ABSTRACT
Background: Many patients do not use inhalers correctly. Inhalers associated with good technique have the potential to improve symptom control and are often preferred by patients. Methods: Inhaler-naïve, adult volunteers were randomized to use empty Spiromax®, Easyhaler®, and Turbuhaler® dry powder inhalers (DPIs) in one of six possible sequences in this single-site, single-visit, crossover study conducted in Sweden. Randomization was stratified by age and gender. Participants attempted to use each device intuitively (no instructions) and after reading the instructions for use from the patient information leaflet. Device preference was surveyed after using all devices. Mastery of device handling (i.e. dose preparation) or inhalation was defined as having no healthcare-professional-observed errors. The primary endpoint was mastery of device handling after reading the instructions. Results: More participants mastered device handling with Spiromax vs Easyhaler or Turbuhaler, both intuitively (44%, 0%, and 10%, respectively) and after reading the instructions (99%, 56%, and 81%, respectively). Fewer participants had ≥1 device-handling error with Spiromax than with the other devices. The percentage of participants still showing inhalation errors after reading the instructions ranged between 21% for Spiromax and 40% for Easyhaler. After reading instructions, mastery of handling and inhalation was numerically lower among older (aged >60 years) vs younger participants across all devices. Most participants preferred Spiromax for device handling (59%) and intuitiveness/ease of use (61%). Conclusion: These findings highlight that important differences exist between DPI devices, which could have implications for disease control when selecting a device for a patient.
Introduction
In the European Union, it has been estimated that over 200 million adults aged 15–44 years have asthma and over 250 million older adults (≥40 years) have chronic obstructive pulmonary disease (COPD) [Citation1]. Asthma was responsible for 82,000 episodes of hospital in-patient care and 380 deaths per year in younger adults, and COPD was responsible for 1.1 million hospitalizations and 150,000 deaths per year in older adults [Citation1]. In Sweden, asthma occurs in approximately 8–10% of the population [Citation2]. According to various diagnostic criteria, another Swedish study found that COPD occurred in 8% (British Thoracic Society criteria), 14% (ERS or Global Initiative for Chronic Obstructive Lung Disease [GOLD] criteria), or 12% (American Thoracic Society clinical criteria) of the population [Citation3].
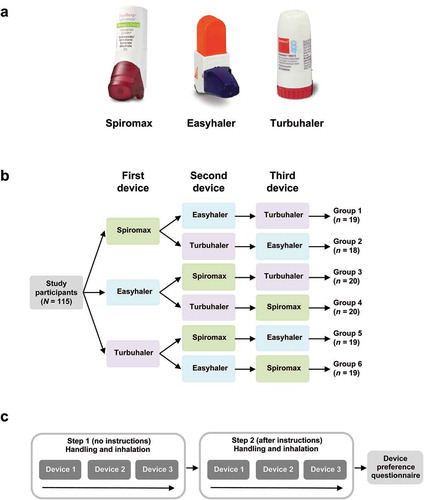
Regular low-dose inhaled corticosteroid (ICS) treatment is appropriate for many patients with asthma [Citation4]. The combination of an ICS and a long-acting β2-agonist (LABA) may be appropriate when symptoms are not controlled by ICS alone, as initial treatment for patients with moderate to severe symptoms or patients with frequent exacerbations [Citation5]. A fixed-dose combination of budesonide and the rapid-onset LABA formoterol (BF) is a commonly used ICS/LABA combination in Europe and the most commonly used ICS/LABA combination in Sweden. This combination may also be appropriate as both maintenance and reliever therapy in asthma [Citation5]. In Europe, although there are some differences between countries, BF is currently available in three dry powder inhalers (DPIs): DuoResp (BF) Spiromax® (Teva Pharmaceutical Industries Ltd, Petach Tikva, Israel), Bufomix (BF) Easyhaler® (Orion Corporation, Espoo, Finland), and Symbicort (BF) Turbuhaler® (AstraZeneca PLC, London, UK) ()).
Figure 1. Study design: (a) devices used in this study; (b) participants were randomised to use empty devices in one of six counter-balanced orders; (c) for each device, participants were asked to intuitively prepare a dose and inhale (no instructions; step 1) and to try again after reading the instructions for use (step 2).
Due to the need for homogeneous patient populations, randomized controlled trials (RCTs) are not representative of the real-life clinical population. This is particularly true in respiratory medicine, where patients use inhalers to administer a variety of treatments. Many patients in routine clinical practice do not use their inhalers correctly. This may be due to a lack of training or inappropriate device choice, and poor inhaler technique correlates with both poor control of symptoms and increased exacerbations [Citation5–Citation7]. In addition, approximately 50% of adults and children do not take their medications as prescribed [Citation5]. There is evidence that low percentages of patients with asthma and patients with COPD are eligible for RCTs [Citation8]. Further differences from clinical reality relate to methods followed during RCTs: for example, study participants frequently receive treatment reminders and training.
Suboptimal adherence and inhaler mishandling have adverse effects on disease control, while satisfaction with inhaler devices is associated with improved adherence, improved clinical outcomes and reduced cost [Citation9–Citation11]. In turn, devices associated with the lowest numbers of handling errors have the highest patient preference ratings [Citation12,Citation13], suggesting that a patient’s acceptance of a device may be correlated with ease of handling. Therefore, device handling, correct inhaler technique, patient preference, and adherence are intertwined factors that all contribute to good symptom control.
The available DPIs may differ in ease of handling and patient preference [Citation13] as well as in estimated lung deposition of the BF powder [Citation14] and lung function [Citation15]. Therefore, we aimed to test DPI technique and preference in device-naïve Swedish volunteers, to mirror newly diagnosed patients with asthma or COPD and to avoid any confounding influence of prior inhaler training.
Methods
Study design and participants
Inhaler-naïve, adult Swedish volunteers were randomized to use empty Spiromax, Easyhaler, and Turbuhaler in one of six possible sequences ()) in this single-site, single-visit, crossover study. Three equally sized age groups (18–40 years, 41–60 years, and ≥61 years) were recruited with equal numbers of men and women in each age group. Microsoft Excel was used to randomize participants to each of the six device sequences, with balancing by age group and gender. For each DPI, mastery of device handling was evaluated using a two-step approach ()): in step 1, participants attempted to use the three devices without instructions (intuitive use); in step 2, participants were asked to read the ‘instructions for use’ section of the patient information leaflets (Supplementary Table S1) and to use the devices again. Device preference was surveyed after step 2. Inhalation technique was videotaped and assessed by independent healthcare professionals (HCPs), based on pre-defined error checklists (Supplementary Tables S2–S4).
Only individuals with no prior use of, knowledge of or training with the study devices were included. Potential study participants were excluded if they used any kind of inhalation treatment or had asthma, COPD or any condition that could affect cognitive function.
The study synopsis, together with various study-related documents, was approved by an Independent Ethics Committee. Participants provided written informed consent. The study was not included in a clinical registry because the local ethics committee did not consider it to be a clinical trial (there was no active drug or excipient and, therefore, no intervention).
Assessments
A visual record (video) was made of each participant’s attempt to use each device. Mastery of handling was defined as the absence of HCP-observed dose-preparation errors according to a device-specific handling-error checklist (DSHEC; Supplementary Table S2), and mastery of inhalation according to a device-independent inhalation-error checklist (DIIEC; Supplementary Table S3). The DSHEC was developed from the patient information leaflet for each device, based on available information and our clinical judgement, and was designed to identify preparation errors. The DIIEC included standard questions used in other studies [Citation13] to identify errors during and after inhalation that are common to all study devices. Errors were scored by more than one reviewer to avoid bias: where there were differences in judgment between two reviewers, the opinion of a third HCP was taken into account. Device preferences were assessed using a device preference questionnaire (Supplementary Table S4).
Statistical analysis
The primary endpoint was the proportion of participants demonstrating mastery of device handling (absence of HCP-observed dose-preparation errors using the DSHEC) for each device in step 2, after reading the instructions for use. Secondary endpoints included: the proportion of participants demonstrating intuitive mastery of device handling (no instructions; step 1); the proportion of participants demonstrating mastery of inhalation; characterization of handling, and inhalation errors; device preferences.
The study was powered to test superiority of Spiromax vs Easyhaler and Spiromax vs Turbuhaler for the primary endpoint. A sample size of 117 participants was estimated to have 90% power to detect a difference of 20% for the primary endpoint with the expected proportion of discordant pairs (45%) when analyzed by McNemar’s test of equality of paired proportions with a 0.050 two-sided significance level.
For mastery of device handling and inhalation, McNemar’s test (as described above) was used to produce P-values. Using this pre-specified testing method, P-values could only be calculated when at least one participant achieved device-handling mastery with Easyhaler or Turbuhaler but not with Spiromax. This was not the case for the comparison of Easyhaler with Spiromax, so no P-value could be calculated for this comparison using the pre-specified approach. An alternative Bayesian approach, using a non-informative prior (Jeffrey’s prior), was used to calculate this P-value. Characterization of handling and inhalation errors, and device preferences, were summarized using descriptive statistics only.
Results
Overall, 117 volunteers took part in the study. Two participants were excluded from the analysis due to protocol violations. Although they were screened as having no experience with inhalers, when they attended the test, they realized and told the interviewer that they had seen one of the inhalers being used. Therefore, the analysis included 115 participants.
Participants were well distributed with respect to age and gender (). Half of the participants were males, and the mean age was 50 years (standard deviation, 14.8). Most participants (60%) had attended college or university.
Table 1. Demographic characteristics of the study participants (N = 115).
Device mastery
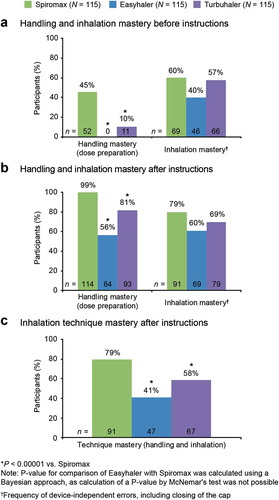
Intuitive mastery of device handling (no instructions; step 1) was achieved by more participants with Spiromax (45%) vs Easyhaler (0%) or Turbuhaler (10%; P < 0.00001) ()). Device-handling mastery, defined as having no HCP-observed dose-preparation errors, was achieved after reading the instructions (step 2) by 99% of volunteers with Spiromax, 56% with Easyhaler and 81% with Turbuhaler (primary endpoint) ()). The difference between Spiromax and Turbuhaler was statistically significant (P < 0.00001). None of the participants who mastered device handling with Easyhaler failed to do so with Spiromax; therefore, P-values could not be calculated for that comparison using the pre-specified frequentist approach. Using a Bayesian approach (see Methods for details) the difference between Spiromax and Easyhaler was found to be statistically significant (P = 0.000000121). The point estimate of the odds ratio was 198.5 (95% CI: 6.73–17660). The percentage of participants achieving mastery of both dose preparation and inhalation after reading instructions was higher with Spiromax (79%) vs Easyhaler (41%; P < 0.00001) or Turbuhaler (58%; P < 0.00001) ()).
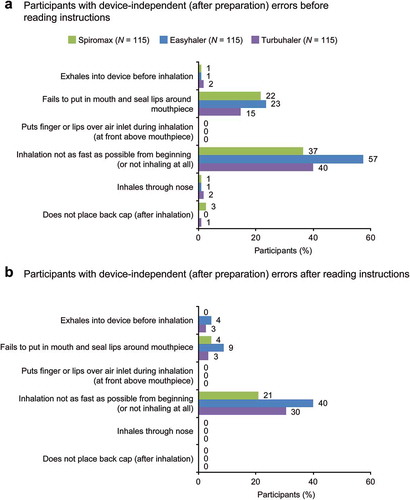
Figure 2. Summary of device mastery: (a) before reading instructions (no information; step 1) and (b) after reading instructions (step 2). Primary endpoint was the percentage of participants achieving handling mastery after reading instructions. *P < 0.00001 vs Spiromax. Where a P-value is not stated then it was not calculated. P-values were not calculated for inhalation errors. †Frequency of device-independent errors, including closing of the cap.
There was no consistent pattern across gender or education subgroups for mastery of device handling or inhalation after reading the instructions (Supplementary Table S5). However, across all devices, mastery of handling and inhalation was numerically lower among older (aged >60 years) vs younger participants. There was no consistent pattern across any subgroup for intuitive mastery of device handling (Supplementary Table S5). Intuitive mastery of device handling did not increase after participants had used other devices; a similar proportion of participants achieved this endpoint when the device was used first, second, or third (Supplementary Figure S1). Mastery of handling with Spiromax or Turbuhaler after reading instructions (step 2) did not increase with increased device experience. However, mastery of handling with Easyhaler after reading the instructions was numerically higher after the use of other devices (Supplementary Figure S1).
Characterization of device-handling errors and inhalation errors
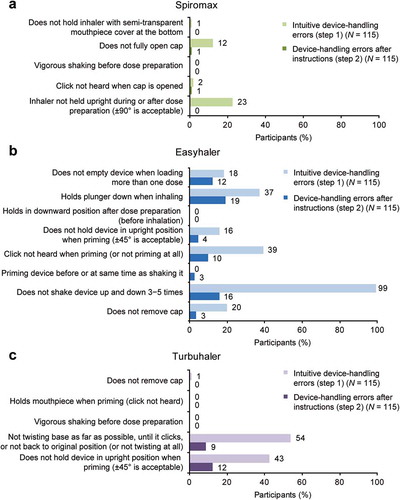
As illustrated by the data in , the percentage of study participants with ≥1 device-handling (dose-preparation) error was lower with Spiromax vs Easyhaler or Turbuhaler, both intuitively and after reading the instructions. The percentage of participants with ≥1 handling error after reading the instructions was 1% with Spiromax, 44% with Easyhaler, and 19% with Turbuhaler. The most frequent intuitive device-handling errors were: not holding the device in a sufficiently upright position for Spiromax; not shaking the device correctly for Easyhaler; and not twisting the base correctly for Turbuhaler (). Not holding the device sufficiently upright was the second most common error with Turbuhaler. After reading instructions, the frequency of errors was reduced with all three devices. The only device-handling errors with Spiromax after reading the instructions were not fully opening the cap and the click not being heard when opening the cap, both occurring in 1% of participants. For Turbuhaler, the corresponding errors were not holding the device sufficiently upright (12% of participants) and not twisting the base correctly (9%). With Easyhaler, four different device-handling errors were seen in ≥10% of participants after reading the instructions. Spiromax was the only device which all participants held sufficiently upright after reading the instructions.
Figure 3. Device-specific handling errors: participants with each handling error assessed by a device-specific handling-error checklist intuitively (no instructions) and after reading instructions for (a) Spiromax, (b) Easyhaler, and (c) Turbuhaler.
As with device-handling errors, the percentage of participants with ≥1 inhalation error was lowest with Spiromax intuitively and after reading the instructions. At least one inhalation error was observed after reading the instructions in 21% of participants with Spiromax, 40% with Easyhaler and 31% with Turbuhaler. The most common inhalation error was not inhaling as fast as possible from the beginning (or not inhaling at all), across all devices and both before and after reading the instructions (). Some participants exhaled into the device after inhalation which could impair subsequent device performance in real-life use.
Device preference
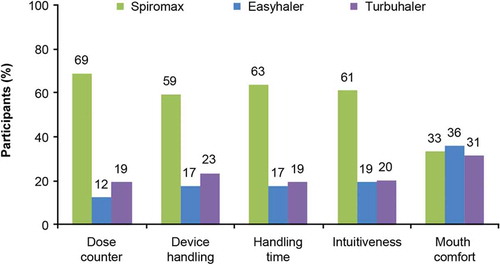
Spiromax was preferred by most participants when considering device handling, handling time, dose counter, and intuitiveness or easiness to learn and use (). A similar proportion of participants preferred each device when considering mouthpiece comfort.
Discussion
Main findings
More DPI-naïve volunteers mastered device handling (i.e. dose preparation) with Spiromax vs Easyhaler or Turbuhaler, both intuitively (no instructions) and after having read the instructions for use. Correspondingly, the number of participants with ≥1 device-handling error was generally lower with Spiromax than with the other devices. Not surprisingly, therefore, most participants preferred Spiromax in terms of device handling and intuitiveness/ease of use. Mastery of inhalation was also greater with Spiromax than with the other devices and the number of participants with ≥1 inhalation error was generally lower. The percentage of participants with inhalation errors (both preparation and inhalation) after reading the instructions ranged between 21% for Spiromax and 40% for Easyhaler.
Not holding the device in a sufficiently upright position was observed as an intuitive handling error with all three devices. After reading the instructions, it was not seen with Spiromax but it persisted with the other two devices (albeit with reduced frequency than before reading the instructions). This could be related to the fact that a wider range of angles is permissible with Spiromax (±90 degrees, compared to ±45 degrees with Easyhaler and Turbuhaler) or, perhaps, to the way this instruction is written in the different patient leaflets. Intuitively, the ±90 degree administration angle permitted with Spiromax use provides a more forgiving application angle than Easyhaler and Turbuhaler. To our knowledge, the drop in delivered dose resulting from actuation of Turbuhaler or Easyhaler in a non-upright manner has not been reported, with the exception of one study that described an 8% drop resulting from horizontal actuation of the Turbuhaler [Citation16]. However, reports of several studies of inhaler technique have underscored the importance of holding the Turbuhaler upright [Citation6, Citation7, Citation11, Citation17–Citation20]
Spiromax had the lowest percentage of patients with ≥1 inhalation error, both before and after reading the instructions. When considering specific inhalation errors, with all devices, not inhaling as fast as possible from the beginning was the most common error. The second most frequent inhalation error observed, before and after reading the instructions – was failure to put the device into the mouth and seal lips around the mouthpiece.
Interpretation of findings in relation to previously published work
The finding that device-handling (dose preparation) technique was generally better with Spiromax than either Easyhaler or Turbuhaler, both intuitively and after reading the instructions, and that Spiromax was generally preferred, is in line with several previous studies [Citation13,Citation[16]Citation21–Citation23Citation[18]]. A recent study by Sandler et al. was similar to the present one, focusing on patients’ mastery with the Spiromax, Easyhaler, and Turbuhaler devices [Citation13]. Overall, the results of the two studies are similar in showing higher levels of device mastery with Spiromax vs the comparator devices, both intuitively and after reading the instructions. However, slightly lower percentages of participants in the study by Sandler et al. mastered handling of Spiromax than in our study; this might be related to the use of unlabeled Spiromax devices in that study, while both Turbuhaler and Easyhaler were labeled. All the devices used in our study were labeled and similar to those used in clinical practice in Sweden, which we hope ensures good applicability of our results to real patients.
Overall, the patterns of device-handling errors in this study were in line with those from the study by Sandler et al. [Citation13]. However, we were surprised by the large number of device-handling errors with Easyhaler in our study. It is difficult to know whether this truly reflects the usability of the Easyhaler device or the clarity of the instructions in Swedish. For example, some participants were observed carelessly shaking the Easyhaler device after reading the instructions, instead of vigorously shaking up and down 3–5 times, which could be attributed to the instructions not providing clear guidance. Furthermore, Easyhaler was the only device for which mastery of handling was higher in participants who had used one or both of the other devices, even when instructions were provided.
After preparing the dose, the inhalation maneuver is similar for all DPIs. Therefore, the fact that the same specific inhalation errors were most common with each device was to be expected. However, it was surprising that inhalation technique after reading the instructions was generally better with Spiromax than with the other devices. As with device handling, this could be related to the clarity of the instructions, but the influence of inhaler design cannot be ruled out. Mastery of inhalation technique was notably lower than in the previously mentioned study [Citation13], although insufficient inspiratory flow rate was the major error seen in both. This might reflect the inclusion of a higher percentage (35%) of participants aged >60 years. Across all devices after reading the instructions, we saw a tendency towards decreased mastery of inhalation technique among this age group vs younger participants, primarily due to apparently insufficient inspiratory efforts. There was also a trend for fewer participants aged >60 years to master handling of Easyhaler and Turbuhaler after reading instructions compared with younger participants in our study, and the only participant who did not master dose preparation with Spiromax was in the older age group.
Strengths and limitations of this study
It is not possible to conduct a placebo-controlled, blinded study to assess device handling, and an open-label study will always have the potential for bias. However, the randomized, sequential crossover design that we used is accepted as one of the best possible approaches for this type of study. There is also the potential for bias based in the study population; however, the inclusion of older adults and those without university education hopefully ensures good applicability to the population using the study devices in clinical practice. For this study type, the data are only as good as the questions, and our device-handling checklist was designed to encompass all expected and relevant handling errors based on the available information and our clinical experience. Errors were not pre-defined as critical vs non-critical. Although this could be considered as a limitation, all errors assessed in the study could result in a lower dose or no dose at all being delivered. There is also the potential for bias in the designation of what is and is not counted as an error; however, we minimized this by making a visual record (video) of participants using each device and having more than one experienced HCP complete the checklist for each volunteer. Unfortunately, some data had to be discounted during the analysis, as it became clear that some study participants had prior knowledge of inhalation devices or had been instructed incorrectly. In addition, some participants received a Turbuhaler device with a differently colored base to that stated in the instructions, resulting in a few participants taking some additional time to understand the text. This specific logistical error could have been avoided, but potentially biased data were excluded from the analysis as far as possible. Finally, the pre-specified statistical analysis that we used is standard best practice for this type of study; however, it was mathematically impossible to generate P-values for many of the planned comparisons using a frequentist approach, due to the fact that all study participants achieving mastery with Easyhaler also achieved mastery with Spiromax. Although not pre-specified, the use of an exact Bayesian approach with non-informative priors enabled a Bayesian confidence interval to be constructed and a P-value for the comparison to be calculated.
Implications for practice, policy, and future research
Device intuitiveness/ease of use, correct inhaler technique, patient preference and adherence are intertwined factors that all contribute to good symptom control in asthma and COPD. Therefore, when a first or different inhaler is needed, it is logical that selecting an intuitive, easy-to-handle device would be most likely to produce good inhaler technique and good control of symptoms. Comorbidities which could impact inhaler technique, such as arthritis and visual problems, should also be borne in mind when selecting a delivery device for inhaled medications. This is particularly pertinent in the elderly, who comprise a large proportion of the COPD patient population [Citation24]. However, it is also important to bear in mind that switching devices in patients with stable and controlled disease, particularly without patient consent and/or training, is associated with poor inhaler technique, poor patient compliance and a loss of symptom control [Citation25–Citation27].
This study highlighted several key device-handling errors that warrant special attention when explaining or demonstrating inhaler technique to patients. Before reading the instructions, the most common errors were not holding the device sufficiently upright (Spiromax), failure to shake the device as required (Easyhaler) and not twisting the base correctly (Turbuhaler). After reading instructions, very few errors were observed with Spiromax. Incorrect twisting of the base and not holding the device sufficiently upright both occurred in around 10% of participants with Turbuhaler. Several different errors were seen with Easyhaler after the instructions had been read, suggesting that careful training and frequent reviewing of patient technique are particularly important when this device is used. Our study also showed that not inhaling as fast as possible from the beginning was a frequent inhalation error, suggesting that this warrants special attention when discussing inhaler technique with patients using DPIs. Finally, we noted a trend for more participants of older age (>60 years) to demonstrate low inspiratory flow rates and to show device-handling errors compared with younger individuals, across all devices. These potential associations are highly speculative and require confirmation in further studies, but they suggest that targeted training and reviewing of inhaler technique may be beneficial for this age group. Furthermore, other patient groups with low inspiratory flow rates, including patients with severe asthma and COPD may also benefit from such targeted training and review of technique. Previously, in vitro comparisons showed DuoResp Spiromax to have greater consistency than Symbicort Turbuhaler in terms of fine particle dose (the amount of the aerosolized drug particles which have an aerodynamic diameter < 5 μm), over a range of inspiratory flow profiles, including low flows [Citation14].
Conclusions
This study highlights important differences between DPI devices in intuitiveness and ease of use. Handling (dose-preparation) errors and inhalation errors were least common with Spiromax and most common with Easyhaler, both before and after the instructions had been read. These data suggest that the need for reviewing patients’ device handling and inhalation technique varies between devices, and there could be implications for disease control. Therefore, intuitiveness and ease of use are important considerations when selecting a device for a patient.
Disclosure of interest
PR has given lectures at events sponsored by Teva Pharmaceuticals. BR has received remuneration for the audit of studies. GS is an employee of Teva Pharmaceuticals. SM has declared no conflicts of interest. LJ has attended advisory boards and given lectures at events sponsored by Teva Pharmaceuticals.
Supplemental Material
Download MS Word (89.1 KB)Acknowledgments
Medical writing support was provided by Jo Swainston, PhD, and Victoria A. Robb, PhD, of GeoMed, an Ashfield company, part of UDG Healthcare plc, and was funded by Teva Europe. Teva provided a full review of the article. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
Notes on contributors
Per Rönmark
Per Rönmark is a general practitioner at the Primary Health Care Center of Stadsfjärden, Praktikertjänst in Nyköping, Sweden. He is specialist in family medicine since 1994 and has a special interest in asthma and COPD in primary care.
Guilherme Safioti
Guilherme Safioti, MD, is a specialist in Respiratory Medicine, Global Medical Director for Connected Respiratory, Teva Pharmaceuticals, Amsterdam in the Netherlands. His research focus is inhalation therapy, digital interventions and predictive analytics in asthma and COPD.
Leif Bjermer
Leif Bjermer, MD, PhD, is a Professor in Pulmonary Medicine and Allergology at Skåne University Hospital, Lund University in Sweden. He is Head of Respiratory Medicine and Allergology, Department of Clinical Sciences in Lund. His primary research area is airway pathophysiology, including immunopharmacological research in patients with asthma and COPD. He runs a clinical trial unit mainly doing phase II and III studies in patients with asthma or COPD and is responsible for the regional competence centre for Asthma, Allergy and COPD.
References
- European Respiratory Society (ERS) White book. Chapter 1: the burden of lung disease. 2013:1–11. Available from: http://www.erswhitebook.org/chapters/the-burden-of-lung-disease/.
- Lötvall J, Ekerljung L, Rönmark EP, et al. West Sweden Asthma Study: prevalence trends over the last 18 years argues no recent increase in asthma. Respir Res. 2009;10:94.
- Lindberg A, Jonsson AC, Rönmark E, et al. Prevalence of chronic obstructive pulmonary disease according to BTS, ERS, GOLD and ATS criteria in relation to doctor’s diagnosis, symptoms, age, gender, and smoking habits. Respiration. 2005;72:471–479.
- Global Initiative For Asthma. Pocket guide for asthma management and prevention (for adults and children older than 5 years). A pocket guide for physicians and nurses. Updated 2015. Available from: http://www.ginasthma.org/wp-content/uploads/2016/01/GINA_Pocket_2015.pdf.
- Global Initiative for Asthma. Global strategy for asthma management and prevention. Updated 2017. Available from: http://www.ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention.
- Lavorini F, Magnan A, Dubus JC, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med. 2008;102:593–604.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49:1601794.
- Herland K, Akselsen JP, Skjønsberg OH, et al. How representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease? Respir Med. 2005;99:11–19.
- Bjermer L. The importance of continuity in inhaler device choice for asthma and chronic obstructive pulmonary disease. Respiration. 2014;88:346–352.
- Mäkelä MJ, Backer V, Hedegaard M, et al. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107:1481–1490.
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–938.
- Schulte M, Osseiran K, Betz R, et al. Handling of and preferences for available dry powder inhaler systems by patients with asthma and COPD. J Aerosol Med Pulm Drug Deliv. 2008;21:321–328.
- Sandler N, Holländer J, Långström D, et al. Evaluation of inhaler handling-errors, inhaler perception and preference with Spiromax, Easyhaler and Turbuhaler devices among healthy Finnish volunteers: a single site, single visit crossover study (Finhaler). BMJ Open Resp Res. 2016;3:e000119.
- Chrystyn H, Safioti G, Keegstra JR, et al. Effect of inhalation profile and throat geometry on predicted lung deposition of budesonide and formoterol (BF) in COPD: an in-vitro comparison of Spiromax with Turbuhaler. Int J Pharm. 2015;491:268–276.
- Cazzola M, Ora J, Di Paolo A, et al. Onset of action of budesonide/formoterol Spiromax® compared with budesonide/formoterol Turbuhaler® in patients with COPD. Pulm Pharmacol Ther. 2016;39:48–53.
- Meakin BJ, Cainey JM, Woodcock PM. Simulated ‘in-use’ and ‘mis-use’ aspects of the delivery of terbutaline sulphate from Bricanyl TurbohalerTM dry powder inhalers. Int J Pharmaceutics. EMJ Respir. 1995;119:103–108.
- Molimard M, Raherison C, Lignot S, et al. Assessment of handling of inhaler devices in real life: An observational study in 3811 patients in primary care. J Aerosol Med. 2003;16:249–254.
- Price D, Bosnic-Anticevich, Briggs A, et al. Inhaler competence in asthma: Common errors, barriers to use and recommended solutions. Resp Med. 2013;107:37–46.
- Basheti IA, Bosnic-Anticevich SZ, Armour CL, et al. Checklists for power inhaler technique: A review and recommendations. Respir Care. 2014;59:1140–1154.
- Mahon J, Fitzgerald A, Glanville J, et al. Misuse and/or treatment delivery failure of inhalers among patients with asthma or COPD: A review and recommendations for the conduct of future research. Respir Med. 2017;129:98–116.
- Dekhuijzen R, Wong G, Virchow JC, et al. Inhaler devices: the past, the present, and the future. EMJ Respir. 2015;3:49–54.
- Virchow JC, Rodriguez-Roisin R, Papi A, et al. A randomized, double-blinded, double-dummy efficacy and safety study of budesonide-formoterol Spiromax® compared to budesonide-formoterol Turbuhaler® in adults and adolescents with persistent asthma. BMC Pulm Med. 2016;16:42.
- Chrystyn H, Dekhuijzen R, Rand C, et al. Evaluation of inhaler technique mastery for budesonide formoterol Spiromax® compared with Symbicort Turbohaler® in adult patients with asthma primary results from the easy low instruction over time [ELIOT] study. Thorax. 2015;70(Suppl 3):A154–A155.
- Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23–30.
- Lavorini F, Braido F, Baiardini I, et al. SIAAC-SIMER. Asthma and COPD: interchangeable use of inhalers. A document of Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC) & Italian Society of Respiratory Medicine (SIMeR). Pulm Pharmacol Ther. 2015;34:25–30.
- Melani AS, Paleari D. Maintaining control of chronic obstructive airway disease: adherence to inhaled therapy and risks and benefits of switching devices. Copd. 2016;13:241–250.
- Ekberg-Jansson A, Svenningsson I, Rågdell P, et al. Budesonide inhaler device switch patterns in an asthma population in Swedish clinical practice (ASSURE). Int J Clin Pract. 2015;69:1171–1178.