ABSTRACT
The diversity and delicate balance of the oral microbiome contribute to oral health, with its disruption leading to oral and systemic diseases. Toothpaste includes elements like traditional additives such as sodium lauryl sulfate (SLS) as well as novel postbiotics derived from probiotics, which are commonly employed for maintaining oral hygiene and a healthy oral cavity. However, the response of the oral microbiota to these treatments remains poorly understood. In this study, we systematically investigated the impact of SLS, and toothpaste containing postbiotics (hereafter, postbiotic toothpaste) across three systems: biofilms, animal models, and clinical populations. SLS was found to kill bacteria in both preformed biofilms (mature biofilms) and developing biofilms (immature biofilms), and disturbed the microbial community structure by increasing the number of pathogenic bacteria. SLS also destroyed periodontal tissue, promoted alveolar bone resorption, and enhanced the extent of inflammatory response level. The postbiotic toothpaste favored bacterial homeostasis and the normal development of the two types of biofilms in vitro, and attenuated periodontitis and gingivitis in vivo via modulation of oral microecology. Importantly, the postbiotic toothpaste mitigated the adverse effects of SLS when used in combination, both in vitro and in vivo. Overall, the findings of this study describe the impact of toothpaste components on oral microflora and stress the necessity for obtaining a comprehensive understanding of oral microbial ecology by considering multiple aspects.
Introduction
The oral microbiome represents the second-most diverse bacterial community in the human body [Citation1]. Oral bacteria are not planktonic; rather, they exist as biofilms in the oral cavity. The biofilms formed in different niches of the oral cavity are diverse in composition and include approximately 1000 species across individuals [Citation2]. The synergy and interactions between of various oral microorganisms protect the human body from invasion by undesirable internal and external perturbations [Citation2]. However, a disruption of the delicate equilibrium within the microbial ecosystem contributes to various oral and systemic diseases. In particular, the highly prevalent periodontal diseases such as gingivitis and periodontitis affect up to 90% of the world population [Citation3]. Therefore, promoting a balanced microbiome is important for the effective maintenance or restoration of oral health.
Regular oral hygiene is effective in controlling the microbial load in the dentition and oral cavity to prevent oral problems [Citation4]. Brushing with toothpaste is the most highly advocated and primary approach to oral hygiene in industrialized countries [Citation5]. A typical toothpaste formulation contains a combination of various antimicrobials, surfactants, polymers, and other components to achieve bactericidal effects and plaque removal [Citation3]. However, the complete elimination of oral microorganisms is not an ideal solution [Citation4]. The traditional approach involving the indiscriminate eradication of the commensal bacteria along with the pathogenic bacteria disturb the oral microecology, with adverse impacts on oral health. The preferred strategies for the prevention of oral diseases emphasize the preservation of bacterial ecology while decreasing its pathogenic properties and ensuring the reconstruction of the bacterial community homeostasis.
Sodium lauryl sulfate (SLS) is a surfactant that has been commonly used as an ingredient of toothpaste for more than 50 years owing to its cleaning and foaming actions. The concentration of SLS in commercially available dentifrices ranges from 1%–3%. SLS can inhibit the growth of many microorganisms [1980]. This antimicrobial activity of SLS is mainly owing to its ability to interact with and penetrate the bacterial cell wall, and to further interact with components of the cell membrane, such as lipids and proteins. The penetration of SLS into the cell membrane leads to enhanced permeability of the bacterial cell wall, which may contribute to the leakage of intracellular components and cytolysis [Citation6,Citation7]. Thus, SLS can act as a non-specific antibacterial agent, capable of killing beneficial bacteria as well. However, SLS continues to be used by leading toothpaste manufacturers to date due to its perfect foaming ability, acceptable taste, and lower cost compared to other surfactants. Therefore, a detailed profiling of the microbial community is warranted to characterize the SLS-induced shift in microbial homeostasis.
The use of probiotics and postbiotics is a novel approach for overcoming the limitations of traditional interventions. An increasing number of studies have shown that probiotics aid in relieving gingivitis, plaque, and alveolar bone loss, besides regulating pro-inflammatory effects [Citation8]. Different Lactobacilli strains, including Lactobacillus paracasei, L. plantarum, Lactobacillus rhamnosus, and Lactobacillus salivarius, have been shown to inhibit disparate microbial species and balance the microflora [Citation9]. However, certain worrying limitations are associated with the use of probiotics, including the negative effects of consuming live bacteria on immunocompromised patients, possibility of horizontal transfer of antibiotic resistance genes to pathogenic microorganisms, appearance of antibiotic resistance, and the high cost associated with the maintenance of bacterial viability during manufacture, storage, and distribution of the probiotics. Therefore, postbiotics, a new category of antibiotics with no need for viable cells and beneficial therapeutic effects, may be safe and cost-effective alternatives to probiotics. The components of postbiotics include short-chain fatty acids, exopolysaccharides, vitamins, teichoic acids, bacteriocins, enzymes and peptides in a non-purified inactivated cell preparation. Previous studies have suggested that postbiotic preparations of Lactococcus lactis that include the bacteriocin Nisin can be potentially employed for therapeutic applications in humans to support a healthy oral microbiome [Citation10–12]. Accordingly, the use of postbiotics is a potential strategy for the prevention and treatment of bacterial diseases, allowing the recovery of oral biodiversity and restoration of the ecological balance of the oral microbiome.
In the present study, the effects of the traditional additive SLS as well as promising postbiotics incorporated in toothpaste were evaluated using biofilm model, rat model of periodontitis and individuals with gingivitis. To the best of our knowledge, this is the first study to explore oral microecology by simultaneously employing three different systems: biofilms, animal models, and humans.
Materials and methods
In vitro biofilm model study
A postbiotic toothpaste containing 20% Lactobacilli fermentation extract derived from Lactobacilli sp. BZ was utilized in this study. The other ingredients of the toothpaste include calcium dihydrogen phosphate, glycerin, water, cellulose, pectin, xanthan gum, gellan gum, curdlan gum, and agar. As shown in , the Amsterdam Active Attachment Model (AAA-model) was employed to establish an in vitro biofilm model. Saliva samples were collected from five healthy individuals (two males and three females) aged 21–26 years, with the approval of the Ethics Committee of Tianjin Stomatological Hospital (approval number 2021-006-01). The collected saliva samples were pooled together and centrifuged at 2,600 × g for 10 minutes at 4°C to remove large fragments and eukaryotic cells. Next, the inoculation medium was prepared in a 1/50 ratio of saliva to SHI medium. Then, 1.5 mL inoculation medium was added to each well of a 24-well plate, and the previously sterilized cover of the AAA model was transferred to the 24-well plate. The models were incubated for 8 h at 37°C under anaerobic conditions in an automated anaerobic incubator for allowing initial attachment to the holding hydroxyapatite (HAP) discs. The biofilms were cultured for 48 h or 12 h after the initial attachment period to serve as preformed biofilms (mature biofilms) and developing biofilms (immature biofilms), respectively. The biofilms were then treated with ultrapure water (CTL), 0.12% chlorhexidine (CHX), 2% SLS (SLS), a 25% postbiotic toothpaste (PT), and 25% mixture of postbiotic toothpaste with 1.8% SLS (PT+SLS). The treatments were performed every 12 h within 72 h for preformed biofilms and of 36 h for developing biofilms, and lasted for 2 min each time to simulate the daily brushing process. The biofilms were characterized using crystal violet staining, colony forming unit (CFU) assay, quantitative polymerase chain reaction (qPCR), lactic acid content analysis, scanning electron microscopy (SEM), and 16S rRNA sequencing. According to previous studies [Citation13–15], the universal primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806 R (5′-GGACTACVSGGGTATCTAAT-3′) were used for qPCR in this study.
Rat periodontitis model study
Sprague Dawley (SD) rats were used to construct a periodontitis model after one week of adaptation. The rats were anesthetized and the first maxillary molars were ligated with a wire (diameter, 0.2 mm). After ligation, 0.2 mL of LPS (1 mg/mL) was injected into the gingival sulci between the first and second maxillary teeth of rats every day for three consecutive days, and a periodontal probe depth greater than 1 mm was confirmed as successful establishment of the model. SD rats in the non-periodontitis model served as the control group (CTL), and those in the periodontitis model treated with normal saline served as the model group (MD). All treatments were performed every 12 h for 14 days. The gingival index (GI), sulcus bleeding index (SBI), and probing depth (PD) were measured on days 0 and 14 to evaluate the changes after different treatments. Alveolar bone resorption was tested using methylene blue staining, and cemento-enamel junction (CEJ)-alveolar bone crest (ABC) distances were recorded to analyze alveolar bone resorption. Histological changes were assessed by hematoxylin-eosin (H&E) staining, and the content of interleukin 1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor (TNF-α) in serums and tissues were evaluated using enzyme-linked immunosorbent assay (ELISA).
Clinical trial study
This randomized-control, double-blind, parallel-design clinical trial was conducted with an observation period of one month. This study was approved by the Ethics Committee of Tianjin Stomatological Hospital (reference number 2021-006-01). The trial was registered in the Chinese Clinical Trial Registry (Identifier: ChiCTR2400081617). Written informed consent was obtained from all participants. This study was conducted in accordance with the Good Clinical Practice (GCP) guidelines and the principles of the Declaration of Helsinki. In total, 144 participants were recruited, all of whom had been diagnosed with gingivitis. The subjects were randomly assigned to either the PT group (72 subjects) or PT+SLS group (72 subjects). Throughout the experiment, the subjects were instructed to brush their teeth with the test toothpaste twice a day, once each in the morning and evening, using the horizontal vibrating brush method, for 2 minutes each time. The plaque index (PLI) and gingival index (GI) were recorded at the following time points: baseline (day 0), day 7, day 14, day 21, and day 28. Saliva samples from each subject were collected after oral examination on days 0 and 28 and analyzed for microbial communities using 16S rRNA sequencing.
For the extended methods, please refer to the Supplementary Materials and Methods.
Results
Effects of postbiotic toothpaste and SLS on biofilm quantification
To clarify the effects of postbiotic toothpaste and SLS, biofilms at different stages were successfully established based on the AAA model, including preformed and developing biofilms. In this study, multiple methods including crystal violet staining, CFU assays, qPCR analysis, lactic acid determination, and SEM observation, were used to comprehensively evaluate biofilm growth. Each method reflects different aspects of biofilm growth. Crystal violet staining is a non-specific staining method that can quantify all types of biomass, including exopolysaccharides, dead bacteria, and live bacteria [Citation16]. The CFU assay is an effective method for determining the number of viable bacteria. With the assistance of universal primer, qPCR was used to determine the total bacterial load [Citation14]. Further, as lactic acid production is a key indicator of the metabolic activity, the concentration of lactic acid was detected to investigate the metabolic activity. SEM was used to examine the biofilm morphology.
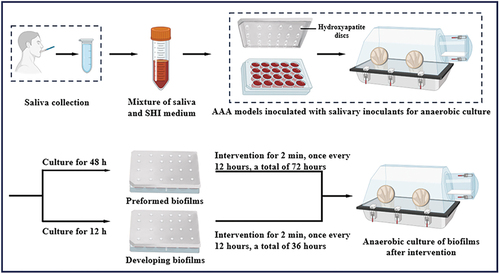
The preformed biofilms were cultured for 48 h until maturity and then subjected to various treatments for further 72 h. As shown in , the biofilm biomass of the SLS group was significantly decreased, whereas that of the PT group was similar to that of control group. Interestingly, the biomass of the PT+SLS group was intermediate between that of the SLS and PT groups, with no significant difference from that of the control group, indicating that postbiotic toothpaste can alleviate the inhibitory effect of SLS on biofilm biomass. Compared with that in the control group, the number of live bacteria was lower in the SLS and positive control CHX groups, but there were many live bacteria in both the PT and PT+SLS groups (). qPCR analysis showed the highest copy number of 3.12 × 107 copies/μL in the PT group, suggesting that postbiotic toothpaste enhanced the number of bacteria in the biofilm (). Crystal violet staining enables quantitative biomass assessment, including live and dead bacteria, as well as extracellular polymer substances [Citation17]. Based on the illustrations in , we propose that SLS demonstrates bactericidal properties and contributes to extracellular polymer substance removal. As shown in , the lactic acid contents of the CHX, SLS, PT and PT+SLS groups were all lower than those of the control group, while those of the SLS group were the lowest (). As shown in the SEM images, the biofilm structures of the control, PT, and PT+SLS groups were tight, and the pores between the different bacterial clumps were small. The bacteria were tightly packed within the extracellular matrix and formed a three-dimensional network structure (). However, the biofilm structure of the SLS group was the same as that of the CHX group, which was sparse and loose, with large gullies. The closeness and thickness of the biofilms were lower than those in the control group, and the number of bacteria in the biofilms was also significantly reduced. These results were consistent with those of the crystal violet staining and CFU counts. In conclusion, these results revealed that the SLS could inhibit the bacteria growth, reduce the biofilm biomass and destroy the three-dimensional network structure of extracellular polymer substances, whereas postbiotic toothpaste contributed to the bacterial homeostasis and normal growth of biofilms.
Figure 2. The effect of postbiotic toothpaste and SLS on the preformed biofilms. (a) The biomass of preformed biofilms after different treatments as measured by crystal violet staining. (b) Colony forming unit (CFU) counts of preformed biofilms (statistics are log-transformed). (c) Quantitative analysis of the preformed biofilms using qPCR after different treatments. (d) The concentration of Lactic acid concentration of the preformed biofilms. (e) Microstructure of the biofilms as observed by scanning electron microscopy (SEM) at 3000× magnification. Scale bar, 5 μm. (f) Representative SEM images of the biofilms at 10,000× magnification. Scale bar, 1 μm.
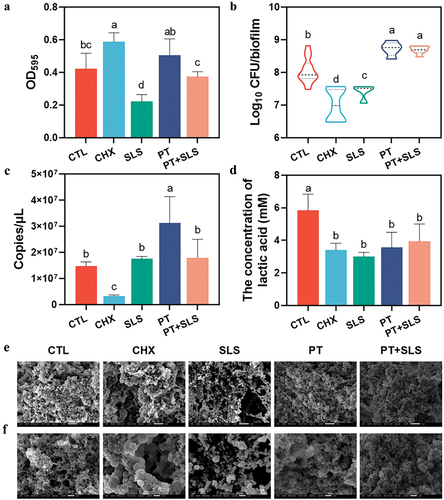
As for developing biofilms, they were only cultured for 12 h to keep them in an immature state, and then subjected to different treatments for further 12 h. As depicted in , crystal violet staining revealed that similar to preformed biofilms, developing biofilms revealed reduced biomass in the SLS group, increased biomass in the PT group, and intermediate biomass in the PT+SLS group. SLS and CHX were also found to inhibit the viable bacteria (), total bacteria () and lactic acid content () of the developing biofilms, whereas the PT group exhibited levels comparable hose in to the control group. Additionally, the postbiotic toothpaste mitigated the negative effects of SLS on total bacterial and lactic acid levels, as demonstrated in the PT+SLS group. The SEM observations revealed that compared with the control group, all other treatments loosened the dense network structure of the developing biofilms, among which the degree of looseness in the PT and PT+SLS groups was relatively low (). In contrast, the biofilms after CHX treatment showed that the extracellular matrix around streptococcus was largely decreased. After SLS treatment, the sticky extracellular matrix covering the bacteria almost disappeared; the bacterial morphology was clearly observed, and most bacteria were found to be spherical. Furthermore, SEM images at higher magnification showed that the bacterial morphology had changed, and many bulged vesicles appeared on the cell membrane (). These results showed that PT and PT+SLS had little effect on the three-dimensional structure of the biofilm during development, whereas SLS significantly damaged the developing biofilms, resulting in complete disappearance of the extracellular matrix and changes in bacterial morphology.
Figure 3. The effect of postbiotic toothpaste and SLS on the developing biofilms. (a) The biomass of developing biofilms after different treatments as measured by crystal violet staining. (b) Colony forming unit (CFU) counts of the developing biofilms (statistics are log-transformed). (c) The quantitative analysis of developing biofilms by qPCR in different treatments. (d) Lactic acid concentrations of the developing biofilms. (e) Microstructure of the biofilms as observed by scanning electron microscopy (SEM) at 3,000× magnification. Scale bar, 5 μm. (f) Representative SEM images of the biofilms at 10,000× magnification. Scale bar, 1 μm.
Effects of postbiotic toothpaste and SLS on the microbiome of biofilms
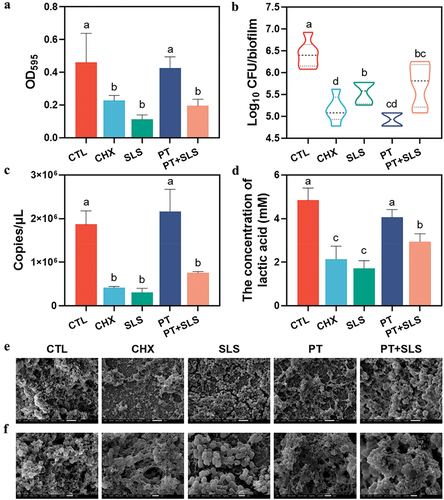
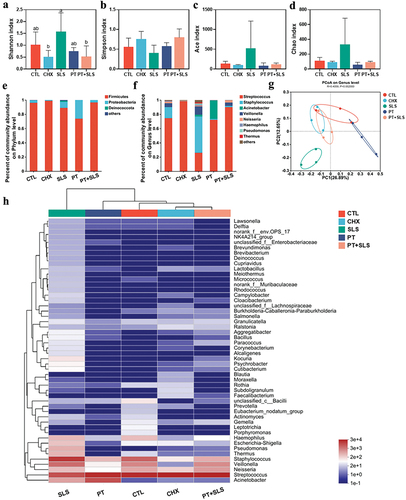
In this study, 16S rRNA sequencing was performed to identify shifts in the microbiota. Alpha diversity was analyzed at the operational taxonomic unit (OTU) level, in which the Shannon and Simpson indices were used to reflect community diversity and the ACE and Chao indices were used to represent community richness (). In terms of preformed biofilms, the Shannon, ACE, and Chao indices in the PT and PT+SLS groups were as high as those in the control group, and the Simpson index was similar to that of the control group, indicating that treatment with postbiotic toothpaste could maintain the microflora diversity. It is obvious that SLS decreased the alpha diversity, as shown by the profoundly lowered Shannon, Ace, Chao indices, and the elevated Simpson index. Beta diversity analysis was performed using principal coordinate analysis (PCoA), which indicated that the SLS and CHX groups were clearly separated from the other groups (). present the differences in microbial composition at the phylum and genus levels. Notably, the biofilms of the SLS group were almost completely composed of Streptococcus and Veillonella, whereas those of the PT and PT+SLS groups contained more diverse bacterial genera. Furthermore, the control, PT and PT+SLS groups were clustered close and farther from the SLS and CHX groups (). These findings suggest that the biofilms after PT and PT+SLS treatments maintained ecological diversity and possessed a microbial community structure similar to that of the control group. Based on the specific bacterial abundance at the genus level, the abundances of Streptococcus, Veillonella and Prevotella in the PT and PT+SLS groups were comparable to those in the control group (Figure S1). However, SLS increased the abundance of Streptococcus to almost twice that in the control, PT and PT+SLS groups, and reduced the abundance of Veillonella to less than one-sixth that in the control, PT, and PT+SLS groups. Notably, a high abundance of Staphylococcus was observed in the SLS group. Moreover, CHX markedly increased the abundance of Lactobacillus and Pseudomonas.
Figure 4. The effect of postbiotic toothpaste and SLS on the preformed biofilms. (a-d) the Shannon, Simpson, ACE and Chao indices of the preformed biofilms after different treatments, respectively. (e-f) Distribution of bacterial groups at the phylum and genus levels. (g) Principal coordinate analysis at the genus level for the preformed biofilms. (h) Community heatmap analysis at the genus level.
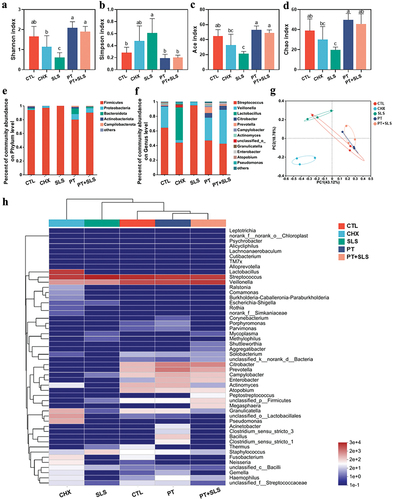
In terms of biofilm development, there were no apparent differences among the control, PT, and PT+SLS groups in the Shannon, Simpson, Ace and Chao indices (). Surprisingly, SLS promoted alpha diversity, as the Shannon index in the SLS group was significantly higher than that in the other groups; and the ACE and Chao indices in the SLS group were also relatively high. Further, PCoA showed a clear separation between the SLS group and the other groups, suggesting significant changes in the microbiota composition of the SLS group (). The percentage of community abundance revealed that Firmicutes and Proteobacteria were dominant at the phylum level in all groups (). The percentage of community abundance at the genus level also indicated high diversity in the SLS group (). As shown in (), the PT+SLS, CHX, control, and PT groups were clustered close and farther from the SLS group. Analysis of significantly changed bacteria shown that SLS decreased the level of Streptococcus and Neisseria, and increased the level of Staphylococcus, Thermus, Pseudomonas, Campylobacter, Bacillus, and Escherichia-Shigella, which explained the enhanced diversity of the SLS group as mentioned previously (Figure S2).
Figure 5. The effect of postbiotic toothpaste and SLS on the developing biofilms. (a-d) the Shannon, Simpson, ACE and Chao indices of the developing biofilms after different treatments, respectively. (e-f) Distribution of bacterial groups at the phylum and genus levels. (g) Principal coordinate analysis at genus level for the developing biofilms. (h) Community heatmap analysis at the genus level.
Effects of the postbiotic toothpaste and SLS on the rat periodontitis model
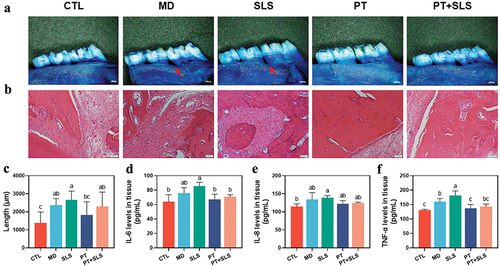
Considering that rat models could contribute detailed information on histopathological and blood biochemical analyses, the rat periodontitis model was successfully established, and clinical indicators were examined and recorded before and after 14 days of intervention. As depicted in , the gingival index (GI) and sulcus bleeding index (SBI) of all groups showed a significantly decreasing trend on day 14, with the highest decrease in the PT group, followed by that in the PT+SLS group. In the MD group, the periodontal pockets continued to deepen on day 14 as reflected by the increase in probing depth (PD), which further confirmed the successful model construction. The PD of the SLS group increased after the intervention, whereas that of the PT+SLS group showed no change, however, the PD of the PT group decreased significantly on the 14th day. Overall, the symptoms of periodontitis in the PT group improved, and those in the PT+SLS group were also alleviated to some extent, whereas those in MD and SLS groups were aggravated.
Table 1. The gingival index, sulcus bleeding index and probing depth of the rats in each group.
The stained alveolar bone is shown in ; it clearly shows that alveolar bone resorption occurred in all groups except for the control group. In particular, alveolar bone loss was heavier in the MD and SLS groups, with tooth roots beginning to become exposed. Quantitative data on alveolar bone resorption also showed no significant difference between the PT and control groups, whereas the other groups showed a significant increase in alveolar bone resorption (). Morphological changes in the periodontium were evaluated by H&E staining (). The control group exhibited the normal tissue structure. Obvious destruction of periodontal tissue, infiltration of inflammatory cells, and increased osteoclasts were detected in the MD and SLS groups, but were alleviated in the PT and PT+SLS groups. We further assessed the concentrations of the pro-inflammatory cytokines, IL-6, IL-8, and TNF-α in the serum and tissues. No significant difference was found in the serum levels of IL-6, IL-8, and TNF-α, suggesting little effect of the various treatments on the serum (Figure S3). As for the inflammatory cytokine levels in tissues, SLS treatment resulted in the highest levels of IL-6, IL-8, and TNF-α, whereas those in the PT group were suppressed to be as low as those in the unmodeled normal control group (). The inflammatory cytokines levels in the PT+SLS group were slightly but not significantly higher than those in the PT group, but were significantly lower than those in the SLS group, indicating the pro-inflammatory effect of SLS and the relieving effect of postbiotic toothpaste.
Figure 6. The effect of postbiotic toothpaste and SLS on periodontitis rats. (a) Methylene blue staining images for evaluating alveolar bone resorption in different groups. (b) Hematoxylin and eosin (h&e) staining images of the periodontium from different groups. (c) Quantitative analysis of alveolar bone resorption from different groups. (d-e) Pro-inflammatory cytokine levels in the periodontium of IL-6, IL-8, and TNF-α, respectively.
Effects of postbiotic toothpaste and SLS on the clinical population with gingivitis
In total, 144 subjects were recruited and randomly assigned to the two groups. During the clinical trial, six subjects (three in the control group and three in the experimental group) dropped out, while 138 subjects completed the trial (69 each in the experimental and control group). PLI and GI were investigated by three examiners for five visits over 30 days. The consistency of the examination standards for PLI and GI among the three examiners was excellent with an intraclass correlation coefficient (ICC) of 0.983, which was much higher than 0.75 (Table S1). Examiner repeatability was also excellent, with weighted kappa values of 0.869, 0.852, and 0.818 for three examiners, which were all greater than 0.8 (Table S2). provides the summary of the PLI and GI of the participants at different times. PLI showed a decreasing trend with prolongation of the PT intervention time and it was significantly different from the baseline on day 21, indicating that PT treatment reduced the plaque. Nevertheless, the PLI of the PT+SLS group increased with extension of the treatment time, showing a significant difference between day 28 and the baseline, indicating that dental plaque increased in the PT+SLS group. Additionally, from day 7 onwards, the GI was significantly lower than that at the baseline in both the PT and PT+SLS groups, with no apparent difference between the two groups. During the trial, 37 subjects were infected with the novel coronavirus; however, the results showed that this had no significant effect on the PLI and GI (Table S3). Further, changes in the PLI and GI between females and males were explored, which demonstrated that males had higher PLI and GI values (Table S4).
Table 2. The plaque index and gingival index of subjects in the PT and PT+SLS groups.
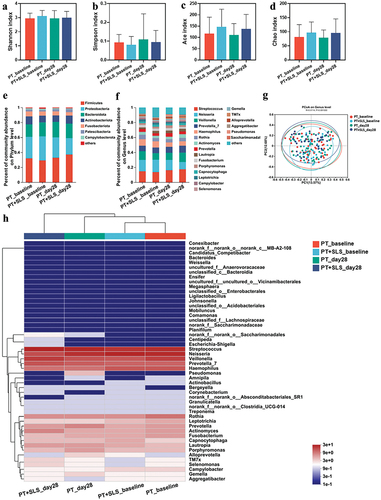
Furthermore, alterations in the bacterial community were evaluated to understand the effects of the postbiotic toothpaste and SLS on the clinical population. Specifically, the Shannon, Simpson, ACE, and Chao indices displayed no remarkable variation between the PT and PT+SLS groups at the baseline and on day 28 (). The beta diversity explored by PCA showed that the microbial structure among the groups was largely overlapped, confirming that PT and PT+SLS did not significantly disturb the microbiota structure (). The bar plots of average relative abundances revealed that Firmicutes, Proteobacteria, Bacteroidota, Actinobacteriota, Fusobacteriota, Patescibacteria, and Campylobacterota were dominant at the phylum level in all groups (). At the genus level, the species were similar among groups, although the abundance of each species varied slightly (). The PT_baseline and PT+SLS_baseline groups clustered together, which verified that the inclusion criteria for the subjects were consistent ().
Figure 7. The effect of postbiotic toothpaste and SLS on the oral microecology of clinical populations. (a-d) the Shannon, Simpson, ACE, and Chao indices of the oral microflora of subjects, respectively. (e-f) Distribution of bacterial groups at the phylum and genus levels. (g) Principal coordinate analysis at the genus level for the oral microflora of subjects. (h) Community heatmap analysis at the genus level.
These results proved that the postbiotic toothpaste had a significant impact on reducing both the PLI and GI, thereby playing a crucial role in mitigating gingival inflammation. The addition of SLS weakened the effect of plaque removal, but did not weaken the effect of improving gingival inflammation. In agreement with the findings of prior researches [Citation18], our study demonstrated that the salivary microbiota was sufficiently robust to withstand the PT and PT+SLS interventions.
Discussion
The oral microbiome is, a complex and diverse oral microbial community that plays a crucial role in maintaining oral and systemic health [Citation19]. In this study, we systematically examined the influence of SLS and postbiotic toothpaste on the oral microbial community at three levels: biofilms, animals and clinical populations. Human saliva-derived biofilm models were established to represent the diversity and overall metabolic functionality of oral microbiota, providing a less cumbersome, more controllable and ethically sound platform for assessing the potential effects of oral product components [Citation20]. The rat periodontitis model could contribute detailed information on histopathological and blood biochemical analyses [Citation21]. In addition, population trials could provide more realistic and reliable research results, allowing us to gain a deeper understanding of the responses in the human body [Citation22]. One of the novel aspects of this research lies in the systematic evaluation of oral microecology modulation by SLS and postbiotic toothpaste in vitro and in vivo by the combined application of the above-mentioned approaches to provide insights into the optimization and use of toothpaste ingredients to maintain oral homeostasis and prevent oral problems.
To simulate the brushing process, short daily exposure to SLS and PT was conducted twice in the preformed and developing biofilm models, using a mature biofilm after preformed culture and an immature biofilm with only a short duration of adhesion culture, respectively. Chlorhexidine, as a positive control, significantly increased the biomass of the biofilm but significantly reduced the number of viable bacteria and the total bacteria in preformed biofilms, indicating that it inhibited bacterial growth. However, the bacteria that could secrete exopolysaccharides accounted for an important proportion of the remnant surviving bacteria. Exopolysaccharides are known to account for approximately 33–85% of mature biofilms [Citation23,Citation24]. SLS significantly decreased the biomass and viable bacterial count in preformed biofilms and reduced the biomass, viable bacterial count, and total bacteria in developing biofilms. It also destroyed the scaffold structure of both biofilms, indicating that SLS intervention exerted a bactericidal effect and demonstrated the ability to reduce exopolysaccharides. This result was consistent with that of SLS, which as a surfactant, has been reported to inhibit bacterial plaque and exert antimicrobial activity [Citation25–27]. However, the postbiotic toothpaste increased the biomass and total bacteria and maintained the normal reticulate structure in both the preformed and developing biofilms, confirming the beneficial impact of postbiotics. Researchers have previously stressed the role of postbiotics in maintaining ecological equilibrium and their effectiveness in improving the health of the oral cavity [Citation28]. The combination of postbiotic toothpaste and SLS was found to attenuate the inhibitory effect of SLS in both biofilm models. The combined use of postbiotic toothpaste and SLS resulted in higher levels of biomass, viable bacteria, and total bacteria than those obtained with the SLS treatment, but lower levels than those with the postbiotic toothpaste treatment, which revealed the pattern of interaction between the postbiotic toothpaste and SLS.
Over-intervention with external factors usually shifts healthy oral microbiota toward microbial dysbiosis, and an increasing number of studies have underlined the significance of preserving the diversity and stability of the oral ecosystem [Citation29–31]. In this study, preformed biofilms were dominated by Streptococcus and Veillonella, in agreement with previous studies [Citation32,Citation33] and, confirmed successful establishment of dental plaque-derived biofilms in healthy subjects [Citation21,Citation34]. Remarkably, SLS was found to disturb the oral flora with opposite patterns in the two biofilm models, where SLS decreased the flora diversity in preformed biofilms and increased the community diversity in developing biofilms. From an ecological standpoint, an increase in diversity has positive implications; however, the composition of the microbiome must be observed carefully to avoid making hasty conclusions. Streptococcus and Veillonella accounted for 99.31% of the preformed biofilms after SLS treatment, among which the abundance of Streptococcus increased to 94.92%, and that of Veillonella decreased to 4.39%. Streptococcus is reported to be the most common bacteria in the oral cavity and plays an important role in maintaining the oral microecological balance and pathogenesis [Citation35]. Streptococcus can ferment carbohydrates into lactic acid, thereby reducing the pH of the medium and creating a cariogenic environment. Veillonella is a key member of the oral microbiota with an abundance lower than Streptococcus in the healthy oral cavity. It cannot metabolize carbohydrates and instead uses the organic acids produced by streptococcus, especially lactic acid, as energy for fermentation; thus, it can reduce the incidence of dental caries by reducing the pH [Citation36]. Therefore, the greatly increased Streptococcus and largely reduced Veillonella may lead to an increased risk of caries in preformed biofilms. SLS substantially increased the abundance of numerous pathogenic bacteria. Staphylococcus, a gram-positive pathogen, can cause many acute and chronic infections and is responsible for various instances of abscesses, septicemia, arthritis, and endocarditis [Citation37,Citation38]. The abundance of Staphylococcus was increased in both biofilm models, suggesting a negative effect of SLS. Other pathogenic bacteria, such as Neisseria, Thermus, Pseudomonas, Campylobacter, Bacillus, and Escherichia-Shigella, also appeared in developing biofilms, which could explain the higher diversity upon SLS treatment. The bacterial profiles of the PT and PT+SLS treatment groups were similar to those of the control group, implying that the postbiotic toothpaste had little influence on oral homeostasis, indicating its high suitability. Furthermore, PT appeared to counteract the disruptive effects of SLS on oral biofilms.
Periodontitis, a disease closely associated with oral flora, can be fueled by the altered relationship between the host and resident microbiota, from symbiosis to dysbiosis, owing to structural and functional variations in the oral microflora [Citation39,Citation40]. The use of a rat periodontitis model facilitated exploration of the effects of SLS and postbiotic toothpaste on oral clinical indicators and histopathological morphology. Periodontitis is considered a chronic, irreversible inflammatory disease in which the chronic infiltration of immune cells mediates the destruction of connective tissue and alveolar bone [Citation3]. These signs of periodontitis were clearly observed in the rats used in our study. Obviously, SLS treatment deepened the periodontal pockets, destroyed the periodontal tissue, promoted inflammatory cell infiltration, osteoclastogenesis and alveolar bone resorption, along with high tissue levels of inflammatory cytokines IL-6, IL-8, and TNF-α. SLS has been demonstrated to affect the oral epithelial structure and aggravate more pronounced inflammatory reactions at concentrations exceeding 1% [Citation41,Citation42]. Ahlfors et al. revealed mononuclear cell infiltration in the oral mucosa after application of 2% SLS, with local parts of the superficial epithelium occasionally exhibiting necrosis [Citation43]. These previous results support our findings regarding the SLS-induced inflammation and tissue destruction. Postbiotic toothpaste intervention alleviated gingival bleeding, reduced the depth of the periodontal pocket, decreased alveolar bone absorption, and effectively relieved periodontitis-related inflammation; these effects were also detected in the PT+SLS treatment to a slightly lower degree than that with postbiotic toothpaste intervention.
Furthermore, the impact of postbiotic toothpaste with or without SLS on the clinical manifestations and oral microbiota of patients with gingivitis were examined. Gingivitis is the most common and prevalent form of periodontal disease among adults, and is a reversible inflammatory disease resulting from a resident bacterial plaque generated at the gingival margin [Citation31]. The clinical symptoms of gingivitis include swollen and inflamed gums, as well as spontaneous or probing-induced bleeding. If uncontrolled, gingivitis can progress to periodontitis. The mechanisms of action of postbiotics in oral diseases are derived from parallels with studies on probiotics, including antimicrobial activities (such as organic acids, bacteriocins, free fatty acids, H2O2) [Citation12,Citation44,Citation45] and prevention of biofilm formation [Citation10], as well as immunoregulatory properties [Citation46]. As expected, our results suggest that the postbiotic toothpaste significantly attenuated gingivitis after 28 days, with reduced plaque load, gingival swelling, and bleeding. Thus, regular brushing with an effective toothpaste could maintain dental plaque in an immature state at relatively low levels, which is in agreement with the findings of other researchers [Citation47]. Further, combined use of postbiotic toothpaste and SLS elevated the plaque levels but suppressed gingival swelling and bleeding. Exploration of plaque microbiota responses to external interventions in the human population are needed to understand the underlying mechanism more clearly. Teng et al. found a significant increase in microbial diversity from the baseline to day 21 in subjects from the control group who did not use mouth rinses [Citation48], which corresponded with previous findings from other gingivitis cohorts [Citation14,Citation49,Citation50]. However, subjects with gingivitis who used the tested mouth rinses showed no apparent changes [Citation48]. Considering that health-related plaque is usually immature [Citation32], while gingivitis is related to a more developed and complex microbial community [Citation51,Citation52], Teng et al. considered that the oral microbial diversity in subjects using mouth rinses remained stable and prevented the acquisition of new taxa, providing a significant benefit in balancing the diversity and composition of oral microbiota. This observation consistently showed that diversity in the postbiotic toothpaste treatment group remained stable, indicating that treatment with postbiotic toothpaste restrained the appearance of new microorganisms and maintained the initial biodiversity of the oral microflora. Moreover, there was no obvious difference between the postbiotic toothpaste group and the SLS-containing postbiotic toothpaste group on day 28, indicating a similar balancing effect on the oral microbiome in gingivitis.
Although SLS incorporated into toothpaste may have potential for cleaning, it cannot guarantee transformation of the oral cavity to a healthy state. In contrast, our research showed that SLS could perturb the commensal microbiome, leading to a higher relative abundance of pathogenic taxa. Nielsen et al. revealed that surfactants can increase the permeability of membranes and bind metal ions that participate in many cellular processes, causing growth inhibition and bacterial death [Citation38]. Therefore, indiscriminate targeting of the oral microbiome with SLS may be detrimental. Nevertheless, postbiotics in toothpaste play a vital role in maintaining bacterial homeostasis and oral health, suggesting that postbiotic toothpaste may be a novel approach for the prevention and control of plaque-related diseases, such as gingivitis and periodontitis.
Conclusions
In conclusion, this study characterized the association of SLS and postbiotic toothpaste with oral microecology using in vitro biofilms, periodontitis in rats, and patients with gingivitis. SLS has been demonstrated to inhibit of biofilm growth, disturb community structure, and increase the number of pathogenic bacteria in biofilms in vitro, as well as cause changes in clinical indicators, destruction of periodontal tissue, alveolar bone resorption, and elevated levels of inflammation in vivo. Post-biotic toothpaste exhibited a significant benefit in balancing the oral microbiota, which maintained the development of biofilms in vitro and attenuated periodontitis and gingivitis in vivo. Moreover, the combined use of a postbiotic toothpaste and SLS alleviated the adverse effects of SLS. Overall, our study highlights the need for reconsidering postbiotics and SLS formulations in toothpaste from a microecological perspective and provides a thoughtful understanding of oral microecology versus oral health.
Contributions
Zhao H., Shi Q., and Lu F. conceived and designed the research. Shi Q., Li F., Chen D., Gao J., Shi T., Tan Y., Chang H., Sun L. and Kang J. performed experiment. Shi Q., Shi T., Tan Y. and Chang H., analyzed the data. Shi Q. wrote the draft and manuscript. Zhao H., Shi Q., and Lu F. revised paper. Zhao H., Huang Z., Liu X. and Lu F. supervised the research. All authors approved the final version of the paper.
Revised supplemental information.docx
Download MS Word (1.3 MB)Acknowledgments
We thank Zhaoying Han for the analysis and interpretation of data in the process of paper revision. We thank Shulin Liu for polishing the language of the article. We thank Congcong Wang and Fanglu Yu for the critically review of the article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20002297.2024.2372224.
Additional information
Funding
References
- Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–15. doi: 10.1038/nature11234
- Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013;69(1):137–143. doi: 10.1016/j.phrs.2012.11.006
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8
- Radaic A, Kapila YL. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput Struct Biotechnol. J. 2021;19:1335–1360. doi: 10.1016/j.csbj.2021.02.010
- Saelzer S, Graetz C, Doerfer CE, et al. Contemporary practices for mechanical oral hygiene to prevent periodontal disease. Periodontol 2000. 2020;84(1):35–44. doi: 10.1111/prd.12332
- Bartnik FG. Interaction of anionic surfactants with proteins, enzymes and membranes. In: Gloxhuber C, Künstler K, editors. Anionic surfactants: biochemistry, toxicology, dermatology. New York: MARCEL DEKKER; 1992. p. 1–42.
- Salton MRJ. Lytic Agents, Cell Permeability, and Monolayer Penetrability. J Gen Physiol. 1968;52(1):227–252. doi: 10.1085/jgp.52.1.227
- Nguyen T, Brody H, Radaic A, et al. Probiotics for periodontal health—Current molecular findings. Periodontol 2000. 2021;87(1):254–267. doi: 10.1111/prd.12382
- Koll-Klais P, Mändar R, Leibur E, et al. Oral lactobacilli in chronic periodontitis and periodontal health: species composition and antimicrobial activity. Oral Microbiol Immunol. 2005;20(6):354–361. doi: 10.1111/j.1399-302X.2005.00239.x
- Radaic A, Ye C, Parks B, et al. Modulation of pathogenic oral biofilms towards health with nisin probiotic. J Oral Microbiol. 2020;12(1):1809302. doi: 10.1080/20002297.2020.1809302
- Gao L, Kuraji R, Zhang MJ, et al. Nisin probiotic prevents inflammatory bone loss while promoting reparative proliferation and a healthy microbiome. NPJ Biofilms Microbiomes. 2022;8(1). doi: 10.1038/s41522-022-00307-x
- Radaic A, Brody H, Contreras F, et al. Nisin and Nisin probiotic disrupt oral pathogenic biofilms and restore their microbiome composition towards healthy control levels in a peri-implantitis setting. Microorganisms. 2022;10(7):1336. doi: 10.3390/microorganisms10071336
- Carda-Diéguez M, Moazzez R, Mira A. Functional changes in the oral microbiome after use of fluoride and arginine containing dentifrices: a metagenomic and metatranscriptomic study. Microbiome. 2022;10(1). doi: 10.1186/s40168-022-01338-4
- Abusleme L, Dupuy AK, Dutzan N, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. Isme J. 2013;7(5):1016–1025. doi: 10.1038/ismej.2012.174
- Nagaraj V, Skillman L, Ho G, et al. Characterisation and comparison of bacterial communities on reverse osmosis membranes of a full-scale desalination plant by bacterial 16S rRNA gene metabarcoding. NPJ Biofilms Microbiomes. 2017;3(1). doi: 10.1038/s41522-017-0021-6
- Cheng X, Liu J, Li J, et al. Comparative effect of a stannous fluoride toothpaste and a sodium fluoride toothpaste on a multispecies biofilm. Arch Oral Biol. 2017;74:5–11. doi: 10.1016/j.archoralbio.2016.10.030
- Magana M, Sereti C, Ioannidis A, et al. Options and limitations in clinical investigation of bacterial biofilms. Clin Microbiol Rev. 2018;31(3). doi: 10.1128/cmr.00084-16
- Wang J, Jia Z, Zhang B, et al. Tracing the accumulation of in vivo human oral microbiota elucidates microbial community dynamics at the gateway to the GI tract. Gut. 2020;69(7):1355–1356. doi: 10.1136/gutjnl-2019-318977
- Pedersen AML, Belstrom D. The role of natural salivary defences in maintaining a healthy oral microbiota. J Dent. 2019;80:S3–S12. doi: 10.1016/j.jdent.2018.08.010
- Chatzigiannidou I, Teughels W, Van de Wiele T, et al. Oral biofilms exposure to chlorhexidine results in altered microbial composition and metabolic profile. NPJ Biofilms Microbiomes. 2020;6(1). doi: 10.1038/s41522-020-0124-3
- Jiang W, Wang Y, Luo J, et al. Antimicrobial peptide GH12 prevents dental caries by regulating dental plaque microbiota. Appl Environ Microbiol. 2020;86(14). doi: 10.1128/aem.00527-20
- Tonetti MS, Lang NP, Cortellini P, et al. Effects of a single topical doxycycline administration adjunctive to mechanical debridement in patients with persistent/recurrent periodontitis but acceptable oral hygiene during supportive periodontal therapy. J Clin Periodontol. 2012;39(5):475–482. doi: 10.1111/j.1600-051X.2012.01864.x
- Flemming H-C, Neu TR, Wozniak DJ. The EPS matrix: The “House of Biofilm cells”. J Bacteriol. 2007;189(22):7945–7947. doi: 10.1128/jb.00858-07
- Sutherland IW. The biofilm matrix – an immobilized but dynamic microbial environment. Trends Microbiol. 2001;9(5):222–227. doi: 10.1016/s0966-842x(01)02012-1
- Waaler SM, Rolla G, Skjorland KK, et al. Effects of oral rinsing with triclosan and sodium lauryl sulfate on dental plaque formation: a pilot study. Eur J Oral Sci. 1993;101(4):192–195. doi: 10.1111/j.1600-0722.1993.tb01103.x
- Jenkins S, Addy M, Newcome R. Triclosan and sodium lauryl sulphate mouthrinses. (II). Effects of 4-day plaque regrowth. J Clin Periodontol. 1991;18(2):145–148. doi: 10.1111/j.1600-051X.1991.tb01704.x
- Giertsen E, Scheie AA, Rolla G. Plaque inhibition by a combination of zinc citrate and sodium lauryl sulfate. Caries Res. 1989;23(4):278–283. doi: 10.1159/000261192
- Scott E, De Paepe K, Van de Wiele T. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules. 2022;12(11):1640. doi: 10.3390/biom12111640
- Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745–759. doi: 10.1038/s41579-018-0089-x
- Hou K, Wu Z-X, Chen X-Y, et al. Microbiota in health and diseases. Sig Transduct Target Ther. 2022;7(1). doi: 10.1038/s41392-022-00974-4
- Zhang Y, Wang X, Li H, et al. Human oral microbiota and its modulation for oral health. Biomed Pharmacother. 2018;99:883–893. doi: 10.1016/j.biopha.2018.01.146
- Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721–5732. doi: 10.1128/jcm.43.11.5721-5732.2005
- Diaz PI, Chalmers NI, Rickard AH, et al. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl Environ Microbiol. 2006;72(4):2837–2848. doi: 10.1128/aem.72.4.2837-2848.2006
- Fernandez y Exterkate M, Buijs MJ, Crielaard W, Zaura E. Effect of mouthwashes on the composition and metabolic activity of oral biofilms grown in vitro. Clin Oral Invest. 2017;21(4):1221–1230. doi: 10.1007/s00784-016-1876-2
- Ramage G, O’Donnell L, Sherry L, et al. Impact of frequency of denture cleaning on microbial and clinical parameters – a bench to chairside approach. J Oral Microbiol. 2019;11(1):1538437. doi: 10.1080/20002297.2018.1538437
- Periasamy S, Kolenbrander PE. Central role of the early colonizer Veillonella sp. in establishing multispecies biofilm communities with initial, middle, and late colonizers of Enamel. J Bacteriol. 2010;192(12):2965–2972. doi: 10.1128/jb.01631-09
- Kenny JG, Ward D, Josefsson E, et al. The Staphylococcus aureus response to unsaturated long chain free fatty acids: survival mechanisms and virulence implications. PLoS One. 2009;4(2):e4344. doi: 10.1371/journal.pone.0004344
- Nielsen CK, Kjems J, Mygind T, et al. Effects of Tween 80 on growth and biofilm formation in laboratory media. Front Microbiol. 2016;7. doi: 10.3389/fmicb.2016.01878
- Belstrom D, Constancias F, Liu Y, et al. Metagenomic and metatranscriptomic analysis of saliva reveals disease-associated microbiota in patients with periodontitis and dental caries. NPJ Biofilms Microbiomes. 2017;3(1). doi: 10.1038/s41522-017-0031-4
- Belstrom D, Constancias F, Drautz-Moses DI, et al. Periodontitis associates with species-specific gene expression of the oral microbiota. NPJ Biofilms Microbiomes. 2021;7(1). doi: 10.1038/s41522-021-00247-y
- Neppelberg E, Costea DE, Vintermyr OK, et al. Dual effects of sodium lauryl sulphate on human oral epithelial structure. Exp Dermatol. 2007;16(7):574–579. doi: 10.1111/j.1600-0625.2007.00567.x
- Aramaki J, Effendy I, Happle R, et al. Which bioengineering assay is appropriate for irritant patch testing with sodium lauryl sulfate? Contact Dermatitis. 2001;45(5):286–290. doi: 10.1034/j.1600-0536.2001.450506.x
- Ahlfors EE, Dahl JE, Lyberg T. The development of T cell-dominated inflammatory responses induced by sodium lauryl sulphate in mouse oral mucosa. Arch Oral Biol. 2012;57(6):796–804. doi: 10.1016/j.archoralbio.2011.11.005
- Ishikawa KH, Bueno MR, Kawamoto D, et al. Lactobacilli postbiotics reduce biofilm formation and alter transcription of virulence genes of Aggregatibacter actinomycetemcomitans. Mol Oral Microbiol. 2021;36(1):92–102. doi: 10.1111/omi.12330
- Yoon BK, Jackman JA, Valle-González ER, et al. Antibacterial free fatty acids and monoglycerides: biological activities, experimental testing, and therapeutic applications. IJMS. 2018;19(4):1114. doi: 10.3390/ijms19041114
- Taguchi C, Arikawa K, Saitou M, et al. Orally ingested Lactobacillus crispatus KT-11 inhibits Porphyromonas gingivalisinfected Alveolar Bone Resorption. IJOMS. 2015;13(3):102–109. doi: 10.5466/ijoms.13.102
- Fischman SL. The history of oral hygiene products: how far have we come in 6000 years? Periodontology 2000. 1997;15(1):7–14. doi: 10.1111/j.1600-0757.1997.tb00099.x
- Schmitter T, Fiebich BL, Fischer JT, et al. Ex vivo anti-inflammatory effects of probiotics for periodontal health. J Oral Microbiol. 2018;10(1):1502027. doi: 10.1080/20002297.2018.1502027
- Griffen AL, Beall CJ, Campbell JH, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. Isme J. 2012;6(6):1176–1185. doi: 10.1038/ismej.2011.191
- Liu Z, Zhang W, Zhang J, et al. Oral hygiene, periodontal health and chronic obstructive pulmonary disease exacerbations. J Clin Periodontol. 2012;39(1):45–52. doi: 10.1111/j.1600-051X.2011.01808.x
- Kistler JO, Booth V, Bradshaw DJ, et al. Bacterial Community Development in Experimental Gingivitis. PLoS One. 2013;8(8):e71227. doi: 10.1371/journal.pone.0071227
- Huang S, Li R, Zeng X, et al. Predictive modeling of gingivitis severity and susceptibility via oral microbiota. Isme J. 2014;8(9):1768–1780. doi: 10.1038/ismej.2014.32