Abstract
Species of Clonostachys and Trichoderma isolated from the surface of bananas were highly antagonistic to crown rot-causing fungal pathogens of banana, such as Lasiodiplodia theobromae, Thielaviopsis paradoxa, Colletotrichum musae, and Fusarium verticillioides. Cultural and morphological examinations revealed that the fungal antagonists were similar to C. byssicola and T. harzianum, with some characters overlapping with closely related species. Molecular identification is recommended since the fungal isolates could not be differentiated adequately by cultural and morphological methods. Accurate taxonomy of these fungal antagonists was essential for the subsequent biological control studies. The tub2 region of β-tubulin genes of Clonostachys and 5.8S rDNA with the ITS regions of Trichoderma isolates were analyzed to determine their phylogenic placement. Molecular and phylogenetic analyses revealed 100% homology of Clonostachys (accession number AB308539) to C. byssicola (accession number AF358154) and 99% identity of Trichoderma (AB308540) to T. harzianum (accession number AY625068, AF443927). The identity of the fungal isolates was confirmed as Clonostachys byssicola Schroers and Trichoderma harzianum Rifai.
Introduction
The best method of obtaining a biocontrol agent is to isolate potential candidates from areas of the plant where it is expected to function in disease control. Isolates collected in this manner can then be screened for biocontrol efficacy (Howell Citation2003). Utilization of natural epiphytes is the most practical approach, because natural resident biocontrol agents colonize food sources in plants without damaging the cells (Janisiewicz et al. Citation1994).
Species of Clonostachys and Trichoderma, such as C. rosea, T. asperellum, T. viride and T. harzianum, are well known biological control agents of various plant pathogens (Elad et al. Citation1980; Papavivas 1985; Howell Citation1987; Harman and Stasz Citation1989; Hjeljord and Tronsmo Citation1998; Krauss and Soberanis Citation2001; Howell Citation2002; Ten Hoopen et al. Citation2003; Batta Citation2007; Jensen et al. Citation2007; Verma Citation2007; Sant et al. Citation2010). Trichoderma and Gliocladium spp. isolated from green banana peel suppressed crown-rot disease complex pathogens, C. musae, L. theobromae and Fusarium moniliforme (Kraus et al. 1998). We isolated Clonostachys and Trichoderma from the surface of diseased bananas imported into Japan from the Philippines (Alvindia et al. Citation2000). These fungi inhibited the growth of crown rot-causing fungal pathogens of banana, such
as Lasiodiplodia theobromae, Thielaviopsis paradoxa, Colletotrichum musae and Fusarium verticillioides in vitro and, remarkably, controlled this disease when applied as postharvest treatment (Alvindia and Natsuaki Citation2008).
Crown rot, the most important postharvest disease of banana, is caused by several fungi, including L. theobromae (Ogawa Citation1970; Johanson and Blazquez Citation1992), C. musae (Finlay and Brown Citation1993), T. paradoxa (Alvindia et al. Citation2002), and a complex of Fusarium spp. (Knight et al. Citation1977; Jimenez et al. Citation1993; Alvindia et al. Citation2000; Hirata et al. Citation2001) . The fungi infect the crown through flesh wounds created after trimming the crown of the banana hand into a crescent shape. The symptoms of crown rot are not visible at packing stations in banana-growing countries, but develop later, during shipment, ripening and storage in consumer countries. During the rainy season, losses of more than 10% have been recorded for Windward Islands bananas arriving in the UK (Krauss and Johanson Citation2000). Losses as high as 86% have been observed in the case of bananas from the Philippines, having undergone no chemical treatment (Alvindia et al. Citation2000).
Identification by cultural and morphological methods showed that Clonostachys and Trichoderma isolated from banana surfaces were similar to C. byssicola and
T. harzianum, respectively. However, overlapping of some cultural and morphological characteristics of our isolates with related Clonostachys and Trichoderma spp. was evident. Obviously, our Clonostachys and Trichoderma isolates could not be differentiated adequately by cultural and morphological methods. Correct identification of these biological materials is important for subsequent biological control studies. In this paper, we report the identification of Clonostachys and Trichoderma isolated from banana surfaces by cultural and morphological methods and confirm the identities by molecular technique. We discuss the cultural and morphological similarities of our isolates with closely related species of Clonostachys and Trichoderma, and construct a phylogenetic tree by neighbor-joining analysis of their DNA sequences.
Materials and methods
Source and maintenance of fungal isolates
Species of Clonostachys and Trichoderma isolated from the surface of diseased Cavendish bananas imported from the Philippines (Alvindia et al. Citation2000) were kept in a store room of Food Protection Division, PhilMech at 25°C. The isolates were periodically maintained in test tube slants of potato dextrose agar (PDA) supplemented with 5% malt extract (ME) for good growth. Representative of the fungal isolates were also deposited in the Genebank National Institute of Agrobiological Sciences (Ibaraki, Japan).
Cultural and morphological observations
PDA and oat meal agar (OA) were used for cultural and morphological observations of Clonostachys, while PDA, OA and corn meal dextrose medium (CMD) were utilize for Trichoderma. Methuen's Handbook of Color (Kornerup and Wanscher Citation1978) was our guide in determining colony colors of the isolates. The works of Chaverri and Samuels (Citation2004), Gams and Bissette (Citation1998), Schroers (Citation2001) and Samuels (Citation2006) were followed for cultural and morphological descriptions.
Molecular and phylogenetic analysis
Mycelia grown on PDA at 25°C were harvested after 1–2 weeks. Genomic DNA was extracted from lyophilized hyphae based on the method of O'Donnel et al. (1997) with some modifications or with DNeasy Plant Mini Kit (Qiagen, Dusseldorf, Germany). For Trichoderma, the nuclear ribosomal internal transcribed spacer (ITS) region was amplified with primer pairs ITS1 and ITS4 (White et al. Citation1990) and the tub2 region of β-tubulin genes was amplified with primer pairs T1 and T224 (O'Donnel and Cigelnik Citation1997) for Clonostachys. Polymerase chain reaction (PCR) amplification of ribosomal DNA (rDNA) was performed with 30 cycles of incubation for 1 min at 96°C, 1 min at 52°C, and 2 min at 72°C, while the tub2 genes were subject to denaturation for 2 min at 95°C followed by 40 cycles of incubation for 35 s at 94°C, 55 s at 52°C, and 2 min at 72°C. Gene amplifications were performed with the TaKaRa ExTaq system (TaKaRa, Otsu, Japan). Sequencing was conducted with the ABI-Prism 377 DNA sequencing system (Applied Biosystems, Foster City, CA, USA) and DNA sequencing kit (Perkin-Elmer, Waltham, MA, USA) following the ABI protocol.
Sequence alignment and homology analysis were carried out using AssemblyLIGNTM 1.0.9c (Accelrys, San Diego, CA, USA) and CLUSTAL W package with McVector 6.5.3 (Accelrys) (Thompson et al. Citation1994). The aligned sequences were analyzed by the neighbor-joining method (Saitou and Nei, Citation1987), using PAUP 4.0b. The distance matrix was calculated using DNADIST with the Kimura's two-parameter method, and the topology was tested with 1000 bootstrap trials.
Results
Clonostachys
Pure cultures of Clonostachys have cottony colonies with a powdery surface on PDA and OA media, measuring 55–58 mm in 7 days at 28°C. The colony surface turned granular with a light orange coloration over time, due to production of sporodochia and conidial masses, and pale yellow in reverse. Morphological examinations showed that isolates had dimorphic conidiophores composed of primary and secondary. Primary verticillium-like conidiophores observed throughout the colony. In primary conidiophores, stipes measured 10–110 × 3.5 μm. Bi- to quinquiesverticilliate, adpressed to divergent secondary conidiophores. Conidia hyaline, smooth to finely roughened surface, broadly rounded, with lateral hilum, 4–12.5 × 1.5–3 μm. Comparison of cultural and morphological characteristics revealed that our Clonostachys isolate was identical to C. byssicola ().
Table 1. Comparison of the cultural and morphological characteristics of the Clonostachys banana isolate and three Clonostachys species
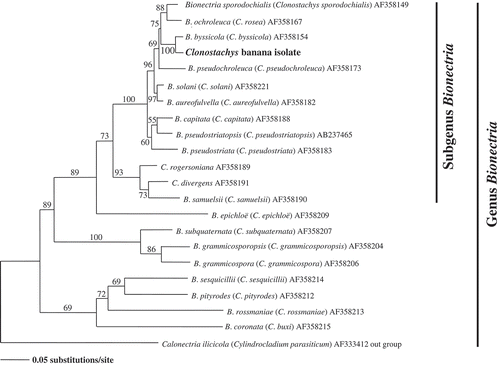
The sequenced nucleotide of tub2 in the β-tubulin region was 568 bp. A rooted molecular phylogenic tree was constructed by neighbor-joining analysis of the aligned sequences of tub2 (). In the phylogenic study, C. byssicola and our Clonostachys isolate were examined with Cylindrocladium parasiticum as outgroup. The topology of Clonostachys banana isolate was identical to C. byssicola (accession number AF358173) with 100% bootstrap values ().
Trichoderma
Pure cultures were cottony colonies that overgrew the 90-mm PDA, OA and CMD plates after 2 days at 28°C. Colonies on PDA and OA were shaded light green with sparse conidiation; powdery to granular colonies on CMA due to dense dark-green conidiation after 7 days. Reverse on all media colorless to dull yellow. Regular verticilliate conidiophores forming a pyramidal structure. Phialides ampulliform, 5.0–8.0 × 2.5–3.5 μm; conidia sub-globose to ovoidal, smooth-walled, subhyaline to pale green, 2.5–4.0 × 2.5–3.0 μm; chlamydospores produced. Comparison of the morphological characteristics between our Trichoderma isolate and three related Trichoderma species showed that the banana isolate was identical to T. harzianum ().
Table 2. Comparison of the morphological characteristics of the Trichoderma banana isolate and three Trichoderma species
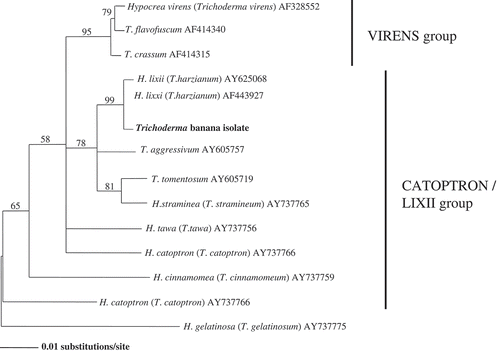
The sequenced nucleotide of the rDNA ITS region was 486 bp. A rooted molecular phylogenic tree was constructed by neighbor-joining analysis of the aligned nucleotide sequences of the ITS and 5.8s regions (). In the phylogenic study, T. harzianum and Trichoderma banana isolate were compared. Based on the results shown (), the topology of Trichoderma banana isolate was identical to T. harzianum (accession numbers AY625068 and AF443927) with 99% bootstrap values.
Discussion
The genus Clonostachys and Trichoderma are well reported and studied biocontrol agents. However, identification of species belonged to these genera are notoriously difficult via traditional method. Cultural and morphological characteristics, such as growth rate, colony color and surface, stipe length, and size of conidia, of the C. byssicola banana isolate were distinctly different with C. byssicola reported in Schroers (Citation2001). Furthermore, some cultural and morphological traits of our Clonostachys isolate overlapped with C. rosea, which made identification more complicated, corroborating Schroers' (Citation2001) observation that C. rosea and C. byssicola can only be distinguished by molecular means. The confusing results achieved by classical methods lead to the molecular identification of our isolates. The 100% homology of our Clonostachys isolate with C. byssicola confirmed the identity of the fungus.
Most published works on Clonostachys as a biocontrol agent has focused on C. rosea, which controls Alternaria brassicicola in broccoli seeds (Sivapalan Citation1993), Bipolaris sorokiniana and F. culmorum in seedlings of barley and wheat (Knudsen et al. Citation1995), Botrytis spp. in chick pea and onion (Burgess et al. Citation1997; Sutton et al. Citation1997), Moniliophthora roreri, Phytoptora palmivora in cacao pods (Krauss and Soberanis Citation2001), Phomopsis sclerotioides in cucumber (Moody and Gindrat Citation1977) and Verticillium dahliae in soil (Keinath et al. Citation1991). Conversely, the biocontrol efficacy of C. byssicola is limited to the work of Martijn ten Hoopen et al. (Citation2006). Hence, our isolate is added to the list, with C. byssicola as a biocontrol agent of crown rot-causing fungal pathogens in banana (Alvindia and Natsuaki Citation2008).
Trichoderma from banana surfaces differs from T. harzianum described in Schroers (Citation2001) and Chaverri and Samuels (Citation2004), with its rapid colony growth, sparse conidiation and non-production of soluble pigment on media. The size of conidia and phialides overlapped with T. catoptron and T. stramenium. The absence of a teleomorphic state made identification more difficulty. Gams and Bissett (Citation1998) showed that variations among Trichoderma spp. could not be differentiated satisfactorily via classical methods, thus making nomenclature placement uncertain. Hence, we only confirmed the taxonomy of our Trichoderma isolate by molecular means. The development of molecular techniques and the use of DNA sequence analysis became the new paradigm in fungal systematics for Trichoderma (Samuels Citation2006) and determined the taxonomical placement of this genus (Gams and Meyer Citation1998; Hermosa et al. Citation2000). The development of molecular tools has also enabled the positive identification of any strain and the development of a phylogenetic tree. Identification of Trichoderma species and species in other economically important and species-rich genera will rely on DNA sequence data as the limits of phenotype to distinguish species are reached (Samuels Citation2006), as in the present study. The identification T. harzianum from banana as a biocontrol agent of crown rot-causing fungal pathogens added to the long lists of published articles on the antagonistic character of this fungus.
Being a highly perishable fruit, banana suffers severe postharvest losses both in terms of quality and quantity. Anthracnose, crown rot and cigar-end rot are common and serious postharvest diseases of banana. Although chemical control is a feasible option to control postharvest diseases, environmental and health risks are high. Therefore, consumer demand is increasing for fruit which has been treated non-chemically for postharvest pathogens, such as biological control.
The effective and safe application of biocontrol agents depend on a host of environmental conditions, target species and the accurate and reliable identification of potential isolates. In addition, the accurate identification of Clonostachys and Trichoderma isolated from banana peel is essentially important in pursuing our researches on the utilization of these fungi as biocontrol agents of banana diseases. We fulfilled one important step in our biological control research by correctly identifying the fungal antagonists. Correct identification will provide information on understanding the interparasitic relationship with target pathogens and the subsequent environmental fate of the antagonist needed for effective application.
Acknowledgements
The senior author is grateful to the Japan Society for the Promotion of Science (JSPS) for a grant under the Postdoctoral Fellowship Program (P06201) to continue research on the development and evaluation of new technology for postharvest disease management of banana.
References
- Alvindia , DG and Natsuaki , KT. 2008 . Evaluation of fungal epiphytes isolated from banana fruit surfaces for biocontrol of banana crown rot disease . Crop Prot , 27 : 1200 – 1207 .
- Alvindia , DG , Kobayashi , T , Yaguchi , Y and Natsuaki , KT. 2000 . Symptoms and the associated fungi of postharvest diseases on non-chemical bananas imported from the Philippines . Jpn J Trop Agric. , 44 : 87 – 93 .
- Alvindia , DG , Kobayashi , T , Yaguchi , Y and Natsuaki , KT. 2002 . Pathogenicity of fungi isolated from non-chemical bananas . Jpn J Trop Agric. , 44 : 215 – 223 .
- Batta , YA. 2007 . Control of postharvest diseases of fruit with an invert emulsion formulation of Trichoderma harzianum Rifai . Postharv Biol Technol. , 43 : 143 – 150 .
- Burgess , DR , Bretag , T and Keane , PJ. 1997 . Biocontrol of seedborne Botrytis cinerea in chickpea with Gliocladium roseum . Plant Pathol. , 46 : 298 – 305 .
- Chaverri , P and Samuels , GJ. 2004 . Hypocrea/Trichoderma (Ascomycota, Hypocreales, Hypocreaceae): Species with green ascospores . Stud Mycol. , 48 : 1 – 116 .
- Elad , Y , Chet , I and Katan , J. 1980 . Trichoderma harzianum: a biocontrol agent against Sclerotium rolfsii and Rhizoctonia solani . Phytopathology , 70 : 119 – 121 .
- Finlay , AR and Brown , EA. 1993 . The relative importance of Colletotrichum musae as a crown rot pathogen on Windward Island bananas . Plant Pathol. , 40 : 568 – 575 .
- Gams , W and Bissett , J. 1998 . “ Morphology and identification of Trichoderma ” . In Trichoderma and Gliocladium , Edited by: Kubicek , CP , Harman , GE. and Editors . Vol. 1 , 1 – 34 . London : Taylor and Francis .
- Gams , W and Meyer , W. 1998 . What exactly is Trichoderma harzianum? . Mycologia , 90 : 904 – 915 .
- Harman , GE and Stasz , TE. 1989 . Combining effective strains of Trichoderma harzianum and solid matrix priming to provide improved biological seed treatment systems . Plant Dis. , 72 : 631 – 637 .
- Hermosa , M , Grondona , I , Iturriaga , E.A , Diaz-Minguez , JM , Castro , C , Monte , E and Garcia-Acha , I. 2000 . Molecular characterization and identification of biocontrol isolates of Trichoderma spp . Appl Environ Microbiol. , 66 : 1890 – 1898 .
- Hirata , T , Kimishima , E , Aoki , T , Nirenberg , HI and O'Donnell , K. 2001 . Morphological and molecular characterization of Fusarium verticillioides from rotten banana imported into Japan . Mycoscience , 42 : 155 – 166 .
- Hjeljord , LG and Tronsmo , A. 1998 . “ Trichoderma and Gliocladium in biological control: an overview ” . In Trichoderma and Gliocladium: Enzymes, Biological Control and Commercial Application , Edited by: Harman , GE and Kubicek , CP. Vol. 2 , 131 – 151 . London : Taylor & Francis .
- Howell , CR. 1987 . Relevance of mycoparasitism in the biological control of Rhizoctonia solani by Gliocladium virens . Phytopathology , 77 : 992 – 994 .
- Howell , CR. 2003 . Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts . Plant Dis. , 87 : 1 – 10 .
- Howell , CR. 2002 . Cotton seedling preemergence damping-off incited by Rhizopus oryzae and Pythium spp. and its biological control with Trichoderma spp . J Phytopathol. , 92 : 177 – 180 .
- Janisiewicz , WJ , Peterson , DL and Bors , R. 1994 . Control of storage rots of apples with Sporobolomyces roseus . Plant Dis. , 78 : 466 – 470 .
- Jensen , DF , Knudsen , IMB , Lübeck , M , Mamarabadi , M , Hockenhull , J and Jensen , B. 2007 . Development of a biocontrol agent for plant disease control with special emphasis on the near commercial fungal antagonist Clonostachys rosea strain ‘IK726’ . Australas Plant Pathol. , 36 : 95 – 101 .
- Jimenez , M , Logrieco , A and Bottalico , A. 1993 . Occurrence and pathogenicity of Fusarium species in banana fruits . J Phytopathol. , 137 : 214 – 220 .
- Johanson , A and Blazquez , B. 1992 . Fungi associated with banana crown rot on field packed fruit from the Windward Islands and assessment of their sensitivity to fungicides thiabendazole, procloraz and imazalil . Crop Prot. , 11 : 79 – 83 .
- Keinath , AP , Fravel , DR and Papavizas , GC. 1991 . Potential of Gliocladium roseum for biocontrol of Verticillium dahliae . J Phytopathol. , 81 : 644 – 648 .
- Knight , C , Cutts , DF and Colhoun , J. 1977 . The role of Fusarium semitectum in causing crown rot of bananas . Phytopathol Z. , 89 : 170 – 176 .
- Knudsen , IMB , Hockenhull , J and Jensen , DF. 1995 . Biocontrol of seedling diseases of barley and wheat caused by Fusarium culmorum and Bipolaris sorokiniana: effects of selected fungal antagonists on growth and yield components . Plant Pathol. , 44 : 467 – 477 .
- Kornerup , A and Wanscher , JH. 1978 . Methuen handbook of colour , 252 London : Eyre Methuen. p .
- Krauss , U and Johanson , A. 2000 . Recent advances in the control of crown rot of banana in the Windward Islands . Crop Prot. , 19 : 151 – 160 .
- Krauss , U and Soberanis , W. 2001 . Biocontrol of cocoa pod diseases with mycoparasite mixtures . Biol Control , 22 : 149 – 158 .
- Krauss , U , Bidwell , R and Ince , J. 1998 . Isolation and preliminary evaluation of mycoparasites as biocontrol agents of crown rot of banana . Biol Control , 13 : 111 – 119 .
- Martijn ten Hoopen , G , Crozier , J , Buddie , A , Flood , J , Astorga , C and Krauss , U. 2006 . “ Genetic population and structure of Clonostachys byssicola and genetic diseases resistance in cocoa (Theobroma cacao L.) ” . In International Permanent Working Group for Cocoa Pests and Diseases, Costa Rica (abstract only)
- Moody , AR and Gindrat , D. 1977 . Biological control of cucumber black root rot by Gliocladium roseum . J Phytopathol. , 67 : 1159 – 1162 .
- O'Donnell , K and Cigelnik , E. 1997 . Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous . Mol Phylogenet Evol. , 7 : 103 – 116 .
- O'Donnell , K , Cigelnik , E , Weber , NJ and Trappe , JM. 1997 . Phylogenetic relationships among ascomycetous truffles and the true and false morels inferred from 18S and 28S ribosomal DNA sequence analysis . Mycologia , 89 : 48 – 65 .
- Ogawa , JM. 1970 . Postharvest diseases of bananas in China (Taiwan) . FAO Plant Prot Bull. , 18 : 31 – 42 .
- Papavivaz , GC. 1985 . Trichoderma and Gliocladium: Biology, ecology, and potential for biocontrol . Annu Rev Phytopathol. , 23 : 23 – 54 .
- Saito , N and Nei , M. 1987 . The neighbor-joining method: a new method for reconstructing phylogenetic trees . Mycol Biol Evol. , 4 : 406 – 425 .
- Samuels , GJ. 2006 . Trichoderma: Systematics, the sexual state, and ecology . J Phytopathol. , 96 : 195 – 206 .
- Sant , D , Casanova , E , Segarra , G , Avilés , M , Reis , M and Trillas , MI. 2010 . Effect of Trichoderma asperellum strain T34 on Fusarium wilt and water usage in carnation grown on compost-based growth medium . Biol Control , 53 : 291 – 296 .
- Schroers , HJ. 2001 . A monograph of Bionectria (Ascomycota, Hypocreales, Bionectriaceae) and its Clonostachys anamorphs . Stud Mycol. , 46 : 1 – 214 .
- Sivapalan , A. 1993 . Fungi associated with broccoli seed and evaluation of fungal antagonists and fungicides for the control of seed-borne Alternaria brassicicola . Seed Sci Technol. , 21 : 237 – 245 .
- Sutton , JC , Li , DW , Peng , G , Yu , H , Zhang , PG and Valdebenito-Sanhueza , RM. 1997 . Gliocladium roseum: A versatile adversary of Botrytis cinerea in crops . Plant Dis. , 81 : 316 – 328 .
- Ten Hoopen , GM , Rees , R , Aisa , P , Stirrup , T and Krauss , U. 2003 . Population dynamics of epiphytic mycoparasites of the genera Clonostachys and Fusarium for the biocontrol of black pod (Phytophthora palmivora) and moniliasis (Moniliophthora roreri) on cocoa (Theobroma cacao) . Mycol Res. , 107 : 587 – 596 .
- Thompson , JD , Higgins , DG and Gibson , TJ. 1994 . Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice . Nucleic Acid Res. , 22 : 4673 – 4680 .
- Verma , M , Brar , SK , Tyagi , RD , Surampally , RY and Valéro , JR. 2007 . Antagonistic fungi, Trichoderma spp.: panoply of biological control . J Biochem Eng. , 37 : 1 – 20 .
- White , TJ , Bruns , T , Lee , S and Taylor , J. 1990 . “ Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics ” . In PCR Protocols , Edited by: Innis , MA , Gelfand , DH , Sninsky , JJ and White , TJ. 315 – 322 . A guide to methods and applications, San Diego : Academic Press .