ABSTRACT
Introduction: China is responsible for more than 60% of global aquaculture production. As the frontiers of food production have expanded, the cultivation of marine organisms in coastal zones and the open ocean has grown rapidly. The dominant mariculture industry in China is suspended mariculture, which uses net cages, ropes, or other structures suspended in the water column to cultivate aquatic organisms. This systematic, quantitative review provides a clear and comprehensive account of research that has investigated the adverse impacts of suspended mariculture in China and reviews research that has applied Integrated Multi-Trophic Aquaculture (IMTA) systems for mitigating impacts. This work builds on 218 peer reviewed papers that have been published in English-language journals.
Outcomes: Eighteen impacts were identified, including chemical, ecological, physical, and socioeconomic impacts. Eighteen measures for improving suspended mariculture were recommended consisting of government department, farm management, and ecological engineering measures. IMTA was the most frequently recommended measure. The capabilities of IMTA for bioremediation and increased farm production were the most frequently studied advantages. Seven other benefits have been explored but remain understudied. The current challenges facing the expansion of commercial IMTA include limited use of new technology, limited skills development, decreasing production of low trophic-level species, biogeographic and temporal barriers, and negative system feedbacks.
Conclusion: Despite challenges, implementing commercial IMTA is a promising measure for reducing the impacts of suspended mariculture because it presents a range of secondary benefits that can improve the overall sustainability of aquaculture in the coastal zone.
Introduction
In the face of climate change, economic uncertainty, and growing competition for natural resources, a pressing issue for science is how to ensure food security for more than 9 billion people by 2050. The fastest growing food production sector in recent years has been aquaculture, which has produced more fish for human consumption than capture fisheries since 2013 (FAO Citation2016). The largest aquaculture producer in the world is China, which is responsible for more than 60% of total world production using freshwater and marine systems (FAO Citation2016).
In marine aquaculture, or mariculture, one perception is that farming aquatic organisms alleviates pressure on wild stocks. In some cases, however, the opposite may be true: the farming of higher trophic-level carnivorous fish species requires large inputs of wild fish for feed. Wild fish stocks are further diminished through habitat modification, wild seedstock collection, waste release, exotic species introductions, and the transmission of aquaculture pathogens (Naylor et al. Citation2000). The farming of lower trophic-level species, in contrast, is considered more environmentally sound because species such as filter feeding mollusks have a higher eco-efficiency and make a substantial net contribution to global seafood supply (Williams Citation1997). In China, suspended mariculture in open ocean water dominates mariculture production; however, the technique uses net cages, ropes, or other structures suspended in open ocean water and so it has been criticized because adverse impacts are freely imposed on the supporting water column. The vast scale of suspended mariculture in China implies that ecosystem-scale impacts are likely, and therefore solutions that limit or mitigate impacts are vital.
The implementation of Integrated Multi-Trophic Aquaculture (IMTA), a form of ecological engineering in aquaculture, has been proposed to help alleviate the impacts of suspended mariculture for some time (Chopin et al. Citation1999; Neori et al. Citation2004; Troell et al. Citation2009). Prototypical IMTA systems aim to integrate extractive (non-fed) aquaculture species with fed species, so that the extractive species assimilate farm waste and generate a harvestable biomass that can be sold for profit. Other economic, environmental, and social benefits include increased product diversity, improved ecosystem services through improved environmental conditions, and the development of associated industries, wider employment opportunities, and social acceptance. IMTA research worldwide has shown that macroalgae, shellfish, and echinoderms can be used to assimilate dissolved nutrients, suspended particulates, and settling particulates, respectively (Chopin Citation2015). The implementation of IMTA is attractive conceptually, but there may be limitations: ecological engineering to enhance food production is complex and dynamic, and requires advanced skills and systems that may not be widely available yet, particularly on a commercial scale.
This paper reviews the current state of research on the impacts of suspended mariculture and the potential applications of IMTA in China. A systematic, quantitative assessment of the literature was conducted to examine impacts, collate recommendations for mitigating impacts, and identify the scope for, and challenges facing, IMTA in China. Details of the methodology employed in the literature search are available in Appendix S1. Chinese aquaculture production data were extracted from the China Fishery Statistics Yearbooks published annually by the Ministry of Agriculture (MoA) of the People’s Republic of China (MOA Citation1981–2016).
Mariculture in China
China has consistently produced more than 60% of the world’s aquaculture products for the past two decades. Recent projections suggest that this proportion will be maintained through 2025 (FAO Citation2016). In 2015, China farmed 49.37 million tons (RMB 828 billion, ~US$120 billion) of aquatic products, of which 18.75 million tons (38% production, 35% value) was produced using mariculture (MOA 2016).
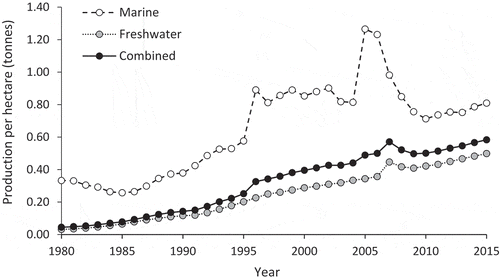
At present, freshwater aquaculture may contribute the bulk of aquaculture production in China, but the practice adds pressure to China’s already overexploited freshwater resources. In northern China, there has been an increase in droughts that is compounding a pre-existing uneven distribution of fresh water across the country. Agriculture, in particular, has suffered heavily (Ye et al. Citation2016). Additionally, freshwater aquaculture in China produces less biomass per hectare than mariculture (). It is therefore unlikely that production from freshwater aquaculture will continue to meet the growing demand for aquatic products over the long term, and so focusing efforts on developing sustainable mariculture is favorable.
Figure 1. Annual production per hectare (tons) from freshwater and marine aquaculture in China from 1980 to 2015. Data were extracted from the China Fishery Statistics Yearbooks (MOA Citation1981–2016).
Mariculture production in 2015 was made up of predominantly low trophic-level species; 13.58 million tons of mollusks, 2.01 million tons of macroalgae, 1.43 million tons of crustaceans, 1.36 million tons of finfish, 0.21 million tons of echinoderms, and 0.07 million tons of other aquatic species such as jellyfish (MOA 2016). In 2014, 12 million tons of bivalve mollusks were farmed – five times higher than those produced in the rest of the world (FAO Citation2016).
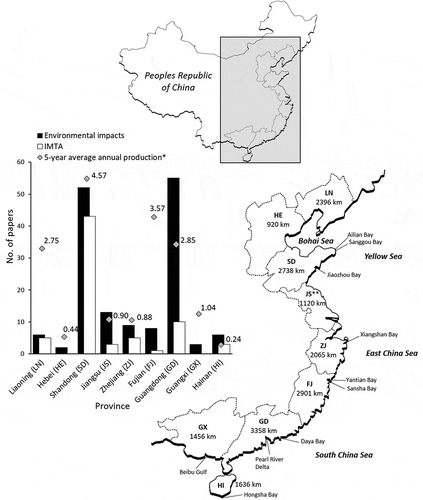
Mariculture production is unevenly distributed along China’s 18,593 km coastline which covers nine coastal provinces. The bulk of mariculture production comes from Liaoning, Shandong, Fujian, and Guangdong, which together represent 61% of the coastline but produced approximately 80% of marine aquatic products in 2015 () (MOA 2016).
Figure 2. Map of China showing the location of the nine coastal provinces and the lengths of their coastlines. The inset graph shows the total number of publications from each province that have investigated the environmental impacts of suspended aquaculture or Integrated Multi-Trophic Aquaculture. Provinces in the inset graph are listed from north to south. * The 5-year average annual mariculture production (× 106 tons) was extracted from the China Fishery Statistics Yearbooks (MOA Citation1981–2016). **The coastline length of Jiangsu includes the 196 km coast of Shanghai.
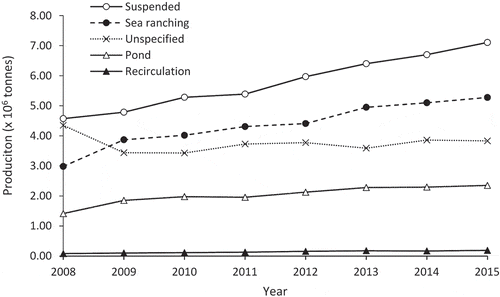
Four mariculture systems are dominant (). Recirculating aquaculture systems and marine ponds are land-based systems that accounted for 0.19 million tons (1.01%) and 2.35 million tons (12.53%) of production in 2015 (). Sea ranching and suspended mariculture can be open-water, coastal or offshore, systems. Sea ranching, or bottom culture, does not require physical aquaculture structures and is practiced by stocking hatchery-reared organisms in the open sea for growout and future harvest (e.g., Wang et al. Citation2017). Suspended mariculture systems require moorings and rigging such that culture organisms can be suspended in the water column. There are two modes of suspended mariculture: longline culture of macroalgae, bivalves or other mollusks, and sea cage, or “fish raft,” culture of fed species. Fish raft systems are sited and moored using methods similar to longline systems but they incorporate floating platforms to facilitate husbandry activities such as feeding and net-cleaning. The surface area of individual fish cages in China is typically small, under 25 m2 each. Sea ranching and suspended mariculture accounted for 5.28 million tons (28.12%) and 7.11 million tons (37.89%) of total mariculture production in 2015 (). Approximately 3.83 million tons (20.43%) of Chinese mariculture production came from unidentified sources that may include the above techniques, as well as pen culture (e.g., Beveridge Citation1984) or other unspecified techniques. Suspended mariculture therefore represents the largest contributor to Chinese mariculture production and is the focus of the present review ().
Figure 3. Total annual production from the different mariculture techniques practiced in China from 2008 to 2015. Data were extracted from the China Fishery Statistics Yearbooks (MOA Citation2009–2016).
Results
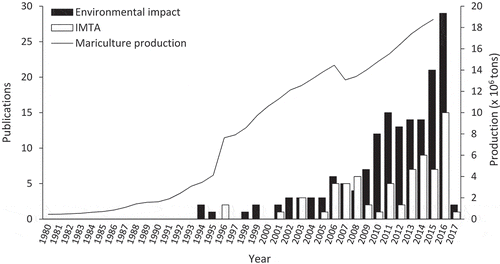
The environmental impacts of suspended mariculture in Chinese waters have been examined in at least 161 papers since 1994 (Appendix S2). The various applications of IMTA in China have been assessed in at least 73 papers since 1996 (Appendix S3). Fifteen (7%) of the papers were relevant to both topics. Most research has been conducted in Shandong and Guangdong (). It is conceivable that 2010 represents the start of modern research on these topics because 155 (71%) of the papers were published between 2010 and 2017 ().
Figure 4. The number, per year, of published papers that have investigated the impact of suspended aquaculture (161 papers) or the applications of Integrated Multi-Trophic Aquaculture (72 papers) in Chinese waters from 1980 to 2015. The solid line represents the total annual mariculture production for the same period.
The environmental impacts of suspended mariculture
Suspended mariculture in China was linked to 18 environmental impacts. Impacts from all trophic levels were characterized as eight chemical, five ecological, two physical, and two socioeconomic impacts ().
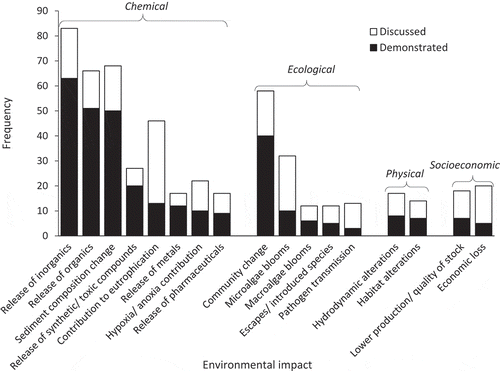
Figure 5. Frequency of the environmental impacts either discussed or demonstrated by at least 161 papers that have studied the impacts of suspended mariculture in Chinese coastal waters since 1994.
Trophic-level impacts
There has been a strong focus on studies investigating the impacts of finfish (86 papers) and shellfish (82 papers) mariculture, while the impacts of macroalgal cultivation have been studied less frequently (42 papers). The impacts of echinoderm culture were studied by five papers that were relevant to this review, but the echinoderms were cultured in pond or sea ranching systems rather than in suspended systems.
Chemical impacts
Chemical impacts included pollution from organic and inorganic nutrients, and pollution from anthropogenic sources including toxic compounds, pharmaceuticals, and metals. Together these can change the composition of sediment, contribute to eutrophication, or cause hypoxic or anoxic conditions. The most frequently discussed and demonstrated chemical and overall impact caused by suspended mariculture in China is the release of inorganic waste (). Although eutrophication was frequently discussed in the literature (46 papers), fewer studies proceeded to demonstrate the contribution of suspended mariculture to eutrophication (13 papers).
Ecological impacts
Ecological impacts have included changes to surrounding ecological communities, the induction of algal blooms, the transmission of pathogens form cultivated stock to wild communities, and the escape of cultivated stock that can disrupt local populations. Changes to community structure were the most frequently studied ecological impact ().
Physical and socioeconomic impacts
The physical and socioeconomic impacts of suspended aquaculture remain relatively understudied. Initial research has shown that physical impacts can include alterations to hydrodynamics and habitat, while socioeconomic impacts can include decreased productivity, decreased stock quality, and economic loss ().
Recommendations to improve suspended mariculture
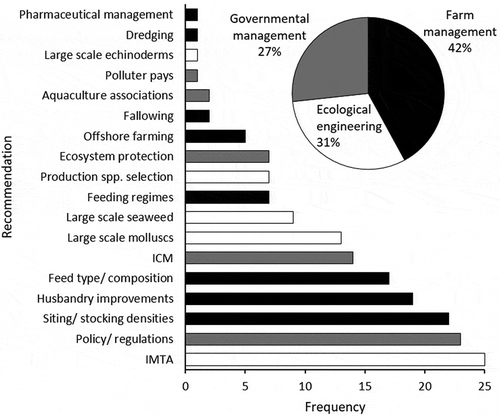
Ninety-two papers that investigated the environmental impacts of suspended mariculture made explicit recommendations for improvement. Eighteen recommendations were categorized as either “farm management” (74 papers, 42%), “government management” (47 papers, 27%), or “ecological engineering” (55 papers, 31%) measures (). The most frequently recommended measure in each category, respectively, was to site farms carefully and enforce appropriate stocking densities, improve policy and regulations, and implement IMTA (). IMTA was the most frequently recommended measure overall (25 papers).
Figure 6. Frequency of the recommendations made in 92 papers for improving the impact of suspended mariculture in Chinese waters. ICM, Integrated Coastal Management; IMTA, Integrated Multi-Trophic Aquaculture. The inset pie chart shows the proportion of recommendations falling into the three relevant management strategies.
The benefits of IMTA in China
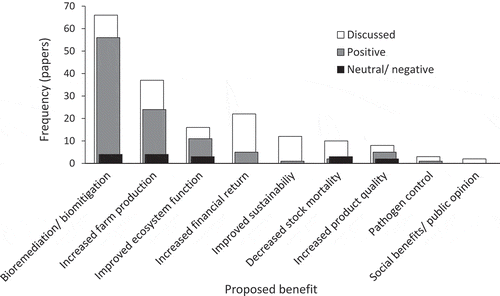
In China, 72 papers show nine potential benefits of IMTA. Forty-eight papers explicitly recommended its implementation. The potential benefits of bioremediation and/or biomitigation, and the possibility of increased farm production are most frequently demonstrated (). Seven other benefits are discussed and/or demonstrated: improved ecosystem function, increased financial return, improved sustainability, decreased stock mortality, increased product quality, potential for pathogen control, and improved public opinion and social benefits. The possibility that IMTA could improve farm financial return was discussed in 22 papers but only 5 papers proceeded to demonstrate a positive financial impact.
Figure 7. Frequency of the proposed benefits of Integrated Multi-Trophic Aquaculture (IMTA) from 73 papers involving studies of IMTA in Chinese coastal waters. Proposed benefits were either “discussed” or “demonstrated.” Studies that demonstrated an effect found a “positive” or “neutral/negative” effect.
Discussion
Suspended mariculture environment
China’s coastline spans 23 degrees of latitude (17–40° N) and 16 degrees of longitude (108–124.5° E) (Xiao et al. Citation2007) and is therefore characterized by a variety of climates, morphological features, biodiversity, and anthropogenic pressure. The high population density of China’s coastal provinces strain the marine environment (Ding, Ge, and Casey Citation2014; Williams et al. Citation2016). All major coastal water bodies are impacted by human activities (Xiao et al. Citation2007). Suspended mariculture sites face pollution from terrestrial runoff, river discharge, and submarine groundwater discharge (He et al. Citation2008). Further challenges include habitat alterations and pollution from large marine industries including shipping, pond aquaculture, and contaminants from atmospheric sources (Ding, Ge, and Casey Citation2014; Hou et al. Citation2016).
Good shelter and wave attenuation are the primary criteria for the development of mariculture in China (Ma et al. Citation2015). Shallow seas with depths up to 10 m cover 10 million ha (Xie et al. Citation2013). The majority of suspended mariculture therefore occurs in the numerous semi-enclosed bays, on mud flats and in shallow seas (Cao et al. Citation2007). Wang (Citation1993) estimated that 1.3 million hectares of inshore area are suitable for mariculture. Xiao et al. (Citation2007) identified 50 major coastal bays, most of which have a >400 km2 surface area and already support suspended mariculture activities.
Bays and islets are often characterized by slow water exchange and so pollutants can accumulate readily in the vicinity of aquaculture operations (Wu et al. Citation1999; Lee, Choi, and Arega Citation2003). Accumulation of effluent is dangerous because in the shallow inshore region the resuspension of toxic substances in adverse weather occurs easily (Wong and Cheung Citation2001; Qi et al. Citation2013). As an alternative, the potential for expanding production from offshore aquaculture has been proposed but offshore systems remain underdeveloped and understudied (Qi et al. Citation2013). Production from circular fish cages sited offshore in China accounted for only 0.56% of total aquaculture production in 2015 (MOA 2016). Understanding the impacts of the environment on aquaculture, and the impacts of aquaculture on the environment, are necessary for achieving sustainable aquaculture (Han, Keesing, and Liu Citation2016).
The environmental impacts of suspended mariculture
While aquaculture has been practiced in China for thousands of years, suspended mariculture started only recently but has expanded rapidly. Suspended fish cage mariculture originated in Hong Kong in the 1960s (Lai and Yu Citation1995). Details on production from suspended mariculture in China in the early years have not been published, but it is known that the mariculture industry boomed from the early 1970s. Production increased from 0.01 million tons in 1950, to 0.18 million tons in 1970 and then to 18.75 million tons in 2015 () (Tseng Citation1993; Zhong and Power Citation1997; MOA 2016). Suspended mariculture is now the biggest contributor to Chinese mariculture production (). Growth of suspended mariculture has been driven largely by shellfish and macroalgal culture in shallow coastal waters (Tang, Zhang, and Fang Citation2011), but the rapid increase of mariculture production has caused a rapid increase in environmental impacts.
Trophic-level impacts
It is well recognized that the impacts of suspended aquaculture are dependent on the culture species, culture method, culture density, feed type, general husbandry practices, and site-specific characteristics including local and regional hydrodynamics (Wu Citation1995; Gao et al. Citation2005). These factors vary widely in China because of the diversity of culture organisms, culture methods, environments, and climates. Despite relatively low finfish production (7.2% by volume in 2015, MOA 2016), the high frequency of studies that have investigated the impacts of suspended finfish culture is probably because finfish have a substantially higher value per kilogram and require feed input that intensifies impacts. The research attention on the impacts of shellfish is probably because the production of shellfish vastly outweighs all other groups in China (72.4% by volume in 2015, MOA 2016) and because the cultivation of shellfish presents a paradox; on the one hand shellfish may help to assimilate suspended particulates but on the other hand shellfish may only partially assimilate particulates and can produce large volumes of psuedofeces and feces that have a high settling velocity and can pollute benthic habitats (Ren and Zhang Citation2016). Macroalgal culture is reputed as an environmentally sound form of mariculture because of its extractive properties that can help to alleviate eutrophication and restore ecosystem services (Edwards Citation2015); however, impacts from suspended macroalgal culture are plausible and include the inhibition of water flow and light penetration, and the deposition of large amounts of tissue from breakage and drop-off (Zhang et al. Citation2012; Zhou Citation2012). The potential for cultivating echinoderms in suspended systems remains experimental and so the impacts caused by suspended echinoderms have not been investigated (e.g., Yu et al. Citation2012). This review grouped the impacts from all trophic levels as either chemical, ecological, physical, or socioeconomic ().
Chemical impacts
Common inorganic substances include nitrogen, phosphorous, and sulfur compounds such as ammonia, nitrate, phosphate, sulfate, and pyrite-S (Cai et al. Citation2016; Duan et al. Citation2016; Kang, Liu, and Ning Citation2016). Organic waste includes fish feed and feces, urea, dead and decaying culture organisms, and methane (Leung, Chu, and Wu Citation1999; Hou et al. Citation2016). When inorganic and organic substances settle, changes in sediment composition can result. Settling biodeposits, synthetic compounds, or metals are commonly recorded from sediment in aquaculture zones (Wang et al. Citation2013; Wang et al. Citation2014a; Ren and Zhang Citation2016). The natural chemical reactions for processing waste from fish farms can give rise to hypoxic or anoxic sediment and water bodies (Zhang, Huang, and Huang Citation2013; Kang, Liu, and Ning Citation2016). Chemical impacts from suspended aquaculture are generally caused by the continuous or pulse release of waste substances associated with normal husbandry activities (Yang et al. Citation2006; Xu et al. Citation2011).
When the release of nutrients is substantial, eutrophic conditions can occur (Cao et al. Citation2007; Xu et al. Citation2008). The disparity between papers that discuss eutrophication as an impact from suspended mariculture and papers that demonstrate it is probably because of the difficulty in identifying the source of nutrients in open coastal systems impacted by several human activities (). Common sources of nutrients in China’s waters include discharge from rivers, discharge from industrial outlets, submarine groundwater discharge, coastal pond aquaculture effluent, wastewater from shipping ports, urban runoff and untreated sewage, pollutants from large-scale sea ranching and pond aquaculture operations, and waste from suspended mariculture. The combined result can cause nutrient concentrations multiple times higher than natural levels and so many coastal areas in China are eutrophic (Zhou et al. Citation2006c; He et al. Citation2008).
The release of synthetic compounds has included polycyclic aromatic hydrocarbons that have been linked to the operation of boats supporting aquaculture infrastructure (Klumpp et al. Citation2002; Yu et al. Citation2012). Various persistent halogenated compounds (PHCs), pesticides such as DDT (dichlorodiphenyltrichloroethane), and pharmaceuticals have originated in feed (Yu et al. Citation2011b; Chen et al. Citation2015; Fang, Bao and Zeng Citation2016). The release of pharmaceuticals from aquaculture in China has been reviewed previously and, in the context of coastal aquaculture, has included high levels of sulfonamides that can cause the occurrence of antibiotic-resistant genes (He et al. Citation2016). Higher concentration of metals such as Zn, Ni, Cu, Cr, Pb, and Ca has been linked to antifouling paints on aquaculture infrastructure and the use of low-quality feeds (Gu et al. Citation2014; Liang et al. Citation2016). If chemical impacts are substantial, ecological impacts can occur.
Ecological impacts
Research in China has shown that suspended aquaculture systems can cause changes to surrounding ecological communities by significantly altering benthic, microbial, planktonic, and fish community dynamics (e.g., Jiang et al. Citation2012; Han et al. Citation2013a; Lu et al. Citation2015; Zhao et al. Citation2016).
Two other widely publicized ecological impacts are micro- and macro-algae blooms, which proliferate and thrive in eutrophic environments. Harmful algal blooms (HABs), comprising microalgae, have caused major stock and economic loss to the aquaculture industry and have also caused large-scale mortality of wild populations for decades (Lai and Yu Citation1995). The frequency and extent of HABs has been increasing in China (Wang et al. Citation2008; Lu et al. Citation2014a). Based on long-term data from Hong Kong, Lee (Citation2016) showed a significant positive correlation between the intensity of coastal mariculture and the occurrence of HABs. Macroalgal blooms have started to occur more recently and have been reported in the northern East China Sea and Yellow Sea since 2007 (Keesing et al. Citation2011). These seaweed blooms have been shown to originate from open water suspended Porphyra culture systems and have spanned more than 40,000 km2 in some instances. Bloom-forming species including Ulva and Enteromorphaspp. have caused major economic loss by inundating waterways and beaches, and have caused widespread asphyxiation of organisms when the blooms biodegrade (Liu et al. Citation2009; Liu et al. Citation2013). A third bloom type, jellyfish blooms, has also been linked to the expansion of suspended mariculture because husbandry infrastructure provides substrate for larval settlement and husbandry activities provide nutrients for proliferation (Dong, Liu, and Keesing Citation2010).
Further ecological impacts are the transmission of pathogens and the introduction of escaped culture organisms that can include invasive species. Pathogens tend to flourish in intensive aquaculture because culture organisms are stocked at high density, are easily stressed, and so are more susceptible to infection (Pang et al. Citation2015; Liu et al. Citation2016). In cultured grouper, it was shown that parasitic gill monogeneans have a higher species richness and diversity in suspended mariculture systems compared to grouper harvested from the wild (Luo and Yang Citation2012). It has been hypothesized that pathogens can be transmitted to natural populations either by direct transfer or through escaped culture organisms as vectors (Bondad-Reantaso et al. Citation2005); however in China, this remains to be demonstrated in suspended mariculture. Lin et al. (Citation2015) reviewed the introduction of non-native species for use in aquaculture in China, but the impact of escaped/released organisms from suspended mariculture requires further investigation. Escaped indigenous species can dilute the genetic diversity of natural populations while introduced species can lead to successful ecological invasions (Wang et al. Citation2007a; Wang et al. Citation2014b).
One ecological impact that remains unstudied is the release of macrowaste (garbage) that can contribute to degrading the ecological condition of mariculture areas. Feng et al. (Citation2004) hypothesized that garbage release from open water farms was likely but did not investigate further. In Hong Kong, open water fish farmers have been observed discarding garbage directly into the surrounding water as normal practice and numerous plastic feed bags have been found on the shoreline up to 4 km from the nearest fish culture zone. It is also common for fish raft ablution facilities to release untreated sewage and cleaning products directly into the water.
Physical impacts
It is known that longlines, rigging, and net cages can physically alter both hydrodynamics and habitat (). Aquaculture structures can significantly change surface current speed and direction, induce downwelling, and reduce water exchange of bays (Grant and Bacher Citation2001; Zeng et al. Citation2015; Lin, Li, and Zhang Citation2016). Shi and Wei (Citation2009) found that suspended aquaculture in Sanggou (Sungo) Bay reduced the average speed of currents by 40%, and the average half-life of water exchange was prolonged by 71%. Physical alterations to habitat can be positive or negative. Wang et al. (Citation2015) noted that floating structures from aquaculture increase the complexity of wild fish assemblages and enhance the populations of local species. However, aquaculture structures provide a substrate for biofouling communities. While biofouling communities can help to process dissolved and particulate aquaculture waste in some cases, Qi et al. (Citation2015) showed that ascidians that colonize scallop cages in Sanggou Bay played an important role in coupling material fluxes from the water column to the sea bed through biodeposits generated by the ascidians and through ascidian drop-off to the sea floor. That study reveals a notable 143 tons of ascidian drop-off into the bay over a growing season.
Socioeconomic impacts
The socioeconomic impacts that have been studied in China generally result from the cumulative effects of multiple chemical, ecological, or physical factors. These factors decrease farm production and increase economic loss through events such as fish kills and reduced product quality (). For example, one consistently devastating challenge facing the Zikong scallop Chlamys farreri industry in northern China is the mass summer mortalities that have occurred since 1996, causing up to 85% loss of stock. It is shown that these mass mortality events are probably caused by a combination of several factors including reproductive stress, high temperature, overcrowding, poor water circulation, opportunistic invaders or predators, and hatchery inbreeding (Xiao et al. Citation2005).
As food safety regulators tighten monitoring and consumers become more responsible, the importance of product quality is becoming a focus; quality indices are shown to successfully identify the origin of cultured scallop Argopecten irradians in northern China (Xu et al. Citation2015). Waste substances that reduce the quality of final products, and are potentially harmful to humans, have been detected in culture organisms. These substances originate from husbandry activities, or in low-quality feed, and can contaminate products and lead to negative socioeconomic consequences. Examples of these substances include selenium, organochlorine pesticides such as hexachlorocyclohexanes, DDTs, and other PHCs (Yu et al. Citation2011a; Yu et al. Citation2011b; Wang et al. Citation2014a; Chang et al. Citation2016). To gain consumer support for suspended mariculture over the long term, it will be important to mitigate these issues through proper monitoring, regulation, and certification.
Recommendations to improve suspended mariculture
To insure the long-term sustainability of suspended mariculture, healthy aquaculture environments sufficiently protected from external stressors are required. The relatively even distribution of papers that have recommended farm management, government management, and ecological engineering measures suggests that a multidisciplinary combination of methods is probably necessary to achieve sustainability ().
Farm management
Farm management measures can be implemented by farm managers at the individual farm level. It is suggested that carefully siting farms is one of the most effective ways to limit environmental impacts because farmed stock should be maintained at or below the carrying capacity of a site (Feng et al. Citation2004). Another recommendation was to move farm operations offshore, away from the semi-enclosed bays that tend to have low carrying capacities (Ferreira et al. Citation2009). Offshore areas are characterized by deep water and higher-order hydrodynamics that can flush farm waste away from farm areas and distribute it over larger areas for assimilation by natural processes (Feng et al. Citation2004).
Other farm management recommendations included improving general husbandry practices, using carefully formulated feeds and optimizing feeding regimes (). In China trash fish is still the most commonly used feed for the culture of fed species (FAO Citation2014). Formulated feeds should replace trash fish because formulated feeds are generally more digestible and will result in lower feed conversion ratios and environmental impact. Formulated feeds should be high quality because many low-quality feeds have low nutritional benefit or contain contaminants that originate in the ingredients used to make the feed (Edwards Citation2015; Liang et al. Citation2016). Improving the use of pharmaceuticals for the treatment of pathogens in suspended mariculture was suggested in only one study (Xie et al. Citation2013). Fallowing of culture sites and dredging to remove contaminated aquaculture sediments were recommended by few studies: Feng et al. (Citation2004) suggested moving fish rafts to new areas and halting culture activities at the original site for 1–2 years to facilitate recovery of impacted sediment. However, fallowing and dredging have not been popular probably because of the associated expense and logistics.
Governmental management
Governmental management measures must be initiated, coordinated, and regulated by relevant government departments and local municipalities. The most frequently recommended measure in this category was to improve policy and/or regulations. Suggestions for policy were centered around implementing well-regulated legislation that facilitates an industry transition to more sustainable practices. The current licensing system in China has been reviewed by (Fang et al. Citation2016a); licenses for sea area use and aquaculture are granted separately and once a farmer is in possession of both they can engage in open water aquaculture. In many cases, licenses have not specified the species, stocking densities, or system layouts and so aquaculture activities have proceeded unrestricted. Prior to the 1990s this system was advantageous and played an important role in the development of suspended mariculture in China. More recently, however, the lack of restrictions has, in many cases, allowed production volumes to far exceed local carrying capacities and has resulted in severe pollution and disease outbreaks (Fang et al. Citation2016a).
Other recommendations for governmental measures included implementing integrated coastal management (ICM), initiating ecosystem protection, establishing aquaculture associations, or implementing regulations based on the polluter pays principle (). ICM refers to a holistic management framework that guides multidisciplinary management measures in the coastal zone (Yu et al. Citation2016a). In aquaculture, this can include efforts to use multidimensional models to guide management decisions across marine sectors and to guide the implementation of suitable policies (Nobre et al. Citation2009). Ecosystem protection measures could include the zoning of protected areas, under various levels of protection, to limit the intensity of aquaculture and support a healthy ecosystem overall (Hu Citation1994; Feng et al. Citation2004; Yu et al. Citation2016b). The formation of aquaculture associations is recommended to help guide farmers on husbandry practices and encourage information sharing between farmers (Liu et al. Citation2009; Xie et al. Citation2013). Implementing the polluter pays principle is also suggested and would require farmers to pay a tax on any pollution they release over and above predetermined levels (Zhou et al. Citation2006a; Neori Citation2008). A polluter pays system could be expanded further to include a nutrient credit system that allows for intra- and inter-industry nutrient credit trading based on net nutrient release or extraction (Ferreira et al. Citation2009; Troell et al. Citation2009). Farmers engaging in the cultivation of extractive species would benefit from a nutrient credit trading system because the economic value of lower trophic-level species would increase.
Ecological engineering
Ecological engineering in aquaculture addresses, quantifies, and facilitates the construction of biological systems that can assist in managing waste as a resource (Troell et al. Citation2009). The implementation of IMTA, large-scale mollusk farming, large-scale seaweed farming, the careful selection of production species, and large-scale echinoderm farming are all forms of ecological engineering that have been recommended in China ().
IMTA and the large-scale cultivation of extractive species can mitigate or remediate aquaculture waste by assimilating it (Chopin et al. Citation2001; Neori et al. Citation2004). These techniques have become popular because, if species are selected carefully, they should provide an economic return when harvested. For example, filter feeding mollusks have been shown to remove suspended particulates, seaweeds can remove dissolved substances, and echinoderms remove settling debris, either directly or indirectly. Direct removal occurs when the extractive species consumes farm waste directly, such as when echinoderms feed on fish feed that has settled to the sea floor (Zhou et al. Citation2006b). Large-scale seaweed cultivation is proposed as a means to reduce the widespread coastal eutrophication in China because macroalgae are highly efficient at directly assimilating dissolved nutrients that are then harvested as seaweed biomass (Feng et al. Citation2004; He et al. Citation2008). Indirect removal of waste occurs when extractive species feed on the additional productivity caused by aquaculture waste, such as when bivalves feed on the increased availability of microalgae present in aquaculture zones due to higher concentrations of nutrients that promote microalgal growth (Lu et al. Citation2014b). Ecological engineering is becoming popular in China because of the recent, rapid expansion of mariculture production and the waste it generates, and because China has a history of cultivating and consuming species from low trophic levels.
One simple ecological engineering measure to improve the sustainability of aquaculture operations is the fundamental step of carefully selecting the species to be cultured (Luo and Yang Citation2012; Zhao et al. Citation2013). This applies to intensive monoculture and polyculture. Monoculture of extractive species such as seaweed and bivalves has been promoted in China because they cause less environmental impact compared to fed finfish (Kang et al. Citation2013). Monoculture of different fed finfish can also have varying degrees of impacts, depending on species diet, metabolism, and trophic level (Wu Citation1995). Herbivorous fish species are favored because of their plant-based diets and comparatively lower environmental impact (Williams Citation1997; Naylor et al. Citation2000). It is important to select species that are well-suited to the environmental conditions in an area because feed conversion ratios are usually more favorable where environmental conditions are optimal (Nordgarden et al. Citation2003). In an ecological engineering context, the selection of species for co-culture is important because the ratios of nutrients released, or assimilated, vary between species within trophic levels.
By implementing selected recommendations simultaneously, multidisciplinary approaches can help to develop a sustainable suspended mariculture industry in China. In particular, ecological engineering approaches such as IMTA can be used to augment farm- and government-level measures. The implementation of IMTA is the most frequently recommended measure for helping to reduce the impacts of suspended mariculture ().
IMTA in China
Development of IMTA
The implementation of various forms of IMTA is happening readily in China partly because there is a growing philosophy that the waste from the production of one resource must become an input into another (Ruddle and Zhong Citation1988; Chopin et al. Citation2001). Because of the early suspended seaweed culture activity in the 1950s, the advent of suspended scallop culture in the 1960s, and the widespread adoption of suspended fish cage culture in the 1980s, IMTA has been described as the most common culture system in the coastal zone (Fang et al. Citation1996; Ren et al. Citation2014; Edwards Citation2015). However, the implementation of IMTA has not been homogenous. Suspended IMTA systems in open water can be characterized under one of three operational regimes: incidental (extensive) IMTA, transitional IMTA, and engineered (intensive) IMTA. Incidental IMTA is the most common and occurs when extractive species are farmed in the same semi-enclosed bay as fed species so that waste assimilation by extractive species occurs naturally (Edwards Citation2015). The semi-enclosed inshore bays of China are favored locations for aquaculture and so connectivity between species is facilitated by natural hydrodynamics (Ma et al. Citation2015). Over the years there has been a sequential development of IMTA from these incidental systems to engineered systems as more data, information, and training become available. Transitional IMTA systems are therefore systems that initially existed as incidental systems, but are being refined by farmers to intentionally optimize operation potential by integrating species from multiple trophic levels to supplement overall farm production. Engineered IMTA systems are uncommon and most examples are experimental rather than commercial (Han et al. Citation2013b; Yu et al. Citation2016c). China’s leading case for a truly commercial, engineered IMTA system is Sanggou Bay in Shandong (37°05ʹ44.5ʹʹN 122°31ʹ39.1ʹʹE). The bay has been well researched in an IMTA context because farmers in Sanggou Bay have intentionally cultured species from multiple trophic levels in combination, and on a large scale, since at least 1996 (Fang et al. Citation1996; Mao et al. Citation2006). Finfish are produced in the inner bay, and scallops and oysters are cultivated in the mid-bay (Mahmood et al. Citation2016b). There is a mixed-culture zone where bivalves and macroalgae are farmed in combination in the outer-mid-bay and then toward the mouth of the bay macroalgal culture is dominant because of the optimal hydrodynamics there (Mahmood et al. Citation2016a). Successful nutrient transfer is shown between trophic levels and more recently scientists and farmers have worked together to improve production ratios by managing trophic levels and assessing the feasibility of various mariculture schemes (Ren et al. Citation2014; Liu and Su Citation2015).
Geographic distribution of IMTA
Research on IMTA has been heavily centered in Shandong province in northern China (), probably because Sanggou Bay has served as a model for large-scale IMTA research since 1996 and because Shandong province is home to more than 70% of the marine research institutions in China (Fang et al. Citation1996; Ding, Ge, and Casey Citation2014). Although few studies have assessed the applications of IMTA in southern China the region is not short of valuable, commercial species from low trophic levels. For example, the seaweed Gracilaria lemaneiformis (Yang et al. Citation2015), scallop Chlamys nobilis (Guo and Luo Citation2006), and oyster Crassostrea hongkongensis (Lam and Morton Citation2003) are all commercially cultivated in southern China and can be tested in large-scale IMTA systems, but so far integrating low trophic level species with fed species remains experimental (e.g., Yu et al. Citation2012; Yu et al. Citation2014b). To elucidate potential climatic influences that may act as drivers to the success of commercial IMTA in northern China, new research could focus on commercial-scale IMTA in provinces south of Shandong. The details of extractive species that have been studied for IMTA systems in China are presented in Appendix S4: Table S1.
The benefits of IMTA in China
The potential benefits of IMTA are well reviewed internationally (e.g., Chopin et al. Citation2001; Neori et al. Citation2007; Troell et al. Citation2009). The benefits of IMTA in China have not been reviewed previously. China’s IMTA research has been directed at the core benefits of bioremediation and/or biomitigation while other benefits have been studied less frequently ().
Bioremediation/biomitigation
Bioremediation or biomitigation of waste products can be achieved by cultivating low trophic-level organisms to assimilate farm waste and convert it to a harvestable biomass (Troell et al. Citation2009). For example, suspension feeding bivalves are suitable for processing suspended particulate wastes, and seaweeds are suitable for absorbing dissolved nutrients (Zhou et al. Citation2006c; Wu et al. Citation2015a). Energy transfer between trophic levels has been confirmed in several studies using stable isotope analysis and/or fatty acid profiling (e.g., Gao et al. Citation2006; Jiang et al. Citation2013; Mahmood et al. Citation2016b). The bioremediation potential of several species has been demonstrated (e.g., He et al. Citation2008; Yu et al. Citation2014a; Appendix S4, Table S1). In Jiaozhou Bay the production of 200,000 tons of Manila clam has been estimated to filter the entire volume of the bay in under 1 week, substantially reducing the occurrence of eutrophication there (Xiao et al. Citation2007). It has been proposed that, when low trophic-level species are favored, top-down control from aquaculture can help to maintain the ecological structure and function of bays (Zhou et al. Citation2006d). In Xiangshan Bay it was shown that a yield of 30.4 tons of G. lemaneiformis removed nearly 94 kg of N and 13 kg of P and several water quality parameters including dissolved oxygen and pH were improved (Xu et al. Citation2008). He et al. (Citation2016) has hypothesized that various algae can be used to extract selected, unwanted pharmaceuticals from the water. Additionally, IMTA systems are also shown to effectively sequester atmospheric CO2 released by factories in the region (Tang, Zhang, and Fang Citation2011).
Increased farm production
Increased farm production is beneficial because culture organisms reach size-at-harvest more rapidly or the total harvestable biomass that can be produced in a site is increased (Neori et al. Citation2004; Sara et al. Citation2012). In Ailian Bay, Shandong, Pacific oyster Crassostrea gigas grew significantly larger shell heights and flesh dry weights at a fish cage area compared to a control area (Jiang et al. Citation2013). In Sanggou Bay, Shandong, sea urchin Hemicentrotus pulcherrimus have been used experimentally to control the biofouling on scallop C. farreri cages and scallop soft tissue grew larger, but shell size did not differ, in the presence of sea urchins (Qi et al. Citation2014). In Daya Bay, Guangdong, Sargassum hemiphyllum and Sargassum henslowianum had significantly faster growth rates and reached a larger size in a fish culture zone compared with naturally occurring wild populations (Yu et al. Citation2014b). Increased production can ultimately help to improve overall farm profitability.
Other benefits
The seven other benefits of IMTA remain poorly demonstrated and require further investigation in China but some preliminary information has been published ().
Improved function of the surrounding ecosystem is achieved when extractive species contribute to maintaining the environmental health of mariculture areas. In Hongsha Bay, Hainan, the macroalga Eucheuma gelatinae and bivalve Gafrarium tumidum were successfully used to reduce eutrophication and maintain microalgal density at acceptable levels (Li, Yu, and Peng Citation2015). Macroalgae can inhibit the growth of microalgae by competition for nutrients, inhibitory allelopathy, or by reducing light penetration (Zhou et al. Citation2006c; Wang et al. Citation2007b; Yang et al. Citation2015).
Improved financial return and improved overall sustainability are secondary benefits of IMTA that can result from increased production, decreased stock mortality, improved product quality, and improved public opinion and social benefits. Decreased mortality of the finfish Sebastodes fuscescens and the scallop C. farreri was achieved by integrating the macroalga G. lemaneiformis because the algae improved the aquaculture environment overall (Zhou et al. Citation2006a; Mao et al. Citation2009). Improved product quality is possible when IMTA systems improve the composition or appearance of products. S. hemiphyllum cultured with fish had a higher crude protein content, favorable for its use as fodder for abalone, compared to samples harvested from the wild (Yu et al. Citation2014b). The yield of premium quality pearls from Pinctada martensi was consistently higher when co-cultivated with the macroalga Kappaphycus alvarezii (Wu et al. Citation2003). The social benefits and improved public opinion associated with IMTA remain undemonstrated in China, but the possibility of these benefits has been discussed and should be investigated properly to help motivate wider implementation of IMTA (Troell et al. Citation2009; Fang et al. Citation2016b; Tang, Ying, and Wu Citation2016).
Soto (Citation2009) proposed the potential for IMTA systems to assist in the control of aquaculture pathogens. In IMTA systems internationally, bivalves can help control luminous bacterial disease in shrimp (Tendencia Citation2007). In China the sponge Hymeniacidon perleve collected from the Yellow Sea effectively removed the common aquaculture pathogens Escherichia coli and Vibrio anguillarum II from seawater (Fu et al. Citation2006). The mechanisms that drive the control of pathogens in IMTA systems remain unclear, but the preliminary evidence presented here warrants further investigation.
Improved social benefits and public opinion have been associated with the implementation of IMTA (Fang et al. Citation2016b). Potential social benefits could result from increased employment on farms that practice IMTA or in industries that support IMTA, such as hatcheries that are necessary to ensure a steady supply of IMTA species seed stock. Improved public opinion could arise because IMTA can have a reputation for being an environmentally friendly farming technique (Alexander et al. Citation2016). This in turn would present opportunities for producers to eco-label premium products. However, in China, the potential for social benefits and improved public opinion associated with IMTA requires further investigation.
Potential challenges to IMTA in China
The challenges facing the development of marine industries in China were reviewed by Ding, Ge, and Casey (Citation2014). There are several challenges facing IMTA that could hinder its implementation.
Limited new technology and skills
Despite China’s role as the global leader of aquaculture production, the country has been slow in adopting new skills and technology to improve aquaculture techniques (Li et al. Citation2011). This is particularly true in the suspended mariculture sector where the focus has been on expanding production without technical progress or improvements in farm efficiency. Husbandry techniques remain largely traditional and inefficient. Engineered IMTA systems, at any scale, require advanced technology and skills for activities like water quality monitoring and regular assessments of stock growth and health (Mao et al. Citation2006). Large-scale IMTA systems are particularly challenging because, in most places, there is not enough information on how the separate system components interact and function as a whole (Fang et al. Citation2016b). To overcome these difficulties, training programs in modern husbandry, recruitment of a younger generation of aquaculturists, and the use of advanced aquaculture technology are recommended. Furthermore, a division in expertise in the suspended mariculture supply chain is encouraged such that individual production components are managed optimally.
Decreasing production of low trophic levels
Globally there has been an increase in the weighted mean annual trophic level of cultivated species, and the number of finfish species under commercial cultivation (Campbell and Pauly Citation2013). There has been a trend of “farming up the food web,” resulting in a decline in the production of low trophic-level species from aquaculture (Tacon et al. Citation2009). Market prices for low trophic-level organisms in China will probably determine the future scale of their culture (Fang et al. Citation1996). Although finfish mariculture production levels remain low, their proportion in total mariculture output has been increasing: from 1994 to 2004, 2014 and 2015 production expanded from 2.93% to 4.42%, 6.56%, and 7.26% of total production (MOA 2016). To prevent a continued move away from low trophic-level species, further research on alternate high-value uses of these organisms is recommended. In addition, the potential for implementing inter-industry nutrient trading schemes based on the extractive capabilities of low trophic-level species should be explored.
Biogeographic and temporal barriers
Biogeographic barriers arise from the wide longitudinal distribution of China’s coastline. Temporal barriers arise from seasonal changes to local conditions. Aquaculture techniques and species that are used in the cold north are not suitable to the tropical south. The environmental implications of aquaculture operations are case specific (Gao et al. Citation2005). In Yantian Bay, in Fujian, no commercial seaweed cultivation occurs in the warm seasons from late spring to early autumn, whereas in north China various species of macroalgae can be cultivated all year (Wu et al. Citation2015a). Optimization of stocking ratios at the species level is key to implementing IMTA systems (Tang et al. Citation2015), but ratios of fish, primary producers, filter feeders, and deposit feeders vary seasonally based on farm production regimes and the metabolic rates of the culture organisms (Ren and Zhang Citation2016). To overcome these barriers, exploration of indigenous species that could hold commercial value must be conducted to expand site- and season-specific lists of IMTA candidate species. Where information is lacking, a precautionary approach is recommended. The cultivation of extractive species should be prioritized while the addition of fed species should proceed cautiously based on recommendations from site-specific environmental monitoring.
Negative feedbacks
Negative feedbacks can result because newly added extractive species can themselves impact the environment or because the interactions between multiple species in a small area can have adverse consequences. In bivalve aquaculture, for example, there are carrying capacity issues to consider due to the consumption of food directly from the water column (Duarte et al. Citation2003; Zhou et al. Citation2006c). In addition, bivalves may only partially assimilate large volumes of suspended particulates that are then converted to settling matter and can substantially contribute to the onset of anoxia in benthic environments (Ren and Zhang Citation2016). In Jiaozhou Bay, Shandong, the strong top-down control of shellfish aquaculture impacts the spawning, nursery and feeding grounds of benthic fish (Xiao et al. Citation2007). Macroalgal cultivation can also impact the environment. In Sanggou Bay Saccharina japonica was shown to lose culture biomass through loss of entire individuals, breakage of thalli, and erosion of distal tissue that resulted in 61% of total carbon and 54% of total nitrogen produced in the tissues being lost to the environment (Zhang et al. Citation2012). There is also the possibility that macroalgae can totally outcompete phytoplankton for essential nutrients, inhibiting the food source of filter feeding bivalves (Duarte et al. Citation2003).
Some interspecies interactions in IMTA are not favorable. Tissue samples from the macroalga S. henslowianum cultivated near fish cages showed significantly higher concentrations of the metals Cr, Pb, and Cd compared with samples from a wild population, probably due to the high concentration of these metals in feed and antifouling paints (Yu et al. Citation2014b). To realistically assess the suitability and benefits of IMTA at local scales, the positive and negative feedbacks of implementing these systems must be studied (Hawkins et al. Citation2002).
Conclusions
In China mariculture produces more biomass per hectare than freshwater aquaculture. Mariculture production in Shandong, Fujian, Guangdong, and Liaoning is well established, but there is scope for the development of mariculture in Hebei, Jiangsu, Zhejiang, Guangxi, and Hainan. While the suspended mariculture industry is still heavily based on traditional and inefficient methods, the likelihood of fully utilizing the space suitable for mariculture development will depend on the implementation of sustainable industry practices. The impacts from suspended mariculture have been well studied in Shandong and Guangdong, but there is limited information for all other provinces. Impacts can be categorized as chemical, ecological, physical, and socioeconomic. Chemical impacts have been well studied but relatively few studies have focused on ecological, physical, or socioeconomic impacts. Minimizing impacts, and preserving the condition of suspended mariculture areas, is probably best achieved by a combination of management measures at government department, farm management, and ecological engineering levels. Of all the recommendations made in published research, implementing IMTA is the most frequently recommended. However, Shandong remains the only province in which the applications of IMTA have been methodically investigated. Further research in all provinces is encouraged. The benefits of bioremediation, biomitigation, and increased farm production have been the most frequently demonstrated, but little information has been published on other potential benefits. The challenges currently facing the expansion of engineered, commercial IMTA include limited implementation of new technology, limited development of new skills, decreasing production of low trophic-level species, biogeographic and temporal barriers, and negative system feedback.
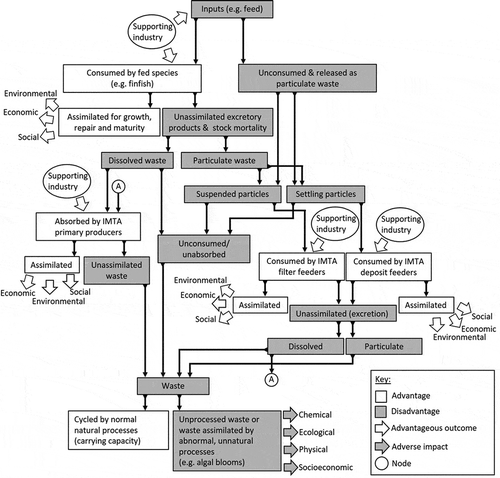
Further research is essential for developing engineered IMTA systems that are well adapted to a variety of species and environmental circumstances. Fundamental research such as the exploration of candidate IMTA species, IMTA system designs, and the microeconomics of IMTA systems is necessary. In addition, forward-looking research into supporting industries and the implementation of affordable technology is recommended. Husbandry practices could move toward automation of basic tasks such as water quality monitoring, feeding, and size sorting. Aquaculture information management systems could be developed for stock health monitoring, regulating food safety, and information sharing amongst aquaculture associations. To facilitate the continued growth of IMTA in China, research should be coordinated around a relevant framework that forms the basis of a research, development, and implementation continuum ().
Figure 8. Integrated Multi-Trophic Aquaculture (IMTA) concept model outlining the various system components in the production process that could be incorporated into a research, development and implementation continuum for the expansion of engineered IMTA in China.
Improving the sustainability of suspended mariculture is a prerequisite to increasing the supply of marine products from China. Despite several clear challenges facing commercial IMTA, its implementation and development are encouraged. A move toward implementing engineered IMTA systems across China could help to alleviate some of the anthropogenic pressure facing China’s coastal zones and increase the environmental, economic, and social sustainability of the suspended mariculture industry.
Appendix_S4.docx
Download MS Word (20.4 KB)Appendix_S3.docx
Download MS Word (24.4 KB)Appendix_S2.docx
Download MS Word (35.2 KB)Appendix_S1.docx
Download MS Word (19.6 KB)Acknowledgment
This work was supported by the Sustainable Ecological Aquaculture project of the City University of Hong Kong (No. 9610320), the Sustainable Fisheries Development Fund of the Hong Kong Agriculture Fisheries and Conservation Department (SFDF-0016) and the Science, Technology and Innovation Commission of the Shenzhen Municipality, China (ZDSYSY20140509155229806). We thank Mr. Khem Limbu, City University of Hong Kong, for assistance collating relevant literature.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Alexander, K. A., D. Angel, S. Freeman, D. Israel, J. Johansen, D. Kletou, M. Meland, et al. 2016. “Improving Sustainability of Aquaculture in Europe: Stakeholder Dialogues on Integrated Multi-Trophic Aquaculture (IMTA).” Environmental Science and Policy 55: 1–20. doi:10.1016/j.envsci.2015.09.006.
- Beveridge, M. C. M. 1984. “Cage and Pen Fish Farming: Carrying Capacity Models and Environmental Impact.” Page FAO Fisheries technical paper (255). Rome: Food and Agriculture Organisation of the United Nations.
- Bondad-Reantaso, M. G., R. P. Subasinghe, J. R. Arthur, K. Ogawa, S. Chinabut, R. Adlard, Z. Tan, and M. Shariff. 2005. “Disease and Health Management in Asian Aquaculture.” Veterinary Parasitology 132: 249–272. doi:10.1016/j.vetpar.2005.07.005.
- Cai, H., L. G. Ross, T. C. Telfer, C. Wu, A. Zhu, S. Zhao, and M. Xu. 2016. “Modelling the Nitrogen Loadings from Large Yellow Croaker (Larimichthys Crocea) Cage Aquaculture.” Environmental Science and Pollution Research 23: 7529–7542. doi:10.1007/s11356-015-6015-0.
- Campbell, B., and D. Pauly. 2013. “Mariculture: A Global Analysis of Production Trends since 1950.” Marine Policy 39: 94–100. doi:10.1016/j.marpol.2012.10.009.
- Cao, L., W. Wang, Y. Yang, C. Yang, Z. Yuan, S. Xiong, and J. Diana. 2007. “Environmental Impact of Aquaculture and Countermeasures to Aquaculture Pollution in China.” Environmental Science and Pollution Research International 14: 452–462. doi:10.1065/espr2007.05.426.
- Chang, Y., J. Zhang, J. Qu, Z. Jiang, and R. Zhang. 2016. “Infuence of Mariculture on the Distribution of Dissolved Inorganic Selenium in Sanggou Bay.” Northern China 8: 247–260.
- Chen, H., S. Liu, X. R. Xu, S. S. Liu, G. J. Zhou, K. F. Sun, J. L. Zhao, and G. G. Ying. 2015. “Antibiotics in Typical Marine Aquaculture Farms Surrounding Hailing Island, South China: Occurrence, Bioaccumulation and Human Dietary Exposure.” Marine Pollution Bulletin 90: 181–187. doi:10.1016/j.marpolbul.2014.10.053.
- Chopin, T. 2015. "Marine Aquaculture In Canada: Well-established monocultures of Finfish and Shellfish and an Emerging Integrated Multi-trophic Aquaculture (Imta) Approach Including seaweeds, Other Invertebrates, and Microbial Communities." Fisheries 40: 28–31. doi: 10.1080/03632415.2014.986571.
- Chopin, T., A. H. Buschmann, C. Halling, M. Troell, N. Kautsky, A. Neori, G. P. Kraemer, J. A. Zertuche-González, C. Yarish, and C. Neefus. 2001. “Integrating Seaweeds into Marine Aquaculture Systems: A Key toward Sustainability.” Journal of Phycology 37: 975–986. doi:10.1046/j.1529-8817.2001.01137.x.
- Chopin, T., C. Yarish, R. Wilkes, E. Belyea, and S. Lu. 1999. “Salmon Integrated Aquaculture for Bioremediation and Diversification of the Aquaculture Industry.” Journal of Applied Phycology 11: 463–472. doi:10.1023/A:1008114112852.
- Ding, J., X. Ge, and R. Casey. 2014. ““Blue Competition” in China: Current Situation and Challenges.” Marine Policy 44: 351–359. doi:10.1016/j.marpol.2013.09.028.
- Dong, Z., D. Liu, and J. K. Keesing. 2010. “Jellyfish Blooms in China: Dominant Species, Causes and Consequences.” Marine Pollution Bulletin 60: 954–963. doi:10.1016/j.marpolbul.2010.04.022.
- Duan, L.-Q., J.-M. Song, H.-M. Yuan, X.-G. Li, and N. Li. 2016. “Distribution, Partitioning and Sources of Dissolved and Particulate Nitrogen and Phosphorus in the North Yellow Sea.” Estuarine, Coastal and Shelf Science 181: 182–195. doi:10.1016/j.ecss.2016.08.044.
- Duarte, P., R. Meneses, A. J. S. Hawkins, M. Zhu, J. Fang, and J. Grant. 2003. “Mathematical Modelling to Assess the Carrying Capacity for Multi-Species Culture within Coastal Waters.” Ecological Modelling 168: 109–143. doi:10.1016/S0304-3800(03)00205-9.
- Edwards, P. 2015. “Aquaculture Environment Interactions: Past, Present and Likely Future Trends.” Aquaculture 447: 2–14. doi:10.1016/j.aquaculture.2015.02.001.
- Fang, J., H. Sun, J. Yan, G. F. Newkirk, and J. Grant. 1996. “Polyculture of Scallop Chlamys Farreri and Kelp Laminaria Japonica in Sungo Bay.” Chinese Journal of Oceanology and Limnology 14: 322–329. doi:10.1007/BF02850552.
- Fang, J., J. Zhang, T. Xiao, D. Huang, and S. Liu. 2016b. “Integrated Multi-Trophic Aquaculture (IMTA) in Sanggou Bay, China.” Aquaculture Environment Interactions 8: 201–205. doi:10.3354/aei00179.
- Fang, J., Z. Li, Z. Jiang, and Q. Wang. 2016a. “Development Strategy for Ecological Aquaculture and New Mode of Aquacultural Farming.” Chinese Journal of Engineering Science (In Chinese) 18: 22–28.
- Fang, S., L. Bao, and E. Y. Zeng. 2016. “Source Apportionment Of Ddts In Maricultured Fish: A Modeling Study in South China.” Environmental Science And Pollution Research 23: 7162–7168.
- FAO. 2014. The State of World Fisheries and Aquaculture 2014. Rome: Food and Agriculture Organisation of the United Nations.
- FAO. 2016. The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All. Rome: Food and Agriculture Organisation of the United Nations.
- Feng, Y. Y., L. C. Hou, N. X. Ping, T. D. Ling, and C. I. Kyo. 2004. “Development of Mariculture and Its Impacts in Chinese Coastal Waters.” Reviews in Fish Biology and Fisheries 14: 1–10. doi:10.1007/s11160-004-3539-7.
- Ferreira, J. G., A. Sequeira, A. J. S. Hawkins, A. Newton, T. D. Nickell, R. Pastres, J. Forte, A. Bodoy, and S. B. Bricker. 2009. “Analysis of Coastal and Offshore Aquaculture: Application of the FARM Model to Multiple Systems and Shellfish Species.” Aquaculture 292: 129–138. doi:10.1016/j.aquaculture.2009.03.039.
- Fu, W., L. Sun, X. Zhang, and W. Zhang. 2006. “Potential of the Marine Sponge Hymeniacidon Perleve as a Bioremediator of Pathogenic Bacteria in Integrated Aquaculture Ecosystems.” Biotechnology and Bioengineering 93: 1112–1122. doi:10.1002/(ISSN)1097-0290.
- Gao, Q. F., K. L. Cheung, S. G. Cheung, and P. K. S. Shin. 2005. “Effects of Nutrient Enrichment Derived from Fish Farming Activities on Macroinvertebrate Assemblages in a Subtropical Region of Hong Kong.” Marine Pollution Bulletin 51: 994–1002. doi:10.1016/j.marpolbul.2005.01.009.
- Gao, Q. F., P. K. S. Shin, G. H. Lin, S. P. Chen, and G. C. Siu. 2006. “Stable Isotope and Fatty Acid Evidence for Uptake of Organic Waste by Green-Lipped Mussels Perna Viridis in a Polyculture Fish Farm System.” Marine Ecology Progress Series 317: 273–283. doi:10.3354/meps317273.
- Grant, J., and C. Bacher. 2001. “A Numerical Model of Flow Modification Induced by Suspended Aquaculture in A Chinese Bay.” Canadian Journal of Fisheries and Aquatic Sciences 58: 1003–1011. doi:10.1139/f01-027.
- Gu, Y. G., Q. Lin, S. J. Jiang, and Z. H. Wang. 2014. “Metal Pollution Status in Zhelin Bay Surface Sediments Inferred from a Sequential Extraction Technique, South China Sea.” Marine Pollution Bulletin 81: 256–261. doi:10.1016/j.marpolbul.2014.01.030.
- Guo, X., and Y. Luo. 2006. “Scallop Culture in China.” In Scallops: Biology, Ecology and Aquaculture, edited by S. E. Shumway and G. J. Parsons, 1143–1161. 2nd ed. Boston: Elsevier Science.
- Han, Q., J. K. Keesing, and D. Liu. 2016. “A Review of Sea Cucumber Aquaculture, Ranching, and Stock Enhancement in China.” Reviews in Fisheries Science & Aquaculture 24: 326–341. doi:10.1080/23308249.2016.1193472.
- Han, Q., Y. Wang, Y. Zhang, J. Keesing, and D. Liu. 2013a. “Effects of Intensive Scallop Mariculture on Macrobenthic Assemblages in Sishili Bay, the Northern Yellow Sea of China.” Hydrobiologia 718: 1–15. doi:10.1007/s10750-013-1590-x.
- Han, T., Z. Jiang, J. Fang, J. Zhang, Y. Mao, J. Zou, Y. Huang, and D. Wang. 2013b. “Carbon Dioxide Fixation by the Seaweed Gracilaria Lemaneiformis in Integrated Multi-Trophic Aquaculture with the Scallop Chlamys Farreri in Sanggou Bay, China.” Aquaculture International 21: 1035–1043. doi:10.1007/s10499-012-9610-9.
- Hawkins, A. J. S., P. Duarte, J. G. Fang, P. L. Pascoe, J. H. Zhang, X. L. Zhang, and M. Y. Zhu. 2002. “A Functional Model of Responsive Suspension-Feeding and Growth in Bivalve Shellfish, Configured and Validated for the Scallop Chlamys Farreri during Culture in China.” Journal of Experimental Marine Biology and Ecology 281: 13–40. doi:10.1016/S0022-0981(02)00408-2.
- He, P., S. Xu, H. Zhang, S. Wen, Y. Dai, S. Lin, and C. Yarish. 2008. “Bioremediation Efficiency in the Removal of Dissolved Inorganic Nutrients by the Red Seaweed, Porphyra yezoensis, Cultivated in the Open Sea.” Water Research 42: 1281–1289. doi:10.1016/j.watres.2007.09.023.
- He, Z., X. Cheng, G. Z. Kyzas, and J. Fu. 2016. “Pharmaceuticals Pollution of Aquaculture and Its Management in China.” Journal of Molecular Liquids 223: 781–789. doi:10.1016/j.molliq.2016.09.005.
- Hou, J., G. Zhang, M. Sun, and W. Ye. 2016. “Methane Distribution, Sources, and Sinks in an Aquaculture Bay (Sanggou Bay, China).” Aquaculture Environment Interactions 8: 481–495.
- Hu, B.-T. 1994. “Cage Culture Development and Its Role in Aquaculture in China.” Aquaculture and Fisheries Management 24: 305–310.
- Jiang, Z., G. Wang, J. Fang, and Y. Mao. 2013. “Growth and Food Sources of Pacific Oyster Crassostrea Gigas Integrated Culture with Sea Bass Lateolabrax japonicus in Ailian Bay, China.” Aquaculture International 21: 45–52. doi:10.1007/s10499-012-9531-7.
- Jiang, Z. B., Q. Z. Chen, J. N. Zeng, Y. B. Liao, L. Shou, and J. Liu. 2012. “Phytoplankton Community Distribution in Relation to Environmental Parameters in Three Aquaculture Systems in a Chinese Subtropical Eutrophic Bay.” Marine Ecology Progress Series 446: 73–89. doi:10.3354/meps09499.
- Kang, X., S. Liu, and X. Ning. 2016. “Reduced Inorganic Sulfur in Sediments of the Mariculture Region of Sanggou Bay, China.” Aquaculture Environment Interactions 8: 233–246. doi:10.3354/aei00154.
- Kang, Y. H., J. R. Hwang, I. K. Chung, and S. R. Park. 2013. “Development of a Seaweed Species-Selection Index for Successful Culture in a Seaweed-Based Integrated Aquaculture System.” Journal of Ocean University of China 12: 125–133. doi:10.1007/s11802-013-1928-z.
- Keesing, J. K., D. Liu, P. Fearns, and R. Garcia. 2011. “Inter- and Intra-Annual Patterns of Ulva Prolifera Green Tides in the Yellow Sea during 2007-2009, Their Origin and Relationship to the Expansion of Coastal Seaweed Aquaculture in China.” Marine Pollution Bulletin 62: 1169–1182. doi:10.1016/j.marpolbul.2011.03.040.
- Klumpp, D. W., H. Huasheng, C. Humphrey, W. Xinhong, and S. Codi. 2002. “Toxic Contaminants and Their Biological Effects in Coastal Waters of Xiamen, China.” I Organic Pollutants in Mussel and Fish Tissues Marine Pollution Bulletin 44: 752–760.
- Lai, L., and T. Yu. 1995. “The “Hong Kong” Solution to the Overfishing Problem: A Study of the Cultured Fish Industry in Hong Kong.” Managerial and Decision Economics 16: 525–535. doi:10.1002/(ISSN)1099-1468.
- Lam, K., and B. Morton. 2003. “Mitochondrial DNA and Morphological Identification of a New Species of Crassostrea (Bivalvia: Ostreidae) Cultured for Centuries in the Pearl River Delta, Hong Kong, China.” Aquaculture 228: 1–13. doi:10.1016/S0044-8486(03)00215-1.
- Lee, J. H. W., K. W. Choi, and F. Arega. 2003. “Environmental Management of Marine Fish Culture in Hong Kong.” Marine Pollution Bulletin 47: 202–210. doi:10.1016/S0025-326X(02)00410-1.
- Lee, S. Y. 2016. “From Blue to Black: Anthropogenic Forcing of Carbon and Nitrogen Influx to Mangrove-Lined Estuaries in the South China Sea.” Marine Pollution Bulletin 109: 682–690. doi:10.1016/j.marpolbul.2016.01.008.
- Leung, K. M. Y., J. C. W. Chu, and R. S. S. Wu. 1999. “Nitrogen Budgets for the Areolated Grouper Epinephelus areolatus Cultured under Laboratory Conditions and in Open-Sea Cages.” Marine Ecology Progress Series 186: 271–281. doi:10.3354/meps186271.
- Li, C., X. Yu, and M. Peng. 2015. “The Roles of Polyculture with Eucheuma gelatinae and Gafrarium tumidum in Purification of Eutrophic Seawater and Control of Algae Bloom.” Marine Pollution Bulletin 101: 750–757. doi:10.1016/j.marpolbul.2015.10.001.
- Li, X., J. Li, Y. Wang, L. Fu, Y. Fu, B. Li, and B. Jiao. 2011. “Aquaculture Industry in China: Current State, Challenges, and Outlook.” Reviews in Fisheries Science 19: 187–200. doi:10.1080/10641262.2011.573597.
- Liang, P., S. C. Wu, J. Zhang, Y. Cao, S. Yu, and M. H. Wong. 2016. “The Effects of Mariculture on Heavy Metal Distribution in Sediments and Cultured Fish around the Pearl River Delta Region, South China.” Chemosphere 148: 171–177. doi:10.1016/j.chemosphere.2015.10.110.
- Lin, J., C. Li, and S. Zhang. 2016. “Hydrodynamic Effect of a Large Offshore Mussel Suspended Aquaculture Farm.” Aquaculture 451: 147–155. doi:10.1016/j.aquaculture.2015.08.039.
- Lin, Y., Z. Gao, and A. Zhan. 2015. “Introduction and Use of Non-Native Species for Aquaculture in China: Status, Risks and Management Solutions.” Reviews in Aquaculture 7: 28–58. doi:10.1111/raq.2015.7.issue-1.
- Liu, D., J. K. Keesing, P. He, Z. Wang, Y. Shi, and Y. Wang. 2013. “The World’s Largest Macroalgal Bloom in the Yellow Sea, China: Formation and Implications.” Estuarine, Coastal and Shelf Science 129: 2–10. doi:10.1016/j.ecss.2013.05.021.
- Liu, D., J. K. Keesing, Q. Xing, and P. Shi. 2009. “World’s Largest Macroalgal Bloom Caused by Expansion of Seaweed Aquaculture in China.” Marine Pollution Bulletin 58: 888–895. doi:10.1016/j.marpolbul.2009.01.013.
- Liu, H., and J. Su. 2015. “Vulnerability of China’s Nearshore Ecosystems under Intensive Mariculture Development.” Environmental Science and Pollution Research. doi:10.1007/s11356-015-5239-3.
- Liu, L., M. Ge, X. Zheng, Z. Tao, S. Zhou, and G. Wang. 2016. “Investigation of Vibrio alginolyticus, V. harveyi, and V. parahaemolyticus in Large Yellow Croaker, Pseudosciaena crocea (Richardson) Reared in Xiangshan Bay, China.” Aquaculture Reports 3: 220–224. doi:10.1016/j.aqrep.2016.04.004.
- Lu, D., Y. Qi, H. Gu, X. Dai, H. Wang, Y. Gao, -P.-P. Shen, Q. Zhang, R. Yu, and S. Lu. 2014a. “Causative Species of Harmful Algal Blooms in Chinese Coastal Waters.” Algological Studies 145/146: 145–168. doi:10.1127/1864-1318/2014/0161.
- Lu, J., L. Huang, T. Xiao, Z. Jiang, and W. Zhang. 2015. “The Effects of Zhikong Scallop (Chlamys farreri) on the Microbial Food Web in a Phosphorus-Deficient Mariculture System in Sanggou Bay, China.” Aquaculture 448: 341–349. doi:10.1016/j.aquaculture.2015.06.021.
- Lu, J., L. Huang, Y. Luo, T. Xiao, Z. Jiang, and L. Wu. 2014b. “Effects of Freshwater Input and Mariculture (Bivalves and Macroalgae) on Spatial Distribution of Nanoflagellates in Sungo Bay, China.” Aquaculture Environment Interactions 6: 191–203. doi:10.3354/aei00124.
- Luo, Y., and T. Yang. 2012. “Seasonal Patterns in the Community of Gill Monogeneans on Wild versus Cultured Orange-Spotted Grouper, Epinephelus coioides Hamilton, 1822 in Daya Bay, South China Sea.” Aquaculture Research 43: 1232–1242. doi:10.1111/j.1365-2109.2011.02927.x.
- Ma, Y., A. Hu, C. P. Yu, Q. Yan, X. Yan, Y. Wang, F. Deng, and H. Xiong. 2015. “Response of Microbial Communities to Bioturbation by Artificially Introducing Macrobenthos to Mudflat Sediments for in Situ Bioremediation in a Typical Semi-Enclosed Bay, Southeast China.” Marine Pollution Bulletin 94: 114–122. doi:10.1016/j.marpolbul.2015.03.003.
- Mahmood, T., J. Fang, Z. Jiang, and J. Zhang. 2016b. “Carbon, Nitrogen Flow and Trophic Relationship among the Cultured Species in an Integrated Multi-Trophic Aquaculture (IMTA) Bay.” Aquaculture Environment Interactions 8: 207–219. doi:10.3354/aei00152.
- Mahmood, T., J. Fang, Z. Jiang, W. Ying, and J. Zhang. 2016a. “Seasonal Distribution, Sources and Sink of Dissolved Organic Carbon in Integrated Aquaculture System in Coastal Waters.” Aquaculture International 25: 1–15.
- Mao, Y., H. Yang, Y. Zhou, N. Ye, and J. Fang. 2009. “Potential of the Seaweed Gracilaria lemaneiformis for Integrated Multi-Trophic Aquaculture with Scallop Chlamys Farreri in North China.” Journal of Applied Phycology 21: 649–656. doi:10.1007/s10811-008-9398-1.
- Mao, Y., Y. Zhou, H. Yang, and R. Wang. 2006. “Seasonal Variation in Metabolism of Cultured Pacific Oyster, Crassostrea Gigas, in Sanggou Bay, China.” Aquaculture 253: 322–333. doi:10.1016/j.aquaculture.2005.05.033.
- MOA. 1981–2016. China Fishery Statistics Yearbook of the Ministry of Agriculture of the People’s Republic of China. Beijing: China Ocean Press.
- MOA. 2009–2016. China Fishery Statistics Yearbook of the Ministry of Agriculture of the People’s Republic of China. Beijing: China Ocean Press.
- Naylor, R. L., R. J. Goldburg, J. H. Primavera, N. Kautsky, M. C. Beveridge, J. Clay, C. Folke, J. Lubchenco, H. Mooney, and M. Troell. 2000. “Effect of Aquaculture on World Fish Supplies.” Nature 405: 1017–1024. doi:10.1038/35016500.
- Neori, A. 2008. “Essential Role of Seaweed Cultivation in Integrated Multi-Trophic Aquaculture Farms for Global Expansion of Mariculture: An Analysis.” Journal of Applied Phycology 20: 567–570. doi:10.1007/s10811-007-9206-3.
- Neori, A., M. Troell, T. Chopin, C. Yarish, A. Critchley, and A. H. Buschmann. 2007. “The Need for a Balanced Ecosystem Approach to Blue Revolution Aquaculture.” Environment: Science and Policy for Sustainable Development 49: 36–43.
- Neori, A., T. Chopin, M. Troell, A. H. Buschmann, G. P. Kraemer, C. Halling, M. Shpigel, and C. Yarish. 2004. “Integrated Aquaculture: Rationale, Evolution and State of the Art Emphasizing Seaweed Biofiltration in Modern Mariculture.” Aquaculture 231: 361–391. doi:10.1016/j.aquaculture.2003.11.015.
- Nobre, A. M., J. K. Musango, M. P. de Wit, and J. G. Ferreira. 2009. “A Dynamic Ecological-Economic Modeling Approach for Aquaculture Management.” Ecological Economics 68: 3007–3017. doi:10.1016/j.ecolecon.2009.06.019.
- Nordgarden, U., F. Oppedal, G. L. Taranger, G. Hemre, and T. Hansen. 2003. “Seasonally Changing Metabolism in Atlantic Salmon (Salmo salar L.) I – Growth and Feed Conversion Ratio.” Aquaculture Nutrition 9: 287–293. doi:10.1046/j.1365-2095.2003.00256.x.
- Pang, T., J. Liu, Q. Liu, H. Li, and J. Li. 2015. “Observations on Pests and Diseases Affecting a Eucheumatoid Farm in China.” Journal of Applied Phycology 27: 1975–1984. doi:10.1007/s10811-014-0507-z.
- Qi, Z., J. Wang, Y. Mao, H. Liu, and J. Fang. 2013. “Feasibility of Offshore Co-Culture of Abalone, Haliotis discus Hannai ino, and Sea Cucumber, Apostichopus japonicus, in a Temperate Zone.” Journal of the World Aquaculture Society 44: 565–573. doi:10.1111/jwas.2013.44.issue-4.
- Qi, Z., J. Wang, Y. Mao, J. Zhang, Z. Jiang, and J. Fang. 2014. “Use of the Sea Urchin Hemicentrotus pulcherrimus for Biological Control of Fouling in Suspended Scallop Cultivation in Northern China.” Aquaculture 420–421: 270–274.
- Qi, Z., T. Han, J. Zhang, H. Huang, Y. Mao, Z. Jiang, and J. Fang. 2015. “First Report on in Situ Biodeposition Rates of Ascidians (Ciona intestinalis and Styela clava) during Summer in Sanggou Bay, Northern China.” Aquaculture Environment Interactions 6: 233–239. doi:10.3354/aei00129.
- Ren, L., and J. Zhang. 2016. “Temporal Variation in Biodeposit Organic Content and Sinking Velocity in Long-Line Shellfish Culture.” Chinese Journal of Oceanology and Limnology 34: 985–991. doi:10.1007/s00343-016-4242-y.
- Ren, L., J. Zhang, J. Fang, Q. Tang, M. Zhang, and M. Du. 2014. “Impact of Shellfish Biodeposits and Rotten Seaweed on the Sediments of Ailian Bay, China.” Aquaculture International 22: 811–819. doi:10.1007/s10499-013-9709-7.
- Ruddle, K., and G. Zhong. 1988. Integrated Agriculture-Aquaculture in the South of China: The Dike-Pond System in the Zhujiang Delta. Cambridge: Cambridge University Press.
- Sara, G., G. K. Reid, A. Rinaldi, V. Palmeri, M. Troell, and S. A. L. M. Kooijman. 2012. “Growth and Reproductive Simulation of Candidate Shellfish Species at Fish Cages in the Southern Mediterranean: Dynamic Energy Budget (DEB) Modelling for Integrated Multi-Trophic Aquaculture.” Aquaculture 324–325: 259–266. doi:10.1016/j.aquaculture.2011.10.042.
- Shi, J., and H. Wei. 2009. “Simulation of Hydrodynamic Structures in a Semi-Enclosed Bay with Dense Raft-Culture.” Periodical of Ocean University of China (In Chinese) 39: 1181–1187.
- Soto, D. 2009. “Integrated Mariculture: A Global Review.” FAO fisheries and aquaculture technical paper No. 529. Rome: Food and Agriculture Organisation of the United Nations.
- Tacon, A. G. J., M. Metian, G. M. Turchini, and S. S. De Silva. 2009. “Responsible Aquaculture and Trophic Level Implications to Global Fish Supply.” Reviews in Fisheries Science 18: 94–105. doi:10.1080/10641260903325680.
- Tang, J. Y., Y. X. Dai, Y. Wang, J. G. Qin, S. S. Su, and Y. M. Li. 2015. “Optimization of Fish to Mussel Stocking Ratio: Development of a State-Of-Art Pearl Production Mode through Fish-Mussel Integration.” Aquacultural Engineering 66: 11–16. doi:10.1016/j.aquaeng.2015.01.002.
- Tang, Q., J. Zhang, and J. Fang. 2011. “Shellfish and Seaweed Mariculture Increase Atmospheric CO2 Absorption by Coastal Ecosystems.” Marine Ecology Progress Series 424: 97–104. doi:10.3354/meps08979.
- Tang, Q., Y. Ying, and Q. Wu. 2016. “The Biomass Yields and Management Challenges for the Yellow Sea Large Marine Ecosystem.” Environmental Development 17: 175–181. doi:10.1016/j.envdev.2015.06.012.
- Tendencia, E. A. 2007. “Polyculture of Green Mussels, Brown Mussels and Oysters with Shrimp Control Luminous Bacterial Disease in a Simulated Culture System.” Aquaculture 272: 188–191. doi:10.1016/j.aquaculture.2007.07.212.
- Troell, M., A. Joyce, T. Chopin, A. Neori, A. H. Buschmann, and J. G. Fang. 2009. “Ecological Engineering in Aquaculture - Potential for Integrated Multi-Trophic Aquaculture (IMTA) in Marine Offshore Systems.” Aquaculture 297: 1–9. doi:10.1016/j.aquaculture.2009.09.010.
- Tseng, C. K. 1993. “Notes on Mariculture in China.” Aquaculture 111: 21–30. doi:10.1016/0044-8486(93)90021-P.
- Wang, H. S., Z. J. Chen, Z. Cheng, J. Du, Y. B. Man, H. M. Leung, J. P. Giesy, C. K. C. Wong, and M. H. Wong. 2014a. “Aquaculture-Derived Enrichment of Hexachlorocyclohexanes (Hchs) and Dichlorodiphenyltrichloroethanes (Ddts) in Coastal Sediments of Hong Kong and Adjacent Mainland China.” Science of the Total Environment 466–467: 214–220. doi:10.1016/j.scitotenv.2013.07.027.
- Wang, L., H. Zhang, L. Song, and X. Guo. 2007a. “Loss of Allele Diversity in Introduced Populations of the Hermaphroditic Bay Scallop Argopecten irradians.” Aquaculture 271: 252–259. doi:10.1016/j.aquaculture.2007.06.020.
- Wang, L., Y. Fan, C. Yan, C. Gao, Z. Xu, and X. Liu. 2017. “Assessing Benthic Ecological Impacts of Bottom Aquaculture Using Macrofaunal Assemblages.” Marine Pollution Bulletin 114: 258–268.
- Wang, S., D. Tang, F. He, Y. Fukuyo, and R. V. Azanza. 2008. “Occurrences of Harmful Algal Blooms (Habs) Associated with Ocean Environments in the South China Sea.” Hydrobiologia 596: 79–93. doi:10.1007/s10750-007-9059-4.
- Wang, S. L., X. R. Xu, Y. X. Sun, J. L. Liu, and H. B. Li. 2013. “Heavy Metal Pollution in Coastal Areas of South China: A Review.” Marine Pollution Bulletin 76: 7–15. doi:10.1016/j.marpolbul.2013.08.025.
- Wang, X. 1993. “Coastal Development and Environmental Protection.” UNEP Industrial Environment 15: 7–10.
- Wang, Y., Z. Yu, X. Song, X. Tang, and S. Zhang. 2007b. “Effects of Macroalgae Ulva pertusa (Chlorophyta) and Gracilaria lemaneiformis (Rhodophyta) on Growth of Four Species of Bloom-Forming Dinoflagellates.” Aquatic Botany 86: 139–147. doi:10.1016/j.aquabot.2006.09.013.
- Wang, Z., C. Chen, Y. Guo, and C. Liu. 2014b. “High Sequence Variation and Low Population Differentiation of Mitochondrial Control Regions of Wild Large Yellow Croaker in South China Sea.” Biochemical Systematics and Ecology 56: 151–157. doi:10.1016/j.bse.2014.05.019.
- Wang, Z., Y. Chen, S. Zhang, K. Wang, J. Zhao, and Q. Xu. 2015. “A Comparative Study of Fish Assemblages near Aquaculture, Artificial and Natural Habitats.” Journal of Ocean University of China 14: 149–160. doi:10.1007/s11802-015-2455-x.
- Williams, G. A., B. Helmuth, B. D. Russell, Y. Dong, V. Thiyagarajan, and L. Seuront. 2016. “Meeting the Climate Change Challenge : Pressing Issues in Southern China and SE Asian Coastal Ecosystems.” Regional Studies in Marine Science 8: 373–381. doi:10.1016/j.rsma.2016.07.002.
- Williams, M. 1997. “Aquaculture and Sustainable Food Security in the Developing World.” In Sustainable Aquaculture, edited by J. E. Bardach, 15–51, New York: Wiley.
- Wong, W. H., and S. G. Cheung. 2001. “Feeding Rates and Scope for Growth of Green Mussels, Perna viridis (L.) and Their Relationship with Food Availability in Kat O, Hong Kong.” Aquaculture 193: 123–137. doi:10.1016/S0044-8486(00)00478-6.
- Wu, H., Y. Huo, M. Hu, Z. Wei, and P. He. 2015a. “Eutrophication Assessment and Bioremediation Strategy Using Seaweeds Co-Cultured with Aquatic Animals in an Enclosed Bay in China.” Marine Pollution Bulletin 95: 342–349. doi:10.1016/j.marpolbul.2015.03.016.
- Wu, M., S. K. K. Mak, X. Zhang, and P. Y. Qian. 2003. “The Effect of Co-Cultivation on the Pearl Yield of Pinctada martensi (Dumker).” Aquaculture 221: 347–356. doi:10.1016/S0044-8486(03)00122-4.
- Wu, R. 1995. “The Environmental Impact of Marine Fish Culture: Towards a Sustainable Future.” Marine Pollution Bulletin 31: 159–166. doi:10.1016/0025-326X(95)00100-2.
- Wu, R. S. S., P. K. S. Shin, D. W. MacKay, M. Mollowney, and D. Johnson. 1999. “Management of Marine Fish Farming in the Sub-Tropical Environment: A Modelling Approach.” Aquaculture 174: 279–298. doi:10.1016/S0044-8486(99)00024-1.
- Xiao, J., S. E. Ford, H. Yang, G. Zhang, F. Zhang, and X. Guo. 2005. “Studies on Mass Summer Mortality of Cultured Zhikong Scallops (Chlamys farreri Jones Et Preston) in China.” Aquaculture 250: 602–615. doi:10.1016/j.aquaculture.2005.05.002.
- Xiao, Y., J. G. Ferreira, S. B. Bricker, J. P. Nunes, M. Zhu, and X. Zhang. 2007. “Trophic Assessment in Chinese Coastal Systems-Review of Methods and Application to the Changjiang (Yangtze) Estuary and Jiaozhou Bay.” Estuaries and Coasts 30: 901–918. doi:10.1007/BF02841384.
- Xie, B., J. Qin, H. Yang, X. Wang, Y. H. Wang, and T. Y. Li. 2013. “Organic Aquaculture in China: A Review from A Global Perspective.” Aquaculture 414–415: 243–253. doi:10.1016/j.aquaculture.2013.08.019.
- Xu, Q., F. Gao, H. Wang, and H. Yang. 2015. “Quality Indices as Potential Markers Indicating the Origin of Cultured Scallop (Argopecten irradians) in the North China Sea.” Journal of Shellfish Research 34: 743–750. doi:10.2983/035.034.0303.
- Xu, S., Z. Chen, S. Li, and P. He. 2011. “Modeling Trophic Structure and Energy Flows in a Coastal Artificial Ecosystem Using Mass-Balance Ecopath Model.” Estuaries and Coasts 34: 351–363. doi:10.1007/s12237-010-9323-0.
- Xu, Y., J. Fang, Q. Tang, J. Lin, G. Le, and L. Liao. 2008. “Improvement of Water Quality by the Macroalga, Gracilaria lemaneiformis (Rhodophyta), near Aquaculture Effluent Outlets.” Journal of the World Aquaculture Society 39: 549–555. doi:10.1111/j.1749-7345.2008.00180.x.
- Yang, Y., Z. Chai, Q. Wang, W. Chen, Z. He, and S. Jiang. 2015. “Cultivation of Seaweed Gracilaria in Chinese Coastal Waters and Its Contribution to Environmental Improvements.” Algal Research 9: 236–244. doi:10.1016/j.algal.2015.03.017.
- Yang, Y. F., X. G. Fei, J. M. Song, H. Y. Hu, G. C. Wang, and I. K. Chung. 2006. “Growth of Gracilaria lemaneiformis under Different Cultivation Conditions and Its Effects on Nutrient Removal in Chinese Coastal Waters.” Aquaculture 254: 248–255. doi:10.1016/j.aquaculture.2005.08.029.
- Ye, T., H. Jia, Y. Lei, P. Shi, and J. Wang. 2016. “Droughts in China.” In Natural disasters in China, edited by P. Shi, 161–186. Berlin: Springer Berlin Heidelberg.
- Yu, F., F. Cai, J. Ren, and J. Liu. 2016a. “Island Beach Management Strategy in China with Different Urbanization Level - Take Examples of Xiamen Island and Pingtan Island.” Ocean and Coastal Management 130: 328–339. doi:10.1016/j.ocecoaman.2016.07.007.
- Yu, H. Y., B. Z. Zhang, J. P. Giesy, and E. Y. Zeng. 2011b. “Persistent Halogenated Compounds in Aquaculture Environments of South China: Implications for Global Consumers’ Health Risk via Fish Consumption.” Environment International 37: 1190–1195. doi:10.1016/j.envint.2011.04.012.
- Yu, H. Y., L. J. Bao, C. S. Wong, Y. Hu, and E. Y. Zeng. 2012. “Sedimentary Loadings and Ecological Significance of Polycyclic Aromatic Hydrocarbons in a Typical Mariculture Zone of South China.” Journal of Environmental Monitoring 14: 2685–2691. doi:10.1039/c2em30292f.
- Yu, H. Y., Y. Guo, L. J. Bao, Y. W. Qiu, and E. Y. Zeng. 2011a. “Persistent Halogenated Compounds in Two Typical Marine Aquaculture Zones of South China.” Marine Pollution Bulletin 63: 572–577. doi:10.1016/j.marpolbul.2010.12.006.
- Yu, Z., S. M. C. Robinson, J. Xia, H. Sun, and C. Hu. 2016c. “Growth, Bioaccumulation and Fodder Potentials of the Seaweed Sargassum hemiphyllum Grown in Oyster and Fish Farms of South China.” Aquaculture 464: 459–468. doi:10.1016/j.aquaculture.2016.07.031.
- Yu, Z., X. Zhu, Y. Jiang, P. Luo, and C. Hu. 2014b. “Bioremediation and Fodder Potentials of Two Sargassum Spp. in Coastal Waters of Shenzhen, South China.” Marine Pollution Bulletin 85: 797–802. doi:10.1016/j.marpolbul.2013.11.018.
- Yu, Z., Y. Zhou, H. Yang, Y. Ma, and C. Hu. 2014a. “Survival, Growth, Food Availability and Assimilation Efficiency of the Sea Cucumber Apostichopus japonicus Bottom-Cultured under a Fish Farm in Southern China.” Aquaculture 426–427: 238–248. doi:10.1016/j.aquaculture.2014.02.013.
- Yu, Z.-L., Q. Lin, Y.-G. Gu, C.-L. Ke, and R.-X. Sun. 2016b. “Spatial–Temporal Trend and Health Implications of Polycyclic Aromatic Hydrocarbons (Pahs) in Resident Oysters, South China Sea: A Case Study of Eastern Guangdong Coast.” Marine Pollution Bulletin 110: 203–211. doi:10.1016/j.marpolbul.2016.06.061.
- Zeng, D., D. Huang, X. Qiao, Y. He, and T. Zhang. 2015. “Effect of Suspended Kelp Culture on Water Exchange as Estimated by in Situ Current Measurement in Sanggou Bay, China.” Journal of Marine Systems 149: 14–24. doi:10.1016/j.jmarsys.2015.04.002.
- Zhang, J., J. Fang, W. Wang, M. Du, Y. Gao, and M. Zhang. 2012. “Growth and Loss of Mariculture Kelp Saccharina japonica in Sungo Bay, China.” Journal of Applied Phycology 24: 1209–1216. doi:10.1007/s10811-011-9762-4.
- Zhang, X., X. Huang, and L. Huang. 2013. “Phytoplankton Community Structure Shaped by Key Environmental Factors in Fish and Shellfish Farms in Daya Bay, South China.” Aquatic Ecosystem Health & Management 16: 300–310.
- Zhao, L., Y. Zhao, J. Xu, W. Zhang, L. Huang, Z. Jiang, J. Fang, and T. Xiao. 2016. “Distribution and Seasonal Variation of Picoplankton in Sanggou Bay, China.” Aquaculture Environment Interactions 8: 261–271. doi:10.3354/aei00168.
- Zhao, S., K. Song, F. Gui, H. Cai, W. Jin, and C. Wu. 2013. “The Emergy Ecological Footprint for Small Fish Farm in China.” Ecological Indicators 29: 62–67. doi:10.1016/j.ecolind.2012.12.009.
- Zhong, Y., and G. Power. 1997. “Fisheries in China: Progress, Problems, and Prospects.” Canadian Journal of Fisheries and Aquatic Sciences 54: 224–238. doi:10.1139/f96-265.
- Zhou, J. 2012. “Impacts of Mariculture Practices on the Temporal Distribution of Macrobenthos in Sandu Bay, South China.” Chinese Journal of Oceanology and Limnology 30: 388–396. doi:10.1007/s00343-012-1150-7.
- Zhou, Y., H. Yang, H. Hu, Y. Liu, Y. Mao, H. Zhou, X. Xu, and F. Zhang. 2006a. “Bioremediation Potential of the Macroalga Gracilaria lemaneiformis (Rhodophyta) Integrated into Fed Fish Culture in Coastal Waters of North China.” Aquaculture 252: 264–276. doi:10.1016/j.aquaculture.2005.06.046.
- Zhou, Y., H. Yang, S. Liu, X. Yuan, Y. Mao, Y. Liu, X. Xu, and F. Zhang. 2006b. “Feeding and Growth on Bivalve Biodeposits by the Deposit Feeder Stichopus Japonicus Selenka (Echinodermata: Holothuroidea) Co-Cultured in Lantern Nets.” Aquaculture 256: 510–520. doi:10.1016/j.aquaculture.2006.02.005.
- Zhou, Y., H. Yang, T. Zhang, P. Qin, X. Xu, and F. Zhang. 2006d. “Density-Dependent Effects on Seston Dynamics and Rates of Filtering and Biodeposition of the Suspension-Cultured Scallop Chlamys farreri in a Eutrophic Bay (Northern China): An Experimental Study in Semi-In Situ Flow-Through Systems.” Journal of Marine Systems 59: 143–158. doi:10.1016/j.jmarsys.2005.11.002.
- Zhou, Y., H. Yang, T. Zhang, S. Liu, S. Zhang, Q. Liu, J. Xiang, and F. Zhang. 2006c. “Influence of Filtering and Biodeposition by the Cultured Scallop Chlamys farreri on Benthic-Pelagic Coupling in a Eutrophic Bay in China.” Marine Ecology Progress Series 317: 127–141. doi:10.3354/meps317127.
