ABSTRACT
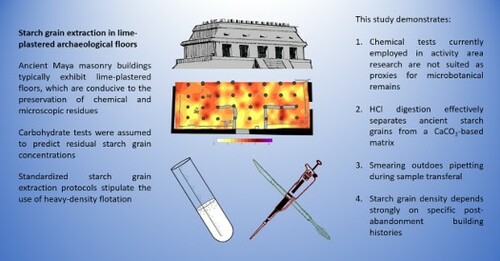
This paper focuses on the recovery of archaeological starch grains from building interiors at pre-Columbian Maya sites in southern Mexico. In an effort to render analytical protocols more effective, it examines the performance of chemical residue tests as prospective tools, proposes a customized extraction procedure for lime-plaster floors, and compares the efficacy of two mounting techniques. While the alleged predictive power of proxies like phosphate and carbohydrate tests could not be confirmed, the customized plaster processing protocol not only simplifies the extraction process but also results in the recovery of dense assemblages of individual starches as well as grain clusters. However, despite increases in protocol efficacy, architectural context and building history continue to be factors of utmost importance for microbotanical analyses.
GRAPHICAL ABSTRACT
1. Introduction
Frequent findings of large grinding stone slabs in outdoor patio areas (Pat Cruz Citation2006), the virtual absence of indoor fireplaces, as well as ethnographically observed patterns in contemporary communities (Rodríguez Canté Citation2017) support the notion that most food preparation among the ancient Maya was carried out in outdoor, open-air spaces. Interiors, nevertheless, also present potential for the recovery of diet-related archaeological data given their use for food consumption as evidenced, among others, by the reception scenes depicted on many polychrome ceramics (Reents-Budet Citation1994). Diehl (Citation2017) contends both food preparation and consumption activities regularly lead to minor spills or small debris being swept away and burned, therefore creating a random sample of continuous activity. In comparison to macrobotanical remains, microscopic residues escape cleaning efforts more easily and sink into porous occupation surfaces such as the typical lime-plastered floors found in the Maya Area. Regularly occurring spills can turn into in situ middens on a miniature scale, offering a palimpsest perspective on consumption patterns over a building’s occupation period.
The palimpsest character is an important aspect to consider when comparing occupation surfaces to artifacts employed in food processing or consumption. Food spills occur regularly over the course of thousands of meals consumed by the inhabitants of a given residential building. Although the ancient Maya generally repaired floors in building interiors over the years (Villaseñor Alonso Citation2009), microbotanical spill-assemblages represent rather long periods of time likely covering the totality of dishes included in the culinary repertoire of the respective settlement and time period. This situation is different for microbotanical assemblages recovered from artifact collections. Although we know the Maya used and still use their metates to grind more than just maize (Searcy Citation2011), we must acknowledge not all vegetable foodstuffs were ground. Flaked lithic tools can provide significant numbers of microbotanical residues (Morell-Hart, Joyce, and Henderson Citation2014; Devio Citation2016). The same seems to hold true for ceramics (Ezra, Acosta, and García Citation2015; Cagnato and Ponce Citation2017; Novelo-Pérez et al. Citation2019). However, not all plant foods need to be cut, nor are we certain about the array of ingredients prepared or served in a given ceramic vessel. Therefore, artifact collections present a lower degree of representability compared to occupation surfaces when it comes to dietary reconstruction. This does not mean, starch grain assemblages from this type of context are free of bias. Both taphonomic and cultural factors continue impacting the archaeobotanical record.
An awareness regarding formation processes is therefore imperative not only for the archaeological materials under study but also the employed proxies. Today, microbotanical research centers around three different indicators: pollen, phytoliths, and starch grains. Palynologic analyses are efficient at tracking grain-based agroecosystems, because Poaceae crops such as wheat (Triticum sp.), barley (Hordeum vulgare), rice (Oryza sp.), or maize (Zea mays) are anemophilous plants producing pollen on a massive scale. Under the appropriate conditions (e.g. water-logged deposits), archaeological strata preserve significant amounts of pollen. Tuber crops, however, create little to no pollen due to their focus on vegetative reproduction or cloning. Thus, even when maize and manioc (Manihot esculenta) fields were in close proximity (e.g. Joya del Ceren in the Maya Highlands, Sheets et al. Citation2012), the local pollen record would be strongly biased toward the Poaceae while the Euphorbiaceae might be close to absent.
While pollen is produced in flowers, and phytoliths are most abundant in stalks, leaves, and rind, starch grains themselves form the center of human attention as they represent the most important energy storage for plants. As a polymeric carbohydrate composed of large numbers of glucose rings, ingested starch fuels the metabolism of countless organisms including humans. In other words, even though pollen and phytolith more readily preserve due to the inertness of sporopollenin and the inorganic character of silicates, respectively, tissues containing one or the other are frequently discarded during the early steps of food production and preparation processes.
Starch deposits, on the other hand, occur principally in seeds, tubers, and rhizomes. They are therefore particularly well suited for tracing staples as they will certainly be present in consumption contexts. Aside from their advantages in terms of formation processes and depositional histories, starch grains also exhibit consistency in morphology across time and ecological setting. Although some morphotypes might only be identified to the genus or family level, often granules can be classified to the species or even subspecies level (Torrence Citation2006). In sum, a broad understanding of human-plant interactions in the past requires multi-proxy approaches and data integration (Morell-Hart Citation2019). Ancient starch grains, however, allow to address particular research questions surrounding plant-based caloric intake such as the specific composition of the C3 photosynthesis crop complex among the pre-Columbian Maya (Somerville, Fauvelle, and Froehle Citation2013).
2. Study sites and archaeological contexts
As a karst platform of Eocene origin, the Yucatan Peninsula offers an abundance of raw material for lime-based products. Early archaeological projects in the Maya Area recreated presumably pre-Columbian lime production techniques reported in the ethnohistorical and ethnographic records (Morris Citation1931). Seligson, Ortiz Ruiz, and Barba Pingarrón (Citation2019) perceive variation in production technologies and organizational schemes on the subregional level based on factors such as demand, fuel availability, and local social and economic trajectories. In recent years, the number of detected pre-Columbian lime kilns across the Maya Area has risen considerably (Seligson, Ortiz Ruiz, and Barba Pingarrón Citation2019) (). Although direct dating through archaeomagnetic techniques is still in its infancy and pilot studies have only confirmed the use of semi-enclosed features during the Terminal Classic (AD 900-1050) and Colonial periods (Goguitchaichvili et al. Citation2020), plastered floors have been observed as early as in Middle Preclassic (1000-400 BCE) contexts (Hammond and Gerhardt Citation1990). In addition to their architectural functions, these formal occupation surfaces provided the ancient Maya with more sanitary spaces in which to develop quotidian activities such as food consumption (Barba Pingarrón Citation2013).
Table 1. Study contexts by site and physiographic district.
In order to assess the impact of regional ecology, chronology, and site hierarchy on ancient Maya staple crop diversity as part of the author’s dissertation research (Zimmermann Citation2019), collections of plaster floor samples were gathered from a total of 19 units corresponding to nine structures, five pre-Columbian settlements, and three physiographic districts (). For this article, contextual description will be limited to differences in sample provenience, structure abandonment, and the temporal proximity between excavation and sample recovery. Lower densities of preserved starch grains were expected in samples retrieved from exterior vs. interior contexts due to increased risk of exposure to taphonomic agents. The opposite was assumed to occur when comparing recently excavated units to those whose floors had laid exposed for decades.
The first two collections belong to Chichen Itza. Structure 2D6 is a gallery-patio located in the northeastern corner of the Great Platform. Its colonnaded hall was excavated in 2009–2010 exhibiting an interior space which extends over roughly 330 m2. Based on ceramic and architectural data, Str. 2D6 was built as part of the last amplification of the Great Platform (Fernández Souza, Hernández Álvarez, and Zimmermann Citation2021). Structure 2D14, in turn, belongs to a series of features identified as part of previous construction phases. This second gallery-patio is located halfway between the Castillo’s eastern stairway and the entrance to the Thousand Columns Group, about 2 m below the current surface. Even though only its northern half was entirely excavated, Str. 2D14 must have been almost square extending over roughly 170 m2.
Materials were also collected during the excavations of Structure 5E-52 at Yaxuna, and Structures 2C3 and 2C6 at Kabah. All three cases represent vaulted range-type constructions with either partially or well-preserved plaster floors located in site centers and associated with the higher status segments of ancient Maya society (Stanton and Magnoni Citation2013; Novelo Rincón Citation2017). Structure CA-5 and CA-6 at Oxkintok correspond to a similar type of multi-chambered building. However, sampling at this site was more opportunistic. Both constructions had been extensively excavated during the 1980s (Vidal Lorenzo Citation1994). Given the high amount of corresponding floor intrusions, intact stretches of plaster floors were limited to two interior units of Str. CA-5, as well as two previously excavated spaces and one room with a remaining debris layer in Str. CA-6.
The Late Classic Chan Chi’ich architectural complex represents a special feature within Calakmul’s urban design. It is a residential compound very similar in style to the diagnostic domestic architecture at the Central-Mexican site of Teotihuacan (Manzanilla Naim Citation2017). The compound spans 3480 m2 and internal communication is guaranteed through several walkways and four shared patios. Samples originate from a set of features belonging to Patio B including three rooms, as well as a series of outside areas. Plaster floor samples representative of open-air spaces were also gathered from Yaxuna. After the excavation of its E-Group – a widely recognized architectural pattern for Preclassic Maya settlements with calendrical and ceremonial significance – Collins (Citation2018) reports eleven distinct floor levels for its plaza. Samples from plastered Floors 2 and 3 were analyzed for this study.
Most collections were recovered by staff members of the corresponding projects. The author designed and carried out sampling at the sites of Chichen Itza and Oxkintok (). Accordingly, control grids ranged from the customized 1.80 × 1.10 m intervals for the Str. 2D6 gallery at Chichen Itza to 0.50 × 0.50 m units at Kabah. Plaster floor samples were extracted with tools as simple as chisel and hammer. The diameter of perforations was kept at about 3 cm to refrain from inflicting excessive damage. If parts of the floor were cracked in the vicinity of a given extraction point, loose fragments were bagged in an effort to maintain solid expanses as intact as possible. The recovered samples weigh generally less than 10 g and provide enough sediment for dozens of tests, therefore eliminating the need for additional samples.
3. Methods
Due to the discrete distribution of domestic activities over interiors and exteriors, the recovery of invisible remains from archaeological floors and occupation surfaces benefits from prospective tools such as chemical residue analyses (CRA) which help visualize distribution patterns. This study examines the suitability of a series of (semi)quantitative, quick, and cost-efficient tests (Barba Citation2007) as indicators for concentrations of archaeological starch grains. After CRA-guided subsampling, an extraction protocol tailored specifically to the lime-plaster floors typical of ancient Maya masonry buildings will be outlined.
3.1. Chemical residue analyses
For this study, phosphate, fatty acid, protein, and carbohydrate tests were conducted as prospective tools for two principal reasons. First, they display the potential to be linked to organic products and foodstuffs in particular. Second, compared to pH or carbonate tests, results for these proxies are not significantly affected by lime plaster as the sample matrix. The use of phosphate concentrations as an indicator of soil organic matter was adopted by archaeologists during the 1940s (Eidt Citation1973). The three remaining analyses expand the level of detail regarding the type of organic matter indicated through the preceding phosphate analyses. Fatty acid tests are designed to trace remains of products such as oils and fats, while protein analyses target blood and meat, and carbohydrate exams can indicate the presence of sugary foods. The carbohydrate test is of particular interest to this study as Zimmermann and Matos Llanes (Citation2015) observe its potential in detecting elevated concentrations of preserved starches thereby offering a possible methodological bridge between chemical residue tests and ensuing microbotanical analyses.
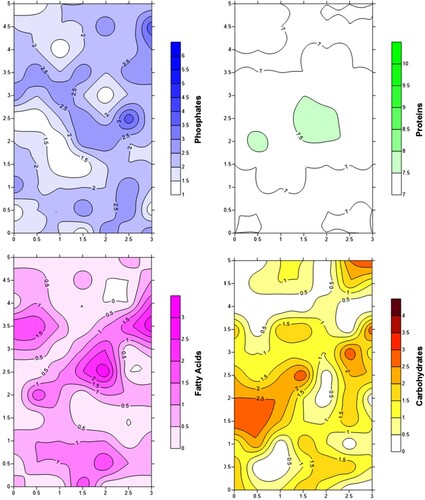
Phosphate analyses for the sites of Chichen Itza, Yaxuna, and Calakmul followed the spot test protocol developed by Barba, Rodríguez, and Córdoba (Citation1991). The Kabah and Oxkintok collections were tested with the hand-held colorimeter Checker HI 717 (HANNA Instruments, Woonsocket, RI). In both cases, the sediment sample is exposed to reagents that split the existing molecular bonds. The targeted phosphate molecule is then combined with a different compound whose linkage leads to a change in optical properties (i.e. blue coloration). In comparison to the visual evaluation of the spot test on a scale from 0 to 6, the colorimeter employs a light sensor and yields continuous quantitative data expressed in densities. Due to the lack of comparable instruments, fatty acid, protein, and carbohydrate analyses were all executed in accordance with the specifications set by Barba (Citation2007). All resulting data were plotted using Server 11 (Golden Software, Golden, CO) to visualize ancient activity areas related, among others, to food preparation and consumption (). Areas showing marked enrichments in plant organic matter – specifically those with high phosphate and carbohydrate concentrations – were then targeted for subsampling and extraction of starch grains.
3.2. Starch grain extraction
Standard extraction protocols for starch grains include the use of heavy density solutions like cesium chloride or sodium polytungstate (SPT) (Callahan Citation1987; Pearsall Citation2015). The goal is to pipette starch grains off when floating atop the supernatant of an immersed sediment sample. Fresh starch typically has a density of 1.5 g/ml and the specific gravity of SPT tends to be set to 1.7 g/ml (Coil et al. Citation2003). In this study, however, heavy-density solutions were not employed in an effort to reduce costs as well as the number of sample transferals. From a broader perspective, the protocol customization follows Coil et al.’s (Citation2003) recommendations on balancing three factors: safety, economics, and efficacy.
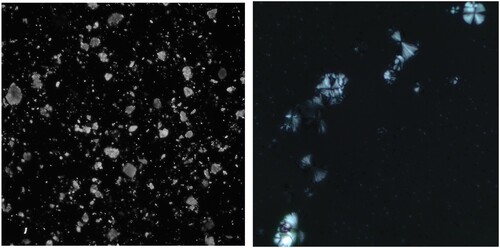
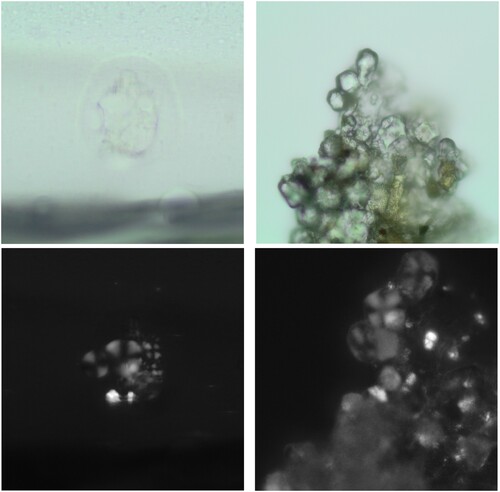
For this study the following extraction procedure was applied: First, small fragments of intact floor surface were ground. Approximately 0.25 ml of sediment were then transferred to 2 ml microvials where they were exposed to 10% hydrochloric acid (HCl). Previous experimentation with modern starch from different species had shown no significant morphometric differences between samples treated with diluted HCl and untreated preparations. The objective of the HCl digestion was to dissolve the calcium carbonate (CaCO3) crystals representing the matrix of lime-plastered floors. When present, these crystals pose a significant obstacle to the observation of starch grains as they appear as bright flecks under polarized light ((a)).
Given the amount of CaCO3 in plaster preparations, reactions are very violent at the start and even diluted acid must be added with care to prevent the sample from spilling. In most cases, bubbling did not stop before close to one hour had passed. Once this point was reached, the microvials were centrifuged for 10 min at 3500 r/min. The acid was then decanted, and the vials filled again with deionized water. The consolidated sediment was agitated to further dilute remaining amounts of acid before being centrifuged for another 10 min and decanted. The rinse with deionized water was then repeated twice more. After the last rinse, approximately 0.5 ml of solution was kept in the microvials. Despite the application of multiple rinses, HCl-based digestion saves several hours of processing time compared to the deflocculation of non-carbonate-based sediments.
Without the HCl digestion, samples might not only contain the irregularly shaped crystals shown in (a), but also the spherulites present in (b). Canti (Citation1997, 219) defines those as “crystal aggregations with an approximately circular outline and a permanent cross of extinction in crossed polarized light.” The major component of spherulites is CaCO3 and their fibrous nature can usually be observed under higher magnification (Canti Citation1997).
Once calcium crystals were removed, the sediment was prepared for microscopic observation. Two different techniques of transferal from vial to microscope slide were tested. For some collections, a micropipette and the corresponding disposable pipette tips were used. Here, the pipette was introduced into the extract, 20 μL of solution were sucked into the tip and released again into the remaining extract. This initial step was used to stir up the extract in order to obtain a homogenous solution with heavier particles in suspension. Two drops of the solution were then transferred to the microscope slides. For other collections, the tip of a disposable plastic toothpick was immersed in the extract and used to stir it. Once the sediment was in suspension, the toothpick was retrieved and the material on it was smeared onto the slide. For this procedure, the transferal process needed to be repeated several times before a reasonable amount of sediment was deposited. Independent of the transferal technique, two to three drops of a glycerol:water solution [1:1] were added as a mounting medium.
In order to reduce and control for contamination in the laboratory (Crowther et al. Citation2014), several measures were taken in regard to cleaning protocols, use of space, and consumables. All working surfaces were kept free from collection materials not immediately involved in processing and were regularly washed down with vinegar, a natural caustic agent. Paper towels, Calgon, and powdered gloves were eliminated from the lab. Powder-free gloves were only used for application of chemicals during sample processing, but not at the mounting stage. Microscope slides and cover slips were boiled for 30 min in vinegar. Similarly, the plastic toothpicks were set in hot vinegar before use. Air vents were turned off during mounting. Protocol cleanliness was repeatedly assessed by blank microscopic preparations in which only the mounting medium was deposited between slide and coverslip. Moreover, a proportion of 36% (118/332) of zero-count slides among the archaeological samples suggests that contamination potentials were controlled appropriately.
4. Results
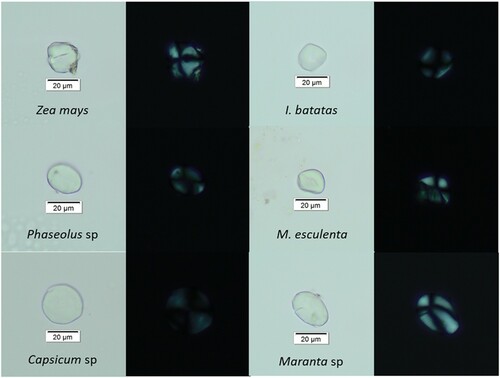
After initial CRA, 123 plaster samples were selected for microscopic analyses. The corresponding preparations yielded 1149 individual starch grain specimens. In addition, several samples produced one or more starch grain clusters in excess of 10 specimens each. Morphological analyses led to the identification of six recurrently present species or taxonomic groups including maize (Zea mays [n = 458/39.9%]), American beans (Phaseolus sp. [n = 100/8.7%]), chilies (Capsicum sp. [n = 174/15.1%]), sweet potato (Ipomoea batatas [n = 143/12.4%]), manioc (Manihot esculenta [n = 64/5.6%]), and arrowroot (Maranta arundinacea [n = 70/6.1%]) ().
4.1. Prospective potential of CRA proxies
The results of this study did not confirm the hypothesis of carbohydrate tests serving as a prospective tool for microbotanical extractions as suggested by Zimmermann and Matos Llanes (Citation2015). demonstrates that the average values for carbohydrate residues do not vary significantly by context. Floor 3 of the E-Group plaza at Yaxuna and the Chan Chi’ich residential compound at Calakmul represent exceptions which completely lacked high or intermediate carbohydrate enrichments (the corresponding scale ranges from 0 for absence to 4 for dense concentrations of residues (Barba Citation2007)).
Table 2. Total counts, starch grain density, and carbohydrate average by context.
Despite relatively homogeneous intermediate carbohydrate values among the remaining contexts, specimen density is highly variable. Structure 2C3 at Kabah, for example, presents an average carbohydrate value of 2 and at the same time the lowest specimen density, except for the third floor of Yaxuna’s E-Group plaza. To the contrary, specimen density is highest for Structure 2D14 at Chichen Itza, although its carbohydrate average is essentially identical to Kabah’s Structure 2C3. Given the ordinal character of the carbohydrate test scale, it is not feasible to further evaluate this relationship statistically.
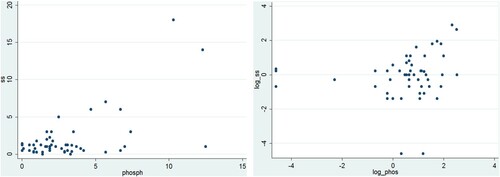
On the other hand, the application of a fully quantitative technique for the measurement of residual phosphate concentrations at Kabah and Oxkintok allows for the exploration of a possible correlation for this proxy. Phosphate data in parts per million (ppm) and starch grain density are available for a total of 53 samples. (a) shows a possible weak linear association between both variables. However, a subsequent Shapiro–Wilk test rejected the assumption of a normal distribution for either. To avoid the effects of skewed distributions, both variables were logarithmically transformed. However, the scatterplot of the resulting new variables does not maintain evidence for a linear relationship, with extreme outliers surrounding a central cloud of points along both axes ((b)). In conclusion, the data obtained in this study do not support the usage of either carbohydrate or phosphate residue tests as prospective tools in the detection of starch grain residues.
Figure 5. Scatterplots for (a) unmodified phosphate and density data, and (b) logarithmically transformed data.
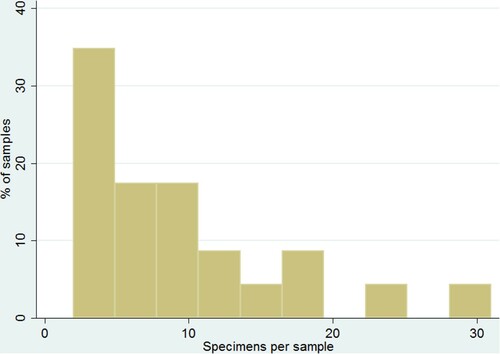
Although neither carbohydrate nor phosphate tests are efficient at predicting concentrations of starchy remains, it is worth discussing the potential of microbotanical assemblages themselves as indicators for the spatial distribution of activities. Both pollen (Hill and Hevly Citation1968) and phytolith data (González et al. Citation1993) have been used in the past by archaeologists to discern spatial patterns on occupational surfaces. I contend starch grain remains present a similar potential. presents the skewed, leptokurtic distribution of specimens recovered per sample from the context with the highest number of processed samples (n = 23): Str. 2D6 at Chichen Itza.
Although the selected elements only represent about 10% of the total number of floor samples recovered at Str. 2D6 and despite the biased character of the sampling procedure, the specifics of the skewedness predict the aspect of a distribution map based on starch grain counts for an entirely analyzed surface. Among the samples chosen for microbotanical analysis, more than 30% yielded either zero or very low counts of up to five specimens. 17 of 23 (74%) samples present counts of eleven or less specimens. The left-shifted weight means large expanses of a contour map would remain relatively void. The steady decline and long tail, in turn, imply the emergence of a relatively small number of distinctively enriched areas with only a few zones exhibiting peak concentrations.
In other words, if carried out for complete collections of surface sediments, starch grain analyses will in all likelihood provide useful data for the creation of distribution maps. Absolute counts as well as specimen averages per preparation could then be used in the reconstruction of food-related activities. Nevertheless, caution is warranted regarding the homogeneity of the surface under study. As discussed in Section 4.3 starch grain deposits might vary due to a series of taphonomic rather than functional factors. Hence, resulting counts should be scaled separately for interior vs. exterior areas, or recently excavated units vs. spaces which were intervened some time ago.
4.2. Specimen density and mounting techniques
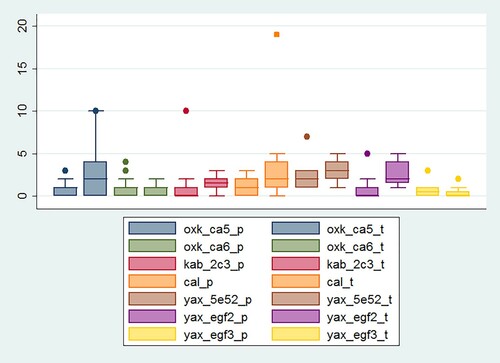
In order to employ starch grain data for spatial analyses, total counts must be high enough for significant variation to emerge within a given collection of samples. presents considerable differences in specimen counts per slide by context. Collections with high or intermediate density values allowed to reach the minimum total count of 50 grains per unit in an efficient manner. Others resulted in the scanning of large amounts of microscopic preparations and, therefore, an important investment in time. Moreover, the Floor 3 assemblage from the E-Group plaza at Yaxuna was not considered for microbotanical analysis due to its extremely low specimen density. Fortunately, technical experimentation over the course of this study brought to light another relevant factor in the pursuit of high specimen counts.
highlights the differences in specimen density when comparing the two extract transferal techniques described above. The chart captures data originating from all collections for which both techniques were employed. With the exceptions of Structure CA-6 from Oxkintok and Floor 3 of the E-Group plaza at Yaxuna, the box plot exhibits a strong tendency toward higher yields when plastic toothpicks rather than the micropipette were employed. The reasons for the notable difference between both techniques remain elusive. The higher surface-to-volume ratio inside a micropipette tip might result in a higher number of specimens sticking to the plastic instead of being flushed out and deposited on the microscope slide. The disposable toothpicks employed in this study lend some support to this hypothesis. Their white color led to the observation that some sediment stuck on the spades instead of being dropped onto the slides. Further testing and quantitative evaluation of processing techniques are necessary for protocols to become as efficient as possible.
4.3. Context-dependent variation in starch grain assemblages
Nonetheless, differences between processing techniques were not the only factors causing variations in starch grain density. Considering the impact of taphonomic processes on organic archaeological remains, it was expected exterior areas were less likely to yield larger concentrations of microbotanical remains compared to the interior spaces in large, vaulted structures. The data obtained throughout this study provide only inconclusive support for this assumption ().
The assemblages recovered from Yaxuna effectively show a higher specimen density corresponding to the interior of Str. 5E-52 (2.7 specimens/slide[s/s]) compared to the E-Group plaza floors. However, there also is a notable difference among both E-Group plaza floors: F2 = 1.9 s/s vs. F3 = 0.6 s/s. In comparison, within the residential compound Chan Chi’ich at Calakmul differences emerge at the unit level. Samples retrieved from Rooms 6 (1.6 s/s) and 7 (2.1 s/s) were considerably more productive than their counterparts from Room 5 (0.9 s/s) and an outside area (1.2 s/s). This set of cases demonstrates exterior areas consistently scoring below two specimens per slide. However, it also testifies to the fact that some interior spaces might yield densities of less than one specimen per slide.
One possible explanation for the low densities at Chan Chi’ich might be the lack of vaulted roofs atop the interior surfaces. Although the ancient inhabitants of this residential compound built perishable roofs protecting their living spaces from the weather, once abandoned, these readily decaying covers provided less shelter for organic remains left behind. This situation is expected to be different for buildings with vaulted roofs. Here, it is not uncommon for larger parts of the ceiling to collapse into the open spaces beneath, essentially burying past occupation surfaces under thick layers of rubble, therefore providing more protection from taphonomic agents (e.g. changing moisture levels, detritivore insects).
Exhibiting the lowest reported density values, the Oxkintok assemblages seem to work against this hypothesis. However, data from Structures CA-5 and CA-6 must be examined with extra caution as some plaster samples were recovered about 30 years after initial excavation. Room 1 of Str. CA-6 had been excavated and its vaulted roof was restored. Room 2C contained a debris layer approximately 0.30 m thick prior to excavation and sampling in 2017, while the floor of Room 2E had also already been excavated yet laid exposed to the elements without a restored roof. The latter situation also applied to the preserved stretches of floor in Rooms 1 and 4 of Str. CA-5. Withal, Room 4 is the only one exhibiting averages of more than one specimen per microscopic preparation.
The impact of exposure of organic remains, or lack thereof, is best exemplified by Structure 2D14 from Chichen Itza. It is the only building included in this study which was not part of architectonic vestiges observed on the latest or current surface level. In other words, Str. 2D14 lies below the Great North Platform of Chichen Itza in its latest configuration. The colonnaded hall was dismantled, leveled to a height of approximately 0.30 m, and then buried under 2 m of fill materials. Given the trends in density values emerging from this study, I strongly believe Str. 2D14 was by far the most productive context because all microscopic remains deposited over the course of its occupation were sealed and, therefore, protected from the taphonomic factors which affected the other locales and led to significant losses of starchy residues.
4.4. Starch grain clusters
Aside from yielding single-grain assemblages as dense as 50 specimens per microscopic preparation, the customized starch grain extraction protocol for lime-plastered floors also produced a considerable number of starch clusters. Conglomerations in excess of 10 specimens each in which single granules are fused and often overlap in a 2D field of vision were considered as such. So far, reports of such conglomerates of granules have largely been limited to the grey literature (Devio Citation2016; Morell-Hart et al. Citation2018). Pagán-Jiménez and Carlson (Citation2014) contribute a notable exception reporting on evidence for the South American snuff cojoba (Anadenanthera peregrina) at a pre-Columbian site in Puerto Rico.
In best-case scenarios, the presence of starch grain clusters might allow for the recognition of foodstuffs combined in a single meal, thereby elevating the precision in the reconstruction of ancient diets. In this study, however, chili starches are overrepresented as the constituting taxon of granule clusters ((a)). Out of the 34 clusters observed across all contexts, 16 are either exclusive or dominantly composed of chili starch grains. This represents a proportion of 39%, which is considerably higher than its single-grain frequency of 15%. Moreover, the only contexts containing non-Capsicum clusters are Structures 2D6 and 2D14 from Chichen Itza ((b)) and Structure 2C6 from Kabah. In other words, only highly productive collections yielded clusters formed by starch remains belonging to other species.
5. Conclusions
The experimentation with a customized protocol for processing samples from lime-plastered occupation surfaces has demonstrated its value to research on ancient starch grain assemblages. Despite the investment in time, techniques crafted specifically for the typical floors of the Maya Area help maintain data quality while the reduction in both processing steps and employed reagents during starch grain extraction accelerates turnover and reduces associated costs. Additionally, a comparison of pipetting and smearing procedures for sample mounting culminated in the latter being more reliable.
The acknowledgment of a lack of correlation between carbohydrate concentrations and starchy residues as proposed by Zimmermann and Matos Llanes (Citation2015) is less inspiring. Residual carbohydrate concentrations were assumed to predict the presence of ancient starch. The chemical test seems to pick up too many polysaccharide compounds (e.g. cellulose) to become an adequate prospective tool for this particular type of microbotanical proxy. The fully quantitative phosphate residue datasets mirrored this lack of correlation. Until a different, more accurate method is found, sampling strategies for microbotanical remains must be deployed following other principles or assumptions (D’Alpoim Guedes and Spengler Citation2014).
This study also provided new insights into the impact of taphonomic processes on the archaeobotanical record. Although it is possible to recover ancient starch grains in low concentrations from building exteriors and occupation surfaces which have laid exposed for several decades, it is much more likely to obtain robust datasets when samples are retrieved from interiors and directly during excavation. Buildings which did not decay over time after abandonment but were razed and covered succinctly as part of architectural modifications appear to hold especially high potential for the recovery of dense assemblages of starchy remains.
Acknowledgements
I acknowledge the Instituto Nacional de Antropología e Historia, Mexico, for permissions to carry out chemical residue and microbotanical research as part of the larger archaeological projects conducted at the study sites. I also want to specifically thank the corresponding project directors for allowing me to contribute to their research: Rafael Cobos Palma, Travis W. Stanton, Aline Magnoni, Traci Ardren, Luis R. Pantoja Díaz, Ma. Lourdes Toscano Hernández, and Ramón Carrasco Vargas. Similarly, I extend my gratitude toward Lilia Fernández Souza and Héctor A. Hernández Álvarez who manage the Archaeology Laboratory at UADY and provided significant aid during chemical residue analysis and microbotanical extraction. I also acknowledge my dissertation committee members, Erin K. Thornton, Shannon Tushingham, and Jade D’Alpoim Guedes, for their guidance. Special thanks are directed to my advisor, the late Dr. Steven A. Weber, for his knowledge, patience, and mentoring. This manuscript has strongly benefitted from the insights and constructive criticisms provided by Cassady Fairlane, Moisés Herrera Parra, Jessica Barrios, and María J. Novelo Pérez on previous versions. Finally, I want to acknowledge the stimulating and forthcoming comments made by three anonymous reviewers.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Mario Zimmermann
Mario Zimmermann is a postdoctoral research associate at Washington State University. In my current position, I design and oversee metabolomics-based studies in the WSU Gang Lab and TARL. Our joint-labs’ research focuses on the recovery of alkaloid residues from archaeological artifacts and human remains. We are particularly interested in smoking practices among Native American communities, the spread of caffeinated beverages in the Americas, and the arrival of Old World drugs as part of the Columbian exchange. My doctoral dissertation, conducted in consultation with Dr. Steven A. Weber, examines staple crop diversity in pre-Columbian Maya cities as an expression of ecological adaptation and food security. I developed methodological approaches and employed analytical tools suitable for answering subsistence-related questions based on microbotanical data. The results of my work provide a basis for outreach efforts beyond the academic sphere to include present-day farmers, children, and policy makers.
References
- Barba, L. 2007. “Chemical Residues in Lime-Plastered Archaeological Floors.” Geoarchaeology 22 (4): 439–452. doi:https://doi.org/10.1002/gea.20160.
- Barba, L., R. Rodríguez, and J. Córdoba. 1991. Manual de Técnicas Microquímicas de Campo Para La Arqueología. Mexico City: IIA UNAM.
- Barba Pingarrón, L. 2013. “El Uso de La Cal En El Mundo Prehispánico Mesoamericano.” In La Cal: Historia, Propiedades y Usos, edited by L. Barba Pingarrón and I. Villaseñor, 19–46. Mexico City: UNAM.
- Cagnato, C., and J. M. Ponce. 2017. “Ancient Maya Manioc (Manihot Esculenta Crantz) Consumption: Starch Grain Evidence from Late to Terminal Classic (8th–9th Century CE) Occupation at La Corona, Northwestern Petén, Guatemala.” Journal of Archaeological Science: Reports 16 (May): 276–286. doi:https://doi.org/10.1016/j.jasrep.2017.09.035.
- Callahan, J. 1987. “A Nontoxic Heavy Liquid and Inexpensive Filters for Separation of Mineral Grains.” Journal of Sedimentary Research 57 (4): 765–766.
- Canti, M. G. 1997. “An Investigation of Microscopic Calcareous Spherulites from Herbivore Dung.” Journal of Archaeological Science 24: 219–231. doi:https://doi.org/10.1006/jasc.1996.0105.
- Coil, J., M. A. Korstanje, S. Archer, and C. A. Hastorf. 2003. “Laboratory Goals and Considerations for Multiple Microfossil Extraction in Archaeology.” Journal of Archaeological Science 30 (8): 991–1008. doi:https://doi.org/10.1016/S0305-4403(02)00285-6.
- Collins, R. 2018. From Sedentism to Sprawl: Early Urban Process at Yaxuná. Yucatan, Mexico 1000 BCE to 250 CE: Brandeis University.
- Crowther, A., M. Haslam, N. Oakden, D. Walde, and J. Mercader. 2014. “Documenting Contamination in Ancient Starch Laboratories.” Journal of Archaeological Science 49: 90–104.
- D’Alpoim Guedes, J., and R. Spengler. 2014. “Sampling Strategies in Paleoethnobotanical Analysis.” In Method and Theory in Paleoethnobotany, edited by J. M. Marston, J. D’Alpoim Guedes, and C. Warinner, 77–94. Boulder: University of Colorado Press.
- Devio, J. 2016. Reconstructing Late Classic Food Preparation at Xunantunich. Belize: Using Starch Grain Analysis, The University of Texas at San Antonio.
- Diehl, M. W. 2017. “Adequacy and Ubiquity An Example from the American Southwest.” Advances in Archaeological Practice 5 (2): 196–205. doi:https://doi.org/10.1017/aap.2017.5.
- Eidt, R. C. 1973. “A Rapid Chemical Field Test for Archaeological Site Surveying.” American Antiquity 38 (2): 206–210.
- Ezra, J., G. Acosta, and V. H. García. 2015. “Análisis de Los Granos de Almidón Extraídos de Metates y Vasijas de Xochicalco.” Rev. Investig. Arqueométricas 2 (2): 1–9.
- Fernández Souza, L., H. A. Hernández Álvarez, and M. Zimmermann. 2021. “The Construction of Masculinities at Chichén Itzá: A Functional Interpretation of Structure 2D6.” Anc. Mesoamerica.
- Goguitchaichvili, A., S. Ortiz-Ruiz, J. Morales, V. A. Kravchinsky, O. de Lucio, R. Cejudo, R. Garcia, E. Uc González, J. L. Ruvalcaba, and L. Barba Pingarrón. 2020. “Pyrotechnological Knowledge in the Pre-Hispanic Maya Society: Magnetic and Infrared Spectrometry Surveys of Limekilns in the Western Yucatan Peninsula (Mexico).” Journal of Archaeological Science: Reports 33 (June), doi:https://doi.org/10.1016/j.jasrep.2020.102457.
- González, J., E. Ibarra Morales, J. Zurita Noguera, McClung de Tapia, E. Tapia Recillas, and H. Macrofósiles Botánicos. 1993. “Macrofósiles Botánicos, Fitolitos y Polen.” In Anatomía de un conjunto residencial teotihuacano en Oztoyahualco, edited by L. R. Manzanilla Naim, 661–728. Mexico City: UNAM.
- Hammond, N., and J. C. Gerhardt. 1990. “Early Maya Architectural Innovation at Cuello, Belize.” World Archaeology 21: 461–481.
- Hill, J. N., and R. H. Hevly. 1968. “Pollen at Broken K Pueblo: Some New Interpretations.” American Antiquity 33 (2): 200–210.
- Manzanilla Naim, L. R. 2017. Teotihuacán, Ciudad Excepcional de Mesoamérica. Mexico City: El Colegio Nacional.
- Morell-Hart, S. 2019. “Techniques for Integrating Macrobotanical and Microbotanical Datasets: Examples from Pre-Hispanic Northwestern Honduras.” Journal of Field Archaeology 44 (4): 234–249. doi:https://doi.org/10.1080/00934690.2019.1591917.
- Morell-Hart, S., H. Dine, S. Watson, and S. Teesdale. 2018. Análisis Paleoetnobotánicos de Macabilero y Piedras Negras (2016–2018).
- Morell-Hart, S., R. A. Joyce, and J. S. Henderson. 2014. “Multi-Proxy Analysis of Plant Use at Formative Period Los Naranjos, Honduras.” Latin American Antiquity 25 (1): 65–81.
- Morris, E. H. 1931. The Temple of the Warriors. New York: Charles Scribner’s Sons.
- Novelo-Pérez, M. J., E. M. Herrera-Parra, L. Fernández-Souza, I. Ancona-Aragón, and S. Jiménez-Álvarez. 2019. “Pre-Columbian Culinary Landscapes: Reconstructing Elite Gastronomy at Sihó, Yucatán.” STAR: Science & Technology of Archaeological Research 5 (2): 85–97. doi:https://doi.org/10.1080/20548923.2019.1674508.
- Novelo Rincón, G. 2017. “Investigación y Restauración Arquitectónicas En El Codz Pop de Kabah, Yucatán.” In Recent Investigations in the Puuc Region of Yucatán, edited by M. Rubenstein, 71–80. Oxford: Archaeopress.
- Pagán-Jiménez, J. R., and L. A. Carlson. 2014. “Recent Archaeobotanical Findings of the Hallucinogenic Snuff Cojoba (Anadenanthera Peregrina (L.) SPEG.) in Precolonial Puerto Rico.” Latin American Antiquity 25 (1): 101–116.
- Pat Cruz, E. D. 2006. Análisis de Las Piedras de Molienda de Sihó. Mérida: Universidad Autonoma de Yucatan.
- Pearsall, D. M. 2015. Paleoethnobotany: A Handbook of Procedures. 3rd ed. London: Routledge.
- Reents-Budet, D. 1994. Painting the Maya Universe: Royal Ceramics of the Classic Period. Durham: Duke University Press.
- Rodríguez Canté, B. D. 2017. Identificación de Áreas de Actividad En Grupos Domésticos de Sihó: Un Enfoque Arqueológico. Mérida: Universidad Autónoma de Yucatán.
- Searcy, M. T. 2011. The Life-Giving Stone: Ethnoarchaeology of Maya Metates. Tucson: University of Arizona Press.
- Seligson, K. E., S. Ortiz Ruiz, and L. Barba Pingarrón. 2019. “Prehispanic Maya Burnt Lime Industries: Previous Studies and Future Directions.” Ancient Mesoamerica 30 (2): 199–219. doi:https://doi.org/10.1017/S0956536117000347.
- Sheets, P., D. L. Lentz, D. R. Piperno, J. G. Jones, C. Dixon, G. Maloof, and A. Hood. 2012. “Ancient Manioc Agriculture South of the Ceren Village, El Salvador.” Lat. Am. Antiq. 75 (1): 61–80.
- Somerville, A. D., M. Fauvelle, and A. W. Froehle. 2013. “Applying New Approaches to Modeling Diet and Status : Isotopic Evidence for Commoner Resiliency and Elite Variability in the Classic Maya Lowlands.” Journal of Archaeological Science 40: 1539–1553.
- Stanton, T., and A. Magnoni. 2013. Informe Global: Proyecto de Interacción Política Del Centro de Yucatán: Temporadas 2007, 2008, 2009 y 2011. Mexico City: Instituto Nacional de Antropología e Historia.
- Torrence, R. 2006. “Description, Classification, and Identification.” In Ancient Starch Research, edited by H. Barton and R. Torrence, 115–143. Walnut Creek: Left Coast Press.
- Vidal Lorenzo, M. C. 1994. El Grupo Ah Canul de La Ciudad Maya Yucateca de Oxkintok. Madrid: Universidad Complutense de Madrid.
- Villaseñor Alonso, M. I. 2009. Lowland Maya Lime Plaster Technology: A Diachronic Approach. London: University College London.
- Zimmermann, M. 2019. Subsistence of Pre-Columbian Maya Urban Communities: A Microscopic View on Alternative Staples. Pullman: Washington State University.
- Zimmermann, M., and C. Matos Llanes. 2015. “La Prueba de Carbohidratos Como Herramienta Prospectiva Para La Paleoetnobotánica.” Rev. Investig. Arqueométricas 2 (2): 1–13.