ABSTRACT
Introduction: Alpha lipoic acid (ALA) is an antioxidant used in the treatment of neuro-inflammation, diabetes and diabetic nephropathy. The current study aiming to gauge the effect of oral ALA on diabetic peripheral neuropathy, glycemic control, LDL-C, and HDL-C. Methods: This is a prospective, interventional study carried out on patients with type 2 diabetes mellitus (DM) who were following at the outpatient internal medicine & diabetes clinics at Benha University Hospital. Treatment with ALA for 3 months was given to patient with diabetic peripheral neuropathy. Data in the form of age, sex, body mass index (BMI), duration & treatment of DM, manifestations of peripheral neuropathy were collected. LDL-C, HDL-C, HbA1c, TSH, ALT, AST were measured before and after intervention. Peripheral neuropathy symptoms, nerve conduction velocities, cardiovascular (CV) tests of autonomic neuropathy, and cross-section area of the posterior tibial nerve were performed before and after treatment intervention. Results: 90 adult diabetic patients were recruited in the study, 42.2% were females and 57.8% were males with a median age of 50–60.3 years (IQR = 52). A statistically significant improvements of neuropathic symptoms, nerve conduction velocity, and cardiovascular autonomic neuropathy were noted after 3 months of administration of ALA (p ˂0.001). However, the cross-section area of the posterior tibial nerve at baseline and after treatment did not change significantly (p value of 0.84). There was a significant improvement in the BMI, HDL-C, LDL-C, HbA1c (p ˂ 0.001). Conclusion: Oral treatment with ALA might cause ameliorations of peripheral neuropathy, HbA1c, and LDL-C & HDL-C levels in diabetic patients. Our result failed to proof effect of ALA on nerve cross-section area. The global data encourage further studies with this medication as an ancillary treatment of DM2.
Clinical trial registration: It was registered in clinical trial website; ClinicalTrials.gov Identifier (NCT number): NCT04322240.
1. Introduction
Diabetes mellitus (DM) is estimated as a considerable health issue as a result of its great occurrence [Citation1]. Peripheral neuropathy is a widespread problem in diabetic population, with a prevalence rate of 5.3–47.6% [Citation2,Citation3]. Alpha lipoic acid (ALA), an organo-sulfur compound derived from octanoic acid, was studied as an antioxidant agent in the treatment of obstructive nephropathy, neuro-inflammation, diabetes and diabetic nephropathy [Citation4]. Clinical trials have shown hopeful outcomes of ALA on neuropathy manifestations [Citation5] with significantly improved nerve conduction velocity [Citation6]. Moreover, some studies found a favorable results of ALA supplementation on lipid profile [Citation7]. However, other studies did not recognize any considerable relations [Citation8]. Furthermore, parenteral treatment with ALA causes improvement in the glucose levels in patients with type 2 DM [Citation9]. The aim of the current study was to gauge the effect of oral administration of ALA on diabetic peripheral neuropathy, glycemic control, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C).
2. Subject and method
This is a prospective, interventional study on patients with type 2 DM who were following up at the outpatient internal medicine & diabetes clinics in Benha University Hospitals for 3 months. The following criteria were included; [1] patient’s consent to participate; [2] patient diagnosed as diabetic peripheral neuropathy and ≥ one classic painful neuropathic symptom as burning, paresthesia, shooting pain, muscle cramps or allodynia, in the feet, for ˃6 months, that affect the daily life or sleep; and [3] patients were not allowed to stop ALA during the follow up period. The following criteria were excluded: [1] peripheral neuropathy as a result of chronic liver diseases, chronic alcohol abuse, vitamin B12 deficiency, drug induced neuropathy, hypothyroidism, truncal neuropathy or severe neurological diseases; [2] severe renal impairment with an estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2 according to the Modification of Diet in Renal Disease formula (MDRD) [Citation10]; [3] recent treatment for cancer; [4] peripheral vascular disease identified by absent peripheral pulse in feet &/or intermittent claudication; [5] medication used in the last 3 months that may affect our results, such as medications used for treatment of diabetic peripheral neuropathy (DPN), vitamin B complexes, antioxidants, or opiates; [6] pregnant or lactating patients, or female patients without proper contraception method. Participants were prescribed 600 mg of oral ALA once daily before meal, for 3 months, and were advised not to discontinue anti-diabetic drugs, medications used for managing arterial hypertension, and dyslipidemia medications during the study.
2.1. Data collection and follow-up
Two visits were scheduled for data collection, physical examination and laboratory testing of the patients: the first prior to initiation of ALA administration (baseline visit) and the second at the end of the third month following initiation of ALA (2nd visit). Participants were exposed to careful history and clinical examination with special stress on: Age, Gender, Duration of DM; Type of the treatment; manifestations of peripheral neuropathy (sensory & motor); CVS manifestation of autonomic neuropathy; Other manifestations of autonomic neuropathy; BMI.
2.2. Laboratory assay
Blood samples from the patients were withdrawn after overnight fasting before and after intervention. Serum creatinine; hemoglobin A1c (HbA1c); HDL-C; LDL-C; thyroid stimulating hormone (TSH); alanine aminotransferase (ALT), and Aspartate Aminotransferase (AST) were assayed by routine biochemical methods.
2.3. Assessment of peripheral neuropathy
DPN status was evaluated using symptoms of peripheral neuropathy, monofilament test, vibration perception threshold (VPT), ankle reflexes, and nerve conduction studies. NCV (nerve conduction velocity) were carried out using the Neurowerk, EMG (electromyography) (sigma, Germany) machine for both lower limbs. Motor nerve conduction parameters including Compound Muscle Action Potential (CMAP) amplitudes, distal latency and motor conduction velocity was measured in common peroneal and posterior tibial nerves. Sensory conduction studies included measurement of peak latency and amplitude of SNAPs (sensory nerve action potentials). To ensure adequate uniformity during the procedure, we kept the stimulating and recording parameters constant in all subjects. At the end of the twelfth week, an increase of amplitude of ≥1 mv, latency ≥1 m/s, and conduction velocity ≥10 m/s from baseline were considered an improvement in NCV in motor nerve. While, for sensory nerves; improvement of amplitude ≥5 µV, latency ≥1 m/s, and conduction velocity ≥10 m/s [Citation11]. Tests for cardiac parasympathetic neuropathy including Valsalva ratio, and HR (Heart rate) variation during deep breathing. Valsalva ratio of >1.21, and HR response during deep breathing of >15 beats/min were criteria of improvements. Tests for cardiac sympathetic neuropathy including BP response to sudden standing, and BP response to sustained handgrip. BP response to sudden standing of ˂ 10 mm Hg and after exercise hand grip of >16 mmHg were criteria of improvements. Ultrasound on nervewas done by measuring the cross-sectional area of the nerve as assessment of improvement of DPN before& after use of ALA. The patients were examined in supine position, and the foot was bolstered with a pillow to show the lower leg and foot. The transducer was placed over posterior tibial nerve in both transverse (short axis) and in longitudinal (long axis) views. The 5.0–12.0-MHz multifrequency linear array probe was used for posterior tibial nerve scanning. Logiq P9 ultrasound scanner (General Electric, USA) was used. The study was approved by the Ethics Committee of Benha Faculty of Medicine, Benha University with written informed consent was obtained from all participants. It was registered in clinical trial website; ClinicalTrials.gov Identifier (NCT number): NCT04322240.
2.4. Calculation of sample size
Version 16.1 of MedCalc software (© 1993–2016 MedCalc Software) was used to calculate the required sample size using the average percentage of improvement (35%) in neuropathic symptoms according to Agathos et al. [Citation12].The following variables were entered; Level of significance (type I error) = 0.05, Type II error (1-level of power) = 0.2, Average % of neuropathic improvement = 35%, Null hypothesis percentage = 50%. So, the least sample size = 85 diabetic patients. It was increased to 100 patients to safeguard against drop out during follow up of the study. Ninety cases have completed the study. Ten dropouts were excluded. The data were analyzed using SPSS version 16 software (Spss Inc, Chicago, ILL Company). Categorical data were presented as number and percentages, Mc-Nemar’s test was used for analysis paired proportions. Shapiro-Wilks test were performed for quantitative data assuming normality at (P > 0.05). Normally distributed variables were presented as (mean ± standard deviation) and analyzed by paired “t” test for paired samples. Non-parametric data were expressed as median and inter-quartile range (IQR) and tested by Wilcoxon test considering a significant P value ≤ 0. 05.
3. Results
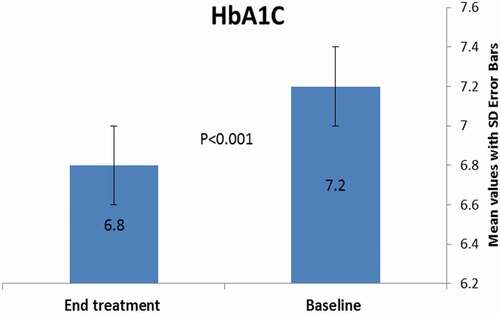
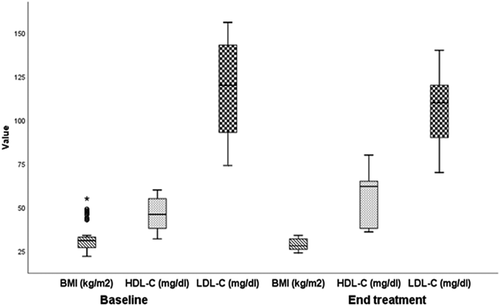
This is a prospective interventional study, conducted at Benha University Hospital, Egypt, between March 2020 and December 2020. The study included 90 type 2 adult diabetic patients. The demographic and laboratory findings of the studied patients at baseline were demonstrated in .The results showed no statistically significant association (P > 0.05) among age, sex, type of treatment, duration of DM, and age of onset of diabetes before and after the intervention. In terms of diabetic peripheral neuropathy parameters, neuropathic symptoms were improved significantly at the end of the follow up (p ˂0.001; ). Twenty out of 74 patients showed improvement of the monofilament test. There was a significant improvement of nerve conduction velocity (p ˂0.001) after intervention treatment by ALA. Additionally, there was a significant improvement in the cardiovascular autonomic neuropathy. However, no significant change in the cross-section area of the posterior tibial nerve at the end of treatment (p value of 0.84) (). Regarding the metabolic parameters, there were a non-significant difference among TSH, ALT, AST levels (). However, HbA1c improved significantly after the intervention (mean± SD; 6. 8 ± 0. 45%) compared to the baseline (mean± SD; 7. 2 ± 0. 54%) (& ). Additionally, there was a significant improvement in the BMI, HDL-C, LDL-C (p value ˂ 0.001) (& ).
Table 1. Baseline clinical data of the studied type 2 diabetic patients
Table 2. Comparison between neuropathy parameters before and after alpha lipoic acid treatment intervention in patients with T2 diabetes mellitus
Table 3. Comparison between different metabolic parameters before and after alpha lipoic acid treatment intervention in patients with T2 diabetes mellitus
4. Discussion
Diabetic neuropathy (DN) is the most widespread sequalae of diabetes. Pathogenesis of DN is linked to chronic hyperglycemia, either by accumulation of free radicals or advanced glycation end products (AGE), which trigger inflammatory cascades causing cell damage and cell death [Citation1]. The purpose of the current study was to investigate prospectively the effect of oral ALA on diabetic peripheral neuropathy, glycemic control, and lipid profiles. Our study, concluded that there were a significant improvements of diabetic peripheral neuropathy manifestations, nerve conduction velocity, and cardiovascular autonomic tests after 3 months of intervention. ZIEGLER et al. found similar results, that is to say, a 600 mg/d orally was found to provide the optimal risk-benefit ratio [Citation13]. Additionally, significant reductions in neuropathic symptoms were shown at a dose of 600 mg/d of ALA at day 40 versus baseline in another study [Citation12]. Mijnhout et al. in a systemic review, concluded that the intravenous daily dose of 600 mg of ALA for 3 weeks, led to a significant improvement in the neuropathic pain [Citation14]. However, he found that the improvement of the clinical symptoms noted after 3–5 weeks of oral ALA in a dose of 600 mg/day, was unclear [Citation15]. It was revealed that, intravenous administration of ALA in patient with diabetic polyneuropathy yielded a quick result on microcirculation [Citation16]. It was recognized that, increased vascular oxidative stress seen in diabetics, resulted in impairment of nitric oxide-mediated vasodilation. At this time, intravenous administration of ALA enhanced nitric oxide–mediated endothelium vasodilation [Citation17]. The rationale for improving diabetic neuropathy symptoms, following treatment with ALA, is mostly due to its antioxidant action. ALA, and its reduced form (dihydrolipoic acid) act as antioxidants by neutralization of reactive oxygen species, inhibition of reactive-oxygen generators, and restoration of damage caused by other oxidants [Citation12]. Furthermore, studies support that ALA increases glutathione levels, and hampers lipid peroxidation [Citation17] & [Citation18]. In our results, the body mass index decreased significantly at the end of the third month. These findings are consistent with a previous study which showed that ALA produced a reduction in BMI, and fasting blood sugar levels in patients suffering from chronic spinal cord injury after administration of 600 mg of alpha lipoic acid daily [Citation19]. In another study, a dose of 1800 mg daily produced a moderate weight loss in obese patients [Citation20]. Li et al. disclosed that an oral dose of 1200 mg daily of ALA for 8 weeks induced mild weight loss [Citation21].Udupa et al. found that, a dose of 300 mg of alpha lipoic acid resulted in a significant reduction in the BMI in type 2 DM after 90 days of the intervention [Citation22]. Further studies will be required to gauge the dose of alpha-lipoic acid that can cause weight loss, and the long-term safety of this agent. The current study revealed that HbA1c decreased significantly at the end of the treatment. In line with our result, one study concluded that, ALA improved peripheral insulin sensitivity in type 2 diabetes mellitus [Citation9]. One meta-analysis showed a significant lower levels of serum glucose after ALA supplementation in patients with stroke [Citation23]. The beneficial role of alpha lipoic acid in lowering the fasting plasma glucose may be connected to its effect in modulating adenosine monophosphate-activated protein kinase (AMPK) [Citation24] in skeletal muscle and beta-cells [Citation25]&[Citation26], which subsequently potentiates the insulin-secretory response of β cells to glucose [Citation27]. Consistent with previous studies, it was declared that ALA can decrease FBS and HbA1C level, possibly by increasing Glucose transporter type 4 (GLUT-4) transportation to fat and muscle cell membranes [Citation19], and increasing the skeletal muscle glucose transport activity [Citation28]. In addition, ALA appears to suppress gluconeogenesis in the liver [Citation29]. It is stated that ALA augments the activity of some proteins of the insulin signaling pathway such as insulin receptor (IR), insulin receptor substrate 1 (IRS1), protein kinase B (AKT), and phosphatidylinositide 3-kinase (PI3K) [Citation30]. According to this, ALA is considered an insulin-mimetic agent [Citation31]. In the current study, HDL-C & LDL-C improved significantly at the end of follow up. One study found, a significant decrease in total cholesterol, and LDL-C and higher HDL-C following 12 weeks of ALA [Citation32]. One meta-analysis concluded that ALA supplementation might be beneficial in lowering total cholesterol levels in subjects with stroke [Citation22]. In contrast, another study did not detect any change in lipid profile following intake of 600 mg ALA/day for 8 weeks in subjects with ESRD [Citation33]. In addition, another study, did not reveal a significant improvement of serum total cholesterol, triglyceride, HDL, and LDL after alpha lipoic acid [Citation34]. In our study, we show that the improvement of LDL-C& HDL-C might be related to the improvement of BMI and glycemic control. Different study design, sample size, dosages of ALA, and the participants characteristics might clarify the inconsistencies among studies. The probable lipid-lowering effects of alpha lipoic acid; firstly, the beneficial effects of ALA on β oxidation of fatty acids in the mitochondria via activation of AMP-activated protein kinase [Citation35]. Secondly, it might be related to the role of ALA in lowering blood glucose levels [Citation36]. Thirdly, ALA administration might decrease the expression of acetyl-CoA carboxylase and fatty acid synthase (enzymes in fatty acid synthesis) [Citation37]. Other possible mechanisms of lowering TC or LDL after administration of ALA include: (i) augmented activity of lipoprotein lipase, (ii) increased synthesis of LDL receptors in the liver which transfer cholesterol to the hepatic system [Citation38], (iii) increase plasma adiponectin levels which enhanced FFAs β-oxidation [Citation39]. We did not report any significant reduction in the cross-section area of the posterior tibial nerve after the intervention. Singh et al. found that, morphological changes of the tibial nerves in diabetic subjects can be detected by ultrasonography, even before the clinical onset of peripheral neuropathy [Citation40].Watanabe and colleagues, indicated the possibility of using US for the diagnosis of DPN with sensitivity of 80% and specificity of 94% [Citation41]. A study performed by Riazi et al. detected lower sensitivity and specificity (69 and 77%, respectively) [Citation42]. To our knowledge, there are little studies comparing the effect of ALA on the cross-sectional area of the nerve. It may need longer duration and larger numbers of participants to assess the cross-section area in a follow up prospective study after treatment intervention with alpha lipoic acid.
5. Conclusion
Oral supplements of ALA improved peripheral neuropathy, glycemic control, and LDL-C & HDL-C levels in diabetic patients. However, there were conflicting studies about the suitable dose, duration and route of administration of ALA. Our results fail to prove the effect of ALA on the nerve cross-section area.
6. Limitations of the study
Firstly, it was unclear whether the improvements of neuropathy and lipid parameters is related to the drug ALA or it is a sequalae of improvement of glycemic control. Secondly, serum levels of vitamin B12 should be measured to exclude the true cases of vitamin B 12 deficiency. Thirdly, the degree of improvements of peripheral neuropathy symptoms need to be evaluated according to a severity score such as Neuropathy Symptoms Score (NSS). Fourthly, additional research, placebo-controlled trials with a multivariate regression analysis are needed, with longer duration, different doses of ALA to judge the effective dose on body weight, glucose control, and different lipid fractions as well as safety on long-term before using this agent as an ancillary treatment of DM2.
Sources of funding
This study did not obtain any specific contribution from funding agencies in the commercial, public, or not-for-profit sectors.
Ethical approval
The study was approved by the Ethics Committee of Benha faculty of medicine, Benha University with written informed consent was obtained from all participants.
Acknowledgments
We would like to thank all the nurses, and the patients who contributed in this study. We would like to express our very great appreciation to Clinical Pathology Department, Benha Faculty of Medicine, for performing laboratory investigations to our patients & Rheumatology Departments, Benha Faculty of Medicine, for sharing in nerve conduction velocities and ultrasonography on nerves.
Disclosure statement
All author(s) stated that they do not have any conflict of interests.
Additional information
Notes on contributors
Ayman M Elbadawy
Ayman M Elbadawy was post-graduated by Medical Doctora in 2006. He is working as assistant professor of internal medicine, in Benha Univeristy. He is interested in internal medicine, gastro-enterology, and hepatology with clinical experience since 1996 in clinical cases management and teaching in Faculty of medicine, Benha Univeristy.
Rasha O. Abd Elmoniem
Rasha O. Abd Elmoniem was post-graduated by Medical Doctora in 2016. she is working as lecturer of internal medicine, in Benha Univeristy. She is interested in internal medicine,and endocrine with clinical experience since 2005 in clinical cases management and teaching in Faculty of medicine, Benha Univeristy.
Amira M. Elsayed
Amira M. Elsayed is originally from Benha city, Qalubia governerate where she attended faculty of medicine, Benha University for her undergraduate degrees. She was graduated in M.B.B.Ch in medicine and Surgey in 2000 then as trainee till 2001 in Benha University Hospitals. She was assigned for work in Egyptian ministry of Health for 2 years as general practational, then she joined again Benha University Hospitals where postgraduated by Master degree & Medical Doctor in 2006 & 2011, respectively. Till now, she is working as assistant professor of internal medicine, endocrinology & diabetes. She spent seven years abroad in gulf area working as physician in internal medicine, endocrine, and diabetes where she experiences in management on general emergency cases, internal medicine, endocrinology and diabetes. In 2019, she got Specialty Certificate Examinations (SCE in endocrinology & diabetes) from Royal Colleges of Physicians of the United Kingdom. she focuses on diabetes and endocrinology cases and researches. You can follow her work here [[email protected]].
References
- Bruschi LKM, Rocha DA, Filho ELG, et al. Diabetes mellitus and diabetic peripheral neuropathy. Open J Endocr Metabol Dis. 2017;7(1):12–21
- Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American diabetes association. Diabetes Care. 2005;28(4):956–962.
- D’ Souza M, Kulkarni V, Bhaskaran U, et al. Diabetic peripheral neuropathy and its determinants among patients attending a tertiary health care centre in Mangalore, India. J Public Health Res. 2015;4:450.
- Li N, Yan W, Hu X, et al. Effects of oral α-lipoic acid administration on body weight in overweight or obese subjects: a crossover randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf). 2017 May;86(5):680–687.
- Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with α-Lipoic acid over 4 years in diabetic polyneuropathy. Diabetes Care. 2011;34(9):2054–2060.
- Han T, Bai J, Liu W, et al. THERAPY OF ENDOCRINE DISEASE: a systematic review and meta-analysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. Eur J Endocrinol. 2012;167(4):465–471.
- Okanovid A, Prnjavorac B, Jusufovid E, et al. Alpha-lipoic acid reduces body weight and regulates triglycerides in obese patients with diabetes mellitus. Med Glas. 2015;12:2.
- Sun Y-D, Dong Y-D, Fan R, et al. Effect of (R)-α-lipoic acid supplementation on serum lipids and antioxidative ability in patients with age-related macular degeneration. Ann Nutr Metab. 2012;60(4):293–297.
- Kamenova P. Improvement of insulin sensitivity in patients with type 2 diabetes mellitus after oral administration of alpha-lipoic acid. Hormones (Athens). 2006 Oct-Dec;5(4):251–258.
- As L, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. modification of diet in renal disease study group. Ann Inter Med. 1999;130(6):461–470.
- Ma B, Afify HE, Sabri N, et al. Comparative Study of Vitamin B complex combined with alpha Lipoic acid versus Vitamin B complex in treatment of diabetic polyneuropathy in type 2 diabetic patients. Clin Exp Pharmacol. 2017;7(4):241.
- Agathos E, Tentolouris A, Eleftheriadou I, et al. Effect of α-lipoic acid on symptoms and quality of life in patients with painful diabetic neuropathy. J Int Med Res. 2018 May;46(5):1779–1790; Epub 2018 Mar 8. PMID: 29517942; PMCID: PMC5991249.
- Ziegler D, Ametov A, Barinov A, et al. Oral treatment With -Lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006 Nov;29(11):2365–2370. PMID: 17065669
- Mijnhout GS, Kollen BJ, Alkhalaf A, et al. Alpha lipoic Acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2012; Epub 2012 Jan 26:456279. DOI:https://doi.org/10.1155/2012/456279. PMID: 22331979; PMCID: PMC3272801.
- Haak E, Usadel KH, Kusterer K, et al. Effects of alpha-lipoic acid on microcirculation in patients with peripheral diabetic neuropathy. Exp Clin Endocrinol Diabetes. 2000;108(3):168–174. PMID: 10926311
- Heitzer T, Finckh B, Albers S, et al. Beneficial effects of alpha-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: relation to parameters of oxidative stress. Free Radic Biol Med. 2001 Jul 1;31(1):53–61. PMID: 11425490.
- Stevens MJ, Obrosova I, Cao X, et al. Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes. 2000 Jun;49(6):1006–1015. PMID: 10866054
- Nagamatsu M, Nickander KK, Schmelzer JD, et al. Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care. 1995 Aug;18(8):1160–1167. PMID: 7587852
- Mohammadi V, Khalili M, Eghtesadi S, et al. The effect of alpha-lipoic acid (ALA) supplementation on cardiovascular risk factors in men with chronic spinal cord injury: a clinical trial. Spinal Cord. 2015 Aug;53(8):621–624; Epub 2015 Mar 10. PMID: 25753493.
- Koh EH, Lee WJ, Lee SA, et al. Effects of alpha-lipoic acid on body weight in obese subjects. Am J Med. 2011 Jan;124(1):85.e1-8.
- Li N, Yan W, Hu X, et al. Effects of oral α-lipoic acid administration on body weight in overweight or obese subjects: a crossover randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf). 2017 May;86(5):680–687; Epub 2017 Feb 26. PMID: 28239907.
- Udupa AS, Nahar PS, Shah SH, et al. Study of comparative effects of antioxidants on insulin sensitivity in type 2 diabetes mellitus. J Clin Diagn Res. 2012;6(9):1469–1473.
- Tabrizi R, Borhani-Haghighi A, Mirhosseini N, et al. The effects of alpha-lipoic acid supplementation on fasting glucose and lipid profiles among patients with stroke: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Metab Disord. 2019 Jul 29;18(2):585–595. PMID: 31890685; PMCID: PMC6915248.
- Karadağ C, Yoldemir T, Yavuz DG. Effects of vitamin D supplementation on insulin sensitivity and androgen levels in vitamin-D-deficient polycystic ovary syndrome patients. J Obstet Gynaecol Res. 2018 Feb 2; Epub 2017 Nov 2. 44(2):270–277. PMID: 29094433.
- Hoseini R, Damirchi A, Babaei P. Vitamin D increases PPARγ expression and promotes beneficial effects of physical activity in metabolic syndrome. Nutrition. 2017 Apr;36:54–59. PMID: 28336108. Epub 2016 Jun 29.
- Mirmasoumi G, Fazilati M, Foroozanfard F, et al. The effects of flaxseed oil omega-3 fatty acids supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp Clin Endocrinol Diabetes. 2018 Apr;126(4):222–228; Epub 2017 Nov 8. PMID: 29117618.
- Szkudelski T, Szkudelska K. The relevance of AMP-activated protein kinase in insulin-secreting β cells: a potential target for improving β cell function? J Physiol Biochem. 2019 Nov;75(4):423–432; Epub 2019 Nov 5. PMID: 31691163; PMCID: PMC6920233.
- Saengsirisuwan V, Kinnick TR, Schmit MB, et al. Interactions of exercise training and lipoic acid on skeletal muscle glucose transport in obese Zucker rats. 1985;2001(91):145e53. J Appl Physiol.
- Lee WJ, Song KH, Koh EH, et al. α-Lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun. 2005 Jul 8;332(3):885–891. PMID: 15913551.
- Bast A, Haenen GR. Lipoic acid: a multifunctional antioxidant. Biofactors. 2003;17(1–4):207–213. PMID: 12897442
- Singh U, Jialal I. Retracted: alpha-lipoic acid supplementation and diabetes. Nutr Rev. 2008 Nov;66(11):646–657. PMID: 19019027; PMCID: PMC2657658
- Kim E, Park DW, Choi SH, et al. A preliminary investigation of α-Lipoic acid treatment of antipsychotic drug-induced weight gain in patients with schizophrenia. J Clin Psychopharmacol. 2008 Apr;28(2):138–146. PMID: 18344723
- Khabbazi T, Mahdavi R, Safa J, et al. Effects of alpha-lipoic acid supplementation on inflammation, oxidative stress, and serum lipid profile levels in patients with end-stage renal disease on hemodialysis. J Ren Nutr. 2012 Mar;22(2):244–250; Epub 2011 Sep 10. PMID: 21908204.
- Sun YD, Dong YD, Fan R, et al. Effect of (R)-α-lipoic acid supplementation on serum lipids and antioxidative ability in patients with age-related macular degeneration. Ann Nutr Metab. 2012;60(4):293–297. Epub 2012 Jun 1. PMID: 22678104.
- Chen W-L, Kang C-H, Wang S-G, et al. α-Lipoic acid regulates lipid metabolism through induction of sirtuin 1 (SIRT1) and activation of AMP-activated protein kinase. Diabetologia. 2012;55(6):1824–1835.
- Ansar H, Mazloom Z, Kazemi F, et al. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med J. 2011;32(6):584–588.
- Seo EY, Ha AW, Kim WK. α-Lipoic acid reduced weight gain and improved the lipid profile in rats fed with high fat diet. Nutr Res Pract. 2012;6(3):195–200.
- Ichikawa T, Liang J, Kitajima S, et al. Macrophage-derived lipoprotein lipase increases aortic atherosclerosis in cholesterol-fed Tg rabbits. Atherosclerosis. 2005;179(1):87–95.
- Vidovic B, Milovanovic S, Stefanovic A, et al. Effects of Alpha-Lipoic acid supplementation on plasma adiponectin levels and some metabolic risk factors in patients with schizophrenia. J Med Food. 2017;20(1):79–85.
- Singh K, Gupta K, Kaur S. High resolution ultrasonography of the tibial nerve in diabetic peripheral neuropathy. J Ultrason. 2017 Dec;17(71):246–252; Epub 2017 Dec 29. PMID: 29375899; PMCID: PMC5769664.
- Watanabe H, Ito A, Morita Y, et al. Sonographic evaluation of the median nerve in diabetic patients, comparison with nerve conduction studies. J Ultrasound Med. 2009;28(6):727–734.
- Riazi S, Bril V, Perkins BA, et al. Can ultrasound of the tibial nerve detect diabetic peripheral neuropathy?: a cross-sectional study. Diabetes Care. 2012;35(12):2575–2579.