ABSTRACT
Non-conventional wine yeasts are extensively studied as promising producers of hydrolytic enzymes and as potential starter cultures in winemaking due to their ability to improve organoleptic properties of wine. Thirty-six yeast strains of enological and brewery origin from the Ukrainian Collection of Microorganisms belonging to Torulaspora, Kloeckera, Candida, Metschnikowia, Pichia, and Zygosaccharomyces genera have been screened for the production of extracellular hydrolases, stress tolerance, fermentative activity, and other traits of enological interest. This study revealed the high incidence of lipolytic, proteolytic, and β-glucosidase activities among the yeasts, while no pectinase activity was detected. Esterase, cellulase and glucanase activities were found in a small proportion of yeasts (8.33–16.66%). Several Pichia anomala, Kloekera javanica, Pichia membranifaciens, and Metschnikowia pulcherrima strains demonstrated a wide range of hydrolytic activities. High tolerance to stress factors (ethanol, osmotic, and oxidative stress) present during alcoholic fermentation was detected in P. anomala and M. pulcherrima strains. Fermentative activity of several yeast strains was evaluated in microfermentations in a model semi-synthetic medium. Strain P. anomala UCM Y-216 was selected as the most promising culture for winemaking due to its hydrolytic activities, tolerance to stress factors and other valuable metabolic traits. This study represents the first step for selecting a non-conventional yeast strain of enological origin as a potential co-culture for winemaking.
Introduction
Most studies researching biodiversity and biotechnological application of yeasts in winemaking and brewing focus on Saccharomyces cerevisiae – yeasts traditionally used in these industries. It is a well-known fact that Saccharomyces yeasts are found considerably rarer and in lesser numbers on grapes surface compared to other yeast species (Barata et al. Citation2012). If wine is produced by spontaneous fermentation there is a greater diversity of aerobic and weakly fermenting yeasts that are predominant in grapes juice or must that include Cryptococcus, Debaryomyces, Issatchenkia, Kluyveromyces, Pichia, Rhodotorula, and Zygosaccharomyces (Varela and Borneman Citation2017). However, most of these yeasts are gradually replaced by more ethanol-tolerant yeasts with high fermenting activity. Some non-Saccharomyces yeasts can still survive at high ethanol concentrations and take part in fermentation, however, the major part of ethanolic fermentation at later stages is conducted by Saccharomyces yeasts (Fleet Citation2003). Nowadays, specially selected S. cerevisiae strains are used in alcoholic fermentation to reduce the participation of the wild yeast microbiota that can produce undesirable compounds such as biogenic amines or excessive amounts of organic acids and often are the cause of wine spoilage (Padilla et al. Citation2016). However, non-Saccharomyces yeasts can also produce a number of secondary metabolites that can considerably improve the organoleptic characteristics of the final product (Ivit and Kemp Citation2018).
Grapes as a substrate for alcoholic fermentation contain a broad spectrum of various chemical compounds including pectins, cellulose, hemicelluloses, glucans, proteins, lignin, phenolic substances, various aromatic precursors (Claus and Mojsov Citation2018). The degradation of these components could lead to the improvement of wine clarity, taste and aroma. The ability to degrade a wide range of organic polymers present in grapes would be an important and desired characteristic for the potential wine yeast culture (Jolly et al. Citation2014). Many non-conventional yeasts possess hydrolytic enzymes that are lacking in Saccharomyces yeasts that allow them to improve the taste and aroma, and, as a result, the complexity of wine (Escribano et al. Citation2017).
Recently non-conventional yeasts attracted considerable attention due to their killer and enzymatic acivities and their role in the formation of aroma and flavor in wine . A number of non-Saccharomyces yeasts were investigated as potential co-starter cultures in winemaking including Metschnikowia pulcherrima (Barbosa et al. Citation2018), Saccharomycodes ludwigii (Esteves et al. Citation2019), Torulaspora delbrueckii (Tataridis et al. Citation2013), Hanseniaspora osmophila (Viana et al. Citation2008), Wickerhamomyces anomalus (Izquierdo Cañas et al. Citation2014) and some others. Several yeast strains of enological origin were proposed as potential starter cultures to obtain wines with reduced ethanol content: Hanseniaspora uvarum, H. osmophila, Starmerella bacillaris and Candida membranaefaciens (Mestre Furlani et al. Citation2017), M. pulcherrima, Schizosaccharomyces malidevorans, Candida stellata (Contreras et al. Citation2014), M. pulcherrima and Saccharomyces uvarum (Varela and Borneman Citation2017). Also non-conventional yeasts of enological origin could be exploited to lower wine acidity (Vilela Citation2019). Consequently, there is notable interest in the search and selection of promising non-conventional wine yeasts, and investigating their enological characteristics, i.e. enzymatic and metabolic characteristics, tolerance to various stress factors that could be present during fermentation process, fermenting activity, safety, killer activity, etc. (Jolly et al. Citation2014).
The Ukrainian Collection of Microorganisms contains almost 1500 yeast strains isolated from various sources which include grapes, grape juice, must, wine, beer and, wort. Non-conventional yeasts isolated from these habitats could possess valuable enological characteristics that could be exploited for the production of wines, especially with reduced ethanol content as most non-Saccharomyces yeasts possess lower fermentative power compared to S. cerevisiae (Ciani et al. Citation2016).
The aim of this study was to evaluate non-conventional strains of enological and brewery origin from the Ukrainian Collection of Microorganisms for traits of enological interest as potential starter cultures or co-cultures in winemaking.
Materials and methods
Yeast strains and inoculum preparation
36 yeast strains of enological and brewery origin used in this study are listed in . They were obtained from the Ukrainian Collection of Microorganisms, Institute of Microbiology and Virology, Kyiv, Ukraine. The yeast strains were maintained by subculturing every 8–12 months on malt agar medium (Kurtzman et al. Citation2011) and stored at 4–6°C.
Table 1. Yeast strains used in the study
For killer activity yeast strains S. cerevisiae UCM Y-554 and Kluyveromyces marxianus UCM Y-1591 (CBS Y-712) were used as killer-sensitive strains and yeast strains S. cerevisiae UCM Y-2505 and UCM Y-522 were used as positive controls for killer activity. Yeast strains were obtained from the Ukrainian Collection of Microorganisms.
Yeast strains were cultivated on YPD agar containing 1% yeast extract, 2% glucose, 2% peptone, and 2% agar (w/v) for 2–3 days at 25–26°C and yeast suspensions were made in sterile 0.9% NaCl solution to a final cell concentration of approximately 106 CFU/ml and were replica plated on the appropriate media using a multi-point steel inoculator.
Qualitative determination of extracellular hydrolytic activities
Proteolytic activity was assessed using gelatin as a substrate. Gelatin hydrolysis was assessed in Yeast Nitrogen Base (YNB) broth containing 0.5% glucose and 10% gelatin for 3 weeks at 25–26°C (Kurtzman et al. Citation2011). The proteolytic activity was examined every week by placing gelatin tubes into the fridge for 1 h until the solidification of the medium and checking afterwards for the signs of gelatin liquefaction. The liquefaction of gelatin indicated the presence of proteolytic activity.
Cellulolytic activity was determined on YPD agar supplemented with 0.5% carboxymethylcellulose (CMC), pH 6.0. Plates were incubated at 25–26°C for 7 days and stained with 0.03% Congo Red followed by destaining with 1 M NaCl. The formation of the hydrolysis zone around colonies indicated the presence of cellulolytic activity (Strauss et al. Citation2001).
Lipolytic activity was determined on tributyrin agar (0.5% peptone, 0.3% yeast extract, 1% tributyrin, 1.5% agar, final pH 6.0). Plates were incubated for 7 days at 25–26°C (Brizzio et al. Citation2007). The appearance of the clear zone of more than 1 mm from the edge of the yeast colony indicated positive lipolytic activity, the appearance of the clearing less than 1 mm from the colony edge or under the colony was considered as a weak activity, while the absence of any changes indicated a negative result (Charoenchai et al. Citation1997).
Esterase activity (the ability to hydrolyse long-chain esters) was assessed by plating yeasts on tween-80 agar (1% peptone, 0.5% NaCl, 0.01% CaCl2х2H2O, 1% tween-80, 2% agar). Plates were incubated at 25–26°C for 1 week. The formation of precipitate around yeast colonies more than 1 mm in width demonstrated positive esterase reaction, the appearance of the precipitation zone less than 1 mm from the edge of the colony was considered as weak activity, the absence of the zone indicated the lack of esterase activity (Charoenchai et al. Citation1997).
Xylanase activity was determined according to Strauss et al. (Citation2001) with some modifications on YNB agar containing 1% xylan. Plates were incubated at 25–26°C for 7 days and flooded with iodine solution. The formation of a hydrolysis zone (clearing) around colonies indicated the presence of xylanase activity.
Pectinase activity was determined on YNB agar supplemented with 1% citrus pectin (Brizzio et al. Citation2007). Plates were incubated at 25–26°C for 7 days and flooded with iodine solution (Martinez et al. Citation2016). A clear halo (hydrolysis zone) around yeast colonies indicated the presence of pectinase activity.
β-glucosidase activity was determined on an agar medium containing 0.5% arbutin, 1% yeast extract, 2% agar. After sterilisation, 2 ml of 1% ammonium ferric citrate solution was added to the 100 ml medium (Kurtzman et al. Citation2011). 100 µl yeast suspension, prepared as indicated earlier, was added to the tubes containing the agar medium. Tubes were incubated at 25–26°C for 5–7 days. The change in the colour of the medium to dark purple-brownish was considered as a positive reaction, while the change to light-medium brown was considered as weak activity. The test for β-glucosidase activity was conducted in 10 ml tubes, as incubation of yeasts on agar plates led to false positive results.
Tolerance to stress factors
Yeast suspensions were replica plated on agar medium containing the corresponding stress factor (glucose, ethanol, copper sulphate). YPD medium without stress factor was used as a positive control.
Osmotolerance of yeasts was determined on 50% glucose YPD agar (Kurtzman et al. Citation2011). Plates were incubated for 2 weeks at 25–26°C. Ethanol tolerance was determined according to Barbosa et al. (Citation2018), with some modifications: on YPD agar containing ethanol at 6%, 9%, 12% and 16% concentrations at pH 6.0 and 3.5, respectively, to imitate the conditions of alcoholic fermentation. Plates were incubated for 1 week at 25–26°C. Yeast tolerance to copper was evaluated on YPD agar containing 200 and 400 µM CuSO4, pH 6.0 and 3.5 (Capece et al. Citation2018). Actively growing strains under such conditions were considered tolerant, while poor yeast growth was regarded as weak tolerance and the absence of the growth indicated the lack of tolerance to the corresponding stress factor.
Oxidative stress tolerance of yeast strains was evaluated by testing yeast tolerance to hydrogen peroxide according to Mestre Furlani et al. (Citation2017), with some modifications. 0.1 ml of yeast suspensions was spread on the surface of YPD agar plate. Wells (7 mm in diameter) were made using a sterilised borer and 50 µl of H2O2 at concentrations of 250 μM, 500 μM and 1 mM, respectively, were added into each well. Plates were incubated for 48 h at 25–26°C and the average diameter (mm) of the inhibition zone around the wells was measured.
Assessment of enological traits of yeasts
Acid production from glucose by yeast strains was evaluated on Causter’s chalk agar containing 5% glucose, 0.5% yeast extract, 0.5% CaCO3, 2% agar (Kurtzman et al. Citation2011). Plates were incubated for 1 week at 25–26°C. The appearance of the clearing zone as a result of chalk solubilisation indicated the ability of yeasts to produce organic acids.
Production of biogenic amines by yeast strains was tested on YPD agar medium containing a mix of amino acids with a total concentration of 1% or 2% and 0.0015% bromocresol purple, final pH 5.2 (Aslankoohi et al. Citation2016). The following amino acids were added to the medium at equal ratios: tyrosine, histidine, phenylalanine, leucine, tryptophan, arginine, and lysine. Plates were incubated for 7 days at 25–26 °C. The medium lacking amino acids and inoculated with yeasts was used as a negative control. Biogenic amine production was indicated by the appearance of purple halo around the colonies. Yeasts were qualitatively defined as weak or positive biogenic amine producers according to the intensity of the produced colour.
Malic and acetic acid assimilation by yeast strains was tested according to Šuranská et al. (Citation2016) on YNB agar (0.67%) containing 0.5% malic acid and 0.25% (w/v) acetic acid. Yeasts were cultivated for up to 3 weeks at 25–26°C.
The killer phenotype of the yeast strains was assessed according to Raymond Eder et al. (Citation2017), with some modifications. Killer-sensitive yeast strains S. cerevisiae UCM Y-554 and K. marxianus UCM Y-1591 were inoculated into YPD-methylene blue agar containing 0.003% (w/v) methylene blue, pH 4.5 at the final cell concentration of approximately 105 CFU/ml. Yeast strains were streaked on the agar seeded with the sensitive test strain and incubated at 25–26°C for 48–72 h. Killer activity was considered positive if a zone of growth inhibition or a region of methylene blue-stained dead yeast cells around the streaks of the tested yeast strains were observed.
Fermentative activity
The fermentative potential of yeast strains was preliminarily tested in bent fermentation tubes filled with semi-synthetic fermentation medium containing 20% sucrose, 1% yeast extract, 0.1% (NH4)2SO4, 0.1% KH2PO4, 0.1% MgSO4 (Lopes et al. Citation2007). The pH of the medium was adjusted to 3.5, similar to wine, using tartaric acid. Tubes were incubated at 25–26°C for 7–14 days, and fermentation activity of yeast strains was qualitatively assessed by the amount of carbon dioxide gas accumulated in the closed arm of the fermentation tube.
Microfermentations were conducted in 150 ml Erlenmeyer flasks containing 100 ml of the same semi-synthetic fermentation medium adjusted to pH 3.5. Yeast inoculum for microfermentations was obtained from 48 h yeast cultures grown in YPD broth. Flasks were inoculated with the selected yeast strains to give the final cell density of approximately 106 CFU/ml and stoppered with glass fermentation traps containing 40% sulphuric acid to allow only CO2 to escape the fermentation medium. Fermentation was conducted under static conditions (without shaking) at 25°C. The weight loss of the flasks due to CO2 production was measured daily. Microfermentations for each strain were conducted in triplicate.
Statistics
The qualitative tests were done in triplicate and the results were assessed as positive, weak, or negative if at least 2 out of 3 replicates produced the same result. The results of the quantitative experiments are presented as means of triplicates with the corresponding standard deviation (± SD). The means were compared by the one-way analysis of variance (ANOVA), p < 0.05 was regarded as statistically significant.
Results and discussion
In this work, 36 yeast strains isolated from sites of enological and brewery origin and maintained in the Ukrainian Collection of Microorganisms were screened for valuable biotechnological traits for winemaking, including the production of extracellular hydrolases, stress tolerance, production of organic acids, biogenic amines, killer activity and fermentative activity.
The results of the screening of 36 yeast strains for the production of extracellular hydrolytic enzymes are summarised in . One of the most relevant enzymes of the enological value is β-glucosidase that breaks down glycosidic complexes, thus releasing terpenes and other volatile compounds (Claus and Mojsov Citation2018). β-glucosidase activity was demonstrated by the hydrolysis of arbutin that resulted in the appearance of light or dark brown-purple colour in the medium. As neighbouring colonies with strong β-glucosidase activity very often caused the darkening of the surrounding medium and to false positive results, tests were conducted in 10 ml tubes. Thirteen out of 36 yeast strains belonging to Zygosaccharomyces fermentati, Z. bailii, Pichia anomala, P. membranifaciens, M. pulcherrima, C. inconspicua, K. javanica, and K. apiculata possessed strong β-glucosidase activity. Eighteen out of 36 yeast strains did not exhibit β-glucosidase activity, 5 strains demonstrated weak activity.
Figure 1. Extracellular hydrolytic activities of yeast strains of enological and brewery origin: PrA – proteolytic activity (gelatin hydrolysis), LiA – lipolytic activity (tributyrin hydrolysis), EsA – esterase activity (tween-80 hydrolysis), CelA – cellulolytic activity (CMC hydrolysis), PecA – pectinase activity, GluA – β-glucosidase activity
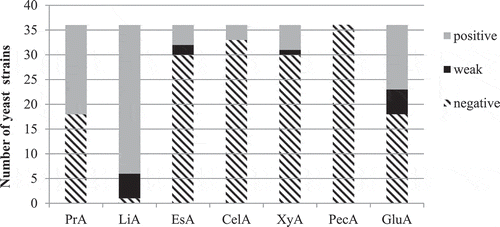
A high incidence of β-glucosidase activity among yeasts of enological origin was observed in several works, especially among Hanseniaspora spp, Meyerozyma (Pichia) guilliermondii, Wickerhamomyces (Pichia) anomalus, Metchnikowia spp. and Rhodosporidium toruloides (Belda et al. Citation2016), T. delbrueckii, Lachancea thermotolerans, and M. pulcherrima (Comitini et al. Citation2011), Hanseniaspora guilliermondii, T. delbrueckii, M. pulcherrima, and Saccharomycodes ludwigii (Grazia et al. Citation2019). Among 97 non-conventional wine yeasts, the highest proportion of strains with β-glucosidase activity was detected in M. pulcherrima (63%) and Cryptococcus spp. (60%) (Escribano et al. Citation2017). However, interestingly none of 245 wine yeasts possessed the ability to hydrolyse arbutin as reported by Strauss et al. (Citation2001). Fernández et al. (Citation2000) detected β-glucosidase activity only in 14% wine yeasts, predominantly in M. pulcherrima strains. So we can conclude that, while some non-Saccharomyces yeast species like M. pulcherrima tend to possess β-glucosidase activity, this characteristic is mostly strain-dependent.
The most commonly detected extracellular enzyme in this study was lipase. Lipolytic activity was detected in 35 out of 36 yeast strains, only one T. delbrueckii strain lacked the ability to hydrolyse tributyrin. The most strongly lipolytic strains belonged to P. anomala, producing hydrolysis zones that were 38–50 mm in diameter (data not shown). A much smaller proportion of the yeasts (16.67%) exhibited esterase activity, i.e. the ability to hydrolyse short-chain esters using tween-80 as a substrate, strains belonging to P. anomala, P. membranifaciens, C. vini, and K. javanica. These results are in agreement with other studies, which reported a high incidence of lypolytic activity and the comparable frequency of esterase activity in ascomyceteous yeasts isolated from tropical environments (Buzzini and Martini Citation2002) and Pichia/Wickerhamomyces yeasts isolated from enological ecosystems (Madrigal et al. Citation2013). However, in another study the ability to hydrolyse tributyrin was observed only in 27.27% non-conventional yeasts belonging to T. delbrueckii, C. pulcherrima, C. stellata and C. krusei (Charoenchai et al. Citation1997). Although the possession of lipolytic activity is not essential for the promising wine culturew, lipolytic enzymes could degrade lipids that originated from grapes or were the result of autolysis of yeasts thus releasing free fatty acids into wine and improving the quality of wine (Claus and Mojsov Citation2018).
Half of the yeast strains demonstrated the ability to hydrolyse gelatin, i.e. proteolytic activity, while none of P. anomala, Z. bailii, and Z. bisporus strains were proteolytic. The most strongly proteolytic strains were M. pulcherrima Y-333, T. delbrueckii Y-2737 and Y-2741, P. membranifaciens Y-473, and completely hydrolysed gelatin by the fourth day of cultivation. In a large study conducted with 770 wine yeast strains protease activity together with β-glucosidase activity was the most frequently observed enzymatic activity, and mostly in Metschnikowia and Hanseniaspora strains (Belda et al. Citation2016). Similar findings were reported by Binati et al. (Citation2019) for 104 yeast strains from high-sugar habitats belonging to Starmerella, Lachancea and Metschnikowia, where 90% of Metschnikowia isolates possessed proteolytic activity. However, a low incidence of proteolytic activity was observed by Fernández et al. (Citation2000) in 182 non-conventional wine yeasts, mostly in P. membranifaciens and M. pulcherrima, while none of 34 wine yeasts of the genera Candida, Lachancea (Kluyveromyces), Metschnikowia, and Torulaspora exhibited proteolytic activity on milk agar (Comitini et al. Citation2011). Such discrepancies could be partly explained by the use of different substrates and media in different studies. For example, in the study performed by Mautone et al. (Citation2010) on 446 yeast and yeast-like strains isolated from phylloplane, 170 yeast strains hydrolysed casein, while only 72 yeasts possessed gelatinase. Proteolytic enzymes of non-Saccharomyces yeasts can play an important role in reducing haze caused by proteins in wine and beer, as S. cerevisiae usually does not possess extracellular proteolytic activity (Claus and Mojsov Citation2018).
Cellulose and hemicellulose are the key structural components of the cell wall of plants so their hydrolysis would result in the release of various aromatic and pigmented compounds from the grape skin and improvement of aroma and colour of wine (Claus and Mojsov Citation2018). Cellulolytic and xylanolytic activities are rarely observed in yeasts and in this study only a small proportion of strains exhibited the ability to degrade carboxymethylcellulose (8.33%), i.e. K. apiculata, K. javanica and P. membranifaciens, and xylan (16.66%) – P. anomala and M. pulcherrima strains, thus confirming this fact. Similarly, Belda et al. (Citation2016) detected cellulase activity only in Aureobasidium pullulans strains, and Strauss et al. (Citation2001) detected xylanase activity only in 6 out of 245 yeasts of enological origin (C. stellata, C. oleophila, C. pulcherrima, C. pelliculosa and K. apiculata), while cellulase activity was found in 11 isolates (C. stellata, C. pulcherrima and K. apiculata). Among 17 Pichia/Wickerhamomyces enological isolates, 8 strains possessed xylanase activity (Madrigal et al. Citation2013). A large screening for extracellular hydrolases among yeasts isolated from the malting ecosystem revealed that among ascomycetous yeasts xylanase activity was found only in A. pullulans strains, while cellulase was detected in A. pullulans, Geotrichum silvicola and Exophiala dermatidis. Overall the ability to degrade complex polysaccharides was mostly restricted to basidiomycetes (Laitila et al. Citation2006). As the current study employed only ascomycetous yeasts the low incidence of cellulase and xylanase activity is not surprising.
Pectinolytic enzymes can play role in the degradation of polysaccharides of the plant cell wall of the grape skin and pulp improving clarification of wine and releasing aromatic and pigmented compounds (Claus and Mojsov Citation2018). None of the studied yeast strains possessed pectinolytic activity. Pectinolytic activity is rarely found in yeasts, especially in ascomycetes as used in this study. Out of 48 yeasts isolated from grapes only 11 belonging to A. pullulans and basidiomycetous yeasts were pectinolytic (Merín et al. Citation2015). None of the 22 wine yeasts belonging to Candida, Debaryomyces, Hanseniaspora, Hansenula, Kloeckera, Metschnikowia, Pichia, Saccharomyces and Torulaspora exhibited pectinase activity (Charoenchai et al. Citation1997).
All the yeast strains possessed at least one extracellular activity of enological interest, while the majority of yeasts exhibited two or more hydrolytic activities (). The most frequent combination of enzymatic activities was lipolytic + proteolytic activities and was found in 10 yeast strains (27.7%) of P. membranifaciens, C. valida, C. lambica, and T. delbrueckii. 15 yeast strains possessed 3 or 4 hydrolytic activities, 3 out of 5 P. anomala strains exhibiting lipase, esterase, xylanase, and β-glucosidase activity. Similar findings were reported by Fernández et al. (Citation2000) and Belda et al. (Citation2016) in large screening studies of non-conventional yeasts of enological origin, although the prevalence of other enzymatic activities is reported. A wide range of extracellular hydrolases was demonstrated by Pichia/Wickerhamomyces enological isolates, including β-glucosidase, protease, esterase, pectinase and xylanase (Madrigal et al. Citation2013).
Table 2. Distribution of enzymatic activities among different species of yeasts of enological and brewery origin
Yeast strains were assessed for the ability to withstand several stress factors. The ability to tolerate ethanol at 6–16% (v/v) at pH 6.0 and 3.5 (to imitate conditions of alcoholic fermentation) was studied on YPD agar plates. The ability of yeast strains to grow at pH 3.5 was tested and, all the yeasts grew well at low pH (data not shown). The current study showed some variability in ethanol tolerance in different non-conventional yeast species. All the yeast strains could tolerate ethanol at 6% at pH 6.0 and all but one at pH 3.5 (). 25 out of 36 strains in this study managed to survive at ethanol concentrations typically found by the end of alcoholic fermentation (12–16%) at pH 6.0; however, at pH 3.5 this number decreased to 22 strains, mostly Pichia and T. delbrueckii strains. K. apiculata, K. javanica, and C. vini strains were the least tolerant to ethanol, while the most tolerant strain was Z. bisporus Y-2730 that tolerated 16% ethanol at pH 6.0 and 3.5.
Table 3. Ethanol tolerance of non-conventional yeast strains
Many non-conventional wine yeasts lack the ability to withstand high ethanol levels that allows S. cerevisiae strains to survive and dominate during grape fermentation (Fleet Citation2003). A large study of phenotypic diversity among non-Saccharomyces yeasts demonstrated high ethanol tolerance of T. delbrueckii, W. anomalus and Z. bailii species (Mukherjee et al. Citation2017), which is in agreement with the results reported in this study. W. anomalus is well known as a ubiquitous yeast species able to survive under a wide range of extreme environmental conditions including pH, temperature and osmotic stress (Mukherjee et al. Citation2017).
Important criteria for potential starter cultures for winemaking also include tolerance to osmotic stress, oxidative stress and copper sulfate that is often used to fight against fungal diseases of vine. Tolerance to oxidative stress was tested by the addition of different concentrations of hydrogen peroxide and was mostly species-dependent (). A single strain of M. pulcherrima was the most tolerant among 36 strains used in this study. Similar findings regarding M. pulcherrima tolerance to hydrogen peroxide are reported by Grazia et al. (Citation2019) and Mestre Furlani et al. (Citation2017). P. anomala and Kloeckera strains also exhibited high tolerance to peroxide stress in this study, which is in agreement with the data reported by Mestre Furlani et al. (Citation2017).
Table 4. Tolerance of non-conventional yeast strains to hydrogen peroxide
All P. anomala and T. delbrueckii strains and a single strain of M. pulcherrima were tolerant to osmotic stress caused by 50% glucose (). This is consistent with the findings in the study by Mukherjee et al. (Citation2017) that indicated the high osmotic tolerance in these species. Seventeen out of 36 yeast strains (P. membranifaciens and several strains of Candida and Zygosaccharomyces) used in the current study lacked the ability to grow at 50% glucose, which is similar to the results obtained by Grazia et al. (Citation2019). These authors reported that 13 out of 29 non-Saccharomyces wine yeasts could not grow in the medium containing 40% glucose.
Table 5. Enological traits of non-conventional yeast strains
Copper-containing chemicals are widely used as traditional fungicides in agriculture and can be toxic to yeasts resulting in the inhibition of growth and activity during alcoholic fermentation (Capece et al. Citation2018). Consequently, tolerance to copper would be a valuable characteristic for yeasts used in fermentation. Most yeast strains used in this study were tolerant to copper sulfate at 200 and 400 µM at pH 6.0 and 3.5, while T. delbrueckii, C. vini and Z. bisporus strains lacked tolerance to copper (). Grazia et al. (Citation2019) reported lower levels of copper tolerance in non-conventional yeasts of enological origin, none of the isolates tolerated CuSO4 above 300 µM. However, most M. pulcherrima strains isolated from wineries tolerated up to 2 mM copper (Barbosa et al. Citation2018) and high resistance to copper up to 10 mM was reported for Starmerella bacillaris and Metschnikowia yeasts isolated from high-sugar habitats (Binati et al. Citation2019). The low copper tolerance of T. delbrueckii strains demonstrated in this study is in accordance with data reported by Gava et al. (Citation2016).
Killer activity in wine yeasts could be a beneficial trait helping starter cultures to inhibit the undesirable fungal microbiota in must (Zagorc et al. Citation2001). Several studies have focused on the killer potential of non-Saccharomyces yeasts as a way to prevent unwanted yeast growth (Yap et al. Citation2000; Comitini et al. Citation2011). However, none among 36 yeast strains exhibited a pronounced killer activity against two killer-sensitive strains S. cerevisiae and K. marxianus (). Weak killer activity was detected in several yeasts, mostly P. anomala strains. Pichia/Wickerhamomyces anomalus strains of enological origin were reported to possess killer activity in several studies (Yap et al. Citation2000; Zagorc et al. Citation2001; Sangorrín et al. Citation2008).
Wine yeasts should lack the ability to produce high quantities of organic acids, including acetic, low levels of volatile organic acids are especially important during the production of botrytized wines (Magyar and Tóth Citation2011). Thirteen out of 36 yeast strains produced a visible zone of solubilisation on carbonate agar, which included all P. anomala strains, 2 strains of C. valida and P. membranifaciens, 1 strain of T. delbrueckii, K. javanica, C. vini, and Z. bisporus (). Low incidence of organic acid production by wine yeasts was also reported in other studies (Suárez Valles et al. Citation2008; Di Maio et al. Citation2012). However, the production of organic acids by several yeast strains in this study could be, in turn, mitigated by the ability of these strains to utilize organic acids (malic and acetic). Most P. membranifaciens strains and all C. valida and P. anomala strains could assimilate malic and acetic acids as a sole source of carbon.
Some non-Saccharomyces wine yeasts are known to produce undesirable compounds such as biogenic amines that are at high levels toxic for humans (Ivit and Kemp Citation2018). Weak ability to produce biogenic amines from a mixture of seven amino acids was detected in six out of 36 strains – C. lambica, C. vini and P. anomala (). Non-conventional wine isolates as well as wine yeasts S. cerevisiae are capable of producing biogenic amines during wine production (Guo et al. Citation2015). The quantitative determination of main biogenic amines (histamine, tyramine, putrescine, cadaverine and phenylethylamine) generated during fermentation by the selected yeast strain or strains would be required in order to guarantee the safety of the final product.
The ability of non-conventional yeasts to carry out fermentation at low pH was mostly species-dependent (), as all T. delbrueckii, Zygosaccharomyces and P. anomala strains displayed strong fermentative activity in the preliminary screening, while all C. valida and C. vini strains were non-fermenting. Many non-conventional yeasts are weakly fermentative or lack the ability to ferment sugars altogether (Padilla et al. Citation2016). Similarly, comparatively high fermentation power was detected in T. delbrueckii strains by Comitini et al. (Citation2011) and Vigentini et al. (Citation2016), and in Zygosaccharomyces yeasts by Domizio et al. (Citation2011), while weak fermentative power was demonstrated by Candida yeasts (Comitini et al. Citation2011). Interestingly, in the preliminary screening, we observed high fermentative activity in P. anomala strains, although Pichia yeasts are usually regarded as weakly fermentative (Padilla et al. Citation2018).
Seven non-conventional yeast strains P. anomala Y-212, Y-213, Y-215, Y-216, Y-217, Z. fermentati Y-658 and K. javanica Y-2693 were further characterised in microfermentation experiments as they possessed tolerance to ethanol, osmotic and peroxide stress, extracellular hydrolases of enological value (β-glucosidase, protease, esterase, xylanase) and high fermentative activity in the preliminary screenings. Strain M. pulcherrima was excluded from microfermentation trials due its poor ethanol tolerance at low pH, while T. delbrueckii and Zygosaccharomyces strains mostly did not display extracellular hydrolases of enological value. All tested strains produced comparatively low levels of CO2 (1.17–2.63 g per 100 ml after 16 days of fermentation) (). The highest fermentative activity in semi-synthetic media was demonstrated by P. anomala Y-216 (2.63 ± 0.01 g of CO2, p < 0.05) that corresponds to 3.29% (v/v) ethanol in the medium (Vaughan-Martini and Martini Citation1998).
Figure 2. CO2 production by non-conventional yeasts in semi-synthetic medium. Results are presented as means of triplicates with the corresponding standard deviation (± SD)
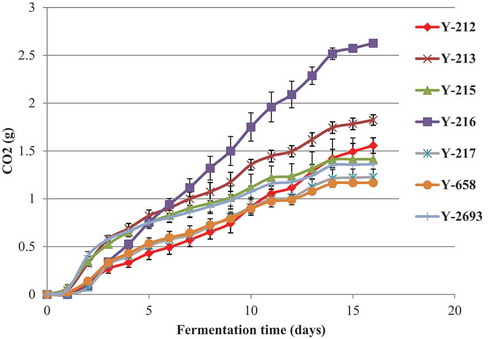
As the obtained data indicate that the selected yeast strains lack the ability to complete fermentation on their own, the use of these yeasts in co-culture with S. cerevisiae strain in the mixed wine fermentation would be advisable. As yeast strain P. anomala Y-216 displayed high tolerance to ethanol, copper sulfate, osmotic and peroxide stress, strong lipase, esterase, xylanase, and β-glucosidase activities and also the highest fermentative activity among the tested yeasts, it was selected in this preliminary screening work as a potential co-culture to use alongside S. cerevisiae in wine fermentation.
Conclusion
S. cerevisiae yeasts are the principal performers of alcoholic fermentation during wine production, but are not usually considered as strong producers of many extracellular hydrolases, including proteases, lipases, esterases, cellulases and some others. This study revealed that non-conventional yeasts of enological origin could potentially be exploited as producers of several extracellular hydrolases. P. anomala strains were especially promising in this aspect exhibiting strong lipase, esterase, xylanase and β-glucosidase activities and M. pulcherrima as a producer of lipase, protease and β-glucosidase. Several P. anomala strains demonstrated valuable enological traits, such as a wide range of enzymatic activities, high stress tolerance, killer activity, and utilization of organic acids. However, due to their limited fermentative efficiency, the use of mixed cultures with highly fermentative S. cerevisiae yeasts would be more preferable. In conclusion, this study represents the first step for selecting a non-conventional yeast strain of enological origin as a potential co-culture for winemaking.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aslankoohi E, Herrera-Malaver B, Rezaei MN, Steensels J, Courtin CM, Verstrepen KJ. 2016. Non-conventional yeast strains increase the aroma complexity of bread. PLoS ONE. 11(10):e0165126. doi:https://doi.org/10.1371/journal.pone.0165126.
- Barata A, Malfeito-Ferreira M, Loureiro V. 2012. The microbial ecology of wine grape berries. Int J Food Microbiol. 153(3):243–259. doi:https://doi.org/10.1016/j.ijfoodmicro.2011.11.025.
- Barbosa C, Lage P, Esteves M, Chambel L, Mendes-Faia A, Mendes-Ferreira A. 2018. Molecular and phenotypic characterization of Metschnikowia pulcherrima strains from Douro wine region. Fermentation. 4(1):8. doi:https://doi.org/10.3390/fermentation4010008.
- Belda I, Ruiz J, Alastruey-Izquierdo A, Navascués E, Marquina D, Santos A. 2016. Unraveling the enzymatic basis of wine “flavorome”: aphylo-functional study of wine related yeast species. Front Microbiol. 7:12. doi:https://doi.org/10.3389/fmicb.2016.00012.
- Binati RL, Innocente G, Gatto V, Celebrin A, Polo M, Felis GE, Torriani S. 2019. Exploring the diversity of a collection of native non-Saccharomyces yeasts to develop co-starter cultures for winemaking. Food Res Int. 122:432–442. doi:https://doi.org/10.1016/j.foodres.2019.04.043.
- Brizzio S, Turchetti B, de García V, Libkind D, Buzzini P, van Broock M. 2007. Extracellular enzymatic activities of basidiomycetous yeasts isolated from glacial and subglacial waters of northwest Patagonia (Argentina). Can J Microbiol. 53(4):519–525. doi:https://doi.org/10.1139/W07-010.
- Buzzini P, Martini A. 2002. Extracellular enzymatic activity profiles in yeast and yeast-like strains isolated from tropical environments. J Appl Microbiol. 93(6):1020–1025. doi:https://doi.org/10.1046/j.1365-2672.2002.01783.x.
- Capece A, Romaniello R, Scrano L, Siesto G, Romano P. 2018. Yeast starter as a biotechnological tool for reducing copper content in wine. Front Microbiol. 8:2632. doi:https://doi.org/10.3389/fmicb.2017.02632.
- Charoenchai C, Fleet GH, Henschke PA, Todd BENT. 1997. Screening of non-Saccharomyces wine yeasts for the presence of extracellular hydrolytic enzymes. Aust J Grape Wine Res. 3(1):2–8. doi:https://doi.org/10.1111/j.1755-0238.1997.tb00109.x.
- Ciani M, Morales P, Comitini F, Tronchoni J, Canonico L, Curiel JA, Oro L, Rodrigues AJ, Gonzalez R. 2016. Non-conventional yeast species for lowering ethanol content of wines. Front Microbiol. 7:642. doi:https://doi.org/10.3389/fmicb.2016.00642.
- Claus H, Mojsov K. 2018. Enzymes for wine fermentation: current and perspective applications. Fermentation. 4(3):52. doi:https://doi.org/10.3390/fermentation4030052.
- Comitini F, Gobbi M, Domizio P, Romani C, Lencioni L, Mannazzu I, Ciani M. 2011. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 28(5):873–882. doi:https://doi.org/10.1016/j.fm.2010.12.001.
- Contreras A, Hidalgo C, Henschke PA, Chambers PJ, Curtin C, Varela C. 2014. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl Environ Microbiol. 80(5):1670–1678. doi:https://doi.org/10.1128/AEM.03780-13.
- Di Maio S, Polizzotto G, Di Gangi E, Foresta G, Genna G, Verzera A, Scacco A, Amore G, Oliva D. 2012. Biodiversity of indigenous Saccharomyces populations from old wineries of south-eastern Sicily (Italy): preservation and economic potential. PLoS ONE. 7(2):e30428. doi:https://doi.org/10.1371/journal.pone.0030428.
- Domizio P, Romani C, Lencioni L, Comitini F, Gobbi M, Mannazzu I, Ciani M. 2011. Outlining a future for non-Saccharomyces yeasts: selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int J Food Microbiol. 147(3):170–180. doi:https://doi.org/10.1016/j.ijfoodmicro.2011.03.020.
- Escribano R, González-Arenzana L, Garijo P, Berlanas C, López-Alfaro I, López R, Gutiérrez AR, Santamaría P. 2017. Screening of enzymatic activities within different enological non-Saccharomyces yeasts. J Food Sci Technol. 54(6):1555–1564. doi:https://doi.org/10.1007/s13197-017-2587-7.
- Esteves M, Barbosa C, Vasconcelos I, Tavares MJ, Mendes-Faia A, Pereira Mira N, Mendes-Ferreira A. 2019. Characterizing the potential of the non-conventional yeast Saccharomycodes ludwigii UTAD17 in winemaking. Microorganisms. 7(11):478. doi:https://doi.org/10.3390/microorganisms7110478.
- Fernández M, Ubeda JF, Briones AI. 2000. Typing of non-Saccharomyces yeasts with enzymatic activities of interest in wine-making. Int J Food Microbiol. 59(1–2):29–36. doi:https://doi.org/10.1016/S0168-1605(00)00283-X.
- Fleet GH. 2003. Yeast interactions and wine flavour. Int J Food Microbiol. 86(1–2):11–22. doi:https://doi.org/10.1016/S0168-1605(03)00245-9.
- Gava A, Ficagna E, Rossato SB, Cisilotto B, Miotto SPS, Gabbi HT. 2016. Change in kinetic parameters of commercial yeast in the presence of copper fungicides. BIO Web Conf. 7:02029. doi:https://doi.org/10.1051/bioconf/20160702029.
- Grazia A, Pietrafesa A, Capece A, Pietrafesa R, Siesto G, Romano P. 2019. Exploitation of technological variability among wild non-Saccharomyces yeasts to select mixed starters for the production of low alcohol wines. BIO Web Conf. 15:02031. doi:https://doi.org/10.1051/bioconf/20191502031.
- Guo -Y-Y, Yang Y-P, Peng Q, Han Y. 2015. Biogenic amines in wine: a review. Int J Food Sci Technol. 50(7):1523–1532. doi:https://doi.org/10.1111/ijfs.12833.
- Ivit NN, Kemp B. 2018. The impact of non-Saccharomyces yeast on traditional method sparkling wine. Fermentation. 4(3):73. doi:https://doi.org/10.3390/fermentation4030073.
- Izquierdo Cañas PM, García-Romero E, Heras Manso JM, Fernández-González M. 2014. Influence of sequential inoculation of Wickerhamomyces anomalus and Saccharomyces cerevisiae in the quality of red wines. Eur Food Res Technol. 239(2):279–286. doi:https://doi.org/10.1007/s00217-014-2220-1.
- Jolly NP, Varela C, Pretorius IS. 2014. Not your ordinary yeast: non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 14(2):215–237.
- Kurtzman CP, Fell JW, Boekhout T, Robert V. 2011. Methods for isolation, phenotypic characterization and maintenance of yeasts. In: Kurtzman CP, Fell JW, Boekhout T, editors. The yeasts: a taxonomic study. 5th ed. Vol. 1. Amsterdam: Elsevier; p. 87–110.
- Laitila A, Wilhelmson A, Kotaviita E, Olkku J, Home S, Juvonen R. 2006. Yeasts in an industrial malting ecosystem. J Ind Microbiol Biotechnol. 33(11):953–966. doi:https://doi.org/10.1007/s10295-006-0150-z.
- Lopes CA, Rodríguez ME, Sangorrín M, Querol A, Caballero AC. 2007. Patagonian wines: the selection of an indigenous yeast starter. J Ind Microbiol Biotechnol. 34(8):539–546. doi:https://doi.org/10.1007/s10295-007-0227-3.
- Madrigal T, Maicas S, Tolosa JJM. 2013. Glucose and ethanol tolerant enzymes produced by Pichia (Wickerhamomyces) isolates from enological ecosystems. Am J Enol Vitic. 64(1):126–133. doi:https://doi.org/10.5344/ajev.2012.12077.
- Magyar I, Tóth T. 2011. Comparative evaluation of some oenological properties in wine strains of Candida stellata, Candida zemplinina, Saccharomyces uvarum and Saccharomyces cerevisiae. Food Microbiol. 28(1):94–100. doi:https://doi.org/10.1016/j.fm.2010.08.011.
- Martinez A, Cavello I, Garmendia G, Rufo C, Cavalitto S, Vero S. 2016. Yeasts from sub-Antarctic region: biodiversity, enzymatic activities and their potential as oleaginous microorganisms. Extremophiles. 20(5):759–769. doi:https://doi.org/10.1007/s00792-016-0865-3.
- Mautone JN, Landell MF, Fuentefria AM, Valente P. 2010. Phylloplane yeasts as a source of industrially interesting enzymes. R Bras Biosci. 8(2):169–173.
- Merín MG, Martín MC, Rantsiou K, Cocolin L, de Ambrosini VIM. 2015. Characterization of pectinase activity for enology from yeasts occurring in Argentine Bonarda grape. Braz J Microbiol. 46(3):815–823. doi:https://doi.org/10.1590/S1517-838246320140160.
- Mestre Furlani MV, Maturano YP, Combina M, Mercado LA, Toro ME, Vazquez F. 2017. Selection of non-Saccharomyces yeasts to be used in grape musts with high alcoholic potential: a strategy to obtain wines with reduced ethanol content. FEMS Yeast Res. 17(2). doi:https://doi.org/10.1093/femsyr/fox010.
- Mukherjee V, Radecka D, Aerts G, Verstrepen KJ, Lievens B, Thevelein JM. 2017. Phenotypic landscape of non-conventional yeast species for different stress tolerance traits desirable in bioethanol fermentation. Biotechnol Biofuels. 10:216.
- Padilla B, Gil JV, Manzanares P. 2016. Past and future of non-Saccharomyces yeasts: from spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front Microbiol. 7:441. doi:https://doi.org/10.3389/fmicb.2016.00411.
- Padilla B, Gil JV, Manzanares P. 2018. Challenges of the non-conventional yeast Wickerhamomyces anomalus in winemaking. Fermentation. 4(3):68.
- Raymond Eder ML, Reynoso C, Lauret SC, Rosa AL. 2017. Isolation and identification of the indigenous yeast population during spontaneous fermentation of Isabella (Vitis labrusca L.) grape must. Front Microbiol. 8:532. doi:https://doi.org/10.3389/fmicb.2017.00532.
- Sangorrín MP, Lopes CA, Jofré V, Querol A, Caballero AC. 2008. Spoilage yeasts from Patagonian cellars: characterization and potential biocontrol based on killer interactions. World J Microbiol Biotechnol. 24(7):945–953. doi:https://doi.org/10.1007/s11274-007-9557-6.
- Strauss ML, Jolly NP, Lambrechts MG, van Rensburg P. 2001. Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. J Appl Microbiol. 91(1):182–190. doi:https://doi.org/10.1046/j.1365-2672.2001.01379.x.
- Suárez Valles B, Pando Bedriñana R, Lastra Queipo A, Mangas Alonso JJ. 2008. Screening of cider yeasts for sparkling cider production (Champenoise method). Food Microbiol. 25(5):690–697. doi:https://doi.org/10.1016/j.fm.2008.03.004.
- Šuranská H, Vránová D, Omelková J. 2016. Isolation, identification and characterization of regional indigenous Saccharomyces cerevisiae strains. Braz J Microbiol. 47(1):181–190. doi:https://doi.org/10.1016/j.bjm.2015.11.010.
- Tataridis P, Kanellis A, Logothetis S, Nerantzis E. 2013. Use of non-Saccharomyces Torulaspora delbrueckii yeast strains in winemaking and brewing. Jour Nat Sci, Matica Srpska Novi Sad. 124:415–426.
- Varela C, Borneman AR. 2017. Yeasts found in vineyards and wineries. Yeast. 34(3):111–128.
- Vaughan-Martini A, Martini A. 1998. Determination of ethanol production. In: Kurtzman CP, Fell JW, editors. The yeasts. 4th ed. Amsterdam: Elsevier; p. 107.
- Viana F, Gil JV, Genovés S, Vallés S, Manzanares P. 2008. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 25(6):778–785. doi:https://doi.org/10.1016/j.fm.2008.04.015.
- Vigentini I, Maghradze D, Petrozziello M, Bonello F, Mezzapelle V, Valdetara F, Failla O, Foschino R. 2016. Indigenous georgian wine-associated yeasts and grape cultivars to edit the wine quality in a precision oenology perspective. Front Microbiol. 7:352.
- Vilela A. 2019. Use of nonconventional yeasts for modulating wine acidity. Fermentation. 5(1):27. doi:https://doi.org/10.3390/fermentation5010027.
- Yap NA, de Barros Lopes M, Langridge P, Henschke PA. 2000. The incidence of killer activity of non-Saccharomyces yeasts towards indigenous yeast species of grape must: potential application in wine fermentation. J Appl Microbiol. 89(3):381–389. doi:https://doi.org/10.1046/j.1365-2672.2000.01124.x.
- Zagorc T, Maráz A, Cadez N, Jemec KP, Péter G, Resnik M, Nemanič J, Raspor P. 2001. Indigenous wine killer yeasts and their application as a starter culture in wine fermentation. Food Microbiol. 18(4):441–451. doi:https://doi.org/10.1006/fmic.2001.0422.
