017;96(12):1353–1360.
- Susheela AK. Fluorosis and iodine deficiency disorders in India. Current Sci. 2018;115(5):860–867.
- Zuo H, Chen L, Kong M, et al. Toxic effects of fluoride on organisms. Life Sci. 2018;198:18–24.
- Mai S, Mauger MT, Niu L-N, et al. Potential applications of antimicrobial peptides and their mimics in combating caries and pulpal infections. Acta Biomater. 2017;49:16–35.
- Kreling PF, Aida KL, Massunari L, et al. Cytotoxicity and the effect of cationic peptide fragments against cariogenic bacteria under planktonic and biofilm conditions. Biofouling. 2016;32(9):995–1006.
- Taniguchi M, Ochiai A, Takahashi K, et al. Antimicrobial activity and mechanism of action of a novel cationic α-helical octadecapeptide derived from α-amylase of rice. Biopolymers. 2015;104(2):73–83.
- Zhang M, Wei W, Sun Y, et al. Pleurocidin congeners demonstrate activity against Streptococcus and low toxicity on gingival fibroblasts. Arch Oral Biol. 2016;70:79–87.
- Ahn KB, Kim AR, Kum K-Y, et al. The synthetic human beta-defensin-3 C15 peptide exhibits antimicrobial activity against Streptococcus mutans, both alone and in combination with dental disinfectants. J Microbiol. 2017;55(10):830–836.
- Guilhelmelli F, Vilela N, Albuquerque P, et al. Antibiotic development challenges: the various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front Microbiol. 2013;4:353.
- Wang Y, Fan Y, Zhou Z, et al. De novo synthetic short antimicrobial peptides against cariogenic bacteria. Arch Oral Biol. 2017;80:41–50.
- Tu H, Fan Y, Lv X, et al. Activity of synthetic antimicrobial peptide GH12 against oral streptococci. Caries Res. 2016;50(1):48–61.
- Wang Y, Wang X, Jiang W, et al. Antimicrobial peptide GH12 suppresses cariogenic virulence factors of Streptococcus mutans. J Oral Microbiol. 2018;10(1):1442089.
- Kanamoto T, Nonaka K, Nakata M. Genetic variation in experimental dental caries in four inbred strains of rats. Caries Res. 1994;28(3):156–160.
- Kurihara Y, Naito T, Obayashi K, et al. Caries susceptibility in inbred mouse strains and inheritance patterns in F1 and backcross (N2) progeny from strains with high and low caries susceptibility. Caries Res. 1991;25(5):341–346.
- Marsh PD, Thuy D, Beighton D, et al. Influence of saliva on the oral microbiota. Periodontol 2000. 2016;70(1):80–92.
- Nobbs AH, Jenkinson HF, Jakubovics NS. Stick to your gums: mechanisms of oral microbial adherence. J Dent Res. 2011;90(11):1271–1278.
- Na DH, Faraj J, Capan Y, et al. Chewing gum of antimicrobial decapeptide (KSL) as a sustained antiplaque agent: preformulation study. J Control Release. 2005;107(1):122–130.
- Tao R, Tong Z, Lin Y, et al. Antimicrobial and antibiofilm activity of pleurocidin against cariogenic microorganisms. Peptides. 2011;32(8):1748–1754.
- Cheng L, Weir MD, Xu HHK, et al. Antibacterial and physical properties of calcium-phosphate and calcium-fluoride nanocomposites with chlorhexidine. Dent Mater. 2012;28(5):573–583.
- Koo H, Pearson SK, Scott-Anne K, et al. Effects of apigenin and tt-farnesol on glucosyltransferase activity, biofilm viability and caries development in rats. Oral Microbiol Immunol. 2002;17(6):337–343.
- Yang H, Bi Y, Shang X, et al. Antibiofilm activities of a novel chimeolysin against Streptococcus mutans under physiological and cariogenic conditions. Antimicrob Agents Chemother. 2016;60(12):7436–7443.
- ISO/TC194. Biological evaluation of medical devices — part 10: tests for irritation and skin sensitization. Standard Number: ISO 10993-10:2010. 2010. Geneva, Switzerland. International Organization for Standardization.
- Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):5.
- Keyes PH. Dental caries in the molar teeth of rats. II. A method for diagnosing and scoring several types of lesions simultaneously. J Dent Res. 1958;37(6):1088–1099.
- Zhang K, Wang S, Zhou X, et al. Effect of antibacterial dental adhesive on multispecies biofilms formation. J Dent Res. 2015;94(4):622–629.
- Koo H, Hayacibara MF, Schobel BD, et al. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemoth. 2003;52(5):782–789.
- Bowen WH. Rodent model in caries research. Odontology. 2013;101(1):9–14.
- Ten Cate JM. Contemporary perspective on the use of fluoride products in caries prevention. British Dent J. 2013;214(4):161–167.
- Koo H, Schobel B, Scott-Anne K, et al. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J Dent Res. 2005;84(11):1016–1020.
- Ren Z, Cui T, Zeng JM, et al. Molecule targeting glucosyltransferase inhibits Streptococcus mutans biofilm formation and virulence. Antimicrob Agents Chemother. 2016;60(1):126–135.
- McComb D, Tam LE. Diagnosis of occlusal caries: part I. Conventional methods. J Can Dent Assoc. 2001;67(8):454–457.
- Klein MI, Scott-Anne KM, Gregoire S, et al. Molecular approaches for viable bacterial population and transcriptional analyses in a rodent model of dental caries. Mol Oral Microbiol. 2012;27(5):350–361.
- Twetman S. Antimicrobials in future caries control? A review with special reference to chlorhexidine treatment. Caries Res. 2004;38(3):223–229.
- Tang X, Sensat ML, Stoltenberg JL. The antimicrobial effect of chlorhexidine varnish on mutans streptococci in patients with fixed orthodontic appliances: a systematic review of clinical efficacy. Int J Dent Hyg. 2016;14(1):53–61.
- Dong L, Tong Z, Linghu D, et al. Effects of sub-minimum inhibitory concentrations of antimicrobial agents on Streptococcus mutans biofilm formation. Int J Antimicrob Agents. 2012;39(5):390–395.
- Herrera D, Roldan S, Santacruz I, et al. Differences in antimicrobial activity of four commercial 0.12% chlorhexidine mouthrinse formulations: an in vitro contact test and salivary bacterial counts study. J Clin Periodontol. 2003;30(4):307–314.
- Consuelo Cousido M, Tomas Carmona I, Garcia-Caballero L, et al. In vivo substantivity of 0.12% and 0.2% chlorhexidine mouthrinses on salivary bacteria. Clin Oral Invest. 2010;14(4):397–402.